Abstract
Background
There has been an increase in the use of pulmonary artery (PA) catheters in heart failure (HF) in the United States in recent years. However, patterns of hospital-use and trends in patient outcomes are not known.
Methods and Results
In the National Inpatient Sample 2001–2012, using ICD-9 codes we identified 11,888,525 adult (≥18 years) HF hospitalizations nationally, of which an estimated 75,209 (SE 0.6%) received a PA catheter. In 2001, the number of hospitals with ≥1 PA catheterization was 1753, decreasing to 1183 in 2011. The mean PA catheter use per hospital trended from 4.9/year in 2001 (limits 1–133) to 3.8/year in 2007 (limits 1–46), but increased to 5.5/year in 2011 (limits 1–70). During 2001–2006, PA catheterization declined across hospitals; however, in 2007–2012 there has been a disproportionate increase at hospitals with large bedsize, teaching programs, and advanced HF capabilities. The overall in-hospital mortality with PA catheter use was higher than without PA catheter use (13.1% vs. 3.4%, P<0.0001), however, in propensity-matched analysis, differences in mortality between these groups have attenuated over time – risk-adjusted odds ratio for mortality for PA-catheterization, 1.66 (95% CI 1.60–1.74) in 2001–2003 down to 1.04 (95% CI 0.97– 1.12) in 2010–2012.
Conclusions
There is substantial hospital-level variability in PA catheterization in HF along with increasing volume at fewer hospitals overrepresented by large, academic hospitals with advanced HF capabilities. This is accompanied by a decline in excess mortality associated with PA catheterization.
Keywords: heart failure, resource utilization, catheterization, variation
Pulmonary artery (PA) catheters have been traditionally used for invasive bedside hemodynamic monitoring in heart failure (HF) both to guide treatment decisions and to evaluate the hemodynamic responses to various therapies such as intravenous diuretics, vasodilators, and inotropes.1–3 While early data on efficacy of invasive hemodynamic monitoring with PA catheter in HF were encouraging,4–6 the ESCAPE trial, a large randomized trial evaluating PA catheterization in routine HF care published in October 2005, provided evidence against their utility in the routine management of HF.7 The current American College of Cardiology (ACC)/American Heart Association (AHA) guidelines support the use of PA catheter in HF patients with cardiogenic shock or with respiratory failure requiring mechanical ventilation (Class IA). Routine use of PA catheter for management of acute HF is not recommended (Class III).8 However, despite these recommendations, we recently reported a significant increase in the use of PA catheters in HF hospitalization in the United States over the past few years, particularly in patients without cardiogenic shock or respiratory failure.9 The hospital- and patient-level factors underlying this temporal increase in PA catheter use in HF are not well understood. Furthermore, with significant advances in the management of HF in recent years, changes in utilization of PA catheterization in HF care and its association with clinical outcomes in not known. Against this background, we aim to evaluate hospital variation, temporal trends, and factors associated with PA catheter use in HF care and assess its association with patient outcomes over time.
METHODS
Data sources
We used the National Inpatient Sample (NIS), the largest all payer database of hospitalized patients in the United States, which is managed under the Healthcare Cost and Utilization Project by the Agency for Healthcare Research and Quality.10 It is provided as an annual data set comprised of a 20% sample of all inpatient hospitalizations drawn from participating states in a given year and consists of de-identified data on demographics, admission diagnoses, procedures, comorbidities and outcomes of hospitalization. We included data for years 2001 through 2012 in our study.
The design of NIS has been described in previous studies.11, 12 During 2001–2011, the NIS was constructed by including 100% of acute inpatient hospitalizations from a 20% random sample of all reporting hospitals stratified by bed-size, teaching status and location. Patient admissions under an observational status and those from rehabilitation hospitals, long-term non–acute care hospitals, psychiatric hospitals, and chemical dependency units were not included. In 2012, the NIS was redesigned to include a random 20% sample of patient discharges selected from 100% of the participating hospitals in 2012, with all discharges stratified by hospital bed-size, teaching status and location.13, 14 Moreover, compared to 2001–2011 when hospitals were identified using the American hospital association survey, the hospital systems in 2012 were identified using the state inpatient database (SID). The changes to the NIS 2012 improved the precision of national discharge estimates. To account for changes in sampling methodology a revised set of discharge weights were provided, called ‘trend weights’ to be use for all studies spanning 2012.13 We used the provided trend weights for all patient-level analyses. However, since only 20% of discharges from sampled hospitals were available for the year 2012, as opposed to 100% hospital discharge volume in 2001–2011, hospital-level estimates which relied on census of hospital discharges for a given year were limited to years 2001–2011. Further details are available in HCUP’s NIS redesign report.13
Study Population & Variables
The NIS provides patient- and hospital-level information on each patient discharge, including patient demographics (including age, sex, race, etc.), primary and up to 24 secondary discharge diagnoses, and a maximum of 15 procedures performed during the index hospitalization. The information on both diagnoses and procedures is available as International Classification of Diseases – 9th Clinical Modification (ICD-9) codes as well as their validated combinations into broad categories, the Clinical Classification of Diseases Software (CCS) codes developed by Elixhauser et al.15 We used a combination of ICD-9 and CCS codes to identify specific comorbidities and procedures. Using the provided hospital identification number, each hospitalization was linked to corresponding hospital characteristics, including bed strength, teaching status, urban vs. rural location and hospital census region.
