Summary
The human endocrine pancreas consists of multiple cell types and plays a critical role in glucose homeostasis. Here, we apply mass cytometry technology to measure all major islet hormones, proliferative markers, and readouts of signaling pathways involved in proliferation at single-cell resolution. Using this innovative technology, we simultaneously examined baseline proliferation levels of all endocrine cell types from birth through adulthood, as well as in response to the mitogen harmine. High-dimensional analysis of our marker protein expression revealed three major clusters of beta-cells within individuals. Proliferating beta-cells are confined to two of the clusters.
Graphical Abstract
Introduction
The endocrine pancreas is organized into the islets of Langerhans and consists of at least five different endocrine cell types, each characterized by the production of a major hormone (Andralojc et al., 2009; Stefan et al., 1982). Cellular heterogeneity exists within each endocrine cell type. For instance, the insulin-producing beta-cells differ considerably in their metabolic responsiveness to glucose stimulation (Kiekens et al., 1992; Schuit et al., 1988; Van Schravendijk et al., 1992). Traditional methods to evaluate human pancreatic endocrine cell composition and function are either laborious, lack resolution, or both. While fluorescence-activated cell sorting captures a set of cellular parameters (Davey and Kell, 1996; Perfetto et al., 2004), spectral overlap limits multiplexing capability (Perfetto et al., 2004).
The recently developed mass cytometry technology greatly facilitates high-dimensional, quantitative analysis of biological samples at the single-cell level in a high throughput fashion (Bandura et al., 2009; Bendall et al., 2011; Ornatsky et al., 2010). In mass cytometry, antibodies are conjugated with lanthanide heavy metals instead of fluorophores, and their abundances are measured as discrete isotope masses (Bandura et al., 2009). As a result, mass cytometry is free of fluorescent bleeding and limited only by the number of unique elemental tags available within the detection range of the instrument (Bandura et al., 2009). Furthermore, the use of rare earth metals reduces background signal, and thus mitigates the issue of “autofluorescence” (Bendall et al., 2011). Since its introduction in 2011, mass cytometry has been employed in the field of immunology to great benefit (Bendall et al., 2011; Horowitz et al., 2013; Newell et al., 2012). Here, we adapt mass cytometry to examine cellular heterogeneity within the human endocrine pancreas at the molecular level.
Results
Overview of mass cytometry technology applied to human islets
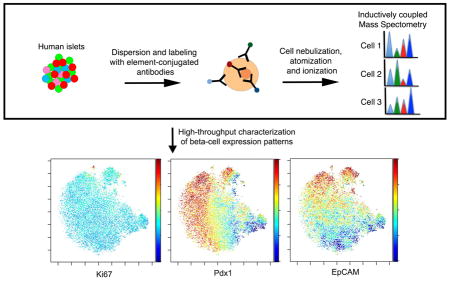
Human pancreatic islet cells and cells isolated along with the islets were labeled with a total of 24 antibodies that passed quality-control (Figures 1A and S1). The targets of these antibodies include the following groups: (1) markers of pancreatic subpopulations, such as C-PEPTIDE (beta cells), GLUCAGON (alpha cells), SOMATOSTATIN (delta cells), POLYPEPTIDE (PP cells), GASTRIN (GASTRIN cells), GHRELIN (epsilon cells), PDX1 (beta and delta cells), HNF1B (ductal cells) and CD49F (Integrin α6, acinar, ductal and subgroups of endocrine cells) (Sugiyama et al., 2007; Wang et al., 2014); (2) a replication marker, Ki67; (3) markers associated with beta-cell proliferation and metabolic activities, such as PDGFRA (Chen et al., 2011), pCREB (Hussain et al., 2006; Jhala et al., 2003), pERK1/2 (Bernal-Mizrachi et al., 2014), pS6 (Balcazar et al., 2009), pSTAT3 (Saxena et al., 2007), pSTAT5 (Jackerott et al., 2006; Nielsen et al., 2001); (4) signaling pathway reporters, such as AXIN2 for WNT signaling, which functions during pancreas development, beta-cell proliferation, and pathophysiology of diabetes (Dabernat et al., 2009; Jho et al., 2002; Rulifson et al., 2007; Sladek et al., 2007), Cleaved-CASPASE3 (Cl-CASPASE3) for apoptosis, CPY26A1 for the retinoic acid pathway, which plays an important role in beta-cell maturation (Loudig et al., 2005; Micallef et al., 2005; Ostrom et al., 2008), and GATA2 for variability in chromatin accessibility (Buenrostro et al., 2015); and (5) markers of beta-cell heterogeneity, such as CD9 and ST8SIA1 (Dorrell et al., 2016) (Figure S2; Tables S1 and S2). In addition, an iridium-containing DNA interchelator was used as a cell indicator and cisplatin as a viability marker (Table S1) (Fienberg et al., 2012; Ornatsky et al., 2008). Data were analyzed using both traditional two-dimensional maps and multi-parametric analysis algorithms (Figure 1B).
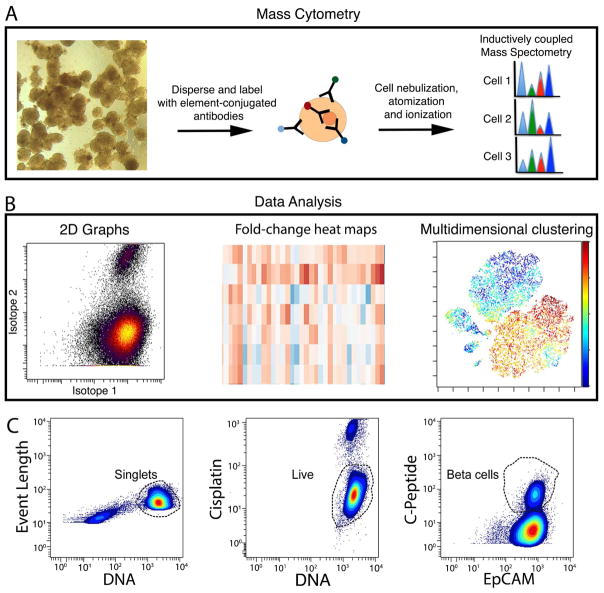
Figure 1. Overview of experimental procedure.
(A) Workflow for sample processing and data analysis. Whole islets were dispersed and labeled with metal conjugated antibodies before loading onto a CyTOF2 instrument. Following nebulization, atomization and ionization, the abundance of different metal-conjugated antibodies within each cell was determined. (B) 2-D biaxial plots, hierarchical clustering, and t-SNE dimension reduction algorithm were employed in downstream data analysis. (C) All events were gated first on singlets, according to DNA content and event length (left). Subsequently, live cells were gated based on cisplatin exclusion (middle). After gating, individual channels were visualized in biaxial plots. An example of C-PEPTIDE versus EpCAM is shown (right). See Table S1 for antibodies used in the current study and Table S2 for antibodies that failed quality control. See Figure S1 for antibody validation and Figure S2A for biaxial plots of individual antibody channel.
Biaxial maps were used for initial gating and assessment of antibody labeling efficiency and specificity (Figures 1C and S2A). Event length, the DNA intercalator iridium, and cisplatin exclusion were used to gate live single cells for downstream analysis (Figure 1C). Islet cells from 20 different donors, covering ages from 18 days to 65 years, were examined (Table S3). Four donors were at or below the age of two, two were 17, and 14 donors were 20 years of age or older. Of donors 17 and older, 13 displayed normal blood glucose homeostasis and 3 had been diagnosed with type 2 diabetes (T2D) (Table S3).
