Abstract
Immune tolerance between the fetus and mother represents an active process by which the developing fetus must not mount immune responses to non-inherited antigens on chimeric maternal cells that reside in fetal tissue. This is, in part, mediated by the suppressive influence of CD4+FoxP3+CD25+ regulatory T cells (Tregs). Fetal secondary lymphoid organs have an increased frequency of Tregs and, as compared to adult T cells, fetal naïve CD4+ T cells exhibit a strong predisposition to differentiate into Tregs when stimulated. This effect is mediated by the T cell receptor (TCR) and TGF-β pathways, and fetal T cells show significantly increased Treg differentiation in response to anti-CD3 and TGF-β stimulation. Naïve fetal T cells also exhibit increased signaling through the TGF-β pathway, with these cells demonstrating increased expression of the signaling mediators TGF-βRI, TGF-βRIII, and SMAD2, and higher levels of SMAD2/SMAD3 phosphorylation. Increased fetal Treg differentiation is mediated by the RNA-binding protein Lin28b, which is overexpressed in fetal T cells as compared to adult cells. When Lin28b expression is decreased in naïve fetal T cells, they exhibit decreased Treg differentiation that is associated with decreased TGF-β signaling and lowered expression of TGF-βRI, TGF-βRIII, and SMAD2. Lin28b regulates the maturation of let-7 microRNAs (miRNAs) and these TGF-β signaling mediators are let-7 targets. We hypothesize that loss of Lin28b expression in fetal T cells leads to increased mature let-7, which causes decreased expression of TGF-βRI, TGF-βRIII, and SMAD2 proteins. A reduction in TGF-β signaling leads to reduced Treg numbers.
Introduction
Human gestation represents a fascinating challenge to classical mechanisms of immune recognition, tolerance, and rejection. The developing mammalian fetus expresses a set of polymorphic major histocompatibility complex (MHC) molecules inherited from both its mother and father, meaning that up to half of the fetal MHC molecules may be recognized by the maternal immune system as allogeneic foreign tissue. Pregnancy also results in immune microchimerism, whereby fetal cells reside in maternal tissues; chimerism also occurs in the opposite direction and maternal cells have been found to reside in fetal tissues. A large body of research has focused on how the maternal immune system deals with this antigen mismatch in order to avoid immune rejection of the developing fetus (1–3). Less investigation has gone into the reciprocal problem of how the fetal immune system develops in a semi-allogeneic host. While it was previously thought that the fetal adaptive immune system avoids rejection of the mother because it is inert or functionally impaired, it is now clear that the fetal immune system actively contributes to tolerance of maternal antigens (4, 5).
Fetal secondary lymphoid immune organs have a significantly increased frequency of CD4+FoxP3+CD25+ regulatory T cells (Tregs) as compared to any other time in development (4, 6–8). This abundance of Tregs is not reflected in the thymus of equivalent gestational age, where the frequency of CD25+FoxP3+ single CD4+ thymocytes is comparable to the infant thymus (8). This suggests that a significant portion of fetal Tregs are derived from expansion of natural Tregs or are generated from conventional CD4+FoxP3- T cells in response to antigen. When fetal naïve CD4+ T cells are isolated and stimulated with alloantigen, they exhibit a strong predisposition to differentiate into Tregs, as compared to adult naïve CD4+ T cells (5). These Tregs are functional and can mediate alloantigen-specific suppression. Further, this effect is dependent on TGF-β, and fetal lymph nodes express significantly higher levels of TGF-β family members, as compared to adult lymph nodes. Given the likely crucial role that fetal Tregs play in tolerance to maternal antigens in utero we sought to determine the mechanism by which fetal naïve CD4+ T cells preferentially differentiate into Tregs.
We hypothesized that the RNA-binding protein Lin28b could be involved in fetal T cell differentiation. Lin28b is a highly evolutionarily-conserved protein, whose expression is associated with undifferentiated cell states in C. elegans, mice, and humans (9–11). Lin28b acts as both a negative regulator of let-7 miRNA biogenesis and a post-transcriptional regulator of mRNA translation (10, 12, 13). Through direct interactions with mRNAs, regulation of numerous splicing factors, and modulation of let-7 activity, Lin28b regulates the expression of thousands of genes, many of which are involved in cellular growth, self-renewal, and proliferation (14–17). Lin28b is highly expressed in human fetal hematopoietic tissues, such as fetal liver and thymus, but not in adult bone marrow and thymus (18). Further, Lin28b overexpression in mouse adult bone marrow-derived hematopoietic stem cells leads to development of a fetal-like immune system, consisting of increased numbers of B-1a B cells, gamma/delta T cells, and natural killer T cells. Lin28b can also drive expression of fetal hemoglobin when overexpressed in adult erythroblasts (19).
Based on these observations, we asked whether Lin28b could act as a regulator of human fetal T cell differentiation. We knocked down Lin28b expression in naïve fetal CD4+ T cells and assessed their differentiation into FoxP3+CD25+ Tregs. In the context of Lin28b knockdown, fetal T cells exhibited decreased Treg differentiation, mediated in part through regulation of the TGF-β pathway.
Materials and Methods
Isolation and preparation of human T samples
Fetal spleen and mesentery were obtained from 18- to 22-gestational-week specimens obtained under the auspices of a protocol approved by the University of California San Francisco Committee on Human Research. Fetal samples were obtained after elective termination of pregnancy, and samples were excluded in the case of (1) known maternal infection, (2) intrauterine fetal demise, and/or (3) known or suspected chromosomal abnormality. Fetal tissues were extensively washed with sterile phosphate buffered saline (PBS) and incubated with collagenase type IV (0.2 mg/mL, Sigma Aldrich) for one hour at 37°C. The tissues were dissociated and strained through a 70 um filter. Adult T cells were isolated from peripheral blood mononuclear cells (PBMC). Mononuclear cells were isolated from fetal spleen and PBMCs by density centrifugation over a Ficoll-Hypaque gradient (Amersham Biosciences). All samples, both fetal and adult, were viably cryopreserved and thawed prior to use. All methods were carried out in accordance with approved guidelines. Informed consent was obtained from all subjects.
