Abstract
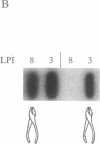
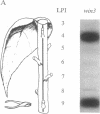
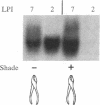
When a single leaf on a young poplar tree is mechanically wounded, wound-induced (win) mRNAs are detected in the unwounded portion of that leaf and in specific leaves that are remote from the wounded leaf. Shortly after wounding (6-8 hr), the remote leaves in which win genes are expressed can be predicted by a knowledge of photoassimilate movement patterns in vivo. When assimilate movement from a wounded leaf is blocked or the direction of assimilate movement is altered by shading, win gene expression in remote leaves is similarly blocked or altered. These data illustrate how the long-distance transduction of wound-induced signals can be manipulated in plants by altering carbon allocation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop P. D., Pearce G., Bryant J. E., Ryan C. A. Isolation and characterization of the proteinase inhibitor-inducing factor from tomato leaves. Identity and activity of poly- and oligogalacturonide fragments. J Biol Chem. 1984 Nov 10;259(21):13172–13177. [PubMed] [Google Scholar]
- Bradshaw H. D., Jr, Hollick J. B., Parsons T. J., Clarke H. R., Gordon M. P. Systemically wound-responsive genes in poplar trees encode proteins similar to sweet potato sporamins and legume Kunitz trypsin inhibitors. Plant Mol Biol. 1990 Jan;14(1):51–59. doi: 10.1007/BF00015654. [DOI] [PubMed] [Google Scholar]
- Dickson R. E. Diurnal changes in leaf chemical constituents and (14)C partitioning in cottonwood. Tree Physiol. 1987 Jun;3(2):157–171. doi: 10.1093/treephys/3.2.157. [DOI] [PubMed] [Google Scholar]
- Farmer E. E., Pearce G., Ryan C. A. In vitro phosphorylation of plant plasma membrane proteins in response to the proteinase inhibitor inducing factor. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1539–1542. doi: 10.1073/pnas.86.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Johnson R., Ryan C. A. Wound-inducible potato inhibitor II genes: enhancement of expression by sucrose. Plant Mol Biol. 1990 Apr;14(4):527–536. doi: 10.1007/BF00027498. [DOI] [PubMed] [Google Scholar]
- Keil M., Sánchez-Serrano J. J., Willmitzer L. Both wound-inducible and tuber-specific expression are mediated by the promoter of a single member of the potato proteinase inhibitor II gene family. EMBO J. 1989 May;8(5):1323–1330. doi: 10.1002/j.1460-2075.1989.tb03512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason H. S., Mullet J. E. Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell. 1990 Jun;2(6):569–579. doi: 10.1105/tpc.2.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T. J., Bradshaw H. D., Jr, Gordon M. P. Systemic accumulation of specific mRNAs in response to wounding in poplar trees. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7895–7899. doi: 10.1073/pnas.86.20.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pēna-Cortés H., Sánchez-Serrano J. J., Mertens R., Willmitzer L., Prat S. Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9851–9855. doi: 10.1073/pnas.86.24.9851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. Assay and Biochemical Properties of the Proteinase Inhibitor-inducing Factor, a Wound Hormone. Plant Physiol. 1974 Sep;54(3):328–332. doi: 10.1104/pp.54.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit B., Stachowiak M. Effects of hypoxia and elevated carbon dioxide concentration on water flux through Populus roots. Tree Physiol. 1988 Jun;4(2):153–165. doi: 10.1093/treephys/4.2.153. [DOI] [PubMed] [Google Scholar]
- Stanford A. C., Northcote D. H., Bevan M. W. Spatial and temporal patterns of transcription of a wound-induced gene in potato. EMBO J. 1990 Mar;9(3):593–603. doi: 10.1002/j.1460-2075.1990.tb08151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Novel Regulation of Vegetative Storage Protein Genes. Plant Cell. 1990 Jan;2(1):1–6. doi: 10.1105/tpc.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]