We identified all adults (>18 years) admitted with a primary discharge diagnosis of heart failure (HF) using ICD-9 codes 428.x, 402.x1, 404.x1, and 404.x3 which have been previously validated and used to identify heart failure hospitalization in the American Heart Association’s Get With The Guidelines (GWTG) – Heart Failure registry and administrative databases.16, 17 We then identified patients admitted for HF who underwent placement of a pulmonary artery catheter using the ICD-9 codes 89.63 (pulmonary artery pressure monitoring), 89.64 (pulmonary artery wedge monitoring), 89.66 (measurement of mixed venous blood gases), 89.67 (monitoring of cardiac output by oxygen consumption technique [Fick method]) and 89.68 (monitoring of cardiac output by other technique [thermodilution indicator]) used in prior studies.18
In order to limit our analysis to use of PA catheters in the management of HF, we excluded hospitalizations where the reported PA catheter may have been used for an alternative indication. First, to exclude patients where the recorded PA catheter may have been used for operative monitoring, we excluded patients who underwent any major surgical procedure during the index hospitalization, as done previously.18 For this, surgery flag software developed by HCUP were used to identify if any of the listed procedures on a patient record represented major invasive procedures, defined as “an invasive therapeutic surgical procedure involving incision, excision, manipulation, or suturing of tissue that penetrates or breaks the skin; typically requires use of an operating room; and also requires regional anesthesia, general anesthesia, or sedation to control pain”. All hospitalization with ≥1 of these procedures were excluded. Second, to exclude patients who may have undergone placement of PA catheter during emergent management of hemodynamic instability, we excluded patients who also underwent placement of a temporary cardiac assist device (e.g. intra-aortic balloon pump, percutaneous ventricular assist devices) during the index hospitalization. Using our exclusion criteria above, we excluded 3443 cases (weighted N = 16,532) from our study.
Statistical analysis
First, we compared patient characteristics for those with vs. without PA catheter use during a HF hospitalization. Second, for each study-year we identified hospitals using ≥1 PA catheters in a HF hospitalization. Since 100% of the discharge volume for each included hospital in a year was included in the sample for years 2001–2011, analysis of hospital PA catheter volume was limited to these years. To describe hospital-level variability in PA catheter utilization, we examined proportion of HF admissions with PA catheter use at hospitals with >10 HF admissions for the most recent year with complete hospital data (2011). For this analysis, HF admissions were further stratified by HF with class I indications for PA catheter use (cardiogenic shock, respiratory failure requiring mechanical ventilation) and HF without these indications.
Next, to better understand the hospital-level variation in use of PA catheters over time, we assessed the temporal trend in the proportion of hospitals using 1 or more PA catheters in a HF hospitalization and the mean PA catheter volume per hospital during the study period. Furthermore, we also compared the trends in PA catheter use over time across different hospital subgroups available in the HCUP hospital dataset, defined by hospital bedsize, teaching status, and urban vs. rural location. Bedsize designations (small, medium or large) were based on defined cutoffs specific for the hospital’s location and teaching status.19 Teaching hospitals were defined by a ratio of ≥0.25 of full-time equivalent interns/residents to non-nursing home beds.20 Urban vs. rural status were assigned based on the census designation of the geographical location of the hospital.21 We further identified hospitals as advanced HF centers if they performed ≥1 LVAD implantation or heart transplantation in a given year. Temporal trends were further assessed in the years before (2001–2006) and after (2007–2012) the ESCAPE trial.
As recommended by the AHRQ, we performed all analyses using a survey methodology while accounting for clustering and stratification of patients. For comparative and subgroup analysis, we used domain analysis to ensure that the estimated population statistics and measures of variance are accurate.22 Trend analysis was performed using the Cochran-Armitage test for categorical variables and survey-specific linear regression for continuous variables. Subgroup comparisons were tested with the Rao-Scott chi-square test for categorical variables and survey ANOVA test for continuous variables.
Next, we examined predictors of PA catheter use at hospitals across the US. For this, we performed survey-specific logistic regression analysis with PA catheter use as a dependent variable and patient and hospital characteristics as independent predictor variables. Patient characteristics included in the model were demographics, discharge diagnoses, comorbidity burden (using the Charlson Comorbidity Index [CCI] score), comorbidities (like diabetes, hypertension, etc.) and inpatient procedures (mechanical ventilation and vasopressor use). Hospital characteristics included advanced HF facility, teaching status, bedsize, rural-urban location and geographic region.
Last, we assessed for the association of PA catheter use with in-hospital mortality. For this, we first compared differences in patient characteristics and unadjusted outcomes for patients with and without PA catheter use when admitted for HF to hospitals using at least 1 PA catheter in HF patients in a given year. To account for confounding by indication, we performed propensity-matched analysis comparing HF patients with vs. without PA catheter use. We first created a non-parsimonious logistic regression model to calculate each patient’s propensity (or likelihood) of receiving a PA catheter based on his/her clinical characteristics. The variables included in our model were patient age, sex, secondary cardiovascular diagnoses (cardiogenic shock, cardiac arrest, AMI, coronary artery disease, peripheral artery disease, cerebrovascular disease, valvular heart disease, cardiac arrhythmia and conduction disorders), comorbid conditions (hypertension, diabetes, dyslipidemia, cancer, liver disease, chronic kidney disease, acute kidney injury, fluid-electrolyte disorder, chronic obstructive pulmonary disease, coagulopathy, tobacco abuse and substance-use disorder), CCI score, procedures (mechanical ventilation and vasopressor use) and nature of admission (non-elective vs. elective). In addition to the variables above we included the trend weight in estimation of the propensity score above as recommended for propensity-matched in studies with a survey design.
Using the propensity scores calculated above, we performed 1:2 matching between HF patients with PA catheter use and those without PA catheter use. Each selected patient with PA catheter use (case) was first matched with as many patients without PA catheter use (controls) whose propensity scores were within the pre-specified caliper width of one-quarter of the standard deviation of the logit of the propensity score of the case. Cases without any corresponding matched controls were excluded at this step. To further select controls that were the closest match to a selected case, we chose those two controls with smallest value of the Mahalanobis metric for the case. The Mahalanobis metric measures the degree of closeness between 2 observations and is their multivariate distance based on the mean, variance and the covariance of the pre-specified variables.23, 24 Mahalanobis metric was calculated using pre-specified characteristics - age, presence of cardiogenic shock, ventilator use and AMI.23 To test the success of our matching algorithm in achieving covariate balance, we calculated standardized differences between case and control groups for all covariates in our model before and after matching. A standardized difference of <10% for all covariates after matching is indicative of a successful match.25 We used the Cochran–Mantel–Haenszel test for matched data to compare the association of PA catheter use with in-hospital mortality and included the discharge weight in the analysis to account for the survey nature of our data.
