Abstract
Designing modern vaccine adjuvants depends on understanding the cellular and molecular events that connect innate and adaptive immune responses. The synthetic TLR4 agonist GLA formulated in a stable emulsion (GLA-SE) augments both cellular and humoral immune responses to vaccine antigens. This adjuvant is currently included in several vaccines undergoing clinical evaluation including those for tuberculosis, leishmaniasis, and influenza. Delineation of the mechanisms of adjuvant activity will enable more informative evaluation of clinical trials. Early after injection, GLA-SE induces substantially more antigen-specific B cells, higher serum antibody titers and greater numbers of T follicular helper (TFH) and TH1 cells than alum, the squalene-in-water emulsion (SE) alone, or GLA without SE. GLA-SE augments antigen-specific B cell differentiation into germinal center and memory precursor B cells as well as pre-plasmablasts that rapidly secrete antibodies. CD169+ SIGNR1+ subcapsular medullary macrophages are the primary cells to take up GLA-SE after immunization and are critical for the innate immune responses, including rapid IL-18 production, induced by GLA-SE. Depletion of subcapsular macrophages (SCMϕ) or abrogation of IL-18 signaling dramatically impairs the antigen-specific B cell and antibody responses augmented by GLA-SE. Depletion of SCMϕ also drastically reduces the TH1 but not TFH response. Thus the GLA-SE adjuvant operates through interaction with IL-18-producing SCMϕ for the rapid induction of B cell expansion and differentiation, antibody secretion and TH1 responses, whereas augmentation of TFH numbers by GLA-SE is independent of SCMϕ.
Introduction
Augmentation of antigen-specific B cell responses and subsequent antibody production is central to the development of effective vaccines. In settings such as the emergence of new pandemics, intentional release of bioterror agents, and on-demand traveler’s vaccines, the rapid initiation of humoral immunity with practical vaccine approaches is highly desirable. The last decade has seen the licensure of several vaccine adjuvants that augment humoral immunity, including the squalene oil-in-water adjuvants, MF59 and AS03 and the TLR4 agonist-containing adjuvants AS01 and AS04. The study of B cell responses following immunization with clinically relevant adjuvants has been primarily limited to serum antibody analyses and the identification of antibody secreting cells. Compared to the wealth of information regarding T cell and antibody responses to vaccine adjuvants, much less is known regarding the early events following immunization that induce antigen-specific B cell and TFH responses and the mechanisms by which these adjuvants determine the course of these humoral responses.
The initial wave of secreted IgM and class-switched antibodies is produced by CD138+ pre-plasmablasts residing in extra-follicular spaces. Concurrently, CD95+ GL7+ germinal center B cells differentiate within follicles, resulting in affinity-matured antibodies, memory cells and long-lived plasmablasts. Memory B cells expressing IgM and CD38 are generated early in the response - independently of germinal centers - and are distinguished from the bulk population of naïve B cells by their antigen specificity (1). The production of a B cell response is coordinated by multiple innate and adaptive responses. The earliest encounter of foreign material in the draining lymph node (LN) can occur within minutes via cell-free transport from the infection or injection site to the draining LNs (2). This material is captured by CD169+ subcapsular macrophages (SCMϕ) that reside in lymph node sinusoidal spaces, allowing delivery of material to B cell follicles (3). Subsequent T-dependent B cell differentiation is regulated by multiple subsets of CD4 T helper cells. PD1+ CXCR5+ T follicular helper cells (TFH) expressing the transcription factor Bcl-6 and localized within B cell follicle are particularly important for germinal center responses and the generation of long-lived memory responses (4). TFH cells, or perhaps their progenitors, have also been shown to be necessary for extra-follicular responses (5).
The prototypical TLR4 agonist, lipopolysaccharide (LPS), is a powerful stimulant of the innate immune system, but it needs to be detoxified for use in human vaccine preparations. Acid and base treatment of LPS results in deacylation and removal of the polysaccharides and one of the phosphates yielding the biological product monophosphoryl lipid A (MPL), which is safe and an effective immune stimulant and which is present in commercial vaccines produced by GlaxoSmithKline. GLA is a synthetic monophosphorylated lipid A analog with six acyl chains and which like MPL is a safe and effective TLR4 agonist for use in adjuvant preparations (6). GLA is an effective vaccine adjuvant when formulated in a squalene oil-in-water emulsion. It generates a robust TH1 response and augmented antibody production skewed towards IgG2 class-switching against numerous infectious disease- and cancer-associated antigens (7–14). In humans GLA-SE promotes production of antigen specific TH1 cells making IFN-γ, TNF and IL-2, augmentation of serum antibody titers, preferential switching to IgG1 and IgG3 subtypes, and increases in neutralizing antibody titers when paired with protein antigens (15, 16). The adjuvant activity of GLA is critically dependent on its formulation. When GLA is formulated as a squalene oil-in-water emulsion (referred to as GLA-SE) compared to aqueous formulations of GLA, unique innate and adaptive features emerge (7, 17, 18). We have previously demonstrated that the TH1 potentiating activity of GLA-SE is mediated in part by MyD88 and TRIF signaling, inflammatory caspases and IL-18 as well as by type I and II interferons, IL-12, and the transcription factor T-bet (18–20). In the present paper we evaluate the cellular and molecular events necessary for augmentation of B cell, antibody and TFH responses by the GLA-SE adjuvant. Defining the cellular and molecular mechanisms of adjuvanticity of GLA-SE will enable more informative clinical evaluation of vaccines containing this adjuvant and provide a rational pathway for the development of next generation vaccine adjuvants by identifying key parameters to optimize during early stage development.
Materials and Methods
Animals and immunizations
Female C57BL/6 mice and IL-18R1 −/− mice aged 6–10 weeks were purchased from The Jackson Laboratory. CD169-DTR mice were provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan (21, 22). All animal experiments and protocols used in this study were approved by IDRI’s IACUC. For the adaptive response assessments mice were immunized subcutaneously via foot pad or base of tail injection with 1.25 or 5 μg of GLA respectively in combination with 1 μg of R-phycoerythrin (Prozyme, Hayward, CA) or 1 μg of the M. tuberculosis antigen ID87 (23) or 2.5 μg of the M. tuberculosis antigen ID97 (24). Squalene oil formulations were emulsified with egg phosphatidylcholine or synthetic dimyristoyl phosphatidylcholine as described (25) and used after dilution in saline for injection to 2% oil. For the innate response assessment mice were immunized via intramuscular in the quadriceps injection with 5μg of DiD-labelled GLA-SE. To label GLA-SE, DiD Oil; DilC18(5) (Molecular Probes) was solubilized in DMSO at 25 mg/mL. DiD was added to GLA-SE at 25 μg/mL, mixed well and incubated at room temperature for 10 minutes. Excess DiD was removed using a PD-10 desalting column (GE).