Islet cell types form distinct clusters
We performed analysis with viSNE, a visualization tool based on the t-SNE (t-distributed stochastic neighbor embedding) algorithm that maps multi-parameter relationships of cellular data into two dimensions (Amir el et al., 2013; Maaten, 2008), on cells from all donors. Cells were mapped by viSNE using all available antibody channels (a total of 24 antibodies, Figure S2 and Table S1). Technical variation between batches and and biological variation between donors prevented clustering of multiple samples on the same viSNE plot. Even when samples were barcoded and processed together, donor variation dominated the t-SNE dimension (Figure S3).
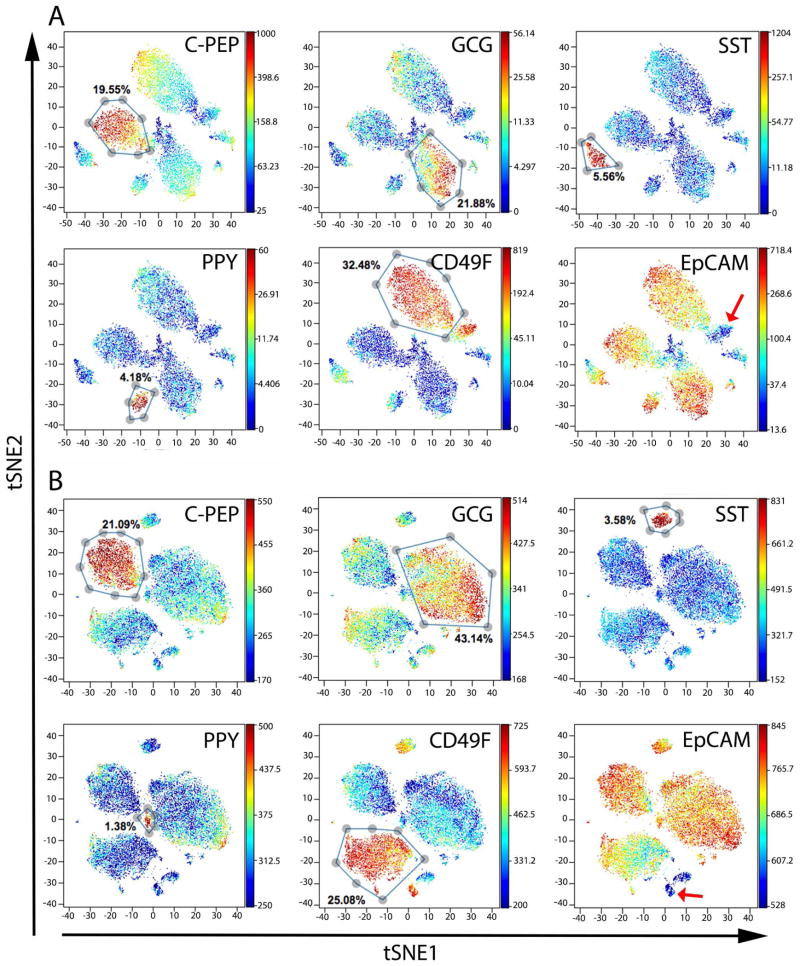
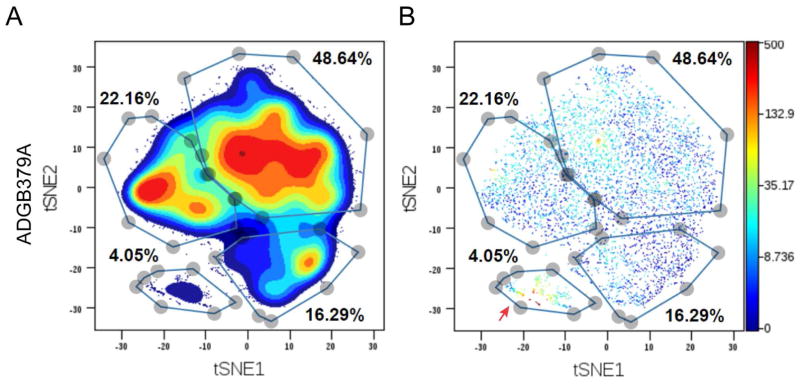
Accordingly, we used viSNE to visualize cells on a per-donor basis. As expected, major cell clusters were formed largely based on expression of the major endocrine hormones produced by each islet cell subtype (Figures 2 and S2B). Both endocrine and exocrine cells exhibited high levels of EpCAM expression, indicating epithelial origin (Figures 2A, 2B and S2B) (Trzpis et al., 2007). In accordance with the literature, CD49F was enriched in acinar and ductal cells as well as in a subset of endocrine cells (Sugiyama et al., 2007; Wang et al., 2014). Additionally, CD49F was expressed in a subset of cells that was negative for EpCAM, suggesting the existence of a previously unidentified mesenchymal subtype within islet preparations (red arrows in Figures 2 and S2B, and Figure S4) (Yu et al., 2012).
Figure 2. viSNE maps show distinct clusters representing different cell types.
t-SNE analysis was performed with all antibody markers used in our experiments. Each dot in the viSNE map represents an individual cell. In all panels, the same viSNE map is shown, colored sequentially by the labeling intensity of C-PEPTIDE (C-PEP, beta cells), GLUCAGON (GCG, alpha cells), SOMATOSTATIN (SST, delta cells), POLYPEPTIDE (PPY, PP cells), CD49F, and EpCAM antibodies. Representative data from two normal adult donors are shown. (A) ACD1098 and (B) ACGZ275. See also Figure S2B for viSNE maps colored by each of the 24 antibodies; Figure S3 for barcoding experiments demonstrating sample-to-sample variation and Figure S4 for biaxial plots of EpCAM and CD49F from all donors. See Table S3 for donor information.
