Abstract
We improved our inductively coupled plasma mass spectrometry (ICP-MS) whole blood method [1] for determination of lead (Pb), cadmium (Cd), and mercury (Hg) by including manganese (Mn) and selenium (Se), and expanding the calibration range of all analytes. The method is validated on a PerkinElmer (PE) ELAN® DRC II ICP-MS (ICP-DRC-MS) and uses the Dynamic Reaction Cell (DRC) technology to attenuate interfering background ion signals via ion-molecule reactions. Methane gas (CH4) eliminates background signal from 40Ar2+ to permit determination of 80Se+, and oxygen gas (O2) eliminates several polyatomic interferences (e.g. 40Ar15N+, 54Fe1H+) on 55Mn+. Hg sensitivity in DRC mode is a factor of two higher than vented mode when measured under the same DRC conditions as Mn due to collisional focusing of the ion beam. To compensate for the expanded method’s longer analysis time (due to DRC mode pause delays), we implemented an SC4-FAST autosampler (ESI Scientific, Omaha, NE), which vacuum loads the sample onto a loop, to keep the sample-to-sample measurement time to less than 5 min, allowing for preparation and analysis of 60 samples in an 8-h work shift. The longer analysis time also resulted in faster breakdown of the hydrocarbon oil in the interface roughing pump. The replacement of the standard roughing pump with a pump using a fluorinated lubricant, Fomblin®, extended the time between pump maintenance. We optimized the diluent and rinse solution components to reduce carryover from high concentration samples and prevent the formation of precipitates. We performed a robust calculation to determine the following limits of detection (LOD) in whole blood: 0.07 μg dL−1 for Pb, 0.10 μg L−1 for Cd, 0.28 μg L−1 for Hg, 0.99 μg L−1 for Mn, and 24.5 μg L−1 for Se.
Keywords: Biomonitoring, Reaction cell, ICP-MS, Whole blood, Blood lead, Manganese, Cadmium, Mercury, Selenium
1. Introduction
The Centers for Disease Control and Prevention’s (CDC) Environmental Health Laboratory at the National Center for Environmental Health (NCEH) uses inductively coupled plasma mass spectrometry (ICP-MS) to measure trace and toxic elements in people’s blood and urine to detect harmful exposures of environmental chemicals in populations [2,3]. These measurements are made as part of ongoing assessments of the U.S. population’s exposure, such as the National Health and Nutrition Examination Survey (NHANES), as well as for emergency response situations due to accidental or intentional acute exposures. In national survey applications, the sensitive, multielement capabilities of ICP-MS permit the efficient low-level quantitation of multiple trace and toxic elements. In emergency response situations, the fast analysis and wide dynamic range of ICP-MS permits the quantification of toxic elements from both chronic and acute exposures.
Lead, cadmium, and mercury are toxic to humans and show only deleterious effects on human health. The Agency for Toxic Substances and Disease Registry (ATSDR) has published Toxicological Profiles for these elements [4–6] which list numerous health effects based on the route of exposure (inhalation, oral, or dermal) including death, chronic diseases, permanent neurological damage, or subclinical effects. The effects of mercury can depend on the type of mercury exposure (inorganic vs. organic) although both are considered toxic. Total blood mercury concentrations, such as those measured in this method, are considered indicative of dietary intake of organic mercury, particularly methyl mercury [7], although inorganic and ethyl mercury can also be measured in blood [8]. Blood lead and blood cadmium measurements are widely accepted as an indicator of recent and long-term exposures [7]. Until 2012, children were identified as having a blood lead “level of concern” if the test result is 10 μg dL−1 or higher of lead in blood. CDC is no longer using the term “level of concern” and is instead using a reference value, currently 5 μg dL−1, to identify children who have been exposed to lead and who require case management [9]. The reference level is based on the 97.5th percentile of the four most recent years of NHANES blood lead data (currently 2007–2010). The method reported here will be used to produce the blood lead data on which the reference value is based.
Selenium and manganese each play an essential role in the human biological system if levels are not deficient or excessive. In humans, selenium is incorporated into selenoproteins, important antioxidant enzymes which help prevent cellular damage caused by free radicals. Free radicals are natural by-products of oxygen metabolism that may contribute to the development of chronic diseases such as cancer and heart disease [10,11]. Other selenoproteins help regulate thyroid function and play a role in the immune system [12–14]. There is evidence that selenium deficiency may contribute to heart disease, hypothyroidism, and a weakened immune system [15,16]. Symptoms of very high exposure to selenium, a condition called selenosis, include gastrointestinal upsets, hair loss, white blotchy nails, garlic breath odor, fatigue, irritability, and mild nerve damage [17]. Manganese, an essential trace element, plays a role in bone mineralization, metabolism, and metabolic regulation. It is part of several metalloenzymes [18] and is ubiquitous in the human body. Elevated manganese levels are known to be neurotoxic and linked to the diagnosis of manganism [18,19]. Environmental human exposures are commonly due to contaminated drinking water [20,21] and potentially due to methylcyclopentadienyl manganese tricarbonyl (MMT), an anti-knocking additive in gasoline [22–24]. Manganese deficiency in humans is rare, but has been associated with impaired growth, reproductive function, and glucose tolerance, and with alterations in carbohydrate and lipid metabolism in various animal species [25]. We added manganese and selenium to the method to establish reference ranges for the U.S. population which have not previously been available.
Our laboratory began using ICP-MS for the determination of Pb, Cd, and Hg in blood starting with the NHANES cycle 2003–2004 [1]. That method used a calibration range optimized for biomonitoring applications where the normal population exposure was also expected to be narrow (i.e., approximately one order of magnitude between the geometric mean and the 95th percentile). A second ICP-MS method was developed in 2008 at the CDC for the analysis of Pb, Cd, and Hg in human blood [26] to be used in state and local public health laboratories to increase emergency response capacity within the U.S. The goal of the work described here was to develop a single method that achieves a wide calibration range suitable for high-throughput biomonitoring and emergency response applications, easily transferable to state and local public health labs, and also to include manganese and selenium.
A review of trace elements in biological fluids by Ivanenko et al. [27] lists four published methods [26,28–30] of whole blood analysis by quadrupole ICP-MS with a collision or reaction cell used to measure at least one of the elements here. Four other publications were identified [22,31–33] in the literature as comparable to our method. Our method has several important advantages. We use a straightforward sample dilution in alkali diluent and the diluted samples can be directly analyzed without extra sonication or centrifugation [31,32]. No lengthy acid digestion is required [22,28]. The diluent makeup is based on the work of Lutz [34] and McShane [26] which minimizes memory effects from high concentration samples to subsequent samples. This method requires a smaller sample volume, 50 μL of whole blood, to complete determination of all five elements, compared to requirements of 100–1 mL in the literature [22,26,28–30,32]. This smaller volume is especially important when a patient sample needs to be split and analyzed by several methods. Our calculation of the method limits of detection (LOD), derived from matrix-matched calibration blanks and standards across numerous runs (n≥60), is more robust and statistically confident than found in the literature and results in comparable values, if not improved.
The severity of the human health effects from exposure to Pb, Cd, Hg, Mn, and Se necessitate an accurate and precise procedure. The analytical method described here quantifies concentrations of Pb, Cd, Hg, Mn, and Se in whole human blood using a PE ELAN® DRC II ICP-MS. The method is applicable to long-term biomonitoring studies to evaluate chronic environmental or other non-occupational exposures or to fast response when acute exposure to these elements is suspected. Discussions of the method will include figures of merit such as accuracy, precision, limit of detection, and ruggedness under routine implementation.
