Abstract
MicroRNA-210 (miR-210) has been implicated in homeostatic adaptation during hypoxia. We hypothesized that miR-210 deficiency impacts feto-placental growth. As expected, mir-210 knockout (ko) mice exhibited markedly reduced placental miR-210 expression, compared to wild-type (wt) mice. Mating of mir-210 heterozygotes resulted in near Mendelian progeny distribution, with insignificant differences between wt and ko animals with regard to embryo or placental weight and gross morphology. Intriguingly, exposure of mice to non-severe hypoxia (O2=12%) between E11.5-E17.5 reduced placental miR-210 expression, with slight expression changes of some miR-210 target mRNAs. Thus, miR-210 is likely dispensable for feto-placental growth in normoxia or non-severe hypoxia.
Keywords: Placenta, Trophoblast, miR-210, Hypoxia, Knockout
Introduction
Diverse miRNA species are expressed in the human or mouse placenta, yet only a few of them have been mechanistically shown to regulate trophoblast function [1-4]. Ample evidence suggests that miR-210 is a critical regulator of tissue response to hypoxia and is likely regulated by HIF1α to drive downregulation of target genes [5-8]. miR-210 has also been associated with hypoxia in placental trophoblasts and shown to be upregulated in diseases attributed to hypoxia, such as preeclampsia, [9, 10]. Luo et al. [11] provide evidence that miR-210 downregulates Thrombospondin Type I Domain Containing 7A (THSD7A) in human placental trophoblasts, possibly contributing to the preeclamptic phenotype. Low oxygen tension also leads to placental mitochondria dysfunction, in which elevated miR-210 levels downregulate the Iron Sulfur Cluster (ISCU) gene and inhibition of miR-210 protects trophoblast cells form oxidative stress [12]. We therefore surmised that miR-210 was crucial for placental response to hypoxia and thus for fetal growth.
Methods
The Institutional Animal Care and Use Committee of the University of Pittsburgh approved all experimental protocols. We used STOCK mir210tm1Mtm/Mmjax female mice that were first crossed with B6.129S4-Gt(ROSA)26Sortm1(FLP1)Dym/RainJ males to delete the Neo cassette, followed by crossing with B6.C-Tg(CMV-cre)1Cgn/J to delete mir-210. (Mice were obtained from Jackson Laboratory, Bar Harbor, ME.) To produce all genotypes, heterozygous males and females were crossed, and pregnant dams were kept either in normal atmosphere or in a hypoxia chamber (Coy Laboratory Products, Grass Lake, MI) in a 12% O2 atmosphere between E11.5-E17.5. On E17.5 the mice were sacrificed, and placentas and embryos were weighed. Genotyping was performed by standard PCR (Veriti, Thermo Fisher, Waltham, MA), using specific primer sets (Supplemental Table S1) and genomic DNA isolated from fetal tail by the HotSHOT (Hot Sodium Hydroxide and Tris) method, adapted from Truett et al [13]. Sex determination of the fetuses was performed based on SRY (Sex-determining Region Y) expression using a specific primer set (Integrated DNA Technologies, Table S1) and standard PCR. Amplification products were visualized on agarose gel with 2% bromide ethidium. Paraffin-embedded sections of 4% paraformaldehyde-fixed placentas were stained with hematoxylin and eosin and inspected under a bright light microscope (Nikon 90i microscope, Nikon, Tokyo, Japan). For analysis of miR-210 and target gene expression by qPCR, we took extra care to trim the placental edges to avoid contamination by decidual cells. Collected placental fragments were soaked in RNA later and frozen at -80°C. Total RNA was isolated using TRI Reagent (Molecular Research Center, Cincinnati, OH) and purified with EconoSpin columns (Epoch Life Science, Missouri City, TX) following the manufacturer's instructions. The quantity and quality of RNA were assessed using a NanoDrop 1000 spectrometer (Thermo Scientific, Wilmington, DE). Reverse transcription and subsequent qPCR for miRNA expression were performed using, respectively, the miScript-II RT Kit and miScript SYBR Green PCR Kit with miScript assay (Qiagen, Valencia, CA), as we have described [14]. For mRNA expression, cDNA was synthesized with the High-Capacity cDNA Reverse Transcription Kit, followed by real-time PCR using SYBR Select Master Mix (Thermo Fisher) and specific primer sets (Integrated DNA Technologies, Coralville, IA; Supplemental Table S2). Relative transcript expression was calculated using the delta delta Ct method [15] and normalized to RNU6 or to mouse ribosomal protein L32. Statistical differences were calculated using two-way ANOVA, followed by the Bonferroni post-hoc test, with significance set at p<0.05 (Prism 6, GraphPad Software, San Diego, CA). All data are shown as mean ± SD.
