Abstract
OBJECTIVE
We examined the function of ATP-binding cassette transporter A1 (ABCA1) in apolipoprotein A-I (ApoA-I) mobilization of cholesterol microdomains deposited into the extracellular matrix by cholesterol-enriched macrophages. We have also determined whether an ApoA-I mimetic peptide without and with complexing to sphingomyelin can mobilize macrophage deposited cholesterol microdomains.
APPROACH AND RESULTS
Extracellular cholesterol microdomains deposited by cholesterol-enriched macrophages were detected with a monoclonal antibody (Mab), 58B1. ApoA-I and an ApoA-I mimetic peptide 5A mobilized cholesterol microdomains deposited by ABCA1+/+ macrophages but not by ABCA1−/− macrophages. In contrast, ApoA-I mimetic peptide 5A complexed with sphingomyelin could mobilize cholesterol microdomains deposited by ABCA1−/− macrophages.
CONCLUSIONS
Our findings show that a unique pool of extracellular cholesterol microdomains deposited by macrophages can be mobilized by both ApoA-I and an ApoA-I mimetic peptide but that mobilization depends on macrophage ABCA1. It is known that ABCA1 complexes ApoA-I and ApoA-I mimetic peptide with phospholipid, a cholesterol solubilizing agent, explaining the requirement for ABCA1 in extracellular cholesterol microdomain mobilization. Importantly, ApoA-I mimetic peptide already complexed with phospholipid can mobilize macrophage deposited extracellular cholesterol microdomains even in the absence of ABCA1.
Keywords: atherosclerosis, reverse cholesterol transport, macrophages, ApoA-I, cholesterol monoclonal antibody, HDL, ABCA1, ABCG1, mimetic peptide
Introduction
Atherosclerotic plaques show accumulation of both intracellular and extracellular lipid. Our earlier studies show that much unesterified cholesterol accumulates within the extracellular matrix of human atherosclerotic plaques1–3. Thus, strategies to decrease lipid accumulation by stimulating reverse cholesterol transport from plaque cholesterol-containing cells, should also address how to mobilize extracellular cholesterol deposits.
Recently, we have shown that macrophages contribute to extracellular deposits of unesterified cholesterol by depositing excess cholesterol into the extracellular matrix4–6. Depending on the macrophage type and culture conditions, macrophages enriched with cholesterol show development of cholesterol microdomains that are associated with the plasma membrane or that macrophages deposit into the extracellular matrix4–6. We have visualized these cholesterol microdomains with a unique monoclonal antibody that immunolabels an ordered array of cholesterol molecules7. Both ATP-binding cassette transporter A1 (ABCA1) and ATP-binding cassette transporter G1 (ABCG1) mediate extracellular deposition of the cholesterol microdomains by mouse bone marrow-derived macrophages, while ABCA1 mediates their deposition by human M-CSF differentiated monocyte-derived macrophages4, 5.
This previously unrecognized pool of extracellular cholesterol microdomains can be mobilized by high-density lipoprotein (HDL), cyclodextrin, and by ApoA-I4, 6. However, in contrast to HDL, ApoA-I mobilization of the cholesterol microdomains depends on the presence of macrophages6. The fact that the cholesterol microdomains deposited by macrophages can be mobilized by HDL and ApoA-I shows that the extracellular cholesterol microdomains can potentially function in the reverse cholesterol transport pathway.
There has been much interest in the development of ApoA-I mimetic peptides that could be administered to patients as treatment for atherosclerosis8–10. ApoA-I mimetics would hopefully promote reverse cholesterol transport, and thus, decrease cholesterol build-up in atherosclerotic plaques. The advantage of administering ApoA-I mimetic peptides over administration of ApoA-I itself would be a lesser cost to produce the mimetic peptides and the possibility that these peptide mimetics could be administered orally. So far, clinical trials of some early ApoA-I mimetics have shown limited efficacy and unexpected toxicities9, 10. However, refinement of the peptides may allow for enhancement of their cholesterol mobilization properties as well as diminished toxicity.