Mass cytometry facilitates accurate and rapid quantification of human islet composition
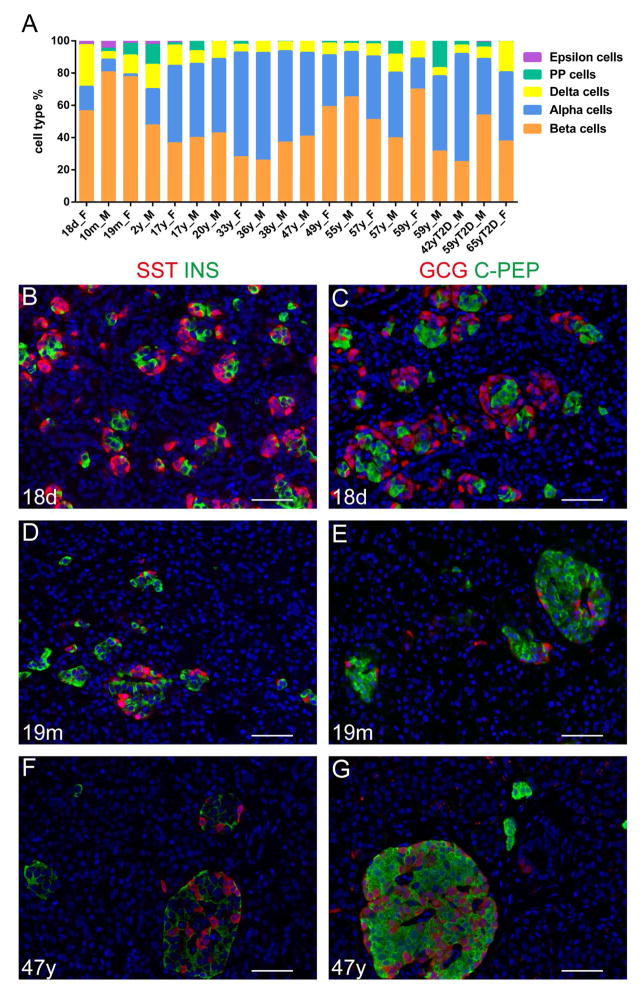
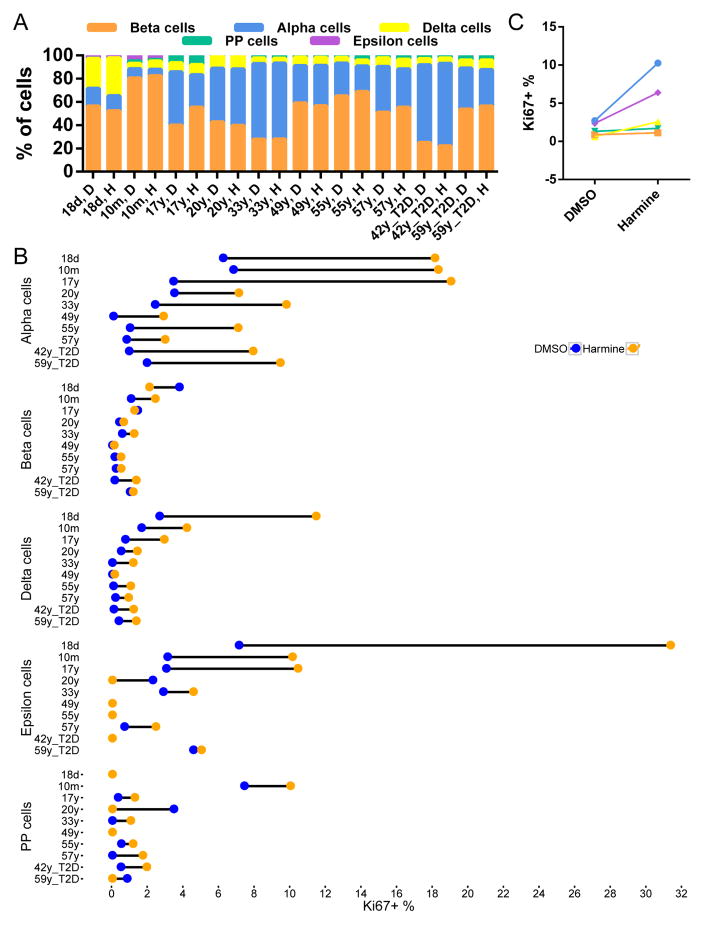
Based on the clusters assembled by the viSNE graph, we gated endocrine subtypes to evaluate the endocrine cell composition for all donors (Figures 2 and 3A). The proportion of endocrine cells varied dramatically from donor to donor, with beta-cell percentages ranging from 25% to 80%, alpha-cell from 2% to 67%, and delta-cell from 5% to 25%. PP and epsilon cells constitute a small proportion of endocrine cells, with epsilon cells only appreciable (more than 2%) in donors less than two years of age (Table S4). The variability in cellular composition was partially age-dependent. Within control donors, delta- and epsilon-cell frequencies displayed a significant negative correlation with age (p=0.042 and 0.00061, respectively, linear regression t-test). In our samples, the percentage of beta-cells plateaued at 19 month old and declined thereafter. The average beta-cell percentage for children was 65.42 ± 16.08 %, for non-diabetic adult donors 42.49 ± 13.81%, and for T2D donors 38.69 ± 14.46%. The age-related rapid decrease of delta- and epsilon-cell percentages and the early plateau of beta-cell abundance are consistent with previous reports (Andralojc et al., 2009; Gregg et al., 2012; Rahier et al., 1981; Stefan et al., 1983). The fractions of cell populations observed by mass cytometry analysis correlated with those seen by immunolabeling from tissue sections of the same donors (Figure 3B–G).
Figure 3. Mass cytometry facilitates quantitative determination of human islet cell-type composition.
(A) Percentage of different endocrine cell types in 20 donors. Donor ages range from 18 days to 65 years of age. Donor gender is indicated by F (female) or M (male). The three diabetic donors are designated as “T2D”. Endocrine cell percentage is listed as total of endocrine cells. (B–G) Representative images from immuno-labeling of SOMATOSTATIN (SST, red) and INSULIN (INS, green) (B, D, and F) or GLUCAGON (GCG, red) and C-PEPTIDE (C-PEP, green) (C, E, and B) in the 18-day (B, C), 19 month (D, E), and 47-year old (F, G) donors. See Table S4 for percentage of endocrine populations in each donor.
Mass cytometry allows simultaneous quantification of proliferation in all endocrine cell types
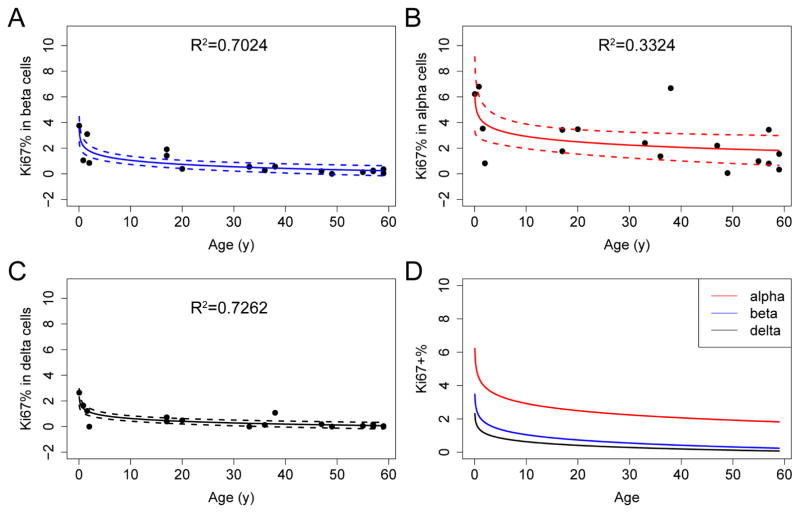
In addition to evaluating the cellular composition of human islets in a high-throughput manner, we also incorporated the proliferation marker Ki67 in our mass cytometry assay (Figure 4). We confirmed that Ki67 was marking cells that had entered the S-phase of the cell cycle by co-labelling cells with both Ki67 and IdU. We detected a high level of correlation between these two proliferation markers (Figure S5). We next examined how proliferation changes with age in each endocrine population. Confirming previous reports, we demonstrated that beta-cell proliferation was highest in the neonatal sample, followed by an exponential decline after childhood (Figure 4A) (Butler et al., 2003; Gregg et al., 2012). Like beta cells, alpha and delta cells displayed diminished proliferation with age (Figures 4B and 4C). Of the three major endocrine cell types, alpha cells had the highest basal replication (p=0.001 between alpha and beta cells; p=3.85×10−5 between alpha- and delta-cells; paired t-test); this phenomenon was observed throughout the human life-span (Figure 4D and Table S5).
Figure 4. Mass cytometry permits the precise and simultaneous assessment of the proliferation index for different endocrine cell types.
