Understanding of the mechanisms underlying acute coronary syndromes (ACS) depended for decades on autopsy studies. The limitations of postmortem studies include death as an inclusion criterion, biasing the studies toward events with fatal outcomes. The more numerous non-mortal events comprise a sizable but heretofore largely hidden denominator of uncertain magnitude. To gain insight into the underlying pathophysiologic basis of the ACS, laboratory researchers have conducted innumerable animal studies on atherosclerosis. Advanced genetic manipulations have led to increasingly refined approaches to probe aspects of experimental atherosclerosis, particularly in mice. Despite their indisputable value for isolating mechanisms, the interpretation and extrapolation of the results of many such mouse studies blithely ignore the yawning gap between the disease in laboratory animals and human patients with atherosclerosis. (1,2) Only under extreme circumstances do mouse or rabbit atheromata actually ever cause thrombosis. Experiments in small animals almost never focus on the coronary arteries, structures that differ strikingly in ontogeny from the human aorta or carotid arteries. Experimental studies routinely refer to plaque “vulnerability,” or “stable” or “unstable” plaque “phenotype” without actually assessing stability, instability, or vulnerability. This leap of faith, albeit routinely accepted, represents a striking suspension of intellectual and scientific rigor to which many turn a blind eye.
Fortunately, we now possess tools of increasing utility and validation for probing the structure of atherosclerotic plaques in living humans. Traditional contrast arteriography provided silhouettes of the lumen but revealed little of the artery wall or the character of atherosclerotic plaques themselves. Intravascular ultrasound provides an outstanding modality for measuring the volume of atherosclerotic plaques. Use of the radiofrequency backscatter from intravascular ultrasound can furnish some rudimentary information regarding tissue characterization. With the advent of optical coherence tomography (OCT), we now possess a remarkable method for near-field imaging of the arterial intima in intact humans. OCT allows visualization of the surface of the plaque and the near-field subjacent intima with almost microscopic resolution.(3) Although OCT does not furnish precise cellular or biochemical information, its practitioners have developed criteria for distinguishing rupture of the plaque’s fibrous cap from non-rupture, and “red” versus “white” thrombus. (4) Either red or white thrombus can provoke myocardial ischemia (recognizable as cyanosis, or blue discoloration of the heart’s surface). There is even evidence that OCT can identify macrophages in intimal lesions, although this feature may not be unambiguous.(5) The judicious application of this powerful imaging modality can provide novel insights regarding the correlation of mechanisms of plaque disruption and thrombosis and clinical outcomes and biomarkers. This rich opportunity points to new ways to probe pathophysiologic pathways in humans and to permit more personalized, precision approaches to therapy.
Nishiguchi and colleagues in this issue of Arteriosclerosis, Thrombosis, and Vascular Biology use OCT to these ends in a series of patients presenting with coronary artery disease.(6) Appropriately, most of the patients requiring acute intervention had ST-segment elevation myocardial infarction (STEMI). These patients underwent manual thrombus aspiration and subsequent sampling of the coronary arterial blood in the region of the culprit lesion before and after deployment of a stent as well as in peripheral blood. The authors report on concentrations of two biomarkers measured in the samples: matrix metalloproteinase–9 (MMP-9) and myeloperoxidase (MPO.) They found a significant elevation in the local versus systemic blood concentrations of MMP-9 in patients with STEMI, albeit with considerable overlap. They furthermore found more thrombi that display OCT–signatures of “red” (presumably fibrin-rich) clots in STEMI than in patients who presented with NSTEMI.
