Summary
Humans easily and flexibly complete a wide variety of tasks. To accomplish this feat, the brain appears to subtly adjust stable brain networks. Here we investigate what regional factors underlie these modifications, asking whether networks are altered at (a) regions activated by a given task, or (b) hubs that interconnect different networks. We used fMRI ‘functional connectivity’ (FC) to compare networks during rest and three distinct tasks requiring semantic judgments, mental rotation, and visual coherence. We found that network modifications during these tasks were independently associated with both regional activation and network hubs. Furthermore, active and hub regions were associated with distinct patterns of network modification (differing in their localization, topography of FC changes, and variability across tasks), with activated hubs exhibiting patterns consistent with task control. These findings indicate that task goals modify brain networks through two separate processes, linked to local brain function and network hubs.
Keywords: fMRI, brain networks, graph theory, task control
Graphical abstract
Introduction
Humans can easily and flexibly complete many different tasks depending on their goals. This ability depends on both specialized processing occurring in individual brain regions and coordinated interactions across distributed regions organized into large-scale networks (also called brain systems). A fundamental question of cognitive neuroscience is how specialized brain regions are able to flexibly link together to perform different tasks.
Using functional Magnetic Resonance Imaging (fMRI), functional networks can be identified even when individuals lie quietly without any explicit task in a “resting state”, based on patterns of correlated fMRI signal between brain regions (i.e., functional connectivity, or FC (Biswal et al., 1995; Power et al., 2011; Yeo et al., 2011)). Recent studies have highlighted the consistent organization of functional networks at rest and during varied tasks (Betti et al., 2013; Cole et al., 2014; Krienen et al., 2014), suggesting that the brain’s large-scale networks are dominated by a fundamentally stable intrinsic backbone (Cole et al., 2014).
However, diverse behavioral states appear to be supported by smaller-scale changes that subtly modify brain networks (Cole et al., 2014; Krienen et al., 2014). Although the magnitude of these changes is small, it is possible to accurately decode the task state of a participant simply from their FC in a task (Alnaes et al., 2015; Gonzalez-Castillo et al., 2015; Shirer et al., 2012). In addition, task performance is related to these modifications of FC (e.g., (Dwyer et al., 2014; Gonzalez-Castillo et al., 2015; Gordon et al., 2014; Hampson et al., 2010; Kelly et al., 2008)), suggesting that the alterations are relevant to behavior.
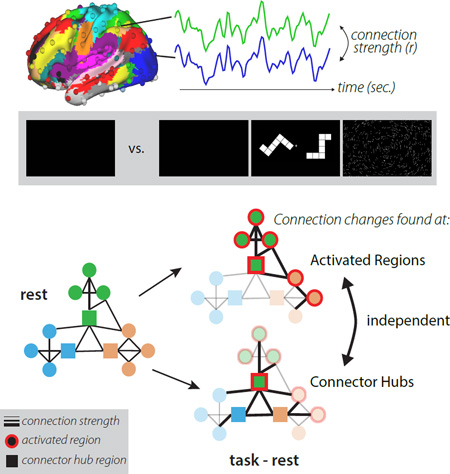
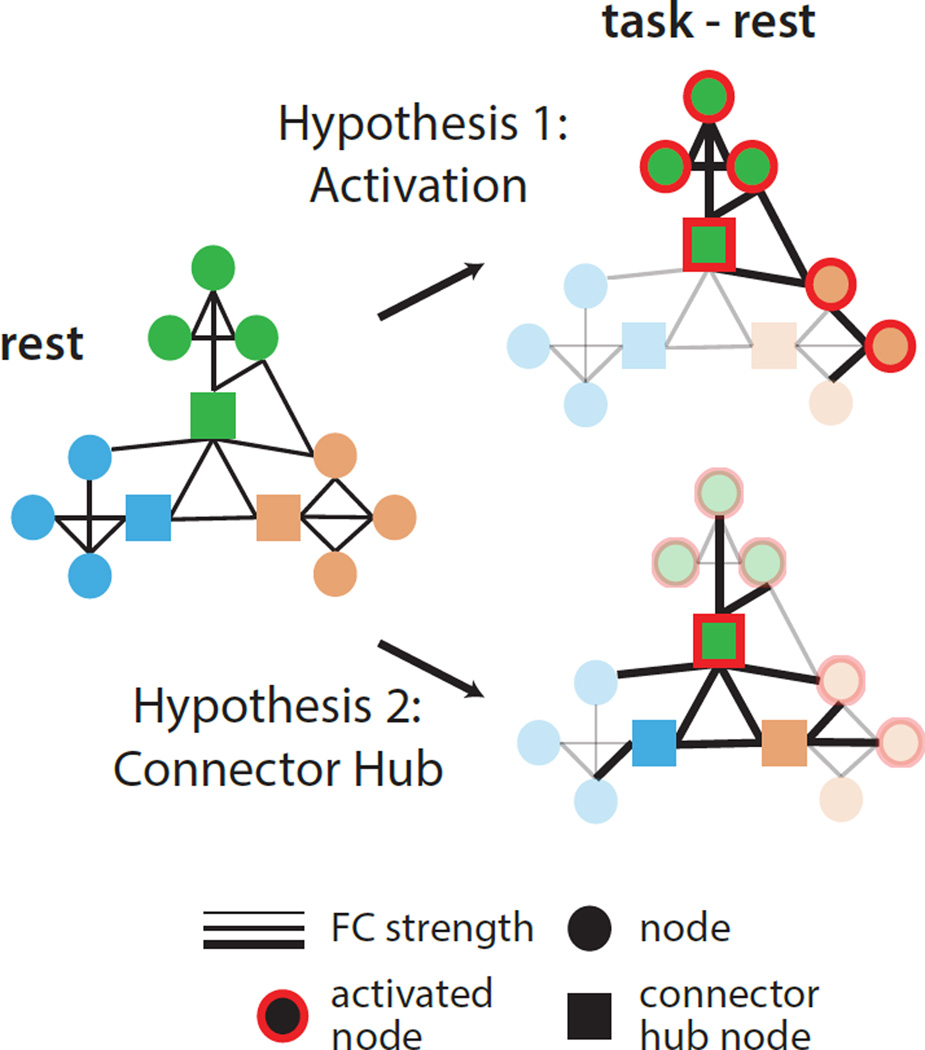
Despite this evidence, previous studies have failed to provide an explanation for where and why network interactions vary during the engagement of a task. Here, we examine two hypotheses inspired by largely distinct literatures on localized and distributed processing: (Figure 1) do networks change 1) because regions are activated in a task or, 2) because of inherent properties of the network’s organization?
Figure 1. Proposed factors contributing to task FC.
Intrinsic network interactions (right) may be modified to accomplish task goals by changing connectivity between regions activated by a task (Hypothesis 1; activated regions shown with red outlines) or by changing connectivity patterns of specialized hub regions (Hypothesis 2; squares) that help connect networks to each other. Regions and connections without changes are faded in the bottom panel to emphasize differences.
The first possibility has motivated a host of studies examining functional connectivity changes among small sets of functionally “relevant” (or task-activated) regions. The logic is that regions specialized for individual cognitive operations are both simultaneously activated and need to interact to complete a complex task. For example, in visual attention tasks, changes in FC have been recorded between activated visual regions and activated attentional-control regions (e.g., (Gazzaley et al., 2007; Spadone et al., 2015)), presumably reflecting the need for control regions to communicate with visual regions. This view proposes that interactions among brain regions are altered in different contexts primarily due to the specialized functions of the individual brain regions.