(A–C) The percentage of Ki67-positive endocrine cells declines with age in all major endocrine lineages. Each dot represents an individual donor. Regression lines and confidence intervals are also shown. Regression is performed with the linear regression command in R, with the model of (Percentage of Ki67+ cells) ~ log (Age). R2 is shown in each panel. (D) Overlay of regression curves of Ki67 indexes for alpha (red), beta (blue), and delta (black) cells. See Figure S5 for correlation between Ki67 and IdU signals in CyTOF. See Table S5 for quantification of Ki67+ cells in each cell type for each donor.
Harmine stimulates proliferation of multiple endocrine cell populations
The multiparametric measurement capability of mass cytometry provides a unique opportunity to monitor a variety of signaling pathways in multiple cell types in response to various stimuli, such as drug treatment. In a proof-of-principle experiment, we treated islets with the DYRK1A inhibitor harmine, which increases human beta-cell replication (Wang et al., 2015a). Employing mass cytometry, we found no significant change in endocrine cell composition upon harmine treatment (paired t-test), likely because the mitogenic effect of harmine is not restricted to one population (Figures 5A, 5B and S6 and Table S6). Intriguingly, alpha cells from control as well as T2D adults maintained a significantly more robust response to mitogenic signals than beta, delta and PP cells (p≤0.001, two-way ANOVA with Tukey’s correction) (Figure 5C). Harmine had a comparable effect in stimulating replication in T2D endocrine cells, indicating that a long-standing impaired metabolic state did not prevent a proliferative response of endocrine cells to this drug (Figure 5B). Due to the low cell numbers of epsilon and PP populations, the measurement of their proliferation in the adult samples were not as accurate (Figure 5B and Table S5).
Figure 5. Alpha cells exhibit a higher baseline replication and are more responsive to the proliferation stimulus harmine than other endocrine cells throughout life.
(A) Harmine treatment does not alter the cell-type percentage. (B) Proliferation in individual donors’ endocrine cells with and without harmine treatment. For each donor, the proliferation rate of baseline (DMSO, blue) and harmine treated cells (Harmine, orange) are connected by dumbbell. (C) Summary of effect of harmine. Each dot represents the mean percentage of proliferating cells from all donors. Different endocrine populations are color-coded as in (A). Alpha cells show a significantly higher response to harmine than beta, delta and PP cells (two-way ANOVA with Tukey’s correction). See Figure S6 for representative dot plots demonstrating the response of individual endocrine populations to harmine treatment. See Table S6 for quantification of Ki67+ cells for each donor.
Mass cytometry reveals multiple beta-cell states
Mass cytometry provides the unique opportunity to interrogate a large number of cells with multiple parameters at the single-cell level in parallel, thus facilitating the discovery of novel biology, such as the identification of rare cell types and the revelation of subtype-specific behavior (Bendall et al., 2011; Bodenmiller et al., 2012). To take advantage of this opportunity, we gated exclusively for beta-cells and then performed viSNE analysis on each donor, using all antibody channels except non-beta hormone markers. This process segregated beta-cells into several groups within each donor, indicating the existence of beta-cell subtypes, or, at a minimum, beta-cell states (Figure 6A). Using contour maps as a guide, we hand-delineated beta-cell clusters (Figure S7A).
Figure 6. Beta-cells contain multiple subtypes, with proliferating cells concentrated within one cluster.
(A) viSNE map displaying subgroups within beta-cells. All antibody channels except non-beta hormone markers were used for t-SNE computing. Colors indicate density of cells. Subgroups were manually gated based on natural occurring groups revealed by different density center. A graph from one representative donor (ADGB379A) is shown. (B) The same viSNE map is shown as in (A), but individual cells are depicted and colored by Ki67 expression level. Red arrows indicate the cluster of Ki67+ cells. See also Figure S7.
The existence of multiple beta-cell states is consistent with a recent finding by means of flow cytometry that multiple subtypes exist within β-cells (Dorrell et al., 2016). Intriguingly, beta cells with high Ki67+ cells often segregated to only one of these subtypes (Figure 6B), even when clustering was performed with the exclusion of the Ki67 channel (data not shown). This result may indicate that a state of beta-cells exists that is poised to enter the cell cycle.
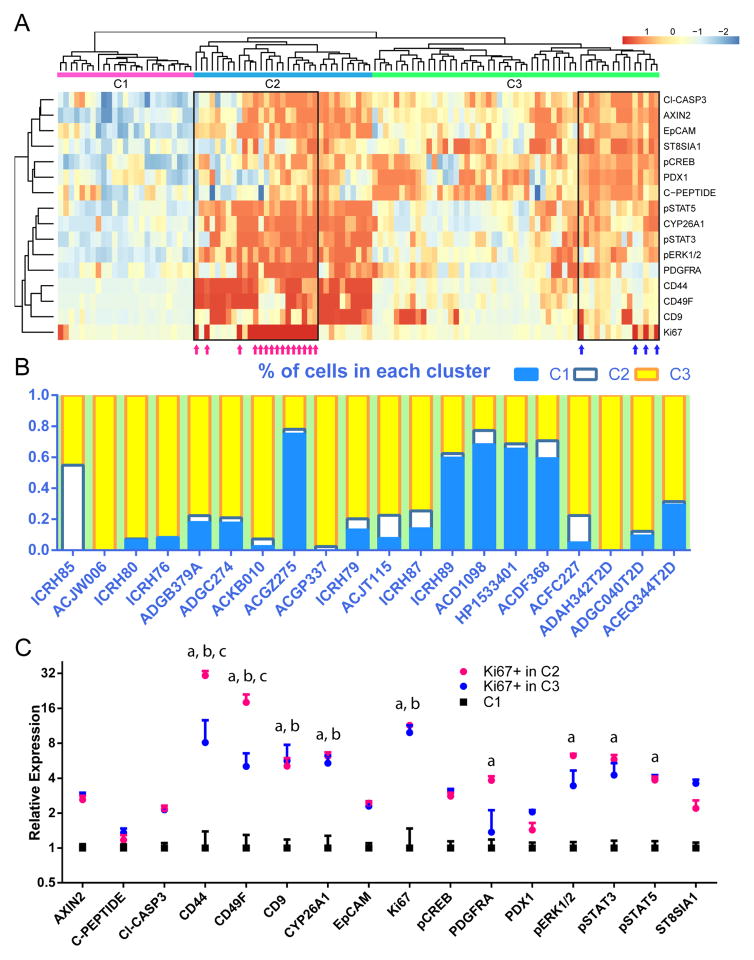
We next asked whether features from these subgroups were consistent among all the donors. To this end, we performed hierarchical clustering on each of the subgroups acquired from viSNE mapping, plus addition groups containing Ki67+ beta cells from every donor (Figure 7A). Normalized median protein expression levels of each beta-cell group were used as the input for this process. Subgroups from all the donors organized into three main clusters, labelled C1, C2 and C3, each with distinct protein expression patterns (Figure 7A). The groups of Ki67+ beta cells segregated to C2 and C3 (magenta and blue arrows, Figure 7A). The three clusters generated by the heatmap mostly corresponded with the original beta-cell group annotations based on cell density separation on viSNE, albeit with fewer clusters apparent in each donor than originally called (Figure S7). The C2 cluster mapped to the group of beta cells containing most Ki67+ cells.
Figure 7. Three main clusters of beta-cells are observed from multiple donors, and cells with relatively high levels of Ki67 separate into two of the clusters.