T cell stimulation and culture
Naïve CD4+ T cells were isolated from fetal tissues and adult PBMCs using the EasySep™ Human Naïve CD4+ T Cell Enrichment Kit (Stem Cell Technologies). 96-welled U-bottom plates were pre-incubated with 0.8 ug/mL anti-CD3 (SP34-2, BD Biosciences) in PBS for four hours at 37°C. Enriched naïve CD4+ T cells were plated in RPMI 1640 (Sigma Aldrich) supplemented with 10% FBS, penicillin, streptomycin, and L-glutamine at 5×105 cells/mL. Cultures were supplemented with α-CD28 (2 ug/mL, Ebioscience), IL-2 (20ng/mL, R&D Systems), or varying concentrations of TGF-β (R&D Systems). Fresh medium with cytokines was added at days 3 and 5. Cells were cultured for 6–7 days before flow cytometric analysis.
Flow cytometry
Mononuclear cell preparations were incubated in fluorescence-activated cell sorting (FACS) staining buffer (phosphate-buffered saline with 2% fetal bovine serum and 2 mM EDTA) with fluorochrome-conjugated, anti-human surface antibodies. Antibodies used included CD3-Alexa Fluor700 (SP34-2, BD Biosciences), CD4-BV605 (RPA-T4, BD Biosciences), ICOS-Alexa Fluor647 (BD Biosciences), CTLA4-PE-CF594 (BNI3, BD Biosciences), CD8 Pe-Cy5 (RPA-T8, BD Biosciences), CD25-PE-Cyanine7 (BC96, eBioscience), TGF-βRII-APC (25508, R&D Systems) and TGF-βRI-PE (141231, R&D Systems). All cells were stained with a live/dead marker (Amine-Aqua/AmCyan, Invitrogen) to exclude dead cells from the analysis. Intracellular staining of FoxP3 was performed using the Foxp3/Transcription Factor Staining Buffer Set (Ebioscience) according to manufacturer’s instructions. Intracellular antibodies included Foxp3-V450 (236A/E7, BD Biosciences). For intracellular TGF-β and SMAD staining, cells were first stained with a live/dead marker prior to fixation/permeabilization and subsequent SMAD staining according to the manufacturer’s protocol (Cytofix Buffer and Permeabilization Buffer III; BD Biosciences). For TGF-β receptor and SMAD2 staining, the mean fluorescence intensity (MFI) of species-matched isotype controls was subtracted from antibody staining. Intracellular antibodies included those against SMAD2 (pS465/pS467)/SMAD3 (pS423/pS425)-Alexa647 (O72-670, BD Biosciences), TGF-βRIII (MM0057-5G9, Abcam), and SMAD2 (D7G7, Cell Signaling) as well as rabbit IgG isotype control (DA1E, Cell Signaling), mouse IgG isotype control (G3A1, Cell Signaling), goat anti-mouse IgG Alexa Fluor647 (Life Technologies), and donkey anti-rabbit Alexa Fluor488 (Life Technologies).
siRNA assays
Naïve CD4+ T cells were isolated from fetal spleen as described above. Cells were plated in 96-well U-bottom plates at 5×105 cells/mL in Accell Delivery Media (GE Dharmacon) supplemented with 2.5% FBS, penicillin, streptomycin, and L-glutamine. Accell siRNAs were resuspended in 1× siRNA buffer to a concentration of 100 uM and used at a final concentration of 0.1 pmol/1×105 cells. Cells were incubated with siRNA for 3 days, at which point they were transferred to 96-well U-bottom plates pre-coated with α-CD3 antibody and stimulated as described above. Additional siRNA was also added to cultures at this time point. siRNAs used were SMARTpool ON-TARGETplus LIN28B (L-028584-01) and ON-TARGETplus Non-targeting Control Pool (D-001810-10) (GE Dharmacon).
miRNA mimic transfection
Jurkat cells (clone E6-1) were cultured in RPMI 1640 (Sigma Aldrich) supplemented with 10% FBS and L-glutamine. Cells were transfected with the Amaxa 4-D Nucleofector system using the Amaxa Cell Line Nucleofector Kit V and the manufacturer’s optimized protocol for Jurkat cells (Lonza). miRNA mimics used were miRIDIAN microRNA Human hsa-let-7g-3p mimic (C-301043-01, GE Dharmacon) and mimic Housekeeping Positive Control #1 (PPIB, CP-001000-01, GE Dharmacon). Jurkats were cultured for 3 days before qRT-PCR analysis.
qRT-PCR
Cells were lysed in Trizol reagent (Life Technologies) and RNA was extracted according to manufacturer’s protocol. The RNA concentration was quantified on a Nanodrop 2000c spectrophotometer (Thermo Scientific). For analysis of mRNA transcripts, reverse transcription was performed using the Omniscript RT Kit (Qiagen) on 300–500 ng total RNA. cDNA was diluted 10-fold and 1/20th total volume used per qPCR reaction. qPCR was performed using TaqMan Gene Expression Master Mix (Life Technologies) and Taqman Gene Expression Assays (Life Technologies) with a StepOnePlus Real-Time PCR Systems (Life Technologies). Assays used were TGF-βRIII (Hs01114253_m1), SMAD2 (Hs00183425_m1), and TGF-βRI (Hs00610320_m1). For analysis of mature miRNAs, reverse transcription was performed using NCode VILO miRNA cDNA Synthesis Kit (Life Technologies). qPCR was performed using PerfeCTa SYBR Green SuperMix with ROX (Quanta Biosciences). SYBR primers used are shown in Table 1.