2. Materials and methods
2.1. Instrumentation
An ELAN® DRC II ICP-MS (PerkinElmer SCIEX, Concord, Ontario, Canada) with quartz cyclonic spray chamber, demountable quartz torch, 2.0 mm i.d. quartz injector, and nickel (or platinum) sampler and skimmer cones (PE, Shelton, CT) was used. The instrument was equipped with an integrated DXi micro peristaltic pump and switching valve, a 1.0 mL sample loop (1.6 mm i.d.), and PolyPro-ST concentric nebulizer (0.25 mm i.d.) as part of the SC-FAST system (Elemental Scientific Inc., Omaha, NB). Peristaltic pump tubing moves the carrier solution through the sample loop to the nebulizer (0.76 mm i.d. “black-black”) and removes waste from the spray chamber (Santoprene 1.30 mm i.d. “grey-grey”) (Meinhard, Golden, CO). The pump was operated at 1.5 rpm, equivalent to a liquid flow rate at the nebulizer of 160 μL min−1. The standard instrument roughing pump, which used general purpose mechanical pump oil (Agilent, Santa Clara, CA), was replaced with a pump that uses Fomblin®, a perfluorinated polyether fluid (PerkinElmer, Shelton, CT), reducing the frequency of pump oil changes from biweekly (see Fig. 1) to annually. An SC-4 DX autosampler (Elemental Scientific Inc., Omaha, NB) was used to access diluted blood specimens and control the FAST sample introduction timing. Sample preparation was performed using a Digiflex™ semiautomatic liquid handler equipped with 10 mL diluting and 200 μL sampling syringes (Titertek, Huntsville, AL). Instrumental parameters used are presented in Table 1, and method parameters are listed in Table 2. All blood sample preparations are carried out in a Class II type A/B biological safety cabinet (BSC) (Nuaire, Plymouth, MN, USA)..
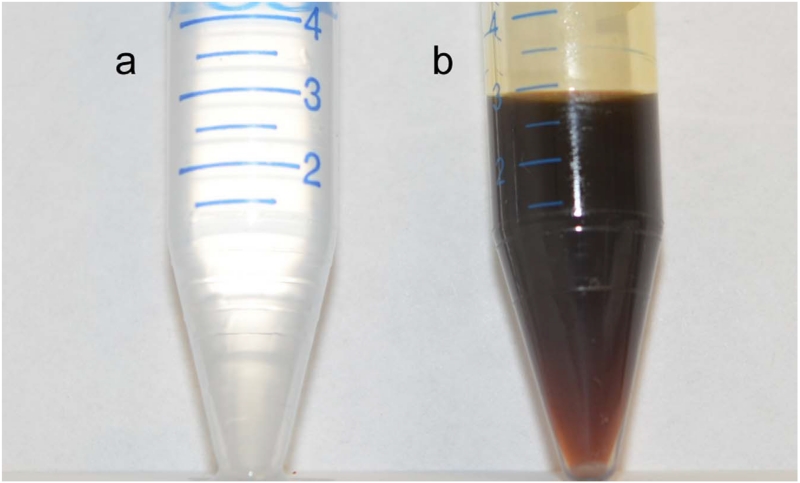
Fig. 1.
Comparison of (a) new hydrocarbon pump oil to (b) hydrocarbon pump oil after 40 days of use (approximately 25 analytical runs).
Table 1.
Instrument parameters for the PE ELAN® DRC II ICP-MS.
| Instrument parameter | Value/setting |
|---|---|
| RF power | 1.45 kW |
| Plasma gas flow (Ar) | 15 L min−1 |
| Auxiliary gas flow (Ar) | 1.2 L min−1 |
| Nebulizer gas flow (Ar) | ~0.90 to 1.0 L min−1 |
| Scan mode | Peak hopping |
| Sweeps/reading | 30 |
| Readings/replicate | 1 |
| Replicates | 3 |
| Dwell time(s) | 100 ms For analytes (Se, Mn, Hg, Cd, Pb) |
| 50 ms For internal standards (Rh, Te, Ir) | |
| Ion lens voltage(s) | AutoLens™ |
| Detector mode | Dual |
| Calibration Regression Type | External, matrix matched, weighted lineara |
| Rinse time | 30 s |
| DRC pressurize delay | 60 s |
| DRC exhaust delay | 30 s |
| DRC channel delay | 30 s |
The ELAN software uses a (1/x2) weighting.
Table 2.
Analyte, internal standards, equations, and DRC parameters.
| Isotope | Internal standard | Equation | Mode (DRC or vented) | Gas | Flow rate (mL min−1) | RPq | RPa |
|---|---|---|---|---|---|---|---|
| 80Se | 130Te | None | DRC, Channel A | CH4 | 0.84 | 0.65 | 0 |
| 55Mn | 103Rh | None | DRC, Channel B | O2 | 1.2 | 0.6 | 0 |
| 202Hg | 130Te | +200Hg | DRC, Channel B | O2 | 1.2 | 0.6 | 0 |
| 114Cd | 193Ir | −0.027250*118Sn | Vented | NA | NA | 0.25 | 0 |
| 208Pb | 193Ir | +206Pb, +207Pb | Vented | NA | NA | 0.25 | 0 |
2.2. Materials and reagents
All rinse, diluent, and standards are prepared with ≥18 MΩ cm deionized (DI) water using a NANOpure® Diamond™ UV water purification system (Barnstead International, Dubuque, Iowa, USA). Concentrated hydrochloric acid (Veritas grade, GFS Chemicals, Columbus, OH, USA), ethylenediaminetetraacetic acid (EDTA) (Fisher Scientific, Fair Lawn, NJ), ammonium pyrrolidinedithiocarbamate (APDC) (laboratory grade, Fisher Scientific, Fairlawn, NJ), ethanol (Pharmco Products, Inc., Brookfield, CT), tetramethylammonium hydroxide (TMAH) (25% w/v, AlfaAesar, Ward Hill, MA), and Triton-X 100™ (J.T. Baker Chemical Co., Phillipsburg, NJ) were used. Single element or custom multi-element stock standards were purchased from various sources (High Purity Standards, Charleston, SC; SPEX CertiPrep, Metuchen, NJ; Inorganic Ventures, Christiansburg, VA) and traceable to the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA). Oxygen (research grade 5.0, 99.999% purity, Airgas South, Atlanta, GA) and methane (research grade 5.0, 99.999% purity, Airgas South, Atlanta, GA) were used in the dynamic reaction cell. We purchased whole human blood to matrix-match calibrators (referred to as base blood) and create quality control materials (Tennessee Blood Services Memphis, TN). Standard Reference Materials (SRMs) were purchased from National Institute for Standards and Technology (NIST) (Gaithersburg, MD), and reference materials from Le Center de toxicology du Quebec (CTQ) (Quebec, Canada), and Wadsworth Center (Albany, NY).
2.3. Sample collection and supplies
Prior to collecting blood samples, supplies (e.g. stainless steel needle, vacutainer, cryovials, and tubes used in analysis) are screened to be free of significant analyte contamination. The screening solution and contact time depends on the intended use of the device. Stainless steel parts and vacutainers are screened with ≥18 MΩ cm deionized (DI) water, while all other devices are screened with 0.5% (v/v) HNO3. Screen solution contact time with needles and pipette tips approximates the normal use of the device, while it is left in sample storage containers overnight. The maximum allowable contribution for an element from a device is based on 10% of the expected population geometric mean adjusted for the volume of sample expected and volume of screening solution used. If the maximum allowable contribution is below the method LOD, then the maximum allowable contribution is set to 1.5 times the LOD. Blood collection tubes contain an appropriate anticoagulant (preferably EDTA).