Results and Discussion
We initially confirmed that miR-210 expression was markedly reduced in mir-210 ko placentas, compared to placentas from wt mice (Fig. 1A). We also confirmed the reduction of miR-210 in the kidneys from ko fetuses (16-fold) and that the expression of miR-210 increased 3-fold in primary human trophoblasts cultured in hypoxic conditions for 48 h compared to those cultured in standard conditions (false discovery rate = 0.0042, RNAseq, data not shown). We found normal Mendelian distribution of fetuses resulting from breeding mir-210 heterozygous males and females (Fig. 1B). The mean litter size was 8.7 embryos (range 7-11) for animals maintained in standard conditions, which was slightly greater than the mean litter size of 7.2 embryos (range 5-10) for mice exposed to hypoxia (n=11 pregnant females/group, p=0.023, unpaired t-test). Embryo and placental weights were not different between wt and ko animals (Fig. 1C, D). This observation was confirmed using analysis of wt vs. ko animals within each litter (not shown).
Fig 1. Characterization of the feto-placental phenotype in mir-210 ko mice exposed to standard conditions (STD) or to moderate hypoxia (HPX, O2=12%).
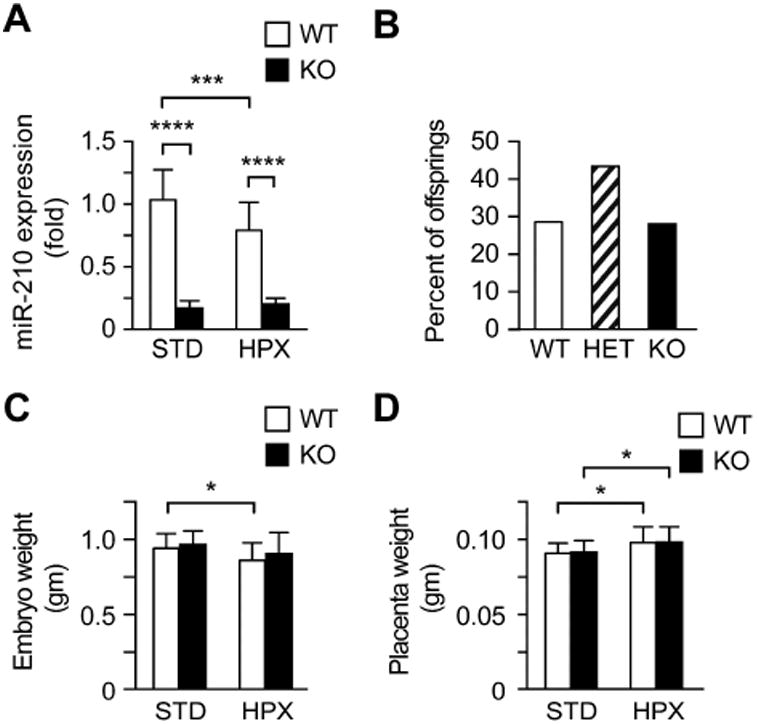
(A) Relative miR-210 expression in wt vs ko placentas, normalized to expression in wt placentas in STD conditions (n=15-17/group). (B) Genotype distribution of fetuses generated by crossing mir-210 heterozygous males and females (n=11 pregnancies, Chi square test p=0.2193). (C) Mean weight (grams) of wt and ko embryos subjected to either STD or HPX (n=18-22 per condition). (D) Mean weight (grams) of wt and ko placentas subjected to either STD or HPX (n=18-22 per condition). Data presented as mean ± SD. * p<0.05, *** p<0.001, **** p<0.0001.