An alternative view proposes that network interactions are guided by the topological structure of brain networks (Sporns, 2011). In this complex systems perspective, brain network properties are determined from the pattern of edges (here, FC) among nodes (brain regions), modeled as a graph, rather than by studying local processing characteristics of brain regions. In the brain graph, specialized connector hub locations are defined by having connections distributed across multiple different networks (Guimera and Amaral, 2005). These regions are seen as critical for coordinating interactions, and thereby information processing, across brain networks (Power et al., 2013). Therefore strong changes in network interactions are predicted to occur at connector hubs. This view is supported by evidence that (a) brain lesions to connector hubs cause widespread disruptions in network organization (Gratton et al., 2012) and behavior (Warren et al., 2014); and (b) connector hubs in the frontoparietal network show malleable connectivity across tasks (Cole et al., 2013). These findings suggest a central role for connector hubs in network interactions, but leave unclear how hubs relate to regional specializations in task control and processing.
We directly contrast these two hypotheses by using fMRI to measure functional brain networks in healthy participants at rest and engaged in three varied tasks. We examined activated and connector hub regions to determine which property most strongly associated with task-related FC alterations.
Results
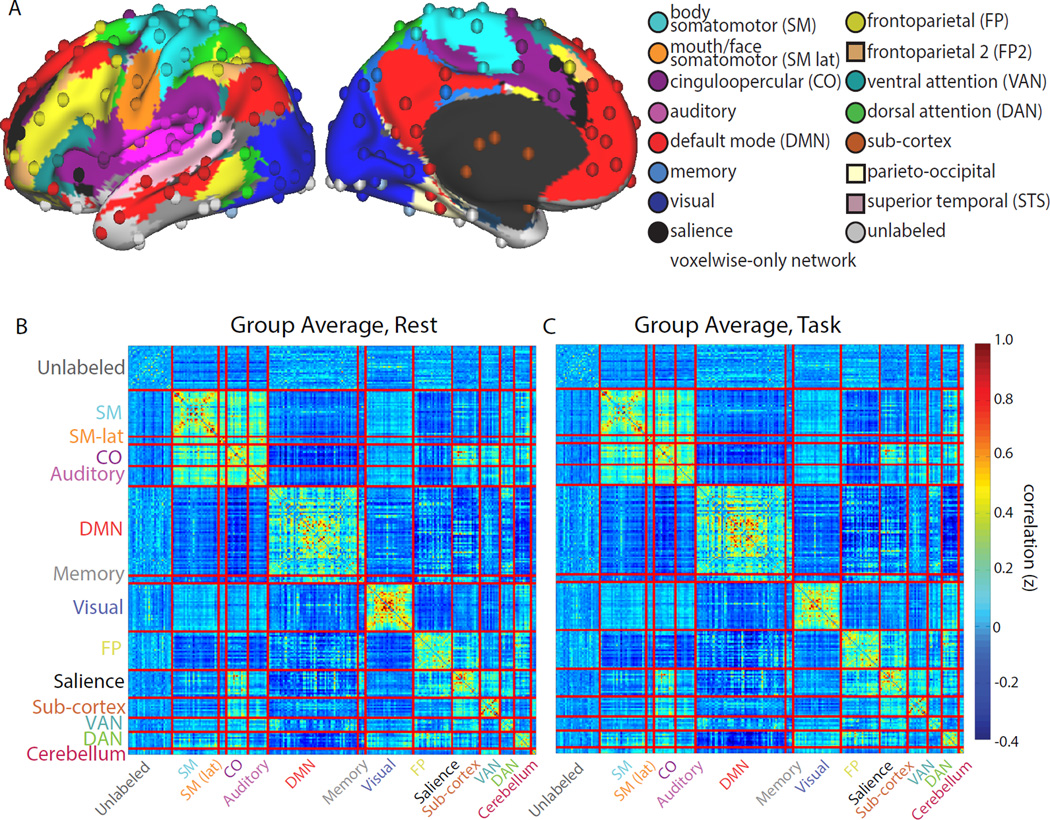
To examine how brain networks are altered during task and rest states, we analyzed fMRI data from 28 participants while they rested quietly or completed three tasks: a semantic task requiring a noun/verb judgment on a presented word, a mental rotation task requiring a same/mirror image judgment on two objects, and a coherence task requiring a judgment of whether dots were arranged concentrically. These tasks are especially well suited to our question, as they included a varied stimuli (including verbal and non-verbal stimuli), and they call upon widely varying cognitive processes (e.g., language, mental manipulation, and perceptual grouping), with differing demands on task control and perceptual resources ((Dubis et al., 2016), e.g., varying in behavioral performance and activation of control systems). We measured functional brain networks in each state by computing correlations across 264 regions arranged into 13 systems (Figure 2A).
Figure 2. A common FC organization is present during task and rest states.
(A) FC was calculated via time-series correlations among 264 cortical and subcortical regions of interest (spheres), distributed across 13 networks (Power et al., 2011) (colors; surface colors represent networks used for voxelwise analyses). FC during rest (B, left) and task (C, right) is very similar, dominated by a strong network structure with high correlations within each system (diagonal) compared to between systems (off-diagonals; similar results were seen for individual tasks, Supp. Fig. 1A).
Network organization is largely similar during task and rest
To evaluate the overall effect of task state on FC network organization, we computed the similarity between task and rest by measuring the correlation between the connectivity matrices in each condition. On average, large-scale networks were very similar between task and rest (Figure 2B,C; “task” data is concatenated across all tasks). The correlation between task and rest group average FC matrices was r = 0.95 (Mantel’s statistic: p<0.001; single subject matrices: r = 0.73, sd = 0.04). High correlations were also seen between rest and single tasks (semantic vs. rest: r = 0.94; mental rotation vs. rest: r = 0.92, coherence vs. rest: r = 0.94, all p<0.001; Supp. Fig. 1A).
Furthermore, network topology was very similar during rest and task states. Data-driven assignment of regions to network communities was substantially unchanged by task engagement and was similar to previously published findings (Power et al., 2011) (quantified with normalized mutual information (NMI): rest vs. task NMI=0.80; rest vs. Power-2011 networks NMI=0.73; task vs. Power-2011 NMI=0.73). In addition, we measured the similarity of connector hub locations across states, by calculating the participation coefficient (PC) metric (Guimera and Amaral, 2005) for each node. This metric measures the distribution of a region’s connections across different systems; regions with high PC are connector hubs. As with network organization, PC values during rest in our subjects were very similar to published findings from a large cohort (Power et al., 2013) (r = 0.88) and to PC during task (r = 0.90) suggesting that connector hub locations did not shift substantially during the performance of tasks. These findings indicate that the core intrinsic network organization is largely unchanged between rest and task states.
Subtle systematic FC differences exist between task and rest
Despite the overall similarity in large-scale network structure, smaller-magnitude differences were present between task and rest. We measured differences by directly contrasting the task and rest FC in a difference matrix (Figure 3A). Differences between the states were reliable: (1) p-value distributions showed a significant enrichment of edges p<0.05 compared with a permuted null distribution (p<0.001), and (2) many individual connections remained significant after FDR correction (Supp. Fig. 2).
Figure 3. Subtle, but reliable, FC differences were present during task and rest states.
Subtle but reliable differences were seen in the direct contrast of task and rest correlation matrices for 264 regions of interest (A) and on average for each voxel to other voxels within its own network (B, left) or voxels in other networks (B, right). FC changed within-system (along the diagonal, e.g., increases within the DMN, decreases within the visual and other sensory/motor systems; red and blue arrows in B) and between-systems (off-diagonal, e.g., increases between visual and subsets of control systems (e.g., CO, FP, DAN); pink and purple arrows in B). These effects were consistent for individual tasks (Supp. Fig. 1B).
FC differences were observed both within and between networks. During tasks, within-network FC decreased within the visual system and, to a lesser extent, in other sensory systems (SM, SM-lat, and auditory) as well as the subcortex. Increases in within-network FC during tasks were seen in the DMN and decreases were seen in the visual system (Fig. 3B, top). By contrast, between-network FC decreased in the DMN and increased for the visual system, as well as subsets of control systems (Fig. 3B, bottom). These gross characteristics were consistent for single tasks (Supp. Fig. 1B), despite their variable cognitive demands.