2.4. Quality control materials
Three levels of blood quality control material pools were prepared by spiking large quantities (~8 L) of whole human blood purchased from Tennessee Blood Services (Memphis, TN) with the analytes of interest. The blood pools are mixed thoroughly and then dispensed into screened HDPE cryovials (FisherScientific, Pittsburg, PA) and stored at ≤−20 °C. One-way analysis of variance (ANOVA) is used to test the hypothesis that the means among trays are equal. The homogeneity of the variance among trays is tested too (Levene’s test). P-values ≥0.05 indicate that there is no statistically significant difference among trays. After homogeneity test, the pools are then characterized. Three characterized QC pools are analyzed at the beginning and ending of each run and the multi-rule quality control system (MRQCS) developed by Caudill et al. [35], are used to determine if runs are in control.
2.5. Calibration preparation and DRC stability time
A custom multi-element stock standard is diluted with 3% (v/v) HCl (S0) in acid-washed Class A, glass, volumetric flasks to prepare eight spiked intermediate calibration standards (see Table 3). Each intermediate calibration standard is then mixed with base blood and diluent using the Digiflex™ pipette to prepare the matrix-matched working calibrators (S0–S8) for an analytical run (see Table 3). The base blood pool is pre-screened and selected to be low in concentration of method analytes. Calibrator 0 is used as the blank for all spiked calibrators. The reagent blank for all patient samples, blood quality controls, and reference materials is prepared using DI water in place of whole blood in the dilution at the Digiflex™. We analyze a bulk preparation of working calibrator 2 for approximately 1 h prior to running the calibration curve to ensure stabilized measurements in DRC mode [36,37].
Table 3.
Reagents, sample composition, calibrator concentrations, and population geometric mean.
| Reagent name | Composition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diluent, rinse, and FAST carrier solutionsa | 0.4% (v/v) TMAH, 1% ethanol, 0.01% APDC, 0.05% Triton X-100™, 5 μg L−1 Rh, Te, Ir | |||||||||
| Sample preparation | 50 μL whole blood sample+50 μL DI water+2400 μL diluent | |||||||||
| Extra dilution sample preparation (2× shown) | 50 μL whole blood sample+150 μL DI water+4800 μL diluent | |||||||||
| Matrix blank and calibrators (S0–S8) | 50 μL base blood+50 μL 3% (v/v) HCl (S0-S8)+2400 μL diluent | |||||||||
| Reagent blank | 100 μL DI water+2400 μL diluent | |||||||||
| Calibrators | Analyte (units) | Spiked concentrations (S1–S8) | Geometric meanb | |||||||
| Cd, Hg (μg L−1) | 0.5 | 1.5 | 3.5 | 5 | 10 | 25 | 75 | 200 | 0.279 (Cd) | |
| 0.703 (Hg) | ||||||||||
| Mn (μg L−1) | 1.5 | 4.5 | 10.5 | 15 | 30 | 75 | 225 | 600 | 9.35 | |
| Se (μg L−1) | 30 | 90 | 210 | 300 | 600 | 1500 | 4500 | 12,000 | 190 | |
| Pb (μg dL−1) | 1 | 3 | 7 | 10 | 20 | 50 | 150 | 400 | 0.973 | |
Rinse does not contain the internal standards (Rh, Te, Ir).
From 2011 to 2012 NHANES [3].
2.6. Sample preparation
During the sample dilution step, a small volume of whole blood is extracted from a larger whole blood patient specimen after the entire specimen is thoroughly mixed (vortexed for several seconds) to create a uniform distribution of cellular components. Sample homogeneity prior to withdrawing a portion is important because some metals (e.g. Pb) are known to be associated mostly with the red blood cells in the specimen [5,38]. Blood with any observable clotting is unsuitable for analysis due to sample inhomogeneity. Whole blood samples are diluted 50× (1+1+48) with DI water and diluent (see Table 3), matching the blood and diluent composition of the working calibrators. Samples that exceed the concentration of the high calibrator are diluted extra (up to 20×) with DI water to bring them within the measurement range. The ratio of the volume of diluent to the total volume of the preparation must be constant across the preparations for the run because the diluent contains the internal standard. Samples which have been diluted 1+1+48 for analysis up to 24 h previously and stored at room temperature can still be analyzed. We observed a significant reduction in measured Hg and Se concentrations in diluted samples after 24 h.
3. Results and discussion
3.1. Spectral interferences and selectivity
In this method Pb, Cd and Hg do not require the use of DRC mode to reduce or remove spectral interferences, but each of these elements is measured with a mathematical equation in the ELAN® software for different reasons (see Table 2). 208Pb signal is summed with the signals from 206Pb and 207Pb to account for variation in relative abundances of lead isotopes in nature. The small natural abundance of 204Pb (1.4%) is not included in the sum because it does not vary and has an isobaric interference from 204Hg. The method uses a mathematical equation to correct for the small isobaric overlap of 114Sn (0.65%) on 114Cd. Molybdenum (Mo), an essential element, is present in human biological samples [7] and could interfere with blood 114Cd analysis as the 98Mo16O polyatomic ion. However, Mo is primarily excreted in the urine [39] and unless a blood sample is drawn within 24 h of an acute Mo exposure [40], we don’t expect a need for interference correction. Tungsten (W), not an essential element, has been measured in biological samples [7] and could interfere with 202Hg analysis as 186W16O and 184W18O polyatomic ions. However, humans primarily excrete W in urine [41], and we don’t expect a need for interference correction in blood samples due to the low reference values for blood W found in the literature (0.4 ng/g [42]). The method measures Hg in DRC mode with O2 gas in the reaction cell (Table 2) to take advantage of collisional focusing that increases the ion signal relative to vented mode (Fig. 2), in addition to summing signal from 202Hg and 200Hg to increase Hg sensitivity. Other isotopes of Hg were excluded because of either isobaric interferences, low natural abundance, or an observed bias when included..
Fig. 2.
202Hg signal versus O2 gas flow rate in the dynamic reaction cell showing collisional focusing of the 202Hg signal (●) 3% (v/v) HCl, (○) 0.5 μg/L Hg in 3% (v/v) HCl, (– –) 202Hg signal normalized to vented mode.
We use the ELAN® ICP-DRC-MS in DRC mode for the remaining two elements in the method, 55Mn and 80Se because of polyatomic interferences. The DRC conditions listed in Table 2 were selected based on the reduction/removal of the most severe spectral overlap for each isotope. Both isotopes suffer from plasma gas-based spectral overlaps: 40Ar2+ on 80Se+, and 40Ar14N1H+ and 38Ar16O1H+ on 55Mn+, among many others. Tables 4 and 5 list the other spectral interferences we considered for 55Mn and 80Se, respectively. This list is not comprehensive for all potential spectral overlaps at m/z 55 and 80. These species were selected based on the expected concentrations of elements in a diluted human blood sample. Several times, the literature reports using CH4 as a reaction gas to remove the 40Ar2+ overlap from 80Se+ [43–45], but we could find only one report on the use of O2 as a reaction gas for measuring 55Mn+ [46] where the selection of O2 was based on the need to detect sulfur as the 32S16O+ product ion. NH3 is more commonly used as the reaction gas in a dynamic reaction cell [33,45], or He gas in a collision cell, for 55Mn+; however, we encountered a significant positive bias when attempting to use NH3 that we avoided by using oxygen as the DRC gas. Praamsma et al. [47] compared Mn results in blood from several sources including our laboratory and found that our Mn results with O2 gas were comparable to those obtained with NH3, sector field (SF)-ICP-MS, and graphite furnace atomic absorption spectrometry (GFAAS).