SCMϕ depletion
Mice were treated with 30 μL of clodronate-loaded liposomes (5mg/mL clodronate disodium salt) (Encapsula Nano Sciences, Brentwood, TN) via intradermal hock injection. Mice were immunized six days later via footpad, hock or intramuscular injection in the quadriceps.
Selective Deletion of CD169+ Macrophages
CD169-DTR mice were injected intraperitoneally with Diphteria Toxin (Sigma-Aldrich), at 20 ng/g body weight, 2 days prior to subsequent immunization.
Serum endpoint titer determination
Serum endpoint titer ELISA’s were performed on Corning high bind 384 well plates. Plates were coated overnight at 4 degrees with 1μg/mL PE. Detection antibodies were Ig (H+L) HRP and IgG2c HRP (Southern Biotech). Endpoints were set as the minimum dilution at which values were ≤ the mean + 3 s.d. of naïve controls.
LN singles cell suspension preparation
Singles cell suspensions of LN were generated by mechanical homogenization in PBS, 0.5%BSA in the presence of Halt protease inhibitor (ThermoScientific) and 10ug/ml BrefeldinA (BD Bioscience). For Figure 2, LN were homogenized and digested at 37°C for 30 minutes in RPMI in the presence of 8μg/mL Collagenase IV (Worthington) and 20μg/mL DNAse I (Roche).
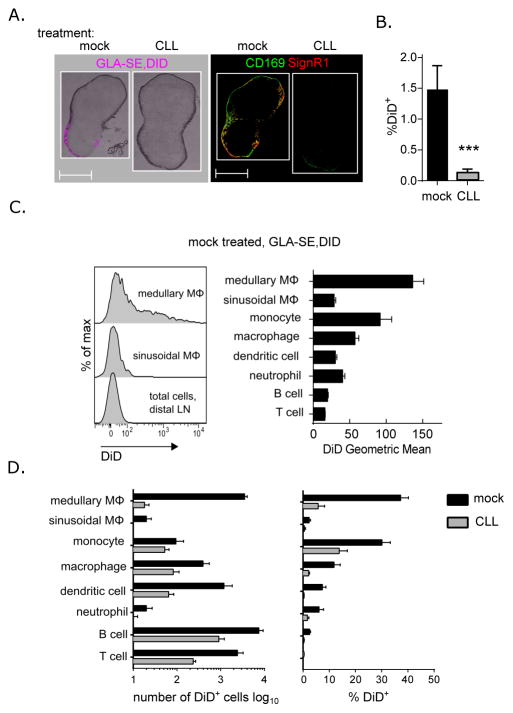
Fig. 2. GLA-SE-DiD cellular uptake after SCMϕ depletion.
Mice were treated with clodronate-loaded liposomes (CLL) or mock treated with saline six days prior to injection with DiD-labelled GLA-SE or saline. Draining inguinal LN were harvested 15 minutes after immunization. (A) Immunofluorescence microscopic images of representative LN sections were taken before and after staining with CD169 and SIGNR1 to assess GLA-SE distribution. (B) FACS analysis of total DiD staining cells per LN were determined. (C) A representative histogram of DiD-GLA-SE staining per cellular population in a mock treated DiD-GLA-SE immunized mouse is shown and DiD-GLA-SE geometric means for each cellular population were plotted. (D) The number of DiD+ cells per population and the percentage of DID+ cells within each population was determined for mock and CLL treated groups. p values were determined by Student t test ***p<0.005. Bars are drawn to the mean + s.e.m.. Data are representative of two independent experiments with four or five animals per group.
Flow cytometry
Samples were prepared by Fc receptor blocking (clone2.4G2). B cells were stained in 1% FBS, 2mM EDTA in PBS with the following: CD95-Bv421 (clone JO2), CD138-Bv605 (clone 16A8), B220-Bv785 (clone RA3-6B2) or CD19-Bv785 (clone 1D3), IgM-PerCP-eFluor710 (clone IL/41), IgD-APC-Cy7 (clone 1126C.2A), CD38-AF700 (clone 90), lineage cocktail: Ly6G-FITC (clone 1A8), CD11b-FITC (clone M1/70), CD11c-FITC (clone N418), F4/80-FITC (clone BM8), Ter119-FITC (clone TER119), CD90.2-FITC (clone OX-7). Cells were fixed and permeabilized with Fix/Perm buffer (BD Biosciences) and stained for intracellular IgG2-Bv510 (clone 5.7). PE-specific B cells were identified using 1 μg/ml PE for surface staining, and 0.1–0.3 μg/ml PE for intracellular staining.
For peptide-MHCII tetramer staining, the Ag85B p25 tetramer (NIH Tetramer Core Facility) was incubated in100 μL with a maximum of 4 × 106 cells at 37° C for 1.5 hours at 13 μg/ml with anti-CD16/32 antibody. Cells were washed and stained for CD4-APC-H7 (clone RM4-5), CD44-AF700 (clone IM7), PD-1-Bv605 (clone 29F1A12), CXCR5-PerCP-710 (clone SPRCL5) and lineage cocktail: CD8a-FITC (clone 53–6.7), CD11b-FITC, CD11c-FITC, Ly6G-FITC, Ter-119-FITC, F4/80-FITC, CD19-FITC at room temperature for 30 minutes. Cells were then fixed and permeabilized as above and stained for Bcl6-AF647 (clone BCL-DWN) and Tbet-Bv-421 (clone 4B10) overnight at 4°C.
Identification of DiD+ cells was performed by surface staining draining lymph node cells by blocking CD16/32 and staining for CD11b-eF450, CD90.2-Bv510 (clone 53–2.1), CD19-Bv785 (clone 6D5), purified SIGNR1 (clone eBio22D1, Armenian hamster host), Ly6C-PerCP-Cy5.5 (clone HK1.4), CD169-PE (clone 3D6.112), Ly6G-PE-CF594, and CD11c-PE-Cy7 at 4°C for 30 minutes. Cells were then washed and stained with a secondary antibody against Armenian hamster coupled to AF488.
Quantitation of cells was carried out by applying the frequencies of cell populations to cells counts obtained with a GUAVA EasyCyteHT (Millipore, Billerica, MA). Data were collected on LSRII or Fortessa flow cytometers (BD). Data were analyzed using FlowJo (Tree Star Inc., Ashland, OR). Statistical analysis was performed using Prism software (GraphPad Software, Inc., La Jolla, CA).
Immunofluorescence microscopy
LNs were collected and embedded in OCT Compound (Tissue-Tek). Cryostat sections (6 um thick) were mounted on Poly-lysine microscope slides (Thermo Scientific). Brightfield and DiD images of cryostat sections were acquired using Nikon Eclipse Ti-5 before acetone fixation. Sections were subsequently dried and fixed in cold acetone for 5 min at −20° C, blocked with 3% BSA in PBS blocking buffer at room temperature for 30 min, and incubated with anti-CD169 (ThermoScientific) and SIGNR1 (eBioSciences) antibodies diluted in the blocking buffer for 1 hour. Primary antibodies were detected with Goat-anti-Rat-AF488 (against CD169, BioLegend) and Goat-Anti-Hamster IgG-568 (against SIGNR1, Abcam). Images were acquired with the Nikon Eclipse Ti-5 microscope, using 4× objective lens and NIS-Elements D3.2 Software and processed with Fiji software.