One such refined ApoA-I mimetic peptide is 5A, which has been shown to stimulate cholesterol efflux from a mouse macrophage cell line and to decrease atherosclerosis in a mouse model of atherosclerosis11. Peptide 5A is a 37 amino acid-long bi-helical amphipathic peptide that contains an 18A α-helix sequence in the first helix as described previously12, joined by a proline with a modified 18A sequence containing five Ala substitutions in the second helix13. Like ApoA-I and other ApoA-I mimetic peptides, peptide 5A can be complexed with phospholipids such as sphingomyelin to increase their potential to stimulate cellular cholesterol efflux independent of ABCA111, 14.
To further investigate the potential efficacy of peptide 5A for human therapy of atherosclerosis, we have examined whether this ApoA-I mimetic peptide can mobilize the extracellular cholesterol microdomains deposited by cholesterol-enriched human monocyte-derived macrophages. Also, we have tested whether ApoA-I and peptide 5A require ABCA1 function in order for these agents to mobilize the extracellular cholesterol microdomains deposited by macrophages. Lastly, we compared the efficacy of ApoA-I and peptide 5A with peptide 5A complexed with sphingomyelin in mobilization of extracellular cholesterol microdomains. Our findings indicate that while ApoA-I and peptide 5A depend on ABCA1 function for their efficacy in mobilizing extracellular cholesterol microdomains, peptide 5A complexed with sphingomyelin can mobilize extracellular cholesterol microdomains independent of ABCA1 function.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
ApoA-I and ApoA-I mimetic peptide 5A mobilize extracellular cholesterol microdomains deposited by macrophages
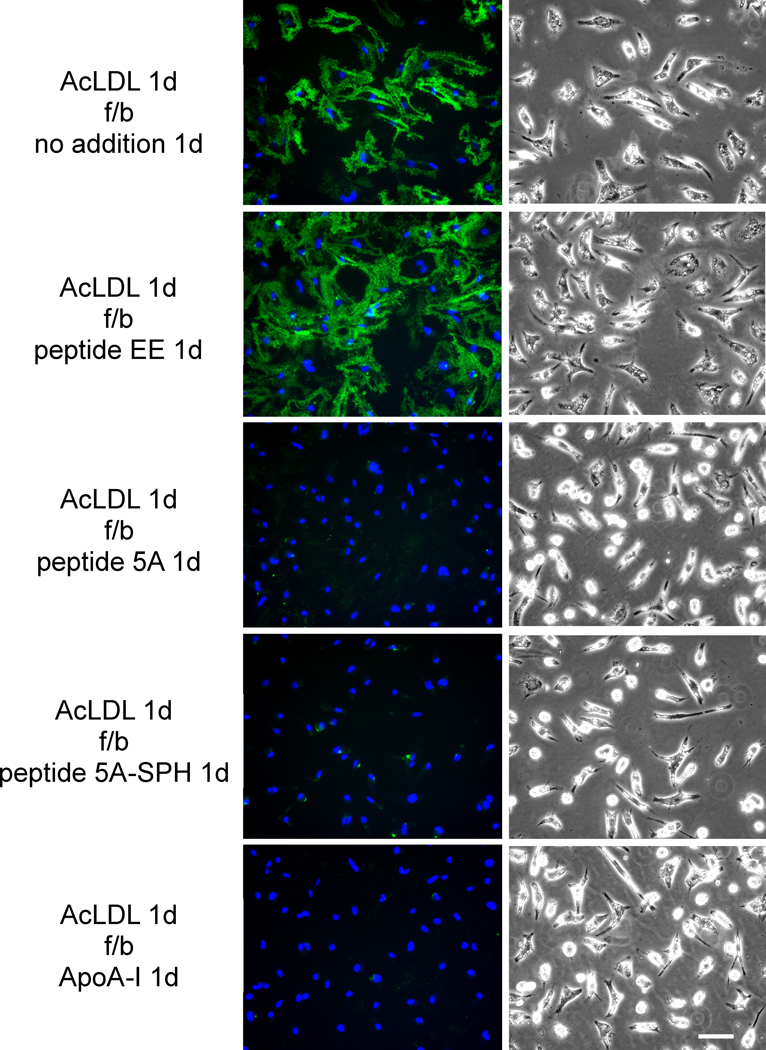
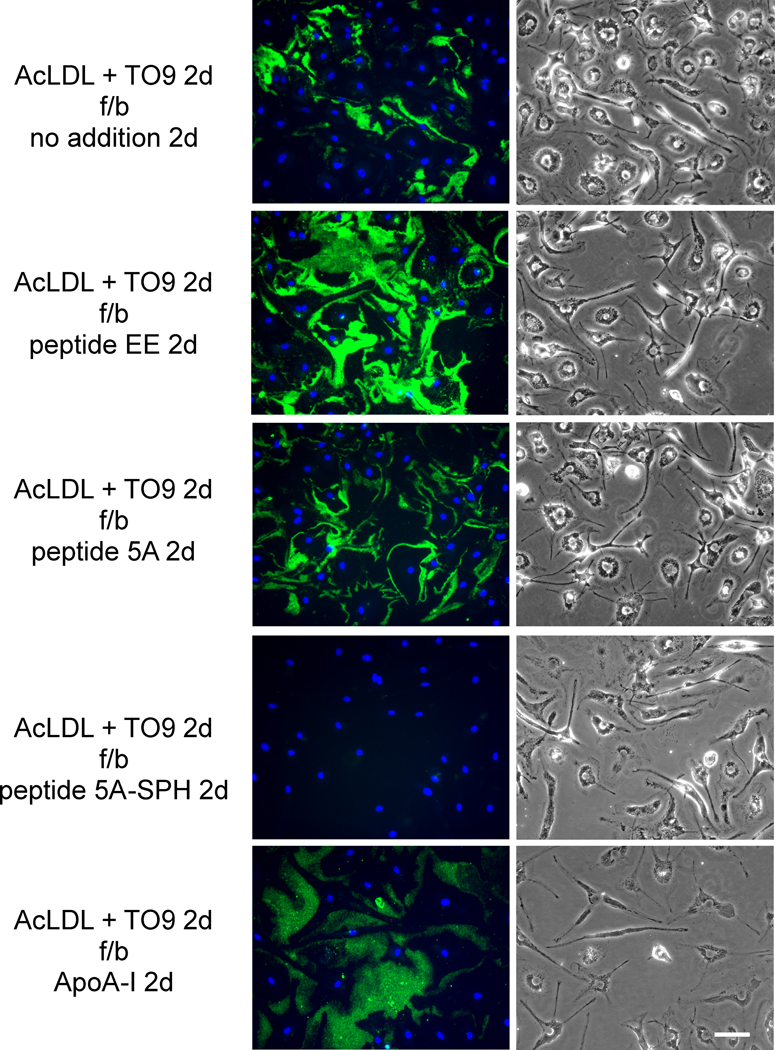
Human M-CSF differentiated monocyte-derived macrophages were cholesterol-enriched by 1-day incubation with AcLDL (50 μg/ml). During this incubation, macrophages deposited cholesterol (detected with Mab 58B1) into the extracellular matrix surrounding the macrophages as we reported previously4–6. Following rinsing of the cultures and subsequent 1-day incubation with peptide 5A, peptide 5A-SPH, or ApoA-I, Mab 58B1 immunostaining was eliminated consistent with mobilization of the extracellular cholesterol microdomains (Figure 1). In contrast, cholesterol was not mobilized when macrophages were incubated with control peptide EE or with no addition. The effect of peptide 5A was concentration dependent with a maximal effect observed at a concentration of 40 μg/ml (Supplemental Figure I). On the contrary, peptide EE sometimes (Figure 1), but not always (Supplemental Figure I) increased extracellular cholesterol microdomain immunostaining for reasons that are not clear to us.
Figure 1. ApoA-I and ApoA-I mimetic peptide 5A mobilization of extracellular cholesterol microdomains deposited by human macrophages.
One-week-old human M-CSF differentiated monocyte-derived macrophage cultures were incubated 1 day with 50 μg/ml AcLDL to induce macrophage deposition of extracellular cholesterol microdomains. Following rinsing, macrophage cultures were incubated 1 day with the indicated additions (20 μg ApoA-I, 100 μg/ml peptide, and 125 μg/ml sphingomyelin complexed with 100 μg/ml peptide 5A) without AcLDL. In the left column, cholesterol microdomains were visualized by fluorescence microscopy using anti-cholesterol microdomain Mab 58B1 (green), and nuclei were imaged with DAPI fluorescence staining (blue). In the right column, macrophages were visualized using phase-contrast microscopy. Left and right columns show corresponding microscopic fields. AcLDL, acetylated low-density lipoprotein; f/b, followed by; 5A-SPH, 5A-sphingomyelin complex. Bar = 50 μm and applies to all.
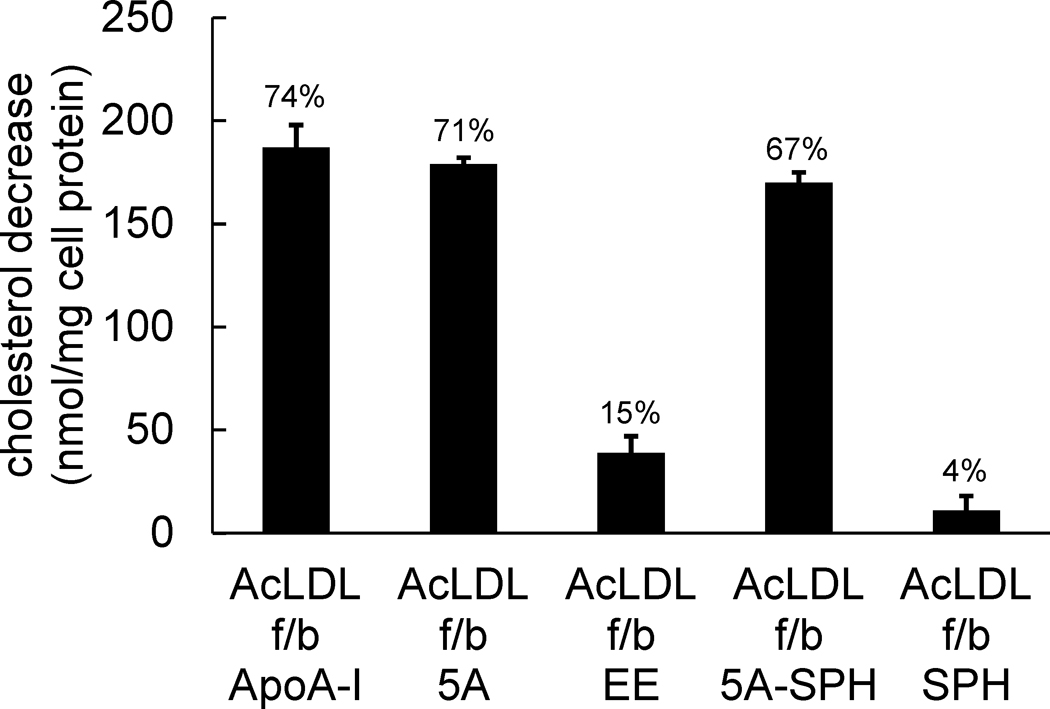
We also assessed the cholesterol content of cholesterol-enriched macrophages following incubation with ApoA-I and the ApoA-I mimetic peptide. Peptide 5A, peptide 5A-SPH, and ApoA-I all caused large and similar decreases in cholesterol content of the cholesterol-enriched human macrophages (about 170–190 nmoles cholesterol/mg cell protein equivalent to about 70% of the accumulated cholesterol) (Figure 2). Sphingomyelin added alone without having been complexed with peptide 5A did not decrease macrophage cholesterol content. Peptide EE showed some decrease in macrophage cholesterol content, but the amount (39 ± 8 nmoles cholesterol/mg cell protein) was much less than that of peptide 5A.