Table 4.
Selectivity testing results for 55Mn+ in the presence of interfering species.
| Spectral interference for 55Mn+ |
Highest anticipated conc. in human whole blood |
Interference concentration | Mn recovery in spiked base blood¥ |
Mn recovery in spiked QMEQAS09B- 02¥ |
|---|---|---|---|---|
| 110Cd++ | 1.30 μg L−1 Cda | 12 μg L−1 Cd | 99% | 98% |
| 39K16O+ | 200 mg L−1Kb | 200 mg L−1 K | 101% | 97% |
| 37Cl18O+ | 3800 mg L−1 Clc | 30,000 mg L−1α Cl | 100% | 99% |
| 54Fe1H+ | 405 mg L−1 Fed | 500 mg L−1 Fe | 103% | 99% |
95th Percentile from NHANES 1999–2002 survey [7].
Calculated from a reference value of 5.1 mmol/L K in adult serum [48].
Calculated from a reference value of 108 mmol/L Cl in plasma [48].
Calculated from reference value of 2700 mg Fe in an adult human [49] 75 mL/kg of blood in an adult (range of 50 – 83 mL/kg) [50]; average adult weight of 88.8 kg [51].
3% HCl matrix (v/v).
Results calculated as measured Mn concentration in spiked sample relative to unspiked base blood or CTQ reference material. Average measured base blood Mn concentration was 8.5 μg L−1; Average measured QMEQAS09B-02 Mn concentration was 10.2 μg L−1.
Table 5.
Selectivity testing results for 80Se+ in the presence of interfering species.
| Spectral interference for80Se+ | Highest anticipated conc. in human whole blood |
Interference concentration |
Se recovery in spiked base blood¥ |
Se recovery in low QC pool¥ |
Se recovery in spiked QMEQAS07B0-09¥ |
|---|---|---|---|---|---|
| 64Ni16O+ | 0.028 mg L−1 Ni [50] | 0.300 mg L−1 Ni | 98% | 96% | 100% |
| 64Ni12C1H4+ | |||||
| 48Ti16O2+ | 0.150 mg L−1 Tia | 1.5 mg L−1 Ti | 100% | 95% | 100% |
| 48Ti(12C1H4)2+ | |||||
| 63MCu17O+ | 1.5 mg L−1 Cu [50] | 15 mg L−1 Cu | 99% | 94% | 102% |
| 64Zn16O+ | 7.18 mg L−1 Znb | 7 mg L−1 Zn | 101% | 95% | 100% |
| 64Zn12C1H4+ | |||||
| 40Ca40Ar+ | 86–100 mg L−1 Ca in serum [50] | 500 mg L−1 Ca | 102% | 97% | 102% |
| 40K40Ar+ | 200 mg L−1 Kc | 200 mg L−1 K | 101% | 97% | 102% |
Calculated from upper value of 0.15 mg/kg (ppm) [52].
Value calculated from reference of 1.22 μg/mL Zn in plasma and plasma containing 17% of the total Zn in whole blood [53].
See Table 4.
Results calculated relative to unspiked base blood, QC, or CTQ reference material. Average measured Se concentration in base blood was 268 μg L−1. Average measured Se concentration in low QC pool was 215 μg L−1. Average measured Se concentration in QMEQAS07B-09 was 169 μg L−1.
We performed selectivity testing for 55Mn and 80Se in the presence of potential spectral interferences by preparing samples in duplicate and adding a small spike of a potential interferent to one and the same volume spike of DI water to the other. We used single element spiking solutions at concentrations sufficiently high so that only a small volume spike (~0.1 mL) was required. We selected biological samples for testing that have a low-normal concentration of the element of interest. The concentrations of some elements can vary greatly in biological samples; therefore, we tested high or elevated but still biologically relevant concentrations. These concentrations were found in the literature in reports of acutely exposed persons, or if known, the 95th percentile of a relevant population. We calculated the percent measured in a spiked sample relative to the unspiked sample. The percent recoveries for 55Mn and 80Se in Tables 4 and 5, respectively, were all within 6% of the non-spiked sample, proving that the DRC conditions are selective for 55Mn and 80Se even in the presence of high concentrations of potential interferents.
3.2. Accuracy and precision
Accuracy of an analytical method is best demonstrated by analysis of standard reference materials (SRM). NIST SRM 955c, “Toxic Metals in Caprine Blood,” is certified at four levels for Pb, Cd, and Hg, but none of the levels are certified for Mn or Se. A consensus value for Mn in Level 1 of 955c has been determined using data from several laboratories, including our own [47]. All four levels of the SRM were analyzed repeatedly over a four-month time period, and the averaged results are shown in Table 6. The accuracy of the measurements with this method are within 5% of the target values. Level 1 is below the method LOD for Cd and Hg; therefore, the results are not listed in Table 6.
Table 6.
Measured results for Pb, Cd, Hg, and Mn in NIST SRM 955c Toxic Metals in Caprine Blood over n=15 measurements.
| NIST 955c SRM |
Analyte | Target Value ( ±Ud) |
Observed Mean Conc. ( ± 1 SD) |
% Bias |
|---|---|---|---|---|
| Level 1 | Pb (μg dL−1) | 0.424 ± 0.011a | 0.441 ± 0.024 | 4.0% |
| Level 2 | 13.95 ± 0.08a | 13.7 ± 0.3 | −1.8% | |
| Level 3 | 27.76 ± 0.16a | 27.4 ± 0.5 | −1.3% | |
| Level 4 | 45.53 ± 0.27a | 44.4 ± 1.3 | −1.9% | |
| Level 2 | Cd (μg L−1) | 2.14 ± 0.24b | 2.11 ± 0.08 | −1.4% |
| Level 3 | 5.201 ± 0.038a | 5.14 ± 0.19 | −1.2% | |
| Level 4 | 9.85 ± 0.17b | 9.98 ± 0.39 | 1.3% | |
| Level 2 | Hg (μg L−1) | 4.95 ± 0.76b | 5.19 ± 0.20 | 4.8% |
| Level 3 | 17.8 ± 1.6a | 18.4 ± 0.7 | 3.3% | |
| Level 4 | 33.9 ± 2.1b | 33.5 ± 1.7 | −1.3% | |
| Level 1 | Mn (μg L−1) | 16.3 ± 0.8c | 16.8 ± 0.6 | 2.8% |
Certified value.
Reference value.
Consensus value.
Expanded uncertainty at approximately 95% confidence level.
When no SRM exists for an element, we use reference materials (RM) which have previously been assigned target values through analysis by multiple labs to validate the accuracy of a method. Results for Mn and Se from analysis of RM samples from two programs are listed in Table 7. These samples were selected to cover a range of concentrations for the two elements. The accuracy of the method is demonstrated in the calculated percent bias of our results, which range from −7.8% to 1.3% for Mn, −6.8% to 3.7% for Se, and an average bias of −2.6% and −1.9%, respectively.
Table 7.