Table 1.
qPCR Primers used for SYBR Green detection of mature miRNAs.
| miRNA SYBR primers | |
|---|---|
| gene | sequence |
| hsa-let7-a | TGAGGTAGTAGGTTGTATAGTT |
| hsa-let7-b | TGAGGTAGTAGGTTGTGTGGTT |
| hsa-let7-c | TGAGGTAGTAGGTTGTATGGTT |
| hsa-let7-d | AGAGGTAGTAGGTTGCATAGTT |
| hsa-let7-e | TGAGGTAGGAGGTTGTATAGTT |
| hsa-let7-f | TGAGGTAGTAGATTGTATAGTT |
| hsa-let7-g | TGAGGTAGTAGTTTGTACAGTT |
| 5.8S | ATCGTAGGCACCGCTACGCCTGTCTG |
Results
The fetal T cell compartment is enriched for regulatory T cells
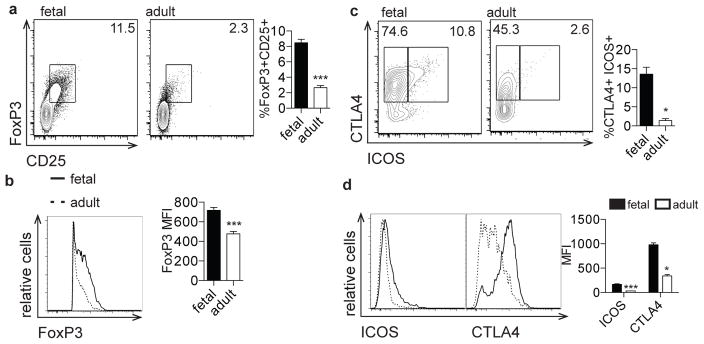
Human fetal Tregs are critical for the suppression of fetal T cell responses to maternal alloantigens [7](20). To further characterize fetal Tregs, we phenotyped fetal and adult T cells based on the functionally significant Treg proteins, FoxP3, ICOS, and CTLA-4 (21–23). Compared to adult PBMCs, fetal spleen and lymph nodes were found to contain a significantly higher percentage of FoxP3+CD25+ Tregs that expressed a significantly higher level of FoxP3 protein (Fig. 1a, b, Supp. Fig. 1). Furthermore, the fetal FoxP3+ Treg population harbored a higher percentage cells that were CTLA4+ and ICOS+ (Fig. 1c) and these proteins were more highly expressed than in adult FoxP3+ cells (Fig. 1d). Of note, increased expression of these three proteins has been associated with increased Treg suppressor activity, suggesting that fetal Tregs may be more suppressive than their adult counterparts (21, 24–27). It is important to note that these analyses compare T cells derived from fetal tissues to adult T cells from peripheral blood. Thus, our findings may reflect both immunological differences associated with ontogeny and age, and experimental procedures used in isolating cells from these distinct anatomical locations.
Figure 1. The fetal T cell compartment is enriched for regulatory T cells.
a. Flow cytometry analysis of staining for intracellular FoxP3 and surface CD25 on fetal and adult CD4+ T cells. Bar graph shows the percentage of FoxP3+CD25+ cells within the CD4+ population. b. Flow cytometry analysis of staining for intracellular FoxP3 on fetal and adult FoxP3+CD4+ T cells. Bar graph shows the mean fluorescence intensity (MFI) of FoxP3 within the FoxP3 population. c. Flow cytometry analysis of staining for ICOS and CTLA4 on fetal and adult FoxP3+CD4+ T cells. Bar graph shows the percentage of ICOS+CTLA4+ cells within the FoxP3+ population. d. Flow cytometry analysis of staining for ICOS and CTLA4 on fetal and adult FoxP3+CD4+ T cells. Bar graph shows the MFI of ICOS and CTLA4 within the FoxP3 population. All plots are pre-gated on cell size, live, CD3+CD8−CD4+ (see Supp. Fig. 1). The data are representative of results from 8 fetal and 8 adult individuals in 3 independent experiments. Statistics refer to Mann-Whitney test. *** p<.001, ** p<.01, * p<.05
Naïve CD4+ T cells isolated from fetal lymphoid organs have an increased propensity to differentiate into FoxP3+CD25+ Tregs when stimulated with antigen presenting cells from an unrelated donor (5). We wondered if similar effects could be seen when naïve CD4+ T cells were stimulated via the T cell receptor (TCR) with anti-CD3 and CD28 antibodies. TCR signal strength plays a critical role in driving the differentiation of naïve T cells into Tregs. During positive selection in the thymus, Tregs are more dependent on strong TCR signals compared with effector T cells (28). In the periphery, however, it has been suggested that weaker TCR stimulation is favorable for FoxP3 expression and for the generation of induced Tregs (29, 30). These studies have largely been conducted in mice and it is unknown which TCR signaling restraints determine Treg differentiation in humans.