We found that hypoxia decreased miR-210 expression level in wt placentas (Fig. 1A). While miR-210 levels were comparable between male and female placentas in standard conditions, in hypoxia the level of miR-210 in the male placentas was approximately 25% lower than the level in female placentas (p<0.05, not shown). As expected, the weight of wt fetuses was reduced when exposed to hypoxia, compared to standard conditions (Fig. 1C, p<0.05). When stratified by fetal sex, the differences were insignificant within each sex (Supplemental Fig. S1A). In contrast, exposure to hypoxia caused a small, yet significant, increase in placental weight in both wt and ko groups (p<0.05; Fig. 1D). This effect was also found in the male placentas, but not in the female placentas (Supplemental Fig. S1B). Placental histomorphology (based on hematoxylin and eosin stain at E18.5) was not different between the groups (not shown). To investigate the possible effect of altered miR-210 expression on known miR-210 targets, we analyzed placental expression of HOXA1, EFNA3, NDUFA4, and ISCU [5, 9, 12]. While we found small differences in mRNA levels between standard and hypoxic conditions, none of the genes were differentially expressed between wt and ko mice (Fig. 2A-D).
Fig 2. The relative expression of selected miR-210 target mRNAs in wt and ko placentas subjected to STD or HPX conditions (n=15-17 placentas in each condition).
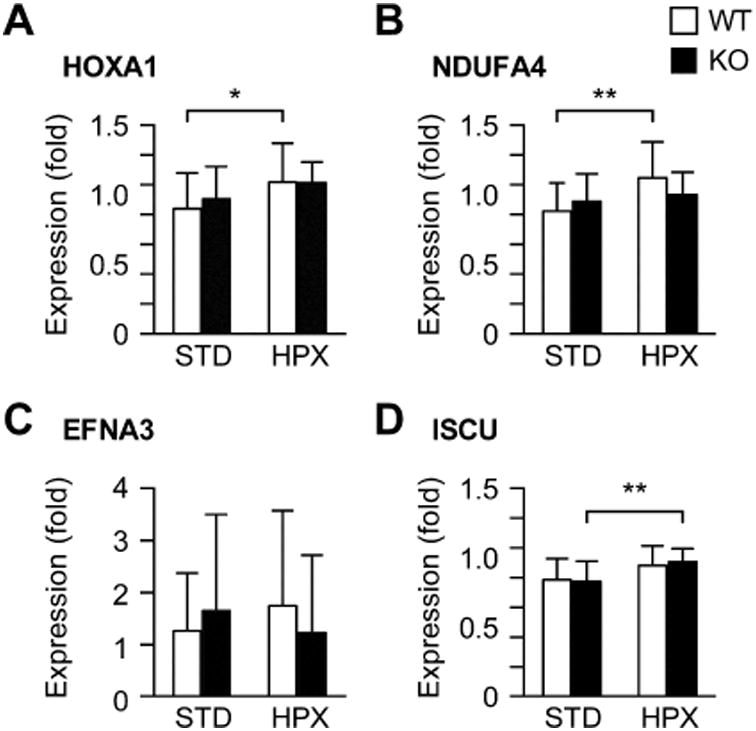
(A) HOXA1; (B) NDUFA4; (C) EFNA3; (D) ISCU. All data presented as mean ± SD, relatively to L32. * p<0.05, ** p<0.01.