To account for temporal changes in HF outcomes, we performed the propensity-matched analysis after dividing patients into four separate 3-year cohorts – 2001–2003, 2004–2006, 2007–2009 and 2010–2012. Within each cohort, we matched each HF patient receiving a PA catheter in a given cohort (‘case’) to two covariate-matched HF patients without PA catheter use from within the same cohort (‘controls’). Second, we performed sensitivity analyses to assess the associations of PA catheter use on the outcomes in patients without a class I indications for use in HF, which included patients without cardiogenic shock or use of mechanical ventilation in the index hospitalization. We compared their outcomes using 1:2 propensity-matched analysis in the four separate time periods, similar to the one described above.
The level of significance was set at a P value of 0.05. All analyses were performed using SAS 9.4 software (SAS institute, Cary, NC), including SAS PROC SURVEYMEANS and PROC SURVEYFREQ for analysis of complex survey data. The study was reviewed by the University of Iowa Institutional Review Board (IRB), which exempted the study from IRB approval and waived the requirement for informed consent since it uses previously collected de-identified data.
Post-hoc analyses
Based on reviewer comments, we performed additional analyses. First, we examined for rates of reported complications –periprocedural bleeding (ICD-9 codes 998.11-998.12) and iatrogenic pneumothorax (ICD9 code 512.1), which are relevant for patients undergoing PA catheterization. Next, we compared mortality and complication rates with PA catheter use in HF by hospital subgroups. Finally, to assess if our selection criteria affected trends, we repeated the analyses of trends in hospital PA catheter utilization after including all patients with a primary diagnosis of HF without excluding any subgroups.
RESULTS
In 2001 through 2012 we identified 2,492, 284 admissions in the NIS with a primary discharge diagnosis of HF, without any major surgical intervention or use of a mechanical circulatory support device during the index hospitalization, which estimates to 11,888,525 HF hospitalizations nationally over the 12-year period. Of these, 15,786 patients or an estimated 75,209 (0.6%) underwent placement of a PA catheter during the index hospitalization. Patients who received a PA catheter appeared to differ substantially from other patients hospitalized for HF (Table). At hospitals with at least 1 PA catheter placement in HF patients in a given year, patients receiving a PA catheter were younger; more commonly men; and more likely to have cardiogenic shock, AMI, cardiac arrest, require mechanical ventilation as compared with patients who did not undergo PA catheterization. Prevalence of comorbid conditions like hypertension, diabetes, malignancies, and COPD were lower in HF patients with vs. without PA catheter use.
Table.
Differences in characteristics for patients with and without use of pulmonary artery (PA) catheter in heart failure at hospitals using at least 1 PA catheter in HF in a given year
| Characteristics* | PA- catheter used | No PA-catheter used | P-value |
|---|---|---|---|
| Estimated number – N (S.D.) | 75,209 (3276) | 8,279,175 (175,167) | |
| Patient characteristics | |||
| Mean Age (SEM) | 64.8 (0.3) | 72.5 (0.1) | <0.0001 |
| Age ≥ 65 year (%) | 54.2 (0.9) | 73.0 (0.4) | <0.0001 |
| Female Sex | 40.8 (0.6) | 52.6 (0.1) | <0.0001 |
| Race | 0.34 | ||
| White | 51.4 (1.7) | 52.7 (0.9) | |
| Black | 15.7 (0.9) | 16.2 (0.6) | |
| Others | 9.6 (0.6) | 9.9 (0.4) | |
| Missing/Unknown | 23.3 (2.1) | 21.2 (1.0) | |
| Secondary cardiovascular discharge diagnoses | |||
| Cardiogenic shock | 9.3 (0.4) | 0.4 (0.0) | <0.0001 |
| AMI | 5.6 (0.2) | 3.1 (0.0) | <0.0001 |