Figure 2. ApoA-I mimetic peptide 5A substantially decreases macrophage cholesterol content.
One-week-old human M-CSF differentiated monocyte-derived macrophage cultures were incubated 1 day with 50 μg/ml AcLDL to enrich macrophages with cholesterol. Following rinsing, macrophage cultures were incubated 1 day with the indicated additions (20 μg/ml ApoA-I, 100 μg/ml peptide, and 125 μg/ml sphingomyelin alone or complexed with100 μg/ml peptide 5A) without AcLDL. Then, the macrophage total cholesterol content was determined, and the decrease in macrophage total cholesterol content induced by the added agent was calculated. Macrophage total cholesterol contents before and after incubation with AcLDL were 98 ± 2 nmoles cholesterol/mg cell protein (5 ± 3% cholesteryl ester) and 305 ± 7 nmoles cholesterol/mg cell protein (30 ± 1% cholesteryl ester), respectively. The percentage decrease in net total cholesterol accumulation is listed above the bar. ApoA-I, 5A, and 5A-SPH were all significantly different than EE and SPH. AcLDL, acetylated low-density lipoprotein; f/b, followed by; ApoA-I, apolipoprotein A-I; 5A, peptide 5A; EE, peptide EE; 5A-SPH, peptide 5A-sphingomyelin complex; SPH, sphingomyelin.
Unless complexed with sphingomyelin, ApoA-I mimetic peptide 5A does not mobilize extracellular cholesterol microdomains in absence of macrophages
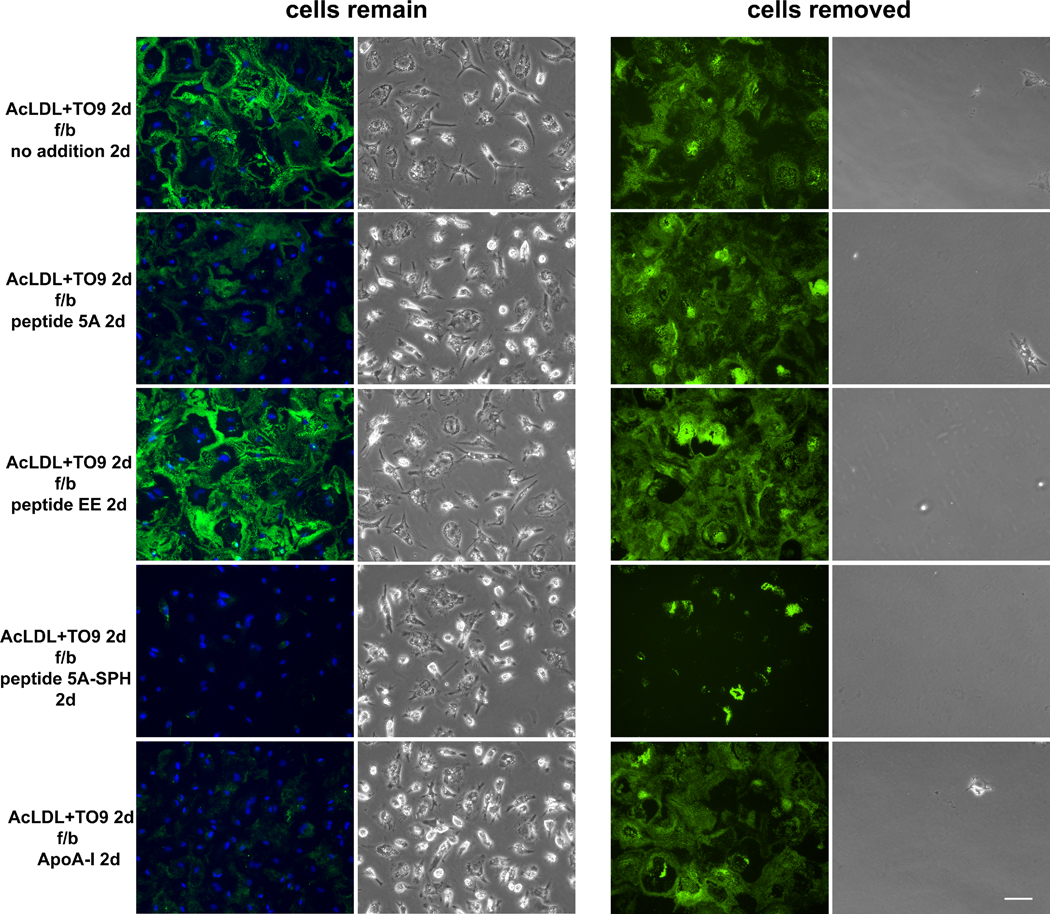
Previously, we reported that ApoA-I could not mobilize extracellular cholesterol microdomains when macrophages were removed from the culture6. Therefore, we examined whether peptide 5A could mobilize extracellular cholesterol microdomains in the absence of macrophages. First, human macrophages were cholesterol-enriched by incubation with AcLDL as described above except that incubation was for 2 days with the LXR agonist, TO901317 (TO9), added to induce maximally ABCA1 and ABCG1 that we previously showed mediate macrophage deposition of extracellular cholesterol microdomains4, 5. Then, macrophages were removed from one set of cultures leaving only the extracellular macrophage deposited cholesterol. Macrophages were not removed in a duplicate set of cultures. Both sets of cultures without and with macrophages were then incubated 1 day without and with the various peptides followed by Mab 58B1 immunostaining of cholesterol microdomains.
Without the presence of macrophages during the incubation with peptides or ApoA-I, only peptide 5A-SPH mobilized cholesterol from the extracellular matrix (Figure 3). However, as compared to its effect with macrophages present, some patches of extracellular Mab58B1 immunostaining remained. This experiment with intensified deposition of cholesterol microdomains showed that with macrophages present, peptide 5A-SPH completely eliminated Mab58B1 immunostaining, while peptide 5A and ApoA-I only partially decreased Mab58B1 immunostaining. This is contrast to the experiment above (Figure 1) where peptide 5A, peptide 5A-SPH, and ApoA-I completely eliminated extracellular Mab58B1immunostaining with macrophages present.
Figure 3. Unless complexed with sphingomyelin, ApoA-I mimetic peptide 5A does not mobilize extracellular cholesterol microdomains in the absence of macrophages.
One-week-old human M-CSF differentiated monocyte-derived macrophage cultures were incubated 2 days with 50 μg/ml AcLDL + 5 μm TO9 to induce macrophage deposition of extracellular cholesterol microdomains. Following rinsing, macrophages remained in a one set of cultures (left) and were removed from a second set of cultures (right). Next, both sets of culture wells were incubated 1 day with the indicated additions (20 μg ApoA-I, 100 μg/ml peptide, and 125 μg/ml sphingomyelin complexed with 100 μg/ml peptide 5A) without AcLDL. Cholesterol microdomains were visualized by fluorescence microscopy using anti-cholesterol microdomain Mab 58B1 (green), and nuclei were imaged with DAPI fluorescence staining (blue). To the right of each fluorescence image is the corresponding, phase-contrast microscopic image. AcLDL, acetylated low-density lipoprotein; TO9, TO901317; f/b, followed by; 5A-SPH, 5A-sphingomyelin complex. Bar = 50 μm and applies to all.
The extracellular cholesterol microdomains that underlie cholesterol-enriched macrophages were not simply cell debris left behind on the culture surface. This was the case because CD14, a macrophage plasma membrane marker15, while present on the macrophage surface, was not present in the extracellular matrix (Supplemental Figure II).
Unless complexed with sphingomyelin, ApoA-I mimetic peptide 5A fails to mobilize extracellular cholesterol microdomains deposited by ABCA−/− mouse macrophages
Peptide 5A mobilization of macrophage deposited cholesterol microdomains depended on the continued presence of macrophages, while peptide 5A-sphingomyelin complex mobilization of extracellular cholesterol microdomains did not depend on macrophages. This suggests that macrophages were necessary for peptide 5A efficacy possibly by providing ABCA1-mediated complexing of macrophage phospholipid with peptide 5A as occurs with ApoA-I16. We examined this possibility.