Cytokine and chemokine protein levels quantitation
The concentration of cytokines and chemokines were measured using a microbead-based ELISA system (ProcartaPlex Mouse, Affymetrix eBioscience), according to the manufacturer’s directions.
Results
GLA-SE induces rapid B cell responses
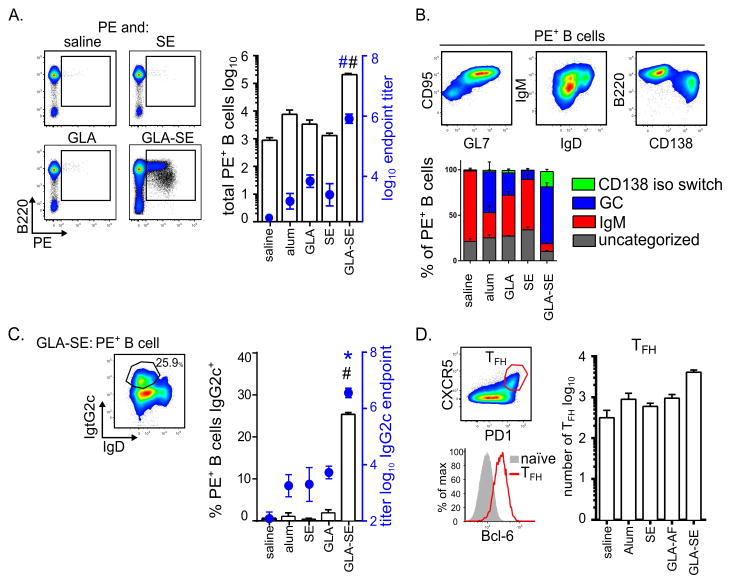
To better understand the B cells responses induced by GLA-SE, we analyzed antigen-specific B cells via immunization with phycoerythrin (PE) either alone or adjuvanted with GLA-SE or its constituent components, SE or GLA, a strategy previously described to identify antigen-specific B cells by flow cytometry (27, 28). We also included alum as a well-established adjuvant with which to benchmark the adjuvanticity of GLA-SE. Seven days after immunization with PE alone, very few antigen-specific B cells were detected in the draining LN (Fig. 1A). These rare PE+ B cells elicited by immunization with unadjuvanted antigen were predominately IgD+, CD38+, IgM+ putative memory B cells (Fig. 1B). Adjuvanting PE with alum, GLA or SE elicited a minor but not statistically significant increase in PE+ B cells with an increase in the proportion of CD95+, GL7+, IgD− germinal center B cells (Fig. 1A and B). In contrast GLA and SE synergized to elicit substantially more PE-specific B cells (Fig. 1A). In addition to inducing germinal center and putative memory B cells, GLA-SE uniquely produced isotype switched pre-plasma blasts (B220lo, CD138+, IgD−, IgM−) which are an early source for circulating antibody (Fig. 1B). Accordingly, early after immunization PE-specific serum antibody titers were dramatically increased with GLA-SE compared to the other immunizations including alum (Fig. 1A). Furthermore, compared to alum, GLA or SE, GLA-SE uniquely augmented the frequency of antigen-specific B cells expressing intracellular IgG2c and the IgG2c serum antibody titers (Fig. 1C).
Fig. 1. Rapid B cell responses elicited by different formulations of the TLR4 adjuvant GLA.
B cell responses in the injection site draining inguinal and axillary LN of mice immunized with the indicated adjuvant and PE were analyzed 7 days after immunization. (A) Antigen specific B cells were enumerated by PE staining and cell populations were determined by cell surface marker staining. Total numbers of PE staining B cells in draining LNs were determined (bars). Overlaid are the total Ig (H+L) serum antibody endpoint titers (circles). (B) FACS plots of GLA-SE induced PE+ B cells are shown. Subsets of B cells were identified by surface marker expression as CD38+ IgM+ memory B cells (IgM), IgD− CD95+ GL7+ germinal center cells (GC), CD138+ B220lo CD38− IgM− IgD− pre-plasmablasts (CD138 iso switch), or uncategorized. The stacked histograms represent the draining LN PE-specific B cell composition for each immunization. (C) Representative FACS plot for the identification of PE+ IgG2c B cells in draining LNs are shown. Frequency of IgG2c+ PE-specific B cells (bars) are overlaid with serum IgG2c endpoint titers (circles). (D) Representative FACS plot for Bcl-6 staining of TFH cells (CXCR5+PD1+) vs naïve cells. Following immunization the numbers of TFH cells per LN were determined. Bars and circles are drawn to the mean values + s.e.m p values were determined by one-way ANOVA/Dunnett’s test #p < 0.001 compared with all other groups, *p<0.05 compared to saline. Data are representative of two independent experiments with three or four animals per group.
Given the importance of TFH cells to humoral immunity (4) we also enumerated TFH cells based on surface expression of CXCR5 and PD1 and expression of the transcription factor Bcl-6. GLA-SE induced more TFH cells in the draining LNs when compared to the antigen alone or adjuvanted with alum, GLA, or SE (Fig. 1D). Taken together, these results suggest that the rapid and dramatic expansion of early B cell responses, especially pre-plasmablasts, antibody production and TFH cells is unique to the combination GLA-SE adjuvant formulation.
SCMϕ are essential for GLA-SE uptake
To better understand how GLA-SE augments rapid B cell responses we identified the cells interacting with GLA-SE immediately after immunization. The cells interacting with the adjuvant were visualized by labeling the adjuvant with the lipophilic fluorescent dye DiD. 15 mins after injection, GLA-SE co-localized with the CD169+ SIGNR1+ medullary macrophage population in the draining lymph node (29) (Fig. 2A and C). Other populations including monocytes (CD11b+ Ly6C+) also took up the adjuvant, but in lesser numbers and with less intense DiD signal intensity (Fig. 2C and D). Of note, DiDdim B cells were the most abundant DiD+ population by absolute numbers, although these cells represented only a small fraction of the total LN B cell population (~3%) (Fig. 2D).
To determine the significance of GLA-SE capture by SCMϕ, including the medullary and sinusoidal macrophage subsets, we depleted these cells by injection of clodronate-loaded liposomes (CLL) and waited for 6 days to allow the re-population of resident phagocytes (monocytes, dendritic cells and neutrophils) but not the SCMϕ (3) as confirmed by analysis of the cellular content of the lymph nodes of CLL-treated animals (Supplemental Fig. 1). SCMϕ depletion dramatically impacted GLA-SE uptake by lymph-node cells with less than 0.2% of the LN cells being DiD+ 15 mins post-injection compared to an average of 1.5% in the mock-treated mice (Fig. 2B). Surprisingly, adjuvant uptake was abrogated in cell populations that were not depleted by CLL treatment including monocytes, macrophages, dendritic cells, T cells and B cells (Fig. 2D). These results indicate that SCMϕ, and most probably the medullary macrophages, are critical to early GLA-SE capture and subsequent distribution in the LN.