Naive fetal T cells have an increased propensity to differentiate into Tregs and exhibit enhanced signaling through the TGF-β pathway
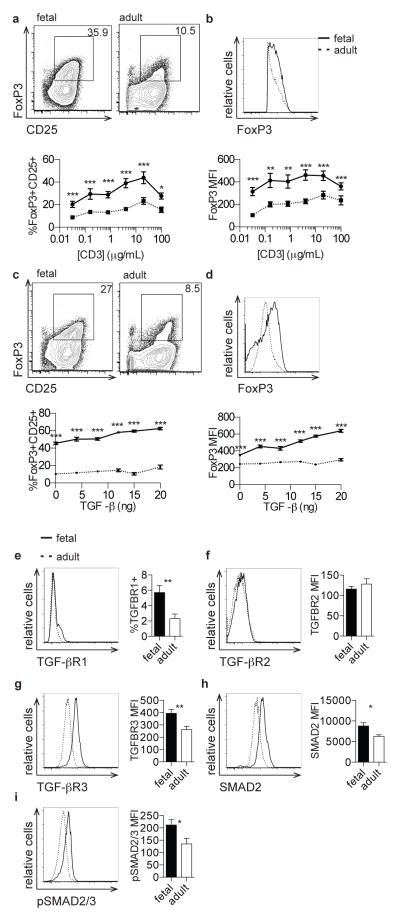
We isolated naive CD4+CD45RA+ T cells from fetal lymphoid organs or adult peripheral blood and stimulated them with a range of concentrations of anti-CD3 monoclonal antibody (Supp. Fig. 2a). While both fetal and adult T cells exhibited increasing percentages of FoxP3+CD25+ Tregs with increasing concentrations of anti-CD3, cultures derived from naïve fetal T cells exhibited significantly increased percentages of Tregs at each concentration of anti-CD3 tested (Fig. 2a). Furthermore, FoxP3+ cells derived from fetal naïve T cells had significantly increased expression of FoxP3 protein compared to those cells derived from adult naïve T cells (Fig. 2b). These data confirm that fetal naïve T cells, in contrast to adult T cells, have a cell-intrinsic propensity to differentiate into Tregs after TCR stimulation.
Figure 2. Naive fetal T cells have an increased propensity to differentiate into Tregs and exhibit enhanced signaling through the TGF-β pathway.
a. Flow cytometry analysis of staining for intracellular FoxP3 and surface CD25 on fetal and adult CD4+ T cells stimulated with 0.8 μg/mL α-CD3. Graph shows percentage of FoxP3+CD25+ cells within the CD4+ population following stimulation across a range of α-CD3 concentrations. b. Flow cytometry analysis of staining for intracellular FoxP3 on fetal and adult FoxP3+CD4+ T cells following stimulation as above. Graph shows FoxP3 MFI within the FoxP3+CD4+ population following stimulation. c. Flow cytometry analysis of staining for intracellular FoxP3 and surface CD25 on fetal and adult CD4+ T cells stimulated with 0.8 μg/mL anti-CD3 in the presence of 8 ng TGF-β. Graph shows percentage of FoxP3+CD25+ cells within the CD4+ population following stimulation across a range of TGF-β concentrations. d. Flow cytometry analysis of staining for intracellular FoxP3 on fetal and adult FoxP3+CD4+ T cells stimulated with 0.8 μg/mL anti-CD3 in the presence of TGF-β. Graph shows FoxP3 MFI within the FoxP3+CD4+ population following stimulation, as above. e–h. Flow cytometry analysis of staining for TGF-βRI, TGF-βRII, TGF-βRIII, and SMAD2 on fetal and adult naïve CD4+ T cells. Bar graphs show percentage of TGF-βRI+ cells and MFI of TGF-βRII, TGF-βRIII, and SMAD2 within the naïve CD4+ population. i. Flow cytometry analysis of staining for intracellular phosphorylated SMAD2/SMAD3 on fetal and adult naïve CD4+ T cells in the presence of 2 ng TGF-β. Bar graph shows phosphorylated SMAD2/SMAD3 MFI within the naïve CD4+ population. Panels a–d are pre-gated on cell size, live, CD3+CD8−CD4+ (see Supp. Fig. 1). Panels e–i are pre-gated on cell size, live, CD3+CD8−CD4+CD45RA+ (see Supp. Fig. 2). The data are representative of results from 8 fetal and 8 adult individuals in 3 independent experiments. Statistics refer to Mann-Whitney test. *** p<.001, ** p<.01, * p<.05
Signaling through TGF-β in CD4+ T cells is absolutely required for the induction of FoxP3 expression, as CD4+ T cells deficient in TGF-β signaling cannot differentiate into Tregs in vitro or in vivo (31–33). Furthermore, human naïve CD4+CD25− cells activated with superantigen or alloantigen along with IL-2 and TGF-β differentiate into FoxP3+CD25+ Tregs with potent in vitro suppressive activity (34, 35). Thus, we asked whether fetal CD4+ T cells stimulated in the presence of TGF-β have an increased propensity to differentiate into Tregs as compared to adult cells. We isolated fetal and adult naïve T cells and stimulated them in the presence of increasing concentrations of TGF-β. While we observed that exogenous TGF-β enhanced FoxP3+CD25+ Treg differentiation in both fetal and adult naïve, fetal T cells exhibited increased Treg frequencies in all conditions (Fig. 2c). Fetal T cells also showed significantly increased expression of the FoxP3 protein in all concentrations of TGF-β compared to adult cells (Fig. 2d).
Based on the critical role that TGF-β signaling plays in Treg differentiation and our observation that fetal T cells exhibit increased Treg differentiation propensity in response to TGF-β, fetal and adult naive T cells were interrogated for the expression of key TGF-β signaling proteins. Fetal naïve CD4+ T cells expressed significantly higher levels of TGF-βRI, TGF-βRIII, and SMAD2 proteins (Fig. 2e, g, h). We observed no significant differences in the expression of TGF-βRII (Fig. 2f). Based on these findings, we investigated whether fetal T cells have alterations in signaling downstream of TGF-β stimulation. We observed that SMAD2/SMAD3 phosphorylation was increased in naive fetal CD4+ T cells at basal conditions and upon stimulation with TGF-β (Fig. 2i, Supp. Fig. 2b,c). When we analyzed the ratio of pSMAD2/3 to total SMAD2 protein, we found no difference between fetal and adult cells. Taken together, these results indicate that fetal naïve CD4+ T cells have higher expression of TGF-β signaling components and increased activation of downstream TGF-β signaling. Fetal lymph nodes have also been shown to express significantly higher levels of TGF-β family members, as compared to adults [7]. Interestingly, naïve fetal T cells express lower levels of latent TGF-β on their surface as compared to adult cells. (Supp. Fig. 2d).