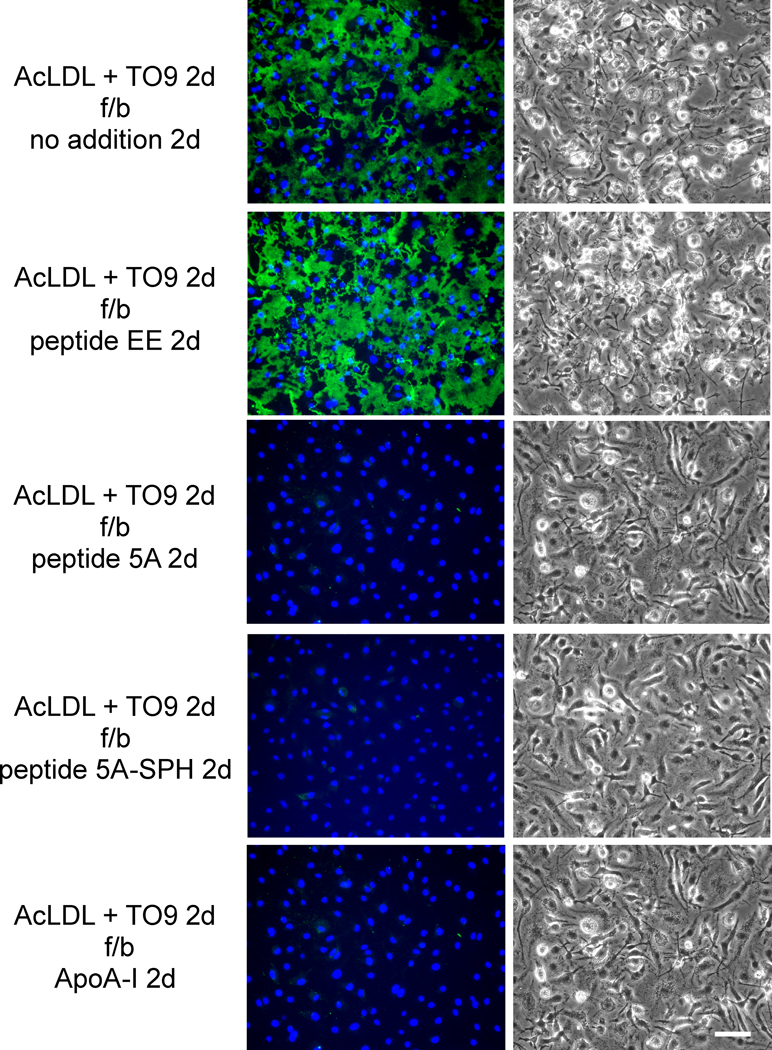
First, we determined the degree of mobilization of macrophage deposited cholesterol microdomains during a 2-day incubation with peptide 5A. In contrast to the 1-day incubation with peptide 5A above in Figure 3 where Mab58B1 immunostaining was only partially decreased, a 2-day incubation showed complete elimination of Mab58B1 immunostaining (Figure 4). Peptide 5A-SPH, and ApoA-I (but not peptide EE) showed a similar elimination of Mab58B1 immunostaining. However, this was not the case when ABCA1−/− mouse macrophages were tested. In this case, only peptide 5A-SPH could mobilize extracellular cholesterol microdomains but peptide 5A and ApoA-I could not (Figure 5). Thus, ABCA1 was necessary for peptide 5A and ApoA-I mobilization of extracellular cholesterol microdomains.
Figure 4. ApoA-I and ApoA-I mimetic peptide 5A mobilize extracellular cholesterol microdomains deposited by ABCA+/+ mouse macrophages.
One-week-old ABCA1+/+ mouse M-CSF differentiated bone marrow-derived macrophage cultures were incubated 2 days with 50 μg/ml AcLDL + 5 μm TO9 to enrich macrophages with cholesterol. Following rinsing, macrophage cultures were incubated 2 days with the indicated additions (20 μg ApoA-I, 100 μg/ml peptide, and 125 μg/ml sphingomyelin complexed with 100 μg/ml peptide 5A) without AcLDL. In the left column, cholesterol microdomains were visualized by fluorescence microscopy using anti-cholesterol microdomain Mab 58B1 (green), and nuclei were imaged with DAPI fluorescence staining (blue). In the right column, macrophages were visualized using phase-contrast microscopy. Left and right columns show corresponding microscopic fields. AcLDL, acetylated low-density lipoprotein; TO9, TO901317; f/b, followed by; 5A-SPH; 5A-sphingomyelin complex. Bar = 50 μm and applies to all.
Figure 5. Unless complexed with sphingomyelin, ApoA-I mimetic peptide 5A fails to mobilize extracellular cholesterol microdomains deposited by ABCA−/− mouse macrophages.
One-week-old ABCA1−/− mouse M-CSF differentiated bone marrow-derived macrophage cultures were incubated 2 days with 50 μg/ml AcLDL + 5 μm TO9 to enrich macrophages with cholesterol. Following rinsing, macrophage cultures were incubated 2 days with the indicated additions (20 μg ApoA-I, 100 μg/ml peptide, and 125 μg/ml sphingomyelin complexed with 100 μg/ml peptide 5A) without AcLDL. In the left column, cholesterol microdomains were visualized by fluorescence microscopy using anti-cholesterol microdomain Mab 58B1 (green), and nuclei were imaged with DAPI fluorescence staining (blue). In the right column, macrophages were visualized using phase-contrast microscopy. Left and right columns show corresponding microscopic fields. AcLDL, acetylated low-density lipoprotein; TO9, TO901317; f/b, followed by; 5A-SPH, 5A-sphingomyelin complex. Bar = 50 μm and applies to all.
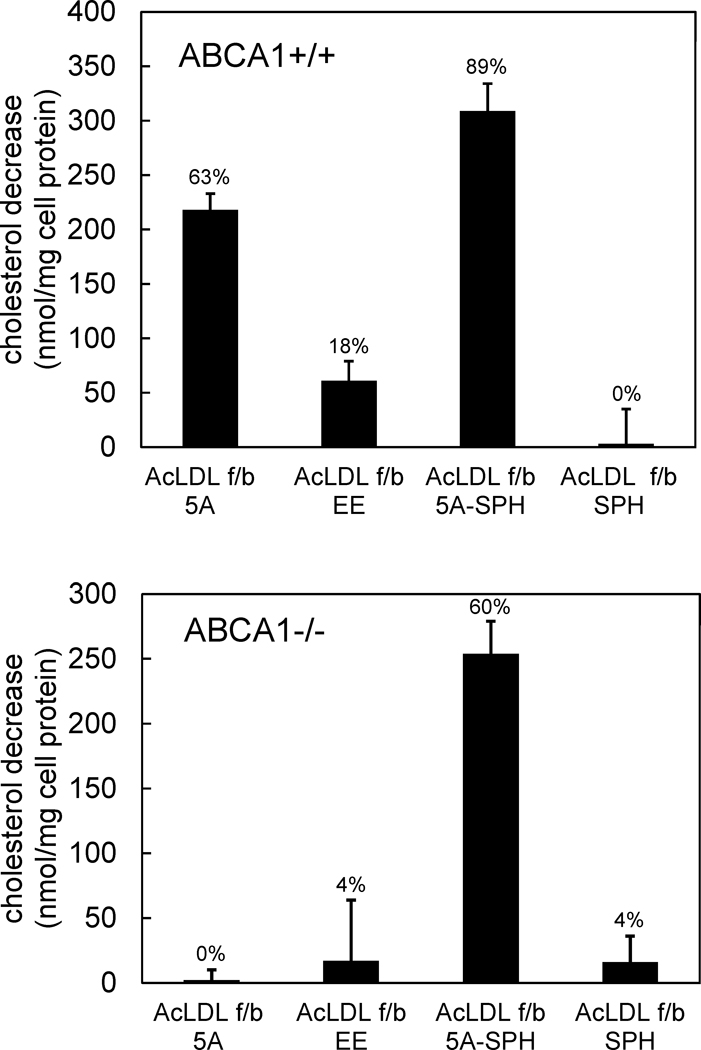
We next assessed the importance of ABCA1 for macrophage cholesterol mass efflux mediated by peptides 5A and 5A-SPH. Similar to human macrophages, peptides 5A and 5A-SPH caused a substantial decrease in cholesterol content (218 ± 15 and 309 ± 25 nmoles cholesterol/mg cell protein, respectively) in cholesterol-enriched ABCA1+/+ (wild-type) mouse macrophages (Figure 6). Also similar to human macrophages, the control peptide EE decreased cholesterol content substantially less (61 ± 18 nmoles cholesterol/mg cell protein) than the ApoA-I mimetic peptide 5A. However, only peptide 5A-SPH significantly decreased the cholesterol content of cholesterol-enriched ABCA1−/− mouse macrophages (Figure 6). This showed that peptide 5A required macrophage ABCA1 in order to stimulate macrophage cholesterol efflux.