SCMϕ are critical for the innate response to GLA-SE
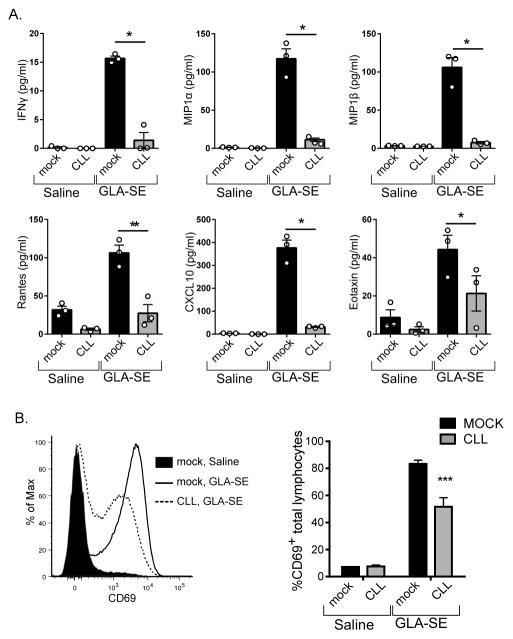
Immunization with GLA-SE initiates a complex innate immune response including production of IFN-γ which is essential for the adjuvanticity of GLA-SE (18) and a number of chemoattractants including RANTES, MIP1α and β, Eotaxin, and CXCL10 (Fig. 3A). Depletion of SCMϕ by CLL treatment severely compromised the innate response to GLA-SE (Fig. 3A). CD69 expression increases upon GLA-SE immunization in a type I and II IFN-dependent manner and is thus a sensitive readout for the response to GLA-SE (18, 20). Treatment with CLL resulted in a substantial decrease in the induction of CD69 on total lymphocytes 18h after injection with GLA-SE (Fig. 3B). The decrease in these early immune responses to GLA-SE after SCMϕ depletion indicated that these cells were critical for the innate immune response to GLA-SE.
Fig. 3. Innate responses to GLA-SE after SCMϕ depletion.
Mice were treated with clodronate-loaded liposomes (CLL) or saline (mock) 6 days prior to injection with GLA-SE or saline and draining LNs were harvested either 4h or 18h after immunization. (A) The concentration of cytokines and chemokines 4h after injection are plotted. (B) A representative histogram of CD69 staining on total lymphocytes 18h after injection is shown and the percentage of CD69+ lymphocytes is plotted. Bars are drawn to the mean + s.e.m.. p values were determined by one-way ANOVA/Dunnett’s test *** p<0.001, **p<0.01, *p<0.05. Data are representative of two independent experiments with three animals per group.
SCMϕ are necessary for B cell and TH1 but not TFH induction by GLA-SE
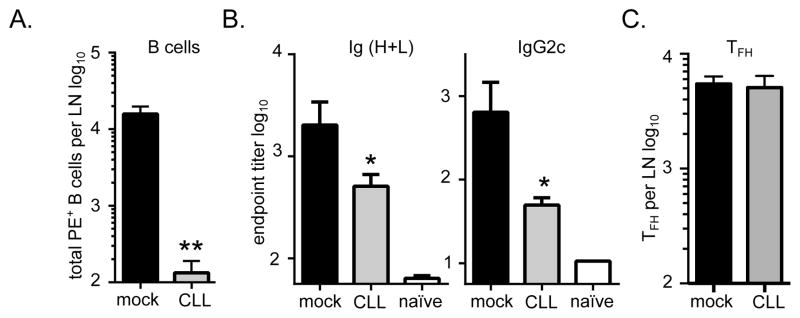
To assess the contribution of the SCMϕ to the adjuvanticity of GLA-SE, we examined the adaptive immune response to PE+GLA-SE immunization in CLL-treated mice. Seven days after immunization, the number of PE-specific B cells were severely diminished by CLL treatment (Fig. 4A). PE-specific Ig (H+L) and IgG2c serum titers were reduced as well (Fig. 4B). This demonstrated that SCMϕ play a central role in the B cell expansion and the antibody responses elicited by immunization with GLA-SE. Surprisingly, we observed no difference in the total numbers of TFH cells in the draining LNs between mock treated and the CLL-treated mice in response to PE+GLA-SE immunization (Fig. 4C).
Fig. 4. B cell and TFH induction via GLA-SE after SCMϕ depletion.
Mice were treated with clodronate-loaded liposomes (CLL) or saline (mock) 6 days prior to immunization with PE+GLA-SE and responses were analyzed 7 days after immunization. (A) Shown are the number of PE+ B cells in draining inguinal lymph nodes, (B) Shown are the total Ig (H+L) and IgG2c endpoint titers. (C) The total numbers of TFH cells (CD4+, CD44+, CXCR5+, PD1+, Bcl-6+) in draining LNs were enumerated. Bars are drawn to the mean + s.e.m.. p values were determined by one-way ANOVA/Dunnett’s test ** p<0.01, * p<0.05. Data are representative of two independent experiments with five animals per group.
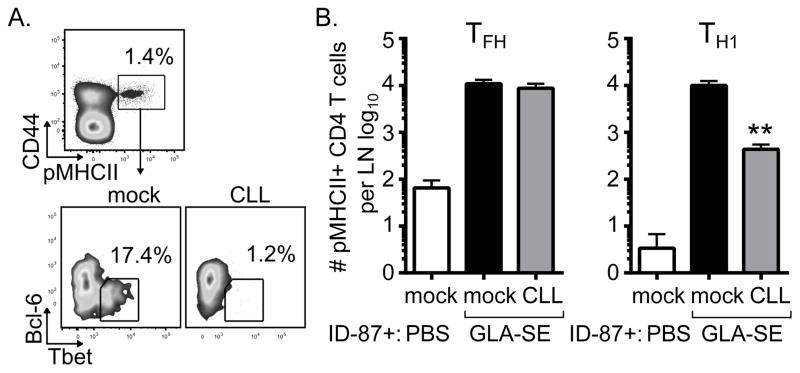
To determine whether antigen-specific TFH numbers are specifically impacted by CLL depletion we immunized mock or CLL-treated mice with the recombinant tuberculosis antigen ID87 which contains the Ag85B P25 epitope (23) and analyzed peptide-MHCII (pMHCII) tetramer staining. The number of antigen specific TFH cells (pMHCII+,CD44+,CXCR5+,PD1+,Bcl-6+) was virtually unaffected by CLL treatment. In contrast, significantly fewer TH1 cells (pMHCII+,CD44+,CXCR5−,PD1−,Tbet+) were present in LNs from CLL treated mice compared to mock treated mice (Fig. 5). To confirm that the observed altered adaptive responses in CLL treated mice were due to the absence of SCMϕ and not unexpected off-target effects of CLL treatment, we also examined responses in CD169-DTR mice (21, 22). Similar to CLL treatment, depletion of CD169+ SCMϕ via diphtheria treatment prior to immunization with GLA-SE reduced the B cells and TH1 responses but not the TFH responses to immunization (Supplemental Fig. 2). These data suggest that antigen-specific TH1 cell numbers, not TFH cell numbers, may be important for the early B cell response in GLA-SE immunized animals.