Lin28b mediates increased Treg differentiation in fetal T cells
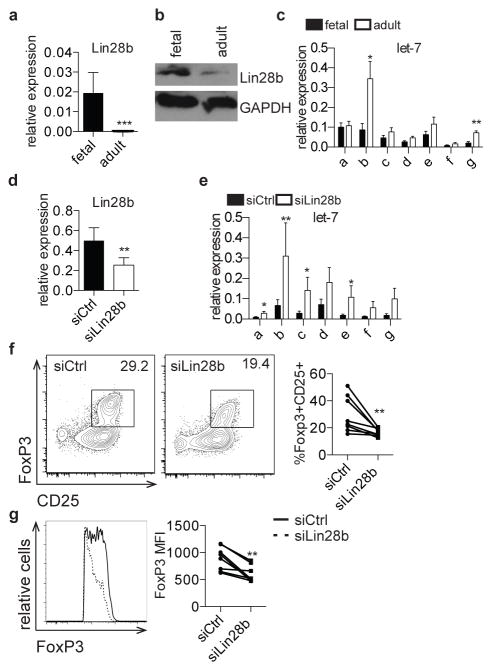
To address the mechanisms underlying these age-dependent differences in TGF-β signaling, we investigated a potential role for the evolutionarily conserved RNA-binding protein, Lin28b, which has key functions in development, metabolism, differentiation, and pluripotency (9, 11, 17, 36).. Lin28b is expressed in undifferentiated cells in C. elegans, mice, and humans (9–11) and in fetal hematopoietic tissues in mice and humans [18], and its overexpression in mouse hematopoietic progenitors redirects them towards fetal-like lymphopoiesis (18, 19). Lin28b functions through binding and directly regulating thousands of mRNAs, and by blocking the biogenesis of let-7 family miRNAs (13–15, 17, 37). Based on the importance of Lin28b in fetal hematopoiesis, we wondered if Lin28b could play a role in human fetal Treg differentiation. We measured Lin28b expression in fetal and adult naïve CD4+ T cells and, in accordance with previous reports, Lin28b mRNA and protein are expressed at much higher levels in fetal T cells as compared to adult cells (Fig. 3a, b) (18). Because Lin28b functions in part through regulation of let-7 biogenesis, we interrogated the expression of let-7 family members in fetal and adult T cells. We observed lower expression of most let-7 members in fetal cells, which reached significance in the case of let-7b and let-7g (Fig. 3c).
Figure 3. Lin28b mediates increased Treg differentiation in fetal T cells.
a. qRT-PCR analysis of Lin28b mRNA expression in fetal and adult naïve CD4+ T cells. Lin28b expression is shown relative to expression of β-actin. b. Western blot analysis of Lin28b protein in fetal and adult naïve CD4+ T cells. GAPDH is shown as a loading control. Blot is cropped and both analyses were performed on the same blot. c. qRT-PCR analysis of let-7 family mature miRNAs (a–g) expression in fetal and adult naïve CD4+ T cells. Let-7 expression is shown relative to expression of 5.8S ribosomal RNA. d. qRT-PCR analysis of Lin28b mRNA expression in fetal CD4+ T cells 3 days following treatment with non-targeting control siRNA (siCtrl) or siRNA targeting Lin28b (siLin28b). Lin28b expression is shown relative to expression of β-actin. e. qRT-PCR analysis of let-7 family mature miRNAs expression in siCtrl or siLin28b fetal CD4+ T cells. Let-7 expression is shown relative to expression of 5.8S ribosomal RNA. f. Flow cytometry analysis of staining for intracellular FoxP3 and surface CD25 on siCtrl and siLin28b fetal CD4+ T cells following 3 days incubation with siRNA and 4 days in vitro Treg differentiation. Graph shows the percentage of FoxP3+CD25+ cells within the CD4+ population. g. Flow cytometry analysis of staining for intracellular FoxP3 on siCtrl and siLin28b FoxP3+CD4+ T cells. Graph shows FoxP3 MFI within the FoxP3 population. The data are representative of results from 8 fetal and 8 adult individuals in 3 independent experiments. Statistics refer to Wilcoxon test. *** p<.001, ** p<.01, * p<.05
To determine if Lin28b drives increased Treg differentiation in fetal T cells, we knocked down Lin28b in naïve fetal CD4+ T cells and assayed resulting Treg frequencies after differentiation in vitro. Cells treated with siRNAs targeting Lin28b (siLin28b) had significantly decreased expression of Lin28b mRNA and increased expression of let-7 members compared to cells treated with non-targeting siRNA cells (siCtrl) (Fig. 3d, e). Furthermore, cultures of siLin28b-treated cells had significantly decreased frequencies of FoxP3+CD25+ Tregs and decreased expression of FoxP3 protein upon stimulation (Fig. 3f, g).