Activated regions alter FC, primarily between networks
Given the reliable changes in FC across participants and tasks, we asked whether changes were systematically related to the properties of individual regions. We examined two potential hypotheses (Figure 1): (1) FC is altered primarily for regions activated by a task or (2) FC is altered primarily for connector hub regions that mediate interactions across different systems (see Supp. Fig. 3 for activated and connector hub nodes).
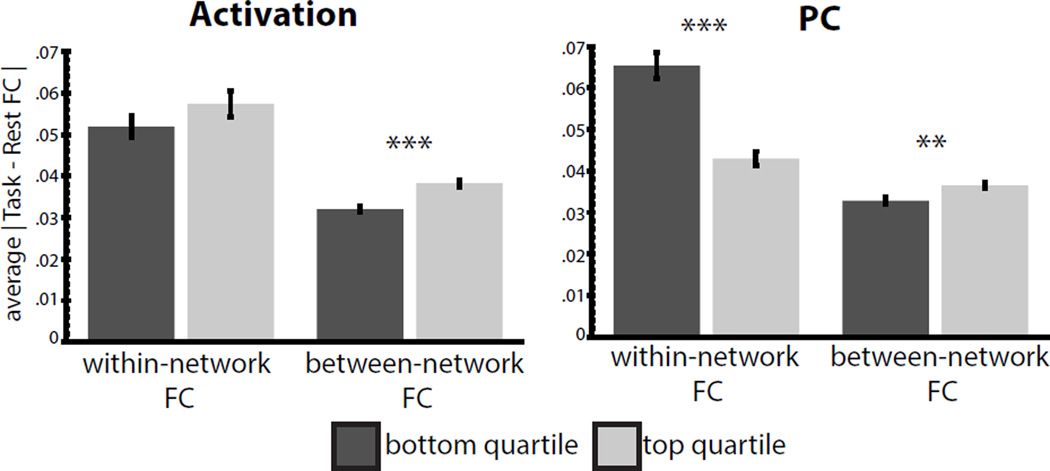
To test the first hypothesis, we examined if FC was altered more in activated regions – that is, those exhibiting large absolute percent signal changes during the task -- than non-activated regions. Note that FC was calculated after removing evoked activations from the task timeseries via regression to reduce spurious inflations of correlation measurements from co-activation as in (Al-Aidroos et al., 2012) (see Supp. Fig. 4 for activation results without task regression; as expected, these statistics were inflated compared with those reported below). We conducted a quartile analysis comparing the absolute changes in FC of regions, grouped based on their activation magnitude (top vs. bottom 25%). We found that FC changed significantly more for activated than non-activated regions (compared with permutation testing here and in following tests: p<0.001; individual tasks: all p[FDR]<0.01 corrected across tasks, Supp. Fig. 5A), providing evidence that the functional specialization of regions relates to their network modulations during tasks.
Next, we asked how activation-related changes in FC were distributed across within- or between-network connections. Compared with non-activated nodes, activated regions showed a greater absolute magnitude of between-network FC changes (Fig 4A, p<0.001; individual tasks: all p[FDR]<0.01, Supp. Fig. 5C), but only a numerical trend to change within their own network (p=n.s.).
Figure 4. FC modulations in activated regions and connector hubs.
Active (A) and connector hub nodes (B) show significantly enhanced modulations in between-network FC, but not within network FC – instead, connector hubs show lower changes in within-system FC than non-connectors nodes. Similar effects were seen for individual tasks (Supp. Fig. 5). ***p<0.001, **p<0.01, error bars represent standard error across ROIs.
Moreover, these findings were robust to variations in our analysis, as follows. We also found a significant linear relationship between activation and FC change if we treated the two measures as continuous variables rather than breaking them into quartile bins (Supp. Fig. 6, all FC: Spearman’s rho =0.37, p<0.001; between FC: rho=0.36, p<0.001, see also Supp Table 1), or if we used other binning thresholds (Supp Table 2). Adopting the Gordon (2016) parcellation or examining activation of the highest and lowest FC-change regions produced analogous results (all FC and between FC, p<0.001). These findings support our first hypothesis: FC of activated regions changes during a task. They also suggest that this effect primarily occurs for between-network connections.
Hubs show complex FC network modulations
Our second hypothesis (Fig. 1) proposes that task-related changes in FC will be seen at connector hubs. We found that the FC of connector hubs (top 25% of PC values) did not change significantly more than non-connectors (bottom 25% of PC values) on average across the brain (permuation p=n.s.). Interestingly, however, significant differences were observed if between- and within-network FC changes were considered separately. Compared to non-connectors, connector hubs showed significantly increased modulation of between-network FC (p<0.001), but significantly reduced modulation of within-network FC (p<0.001; Figure 4B). Individual tasks showed similar effects, with connector hubs exhibiting significantly higher between-system modulations in 2/3 tasks (p[FDR]<0.01 for Mental Rotation and Semantic tasks) and lower within-system FC in all 3 tasks (all p[FDR]<0.05; Supp. Fig. 5B,C). Thus, connector hubs exhibited complex modulation upon entering task states, with a relative suppression of FC changes within a network, but enhanced changes in between network FC.
Again, results were robust to variations in analysis. We found similar relationships if the measures were treated as continuous variables: PC showed a positive correlation with between-network FC change (Supp. Fig. 6, rho=0.25, p<0.001), and a negative correlation with within-network FC changes (rho=−0.34, p<0.001, see also Supp Table 1). Other binning thresholds produced similar results (Supp Table 2). Adopting the Gordon (2016) parcellation, using PC values computed from this dataset, or examining the PC of highest and lowest FC-change regions produced analogous results (all within and between-network comparisons, p<0.01). These findings support our second hypothesis, that FC changes during a task are related to connector hubs, but suggest that hubs differentially modulate different types of connections, showing relatively invariant connectivity within a system, while modulating connections between systems.
Activation and PC are separately related to FC
A linear regression analysis was used to assess the separable influences of activation and connector-hub status on task-state changes in FC, with terms for activation, participation coefficient, and the interaction of both properties (Supp Table 3; activation and PC were not themselves correlated, Spearman’s rho=0.08, p=0.23, Supp. Fig. 3C). As before, activation had a significant positive relationship with task-based changes in FC across the brain (p<0.001), in this case both within- (p<0.01) and between-networks (p<0.001). PC had a significant negative relationship with within-network FC changes (p<0.001) and a positive relationship with between-network FC changes (p<0.001). However, no significant interactions were seen (all p=n.s.). These findings indicate that PC and activation provide separable, additive, contributions to modulations of FC during tasks.