| CAD | 48.6 (0.5) | 52.3 (0.2) | <0.0001 |
| Cardiac arrest | 3.2 (0.2) | 0.7 (0.0) | <0.0001 |
| Cerebrovascular disease | 3.6 (0.2) | 4.5 (0.0) | <0.0001 |
| Arrhythmia | 50.3 (0.6) | 41.7 (0.3) | <0.0001 |
| Comorbid Conditions | |||
| Hypertension | 56.4 (0.8) | 68.3 (0.3) | <0.0001 |
| Diabetes Mellitus | 37.1 (0.5) | 42.2 (0.2) | <0.0001 |
| Dyslipidemia | 26.4 (0.6) | 27.4 (0.3) | 0.0012 |
| Cancer | 7.8 (0.2) | 10.1 (0.1) | <0.0001 |
| COPD | 24.7 (0.5) | 29.2 (0.2) | <0.0001 |
| Pneumonia | 12.5 (0.4) | 9.4 (0.1) | <0.0001 |
| Chronic kidney disease | 22.7 (0.8) | 19.8 (0.3) | <0.0001 |
| Acute kidney injury | 30.5 (0.8) | 11.6 (0.2) | <0.0001 |
| Liver disease | 10.8 (0.3) | 4.8 (0.1) | <0.0001 |
| Fluid/electrolyte disorder | 33.6 (0.6) | 22.5 (0.2) | <0.0001 |
| Anemia | 23.9 (0.6) | 25.4 (0.2) | 0.0051 |
| Coagulation disorder | 7.7 (0.3) | 3.3 (0.0) | <0.0001 |
| Tobacco abuse | 9.0 (0.4) | 8.0 (0.2) | 0.005 |
| Charlson Comorbidity Index | 1.36 | 1.07 | <0.0001 |
| Past history | |||
| Prior myocardial infarction | 11.1 (0.3) | 11.6 (0.2) | 0.17 |
| Prior CABG | 11.5 (0.3) | 14.4 (0.1) | <0.0001 |
| Reported complications | |||
| Iatrogenic pneumothorax | 0.4 (0.1) | 0.1 (0.0) | <0.0001 |
| Bleeding | 1.0 (0.1) | 0.2 (0.0) | <0.0001 |
| Procedures | |||
| Vasopressor use | 1.6 (0.2) | 0.2 (0.0) | <0.0001 |
| Mechanical ventilation | 20.7 (0.6) | 4.9 (0.1) | <0.0001 |
| Elective admission | 15.3 (0.8) | 7.4 (0.2) | <0.0001 |
| Patient outcomes | |||
| Disposition | <0.0001 | ||
| Home or self-care | 48.3 (0.7) | 57.2 (0.3) | |
| Short term hospital | 6.4 (0.3) | 2.9 (0.1) | |
| Skilled care facility | 11.9 (0.4) | 17.8 (0.2) | |
| Home health care | 19.7 (0.6) | 17.4 (0.2) | |
| Missing/AMA | 0.6 (0.1) | 1.2 (0.0) | |
| Died | 13.1 (0.4) | 3.4 (0.0) | |
| Length of stay (days – mean ± SEM) | 9.9 (0.1) | 5.1 (0.0) | <0.0001 |
| Unadjusted mortality | 13.1 (0.4) | 3.4 (0.0) | <0.0001 |
Represents percentages with the corresponding standard errors in parenthesis, unless otherwise specified
Abbreviations: SD – standard deviation, SEM – standard error of mean, C.I. – confidence interval, AMI - acute myocardial infarction, CAD - coronary artery disease, CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease, AMA – against medical advice
P values for positive trends, unless otherwise stated
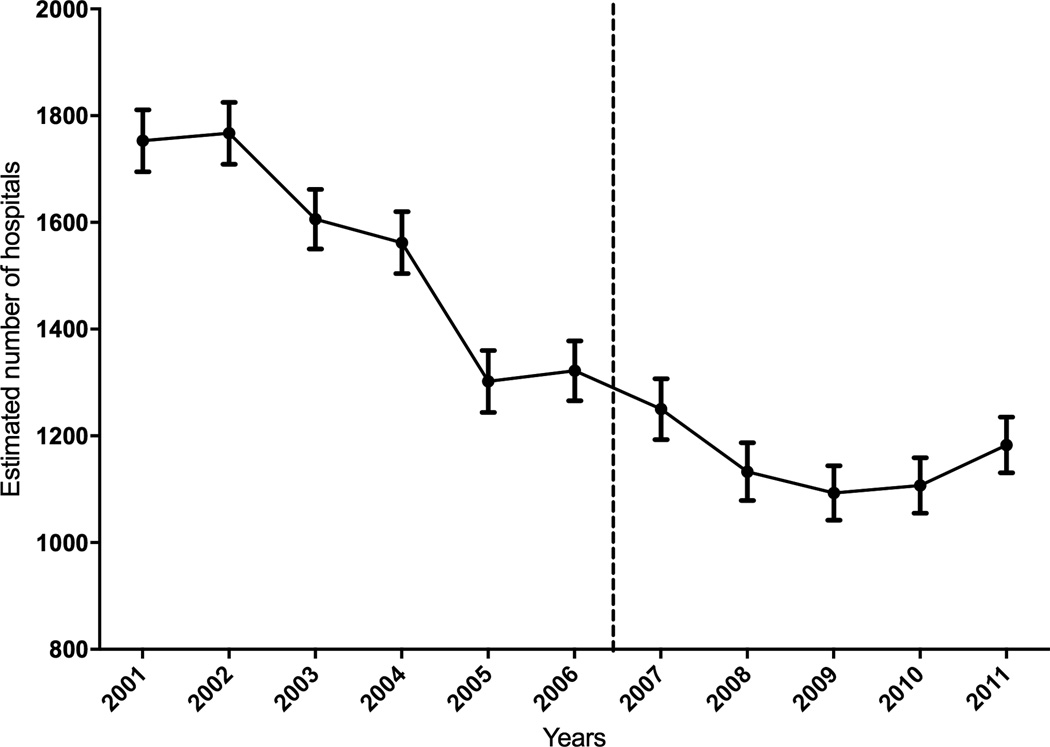
The number of hospitals using at least 1 PA catheter for a HF hospitalization dropped consistently, from an estimated 1753 hospitals in 2001 to 1183 hospitals in 2011 (Figure 1). PA catheter use varied across hospitals, with a mean volume of 15.0/1000 HF hospitalizations in 2001 (limits: 1.0 – 333.3/1000 HF, 10th centile – 2.6/1000 HF; 90th centile 30.0/1000 HF), which decreased to 11.9/1000 HF (limits: 1.1 to 407.4/1000 HF, 10th centile – 2.3/1000 HF; 90th centile 19.9/1000 HF) in 2007, increasing slightly to 12.5/1000 HF (limits: 0.9 to 190.5/1000 HF, 10th centile – 1.9/1000 HF; 90th centile 25.9/1000 HF) in 2011 (Supplemental Figure 1). During this period mean HF admissions/hospital decreased from 413/year in 2001 to 394/year in 2007, increasing thereafter to 475/year in 2011 (Supplemental Figure 2). Overall, the mean PA catheter use per hospital trended down from 4.9/year in 2001 (limits 1–133) to 3.8/year in 2007 (limits 1–46), but increased to 5.5/year in 2011 (limits 1–70) (Supplemental Figure 3). The utilization of PA catheterization varied across hospitals for HF admissions with and without class I indications (cardiogenic shock or respiratory failure) for their use (in 2011 for hospitals >10 HF, class I: mean 63/1000 HF, limits: 5 – 375/1000 HF; and non-class I: mean 10/1000 HF, limits: 0 – 195/1000 HF, Supplemental Figure 4).
Figure 1.
Number of hospitals using PA catheterization in HF patients by study-year.