Lin28b regulates TGFβ signaling in fetal T cells through regulation of let-7
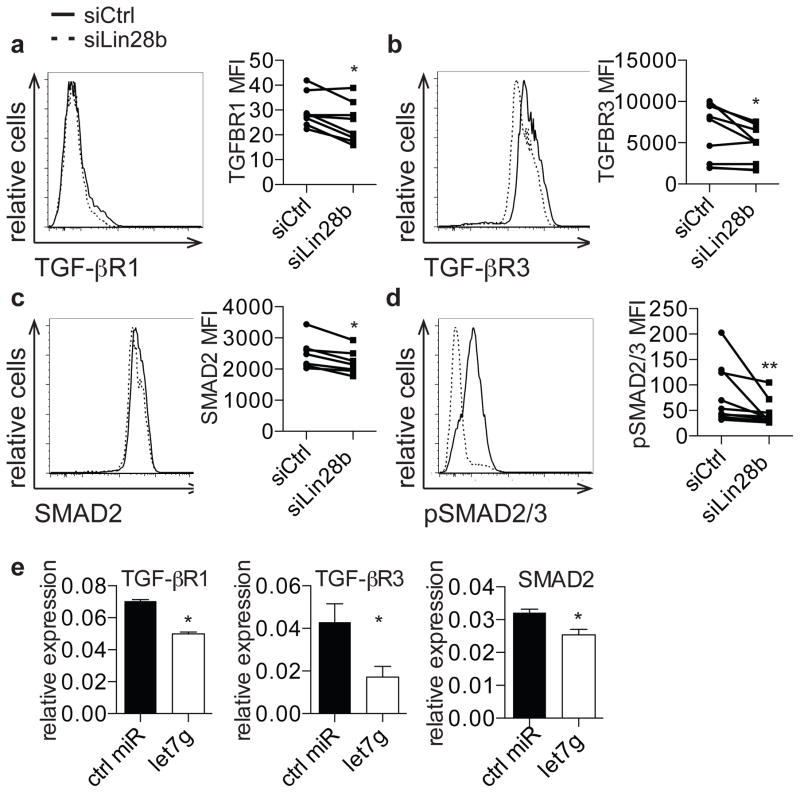
Given that fetal T cells have increased expression of TGF-βRI, TGF-βRIII, and SMAD2, we wondered if siLin28b cells would similarly show decreased expression of these mediators of TGF-β signaling. We observed that cells treated with siLin28b exhibited significantly lower expression of TGF-βRI, TGF-βRIII, and SMAD2 proteins (Fig. 4a, b, c) and that SMAD2/SMAD3 phosphorylation was also impaired in the setting of Lin28b knockdown (Fig. 4d). Finally, we asked if increased let-7 biogenesis underlies the phenotypes observed in the setting of Lin28 knockdown. Because TGF-βRI, TGF-βRIII, and SMAD2 are targets of let-7, we tested whether their expression was effected by let-7. When let-7g was introduced into the Jurkat human T cell line, we observed significantly decreased expression of TGF-βRI, TGF-βRIII, and SMAD2 (Fig. 4e)(38–40). This observation indicates that Lin28b may regulate fetal Treg differentiation through let-7-mediated repression of TGF-β signaling mediators (see model in Supp. Fig. 3).
Figure 4. Lin28b regulates TGFβ signaling in fetal T cells through regulation of let-7.
a–c. Flow cytometry analysis of staining for TGF-βRI, TGF-βRIII, and SMAD2 on siCtrl- and siLin28b-treated fetal CD4+ T cells. Graphs show MFI of TGF-βRI, TGF-βRIII, and SMAD2 on siCtrl- and siLin28b-treated CD4+ T cells. d. Flow cytometry analysis of staining for intracellular phosphorylated SMAD2/SMAD3 on siCtrl- and siLin28b-treated CD4+ T cells in the presence of 2 ng TGF-β. Bar graph shows phosphorylated SMAD2/SMAD3 MFI within the CD4+ population. e. qRT-PCR analysis of TGF-βRI, TGF-βRIII, and SMAD2 mRNA expression in Jurkat cells transfected with control or let-7g miRNA. The data are representative of results from 8 fetal and 8 adult individuals in 3 independent experiments. Statistics refer to Wilcoxon test. *** p<.001, ** p<.01, * p<.05
Discussion
Human fetal secondary lymphoid organs are enriched for regulatory T cells and fetal naïve CD4+ T cells are predisposed to differentiate into FoxP3+ Tregs upon stimulation. The data presented here demonstrate that the RNA-binding protein Lin28b is involved in Treg differentiation in fetal T cells. Naïve fetal T cells exhibit increased expression of key molecules involved in TGF-β signaling, including TGF-βRI, TGF-βRIII, and SMAD2, and show increased phosphorylation of SMAD2/3 upon stimulation with TGF-β. Fetal T cells further show increased differentiation into FoxP3+CD25+ cells across a titration of TCR stimulation and TGF-β concentrations. Lin28b is highly expressed in naïve fetal T cells, as compared to adult cells, and is associated with decreased levels of let-7 family miRNAs. When Lin28b is knocked down in fetal T cells, let-7 miRNAs are upregulated and differentiation into FoxP3+CD25+ cells is significantly decreased. Lin28b knockdown is further associated with decreased expression of TGF-βRI, TGF-βRIII, and SMAD2, and impaired phosphorylation of SMAD2/3 at basal conditions and upon stimulation with TGF-β. These effects may be caused by let-7-mediated repression of TGF-βRI, TGF-βRIII, and SMAD2 expression. Collectively our data support a model in which high expression of Lin28b in fetal CD4+ T cells mediates increased signaling through the TGF-β pathway and differentiation into Tregs.