Measured results for Mn and Se in reference materials from Institut national de santé publique Quebec, and Health Research Inc.
| Reference material ID |
Analyte | Target value ( ± 1 SD) |
Observed mean conc. ( ± 1 SD) |
% Bias | N |
|---|---|---|---|---|---|
| QMEQAS08B-05a | Mn (μg L−1) | 9.3 ± 0.62 | 9.17 ± 0.55 | −1.4% | 15 |
| BE11-03b | 13.2 ± 1.6 | 13.3 ± 0.9 | 0.4% | 8 | |
| QMEQAS08B-08a | 17.7 ± 1.2 | 16.3 ± 0.8 | −7.8% | 15 | |
| QMEQAS10B-03a | 21.6 ± 1.4 | 20.7 ± 1.0 | −4.3% | 15 | |
| QMEQAS10B-06a | 41.2 ± 3.4 | 41.7 ± 2.7 | 1.3% | 8 | |
| BE10-12b | 54.1 ± 4.8 | 54.1 ± 2.9 | −0.1% | 8 | |
| QMEQAS08B-08a | Se (μg L−1) | 165 ± 11 | 157 ± 9 | −5.0% | 15 |
| QMEQAS10B-06a | 239 ± 19 | 248 ± 11 | 3.7% | 8 | |
| QMEQAS08B-05a | 260 ± 17 | 242 ± 12 | −6.8% | 15 | |
| BE10-14b | 367 ± 28 | 372 ± 14 | 1.3% | 8 | |
| BE11-03b | 421 ± 43 | 416 ± 17 | −1.3% | 8 | |
| QMEQAS10B-03a | 627 ± 42 | 606 ± 28 | −3.4% | 15 |
Sample from CTQ (Quebec, Canada).
Sample from the Wadsworth Center (Albany, NY).
We evaluated the run-to-run reproducibility of this method from the analysis of bench QC over 20 runs. The bench QC material is prepared in our laboratory by spiking human blood pools to desired concentrations. During the 20 run characterization process, we attempt to capture what will be normal method variation in our laboratory. This includes rotation of calibrator lots, rotation of the analyst preparing samples, and performing maintenance on the instrument where sample introduction parts are either replaced, or cleaned and reinstalled. Also note that these limits were calculated with data from two different, yet equivalent, ELAN® DRC II ICP-MS instruments. This variation during characterization makes QC limits more rugged. Bench QC limits in Table 8 reflect percent CVs between 1% and 10% with the exception of the Elevated QC pool for Hg which has a percent CV of 14%.
Table 8.
Bench Quality Control (QC) characterized results (N=38–44) for Pb, Cd, Hg, Mn, and Se at three concentration levels.
| Element | Low QC concentration ± 1 SD |
High QC concentration ± 1 SD |
Elevated QC concentration ± 1 SD |
|---|---|---|---|
| Pb (μg dL−1) | 2.11 ± 0.07 | 10.0 ± 0.1 | 88.2 ± 1.5 |
| Cd (μg L−1) | 0.459 ± 0.041 | 3.05 ± 0.09 | 44.8 ± 1.2 |
| Hg (μg L−1) | 0.603 ± 0.056 | 5.89 ± 0.15 | 41.8 ± 5.9 |
| Mn (μg L−1) | 8.44 ± 0.45 | 14.6 ± 0.6 | 42.9 ± 1.8 |
| Se (μg L−1) | 190 ± 6 | 252 ± 8 | 2662 ± 100 |
3.3. Calibration range and limits of detection
The extension of the calibration curve added calibrators S6–S8 (see Table 3) to the method. We determined the concentration of the highest calibrator (S8) after considering input from a CDC medical toxicologist, typical concentrations of proficiency testing challenge samples, and concentrations we measured in our lab due to acute exposures [54]. Because the calibration range for each element spans 2.6 orders of magnitude we found that a weighted linear calibration curve was required to maintain accuracy at the low end of the calibration curve where we typically measure biomonitoring samples (see NHANES geometric mean in Table 3). The ELAN® software uses a 1/x2 weighted linear regression and typical correlation coefficients are greater than 0.99. No external data analysis is used.
Calculated method LODs are listed in Table 9. These values were calculated in a manner equivalent to the recommendations by the Clinical Laboratory Standards Institute (CLSI) which includes both Type I and Type II error in estimates of LOD [55] and further outlined in the DLS Policies and Procedures Manual [56]. These limits are derived from analysis of four low-concentration materials in at least 60 runs over a two-month timeframe. The four low-concentration materials were prepared per the method by spiking a known concentration of Standard 0, 1, 2 or 3 into the base blood matrix. The current and previous method LODs are listed in Table 9 for comparison, and the improvement in LODs for Pb, Cd, and Hg are due to the increase in sensitivity and precision of the new method.
Table 9.
Method limits of detection for Pb, Cd, Hg, Mn, and Se in whole, human blood.
| Element | Limit of Detection | Previous LOD[1] |
|---|---|---|
| Pb | 0.07 μg dL−1 | 0.25 μg dL−1 |
| Cd | 0.10 μg L−1 | 0.20 μg L−1 |
| Hg | 0.28 μg L−1 | 0.33 μg L−1 |
| Mn | 0.99 μg L−1 | N/A |
| Se | 24 μg L−1 | N/A |
3.4. Comparison of washout with two rinse solutions
The expanded calibration range allows for the application of this method to the measurement of Pb, Cd, Hg, Se, and Mn in human blood from acute and normal environmental exposures. Carryover from a high concentration sample might appear as measureable signal in the next sample, potentially making it a false positive result. Long rinse times can be used to minimize carryover, but at the expense of throughput. Proper selection of rinse composition and timing parameters are essential to minimize signal carryover and maximize throughput. The SC-FAST system was installed on the ELAN® DRC II ICP-MS prior to the expanded calibration range and used a method rinse time of only 30 s. We did not want to increase the rinse time to washout elevated samples the method was designed to handle with the expanded calibration range of the method.
We tested two rinse solutions to determine which one best reduced carryover after the system was exposed to high-concentration samples. One rinse solution, referred to here as EDTA+Au, was comprised of 1% Ethanol, 0.25% (v/v) TMAH, 0.05% Triton X-100™, 0.01% EDTA, and 100 μg L−1 Au. It was based on a previous method used in our laboratory [1] which measured only Pb, Cd, and Hg with calibrators up to S5 (see Table 3). The second rinse solution, referred to here as APDC, was comprised of 0.25% (v/v) TMAH, 0.05% Triton X-100™, 0.01% APDC, and 1% isopropanol. It was based on a method [26] which also quantified only Pb, Cd, and Hg but calibrated to higher concentrations: 200 μg dL−1, 100 μg L−1, and 200 μg L−1, respectively. High concentration multi-element standards were prepared with concentrations up to 500 μg dL−1 (Pb), 1000 μg L−1 (Cd and Hg), 3000 μg L−1 (Mn), and 60,000 μg L−1 (Se).