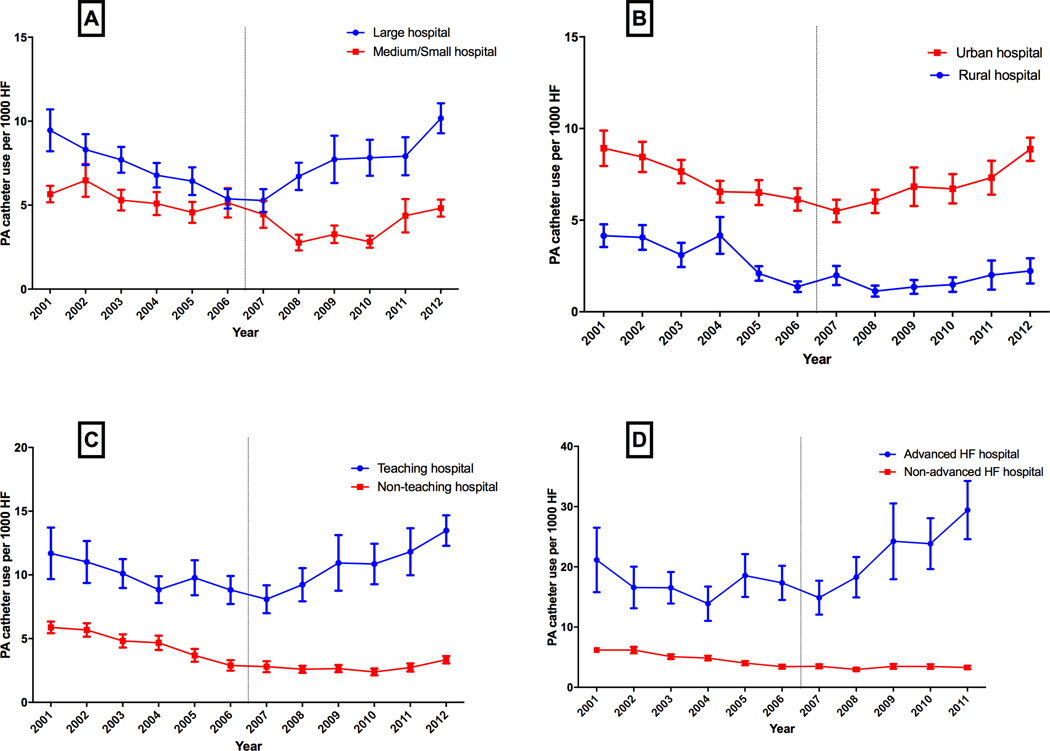
The temporal trends in use of PA catheter varied across different hospital subgroups. There was a significant decline in use of PA catheter in the pre-ESCAPE era across all hospital groups. However, in the post-ESCAPE era, we observed a significant increase in use of PA catheter over time among large bed-size hospitals vs. small or medium size hospitals (Figure 2A), teaching hospitals vs. non-teaching hospitals (Figure 2B), urban centers vs. rural centers (Figure 2C), and advanced HF centers vs. non-advanced HF centers (Figure 2D).
Figure 2.
Trends in PA catheter utilization in HF by hospital characteristics (A) hospital bedsize, (B) urban vs. rural location, (C) teaching status, and (D) advanced heart failure (HF) facilities.
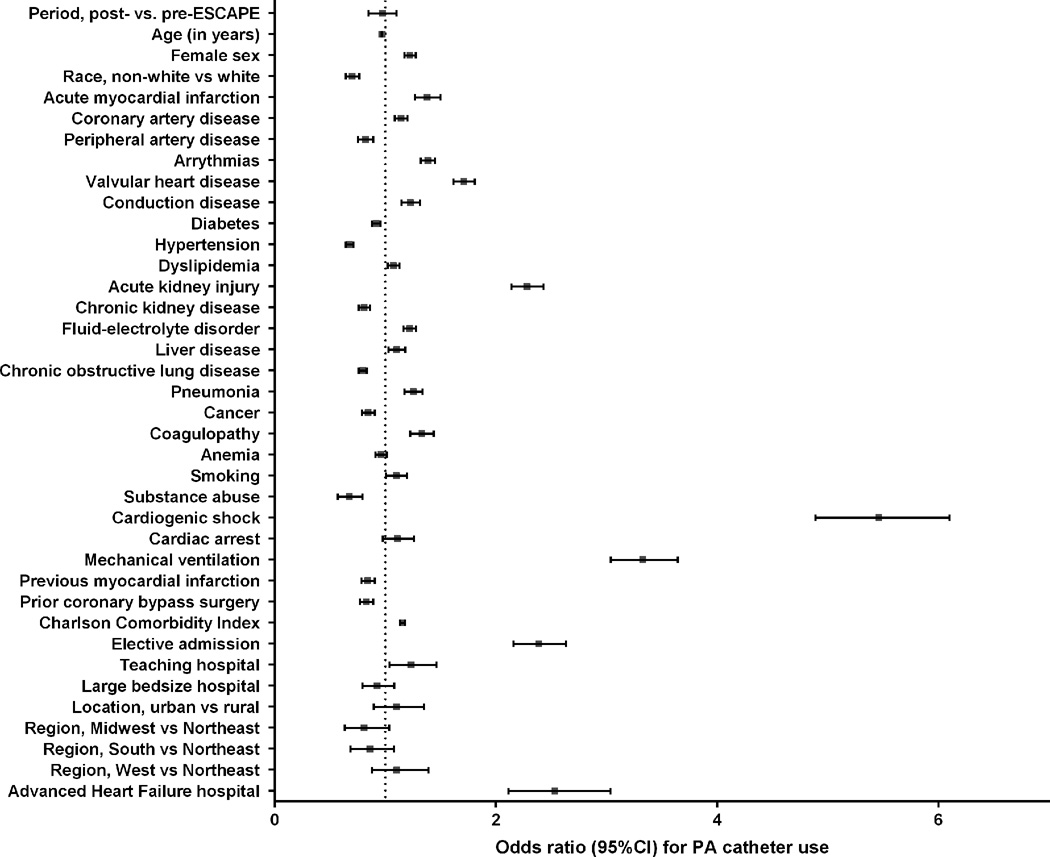
In a stratified multivariable model, patient-level factors independently associated with higher PA catheter use were young age, female sex, elective admission, admission with AMI, cardiogenic shock, respiratory failure requiring mechanical ventilation, acute kidney injury, and valvular heart disease (Figure 3). Hospital characteristics independently associated with higher PA catheter use were advanced HF facilities and teaching status.