The predisposition of fetal T cells to differentiate into Tregs is a dominant feature of fetal and neonatal immune responses, and may contribute to susceptibility to infections and poor vaccine responsiveness. Cellular immunity to vaccination is generally less effective in infants as compared to older children and adults, and is characterized by anti-inflammatory responses (41–43). Based on our finding highlighting Lin28b as a key mediator of the tolerogenic fetal T cell program, it is possible that modulation of Lin28b activity in infants could polarize the neonatal immune response to become more immunoreactive; if so, strategies may be developed to enhance vaccine responsiveness in this age group and confer increased protection. Furthermore, successful pregnancy requires maintenance of immune tolerance between the mother and her fetus, and failure to do so may lead to pregnancy complications such as fetal loss, preterm labor, and preeclampsia (44). Since maternal cells can migrate across the placenta and maternal antigen-specific Tregs are required for prevention of fetal anti-maternal immune activation, modulation of the Lin28b-regulated fetal Treg differentiation pathway may also have impact on outcomes in perinatal medicine (20, 45).
Because of the extreme difficulty in obtaining adult secondary lymphoid organ and fetal peripheral blood samples, these studies compared T cells derived from fetal lymph nodes and spleen to adult T cells from peripheral blood. Thus, our findings may reflect both immunological differences associated with ontogeny and age, and experimental procedures used in isolating cells from these distinct anatomical locations. Future experiments will be required to discriminate between these possibilities. It is further notable that the CD4+CD45RA+ population in adults may contain a small number of TEMRA cells, which may confound our analysis (46).
A number of key questions remain to be addressed. Though we have shown that naïve fetal T cells are predisposed to differentiate into FoxP3+CD25+ cells that also express the key Treg markers, CTLA4 and ICOs, and that such cells have suppressive activity in vitro(20), it will also be important to determine the functional consequences of Lin28b knockdown in fetal T cells. Thus, our data show that loss of Lin28b is associated with decreased differentiation into FoxP3+CD25+ cells and it will be interesting to determine if these cells are also less suppressive. Furthermore, it will be critical to understand whether they instead upregulate proinflammatory cytokines like IFN-γ or IL-2. It has recently been shown that a converse relationship exists wherein TGF-β promotes the transcription of Lin28b (47). Fetal T cells do not exhibit increased expression of TGF-β, thus arguing against a paracrine mechanism wherein fetal Tregs themselves promote SMAD phosphorylation, but fetal lymph nodes do show increased mRNA for numerous TGF-β family cytokines and this may be a mechanism that promotes increased Lin28b transcription in fetal T cells (20).
Supplementary Material
Acknowledgments
We wish to thank Wes Yonemoto, Jillian Jesperson, and Sasha Targ for critical conversation and technical advice. We also thank the staff at the Women’s Options Center, cell and tissue donors, and the NIH/NIAID Infant Immunity Program.
Funding
This work was funded by R01AI100082 and 5T32AI007334-25.
Footnotes
Author Contributions
YB, TDB, and JMM conceived of experiments and wrote the manuscript. YB performed all experiments.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nature immunology. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 2.Mellor AL, Munn DH. Immunology at the maternal-fetal interface: lessons for T cell tolerance and suppression. Annual review of immunology. 2000;18:367–391. doi: 10.1146/annurev.immunol.18.1.367. [DOI] [PubMed] [Google Scholar]
- 3.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 4.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darrasse-Jeze G, Marodon G, Salomon BL, Catala M, Klatzmann D. Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood. 2005;105:4715–4721. doi: 10.1182/blood-2004-10-4051. [DOI] [PubMed] [Google Scholar]
- 7.Cupedo T, Nagasawa M, Weijer K, Blom B, Spits H. Development and activation of regulatory T cells in the human fetus. European journal of immunology. 2005;35:383–390. doi: 10.1002/eji.200425763. [DOI] [PubMed] [Google Scholar]
- 8.Michaelsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176:5741–5748. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- 9.Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88:637–646. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- 10.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 12.Mayr F, Heinemann U. Mechanisms of Lin28-mediated miRNA and mRNA regulation--a structural and functional perspective. International journal of molecular sciences. 2013;14:16532–16553. doi: 10.3390/ijms140816532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilbert ML, Huelga SC, Kapeli K, Stark TJ, Liang TY, Chen SX, Yan BY, Nathanson JL, Hutt KR, Lovci MT, Kazan H, Vu AQ, Massirer KB, Morris Q, Hoon S, Yeo GW. LIN28 binds messenger RNAs at GGAGA motifs and regulates splicing factor abundance. Molecular cell. 2012;48:195–206. doi: 10.1016/j.molcel.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu B, Huang Y. Histone H2a mRNA interacts with Lin28 and contains a Lin28-dependent posttranscriptional regulatory element. Nucleic acids research. 2009;37:4256–4263. doi: 10.1093/nar/gkp372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu B, Zhang K, Huang Y. Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA. 2009;15:357–361. doi: 10.1261/rna.1368009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng S, Chen LL, Lei XX, Yang L, Lin H, Carmichael GG, Huang Y. Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem cells. 2011;29:496–504. doi: 10.1002/stem.591. [DOI] [PubMed] [Google Scholar]