These findings raise a number of interesting issues first with relation to the study itself, its insights into understanding the pathophysiology of ACS, and its implications for personalized therapy. To perform OCT in the safest manner in patients undergoing an ACS, the investigators performed a preliminary thrombus aspiration. Yet, one must ask if this manipulation could itself alter the release or mobilization of the analytes tested. As in accord with the Heisenberg uncertainty principle in quantum physics, could the very act of gazing at the plaque so prepared change the measurements made? The choice of MPO for investigation in this study derives from prior observations that demonstrated that concentrations of this pro-oxidant enzyme rise to a greater extent in ACS caused by superficial erosion than by plaque rupture.(7) As the authors point out, their patient population was biased toward STEMI, as quite appropriately, many NSTEMI patients did not undergo urgent intervention. That feature may explain in part why this study did not find an association between the type of presentation and local MPO concentration. The choice of MMP-9 as a focus for this study is less clear. The finding of predominantly “red” thrombi in this STEMI-slanted population agrees with the notion that lesions that provoke ACS due to superficial erosion may have more platelet-rich, “white” thrombi.(8) Was MMP-9 a prespecified target of this investigation? How many other biomarkers did these investigators analyze that might not have shown significant gradients between coronary arterial and systemic blood? Did the authors analyze MRP 8/14 that we and others have implicated as a biomarker and actual agonist in STEMI?(9,10)
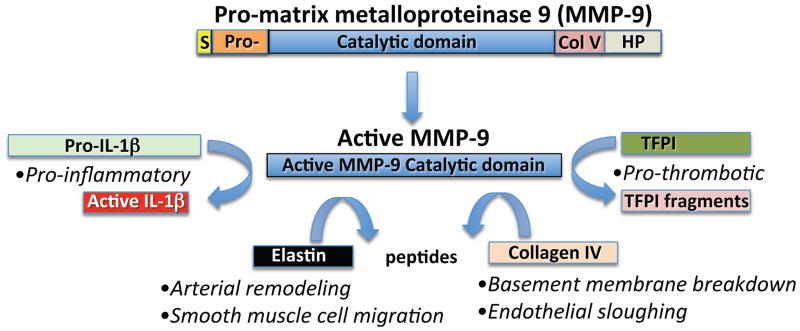
Since MMP-9 did emerge as significantly elevated in the coronary arterial blood sampled at the site of the culprit lesion, the mechanistic implications of this observation merit consideration. Early studies localized this enzyme in human atherosclerotic plaques. (11) This enzyme has many functions that could relate to atherogenesis and plaque disruption (Figure). As a potent elastase, MMP-9 could participate in smooth muscle migration and in outward (Glagovian) geometrical remodeling of plaques during their evolution. As a type IV non-fibrillar collagenase, MMP-9 could degrade the intimal basement membrane, rendering endothelial cells more likely to detach. Sites with impaired or absent endothelial antithrombotic and fibrinolytic and vasodilator function might propagate or aggravate local thrombus accumulation. MMP-9 can cleave pro–interleukin-1 beta to produce the mature, active form of this proinflammatory cytokine. (12) As the authors point out, MMP-9 can also degrade tissue factor pathway inhibitor, removing a homeostatic brake on coagulation. MMP-9’s ability to effect such local changes in atheroma would require the presence of the enzyme’s active form rather than the inactive zymogen precursor proteinase (Figure.) The assay for MMP-9 employed in the study likely could not distinguish between the active and inactive forms of this enzyme, leaving this point unresolved.
Figure 1. Selected actions of matrix metalloproteinase-9 (MMP-9) related to atherothrombosis.
Pro-MMP-9 undergoes cleavage to generate the active MMP-9 catalytic domain. Active MMP-9 in turn can process pro-interleukin-1beta (IL-1β) to form the pro-inflammatory active cytokine. MMP-9 also exhibits elastinolytic activity that can mediate arterial remodeling and smooth muscle cell migration. MMP-9 also possesses gelatinolytic activity that can degrade Type IV collagen, a major constituent of the subendothelial intimal basement membrane. Catabolism of collagen IV may favor endothelial cell sloughing, potentially promoting local thrombus extension. MMP-9 can also inactivate tissue factor pathway inhibitor (TFPI). This action of MMP-9 could promote thrombosis by removing an endogenous inhibitor of the potent procoagulant tissue factor, implicated in coronary artery thrombus formation. The domains of pro-matrix metalloproteinase-9 depicted include the signal sequence (S), the Pro-piece (Pro-), the Collagen V-like domain (Col V), and the hemopexin (HP) domain at the carboxyl terminus of the zymogen.