Figure 3.
Multivariable predictors of PA catheter use.
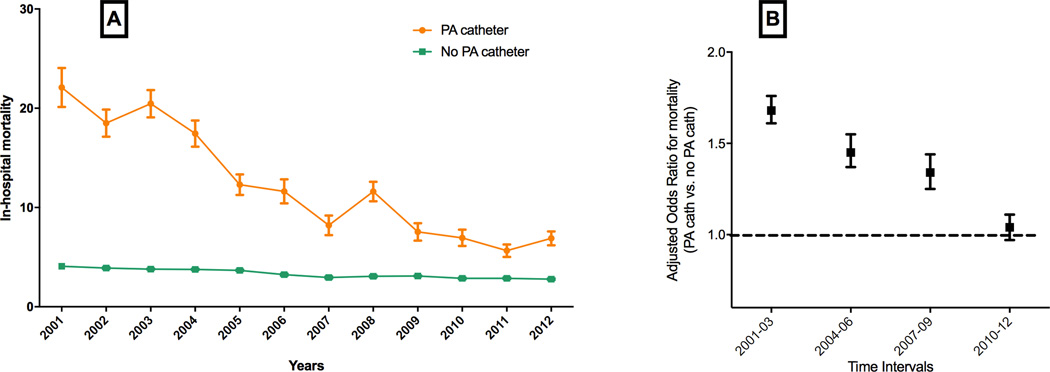
Patients undergoing PA catheterization in a HF hospitalization had higher mortality rates (13.1% vs. 3.4%) and length of stay (9.9 days vs. 5.1 days) compared to those without PA catheter use. Mean length of stay decreased modestly from 10.4 to 9.4 days over the study years and mortality rate decreased substantially from 22.1% in 2001 to 6.9% in 2012. After risk adjustment, temporal trends in mortality continued to be significant (Figure 4A). In 1:2 propensity-matched analyses (Supplemental Figure 5), the risk-adjusted mortality in the PA catheter group was higher than the non-PA catheter group, but the magnitude of difference in mortality attenuated over time, with risk-adjusted odd ratio (OR) for mortality with PA catheter use 1.66 (95% C.I. 1.60 – 1.74) in 2001–2003 to risk-adjusted OR 1.04 (95% C.I. 0.97 – 1.12) in 2010–2012 (Figure 4B). Similar findings were also observed in sensitivity analysis limited to HF patients without cardiogenic shock or use of mechanical ventilation (Supplemental Figure 6).
Figure 4.
Trends in mortality in patients with PA catheterization in HF, (A) overall, and (B) risk-adjusted odds ratios for mortality in propensity-matched analysis in different temporal cohorts.
In post-hoc analyses, rates of reported periprocedural bleeding and iatrogenic pneumothorax were low (≤1%, Supplemental Table 1); and complications, as well as in-hospital mortality did not differ consistently between various hospital subgroups (Supplemental Tables 2–5). Finally, in sensitivity analyses that included all HF admissions, trends in number of hospitals with PA catheter use (Supplemental Figure 7), and average PA catheter utilization – both overall (Supplemental Figure 8) and across hospital subtypes (Supplemental Figure 9) – were consistent with the primary analyses.
DISCUSSION
We observed several important findings in the present study. First, we observed significant hospital-level variation in the utilization of PA catheterization in HF. Second, while there has been a temporal increase in the utilization of PA catheters in HF, this increase appears to be confined to larger, academic, urban hospitals, and centers with advanced HF facilities. Third, significant patient-level factors such as young age, female sex, admission with AMI, and cardiogenic shock were also associated with the use of PA catheters. Finally, HF patients undergoing PA catheter placement had higher in-hospital mortality and longer length of stay as compared with those without PA catheter placement; however, the excess associated mortality risk in the PA catheter group has attenuated substantially over time.
Our study findings provide important insights into the current practice patterns that may be driving the increase in PA catheter use over the past few years. While the total number of hospitals using any PA catheters in HF has declined significantly over the years, the volume of PA catheter use has increased disproportionately among large, urban, academic hospitals with facilities for advanced HF care. Furthermore, in risk-adjusted analysis, we observed that academic status and advanced HF management capabilities were independent hospital-level predictors of increased PA catheter use in our study population. Taken together, our study findings suggest that the temporal increase in PA catheter use in the post-ESCAPE era may be related to its greater utilization at hospitals performing advanced HF therapies such as LVADs and heart transplantation and at other large academic centers, where a proportion of the PA catheter use may represent evaluation for such advanced therapies.
We also identified several patient-level characteristics that were independently associated with use of PA catheter. As expected, presence of class I indications such as cardiogenic shock and respiratory failure requiring mechanical intubation were associated with greater use of PA catheter. Furthermore, presence of AMI, acute kidney injury, and valvular heart disease were also independently associated with greater PA catheter use. This could be related to either more severe disease presentation and need for aggressive monitoring among these patients, or could represent diagnostic evaluation and uncertainty prior to consideration for further therapies in more advanced disease.
PA catheter use was associated with higher mortality risk in the overall study population in the propensity-matched analysis. However, there was a significant temporal decline in the excess mortality risk associated with PA catheter use such that it was not significant among patients treated between 2010–2012. While the improvement in HF mortality over time is consistent with previous reports in the literature and is likely related to the improvement in therapeutic options and quality of care over the past decade,26–29 the reason behind the relative improvement in the PA catheter group could not be ascertained. One potential explanation could be the increasing use of PA catheter among less sick patients as suggested by our prior analysis.9 Alternatively, higher use of PA catheters at large academic hospitals and advanced HF centers may have led to more effective utilization of the hemodynamic data by expert HF physicians to guide clinical management,30 and may have had an impact on patient outcomes.