We found that ablation of mir-210 in the fetus and placenta did not lead to altered intrauterine growth in standard conditions, suggesting that miR-210 is not necessary for intrauterine development in mice. Moreover, there was no upregulation of placental miR-210 in response to moderate maternal hypoxia. Notably, we used a moderate level of hypoxia (O2=12%) because, in our previous studies, we found that this degree of hypoxia caused fetal growth restriction by 10-20%, without excessive fetal loss [16]. In addition, we initiated hypoxia at E11.5, as our goal was to assess the effect of hypoxia on the established placenta and avoid exposure to hypoxia earlier in gestation, when the feto-placental unit develops during physiological hypoxia [17-20]. Indeed, during this period, miR-210 might have a role in attenuating trophoblast invasion [21], as blocking miR-210 in hypoxia increases trophoblast invasion and migration [6]. These processes were not assessed in our study. Thus, while our data cannot be extrapolated to the entire pregnancy or to miR-210 response at any level of hypoxia or other stressors, our results do not support a role for miR-210 in placental adaptation to hypoxia. Interestingly, we recently also found that placenta-specific overexpression of miR-210 had no effect on the mouse fetal and placental weight (Supplemental Fig. S2).
While it has been reproducibly shown by others and by us that miR-210 is upregulated in trophoblasts cultured in hypoxia [6, 22 and Sadovsky et al., unpublished], the evidence for miR-210 function and regulation in vivo is less clear [7, 23, 24]. Although miR-210 expression was increased in severe preeclampsia [10], the levels of this miRNA in patients with mild preeclampsia were lower than in normal pregnancies. It is also possible that the inconsistent results represent the variable level of hypoxia used in the different experiments [19, 25]. Together, our data suggest that compensatory or redundant mechanisms [26-28] regulate placental adaptation to hypoxia in mice and in humans.
Supplementary Material
Highlights.
The weight and morphology of mir-210 knockout placentas and fetuses are indistinguishable from those of wild-type mice.
Exposure of mice to non-severe hypoxia (O2=12%) between E11.5-E17.5 reduced placental miR-210 expression.
miR-210 is likely dispensable for feto-placental growth in normoxia or non-severe hypoxia.
Acknowledgments
We thank Tianjiao Chu for assistance in statistical analysis, Lori Rideout for assistance in manuscript preparation, and Bruce Campbell for editing. This work was supported by NIH grants R01-HD06589 and R21-HD071707 (both to YS).
Grant support: Supported by NIH grants R01-HD065893 and R21-HD071707 (both to YS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keniry A, Oxley D, Monnier P, et al. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell Biol. 2012;14:659–665. doi: 10.1038/ncb2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo L, Ye G, Nadeem L, et al. MicroRNA-378a-5p promotes trophoblast cell survival, migration and invasion by targeting Nodal. J Cell Sci. 2012;125:3124–3132. doi: 10.1242/jcs.096412. [DOI] [PubMed] [Google Scholar]
- 3.Kumar P, Luo Y, Tudela C, et al. The c-Myc-regulated microRNA-17∼92 (miR-17∼92) and miR-106a∼363 clusters target hCYP19A1 and hGCM1 to inhibit human trophoblast differentiation. Mol Cell Biol. 2013;33:1782–1796. doi: 10.1128/MCB.01228-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delorme-Axford E, Donker RB, Mouillet JF, et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci USA. 2013;110:12048–12053. doi: 10.1073/pnas.1304718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang X, Ding L, Bennewith KL, et al. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell. 2009;35:856–867. doi: 10.1016/j.molcel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Fei M, Xue G, et al. Elevated levels of hypoxia-inducible microRNA-210 in pre-eclampsia: new insights into molecular mechanisms for the disease. J Cell Mol Med. 2012;16:249–259. doi: 10.1111/j.1582-4934.2011.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, Feng L, Zhang H, et al. Preeclampsia up-regulates angiogenesis-associated microRNA (i.e., miR-17, -20a, and -20b) that target ephrin-B2 and EPHB4 in human placenta. J Clin Endocrinol Metab. 