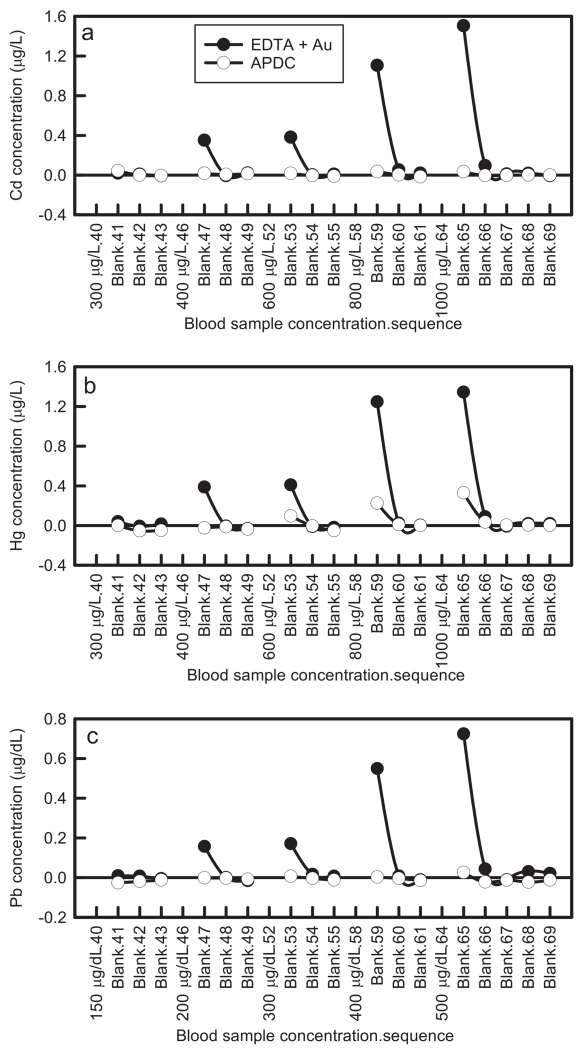
After calibration, we measured several (N=8 or 9) matrix blank samples to determine the average matrix blank response for each element, then alternated between high concentration samples followed by five matrix blanks. We subtracted the averaged matrix blank concentration determined before the high concentration samples from the blanks measured after the high concentration samples to determine if any residual signal was attributable to carryover. The concentrations of the samples for the washout experiment were higher than Standard 8 for all elements. The high sample concentrations of Mn and Se did not exhibit any carryover (results not shown). Results for Pb, Cd, and Hg are displayed in Fig. 3. These results clearly show that the APDC rinse is superior in reducing signal carryover at the method rinse time of 30 s. A small amount of carryover (0.10 – 0.33 μg L−1) was observed after a 600 μg/L Hg or higher spike (three times higher than our highest calibrator). As additional protection against carryover, any sample with a concentration higher than the highest calibrator triggers an extended wash step (200 s) and analysts verify that the run is still in control for lower concentration samples before proceeding..
Fig. 3.
Comparison of (•) EDTA + Au to (○) APDC for washout of high concentrations of (a) Cd, (b) Hg, and (c) Pb; some blank samples in the numerical sequence are not displayed.
This same APDC reagent matrix was successfully adopted as the sample diluent for a short time. However, on occasion a precipitate would form when the calibrators were prepared (i.e. after mixing with diluent and base blood). Feng [57] reported that APDC will co-precipitate metal ions at pH > 4, but not at pH < 4. At pH ≥7 Cd, and Hg did not co-precipitate. We measured the pH of prepared calibrators with the TMAH concentration at 0.25% (v/v) to be between 6.2 and 7.5. By increasing the TMAH concentration to 0.4% (v/v), we increased the pH of the prepared calibrators to > 7, and no precipitates have since been observed.
3.5. Validation of extra dilutions
Extra dilutions of specimens are required if the measured concentration is higher than the concentration of the highest calibrator. Dilutions of biological samples in ICP-MS can be problematic because modifying the matrix may interfere with matrix-matched calibration resulting in bias of observed concentrations. Sometimes extra dilutions are prepared by diluting with a “base” matrix; however, this step adds complications in practice because each level of extra dilution used in the run would require a separate matrix-matched blank.
We performed experiments that tested up to an extra 20× dilution of a blood sample. We spiked a base blood sample to final concentrations of 400 μg dL−1 (Pb), 100 μg L−1 (Cd and Hg), 300 μg L−1 (Mn), and 2000 μg L−1 (Se), and mixed the sample well. The spiked sample was then prepared for analysis at various extra dilution levels (2×–20×) with DI water. The experiment was repeated in separate runs on different days 6–8 times. Each result from an extra dilution (after multiplication by dilution factor) was normalized to the result with no extra dilution from the same run. All normalized results for each dilution level were averaged (see Table 10) and indicate that all analytes of the method (Pb, Cd, Hg, Mn, and Se) can be analyzed at up to a 20× extra dilution without significant effect ( > ± 0.1 i.e. 10% change) to the observed concentration. These results support minimizing the extra dilution necessary to bring a sample within the calibration range.
Table 10.
Normalized observed mean concentrations of each element measured with extra dilution factors. DI water was used to perform the extra dilution.
| Dilution level | Mn | Hg | Se | Cd | Pb |
|---|---|---|---|---|---|
| No Extra (N=8) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2× dilution (N=8) | 1.00 ± 0.01 |
1.03 ± 0.05 |
1.02 ± 0.03 |
1.00 ± 0.01 |
1.01 ± 0.01 |
| 5× dilution (N=6) | 1.01 ± 0.01 |
1.06 ± 0.06 |
1.01 ± 0.02 |
1.01 ± 0.01 |
1.02 ± 0.01 |
| 10× dilution (N=8) |
1.01 ± 0.03 |
1.04 ± 0.06 |
1.04 ± 0.06 |
1.00 ± 0.02 |
1.02 ± 0.02 |
| 20× dilution (N=8) |
1.02 ± 0.04 |
1.09 ± 0.05 |
1.06 ± 0.08 |
1.01 ± 0.03 |
1.02 ± 0.02 |
These results are not intended support a non-matrix matched calibration. We tested extra sample dilutions and determined that the observed effect was acceptable for high concentration samples where medical intervention for the patient would be the same even with the observed effect. However, this effect would be highly significant in the biomonitoring range (i.e. lower concentrations). Matrix matching will result in the best accuracy for biomonitoring studies.
3.6. Transferability to other ICP-MS platforms
Because the ELAN ICP-DRC-MS is no longer produced by PE, we would like to offer our recommended criteria for performing the method described here on other ICP-MS platforms. We believe the information we provide on sample collection, treatment, diluent, and rinse solutions are all transferrable to other platforms. High analyte sensitivity, low background counts, measurement precision, and run-to-run reproducibility are important to achieve the LODs stated here. Low backgrounds for 55Mn and 80Se in human blood samples will only be achieved with the use of an interference removal technique. If a collision or other reaction cell is to be used, the cell must be capable of achieving a consistent sensitivity throughout the run and with varying cell ion densities. The peristaltic pump, or other sample introduction system, must be able to operate at the required low sample uptake rate without introducing noise to the ion signal for the best precision. Lastly, we recommend an instrument with a Fomblin fluid roughing pump to extend the time between required pump maintenance.
4. Conclusion
We developed a rugged method for analysis of whole blood samples for Pb, Cd, Hg, Se, and Mn on a PE ELAN® DRC II ICP-MS, using the vented mode for Pb and Cd, and two DRC modes to remove polyatomic spectral interferences from 55Mn and 80Se, and increase sensitivity for 202Hg. The sample-to-sample time of less than 5 min permits the preparation and analysis of 60 samples/8 h work day; limited by the length of a work shift. The improvements to this method include additional analytes (55Mn and 80Se), expanded calibration range, expanded reportable range using extra dilutions, optimized rinse and diluent components while maintaining short sample-to-sample times using the SC4-FAST system. The analytical metrics supplied demonstrate the method is selective, accurate (less than 8% bias relative to reference materials), and precise (percent CVs less than 14%), with a reportable range than spans more than 4 orders of magnitude, and improved LODs.