Node classes show distinct FC-attributes
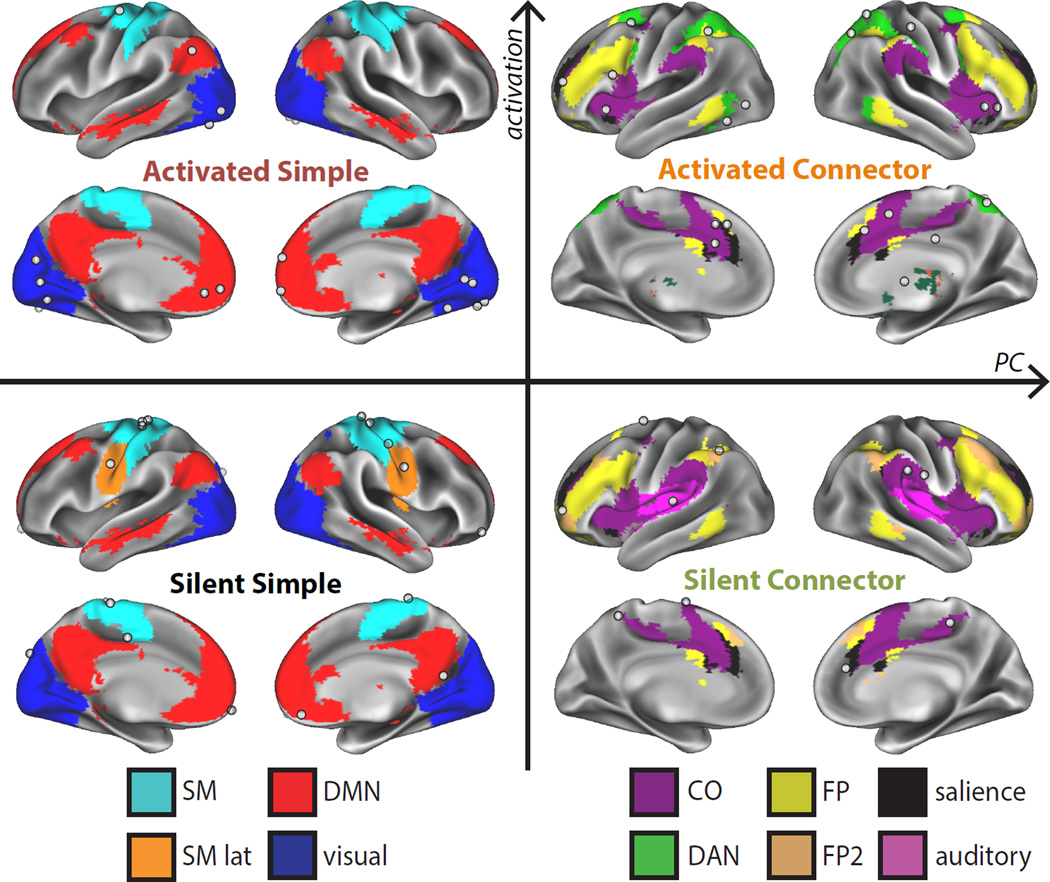
In order to characterize how activation and PC relate to task-control and processing, we identified four classes of nodes from the extremes of each distribution: (1) silent simple nodes (in the bottom 25% of both activation and PC), (2) activated connector nodes (top 25% of activation and PC), (3) activated simple nodes (top 25% activation and bottom 25% PC), and (4) silent connector nodes (top 25% PC and bottom 25% activation). Notably, activation and PC were continuously distributed, but the distributions had heavy tails, suggesting that extreme PC and activation values may exhibit specialized characteristics.
The four classes were found in distinct locations (Figure 5). Activated connectors (N=20) were primarily in top-down control systems, including the FP, DAN, CO, and salience systems. Silent connectors (N=9) were also found in control systems (CO, salience, FP, as well as auditory regions abutting the CO network), but in secondary locations, e.g., posterior insula portions of the CO network and rostral portions of the FP. The activated simple class (N=16) was associated with processing systems (visual, SM, DMN) that were relevant for the present tasks. Finally, the silent simple class (N=14) was also associated with processing systems (SM, SM-lat, and the DMN). The association of the four classes with different networks suggests that they may carry out different roles in task control and processing.
Figure 5. Regions stratified into classes by activated and connector hub characteristics.
Regions were stratified into 4 classes: silent simple (bottom 25% of both activation and PC), activated simple (top 25% activation, bottom 25% PC), silent connector (bottom 25% activation, top 25% PC), and activated connector (top 25% activation and PC) nodes. Node locations are shown as white spheres, overlaid on their systems (colors). Classes were associated with distinct systems.
Furthermore, we examined how classes varied across attributes – the FC change magnitude, topography, and variability across tasks– that would be expected to differ between regions involved in task control and processing. Specifically, regions that enact task control are predicted to show high between-system FC, especially with processing systems relevant for a given task, and flexibility in their patterns across tasks with different goals. Regions involved in basic task processing, instead, are predicted to show high FC-modulations with both control regions and regions within their own system. Additionally, they should show stereotyped FC patterns regardless of task context. We find that each class was associated with distinct FC-attributes, arguing that classes relate to distinct processes for modifying brain networks (summary in Fig. 6D).
Figure 6. Classes differ in the magnitude, topography, and flexibility of their FC patterns.
Node classes had different FC-related attributes. (A) They differed in the absolute magnitude of within and between network FC changes (measured via one-way ANOVA, ***p<0.001). (C) Classes differed in the topography of FC differences across networks, quantified via the FC task-rest difference for a class of regions (source) to each brain network (target; *p(FDR)<0.05; control = CO, Salience, FP, DAN, VAN; relevant processing = visual, SM; processing = SMlat, auditory). (B) Classes also differed in the flexibility of their topography across tasks, measured as the average correlation among FC difference maps for each class. These attributes, and the figures associated with each, are summarized in (D; absolute magnitudes of FC changes are shown with increasing +/− signs relative to silent simple nodes to denote increasingly large differences). Error bars represent standard error across ROIs.
Magnitudes of FC modulations
The four classes differed in the absolute magnitude of FC changes within (F(3,55)=9.67, p<0.001) and between (F(3,55)=10.55, p<0.001) each network (Fig. 6A; tested with a between-group ANOVA of the effect of class on FC change magnitude). Compared with the silent simple class, activated simple nodes had enhanced within- and between-network FC during tasks. Activated connectors, instead, had relatively smaller changes in within-network FC, but the highest levels of between-network FC changes. Finally, silent connector showed the most stable within-network FC, and only modest changes in between-network FC.
Topography of FC modulations
The four classes differed in the topography of their FC changes (measured as the average FC between regions in each class and target systems after removing values <20mm from a seed; Fig. 6C). During the task, the activated connector class had greater FC with control systems and processing regions relevant to the task (i.e., visual and SM regions used for stimulus input and motor output in the tasks), but decreased FC with the DMN. By contrast, the activated simple class exhibited two major patterns: visual regions had decreased task FC with other visual regions and increased FC with control systems, while the DMN regions exhibited increased FC within their own network, but decreased connectivity with control regions. Silent connectors, on average, were not modulated across the major groups of networks (see Fig. 7 for details on a subset). Finally, the silent simple class had decreased FC with processing regions but few increases (see Supp. Fig. 7 for maps and quantification of the dominant patterns exhibited by regions within each class).
