Abstract
Heterocyclic aromatic amines (HAA) are formed in cooked meat, poultry and fish but also arise in tobacco smoke and exhaust gases. HAA are potential human carcinogens, which require metabolic activation to exert their genotoxicity. Human tissues can bioactivate HAA to produce reactive intermediates that bind to DNA. HAA DNA adduct formation occurs in human hepatocytes; however, the potential of HAA to form DNA adducts has not been investigated in human T lymphocytes. In this study, we investigated the ability of human T lymphocytes activated with PMA/Ionomycin or CD3/CD28 to express functional CYP1 activity and bioactivate three major HAA: 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), and 2-amino-9H-pyrido[2,3-b]indole (AαC) to form DNA adducts. Adducts were measured by ultraperformance liquid chromatography-electrospray ionization/multistage scan mass spectrometry. The highest level of DNA adducts occurred for AαC (16 adducts per 109 nucleotides), followed by PhIP (9 adducts per 109 nucleotides). In contrast, DNA adducts formed from MeIQx and the structurally related aromatic amine 4-aminobiphenyl, a known human carcinogen, were below the limit of detection (< 3 adducts per 109 nucleotides). Moreover, we demonstrate that AαC is a potent inducer of CYP1A1 and CYP1B1 activity through a transcriptional mechanism involving the AhR pathway. Overall, our results highlight the capacity of activated human T lymphocytes to more efficiently bioactivate AαC to form DNA adducts than other prominent HAA or 4-ABP.
Keywords: cytochrome P450; carcinogen metabolism; genotoxicity; 2-amino-9H-pyrido[2,3-b]indole (AαC)
Introduction
Environmental exposure to hazardous chemicals is a major risk factor for cancer development [IARC 2010; IARC 2012]. Among the incriminated chemicals, heterocyclic aromatic amines (HAA) are one important class of potential human carcinogens. HAA are mainly formed in cooked meat, poultry and fish [Sugimura et al. 2004], but several HAA also arise in tobacco smoke and exhaust gases [Manabe et al. 1993; Zhang et al. 2011]. More than 25 HAA have been identified and are divided into two major classes: pyrolytic and aminoimidazoarenes. The pyrolytic HAA arise during the high-temperature (>250 °C) pyrolysis of proteins or amino acids such as tryptophan and glutamic acid [Sugimura et al. 2004]. 2-Amino-9H-pyrido[2,3-b]indole (AαC) is by far the most abundant pyrolytic HAA formed in mainstream tobacco smoke, it occurs at amounts up to 258 ng/cigarette [Hoffmann 1998; Zhang et al. 2011]. These amounts are 25 to 100 times higher than those of 4-aminobiphenyl (4-ABP) and benzo(a)pyrene, two human carcinogens [Patrianakos and Hoffmann 1979; Zha et al. 2002]. The aminoimidazoarene HAA are formed by the Maillard reaction at temperatures generally above 150 °C [Jagerstad et al. 1991]. Aminoimidazoarene formation involves the reaction of pyridine or pyrazine, which are derived from heat degradation products of amino acids and hexoses, followed by a condensation reaction with creatinine [Murkovic 2004]. 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) are two of the most abundant aminoimidazoarenes formed in cooked meat and fish; the amounts of MeIQx and PhIP formed are highly dependent upon the type of meat, the temperature, and method of cooking [Ni et al. 2008].