(A) Hierarchical clustering of groups of beta cells from all donors reveal three major clusters, labelled C1, C2 and C3. Ki67+ cells separate into two groups, co-segregating either with C2 or C3 clusters (magenta and blue arrows within black box). Each column of the heatmap represents one subgroup from viSNE map (an example of which is shown in Figure 6(A)), with the addition of populations of Ki67+ beta cells gated directly from individual donor. Each row of the heatmap represents the relative expression level of one protein. 16 antibody channels are utilized in samples from all donors. (B) Census of the percentage of cells in each cluster from individual donors, ordered by age. T2D samples are shown on the right. (C) Expression levels of the 16 proteins from cells in C1, Ki67+ cells in C2, and Ki67+ cells in C3. Each data point represents mean expression + standard error of one of the three populations, normalized to C1. a, b, and c indicate significance as calculated by a two-way ANOVA test with Tukey’s correction. a: comparison between C1 and Ki67+ in C2. b: comparison between C2 and Ki67+ in C3. c: comparison between Ki67+ in C2 and Ki67+ in C3. See also Figure S7 for each cluster mapped back onto a viSNE plot.
Donor-to-donor variations in the percentage of cells contained within each cluster were noticeable (Figure 7B). To further explore factors involved in the partition of cells in each cluster, we built a regression model with age, T2D status, gender, ethnicity and BMI as predictors. The regression model indicated that fraction of cells in C1 positively correlated with age and negatively correlated with BMI, while the percentage of cells in the C2 cluster correlated negatively with T2D status and the fraction of cells in the C3 cluster correlated negatively with age (p<0.05, beta regression).
Subsequently, we compared protein expression between the two Ki67+ groups and the quiescent group of beta cells that did not contain any proliferating beta-cells (C1) (Figure 7C). Both Ki67+ clusters expressed significantly higher levels of the cell-surface markers CD44, CD49F, and CD9 as well as CYP26A1 and, reassuringly, Ki67. The Ki67+ cells in C2 expressed higher levels of PDGFRA, pERK1/2, pSTAT3 and pSTAT5 compared to either the Ki67+ cells in C3 or the quiescent C1 cells. Furthermore, the Ki67+ cells in C2 had even higher levels of CD44 and CD49F compared to those in C3.
Discussion
In this manuscript, we employed mass cytometry to simultaneously examine human endocrine cell composition, proliferation and protein levels in a single assay. We were able to corroborate previously published information showing (1) high variation in the composition of the human islet compartment (Blodgett et al., 2015; Brissova et al., 2005; Cabrera et al., 2006); (2) the decline of human pancreatic endocrine cell replication with age (Cnop et al., 2010; Gregg et al., 2012; Meier et al., 2008; Perl et al., 2010; Reers et al., 2009); and (3) the mitogenic effect of harmine on pancreatic endocrine cells (Wang et al., 2015a). Since we assessed replication in all islet cell types at the same time, we were able to overcome the traditional limitation of fluorescent co-immunolabelling on paraffin sections, for which generally a maximum of four channels can be utilized (Wang et al., 2015b). Because mass cytometry assays each cell individually, this experimental design also bypasses manual counting and sectioning bias, which are both time-consuming processes and may lead to inaccurate measurements (Tang et al., 2012). In addition, the usage of rare earth metals in the conjugation of antibodies for mass cytometry ensures a low background from biological sources, and thus no need to correct for autofluorescence (Bendall et al., 2011).
In the current study, we demonstrate large donor-to-donor variation in protein expression and cellular composition, resulting in inconsistent mapping positions on viSNE, even for demultiplexed samples after barcoding (Figures 2, 3 and S3). This variation could result from bona fide differences between donors, technical variation in islet isolations, technical variations in CyTOF sample preparations, or day-to-day differences in CyTOF2 sensitivity. However, since barcoding experiment controls for technical variation between sample preparations, a major source of variation is likely endogenous differences between samples. Unfortunately, for the analysis of human islets, we are dependent on organ availability and tissue processing at multiple academic medical centers, which contributes to some of the observed variability between samples and which limits the ability to multiplex donors.
Despite donor-to-donor differences, we demonstrated that a population of CD49F+; EpCAM− cells exists in most donors’ islet preparations (Figures 2, S2 and S4). CD49F has been associated with stem cells; the CD49F+; EpCAM− population may thus represent a novel mesenchymal stem cell population isolated along with pancreatic islets (Yu et al., 2012). Furthermore, when we analyzed our single-cell RNA-seq performed on the human pancreas, this population was also observed (Wang et al., 2016)).
Our mass cytometry procedure gives higher values for Ki67+ beta cells compared with immune-labeling methods in pancreatic sections, often obtained from autopsy specimens (Atkinson et al., 2015; Butler et al., 2003; Gregg et al., 2012; Kassem et al., 2000; Wang et al., 2015b). This discrepancy could stem from differences in tissue treatments: our cells were maintained in culture up to the point of analysis, in contrast to most reports of human beta-cell proliferation, in which cadaveric tissues are processed and embedded in paraffin, a process that has recently been shown to have the potential to cause degradation of tissue morphology and protein integrity, leading to an underestimation of the replication rate (Sullivan et al., 2015). In addition, in our study, we assessed on average, tens of thousands of beta-cells in each donor, which facilitates the capture of rare proliferating cells. Alternatively, the higher rate reported here could be a result of the more sensitive quantitative measurement obtained from mass cytometry.
We also demonstrated that of all pancreatic endocrine cell types, alpha-cells have the highest basal replication rate and are most responsive to the mitogen harmine across all donors (Figures 4 and 5). At present, it is unknown whether the life-span of a human alpha-cell is shorter than that of beta-and delta cells. However, it is tempting to speculate that the higher rate of alpha-cell replication, together with the notion of islet cell plasticity, would allow conversion of supernumerary alpha-cells into beta-cells as part of islet homeostasis or in response to metabolic stress. Combined with recent discoveries that alpha-cells maintain both activating and repressing histone-mark bivalency at thousands of loci and can be converted to beta-cells upon genetic or epigenetic manipulations, this finding raises the possibility that the expanding alpha-cell population may serve as a potential therapeutic target for type 1 and type 2 diabetes (Bramswig et al., 2013; Collombat et al., 2009; Thorel et al., 2010; Yang et al., 2011; Ye et al., 2015).
While the Stewart group has already reported an increase in beta-cell replication, along with some proliferation in other endocrine cell types upon treatment with harmine, they did not examine the mitogenic response of endocrine cells from patients with T2D (Wang et al., 2015a). We show that endocrine cells from two donors with T2D increase proliferation in response to harmine in a similar fashion as those from non-diabetic organ donors, with a greater response in alpha-cell than beta-cell proliferation (Figure 5).
Using high-dimensional, multi-parameter analysis, we demonstrate that beta cells form three major groups that may reflect different cellular states. Proliferating beta cells occupy two of these three clusters (Figure 7A). The fraction of cells in C1 is positively correlated with age, while the fraction of cells in C3 is inversely correlated with age (Figure 7B). There are two potential explanations: (1) with age, some of the beta cells switch from a more proliferative state (C3) to a more quiescent state (C1); and (2) the net turnover of C3 population is more negative compared with the other populations. Another interesting discovery is that T2D donors have significantly lower cell percentages in C2, suggesting either that the lack of that this population may contribute to the development of T2D, or that it may be vulnerable to the metabolic toxicity accompanying diabetes. Further study is required to determine the biological significance of these different beta cell groups.