Fig. 5. TFH and TH1 dependency upon SCMϕ.
Peptide-MHCII tetramer staining was used to identify T cell subsets in the context of CLL treatment. Mice were treated with clodronate-loaded liposomes (CLL) or saline (mock) 6 days prior to immunization with GLA-SE and the recombinant protein ID87 and responses were analyzed 7 days after immunization. (A) FACS plots of T-bet and Bcl-6 staining of CD4+pMHCII+ cells from draining lymph nodes. (B) The total numbers of TFH (pMHCII+, CD4+,CD44+,CXCR5+, PD1+, Bcl-6+) and TH1 (pMHCII+,CD4+,CD44+,CXCR5−, PD1−, Tbet+) per LN were enumerated. Bars are drawn to the mean + s.e.m.. p values were determined by one-way ANOVA/Dunnett’s test ** p<0.01. Data are representative of two independent experiments with three or four animals per group.
IL-18R1 contributes to the induction of early B cell responses elicited by GLA-SE
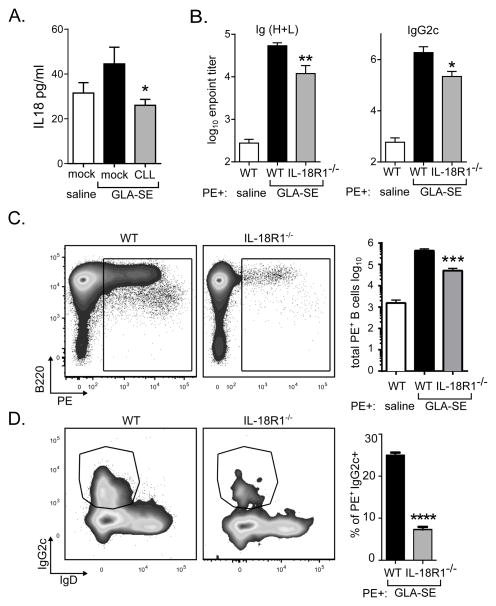
TH1 induction with GLA-SE is dependent on the caspase-1 mediated inflammasome and rapid IL-18 production which is essential for IFN-γ production by memory CD8 T cells and neutrophils upon GLA-SE immunization (18). SCMϕ are an important source of IL-18 in draining lymph nodes (30). Thus, we hypothesized that SCMϕ played a role in the innate and adaptive responses elicited by immunization with GLA-SE in part by producing IL-18. CLL depletion prior to GLA-SE immunization significantly diminished the amount of secreted IL-18 in the draining LN one hour after immunization indicating that the SCMϕ are the main source of IL-18 early after immunization (Fig. 6A). To determine whether IL-18 was important for the induction of antigen-specific B cells and antibody production, we compared responses to immunization in wildtype or IL18R1 deficient (IL-18R1−/−) mice. Compared to wildtype total Ig (H+L) and IgG2c PE-specific serum titers were diminished in IL-18R1−/− mice (Fig. 6B). Concordantly, in the immunized IL-18R1−/− mice significantly fewer PE-specific B cells were present (Fig. 6C) and IgG2c+ class-switching was impaired (Fig. 6D). These data indicate that, similar to CLL or DTR depletion of SCMϕ, ablation of IL-18 signaling impaired the humoral responses elicited by immunization with GLA-SE. Of note DTR or CLL-depletion had a more dramatic impact on adaptive immune responses than IL-18R1 deficiency indicating SCMϕ also control adjuvant driven responses in an IL-18R1-independent manner.
Fig. 6. IL-18 is important for GLA-SE augmentation of B cell responses.
Mice were treated with clodronate-loaded liposomes (CLL) or saline (mock) prior to immunization. (A) Mice were immunized with GLA-SE and IL-18 production in the draining lymph node was assessed 1h post-injection. (B–D) WT or IL18R−/− mice were immunized with PE+/−GLA-SE and responses were analyzed 7 days after immunization. (B) Total Ig (H+L) and IgG2c PE reactive endpoint titers. (C) FACS plots of PE+ staining are shown and the total numbers of PE-specific B cells in the draining LNs are plotted. (D) FACS plots of IgG2c and IgD staining of PE+ B cells are shown and frequencies of IgG2c+ PE+ B cells are plotted. Bars are drawn to the mean + s.e.m.. p values were determined by one-way ANOVA/Dunnett’s test **** p<0.0001, *** p<0.001, **p<0.01, *p<0.05. Data are representative of two independent experiments with four or five animals per group.
Discussion
Rapid antibody production after immunization could mean the difference between protection and infection against a new strain of pathogen such as a pandemic avian influenza virus or biological weapons. As evident in the data presented here, different adjuvants can alter the kinetics and quality of the humoral immune response. Few studies have examined changes in antigen-specific B cell populations elicited by adjuvants, particularly those destined for clinical use. Of note, antigen-staining was recently used to identify germinal center B-cell responses during a prime-boost regimen with the emulsion adjuvant MF59 (31). Overall, those results were consistent with our studies of the similar adjuvant SE. Alum, GLA alone or SE alone only weakly elicited early B cell responses - primarily GC and memory B cells - whereas the combination GLA-SE drove a rapid and robust B cell and antibody response that also included pre-plasmablasts important early antibody secretion. We previously found that a rapid humoral response induced with GLA-SE correlated with early onset protection against the highly pathogenic avian influenza virus H5N1 as early as 4–6 days after immunization, underlining the importance that such an adjuvant could have in a rapidly emerging epidemic (13).
After injection, GLA-SE was predominantly associated with the SIGNR1+ medullary subset of SCMϕ and this correlated with the ability to induce strong B-cell responses. This is consistent with microscopic analysis of cells taking up DiD-labeled MF59 (32). Strikingly, when the SCMϕ population was disrupted by CLL injection, the number of B cells that were associated with labelled GLA-SE fell by nearly ten-fold, suggesting that SCMϕ may be necessary to capture and transfer the GLA-SE adjuvant to B cells. In support of this possibility sinusoidal SCMϕ facilitate transport of virus particles to B cell follicles (3). Presumably this process facilitates the induction of germinal center responses as the disruption of sinusoidal SCMϕ lattice architecture inhibits the induction of germinal center responses (33). Using an intraperitoneal immunization method Nikbakht and colleagues found that CLL depletion of marginal zone macrophages from the spleen also impaired B cell responses to alum adjuvanted immunization (34). In contrast we do not detect substantial antigen-specific B cell responses with alum in our system, likely due to the different route of delivery (i.m. vs i.p.). Nevertheless these findings largely support those of Nikbakht that CLL-dependent macrophages are important for humoral response to adjuvanted immunization. Our studies do not discount the contribution of other cell types to the adjuvanticity of GLA-SE however it is intriguing to note that lymph node resident macrophages but not migratory macrophages that traffic to the immunization site were crucial for B cell responses to a rabies virus vector immunization (35). The importance of these migratory macrophages in the adjuvanticity of GLA-SE remains to be determined.