2012;97:E1051–1059. doi: 10.1210/jc.2011-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang F, Xiong L, Huang X, et al. miR-210 suppresses BNIP3 to protect against the apoptosis of neural progenitor cells. Stem Cell Res. 2013;11:657–667. doi: 10.1016/j.scr.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Pulkkinen K, Malm T, Turunen M, et al. Hypoxia induces microRNA miR-210 in vitro and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially regulated by miR-210. FEBS Lett. 2008;582:2397–2401. doi: 10.1016/j.febslet.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 10.Zhu XM, Han T, Sargent IL, et al. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol. 2009;200:661 e661–667. doi: 10.1016/j.ajog.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 11.Luo R, Wang Y, Xu P, et al. Hypoxia-inducible miR-210 contributes to preeclampsia via targeting thrombospondin type I domain containing 7A. Sci Rep. 2016;6:19588. doi: 10.1038/srep19588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muralimanoharan S, Maloyan A, Mele J, et al. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta. 2012;33:816–823. doi: 10.1016/j.placenta.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truett GE, Heeger P, Mynatt RL, et al. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT) Biotechniques. 2000;29:52, 54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- 14.Mouillet JF, Donker RB, Mishima T, et al. The unique expression and function of miR-424 in human placental trophoblasts. Biol Reprod. 2013;89:25. doi: 10.1095/biolreprod.113.110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Tomlinson TM, Garbow JR, Anderson JR, et al. Magnetic resonance imaging of hypoxic injury to the murine placenta. Am J Physiol Regul Integr Comp Physiol. 2010;298:R312–319. doi: 10.1152/ajpregu.00425.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell JA, Yochim JM. Intrauterine oxygen tension during the estrous cycle in the rat: its relation to uterine respiration and vascular activity. Endocrinology. 1968;83:701–705. doi: 10.1210/endo-83-4-701. [DOI] [PubMed] [Google Scholar]
- 18.Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil. 1993;99:673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- 19.Cuffe JS, Walton SL, Singh RR, et al. Mid- to late term hypoxia in the mouse alters placental morphology, glucocorticoid regulatory pathways and nutrient transporters in a sex-specific manner. J Physiol. 2014;592:3127–3141. doi: 10.1113/jphysiol.2014.272856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matheson H, Veerbeek JH, Charnock-Jones DS, et al. Morphological and molecular changes in the murine placenta exposed to normobaric hypoxia throughout pregnancy. J Physiol. 2016;594:1371–1388. doi: 10.1113/JP271073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anton L, Olarerin-George AO, Schwartz N, et al. miR-210 inhibits trophoblast invasion and is a serum biomarker for preeclampsia. Am J Pathol. 2013;183:1437–1445. doi: 10.1016/j.ajpath.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DC, Romero R, Kim JS, et al. miR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am J Pathol. 2011;179:590–602. doi: 10.1016/j.ajpath.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park CY, Jeker LT, Carver-Moore K, et al. A resource for the conditional ablation of microRNAs in the mouse. Cell Rep. 2012;1:385–391. doi: 10.1016/j.celrep.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mok Y, Schwierzeck V, Thomas DC, et al. MiR-210 is induced by Oct-2, regulates B cells, and inhibits autoantibody production. J Immunol. 2013;191:3037–3048. doi: 10.4049/jimmunol.1301289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai Z, Kalkunte S, Sharma S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2011;57:505–514. doi: 10.1161/HYPERTENSIONAHA.110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulshreshtha R, Davuluri RV, Calin GA, et al. A microRNA component of the hypoxic response. Cell Death Differ. 2008;15:667–671. doi: 10.1038/sj.cdd.4402310. [DOI] [PubMed] [Google Scholar]
- 27.Bertero T, Grosso S, Robbe-Sermesant K, et al. “Seed-Milarity” confers to hsa-miR-210 and hsa-miR-147b similar functional activity. PLoS One. 2012;7:e44919. doi: 10.1371/journal.pone.0044919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park CY, Choi YS, McManus MT. Analysis of microRNA knockouts in mice. Hum Mol Genet. 2010;19:R169–175. doi: 10.1093/hmg/ddq367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