Figure 6. ApoA-I mimetic peptide 5A substantially decreases ABCA+/+ but not ABCA−/− mouse macrophage cholesterol content.
One-week-old mouse M-CSF differentiated bone marrow-derived macrophage cultures were incubated 2 days with 50 μg/ml AcLDL + 5 μm TO9 to enrich macrophages with cholesterol. Following rinsing, macrophage cultures were incubated 2 days with the indicated additions (100 μg/ml peptide and 125 μg/ml sphingomyelin alone or complexed with 100 μg/ml peptide 5A) without AcLDL. Then, the macrophage total cholesterol content was determined, and the decrease in macrophage cholesterol content induced by the added peptide was calculated. Cholesterol contents before and after incubation with AcLDL, respectively, were 107 ± 14 nmoles cholesterol/mg cell protein (10 ± 2% cholesteryl ester) and 453 ± 27 nmoles cholesterol/mg cell protein (70 ± 0% cholesteryl ester) for ABCA1+/+ macrophages, and 146 ± 26 nmoles cholesterol/mg cell protein (25 ± 10% cholesteryl ester) and 571 ± 7 nmoles cholesterol/mg cell protein (75 ± 1% cholesteryl ester) for ABCA1−/− macrophages. For ABCA1+/+ macrophages, 5A and 5A-SPH were significantly different than EE and SPH. For ABCA1−/− macrophages, 5A-SPH was significantly different than 5A, SPH, and EE. AcLDL, acetylated low-density lipoprotein; TO9, TO901317; f/b, followed by; 5A, peptide 5A; EE, peptide EE; 5A-SPH, peptide 5A-sphingomyelin complex; SPH, sphingomyelin.
The isolated cholesterol microdomains contain cholesterol that is unestified
The anti-cholesterol microdomain Mab58B1 shows the presence of cholesterol in the extracellular deposits, and we previously showed that the deposits do not stain with Oil Red O indicating the lack of cholesteryl ester4. However, to confirm that the cholesterol microdomains contain cholesterol in a predominantly unestified form, we analyzed the cholesterol composition of the extracellular cholesterol microdomains. We isolated the cholesterol microdomains by first removing macrophages from culture wells using EDTA, and then releasing the microdomains from the extracellular matrix by trypsin treatment. This was followed by density gradient centrifugation of the released cholesterol microdomains (Supplemental Figure III). The density gradient showed three regions of cholesterol at densities ≤1.005, 1.030 to 1.044, and ≥1.091 g/ml showing 51, 91, and 95 percent of cholesterol that was unesterified, respectively. The cholesteryl ester in the density ≤1.005 region is likely extracellular matrix-bound AcLDL that floats after loss of protein following exposure to protease17. Mab58B1 immunostaining of the isolated cholesterol particles from each cholesterol-rich density gradient region showed that the density 1.030 to 1.044 g/ml cholesterol region was most enriched with cholesterol microdomains that stained with the anti-cholesterol microdomain Mab58B1 (Supplemental Figure III).
Discussion
ApoA-I and ApoA-I mimetic peptide 5A mobilization of extracellular cholesterol microdomains depended on ABCA1. The cholesterol microdomains deposited by macrophages could not be directly solubilized by peptide 5A or ApoA-I. This is presumably because ABCA1 mediates complexing of cell-derived phospholipid with ApoA-I and ApoA-I mimetic peptides16, and it is the phospholipid that solubilizes cholesterol. Under certain conditions, ApoA-I and ApoA-I mimetic peptides can directly solubilize phospholipid and form complexes without ABCA118, 19. However, this depends on the phospholipid type and the amount of cholesterol associated with the phospholipid20. That the cholesterol microdomains could not be directly solubilized by ApoA-I or the ApoA-I mimetic peptide suggests that the cholesterol microdomains are not associated with a cholesterol-solubilizing lipid that is susceptible to amphipathic apolipoprotein-induced micellarization.
Interestingly, an ApoA-I binding site has been described in the extracellular matrix underlying macrophages21. Thus, this binding site localizes ApoA-I to the same location where the extracellular cholesterol microdomains deposit. As the ApoA-I binding site mediates ABCA1-dependent cholesterol efflux, this binding site could be functioning in mobilization of the macrophage deposited cholesterol microdomains.
The cholesterol pool that is mobilized by ApoA-I interaction with ABCA1 has been postulated to be present within the plasma membrane22–27, intracellularly within late endosomes/lysosomes28–33, and within the extracellular matrix as we have shown here. It is possible that efflux from all these cholesterol pools could be occurring simultaneously or individually depending on the cell type under study. Also, intracellular cholesterol pools may be a source of cholesterol that is available for efflux within the plasma membrane or extracellular matrix. In this regard, ABCA1 redistributes intracellular cholesterol to cholesterol oxidase-sensitive pools of cholesterol that could be located within either the plasma membrane, or the extracellular cholesterol microdomains we have described4, 23, 34.
Previous investigations of ApoA-I mediated cellular cholesterol efflux have suggested that cholesterol is concurrently effluxed with the phospholipid that complexes with ApoA-I (one-step process)23, 30, 35, 36. Simultaneous complexing of phospholipid and cholesterol with ApoA-I has been suggested to result from microsolubilization of plasma membrane microdomains37. Both non-raft and raft microdomains, the latter characterized by sphingomyelin and cholesterol enrichment, may contribute to such ApoA-I lipid complexes38–40.
Alternatively, ApoA-I could complex with phospholipid prior to the complex acquiring cholesterol from the cell (two-step process)35, 41–44. The findings that ABCA1-mediated phospholipid efflux can occur without cholesterol efflux, and that cholesterol efflux can be regulated independently of phospholipid efflux in some cell systems could be interpreted to support a two-step process33, 35, 41–47. Our findings show that in the case of extracellular cholesterol microdomains, a two-step process mediates cholesterol efflux from the extracellular matrix. First, macrophages excrete cholesterol microdomains into the extracellular matrix, and subsequently interaction of ApoA-I with macrophage ABCA1 mobilizes the cholesterol microdomains.
We have shown previously that ABCA1 and ABCG1 mediate macrophage deposition of cholesterol microdomains into the extracellular matrix4, 5. Thus, ABCA1 shows two separate functions; one that mediates deposition of the extracellular cholesterol microdomains, and another that mediates ApoA-I’s mobilization of the extracellular microdomains.
Dependence on ABCA1 to mediate complexing of phospholipid with ApoA-I and ApoA-I mimetic peptides could be a rate limiting process in reverse cholesterol transport from atherosclerotic plaques because ABCA1 expression in human atherosclerotic plaques is diminished. Studies have shown that ABCA1 expression in human atherosclerotic plaques is limited due to the differentiation state of intimal smooth muscle cells48, and in the case of macrophages possibly due to the fact that ABCA1 is downregulated by various cytokines likely present within atherosclerotic plaques49. Moreover, much of the cholesterol within human atherosclerotic plaques accumulates in relatively acellular plaque regions where there would not be any cellular expression of ABCA150. In these acellular regions there is no possibility to locally complex either ApoA-I or ApoA-I mimetic peptides with cell-derived phospholipid.