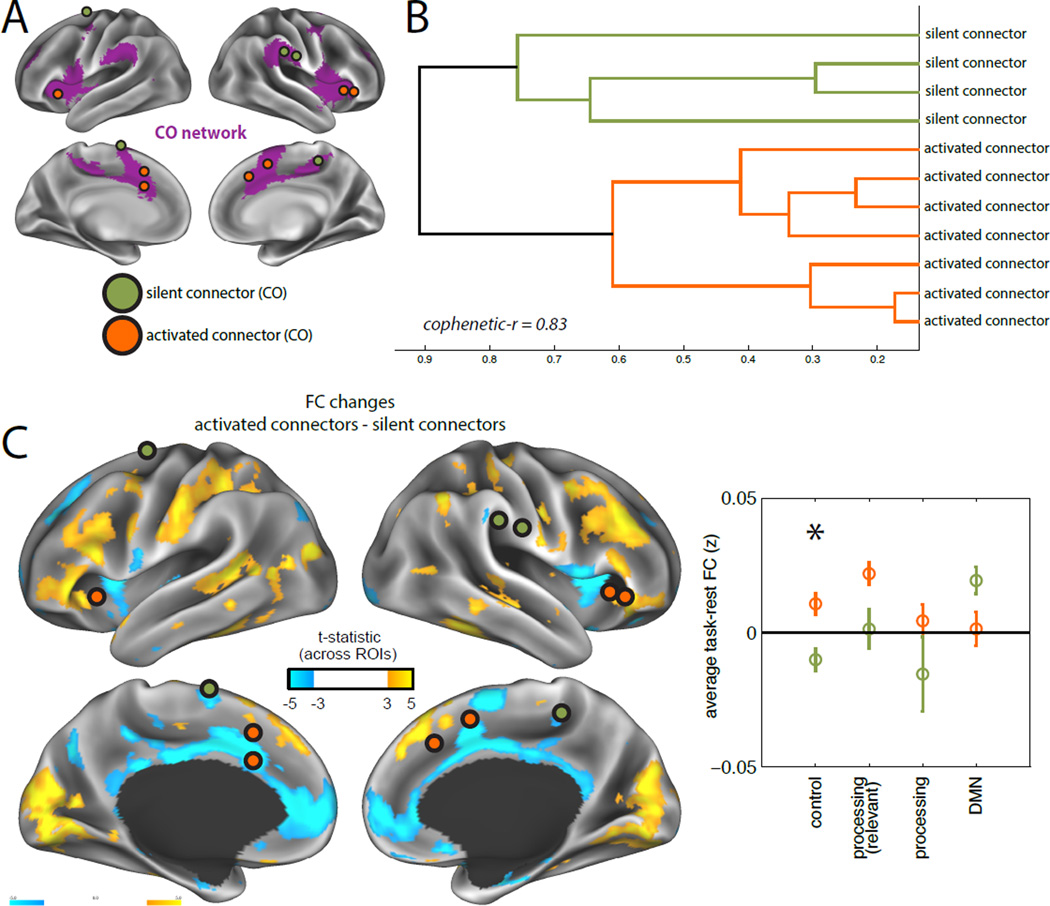
Figure 7. Nodes within the CO network show distinct FC patterns based on their class.
Regions associated with different classes showed distinct patterns of FC modulations, even when they were part of the same network. For example, we contrast the pattern of FC modulations (task - rest) exhibited by activated connectors (N=7, orange) and silent connectors (N=4, green) that are part of the CO network (purple; A). (B) Classes clustered separately from one another based on their FC difference maps. (C) Activated connector CO regions showed increased coupling with FP, DAN, and visual regions relative to silent connector CO regions (quantified in left panel for different types of networks; *p(FDR)<0.05; see Fig. 6 for network groupings). Error bars represent standard error across ROIs.
Flexibility of FC modulations
Finally, we asked if classes were equally ‘flexible’ in adjusting their pattern of connections across task states, as would be predicted for regions that modify distributed brain processing to achieve different goals. ‘Flexibility was quantified by computing the correlation of whole-brain FC differences (task-rest) between the three tasks; lower correlations should indicate relatively more flexibility in FC modulation across tasks. Classes differed significantly in their flexibility (between-group ANOVA: F(3,55)=9.35, p<0.001). The activated simple class had relatively low flexibility in the pattern of changes across tasks, similar to that seen with silent simple nodes. By contrast, activated and silent connectors both showed relatively high flexibility across tasks (Fig. 6B; all 2-sample t-tests between simple and connector classes, p[FDR]<0.01, corrected for 6 comparisons between classes).
Comparing across classes within a single network
Many of the divergent patterns of FC modulation were associated with nodes from distinct networks. However, we observed that, in some cases, regions from the same network, but different classes, also showed systematically different FC changes. For example, we compared activated (N=7) and silent (N=4) connector nodes in the CO network (Fig. 7A; this comparison yielded the highest N in two separate classes). The two classes differed in their pattern of network modulations, clustering separately (Fig. 7B). Moreover, direct comparison showed that activated connectors had relatively higher FC with control systems (FP, DAN) and the visual processing system, but relatively lower FC with the DMN (unpaired t-test; Fig. 7C). This provides evidence that node class, determined by activation and connector-hub status, relates to differences in task-FC even in cases where nodes are from the same network.
Discussion
Despite a largely preserved network organization, we found reliable small-scale differences between task and rest FC. Critically, we tested whether network changes were related to (a) the activation of a region or (b) a region’s topological hub properties. We found evidence that both properties provide separate, additive contributions to changes in FC in tasks varying from semantic judgments, mental rotation, to visual coherence assessments. Activated regions showed higher connectivity than non-activated regions, especially between networks. Connector-hubs also had large modulations of between-network FC, but relatively invariant within-network FC. Regions stratified into different classes based on their activation and connector-hub status were localized to distinct networks and showed significantly different patterns of FC changes, suggesting that they are associated with distinct processes for modulating FC during tasks.
An intrinsic network structure dominates rest and task states, but individual connections show reliable differences
FC networks and network properties were very similar between task and rest states, consistent with past reports (Betti et al., 2013; Cole et al., 2014; Krienen et al., 2014). These findings indicate that functional networks are dominated by stable, intrinsic correlation patterns that do not substantially change under different states of consciousness (Greicius et al., 2008; Larson-Prior et al., 2009) or task engagement (Cole et al., 2014). This stable backbone may be driven by anatomical connectivity between regions as well as the statistical history of co-activations that regions exhibit across the lifespan (Dosenbach et al., 2007).
However, though quite similar, subtle but systematic differences were present between rest and task networks, as suggested by previous examinations of an expanse of tasks (e.g., (Betti et al., 2013; Cole et al., 2014)), including internally-motivated tasks that share many similarities with rest (Krienen et al., 2014; Shirer et al., 2012). We found FC differences both within and between systems, including in processing (e.g., visual), control (e.g., subsets of frontoparietal and cinguloopercular), and default mode systems.
Our tasks differed in detailed aspects of their FC, but prominent changes were consistent across all three tasks. These FC changes were associated with different classes of nodes, defined by nodes’ activation and connector-hub properties, and are discussed in more detail below. Although our tasks varied substantially in their nature (including verbal and non-verbal stimuli, varying levels of perceptual demands, varying levels of difficulty, and varying involvement of control systems (Dubis et al., 2016)), they did not fully sample the space of tasks that humans can complete. All of the tasks had visual inputs, had motor responses, and used a mixed-block/event-related design. Future tests will be needed to establish whether any elements of these findings are dependent on the commonalities present across these tasks and, additionally, what properties may drive network differences between tasks (Cole et al., 2014; Krienen et al., 2014).
Modulations of brain networks are related to both the functional and topological properties of each region
Having found reliable connectivity changes between task and rest, we investigated whether altered FC is more associated with the functional (activation) characteristics of regions (Hypothesis 1) or the topological properties (connector-hub status) of regions within large-scale networks (Hypothesis 2). We found evidence that both the activation of a region and the region's putative hub role was related to changes in FC during a task.
Activation
Intuitively, one might assume that changes in FC will perfectly reflect activation during a task. Indeed, many past studies have focused on studying FC among small sets of activated (or de-activated) areas. For example, in visual attention tasks, interactions are altered among visual association regions and frontoparietal cortex (Gazzaley et al., 2007), the default mode (Chadick and Gazzaley, 2011), and other visual areas (Al-Aidroos et al., 2012), that all show either enhanced or suppressed activity during the task. Analogously, other studies have examined FC among regions activated in long-term memory (King et al., 2015), executive function (Elton and Gao, 2014) and working memory tasks (Cohen et al., 2014; Fransson, 2006; Hampson et al., 2006; Newton et al., 2011).
We examined the assumption adopted in these studies, that task-active regions will show significant changes in FC, by systematically measuring the relationship between activation in these three tasks and FC throughout the brain. Activated regions had greater changes in FC during tasks than non-activated regions, especially between networks. This effect was consistent across our tasks, various analysis approaches, and parcellation schemes, extending the generalizability of our results beyond previous findings limited to a small number of connections in a single task context (here to tasks spanning semantic judgments, mental rotations, and visual coherence). These findings indicate that system-level network changes accompany, and may facilitate, local processing during tasks (see also (Bassett et al., 2012; Siebenhuhner et al., 2013; Zalesky et al., 2012)).