We identify heterogeneity within the proliferating beta cells as well. Among the markers that are more highly expressed in both of these two groups of Ki67+ cells, CD44 and CD49F have been reported to mark highly proliferative pancreatic progenitor cells and pancreatic cancer initiating cells (Figure 7C) (Li et al., 2007; Sugiyama et al., 2007). In addition, proteins that are upregulated significantly in the C2 Ki67+ cells, i.e. PDGFRA, pERK1/2, pSTAT3 and pSTAT5, have previously reported roles in beta-cell proliferation (Figure 7C) (Bernal-Mizrachi et al., 2014; Chen et al., 2011; Jackerott et al., 2006; Nielsen et al., 2001; Saxena et al., 2007).
In conclusion, this study reports the first use of mass cytometry in a solid tissue. The high-throughput, multi-parametric nature of mass cytometry, combined with its single-cell resolution and direct determination of relative protein levels, will greatly facilitate the understanding of biology in several fields. Using this technology, we found that of all the major endocrine cell types, alpha-cells have both the highest baseline level of replication as well as the highest mitotic response to harmine. We further document that human beta-cells exist in distinct cellular states or subtypes, confirming recent findings (Dorrell et al., 2016). In summary, mass cytometry represents a powerful tool for diabetes researchers.
Experimental Procedures
Antibody conjugation
Antibodies were conjugated according to the manufacturer’s protocol (Fluidigm Multi-Metal Maxpar® Kit). Briefly, the supplied polymer was linked with a lanthanide metal of choice in buffer L at 37°C for 30 to 40 min. The antibodies were partially reduced in buffer R plus TCEP and then purified by buffer exchange using a 50 kDa Amicon filter (Millipore, UFC505096). The metal-loaded polymers were concentrated on a 3 kDa Amicon filter (Millipore, UFC500396), added to the reduced antibody, and conjugated at 37°C for 1.5–2 hours. Conjugated antibodies were then washed in buffer W and diluted 1:1 in Antibody Stabilizer (Candor Biosciences, 131050).
Islet acquisition and culture
Human islets were acquired from the Diabetes Research Center of the University of Pennsylvania (NIH DK 19525), Prodo Labs, and the Integrated Islet Distribution Program (IIDP) (http://iidp.coh.org/). Islets were cultured in Prodo Islet Media (PIMS® Standard) with 5% human albumin serum and a glucose concentration of 5.8 mM. For stimulation of proliferation, islets were incubated with 10 μM harmine or the vehicle DMSO (Sigma, 286044) for 72–96 hours.
Isolation and labeling of dissociated cells
Approximately 20,000 human islet equivalents (IEQs) were dissociated using 0.05% trypsin; trypsin was neutralized by the addition of an equal volume of 100% FBS. Cells were passed through a BD FACS tube with a 40 μm strainer top (BD Biosciences, 352235). Dissociated cells were washed once with PBS containing 10% FBS and once with PBS. Cells were resuspended at a density of 10x106/ml in PBS with cisplatin diluted to 1:4000 and incubated at room temperature for 5 min. Cisplatin labelling was stopped by the addition of a 5x volume of PBS with 10% FBS. The cells were then washed twice with PBS with 10% FBS. Dissociated cells were fixed with FoxP3 Fixation/Permeabilization buffer (eBioscience, 00-5123 and 00-5223) at room temperature (RT) for 2 hours followed by twice washing with FoxP3 permeabilization buffer (eBioscience. 00-8333). Antibody labeling was performed in FoxP3 permeabilization buffer for 8 hours at 4°C at a concentration of up to 2 million cells per 300 μl of antibody cocktail, followed by twice washing with FoxP3 permeabilization buffer. Cells were then incubated with the DNA intercalator Iridium (Fluidigm, 201192A) at a dilution of 1:4000 in Fluidigm Fixation and Permeabilization buffer (Fluidigm, 201067) at RT for 1 hour.
Mass cytometry data acquisition
Mass cytometry data were obtained on CyTOF2 (Fluidigm). Immediately before applying the samples to the CyTOF2 instrument, the cells were washed twice with PBS and twice with MilliQ H2O. Cells were resuspended in MilliQ H2O at a density of 5x105/ml and mixed together with 4-element normalization beads (Fluidigm, 201078). Cell events were acquired at a rate of 300–500 events per second. Bead normalization was performed with the Nolan laboratory bead normalizer (Finck et al., 2013). Only loop 1 of the CyTOF instrument was used throughout the entire study.
Antibody selection and titration
Antibodies were validated by the following three methods. (1) Immunofluorescent labelling (Figure S1A–S1E): the labeling efficiency of each antibody was tested by direct labeling of human islet cells in suspension. Briefly, human pancreatic cells were dissociated and labeled with the test antibodies following the same procedure as when performing mass cytometry. Subsequently, Cy3-conjugated secondary antibodies were applied. Cells were cytospun onto microscope slides and imaged under a fluorescent scope. Only those antibodies displaying the expected staining patterns were used in downstream experiments. (2) Flow cytometry (Figure S1F): cells were dissociated and labelled as in mass cytometry experiment, followed by secondary antibody staining in the Cy3 channel. Cellular events were subsequently acquired by a BD LSRII following a standard flow cytometry protocol. (3) Stimulation followed by CyTOF2 sample acquisition (Figure S1G): human islets were (a) incubated in Retinoic acid at 50 nM final concentration for 72 hours; (b) serum starved overnight, followed by stimulation with 1.25 ng/μl Leptin for 4 hours; or (c) serum starved for 48 hours, followed by stimulation with Prolactin at 200 ng/ml for 30 mins. After stimulation, cells were dissociated and processed following a normal mass cytometry sample preparation protocol. Antibodies that passed initial quality control were titrated in CyTOF with 1:100, 1:200, 1:500, 1:1000 and 1:10000 dilutions. Unlabeled cells were used as a negative control.
Barcoding
Barcoding was performed for select donors following the manufacturer’s protocol (Fluidigm, 101-0804 B1) (Table S3). Briefly, human pancreatic islet cells were dissociated, labelled with cisplatin and fixed as described above. Following fixation, cells were washed twice in permeabilization buffer and incubated with an assigned barcode at RT for 30 mins. Barcoded samples were combined and then processed as above.
Data analysis
viSNE analyses were performed using the Cytobank implement (http://www.cytobank.org/). For hierarchical clustering, expression levels of each protein were normalized by the maximum value of the channel within each donor. Rows and columns were clustered by hclust complete linkage with Euclidean distance. To estimate factors involved in partition of cells into the C1, C2 and C3 clusters, betareg package for r was employed, with age, T2D status, gender, ethnicity and BMI as covariates and a logit link function. Default settings were used for other parameters (Cribari-Neto, 2010). Other statistical analysis methods are described within results and figure legends.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Takuya Ohtani for his excellent technical support in operating the CyTOF, and Drs. Wade Rogers, Bertram Bengsch, Shilpa Rao, Adam Zahn, as well as Amber Wang, for their expertise and insight into data processing. We also want to thank Dr. Andrew Raim for consult on statistical analysis. This study was supported by UC4DK104119 to K.H.K. The CyTOF facility was supported by the VISN4 High Cost High Tech Equipment Fund, the Medical Research Program at the Philadelphia Corporal Michael J. Crescenz VA Medical Center, and the University of Pennsylvania Institute for Immunology.