As the authors argue, biomarkers that could stratify patients with ACS mechanistically and therapeutically might help achieve the goal of a more precision management and personalized approach. While MMP-9 could serve as such a marker, a number of practical obstacles impede its implementation in clinical practice. There was a high degree of overlap in MMP-9 measurements between groups, rendering this analyte difficult to use as a binary biomarker to inform diagnosis and management. MMP-9 measurement in coronary arterial blood requires invasive instrumentation, while an ideal strategy for precision triage of ACS patients would avoid catheterization in a subset of individuals who might be managed without interventional treatment.(13–15) MMP-9’s measurement in the ACS setting would also require near instantaneous point-of-care testing, a potentially feasible undertaking, but one not yet in hand or validated.(16) This study provides important illustration of the principle that biomarkers may serve to discriminate various substrates for ACS that could direct individuals to different management strategies. The concept is powerful, pertinent to precision and personalized medicine, and provides a worthy goal toward which to strive in the future.
Acknowledgments
Sources of Funding
Peter Libby is supported by grants from the National Institute of Health (R01 HL080472).
References
- 1.Libby P. Murine “Model” Monotheism: An Iconoclast at the Altar of Mouse. Circ Res. 2015;117:921–5. doi: 10.1161/CIRCRESAHA.115.307523. [DOI] [PubMed] [Google Scholar]
- 2.Drucker DJ. Never Waste a Good Crisis: Confronting Reproducibility in Translational Research. Cell Metab. 2016;24:348–60. doi: 10.1016/j.cmet.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Jia H, Abtahian F, Aguirre AD, et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–1758. doi: 10.1016/j.jacc.2013.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quadros AS, Cambruzzi E, Sebben J, et al. Red versus white thrombi in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention: clinical and angiographic outcomes. Am Heart J. 2012;164:553–60. doi: 10.1016/j.ahj.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Tearney GJ, Yabushita H, Houser SL, et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–9. doi: 10.1161/01.cir.0000044384.41037.43. [DOI] [PubMed] [Google Scholar]
- 6.Nishiguchi T, Tanaka A, Taruya A, et al. Local Matrix Metalloproteinase 9 Level Determines Early Clinical Presentation of ST-Segment–Elevation Myocardial Infarction. Arteriosclerosis, Thrombosis, and Vascular Biology. 2016 doi: 10.1161/ATVBAHA.116.308099. [DOI] [PubMed] [Google Scholar]
- 7.Ferrante G, Nakano M, Prati F, et al. High levels of systemic myeloperoxidase are associated with coronary plaque erosion in patients with acute coronary syndromes: a clinicopathological study. Circulation. 2010;122:2505–13. doi: 10.1161/CIRCULATIONAHA.110.955302. [DOI] [PubMed] [Google Scholar]
- 8.Libby P, Pasterkamp G. Requiem for the ‘vulnerable plaque’. European Heart Journal. 2015;36:2984–7. doi: 10.1093/eurheartj/ehv349. [DOI] [PubMed] [Google Scholar]
- 9.Healy AM, Pickard MD, Pradhan AD, et al. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation. 2006;113:2278–84. doi: 10.1161/CIRCULATIONAHA.105.607333. [DOI] [PubMed] [Google Scholar]
- 10.Yonekawa K, Neidhart M, Altwegg LA, et al. Myeloid related proteins activate Toll-like receptor 4 in human acute coronary syndromes. Atherosclerosis. 2011;218:486–92. doi: 10.1016/j.atherosclerosis.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Galis Z, Sukhova G, Lark M, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. Journal of Immunology. 1998;161:3340–6. [PubMed] [Google Scholar]
- 13.Braunwald E. Coronary plaque erosion: recognition and management. JACC Cardiovasc Imaging. 2013;6:288–9. doi: 10.1016/j.jcmg.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Kanwar SS, Stone GW, Singh M, et al. Acute coronary syndromes without coronary plaque rupture. Nat Rev Cardiol. 2016;13:257–65. doi: 10.1038/nrcardio.2016.19. [DOI] [PubMed] [Google Scholar]
- 15.Jia H, Dai J, Hou J, et al. Effective Anti-thrombotic Therapy without Stenting: Intravascular OCT-based Management in Plaque Erosion (the EROSION study) European Heart Journal. 2016 doi: 10.1093/eurheartj/ehw381. in press. [DOI] [PubMed] [Google Scholar]
- 16.King KR, Grazette LP, Paltoo DN, et al. Point-of-Care Technologies for Precision Cardiovascular Care and Clinical Research. JACC: Basic to Translational Science. 2016;1:73–86. doi: 10.1016/j.jacbts.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]