In general, it is notable that activation and FC were not perfectly correlated with one another. This finding emphasizes that while activation and correlation measures may be related, they appear to index separable aspects of brain function – one encapsulating first order statistics of local neural responses and the other capturing second order statistics reflecting how variations in neural activity may be related across distributed regions.
Finally, it remains unclear whether the task-based alterations seen in this manuscript reflect sustained state-based changes in network correlations or trial-to-trial variability of evoked responses that is correlated across regions (e.g., (Rissman et al., 2004); or, indeed, if these two hypotheses are dissociable, as fluctuations in activity between intrinsically correlated regions help explain trial-to-trial evoked response variability; (Fox et al., 2007)). Future research will be needed to differentiate between these two possibilities and their implications for functional networks.
Connector hubs
In addition to activation, we demonstrate that the hubs are also central to understanding network modulations in tasks. Connector hubs are defined by having strong connections to multiple brain systems; here we show they also have strong changes in between-system FC during tasks. This ability to modify interactions between distributed systems may be central to completing complex tasks such as the ones examined here (Mesulam, 1990). Our findings link to prior evidence that has also suggested that connector hubs are important for cognition. Brain lesions to connector hubs lead to pervasive behavioral deficits (Warren et al., 2014) and connector hubs show a diverse activation profile across different cognitive processes (Bertolero et al., 2015). Further, Cole et al. (2013) found that the FP network, characterized as having many connector-hubs, showed variable between-system FC across tasks. We demonstrate that connector hubs throughout the brain, in many control systems, show high FC modulation across these three tasks. Moreover, these hub effects are separate from the effects of task activation, and exhibit distinct FC-related attributes, suggesting that hub status and task activation index separate factors for modifying brain networks.
Perhaps surprisingly, connector hubs had significantly less absolute change in their within-network FC. While robust, this result is less clearly predicted from previous literature. Perhaps high within-network invariance allows connector hubs to maintain a more veridical tie to the functional processing of their own network, while mediating malleable interactions with other networks. Regardless of their cause, these findings indicate that connector hubs are able to more finely tune their FC, compared with activated regions, as some connections are selectively modified while others are kept constant.
Dissociable factors for task-based network modulation
Activation and PC provided separable, additive contributions to network changes during the three tasks examined here, suggesting they relate to dissociable factors for network modulation. To characterize these complementary processes, we examined classes of regions, stratified based on their activation and connector hub status. The classes exhibited a number of distinct attributes, including in their locations, the topography of their network changes, and the flexibility of this topography across task contexts. Although we can not identify the specific neural processes employed based on these data, the distinct characteristics exhibited by activated and connector hub nodes argue for the presence of at least two dissociable mechanisms for linking brain regions together during complex tasks such as these. We propose that the classes are differentially linked to enacting task control and to conducting task processing.
Activated connectors
Activated connectors, regions that were both activated and connector hubs, exhibited characteristics consistent with a role in enacting control. During tasks, these regions had the largest absolute magnitude of network changes between systems, and the smallest changes within a system, suggesting a substantial but finely tuned manipulation of network connectivity. Activated connectors were found primarily in “control” systems, which have been implicated in a variety of attention- or top-down related functions, including goal-directed attention (DAN; (Corbetta and Shulman, 2002)), detection of salient events (salience; (Seeley et al., 2007)), and task control at multiple timescales (CO, FP; (Dosenbach et al., 2008; Dosenbach et al., 2007)).
Activated connectors had pronounced increases in FC with processing regions that were relevant for our tasks (visual, SM systems), a topography consistent with “control” systems that exert top-down signaling adjustments on relevant processing systems in the service of task goals (Petersen and Posner, 2012). A separate literature has also found lower FC between control systems (especially the DAN) and the DMN during tasks, as we found here (Bluhm et al., 2011; Kelly et al., 2008; Newton et al., 2011; Wang et al., 2012). Fewer studies have examined the interactions across different control systems during tasks (but see (Cohen et al., 2014)) – we show that subsets of these systems become more integrated, despite their independent pattern at rest (Dosenbach et al., 2007; Nomura et al., 2010).
Finally, activated connectors had variable patterns of FC changes across tasks, consistent with the expectation that control regions should show flexibility in network modulations in different task contexts, given differences in their control and processing demands (see (Dubis et al., 2016) on these tasks).
The attributes of activated connectors were distinct from those of other classes, underscoring the importance of both activation and connector-hub properties for understanding how brain regions interact during tasks.
Activated simple class
This class contained regions in processing systems, including the visual system and a hand-SM region. Unlike activated connectors, this class showed high levels of both within- and between-system modifications, and their pattern of FC changes had low flexibility across tasks. These attributes are consistent with basic task-processing regions that alter interactions both with control regions in other networks and processing regions in their own network, but in a stereotyped way regardless of the specific task context.
In this class, the topography of network changes varied substantially by system. Visual regions had decreased visual correlations, but increased correlations to other (especially control) systems, as in previous reports (Betti et al., 2013; Spadone et al., 2015). DMN regions, instead, had decreased correlations with other, especially control, systems (as in (Bluhm et al., 2011; Kelly et al., 2008; Newton et al., 2011; Wang et al., 2012)) and increased within system correlations during tasks. The literature is mixed on how tasks affect FC within the DMN (e.g., (Betti et al., 2013; Fransson, 2006; Hampson et al., 2006; Newton et al., 2011)), perhaps due to differences in task design or the portions of the DMN examined. We speculate that both the visual and DMN effects reflect a relatively more isolated, modular, state for the system – coherence within the system core and segregation from other systems – when not called upon (i.e., during the task for DMN, during rest for the visual system). Indeed, the placement of DMN regions in the activated simple class and their shared characteristics with visual regions suggests that they are closely related to processing, rather than control, systems.
Silent connectors
Silent connectors shared some attributes with activated connectors (invariant within-network FC, high flexibility in FC patterns across tasks, and localization to control systems). However, relative to activated connectors, silent connectors had weaker magnitudes of between-network FC and a distinct FC topography (e.g., a lack of FC to visual systems and, in CO silent connectors, decreased FC with control systems (Fig. 7). Moreover, silent connectors were found in less well-studied regions of control systems (i.e., the mid-cingulate and posterior insula portions of the CO, rostral frontal portions of the FP). These regions may be associated with control of different types of tasks than we, and others, have examined; alternatively they may have a distinct role in task processing than the well studied “core” sections (Dosenbach et al., 2006) associated with activated connectors.
Silent simple class
Silent simple regions were neither task-activated nor connector hubs; therefore, neither hypothesis would expect strong changes in these regions – indeed, we found weak changes in these regions, primarily associated with decreased FC in processing systems.
Conclusion
Although dominated by a stable intrinsic backbone, large-scale networks differ systematically between three distinct tasks and rest. We tested two hypotheses for which locations would show large changes in functional connectivity: regions activated in a task or regions that serve as connector hubs for transferring information across systems. We found evidence that the properties provide separate contributions to network changes. Furthermore, classes of regions defined by their activation and connector-hub status were located in different networks and exhibited different magnitudes, topography, and variability of FC modulations. In particular, “activated connector” regions exhibited attributes consistent with a role in enacting task control. These findings argue for the presence of at least two dissociable factors related to functional specialization and network hubs that contribute to changes in coordination among distributed brain regions during different task contexts.
Experimental Procedures
Participants
Task and resting-state data were collected from 29 healthy young participants (15 female, avg. age 25, range 21–30). Task data was previously published (Dubis et al., 2016), and a subset of the resting-state data was included in a larger cohort reported on in (Power et al., 2011). After censoring high-motion timepoints (Power et al., 2014), 1 participant was dropped for the task (concatenated data from all task conditions) versus rest comparisons and 4 participants were dropped from examinations of single task data due to insufficient remaining time-points (<120). Written informed consent was obtained from all participants, who were compensated monetarily for their participation. Procedures were approved by the Institutional Review Board at Washington University in St. Louis.