Given that much of the cholesterol in human atherosclerotic plaques accumulates within the extracellular space1–3, 6, mobilizing extracellular cholesterol microdomains may be as important as mobilizing cholesterol stored within atherosclerotic plaque cells. Because of dependence on ABCA1 as discussed above, peptide 5A and ApoA-I could not mobilize extracellular cholesterol microdomains deposited by macrophages when macrophages were removed from the cultures or when macrophages lacked ABCA1. In contrast, peptide 5A complexed with sphingomyelin could mobilize extracellular cholesterol microdomains under these conditions showing the potential usefulness of pre-formed ApoA-I mimetic-phospholipid complexes in mobilizing extracellular cholesterol microdomains even in the absence of ABCA1.
The choice of sphingomyelin as the phospholipid complexed to peptide 5A should be optimal for mobilization of cholesterol as this phospholipid has a relatively high affinity for cholesterol as compared to other common phospholipids51. However, sphingomyelin alone did not show any mobilization of extracellular cholesterol microdomain deposits or stimulate a decrease in macrophage cholesterol content. The sphingomyelin was added as liposomes. Such liposomes previously have been shown to be poor acceptors of cholesterol, most likely due to size constraints limiting access of these relatively large particles to sites of potential cholesterol efflux52, 53. In this regard, efficient delivery of cholesterol acceptors to sites of cholesterol accumulation within atherosclerotic plaques is also an obstacle to attaining plaque reverse cholesterol transport. Efficient delivery of cholesterol acceptors may not only be limited by acceptor size, but also by loss of acceptor efflux potential as the acceptor passes through the blood and picks up cholesterol effluxed from other sites besides plaques such as liver54 and the spleen that processes cholesterol from degraded red cells.
ApoA-I and ApoA-I mimetic peptides show a beneficial effect on the development of atherosclerosis in experimental animal models9, 55–58. The mechanism of this effect may be multifactorial, including anti-inflammatory effects in addition to stimulating reverse cholesterol transport by mobilizing cellular and, as we have demonstrated here, extracellular cholesterol microdomains. If not mobilized, extracellular cholesterol microdomains could promote atherogenesis by promoting inflammation. The antibody used here to detect extracellular deposited cholesterol does not bind individual cholesterol molecules. Rather, this antibody labels a structural domain of organized cholesterol molecules as can occur for example in a cholesterol crystal59, 60. Although the physical form of the extracellular cholesterol microdomains deposited by macrophage has not yet been determined, high concentrations of cholesterol in crystals or liposomes can stimulate complement formation, and thereby promote inflammation61–63. Thus, future studies of the potential inflammatory properties of the extracellular cholesterol microdomains is warranted.
In conclusion, previously we showed that both ABCA1 and ABCG1 mediate macrophage deposition of extracellular cholesterol microdomains4, 5. Our finding here that ABCA1 is necessary for mobilization of this cholesterol demonstrates an interesting concerted action of ABCA1, both to deposit and then effect mobilization of extracellular cholesterol microdomains. However, ApoA-I and ApoA-I mimetic peptides already complexed with phospholipid may show better efficacy for both cellular and extracellular plaque cholesterol removal when ABCA1 expression is limited.
Supplementary Material
Highlights.
Cholesterol-enriched macrophages deposit cholesterol microdomains into the extracellular matrix
ApoA-I and ApoA-I mimetic peptide can mobilize these extracellular cholesterol microdomains by a process mediated by macrophage ABCA1
In the absence of macrophage ABCA1, ApoA-I mimetic peptide can mobilize extracellular cholesterol microdomains if the peptide is first complexed with sphingomyelin
Recognition that macrophages not only store excess cholesterol intracellularly, but also deposit this cholesterol extracellularly is important to our further understanding of reverse cholesterol transport
Acknowledgments
The authors thank the Department of Transfusion Medicine, Clinical Center, National Institutes of Health, for providing elutriated monocytes.
Sources of Funding
This work was supported by the Intramural Research Program, National Heart, Lung, and Blood Institute, National Institutes of Health, and by the US-Israel Binational Science Foundation (Grant 2013045).
Abbreviations
- ABCA1
ATP-binding cassette transporter A1
- ABCG1
ATP-binding cassette transporter G1
- AcLDL
acetylated low-density lipoprotein
- ApoA-I
apolipoprotein A-I
- DPBS
Dulbecco’s phosphate-buffered saline with Ca2+ and Mg2+
- EDTA
ethylenediaminetetraacetic acid
- FBS
fetal bovine serum
- HDL
high-density lipoprotein
- Mab
monoclonal antibody
- M-CSF
macrophage colony-stimulating factor
- SPH
sphingomyelin
- TO9
TO901317
Footnotes
Disclosures
Peptide 5A has been licensed by the National Institutes of Health to KineMed for clinical development.
References
- 1.Kruth HS. Localization of unesterified cholesterol in human atherosclerotic lesions. Demonstration of filipin-positive, oil-red-o-negative particles. Am J Pathol. 1984;114:201–208. [PMC free article] [PubMed] [Google Scholar]
- 2.Kruth HS. Filipin-positive, oil red o-negative particles in atherosclerotic lesions induced by cholesterol feeding. Lab Invest. 1984;50:87–93. [PubMed] [Google Scholar]
- 3.Kruth HS, Fry DL. Histochemical detection and differentiation of free and esterified cholesterol in swine atherosclerosis using filipin. Exp Mol Pathol. 1984;40:288–294. doi: 10.1016/0014-4800(84)90046-7. [DOI] [PubMed] [Google Scholar]
- 4.Freeman SR, Jin X, Anzinger JJ, Xu Q, Purushothaman S, Fessler MB, Addadi L, Kruth HS. Abcg1-mediated generation of extracellular cholesterol microdomains. J Lipid Res. 2014;55:115–127. doi: 10.1194/jlr.M044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin X, Freeman SR, Vaisman B, Liu Y, Chang J, Varsano N, Addadi L, Remaley A, Kruth HS. Abca1 contributes to macrophage deposition of extracellular cholesterol. J Lipid Res. 2015;56:1720–1726. doi: 10.1194/jlr.M060053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong DS, Anzinger JJ, Leyva FJ, Rubin N, Addadi L, Kruth HS. Extracellular cholesterol-rich microdomains generated by human macrophages and their potential function in reverse cholesterol transport. J Lipid Res. 2010;51:2303–2313. doi: 10.1194/jlr.M005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Addadi L, Geva M, Kruth HS. Structural information about organized cholesterol domains from specific antibody recognition. Biochim Biophys Acta. 2003;1610:208–216. doi: 10.1016/s0005-2736(03)00019-1. [DOI] [PubMed] [Google Scholar]