Our study findings have important clinical implications. The disproportionate increase in PA catheter use among academic centers with advanced HF capabilities coupled with the temporal decline in associated mortality risk points towards possibly a more judicious use of PA-catheter in the current clinical practice. In this regard, it is important to establish that the PA-catheter is a diagnostic tool, with its utility directly linked to the interpretation of the information it provides. As there has been an increase in potential advanced HF treatment options, PA-catheter use has become more prevalent, functioning as an adjunct for these advanced therapies, both during the short-term,11, 31 and long-term management of HF.32, 33 Thus, PA-catheter use, may be providing data that, when used in conjunction with novel treatment options, provides benefit rather than harm for patients.
The findings of our study should be interpreted in light of some limitations. First, because of reliance on ICD-9-CM codes, we were unable to determine the physician perceived indication for PA catheter use and indirectly assessed that information from the documented discharge diagnoses. Moreover, the lack of information on the duration a PA catheter was left in situ precluded an indirect assessment of PA catheter use for diagnostic vs. a monitoring indication. Second, data regarding cause of death and procedural complications are not consistently recorded in the NIS, which makes it difficult to determine whether patients died as a result of underlying illness or a complication from the PA catheter use. While procedural complications were identified using ICD-9 codes, these codes for procedural complications have not been independently validated, and may underrepresent true complication rates. Third, as discussed previously, although we used a matched propensity score design to account for indication bias and our matching algorithm was successful in achieving covariate balance, important clinical variables that may be predictors of outcomes, as well as receipt of PA catheter use, were not available and these findings may be subject to confounding. Hence, the findings of our study represent associations and do not imply causation. Fourth, because of the administrative nature of data, we were unable to distinguish comorbidities from complications of hospitalization. Fifth, it is not possible to track patients longitudinally after discharge in NIS and readmissions will be counted as separate admissions. However, burden of HF hospitalizations has been assessed in the NIS using our current approach and correlates with resource utilization in HF, regardless of the ability to track individual patients.
In conclusion, in this study of US adults hospitalized with HF we observed significant hospital-level variation in use of PA catheterization, along with greater increase in use over time among large, academic hospitals, and advance HF centers. Furthermore, there was a significant decline in mortality risk associated with PA catheter over time in our study population.
Supplementary Material
Clinical Perspective.
Pulmonary artery (PA)-catheters have been used for invasive bedside hemodynamic monitoring in heart failure (HF) both to guide treatment decisions and to evaluate the hemodynamic responses to various therapies. Despite guideline recommendations advising limited use, there has been a significant increase in the use of PA-catheters in HF in the United States in recent years. In the present study, we found that there is substantial hospital-level variability in trends in the utilization of PA catheterization in HF. The recent increase in PA catheterization in HF is largely driven by increasing volume at fewer hospitals overrepresented by large, academic hospitals with advanced HF capabilities. While HF patients undergoing PA catheter placement had higher in-hospital mortality compared with those without PA catheter placement, the excess associated mortality risk in the PA-catheter group has attenuated substantially over time. The disproportionate increase in PA-catheter use among academic centers with advanced HF capabilities coupled with the temporal decline in associated mortality risk points towards possibly a more judicious use of PA-catheter in the current clinical practice. In this regard, it is important to establish that the PA-catheter is a diagnostic tool, with its utility directly linked to the interpretation of the information it provides. As there has been an increase in potential advanced HF treatment options, PA-catheter use has become more prevalent, potentially functioning as an adjunct for these advanced therapies.
Acknowledgments
Sources of Funding
Dr. Fonarow receives research funding from the National Institutes of Health and the Agency for Healthcare Research and Quality. Dr. Girotra is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award K08HL122527. Drs. Khera and Pandey received support from the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number UL1TR001105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Dr. Gregg C. Fonarow reports the following disclosures: Research AHRQ (significant), NIH (significant), Consultant: Amgen (modest), Bayer (modest), Gambro (modest), Novartis (significant), Medtronic (modest).
Footnotes
Disclosures
Other authors: No disclosures
REFERENCES
- 1.Chatterjee K. The Swan-Ganz catheters: past, present, and future. A viewpoint. Circulation. 2009;119:147–152. doi: 10.1161/CIRCULATIONAHA.108.811141. [DOI] [PubMed] [Google Scholar]
- 2.Barnett CF, Vaduganathan M, Lan G, Butler J, Gheorghiade M. Critical reappraisal of pulmonary artery catheterization and invasive hemodynamic assessment in acute heart failure. Expert review of cardiovascular therapy. 2013;11:417–424. doi: 10.1586/erc.13.28. [DOI] [PubMed] [Google Scholar]
- 3.Nohria A, Mielniczuk LM, Stevenson LW. Evaluation and monitoring of patients with acute heart failure syndromes. The American journal of cardiology. 2005;96:32G–40G. doi: 10.1016/j.amjcard.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Fonarow GC, Stevenson LW, Walden JA, Livingston NA, Steimle AE, Hamilton MA, Moriguchi J, Tillisch JH, Woo MA. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol. 1997;30:725–732. doi: 10.1016/s0735-1097(97)00208-8. [DOI] [PubMed] [Google Scholar]
- 5.Steimle AE, Stevenson LW, Chelimsky-Fallick C, Fonarow GC, Hamilton MA, Moriguchi JD, Kartashov A, Tillisch JH. Sustained hemodynamic efficacy of therapy tailored to reduce filling pressures in survivors with advanced heart failure. Circulation. 1997;96:1165–1172. doi: 10.1161/01.cir.96.4.1165. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson LW, Dracup KA, Tillisch JH. Efficacy of medical therapy tailored for severe congestive heart failure in patients transferred for urgent cardiac transplantation. The American journal of cardiology. 1989;63:461–464. doi: 10.1016/0002-9149(89)90320-2. [DOI] [PubMed] [Google Scholar]
- 7.Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW Investigators E and Coordinators ES. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 9.Pandey A, Khera R, Kumar N, Golwala H, Girotra S, Fonarow GC. Use of Pulmonary Artery Catheterization in US Patients With Heart Failure, 2001–2012. JAMA Intern Med. 2016;176:129–132. doi: 10.1001/jamainternmed.2015.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HCUP Databases. Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2014. Jul, www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed] [Google Scholar]
- 11.Khera R, Cram P, Lu X, Vyas A, Gerke A, Rosenthal GE, Horwitz PA, Girotra S. Trends in the use of percutaneous ventricular assist devices: analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med. 2015;175:941–950. doi: 10.1001/jamainternmed.2014.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. American heart journal. 2016;172:53–63. doi: 10.1016/j.ahj.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houchens RLRD, Elixhauser A, Jiang J. Nationwide Inpatient Sample redesign: Final report. [accessed 07/20/2014];2014 available at: https://www.hcup-us.ahrq.gov/db/nation/nis/reports/NISRedesignFinalReport040914.pdf.