Footnotes
Author contributions
Conceptualization: K.H.K; Methodology: Y.J.W.; Formal Analysis: Y.J.W., M.L.G., and J.S. Investigation: Y.J.W. and M.L.G. Resources: D.T., A.C.P., A.N., C.L., K-M.C., M.G., and C.D; Writing: Original Draft: Y.J.W. and M.L.G.; Writing: Review and Editing: Y.J.W., M.L.G., D.T., and K.H.K.; Visualization: Y.J.W., M.L.G., and J.S.; Supervision: M.L.G. and K.H.K.; Funding Acquisition: K.H.K.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amir el AD, Davis KL, Tadmor MD, Simonds EF, Levine JH, Bendall SC, Shenfeld DK, Krishnaswamy S, Nolan GP, Pe’er D. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nature biotechnology. 2013;31:545–552. doi: 10.1038/nbt.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andralojc KM, Mercalli A, Nowak KW, Albarello L, Calcagno R, Luzi L, Bonifacio E, Doglioni C, Piemonti L. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia. 2009;52:486–493. doi: 10.1007/s00125-008-1238-y. [DOI] [PubMed] [Google Scholar]
- Atkinson MA, von Herrath M, Powers AC, Clare-Salzler M. Current concepts on the pathogenesis of type 1 diabetes--considerations for attempts to prevent and reverse the disease. Diabetes care. 2015;38:979–988. doi: 10.2337/dc15-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcazar N, Sathyamurthy A, Elghazi L, Gould A, Weiss A, Shiojima I, Walsh K, Bernal-Mizrachi E. mTORC1 activation regulates beta-cell mass and proliferation by modulation of cyclin D2 synthesis and stability. The Journal of biological chemistry. 2009;284:7832–7842. doi: 10.1074/jbc.M807458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura DR, Baranov VI, Ornatsky OI, Antonov A, Kinach R, Lou X, Pavlov S, Vorobiev S, Dick JE, Tanner SD. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Analytical chemistry. 2009;81:6813–6822. doi: 10.1021/ac901049w. [DOI] [PubMed] [Google Scholar]
- Bendall SC, Simonds EF, Qiu P, Amir el AD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Mizrachi E, Kulkarni RN, Scott DK, Mauvais-Jarvis F, Stewart AF, Garcia-Ocana A. Human beta-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map. Diabetes. 2014;63:819–831. doi: 10.2337/db13-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodgett DM, Nowosielska A, Afik S, Pechhold S, Cura AJ, Kennedy NJ, Kim S, Kucukural A, Davis RJ, Kent SC, et al. Novel Observations From Next-Generation RNA Sequencing of Highly Purified Human Adult and Fetal Islet Cell Subsets. Diabetes. 2015;64:3172–3181. doi: 10.2337/db15-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenmiller B, Zunder ER, Finck R, Chen TJ, Savig ES, Bruggner RV, Simonds EF, Bendall SC, Sachs K, Krutzik PO, et al. Multiplexed mass cytometry profiling of cellular states perturbed by small-molecule regulators. Nature biotechnology. 2012;30:858–867. doi: 10.1038/nbt.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, Streeter PR, Naji A, Grompe M, Kaestner KH. Epigenomic plasticity enables human pancreatic alpha to beta cell reprogramming. The Journal of clinical investigation. 2013;123:1275–1284. doi: 10.1172/JCI66514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- Buenrostro JD, Wu B, Litzenburger UM, Ruff D, Gonzales ML, Snyder MP, Chang HY, Greenleaf WJ. Single-cell chromatin accessibility reveals principles of regulatory variation. Nature. 2015;523:486–490. doi: 10.1038/nature14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Gu X, Liu Y, Wang J, Wirt SE, Bottino R, Schorle H, Sage J, Kim SK. PDGF signalling controls age-dependent proliferation in pancreatic beta-cells. Nature. 2011;478:349–355. doi: 10.1038/nature10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Hughes SJ, Igoillo-Esteve M, Hoppa MB, Sayyed F, van de Laar L, Gunter JH, de Koning EJ, Walls GV, Gray DW, et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia. 2010;53:321–330. doi: 10.1007/s00125-009-1562-x. [DOI] [PubMed] [Google Scholar]
- Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribari-Neto F, Zeileis A. Beta Regression in R. Journal of Statistical Software. 2010;34:1–24. [Google Scholar]
- Dabernat S, Secrest P, Peuchant E, Moreau-Gaudry F, Dubus P, Sarvetnick N. Lack of beta-catenin in early life induces abnormal glucose homeostasis in mice. Diabetologia. 2009;52:1608–1617. doi: 10.1007/s00125-009-1411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey HM, Kell DB. Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analyses. Microbiological reviews. 1996;60:641–696. doi: 10.1128/mr.60.4.641-696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirice E, Walpita D, Vetere A, Meier BC, Kahraman S, Hu J, Dancik V, Burns SM, Gilbert TJ, Olson DE, et al. Inhibition of DYRK1A Stimulates Human beta-Cell Proliferation. Diabetes. 2016;65:1660–1671. doi: 10.2337/db15-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, Schug J, Canaday PS, Russ HA, Tarlow BD, Grompe MT, Horton T, Hebrok M, Streeter PR, Kaestner KH, et al. Human islets contain four distinct subtypes of beta cells. Nat Commun. 2016;7:11756. doi: 10.1038/ncomms11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fienberg HG, Simonds EF, Fantl WJ, Nolan GP, Bodenmiller B. A platinum-based covalent viability reagent for single-cell mass cytometry. Cytometry Part A : the journal of the International Society for Analytical Cytology. 2012;81:467–475. doi: 10.1002/cyto.a.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck R, Simonds EF, Jager A, Krishnaswamy S, Sachs K, Fantl W, Pe’er D, Nolan GP, Bendall SC. Normalization of mass cytometry data with bead standards. Cytometry Part A : the journal of the International Society for Analytical Cytology. 2013;83:483–494. doi: 10.1002/cyto.a.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA, Rhodes CJ. Formation of a human beta-cell population within pancreatic islets is set early in life. The Journal of clinical endocrinology and metabolism. 2012;97:3197–3206. doi: 10.1210/jc.2012-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, Dekker CL, Mackey S, Maecker H, Swan GE, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Science translational medicine. 2013;5:208ra145. doi: 10.1126/scitranslmed.3006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain MA, Porras DL, Rowe MH, West JR, Song WJ, Schreiber WE, Wondisford FE. Increased pancreatic beta-cell proliferation mediated by CREB binding protein gene activation. Molecular and cellular biology. 2006;26:7747–7759. doi: 10.1128/MCB.02353-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackerott M, Moldrup A, Thams P, Galsgaard ED, Knudsen J, Lee YC, Nielsen JH. STAT5 activity in pancreatic beta-cells influences the severity of diabetes in animal models of type 1 and 2 diabetes. Diabetes. 2006;55:2705–2712. doi: 10.2337/db06-0244. [DOI] [PubMed] [Google Scholar]
- Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes & development. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Molecular and cellular biology. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes. 2000;49:1325–1333. doi: 10.2337/diabetes.49.8.1325. [DOI] [PubMed] [Google Scholar]