- 8.White CR, Garber DW, Anantharamaiah GM. Anti-inflammatory and cholesterol-reducing properties of apolipoprotein mimetics: A review. J Lipid Res. 2014;55:2007–2021. doi: 10.1194/jlr.R051367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osei-Hwedieh DO, Amar M, Sviridov D, Remaley AT. Apolipoprotein mimetic peptides: Mechanisms of action as anti-atherogenic agents. Pharmacol Ther. 2011;130:83–91. doi: 10.1016/j.pharmthera.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoekenbroek RM, Stroes ES, Hovingh GK. Apoa-i mimetics. Handb Exp Pharmacol. 2015;224:631–648. doi: 10.1007/978-3-319-09665-0_21. [DOI] [PubMed] [Google Scholar]
- 11.Amar MJ, D’Souza W, Turner S, Demosky S, Sviridov D, Stonik J, Luchoomun J, Voogt J, Hellerstein M, Sviridov D, Remaley AT. 5a apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J Pharmacol Exp Ther. 2010;334:634–641. doi: 10.1124/jpet.110.167890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anantharamaiah GM, Jones JL, Brouillette CG, Schmidt CF, Chung BH, Hughes TA, Bhown AS, Segrest JP. Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J Biol Chem. 1985;260:10248–10255. [PubMed] [Google Scholar]
- 13.Sethi AA, Stonik JA, Thomas F, Demosky SJ, Amar M, Neufeld E, Brewer HB, Davidson WS, D’Souza W, Sviridov D, Remaley AT. Asymmetry in the lipid affinity of bihelical amphipathic peptides. A structural determinant for the specificity of abca1-dependent cholesterol efflux by peptides. J Biol Chem. 2008;283:32273–32282. doi: 10.1074/jbc.M804461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwendeman A, Sviridov DO, Yuan W, Guo Y, Morin EE, Yuan Y, Stonik J, Freeman L, Ossoli A, Thacker S, Killion S, Pryor M, Chen YE, Turner S, Remaley AT. The effect of phospholipid composition of reconstituted hdl on its cholesterol efflux and anti-inflammatory properties. J Lipid Res. 2015;56:1727–1737. doi: 10.1194/jlr.M060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldo SW, Li Y, Buono C, Zhao B, Billings EM, Chang J, Kruth HS. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol. 2008;172:1112–1126. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama S. Assembly of high density lipoprotein by the abca1/apolipoprotein pathway. Curr Opin Lipidol. 2005;16:269–279. doi: 10.1097/01.mol.0000169346.15450.90. [DOI] [PubMed] [Google Scholar]
- 17.Piha M, Lindstedt L, Kovanen PT. Fusion of proteolyzed low-density lipoprotein in the fluid phase: A novel mechanism generating atherogenic lipoprotein particles. Biochemistry. 1995;34:10120–10129. doi: 10.1021/bi00032a004. [DOI] [PubMed] [Google Scholar]
- 18.Phillips MC. New insights into the determination of hdl structure by apolipoproteins: Thematic review series: High density lipoprotein structure, function, and metabolism. J Lipid Res. 2013;54:2034–2048. doi: 10.1194/jlr.R034025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sviridov DO, Andrianov AM, Anishchenko IV, Stonik JA, Amar MJ, Turner S, Remaley AT. Hydrophobic amino acids in the hinge region of the 5a apolipoprotein mimetic peptide are essential for promoting cholesterol efflux by the abca1 transporter. J Pharmacol Exp Ther. 2013;344:50–58. doi: 10.1124/jpet.112.198143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuda M, Nakano M, Sriwongsitanont S, Ueno M, Kuroda Y, Handa T. Spontaneous reconstitution of discoidal hdl from sphingomyelin-containing model membranes by apolipoprotein a-i. J Lipid Res. 2007;48:882–889. doi: 10.1194/jlr.M600495-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Burgess JW, Kiss RS, Zheng H, Zachariah S, Marcel YL. Trypsin-sensitive and lipid-containing sites of the macrophage extracellular matrix bind apolipoprotein a-i and participate in abca1-dependent cholesterol efflux. J Biol Chem. 2002;277:31318–31326. doi: 10.1074/jbc.M204200200. [DOI] [PubMed] [Google Scholar]
- 22.Denis M, Landry YD, Zha X. Atp-binding cassette a1-mediated lipidation of apolipoprotein a-i occurs at the plasma membrane and not in the endocytic compartments. J Biol Chem. 2008;283:16178–16186. doi: 10.1074/jbc.M709597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaughan AM, Oram JF. Abca1 redistributes membrane cholesterol independent of apolipoprotein interactions. J Lipid Res. 2003;44:1373–1380. doi: 10.1194/jlr.M300078-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Faulkner LE, Panagotopulos SE, Johnson JD, Woollett LA, Hui DY, Witting SR, Maiorano JN, Davidson WS. An analysis of the role of a retroendocytosis pathway in abca1-mediated cholesterol efflux from macrophages. J Lipid Res. 2008;49:1322–1332. doi: 10.1194/jlr.M800048-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorci-Thomas MG, Owen JS, Fulp B, Bhat S, Zhu X, Parks JS, Shah D, Jerome WG, Gerelus M, Zabalawi M, Thomas MJ. Nascent high density lipoproteins formed by abca1 resemble lipid rafts and are structurally organized by three apoa-i monomers. J Lipid Res. 2012;53:1890–1909. doi: 10.1194/jlr.M026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC. Mechanism of atp-binding cassette transporter a1-mediated cellular lipid efflux to apolipoprotein a-i and formation of high density lipoprotein particles. J BiolChem. 2007;282:25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 27.Gillotte KL, Davidson WS, Lund-Katz S, Rothblat GH, Phillips MC. Removal of cellular cholesterol by pre-beta-hdl involves plasma membrane microsolubilization. J Lipid Res. 1998;39:1918–1928. [PubMed] [Google Scholar]
- 28.Takahashi Y, Smith JD. Cholesterol efflux to apolipoprotein ai involves endocytosis and resecretion in a calcium-dependent pathwa. Proc Natl Acad Sci U S A. 1999;96:11358–11363. doi: 10.1073/pnas.96.20.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W, Sun Y, Welch C, Gorelik A, Leventhal AR, Tabas I, Tall AR. Preferential atp-binding cassette transporter a1-mediated cholesterol efflux from late endosomes/lysosomes. J Biol Chem. 2001;276:43564–43569. doi: 10.1074/jbc.M107938200. [DOI] [PubMed] [Google Scholar]
- 30.Smith JD, Le Goff W, Settle M, Brubaker G, Waelde C, Horwitz A, Oda MN. Abca1 mediates concurrent cholesterol and phospholipid efflux to apolipoprotein a-i. J Lipid Res. 2004;45:635–644. doi: 10.1194/jlr.M300336-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Kojima K, Abe-Dohmae S, Arakawa R, Murakami I, Suzumori K, Yokoyama S. Progesterone inhibits apolipoprotein-mediated cellular lipid release: A putative mechanism for the decrease of high-density lipoprotein. Biochim Biophys Acta. 2001;1532:173–184. doi: 10.1016/s1388-1981(01)00124-x. [DOI] [PubMed] [Google Scholar]
- 32.Neufeld EB, Stonik JA, Demosky SJ, Jr, Knapper CL, Combs CA, Cooney A, Comly M, Dwyer N, Blanchette-Mackie J, Remaley AT, Santamarina-Fojo S, Brewer HB., Jr The abca1 transporter modulates late endocytic trafficking: Insights from the correction of the genetic defect in tangier disease. J Biol Chem. 2004;279:15571–15578. doi: 10.1074/jbc.M314160200. [DOI] [PubMed] [Google Scholar]
- 33.Zheng H, Kiss RS, Franklin V, Wang MD, Haidar B, Marcel YL. Apoa-i lipidation in primary mouse hepatocytes. Separate controls for phospholipid and cholesterol transfers. J Biol Chem. 2005;280:21612–21621. doi: 10.1074/jbc.M502200200. [DOI] [PubMed] [Google Scholar]
- 34.Jin X, Freeman SR, Vaisman B, Liu Y, Chang J, Varsano N, Addadi L, Remaley A, Kruth HS. Abca1 mediates macrophage deposition of cholesterol into the extracellular matrix. Arteriosclerosis, Thrombosis and Vascular Biology I Peripheral Vascular Disease Scientific Sessions 2015. 2015 Abstract #534. [Google Scholar]