- 14.HCUP. AHRQ announces the 2012 Redesign Report. 2014 May; Available at: https://www.hcup-us.ahrq.gov/news/announcements/nisredesign_2012.jsp. 2014.
- 15.Elixhauser A, Steiner C, Palmer L Clinical classifications software (CCS) Book Clinical Classifications Software (CCS) 2008 (Editor ed^ eds) [Google Scholar]
- 16.Association AH. Get With The Guidelines - Heart Failure Fact Sheet. [accessed on June 6, 2015];2015 Available at http://www.heart.org/idc/groups/heart-public/@private/@wcm/@hcm/@gwtg/documents/downloadable/ucm_310967.pdf. [Google Scholar]
- 17.Al-Khatib SM, Hellkamp AS, Fonarow GC, Mark DB, Curtis LH, Hernandez AF, Anstrom KJ, Peterson ED, Sanders GD, Al-Khalidi HR, Hammill BG, Heidenreich PA, Hammill SC. Association between prophylactic implantable cardioverter-defibrillators and survival in patients with left ventricular ejection fraction between 30% and 35% Jama. 2014;311:2209–2215. doi: 10.1001/jama.2014.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993–2004. JAMA. 2007;298:423–429. doi: 10.1001/jama.298.4.423. [DOI] [PubMed] [Google Scholar]
- 19.Rockville, MD: Agency for Healthcare Research and Quality; 2008. Sep, [Accessed July 1. 2016]. HCUP NIS Description of Data Elements. Healthcare Cost and Utilization Project (HCUP) www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nisnote.jsp. [Google Scholar]
- 20.Rockville, MD: Agency for Healthcare Research and Quality; 2008. Sep, [Accessed July 1, 2016]. HCUP NIS Description of Data Elements. Healthcare Cost and Utilization Project (HCUP) www.hcup-us.ahrq.gov/db/vars/hosp_teach/nisnote.jsp. [Google Scholar]
- 21.Rockville, MD: Agency for Healthcare Research and Quality; 2008. Sep, [Accessed July 1, 2016]. HCUP NIS Description of Data Elements. Healthcare Cost and Utilization Project (HCUP) www.hcup-us.ahrq.gov/db/vars/hosp_location/nisnote.jsp. [Google Scholar]
- 22.Houchens REA. HCUP Method Series Report # 2003-02. U.S. Agency for Healthcare Research and Quality; [Accessed 5/13/2014. 2005]. Final Report on Calculating Nationwide Inpatient Sample (NIS) Variances, 2001. ONLINE June 2005 (revised June 6, 2005). Available: http://www.hcup-us.ahrq.gov/reports/methods/CalculatingNISVariances200106092005.pdf. [Google Scholar]
- 23.Feng W, Jun Y, Xu R. A method/macro based on propensity score and Mahalanobis distance to reduce bias in treatment comparison in observational study; SAS PharmaSUG 2006 Conference; 2006. [Google Scholar]
- 24.Radice R, Ramsahai R, Grieve R, Kreif N, Sadique Z, Sekhon JS. Evaluating treatment effectiveness in patient subgroups: a comparison of propensity score methods with an automated matching approach. The international journal of biostatistics. 2012;8:25. doi: 10.1515/1557-4679.1382. [DOI] [PubMed] [Google Scholar]
- 25.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in medicine. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khazanie P, Hammill BG, Patel CB, Eapen ZJ, Peterson ED, Rogers JG, Milano CA, Curtis LH, Hernandez AF. Trends in the use and outcomes of ventricular assist devices among medicare beneficiaries, 2006 through 2011. J Am Coll Cardiol. 2014;63:1395–1404. doi: 10.1016/j.jacc.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonarow GC, Abraham WT, Albert NM, Gattis Stough W, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy CW, Young JB Investigators O-H and Hospitals. Influence of a performance-improvement initiative on quality of care for patients hospitalized with heart failure: results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) Arch Intern Med. 2007;167:1493–1502. doi: 10.1001/archinte.167.14.1493. [DOI] [PubMed] [Google Scholar]
- 29.Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Inge PJ, Mehra MR, O'Connor CM, Reynolds D, Walsh MN, Yancy CW. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF) Circulation. 2010;122:585–596. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
- 30.Angus DC. Ongoing Use of Pulmonary Artery Catheters Despite Negative Trial Findings. JAMA Intern Med. 2016;176:133–134. doi: 10.1001/jamainternmed.2015.6547. [DOI] [PubMed] [Google Scholar]
- 31.Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407–1415. doi: 10.1016/j.jacc.2014.07.958. [DOI] [PubMed] [Google Scholar]
- 32.Lampropulos JF, Kim N, Wang Y, Desai MM, Barreto-Filho JA, Dodson JA, Dries DL, Mangi AA, Krumholz HM. Trends in left ventricular assist device use and outcomes among Medicare beneficiaries, 2004–2011. Open heart. 2014;1:e000109. doi: 10.1136/openhrt-2014-000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancini D, Colombo PC. Left Ventricular Assist Devices: A Rapidly Evolving Alternative to Transplant. J Am Coll Cardiol. 2015;65:2542–2555. doi: 10.1016/j.jacc.2015.04.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.