GLA-SE also uniquely induced pre-plasmablasts which have been shown to localize to medullary regions of the lymph node (36). This result suggested that initial priming could occur at sites distal to the medullary sinus, but it is conceivable that medullary macrophages may promote the activation of B cells and their differentiation into pre-plasmablasts in situ. Furthermore, antibody responses in transiently DC-depleted mice (CD11c-DTR) were mostly unaffected, suggesting that DC are not required for B cell priming (37). Thus we suggest that SCMϕ, and not DC, are critical for adjuvant enhancement of B cell priming upon immunization, by creating a microenvironment conducive to B cell expansion and pre-plasmablast generation (e.g. increases in local cytokines such as IL-18, IFN-γ, and others reduced by SCMϕ ablation) and/or facilitating B cell- antigen interactions. It remains to be determined whether SCMϕ or another cell population such as FDCs or neutrophils are also critical for capturing and transporting unprocessed antigen to the B cells during the priming event or if the B cells are directly capturing the antigen (18, 38). In support of a direct role for antigen presentation to B cells by SCMϕ, direct targeting of antigens to these cells by coupling OVA to anti-CD169 increased the OVA-specific germinal centers and humoral response (39).
SCMϕ were also important for the production of IL-18 upon immunization. We previously demonstrated that IL-18 production mediated by caspase 1 activation was important for the production of IFN-γ by memory CD8 T cells and neutrophils (18). This pathway was also important for the generation of antigen-specific CD4 T cells with GLA-SE. This IL-18 axis was also at least partially responsible for the SCMϕ-dependent expansion of antigen-specific B cells. It remains to be determined whether IL-18 is acting directly on B cells to drive their expansion, or via IL-18 mediated production of IFNγ production. Another possibility is that the IL-18 axis is primarily affecting the ability of CD4 T cells to provide sufficient help to B cells for optimal expansion after immunization, as suggested by our previous findings that IL-18 is important for TH1 expansion upon immunization with GLA-SE (18). Our recent work with Tbet-deficient mice indicates that the IFN-γ axis likely plays a role in humoral responses as loss of TH1 cells in the Tbet-deficient animals correlated with a decrease in antibody responses (20).
Substantial evidence has accumulated describing the relationship between TFH cells and the induction of B cell responses (4). Importantly, TFH and GC B cell numbers correlate during the induction of an immune response by vaccination (40). In agreement with these observations, GLA-SE drives an expansion of antigen-specific TFH cells concordant with the increase in antigen-specific B cells and this is superior in magnitude to the other adjuvants tested. However, the contribution of antigen-specific TFH cells to B cell expansion and early antibody titers during immunization with GLA-SE is unclear. Surprisingly antigen-specific TFH cells were unaffected by SCMϕ depletion, whereas B cell and TH1 responses were dramatically impaired. Canonically TH1 cells are associated with CD8 T cell responses, not B cell responses, although TH1 cells can facilitate B cell priming in some circumstances (41). This underlines the current models suggesting that separate subsets of antigen presenting cells mediate the induction of TH1 cells and TFH cells (42, 43). In support of this we have recently shown that T-bet deficient mice which have no TH1 responses to GLA-SE, do not induce an IgG2c skewed response further supporting a role for TH1 cells in B cell responses (20). Although this does not demonstrate that TFH cells are irrelevant to the early B cell response, it does indicate that TFH cells are insufficient to support all B cell responses.
The unique adjuvant properties of GLA-SE to expand B cell responses, relative to GLA alone or SE alone, are likely due both to its biophysical characteristics, as well as molecular signaling capacities. For example, the innate response to GLA-SE depends not only upon MyD88 and TRIF, which are critical components of TLR4 signaling, but also upon the inflammasome (18, 19), which is only engaged by TLR4 agonists upon cytoplasmic recognition (44, 45). On their own, squalene oil-in-water emulsions such as MF59 or SE promote humoral immunity via activation of canonical and non-canonical inflammasome pathways (46–49). Inflammasome activation could be due to direct recognition of the squalene via distinct, but unknown, receptors. GLA and SE in turn likely collaborate to produce bioactive IL-18 in a two-step process in which TLR4 signaling triggered by GLA increases production of pro-IL-18 and SE engagement of the inflammasome generates active caspase 1 to cleave pro-IL-18 into bioactive IL-18. Additionally, the nanoparticle nature of GLA-SE may result in more efficient delivery to the lymph node. Nevertheless the particulate nature cannot fully explain the enhancement mediate by GLA-SE as other oil-in-water/TLR4 agonist compositions, with similar biophysical properties, do not recapitulate the adjuvanticity of GLA-SE (7, 50).
In summary, when formulated as a squalene oil-in-water emulsion (GLA-SE) the TLR4 agonist GLA induces rapid B cell expansion and differentiation, augmented antibody production and TFH expansion. These responses were superior in magnitude to the widely used adjuvant alum, as well as GLA in an aqueous base or the oil-in-water emulsion alone adjuvant. SCMϕ and IL-18 production were central to optimal immunization with GLA-SE as the innate immune response, B cell expansion and TH1 induction were impaired upon SCMϕ depletion or abrogation of IL-18R signaling. Interestingly, TFH induction after GLA-SE immunization was not affected in the absence of SCMϕ. Thus, GLA-SE may be ideally suited for use in vaccines that require a rapid-onset response with a single immunization.
Our results point to early IL-18 production as candidate biomarker of immunogenicity for immunization with GLA-SE adjuvanted vaccines and may indicate that early IL-18 production could be a hallmark of different classes of vaccine adjuvants. Additionally the role of SCMϕ in the mechanism of action of other adjuvants will help determine whether this subpopulation needs to be targeted by all adjuvants through activation of TLR or other PRRs expressed by SCMϕ or by receptor specific delivery mechanisms such as antigen complexed to SIGNR1, similar to the approaches targeting antigens to DCs via anti-DEC205 (39). Defining the key pathways that determine the activity of clinical stage adjuvants including GLA-SE, MF59, and the AS01–04 series is crucial to rational development of next generation vaccine adjuvants tailored to elicit specific immune responses.
Supplementary Material
Acknowledgments
We thank John Laurance for excellent technical assistance. We acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of pMHCII tetramers. We also thank Kenji Kohno, Ph.D. and Masato Tanaka, Ph.D (RIKEN) for CD169-DTR mice.