- 35.Wang N, Lan D, Gerbod-Giannone M, Linsel-Nitschke P, Jehle AW, Chen W, Martinez LO, Tall AR. Atp-binding cassette transporter a7 (abca7) binds apolipoprotein a-i and mediates cellular phospholipid but not cholesterol efflux. J Biol Chem. 2003;278:42906–42912. doi: 10.1074/jbc.M307831200. [DOI] [PubMed] [Google Scholar]
- 36.Gillotte KL, Zaiou M, Lund-Katz S, Anantharamaiah GM, Holvoet P, Dhoest A, Palgunachari MN, Segrest JP, Weisgraber KH, Rothblat GH, Phillips MC. Apolipoprotein-mediated plasma membrane microsolubilization. Role of lipid affinity and membrane penetration in the efflux of cellular cholesterol and phospholipid. J Biol Chem. 1999;274:2021–2028. doi: 10.1074/jbc.274.4.2021. [DOI] [PubMed] [Google Scholar]
- 37.Phillips MC. Molecular mechanisms of cellular cholesterol efflux. J Biol Chem. 2014;289:24020–24029. doi: 10.1074/jbc.R114.583658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iatan I, Bailey D, Ruel I, Hafiane A, Campbell S, Krimbou L, Genest J. Membrane microdomains modulate oligomeric abca1 function: Impact on apoai-mediated lipid removal and phosphatidylcholine biosynthesis. J Lipid Res. 2011;52:2043–2055. doi: 10.1194/jlr.M016196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendez AJ, Lin G, Wade DP, Lawn RM, Oram JF. Membrane lipid domains distinct from cholesterol/sphingomyelin-rich rafts are involved in the abca1-mediated lipid secretory pathway. J Biol Chem. 2001;276:3158–3166. doi: 10.1074/jbc.M007717200. [DOI] [PubMed] [Google Scholar]
- 40.Sorci-Thomas MG, Thomas MJ. Microdomains, inflammation, and atherosclerosis. Circ Res. 2016;118:679–691. doi: 10.1161/CIRCRESAHA.115.306246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fielding PE, Nagao K, Hakamata H, Chimini G, Fielding CJ. A two-step mechanism for free cholesterol and phospholipid efflux from human vascular cells to apolipoprotein a-1. Biochemistry. 2000;39:14113–14120. doi: 10.1021/bi0004192. [DOI] [PubMed] [Google Scholar]
- 42.Li Q, Tsujita M, Yokoyama S. Selective down-regulation by protein kinase c inhibitors of apolipoprotein-mediated cellular cholesterol efflux in macrophages. Biochemistry. 1997;36:12045–12052. doi: 10.1021/bi970079t. [DOI] [PubMed] [Google Scholar]
- 43.Li Q, Yokoyama S. Independent regulation of cholesterol incorporation into free apolipoprotein-mediated cellular lipid efflux in rat vascular smooth muscle cells. J Biol Chem. 1995;270:26216–26223. doi: 10.1074/jbc.270.44.26216. [DOI] [PubMed] [Google Scholar]
- 44.Arakawa R, Abe-Dohmae S, Asai M, Ito JI, Yokoyama S. Involvement of caveolin-1 in cholesterol enrichment of high density lipoprotein during its assembly by apolipoprotein and thp-1 cells. J Lipid Res. 2000;41:1952–1962. [PubMed] [Google Scholar]
- 45.Kiss RS, Maric J, Marcel YL. Lipid efflux in human and mouse macrophagic cells: Evidence for differential regulation of phospholipid and cholesterol efflux. J Lipid Res. 2005;46:1877–1887. doi: 10.1194/jlr.M400482-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Jessup W, Gelissen IC, Gaus K, Kritharides L. Roles of atp binding cassette transporters a1 and g1, scavenger receptor bi and membrane lipid domains in cholesterol export from macrophages. Curr Opin Lipidol. 2006;17:247–257. doi: 10.1097/01.mol.0000226116.35555.eb. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Komaba A, Yokoyama S. Cholesterol is poorly available for free apolipoprotein-mediated cellular lipid efflux from smooth muscle cells. Biochemistry. 1993;32:4597–4603. doi: 10.1021/bi00068a016. [DOI] [PubMed] [Google Scholar]
- 48.Choi HY, Rahmani M, Wong BW, Allahverdian S, McManus BM, Pickering JG, Chan T, Francis GA. Atp-binding cassette transporter a1 expression and apolipoprotein a-i binding are impaired in intima-type arterial smooth muscle cells. Circulation. 2009;119:3223–3231. doi: 10.1161/CIRCULATIONAHA.108.841130. [DOI] [PubMed] [Google Scholar]
- 49.Schmitz G, Langmann T. Transcriptional regulatory networks in lipid metabolism control abca1 expression. Biochim Biophys Acta. 2005;1735:1–19. doi: 10.1016/j.bbalip.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Guyton JR, Klemp KF. Development of the lipid-rich core in human atherosclerosis. Arterioscler Thromb Vasc Biol. 1996;16:4–11. doi: 10.1161/01.atv.16.1.4. [DOI] [PubMed] [Google Scholar]
- 51.Lund-Katz S, Laboda HM, McLean LR, Phillips MC. Influence of molecular packing and phospholipid type on rates of cholesterol exchange. Biochemistry. 1988;27:3416–3423. doi: 10.1021/bi00409a044. [DOI] [PubMed] [Google Scholar]
- 52.Davidson WS, Rodrigueza WV, Lund-Katz S, Johnson WJ, Rothblat GH, Phillips MC. Effects of acceptor particle size on the efflux of cellular free cholesterol. J Biol Chem. 1995;270:17106–17113. doi: 10.1074/jbc.270.29.17106. [DOI] [PubMed] [Google Scholar]
- 53.Jian B, de la Llera-Moya M, Royer L, Rothblat G, Francone O, Swaney JB. Modification of the cholesterol efflux properties of human serum by enrichment with phospholipid. J Lipid Res. 1997;38:734–744. [PubMed] [Google Scholar]
- 54.Yamamoto S, Tanigawa H, Li X, Komaru Y, Billheimer JT, Rader DJ. Pharmacologic suppression of hepatic atp-binding cassette transporter 1 activity in mice reduces high-density lipoprotein cholesterol levels but promotes reverse cholesterol transport. Circulation. 2011;124:1382–1390. doi: 10.1161/CIRCULATIONAHA.110.009704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leman LJ, Maryanoff BE, Ghadiri MR. Molecules that mimic apolipoprotein a-i: Potential agents for treating atherosclerosis. J Med Chem. 2014;57:2169–2196. doi: 10.1021/jm4005847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Fogelman AM. Apo a-1 mimetic peptides as atheroprotective agents in murine models. Curr Drug Targets. 2008;9:204–209. doi: 10.2174/138945008783755584. [DOI] [PubMed] [Google Scholar]
- 57.Smith JD. Apolipoprotein a-i and its mimetics for the treatment of atherosclerosis. Curr Opin Investig Drugs. 2010;11:989–996. [PMC free article] [PubMed] [Google Scholar]
- 58.Gordon SM, Davidson WS. Apolipoprotein a-i mimetics and high-density lipoprotein function. Curr Opin Endocrinol Diabetes Obes. 2012;19:109–114. doi: 10.1097/MED.0b013e32835056d4. [DOI] [PubMed] [Google Scholar]
- 59.Izhaky D, Addadi L. Pattern recognition by antibodies for two-dimensional arrays of molecules. Adv Mater. 1998;10:1009–1013. [Google Scholar]
- 60.Perl-Treves D, Kessler N, Izhaky D, Addadi L. Monoclonal antibody recognition of cholesterol monohydrate crystal faces. Chem Biol. 1996;3:567–577. doi: 10.1016/s1074-5521(96)90148-9. [DOI] [PubMed] [Google Scholar]
- 61.Seifert PS, Kazatchkine MD. Generation of complement anaphylatoxins and c5b-9 by crystalline cholesterol oxidation derivatives depends on hydroxyl group number and position. Mol Immunol. 1987;24:1303–1308. doi: 10.1016/0161-5890(87)90125-8. [DOI] [PubMed] [Google Scholar]
- 62.Cunningham CM, Kingzette M, Richards RL, Alving CR, Lint TF, Gewurz H. Activation of human complement by liposomes: A model for membrane activation of the alternative pathway. J Immunol. 1979;122:1237–1242. [PubMed] [Google Scholar]
- 63.Alving CR, Richards RL, Guirguis AA. Cholesterol-dependent human complement activation resulting in damage to liposomal model membranes. J Immunol. 1977;118:342–347. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.