Tasks
Participants completed three tasks in a mixed block/event-related design: a semantic task (noun/verb judgment on a word), a mental rotation task (same/mirror image judgment on two 3-D objects), and a coherence judgment task (judgment of whether a set of dots arranged were concentrically). The tasks differed substantially from one another, including either verbal or non-verbal stimuli and requiring different perceptual and control demands (Dubis et al., 2016). Furthermore, a substantial amount of data was present for each task (~23 min. per task, > 1 hour total), providing reliable measures of whole-brain task FC (Laumann et al., 2015). See Supp. Experimental Procedures for details on behavioral paradigms and stimuli.
Resting State
During resting-state scans, participants lay quietly in the scanner while passively viewing a fixation cross. Between 10 and 140 min (avg = 50 min.) of total resting state data were collected from each participant, 10–20 min of which were from the same session as the task data. When available (N=23/29 participants), resting state data was supplemented from other experimental sessions.
Image acquisition parameters
Data was acquired on a Siemens 3T Trio at Washington University in St. Louis, using a 12-channel head coil. A high-resolution structural image was acquired from each participant using a sagittal magnetization-prepared rapid gradient echo (MP-RAGE) sequence (slice time echo = 3.08ms, TR=2.4s, inversion time=1s, flip angle= 8°, 176 slices, 1×1×1mm voxels). Functional images were acquired using an asymmetric spin-echo echo-planar pulse sequence (TR=2.5 s, TE=27ms, flip angle=90°, 4×4mm in-plane resolution). Whole b rain coverage was achieved using 32 contiguous interleaved 4mm slices aligned parallel to the anterior-posterior commissure. These parameters were identical for all task and rest sessions.
Data was initially processed using standard techniques to reduce artifacts (Miezin et al., 2000) (including slice-time correction, alignment, intensity normalization, and transformation to atlas space; see Supp. Experimental. Procedures for details).
FC Processing
Both resting and task data were analyzed using a FC approach. First, task evoked activity was removed from task time series by applying the GLM model described below and extracting the residuals from the model. Importantly, this approach reduces spurious correlations induced by task activations and highlights underlying changes in connectivity that are present throughout a period of task performance (i.e., “background” connectivity (Al-Aidroos et al., 2012)). Note that the overall pattern of FC changes were quite similar whether task FC was calculated based on residuals or raw data (Supp. Fig. 4A), although as expected, estimates of their relationship to activation were inflated (Supp. Fig 4B). Tasks were analyzed both individually and as a unit (concatenated across tasks).
FC processing was applied to both task and resting-state time series. Processing followed Power et al. (2014), including regression of nuisance signals from white matter, cerebral spinal fluid, global signal, and motion parameters, spatial and temporal filtering, and censoring of high motion frames (>0.2 mm.; see Supp. Experimental Procedures). Following this, Pearson correlations were calculated between average time series from regions of interest. In task FC analyses, only frames from relevant task periods were included in the correlations.
Regions and Networks
FC analyses were computed among 264 regions of interest (10mm diameter spheres) across the brain spanning cortical and subcortical locations ((Power et al., 2011), see Figure 2A). These regions are associated with 13 networks based on previous work (Power et al., 2011): somatomotor (SM), lateral somatomotor (lat-SM), cinguloopercular (CO), auditory, default mode (DMN), memory, visual, frontoparietal (FP), salience, sub-cortex, ventral attention (VAN), dorsal attention (DAN), and cerebellum, as well as a group of undefined regions. In this and previous work (Power et al., 2011), regions were sorted into networks using the Infomap random walk clustering algorithm (Rosvall and Bergstrom, 2008) based on weighted correlation matrices across a range of sparsity thresholds (2–10% for 264 ROIs, 0.5–5% for voxelwise networks). To algorithmically define consensus networks from this dataset for comparison to Power et al. (Power et al., 2013), we placed regions in networks using data from the lowest threshold, but excluding small networks (<4 nodes or 400 voxels). Higher thresholds were examined in turn to assign networks to voxels that remained unaffiliated.
Analyses were additionally completed on 333 parcels produced through novel surface based FC boundary mapping methods (Gordon et al., 2016) and on modified voxelwise graphs (Power et al., 2011) to show consistency between approaches. All ROI and voxelwise analyses were conducted on volume-space data, but are projected onto the surface for visualization purposes.
General Linear Model (GLM) of task activation
We modeled task activations using a GLM approach to determine how task-FC was altered in activated regions. Modeling was conducted on individual voxels using in-house imaging software. The GLM model included linear and constant terms for each run to remove baseline and drift effects. In addition, the following task events were modeled: start cues, end cues, trials coded by accuracy and type (i.e., noun and verb for the semantic task, 3 different orientation bins for the mental rotation task, and 4 different coherence conditions for the coherence task), and sustained task responses. Sustained responses were modeled as a block effect. For cue and trial conditions, 10 individual timepoints (25s) were modeled with delta functions to describe the full temporal extent of the hemodynamic response. This approach makes no assumptions about the shape of the hemodynamic response (Ollinger et al., 2001), allowing us to fully model (and subsequently remove) evoked activations even when response shapes may differ. Activations from modeling were expressed as a percent signal change, dividing the magnitude of activation by the baseline term for each run. Average activations for each region were computed as a weighted average of all correct task conditions (cue, trial, and sustained; all conditions were included as FC was examined over the entire task).
Comparing Correlation Matrices
Correlation values were Fisher z-transformed. Similarity between FC matrices was evaluated by correlating FC values and by computing Mantel’s statistic. Differences between FC matrices were quantified using two approaches that provided a mixture of generalized and edge-specific measurements. (1) A paired two-sided t-test was conducted for each unique entry in the FC matrix. The distribution of t-test p-values was compared to a null-distribution determined by permuting task and rest states. (2) Individual t-tests were subjected to false discovery rate (FDR) correction for multiple comparisons to identify connections that significantly differed between conditions.
FC change per region
The average absolute change in FC for a given region was computed by taking the mean of the absolute correlation differences between task and rest for that region to every other region. We also examined within- and between-network changes in FC separately by computing the mean of a region’s absolute FC difference to other regions within its own network (within-network) or to regions in other networks (between network). The majority of analyses were computed using the 264 ROIs and networks introduced above. We also made similar computations for voxelwise graphs, where group-average connectivity differences were computed for each voxel to every other voxel (all connections), all other voxels assigned to the same network (within-network) or voxels assigned to other networks (between-network). Voxelwise summaries were used for qualitative representation of the anatomical locations of effects, not quantification.
Relationship between FC and Activation/PC
We used a quartile analysis to compare activated/connector hub regions (those in the top 25% of the activation/PC distribution – see Supp. Experimental Procedures for PC definition) to low activation/non-connectors (those in the bottom 25% of the activation/PC distribution). Regions with low signal and uncertain network assignment (“unassigned”, (Power et al., 2011)) were excluded from these and following analyses. For each sample of ROIs we compared FC changes using non-parametric permutation tests where ROI labels (top, bottom quartile) were permuted. In a second approach, we correlated FC changes for each region with continuous measures of activation/PC, using Spearman’s correlations. Finally, we used a linear regression analysis, with z-scored regressors for activation and PC as well as their interaction, to jointly examine the two properties. For simplicity, only linear relationships were tested in correlations/regression (scatterplots are available in Supp. Fig. 6); however, quartile analyses do not depend on linear assumptions. For these and following analyses when more than two comparisons were made, p-values were FDR corrected for multiple comparisons (i.e., across tasks, across classes).