Footnotes
This work was funded by grants OPP1055855 and OPP1130379 from the Bill & Melinda Gates Foundation to SGR and DC respectively, and by grant R01-AI-025038 and contract HSN272201400041C from the National Institute of Allergy And Infectious Diseases, National Institutes of Health, Department of Health and Human Services to SGR and CBF respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Taylor JJ, Pape KA, Jenkins MK. A germinal center – independent pathway generates unswitched memory B cells early in the primary response. 2012;209:597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moseman EA, Iannacone M, Bosurgi L, Tonti E, Chevrier N, Tumanov A, Fu YX, Hacohen N, von Andrian UH. B Cell Maintenance of Subcapsular Sinus Macrophages Protects against a Fatal Viral Infection Independent of Adaptive Immunity. Immunity. 2012;36:415–426. doi: 10.1016/j.immuni.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, Van Rooijen N, Mempel TR, Whelan SP, Von Andrian UH, Di Paolo NC, van Rooijen N, von Andrian UH. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–4. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 4.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 5.Lee SK, Rigby RJ, Zotos D, Tsai LM, Kawamoto S, Marshall JL, Ramiscal RR, Chan TD, Gatto D, Brink R, Yu D, Fagarasan S, Tarlinton DM, Cunningham AF, Vinuesa CG. B cell priming for extrafollicular antibody responses requires Bcl-6 expression by T cells. J Exp Med. 2011;208:1377–88. doi: 10.1084/jem.20102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coler RN, Bertholet S, Moutaftsi M, Guderian Ja, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS, Reed SG. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orr MT, Fox CB, Baldwin SL, Sivananthan SJ, Lucas E, Lin S, Phan T, Moon JJ, Vedvick TS, Reed SG, Coler RN. Adjuvant formulation structure and composition are critical for the development of an effective vaccine against tuberculosis. J Control Release. 2013;172:190–200. doi: 10.1016/j.jconrel.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duthie MS, Favila M, Hofmeyer KA, Tutterrow YL, Reed SJ, Laurance JD, Picone A, Guderian J, Bailor HR, Vallur AC, Liang H, Mohamath R, Vergara J, Howard RF, Coler RN, Reed SG. Strategic evaluation of vaccine candidate antigens for the prevention of Visceral Leishmaniasis. Vaccine. 2016;34:2779–2786. doi: 10.1016/j.vaccine.2016.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balam S, Jafarshad A, Servis C, Frank G, Reed S, Pink R, Druilhe P, Spertini F, Corradin G. Immunogenicity of dimorphic and C-terminal fragments of Plasmodium falciparum MSP2 formulated with different adjuvants in mice. Vaccine. 2016;34:1566–1574. doi: 10.1016/j.vaccine.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Karmakar S, Zhang W, Ahmad G, Torben W, Alam MU, Le L, Damian RT, Wolf RF, White GL, Carey DW, Carter D, Reed SG, Siddiqui AA. Cross-species protection: Schistosoma mansoni Sm-p80 vaccine confers protection against Schistosoma haematobium in hamsters and baboons. Vaccine. 2014;32:1296–1303. doi: 10.1016/j.vaccine.2013.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Valentin A, Kulkarni V, Rosati M, Beach RK, Alicea C, Hannaman D, Reed SG, Felber BK, Pavlakis GN. HIV/SIV DNA vaccine combined with protein in a co-immunization protocol elicits highest humoral responses to envelope in mice and macaques. Vaccine. 2013;31:3747–55. doi: 10.1016/j.vaccine.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duthie MS, Sampaio LH, Oliveira RM, Raman VS, O’Donnell J, Bailor HR, Ireton GC, Sousa ALM, Stefani MMa, Reed SG. Development and pre-clinical assessment of a 73kD chimeric fusion protein as a defined sub-unit vaccine for leprosy. Vaccine. 2013;31:813–9. doi: 10.1016/j.vaccine.2012.11.073. [DOI] [PubMed] [Google Scholar]
- 13.Clegg CH, Roque R, Van Hoeven N, Perrone L, Baldwin SL, Rininger Ja, Bowen Ra, Reed SG. Adjuvant solution for pandemic influenza vaccine production. Proc Natl Acad Sci U S A. 2012;109:17585–90. doi: 10.1073/pnas.1207308109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behzad H, Huckriede ALW, Haynes L, Gentleman B, Coyle K, Wilschut JC, Kollmann TR, Reed SG, McElhaney JE. GLA-SE, a synthetic toll-like receptor 4 agonist, enhances T-cell responses to influenza vaccine in older adults. J Infect Dis. 2012;205:466–73. doi: 10.1093/infdis/jir769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coler RN, Duthie MS, Hofmeyer KA, Guderian J, Jayashankar L, Vergara J, Rolf T, Misquith A, Laurance JD, Raman VS, Bailor HR, Cauwelaert ND, Reed SJ, Vallur A, Favila M, Orr MT, Ashman J, Ghosh P, Mondal D, Reed SG. From mouse to man: safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE. Clin Transl Immunol. 2015;4:e35. doi: 10.1038/cti.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treanor JJ, Essink B, Hull S, Reed S, Izikson R, Patriarca P, Goldenthal KL, Kohberger R, Dunkle LM. Evaluation of safety and immunogenicity of recombinant influenza hemagglutinin (H5/Indonesia/05/2005) formulated with and without a stable oil-in-water emulsion containing glucopyranosyl-lipid A (SE+GLA) adjuvant. Vaccine. 2013;31:5760–5. doi: 10.1016/j.vaccine.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 17.Anderson RC, Fox CB, Dutill TS, Shaverdian N, Evers TL, Poshusta GR, Chesko J, Coler RN, Friede M, Reed SG, Vedvick TS. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids Surf B Biointerfaces. 2010;75:123–32. doi: 10.1016/j.colsurfb.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Desbien AL, Reed SJ, Bailor HR, Cauwelaert ND, Laurance JD, Orr MT, Fox CB, Carter D, Reed SG, Duthie MS. Squalene emulsion potentiates the adjuvant activity of the TLR4 agonist, GLA, via inflammatory caspases, IL-18, and IFN-γ. Eur J Immunol. 2014:1–11. doi: 10.1002/eji.201444543. [DOI] [PubMed] [Google Scholar]