Footnotes
Disclosure
The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the U.S. Department of Health and Human Services, or the U.S. Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, or the U.S. Centers for Disease Control and Prevention.
References
- [1].Caldwell KL, Mortensen ME, Jones RL, Caudill SP, Osterloh JD. Total blood mercury concentrations in the US population: 1999–2006. Int. J. Hyg. Environ. Health. 2009;212:588–598. doi: 10.1016/j.ijheh.2009.04.004. [DOI] [PubMed] [Google Scholar]
- [2].Pirkle JL, Osterloh J, Needham LL, Sampson EJ. National exposure measurements for decisions to protect public health from environmental exposures. Int. J. Hyg. Environ. Health. 2005;208:1–5. doi: 10.1016/j.ijheh.2005.01.001. [DOI] [PubMed] [Google Scholar]
- [3].Centers for Disease Control and Prevention . Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: Feb, 2015. [Google Scholar]
- [4].Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for Mercury. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 1999. [Google Scholar]
- [5].Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Lead. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2007. [Google Scholar]
- [6].Agency for Toxic Substances and Disease Registry (ATSDR) In: Toxicological Profile for Cadmium. ATSDR, editor. U.S Department of Health and Human Servies, Public Health Service; Atlanta, GA: 2012. [PubMed] [Google Scholar]
- [7].Centers for Disease Control and Prevention . Fourth Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: [accessed 30.06.16]. 2009. http://www.cdc.gov/exposurereport/ [Google Scholar]
- [8].Sommer YL, Verdon CP, Fresquez MR, Ward CD, Wood EB, Pan Y, Caldwell KL, Jones RL. Measurement of mercury species in human blood using triple spike isotope dilution with SPME-GC-ICP-DRC-MS. Anal. Bioanal. Chem. 2014;406:5039–5047. doi: 10.1007/s00216-014-7907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in “Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention”. Atlanta, GA: Jun 7, 2012. [Google Scholar]
- [10].Goldhaber SB. Trace element risk assessment: essentiality vs. toxicity. Regul. Toxicol. Pharmacol. 2003;38:232–242. doi: 10.1016/s0273-2300(02)00020-x. [DOI] [PubMed] [Google Scholar]
- [11].Combs GF, Gray WP. Chemopreventive agents: selenium. Pharmacol. Ther. 1998;79:179–192. doi: 10.1016/s0163-7258(98)00014-x. [DOI] [PubMed] [Google Scholar]
- [12].Arthur JR. The role of selenium in thyroid-hormone metabolism. Can. J. Physiol. Pharmacol. 1991;69:1648–1652. doi: 10.1139/y91-243. [DOI] [PubMed] [Google Scholar]
- [13].Corvilain B, Contempre B, Longombe AO, Goyens P, Gervydecoster C, Lamy F, Vanderpas JB, Dumont JE. Selenium and the thyroid: how the relationship was established. Am. J. Clin. Nutr. 1993;57:S244–S248. doi: 10.1093/ajcn/57.2.244S. [DOI] [PubMed] [Google Scholar]
- [14].McKenzie RC, Rafferty TS, Beckett GJ. Selenium: an essential element for immune function. Immunol. Today. 1998;19:342–345. doi: 10.1016/s0167-5699(98)01294-8. [DOI] [PubMed] [Google Scholar]
- [15].Combs GE. Food system-based approaches to improving micronutrient nutrition: the case for selenium. Biofactors. 2000;12:39–43. doi: 10.1002/biof.5520120107. [DOI] [PubMed] [Google Scholar]
- [16].Zimmermann MB, Köhrle J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: biochemistry and relevance to public health. Thyroid. 2002;12:867–878. doi: 10.1089/105072502761016494. [DOI] [PubMed] [Google Scholar]
- [17].Agency for Toxic Substances and Disease Registry (ATSDR) In: Toxicological profile for Selenium. ATSDR, editor. U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2003. [Google Scholar]
- [18].Saric M, Lucchini R. Manganese. In: Nordberg GF, Fowler BA, Nordberg M, Friberg LT, editors. Handbook on the Toxicology of Metals. Academic Press; Burlington, MA, USA: 2007. p. 645. [Google Scholar]
- [19].Bader M, Dietz MC, Ihrig A, Triebig G. Biomonitoring of manganese in blood, urine and axillary hair following low-dose exposure during the manufacture of dry cell batteries. Int. Arch. Occup. Environ. Health. 1999;72:521–527. doi: 10.1007/s004200050410. [DOI] [PubMed] [Google Scholar]
- [20].Woolf A, Wright R, Amarasiriwardena C, Bellinger D. A child with chronic manganese exposure from drinking water. Environ. Health Perspect. 2002;110:613–616. doi: 10.1289/ehp.02110613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wasserman GA, Liu XH, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, Lolacono NJ, Cheng ZQ, Zheng Y, Graziano JH. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bazzi A, Nriagu JO, Linder AM. Determination of toxic and essential elements in children’s blood with inductively coupled plasma-mass spectrometry. J. Environ. Monit. 2008;10:1226–1232. doi: 10.1039/b809465a. [DOI] [PubMed] [Google Scholar]
- [23].Rollin H, Mathee A, Levin J, Theodorou P, Wewers F. Blood manganese concentrations among first-grade schoolchildren in two South African cities. Environ. Res. 2005;97:93–99. doi: 10.1016/j.envres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- [24].Davis JM, Jarabek AM, Mage DT, Graham JA. The EPA health risk assessment of methylcyclopentadienyl manganese tricarbonyl (MMT) Risk Anal. 1998;18:57–70. doi: 10.1111/j.1539-6924.1998.tb00916.x. [DOI] [PubMed] [Google Scholar]
- [25].Institute of Medicine (US) Panel on Micronutrients . Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press (US); Washington, DC: [accessed 30.06.16]. 2001. pp. 394–419. Available from: 〈 http://www.ncbi.nlm.nih.gov/books/NBK222310/〉 http://:dx.doi.org/10.17226/10026. [PubMed] [Google Scholar]
- [26].McShane WJ, Pappas RS, Wilson-McElprang V, Paschal D. A rugged and transferable method for determining blood cadmium, mercury, and lead with inductively coupled plasma-mass spectrometry, Spectrochim. Acta Part B:- At. Spectrosc. 2008;63:638–644. [Google Scholar]
- [27].Ivanenko NB, Ganeev AA, Solovyev ND, Moskvin LN. Determination of trace elements in biological fluids. J. Anal. Chem. 2011;66:784–799. [Google Scholar]
- [28].D’Ilio S, Violante N, Di Gregorio M, Senofonte O, Petrucci F. Simultaneous quantification of 17 trace elements in blood by dynamic reaction cell inductively coupled plasma mass spectrometry (DRC-ICP-MS) equipped with a high-efficiency sample introduction system. Anal. Chim. Acta. 2006;579:202–208. doi: 10.1016/j.aca.2006.07.027. [DOI] [PubMed] [Google Scholar]
- [29].Palmer CD, Lewis ME, Jr, Geraghty CM, Barbosa F, Jr, Parsons PJ. Determination of lead, cadmium and mercury in blood for assessment of environmental exposure: a comparison between inductively coupled plasma-mass spectrometry and atomic absorption spectrometry. Spectrochimica Acta - Part B, At. Spectrosc. 2006;61:980–990. [Google Scholar]