- 17.Zhu H, Shyh-Chang N, Segre AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, Urbach A, Thornton JE, Triboulet R, Gregory RI, Altshuler D, Daley GQ. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147:81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335:1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YT, de Vasconcellos JF, Yuan J, Byrnes C, Noh SJ, Meier ER, Kim KS, Rabel A, Kaushal M, Muljo SA, Miller JL. LIN28B-mediated expression of fetal hemoglobin and production of fetal-like erythrocytes from adult human erythroblasts ex vivo. Blood. 2013;122:1034–1041. doi: 10.1182/blood-2012-12-472308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mold JE, Michaelsson J, Burt TD, McCune JM. Maternal Alloantigens Promote the Development of Tolerogenic Fetal Regulatory T Cells in Utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182:148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Y, Manzotti CN, Burke F, Dussably L, Qureshi O, Walker LS, Sansom DM. Acquisition of suppressive function by activated human CD4+ CD25- T cells is associated with the expression of CTLA-4 not FoxP3. J Immunol. 2008;181:1683–1691. doi: 10.4049/jimmunol.181.3.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudd CE. CTLA-4 co-receptor impacts on the function of Treg and CD8+ T-cell subsets. European journal of immunology. 2009;39:687–690. doi: 10.1002/eji.200939261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng J, Chan PL, Liu Y, Qin G, Xiang Z, Lam KT, Lewis DB, Lau YL, Tu W. ICOS regulates the generation and function of human CD4+ Treg in a CTLA-4 dependent manner. PloS one. 2013;8:e82203. doi: 10.1371/journal.pone.0082203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, Feigenbaum L, Singer A. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood. 2012;119:5155–5163. doi: 10.1182/blood-2011-11-388918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang AL, Teijaro JR, Njau MN, Chandran SS, Azimzadeh A, Nadler SG, Rothstein DM, Farber DL. CTLA4 expression is an indicator and regulator of steady-state CD4+ FoxP3+ T cell homeostasis. J Immunol. 2008;181:1806–1813. doi: 10.4049/jimmunol.181.3.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vocanson M, Rozieres A, Hennino A, Poyet G, Gaillard V, Renaudineau S, Achachi A, Benetiere J, Kaiserlian D, Dubois B, Nicolas JF. Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. The Journal of allergy and clinical immunology. 2010;126:280–289. 289 e281–287. doi: 10.1016/j.jaci.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka S, Maeda S, Hashimoto M, Fujimori C, Ito Y, Teradaira S, Hirota K, Yoshitomi H, Katakai T, Shimizu A, Nomura T, Sakaguchi N, Sakaguchi S. Graded attenuation of TCR signaling elicits distinct autoimmune diseases by altering thymic T cell selection and regulatory T cell function. J Immunol. 2010;185:2295–2305. doi: 10.4049/jimmunol.1000848. [DOI] [PubMed] [Google Scholar]
- 29.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nature immunology. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 30.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 32.Luo X, Yang H, Kim IS, Saint-Hilaire F, Thomas DA, De BP, Ozkaynak E, Muthukumar T, Hancock WW, Crystal RG, Suthanthiran M. Systemic transforming growth factor-beta1 gene therapy induces Foxp3+ regulatory cells, restores self-tolerance, and facilitates regeneration of beta cell function in overtly diabetic nonobese diabetic mice. Transplantation. 2005;79:1091–1096. doi: 10.1097/01.tp.0000161223.54452.a2. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nature immunology. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 34.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 35.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25- precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 36.West JA, Viswanathan SR, Yabuuchi A, Cunniff K, Takeuchi A, Park IH, Sero JE, Zhu H, Perez-Atayde A, Frazier AL, Surani MA, Daley GQ. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature. 2009;460:909–913. doi: 10.1038/nature08210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Tzur G, Israel A, Levy A, Benjamin H, Meiri E, Shufaro Y, Meir K, Khvalevsky E, Spector Y, Rojansky N, Bentwich Z, Reubinoff BE, Galun E. Comprehensive gene and microRNA expression profiling reveals a role for microRNAs in human liver development. PloS one. 2009;4:e7511. doi: 10.1371/journal.pone.0007511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar MS, Armenteros-Monterroso E, East P, Chakravorty P, Matthews N, Winslow MM, Downward J. HMGA2 functions as a competing endogenous RNA to promote lung cancer progression. Nature. 2014;505:212–217. doi: 10.1038/nature12785. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Colas AR, McKeithan WL, Cunningham TJ, Bushway PJ, Garmire LX, Duester G, Subramaniam S, Mercola M. Whole-genome microRNA screening identifies let-7 and mir-18 as regulators of germ layer formation during early embryogenesis. Genes & development. 2012;26:2567–2579. doi: 10.1101/gad.200758.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rowe J, Macaubas C, Monger T, Holt BJ, Harvey J, Poolman JT, Loh R, Sly PD, Holt PG. Heterogeneity in diphtheria-tetanus-acellular pertussis vaccine-specific cellular immunity during infancy: relationship to variations in the kinetics of postnatal maturation of systemic th1 function. The Journal of infectious diseases. 2001;184:80–88. doi: 10.1086/320996. [DOI] [PubMed] [Google Scholar]
- 42.Zepp F, Knuf M, Habermehl P, Schmitt JH, Rebsch C, Schmidtke P, Clemens R, Slaoui M. Pertussis-specific cell-mediated immunity in infants after vaccination with a tricomponent acellular pertussis vaccine. Infection and immunity. 1996;64:4078–4084. doi: 10.1128/iai.64.10.4078-4084.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowe J, Macaubas C, Monger TM, Holt BJ, Harvey J, Poolman JT, Sly PD, Holt PG. Antigen-specific responses to diphtheria-tetanus-acellular pertussis vaccine in human infants are initially Th2 polarized. Infection and immunity. 2000;68:3873–3877. doi: 10.1128/iai.68.7.3873-3877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nature medicine. 2013;19:548–556. doi: 10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
- 45.Lo YM, Lau TK, Chan LY, Leung TN, Chang AM. Quantitative analysis of the bidirectional fetomaternal transfer of nucleated cells and plasma DNA. Clinical chemistry. 2000;46:1301–1309. [PubMed] [Google Scholar]
- 46.Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immunity & ageing : I & A. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park JT, Kato M, Lanting L, Castro N, Nam BY, Wang M, Kang SW, Natarajan R. Repression of let-7 by transforming growth factor-beta1-induced Lin28 upregulates collagen expression in glomerular mesangial cells under diabetic conditions. American journal of physiology Renal physiology. 2014;307:F1390–1403. doi: 10.1152/ajprenal.00458.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.