We also examined the attributes of classes of regions with combinations of different properties: (a) “activated connectors” (top 25% of both PC and activation), (b) “silent connectors” regions (top 25% of PC, bottom 25% activation), (c) “activated simple nodes” (top 25% activation, bottom 25% PC), and (d) “silent simple nodes” (bottom 25% of both activation and PC). We (1) determined the network identities of nodes in each class, (2) measured the absolute magnitude of within and between FC changes, (3) measured the topography and (4) flexibility of FC changes. Results were compared across classes using one-way between-factor ANOVAs and post-hoc using two-sample t-tests FDR corrected for multiple comparisons. See Supp. Experimental Procedures for details on these analyses and on analyses comparing activated and silent connectors in the CO network.
Supplementary Material
Acknowledgments
We thank Joe Dubis, Rebecca Coalson, Fran Miezin, and Mark McAvoy for technical assistance. This research was supported by a McDonnell Foundation Collaborative Activity Award (SEP), NIH R01NS32979 (SEP), NIH R01NS06424 (SEP), NIH T32NS0007205-33 and F32NS092290 (CG) and was supported in part by the Neuroimaging Informatics and Analysis Center (1P30NS098577).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
C.G. and S.E.P. conceived of the study and secured funding. C.G. performed analyses. T.O.L., E.M.G., and B.A. provided software and feedback. C.G., T.O.L., E.M.G., B.A., and S.E.P. wrote the manuscript.
References
- Al-Aidroos N, Said CP, Turk-Browne NB. Top-down attention switches coupling between low-level and high-level areas of human visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14675–14680. doi: 10.1073/pnas.1202095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnaes D, Kaufmann T, Richard G, Duff EP, Sneve MH, Endestad T, Nordvik JE, Andreassen OA, Smith SM, Westlye LT. Attentional load modulates large-scale functional brain connectivity beyond the core attention networks. NeuroImage. 2015;109:260–272. doi: 10.1016/j.neuroimage.2015.01.026. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Nelson BG, Mueller BA, Camchong J, Lim KO. Altered resting state complexity in schizophrenia. NeuroImage. 2012;59:2196–2207. doi: 10.1016/j.neuroimage.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolero MA, Yeo BT, D'Esposito M. The modular and integrative functional architecture of the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E6798–E6807. doi: 10.1073/pnas.1510619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betti V, Della Penna S, de Pasquale F, Mantini D, Marzetti L, Romani GL, Corbetta M. Natural scenes viewing alters the dynamics of functional connectivity in the human brain. Neuron. 2013;79:782–797. doi: 10.1016/j.neuron.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Clark CR, McFarlane AC, Moores KA, Shaw ME, Lanius RA. Default network connectivity during a working memory task. Human brain mapping. 2011;32:1029–1035. doi: 10.1002/hbm.21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadick JZ, Gazzaley A. Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nature neuroscience. 2011;14:830–832. doi: 10.1038/nn.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Gallen CL, Jacobs EG, Lee TG, D'Esposito M. Quantifying the reconfiguration of intrinsic networks during working memory. PloS one. 2014;9:e106636. doi: 10.1371/journal.pone.0106636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nature neuroscience. 2013;16:1348–1355. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in cognitive sciences. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubis JW, Siegel JS, Neta M, Visscher KM, Petersen SE. Tasks Driven by Perceptual Information Do Not Recruit Sustained BOLD Activity in Cingulo-Opercular Regions. Cereb Cortex. 2016;26:192–201. doi: 10.1093/cercor/bhu187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DB, Harrison BJ, Yucel M, Whittle S, Zalesky A, Pantelis C, Allen NB, Fornito A. Large-scale brain network dynamics supporting adolescent cognitive control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:14096–14107. doi: 10.1523/JNEUROSCI.1634-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Gao W. Divergent task-dependent functional connectivity of executive control and salience networks. Cortex; a journal devoted to the study of the nervous system and behavior. 2014;51:56–66. doi: 10.1016/j.cortex.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, D'Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17(Suppl 1):i125–i135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Castillo J, Hoy CW, Handwerker DA, Robinson ME, Buchanan LC, Saad ZS, Bandettini PA. Tracking ongoing cognition in individuals using brief, whole-brain functional connectivity patterns. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:8762–8767. doi: 10.1073/pnas.1501242112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Breeden AL, Bean SE, Vaidya CJ. Working memory-related changes in functional connectivity persist beyond task disengagement. Human brain mapping. 2014;35:1004–1017. doi: 10.1002/hbm.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb Cortex. 2016;26:288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Nomura EM, Perez F, D'Esposito M. Focal brain lesions to critical locations cause widespread disruption of the modular organization of the brain. Journal of cognitive neuroscience. 2012;24:1275–1285. doi: 10.1162/jocn_a_00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, Menon V. Persistent default-mode network connectivity during light sedation. Human brain mapping. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimera R, Amaral LA. Cartography of complex networks: modules and universal roles. J Stat Mech. 2005;2005 doi: 10.1088/1742-5468/2005/02/P02001. nihpa35573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magnetic resonance imaging. 2010;28:1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca's area varies with reading ability. NeuroImage. 2006;31:513–519. doi: 10.1016/j.neuroimage.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- King DR, de Chastelaine M, Elward RL, Wang TH, Rugg MD. Recollection-related increases in functional connectivity predict individual differences in memory accuracy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:1763–1772. doi: 10.1523/JNEUROSCI.3219-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Yeo BT, Buckner RL. Reconfigurable task-dependent functional coupling modes cluster around a core functional architecture. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2014;369 doi: 10.1098/rstb.2013.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann TO, Gordon EM, Adeyemo B, Snyder AZ, Joo SJ, Chen MY, Gilmore AW, McDermott KB, Nelson SM, Dosenbach NU, et al. Functional System and Areal Organization of a Highly Sampled Individual Human Brain. Neuron. 2015;87:657–670. doi: 10.1016/j.neuron.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of neurology. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. NeuroImage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Newton AT, Morgan VL, Rogers BP, Gore JC. Modulation of steady state functional connectivity in the default mode and working memory networks by cognitive load. Human brain mapping. 2011;32:1649–1659. doi: 10.1002/hbm.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura EM, Gratton C, Visser RM, Kayser A, Perez F, D'Esposito M. Double dissociation of two cognitive control networks in patients with focal brain lesions. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12017–12022. doi: 10.1073/pnas.1002431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. NeuroImage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annual review of neuroscience. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Lessov-Schlaggar CN, Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013;79:798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rosvall M, Bergstrom CT. Maps of random walks on complex networks reveal community structure. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1118–1123. doi: 10.1073/pnas.0706851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenhuhner F, Weiss SA, Coppola R, Weinberger DR, Bassett DS. Intra- and inter-frequency brain network structure in health and schizophrenia. PloS one. 2013;8:e72351. doi: 10.1371/journal.pone.0072351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadone S, Della Penna S, Sestieri C, Betti V, Tosoni A, Perrucci MG, Romani GL, Corbetta M. Dynamic reorganization of human resting-state networks during visuospatial attention. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:8112–8117. doi: 10.1073/pnas.1415439112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Networks of the brain. Cambridge, Mass.: MIT Press; 2011. [Google Scholar]
- Wang Z, Liu J, Zhong N, Qin Y, Zhou H, Li K. Changes in the brain intrinsic organization in both on-task state and post-task resting state. NeuroImage. 2012;62:394–407. doi: 10.1016/j.neuroimage.2012.04.051. [DOI] [PubMed] [Google Scholar]
- Warren DE, Power JD, Bruss J, Denburg NL, Waldron EJ, Sun H, Petersen SE, Tranel D. Network measures predict neuropsychological outcome after brain injury. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:14247–14252. doi: 10.1073/pnas.1322173111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Egan GF, Pantelis C, Bullmore ET. The relationship between regional and inter-regional functional connectivity deficits in schizophrenia. Human brain mapping. 2012;33:2535–2549. doi: 10.1002/hbm.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.