- [30].Heitland P, Koster HD. Biomonitoring of 37 trace elements in blood samples from inhabitants of northern Germany by ICP-MS. J. Trace Elem. Med. Biol. 2006;20:253–262. doi: 10.1016/j.jtemb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- [31].Nixon DE, Neubauer KR, Eckdahl SJ, Butz JA, Burritt MF. Comparison of tunable bandpass reaction cell inductively coupled plasma mass spectrometry with conventional inductively coupled plasma mass spectrometry for the determination of heavy metals in whole blood and urine. Spectrochim. Acta Part B: At. Spectrosc. 2004;59:1377–1387. [Google Scholar]
- [32].Lu Y, Kippler M, Harari F, Grandér M, Palm B, Nordqvist H, Vahter M. Alkali dilution of blood samples for high throughput ICP-MS analysis—comparison with acid digestion. Clin. Biochem. 2015;48:140–147. doi: 10.1016/j.clinbiochem.2014.12.003. [DOI] [PubMed] [Google Scholar]
- [33].Praamsma ML, Arnason JG, Parsons PJ. Monitoring Mn in whole blood and urine: a comparison between electrothermal atomic absorption and inorganic mass spectrometry. J. Anal. At. Spectrom. 2011;26:1224–1232. [Google Scholar]
- [34].Lutz TM, Nirel PMV, Schmidt B. Whole blood analysis by ICP-MS, applications of plasma source mass spectrometry. R. Soc. Chem. 1991:96–100. [Google Scholar]
- [35].Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat. Med. 2008;27:4094–4106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- [36].Jarrett JM, Jones RL, Caldwell KL, Verdon CP. Total urine arsenic measurements using inductively coupled plasma mass spectrometry with a dynamic reaction cell. At. Spectrosc. 2007;28:113–122. [Google Scholar]
- [37].McShane WJ, Pappas RS, Paschal D. Analysis of total arsenic, total selenium and total chromium in urine by inductively coupled plasma-dynamic reaction cell-mass spectrometry. J. Anal. At. Spectrom. 2007;22:630–635. [Google Scholar]
- [38].Barbosa F, Jr, Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ. Health Perspect. 2005;113:1669–1674. doi: 10.1289/ehp.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Turnlund JR, Friberg LT. Molybdenum. In: Nordberg GF, Fowler BA, Nordberg M, Friberg LT, editors. Handbook on the Toxicology of Metals. Academic Press; Burlington, MA, USA: 2007. pp. 731–741. [Google Scholar]
- [40].Carson B, Ellis HI, McCann J. Toxicology and Biological Monitoring of Metals in Humans. Lewis Publishers, Inc.; Chelsea, MI: 1986. [Google Scholar]
- [41].Kazantzi G, Leffer P. Tungsten. In: Nordberg GF, Fowler BA, Nordberg M, Friberg LT, editors. Handbook on the Toxicology of Metals. Academic Press; Burlington, MA, USA: 2007. pp. 871–879. [Google Scholar]
- [42].Leroy MJF, Lagarde F. TungstenHandbook on Metals in Clinical and Analytical Chemistry. Marcel Dekker, Inc.; 1994. [Google Scholar]
- [43].Rowan JT, Houk RS. Attenuation of polyatomic ion interferences in inductively coupled plasma mass-spectrometry by gas-phase collisions. Appl. Spectrosc. 1989;43:976–980. [Google Scholar]
- [44].Sloth JJ, Larsen EH. Application of inductively coupled plasma dynamic reaction cell mass spectrometry for measurement of selenium isotopes, isotope ratios and chromatographic detection of selenoamino acids. J. Anal. At. Spectrom. 2000;15:669–672. [Google Scholar]
- [45].Fong BMW, Siu TS, Lee JSK, Tam S. Multi-elements (aluminium, copper, magnesium, manganese, selenium and zinc) determination in serum by dynamic reaction cell-inductively coupled plasma-mass spectrometry. Clin. Chem. Lab. Med. 2009;47:75–78. doi: 10.1515/CCLM.2009.006. [DOI] [PubMed] [Google Scholar]
- [46].Hann S, Koellensperger G, Obinger C, Furtmüller PG, Stingeder G. SEC-ICP-DRCMS and SEC-ICP-SFMS for determination of metal-sulfur ratios in metallo-proteins. J. Anal. At. Spectrom. 2004;19:74–79. [Google Scholar]
- [47].Praamsma ML, Jones DR, Jarrett JM, Dumas P, Cirtiu CM, Parsons PJ. A comparison of clinical laboratory data for assigning a consensus value for manganese in a caprine blood reference material. J. Anal. At. Spectrom. 2012;27:1975–1982. doi: 10.1039/C2JA30142C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Scott MG, Heusel JW, LeGrys VA, Siggaard-Andersen O. Electrolytes and Blood gases. In: Burtis CA, Ashwood ER, editors. Tietz Textbook of Clinical Chemistry. W.B. Saunders Company; 1999. pp. 1056–1092. [Google Scholar]
- [49].Alexander NM. Iron. In: Seiler HG, Sigel A, Sigel H, editors. Handbook on Metals in Clinical and Analytical Chemistry. Marcel Dekker, Inc.; New York, New York: 1994. pp. 411–421. [Google Scholar]
- [50].Painter PC, Cope JY, Smith JL. Reference information for the clinical laboratory. In: Burtis CA, Ashwood ER, editors. Tietz Textbook of Clinical Chemistry. W.B. Saunders Company; 1999. pp. 1788–1846. [Google Scholar]
- [51].Centers for Disease Control and Prevention, National Center for Health Statistics . Measured average height, weight, and waist circumference for adults ages 20 years and over. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: [accessed 12.12.14]. 2014. http://www.cdc.gov/nchs/fastats/body-measurements.htm. [Google Scholar]
- [52].Jin T, Berlin M. Titanium. In: Nordberg GF, Fowler BA, Nordberg M, Friberg LT, editors. Handbook on the Toxicology of Metals. Academic Press; Burlington, MA, USA: 2007. pp. 861–870. [Google Scholar]
- [53].Thunus L, Lejeune R. Zinc. In: Seiler HG, Sigel A, Sigel H, editors. Handbook on Metals in Clinical and Analytical Chemistry. Marcel Dekker, Inc.; New York, New York: 1994. pp. 667–674. [Google Scholar]
- [54].Dooyema CA, Neri A, Lo YC, Durant J, Dargan PI, Swarthout T, Biya O, Gidado SO, Haladu S, Sani-Gwarzo N, Nguku PM, Akpan H, Idris S, Bashir AM, Brown MJ. Outbreak of fatal childhood lead poisoning related to artisanal gold mining in northwestern Nigeria, 2010. Environ. Health Perspect. 2012;120:601–607. doi: 10.1289/ehp.1103965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].CLSI . Evaluation of Detection Capability for Clinical Laboratory Measurements Procedures; Approved Guideline – Second Edition. In: Pierson-Perry JF, Vaks JE, Durham AP, Fischer C, Gutenbrunner C, Hillyard D, Kondratovich MV, Ladwig P, Middleberg RA, editors. CLSI document EP17-A2. Clinical and Laboratory Standards Institute; Wayne, PA: 2012. [Google Scholar]
- [56].Centers for Disease Control and Prevention . Division of Laboratory Sciences Policies and Procedures Manual. Atlanta, GA: May, 2015. Available from: 〈 http://intranet.cdc.gov/nceh-atsdr/dls/pdf/05/DLS_Policies_and_Procedures_Manual.pdf〉. [Google Scholar]
- [57].Feng X, Ryan DE. Combination collectors in adsorption colloid flotation for multielement determination in waters by neutron activation. Anal. Chim. Acta. 1984;162:47–55. [Google Scholar]