- Kiekens R, In ’t Veld P, Mahler T, Schuit F, Van De Winkel M, Pipeleers D. Differences in glucose recognition by individual rat pancreatic B cells are associated with intercellular differences in glucose-induced biosynthetic activity. The Journal of clinical investigation. 1992;89:117–125. doi: 10.1172/JCI115551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer research. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Loudig O, Maclean GA, Dore NL, Luu L, Petkovich M. Transcriptional cooperativity between distant retinoic acid response elements in regulation of Cyp26A1 inducibility. The Biochemical journal. 2005;392:241–248. doi: 10.1042/BJ20050874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaten L, Hinton G. Visualizing Data using t-SNE. Journal of Machine Learning Research. 2008;9:2579–2605. [Google Scholar]
- Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef SJ, Janes ME, Knezevic K, Davis RP, Elefanty AG, Stanley EG. Retinoic acid induces Pdx1-positive endoderm in differentiating mouse embryonic stem cells. Diabetes. 2005;54:301–305. doi: 10.2337/diabetes.54.2.301. [DOI] [PubMed] [Google Scholar]
- Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity. 2012;36:142–152. doi: 10.1016/j.immuni.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JH, Galsgaard ED, Moldrup A, Friedrichsen BN, Billestrup N, Hansen JA, Lee YC, Carlsson C. Regulation of beta-cell mass by hormones and growth factors. Diabetes. 2001;50(Suppl 1):S25–29. doi: 10.2337/diabetes.50.2007.s25. [DOI] [PubMed] [Google Scholar]
- Ornatsky O, Bandura D, Baranov V, Nitz M, Winnik MA, Tanner S. Highly multiparametric analysis by mass cytometry. Journal of immunological methods. 2010;361:1–20. doi: 10.1016/j.jim.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Ornatsky OI, Lou X, Nitz M, Schafer S, Sheldrick WS, Baranov VI, Bandura DR, Tanner SD. Study of cell antigens and intracellular DNA by identification of element-containing labels and metallointercalators using inductively coupled plasma mass spectrometry. Analytical chemistry. 2008;80:2539–2547. doi: 10.1021/ac702128m. [DOI] [PubMed] [Google Scholar]
- Ostrom M, Loffler KA, Edfalk S, Selander L, Dahl U, Ricordi C, Jeon J, Correa-Medina M, Diez J, Edlund H. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into beta-cells. PloS one. 2008;3:e2841. doi: 10.1371/journal.pone.0002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetto SP, Chattopadhyay PK, Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Nature reviews Immunology. 2004;4:648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- Perl S, Kushner JA, Buchholz BA, Meeker AK, Stein GM, Hsieh M, Kirby M, Pechhold S, Liu EH, Harlan DM, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. The Journal of clinical endocrinology and metabolism. 2010;95:E234–239. doi: 10.1210/jc.2010-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahier J, Wallon J, Henquin JC. Cell populations in the endocrine pancreas of human neonates and infants. Diabetologia. 1981;20:540–546. doi: 10.1007/BF00252762. [DOI] [PubMed] [Google Scholar]
- Reers C, Erbel S, Esposito I, Schmied B, Buchler MW, Nawroth PP, Ritzel RA. Impaired islet turnover in human donor pancreata with aging. European journal of endocrinology/European Federation of Endocrine Societies. 2009;160:185–191. doi: 10.1530/EJE-08-0596. [DOI] [PubMed] [Google Scholar]
- Rulifson IC, Karnik SK, Heiser PW, ten Berge D, Chen H, Gu X, Taketo MM, Nusse R, Hebrok M, Kim SK. Wnt signaling regulates pancreatic beta cell proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6247–6252. doi: 10.1073/pnas.0701509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NK, Vertino PM, Anania FA, Sharma D. leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. The Journal of biological chemistry. 2007;282:13316–13325. doi: 10.1074/jbc.M609798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit FC, In’t Veld PA, Pipeleers DG. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:3865–3869. doi: 10.1073/pnas.85.11.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Taylor B, Jin Q, Nguyen-Tran V, Meeusen S, Zhang YQ, Kamireddy A, Swafford A, Powers AF, Walker J, et al. Inhibition of DYRK1A and GSK3B induces human beta-cell proliferation. Nat Commun. 2015;6:8372. doi: 10.1038/ncomms9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- Stefan Y, Grasso S, Perrelet A, Orci L. A quantitative immunofluorescent study of the endocrine cell populations in the developing human pancreas. Diabetes. 1983;32:293–301. doi: 10.2337/diab.32.4.293. [DOI] [PubMed] [Google Scholar]
- Stefan Y, Orci L, Malaisse-Lagae F, Perrelet A, Patel Y, Unger RH. Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. Diabetes. 1982;31:694–700. doi: 10.2337/diab.31.8.694. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Rodriguez RT, McLean GW, Kim SK. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:175–180. doi: 10.1073/pnas.0609490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BA, Hollister-Lock J, Bonner-Weir S, Weir GC. Reduced Ki67 Staining in the Postmortem State Calls Into Question Past Conclusions About the Lack of Turnover of Adult Human beta-Cells. Diabetes. 2015;64:1698–1702. doi: 10.2337/db14-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang LH, Gonen M, Hedvat C, Modlin IM, Klimstra DS. Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: a comparison of digital image analysis with manual methods. The American journal of surgical pathology. 2012;36:1761–1770. doi: 10.1097/PAS.0b013e318263207c. [DOI] [PubMed] [Google Scholar]
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. The American journal of pathology. 2007;171:386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schravendijk CF, Kiekens R, Pipeleers DG. Pancreatic beta cell heterogeneity in glucose-induced insulin secretion. The Journal of biological chemistry. 1992;267:21344–21348. [PubMed] [Google Scholar]
- Wang P, Alvarez-Perez JC, Felsenfeld DP, Liu H, Sivendran S, Bender A, Kumar A, Sanchez R, Scott DK, Garcia-Ocana A, et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nature medicine. 2015a;21:383–388. doi: 10.1038/nm.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Fiaschi-Taesch NM, Vasavada RC, Scott DK, Garcia-Ocana A, Stewart AF. Diabetes mellitus--advances and challenges in human beta-cell proliferation. Nature reviews Endocrinology. 2015b;11:201–212. doi: 10.1038/nrendo.2015.9. [DOI] [PubMed] [Google Scholar]
- Wang YJ, McAllister F, Bailey JM, Scott SG, Hendley AM, Leach SD, Ghosh B. Dicer is required for maintenance of adult pancreatic acinar cell identity and plays a role in Kras-driven pancreatic neoplasia. PloS one. 2014;9:e113127. doi: 10.1371/journal.pone.0113127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Schug J, Won KJ, Liu C, Naji A, Avrahami D, Golson ML, Kaestner KH. Single cell transcriptomics of the human endocrine pancreas. Diabetes. 2016 doi: 10.2337/db16-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YP, Thorel F, Boyer DF, Herrera PL, Wright CV. Context-specific alpha- to-beta-cell reprogramming by forced Pdx1 expression. Genes & development. 2011;25:1680–1685. doi: 10.1101/gad.16875711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Robertson MA, Hesselson D, Stainier DY, Anderson RM. Glucagon is essential for alpha cell transdifferentiation and beta cell neogenesis. Development. 2015;142:1407–1417. doi: 10.1242/dev.117911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu KR, Yang SR, Jung JW, Kim H, Ko K, Han DW, Park SB, Choi SW, Kang SK, Scholer H, et al. CD49f enhances multipotency and maintains stemness through the direct regulation of OCT4 and SOX2. Stem cells. 2012;30:876–887. doi: 10.1002/stem.1052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.