- 19.Orr MT, Fox CB, Baldwin SL, Sivananthan SJ, Lucas E, Lin S, Phan T, Moon JJ, Vedvick TS, Reed SG, Coler RN. Adjuvant formulation structure and composition are critical for the development of an effective vaccine against tuberculosis. J Control Release. 2013;172:190–200. doi: 10.1016/j.jconrel.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orr MT, Duthie MS, Windish HP, Lucas EA, Guderian JA, Hudson TE, Shaverdian N, O’Donnell J, Desbien AL, Reed SG, Coler RN. MyD88 and TRIF synergistic interaction is required for TH1-cell polarization with a synthetic TLR4 agonist adjuvant. Eur J Immunol. 2013 doi: 10.1002/eji.201243124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cauwelaert ND, Desbien AL, Hudson TE, Pine SO, Reed SG, Coler RN, Orr MT. The TLR4 Agonist Vaccine Adjuvant, GLA-SE, Requires Canonical and Atypical Mechanisms of Action for TH1 Induction. PLoS One. 2016;11:e0146372. doi: 10.1371/journal.pone.0146372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117:2268–78. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19:746–50. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 24.Windish HP, Duthie MS, Misquith A, Ireton G, Lucas E, Laurance JD, Bailor RH, Coler RN, Reed SG. Protection of mice from Mycobacterium tuberculosis by ID87/GLA-SE, a novel tuberculosis subunit vaccine candidate. Vaccine. 2011;29:7842–8. doi: 10.1016/j.vaccine.2011.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orr MT, Ireton GC, Beebe EA, Huang P-WD, Reese VA, Argilla D, Coler RN, Reed SG. Immune subdominant antigens as vaccine candidates against Mycobacterium tuberculosis. J Immunol. 2014;193:2911–8. doi: 10.4049/jimmunol.1401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox CB, Baldwin SL, Vedvick TS, Angov E, Reed SG. Effects on immunogenicity by formulations of emulsion-based adjuvants for malaria vaccines. Clin Vaccine Immunol. 2012;19:1633–40. doi: 10.1128/CVI.00235-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pape Ka, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–7. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayakawa K, Ishii R, Yamasaki K, Kishimoto T, Hardy RR. Isolation of high-affinity memory B cells: phycoerythrin as a probe for antigen-binding cells. Proc Natl Acad Sci U S A. 1987;84:1379–83. doi: 10.1073/pnas.84.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray EE, Cyster JG. Lymph Node Macrophages. 2013;4:424–436. doi: 10.1159/000337007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kastenmüller W, Torabi-Parizi P. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. 2012;150:1235–1248. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lofano G, Mancini F, Salvatore G, Cantisani R, Monaci E, Carrisi C, Tavarini S, Sammicheli C, Rossi Paccani S, Soldaini E, Laera D, Finco O, Nuti S, Rappuoli R, De Gregorio E, Bagnoli F, Bertholet S. Oil-in-Water Emulsion MF59 Increases Germinal Center B Cell Differentiation and Persistence in Response to Vaccination. J Immunol. 2015 doi: 10.4049/jimmunol.1402604. [DOI] [PubMed] [Google Scholar]
- 32.Cantisani R, Pezzicoli a, Cioncada R, Malzone C, De Gregorio E, D’Oro U, Piccioli D. Vaccine Adjuvant MF59 Promotes Retention of Unprocessed Antigen on Lymph Node Macrophage Compartments and Follicular Dendritic Cells. J Immunol. 2015 doi: 10.4049/jimmunol.1400623. [DOI] [PubMed] [Google Scholar]
- 33.Gaya M, Castello A, Montaner B, Rogers N, Reis e Sousa C, Bruckbauer A, Batista FD. Inflammation-induced disruption of SCS macrophages impairs B cell responses to secondary infection. Science (80- ) 2015;347:667–672. doi: 10.1126/science.aaa1300. [DOI] [PubMed] [Google Scholar]
- 34.Nikbakht N, Shen S, Manser T. Cutting edge: Macrophages are required for localization of antigen-activated B cells to the follicular perimeter and the subsequent germinal center response. J Immunol. 2013;190:4923–7. doi: 10.4049/jimmunol.1300350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lytle AG, Shen S, McGettigan JP. Lymph node but not intradermal injection site macrophages are critical for germinal center formation and antibody responses to rabies vaccination. J Virol. 2015;89:2842–8. doi: 10.1128/JVI.03409-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fooksman DR, Schwickert TA, Victora GD, Dustin ML, Nussenzweig MC, Skokos D. Development and Migration of Plasma Cells in the Mouse Lymph Node. Immunity. 2010;33:118–127. doi: 10.1016/j.immuni.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pantel A, Cheong C, Dandamudi D, Shrestha E, Mehandru S, Brane L, Ruane D, Teixeira A, Bozzacco L, Steinman RM, Longhi MP. A new synthetic TLR4 agonist, GLA, allows dendritic cells targeted with antigen to elicit Th1 T-cell immunity in vivo. Eur J Immunol. 2012;42:101–109. doi: 10.1002/eji.201141855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–71. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Veninga H, Borg EGF, Vreeman K, Taylor PR, Kalay H, van Kooyk Y, Kraal G, Martinez-Pomares L, den Haan JMM. Antigen targeting reveals splenic CD169+ macrophages as promoters of germinal center B-cell responses. Eur J Immunol. 2015;45:747–57. doi: 10.1002/eji.201444983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, Haberman AM. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011;34:947–60. doi: 10.1016/j.immuni.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith KM, Pottage L, Thomas ER, Leishman aJ, Doig TN, Xu D, Liew FY, Garside P. Th1 and Th2 CD4+ T cells provide help for B cell clonal expansion and antibody synthesis in a similar manner in vivo. J Immunol. 2000;165:3136–44. doi: 10.4049/jimmunol.165.6.3136. [DOI] [PubMed] [Google Scholar]
- 42.Groom JR, Richmond J, Murooka TT, Sorensen EW, Sung JH, Bankert K, von Andrian UH, Moon JJ, Mempel TR, Luster AD. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity. 2012;37:1091–103. doi: 10.1016/j.immuni.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012;209:1241–1253. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagar Ja, Powell Da, Aachoui Y, Ernst RK, Miao Ea. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–3. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014 doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 46.O’Hagan DT, Ott GS, De Gregorio E, Seubert a. The mechanism of action of MF59 - an innately attractive adjuvant formulation. Vaccine. 2012;30:4341–8. doi: 10.1016/j.vaccine.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 47.Seubert A, Calabro S, Santini L, Galli B, Genovese A, Valentini S, Aprea S, Colaprico A, D’Oro U, Giuliani MM, Pallaoro M, Pizza M, O’Hagan DT, Wack A, Rappuoli R, De Gregorio E. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci U S A. 2011;108:11169–74. doi: 10.1073/pnas.1107941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vono M, Taccone M, Caccin P, Gallotta M, Donvito G, Falzoni S, Palmieri E, Pallaoro M, Rappuoli R, Di Virgilio F, De Gregorio E, Montecucco C, Seubert A. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc Natl Acad Sci U S A. 2013;110:21095–100. doi: 10.1073/pnas.1319784110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellebedy AH, Lupfer C, Ghoneim HE, DeBeauchamp J, Kanneganti T-D, Webby RJ. Inflammasome-independent role of the apoptosis-associated speck-like protein containing CARD (ASC) in the adjuvant effect of MF59. Proc Natl Acad Sci U S A. 2011;108:2927–32. doi: 10.1073/pnas.1012455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox CB, Baldwin SL, Duthie MS, Reed SG, Vedvick TS. Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions. Vaccine. 2011;29:9563–72. doi: 10.1016/j.vaccine.2011.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.