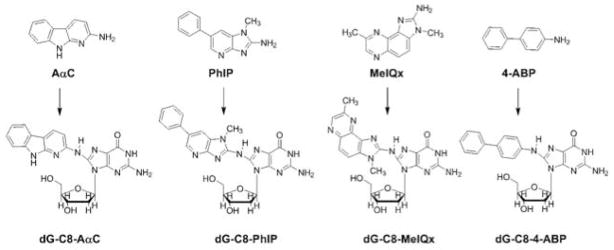
HAA require metabolic activation, which is carried out by N-oxidation of the exocyclic amine group by cytochrome P450 (CYPs) or other oxidases [Turesky and Le Marchand 2011]. The N-oxidation reaction of HAA is mainly catalyzed by CYP1A2 in the liver, and depending upon the HAA substrate, the N-oxidation reaction can be catalyzed by CYP1A1, CYP1B1, and several CYPs other than those in the Family 1 in extra-hepatic tissues [Josephy et al. 2001; Kim and Guengerich 2005; Turesky and Le Marchand 2011; Shimada et al. 2013]. CYP1A1 and CYP1B1 are the most active enzymes that carry out this reaction in extra-hepatic tissues [Kim and Guengerich 2005]. The N-hydroxylated HAA metabolites are substrates for N-acetyltransferases (NAT) or sulfotransferases (SULT), and form unstable esters [Turesky and Le Marchand 2011]. These intermediates undergo heterolytic cleavage to form the proposed reactive nitrenium/carbenium ions, which covalently bind to DNA [Turesky and Le Marchand 2011]. HAA form DNA adducts primarily at the C8 position of guanine (Figure 1) and also at lower amounts at the N2 position of guanine and N6 position of adenine [Turesky and Vouros 2004; Bessette et al. 2009].
Figure 1. Chemical structures of HAA and 4-ABP and their DNA adducts.
HAA are mutagenic in bacteria and mammalian cells, and all HAA assayed thus far are carcinogenic in animals [Sugimura et al. 2004]. DNA adducts are regarded as an important factor in chemical carcinogenesis, and DNA adducts derived from HAA are believed to induce tumor formation by producing mutations in oncogenes and tumor suppressor genes such as H-ras, p53, Apc and β-Catenin [Turesky 2002; Sugimura et al. 2004]. Among the mutations induced by HAA in these tumor-related genes or lacl or lacZ genes of transgenic mice, GC to TA transversions and GC deletions are most frequent [Sugimura et al. 2004]. In addition to liver, colon and prostate, some HAA induce cancer in lymphoid tissue and the hematopoietic system in rodents [Sugimura et al. 2004]. Some epidemiological studies have reported that a high intake of red meat is associated with an increased risk in colon and prostate cancers, but also for the development of lymphoma cancer [Zheng and Lee 2009; John et al. 2011; Ferrucci et al. 2012; Yang et al. 2015].
Human lymphocytes express several CYPs enzymes including CYP1A1 and CYP1B1, mainly in response to their activation [Siest et al. 2008]. CYP1B1 mRNA expression is barely detected in quiescent cells; however, both CYP1A1 and CYP1B1 mRNA are expressed at high levels in activated cells [Crawford et al. 1997; van Duursen et al. 2005; Prigent et al. 2014]. Human T lymphocytes are physiologically activated by a double signal that involves T cell receptor (TCR) engagement and stimulation of the CD28 receptor [Smith-Garvin et al. 2009]. In vitro these cells can be activated by anti-CD3 and anti-CD28 antibodies, which mimic physiological activation by targeting TCR and CD28 receptors [Trickett and Kwan 2003]; or by the combination of Phorbol 12-Myristate 13-Acetate and Ionomycin (PMA/Iono), which target the same signal pathways but bypass the TCR [Truneh et al. 1985].
In this study, we examine the capacity of activated primary human T lymphocytes to express functional CYP1 activity and bioactivate HAA to form DNA adducts. The level of CYP1 activity was determined, and the DNA adducts derived from the N-oxidized metabolites of PhIP, MeIQx and AαC, three major HAA present in cooked meat or tobacco smoke, were measured by ultraperformance liquid chromatography-electrospray ionization/multistage scan mass spectrometry (UPLC-ESI/MS3). DNA adducts levels were compared to those formed with 4-aminobiphenyl (4-ABP), a structurally related aromatic amine present in tobacco smoke and a recognized human bladder carcinogen [IARC 2010]. Our results demonstrate that AαC formed the highest level of DNA adducts, followed by PhIP, whereas DNA adducts of MeIQx and 4-ABP were not detected. Moreover, our results demonstrate that AαC induced CYP1A1 and CYP1B1 activity through the AhR pathway. Overall, our findings highlight the ability of activated human T lymphocytes to efficiently form DNA adducts of HAA, particularly with AαC.
Materials and Methods
Caution
AαC, 4-ABP, MeIQx, and PhIP are potential human carcinogens, and they should be handled only in a well-ventilated fume hood while wearing appropriate protective clothing.
Chemicals and Reagents
Phorbol-12-Myristate-13-Acetate (PMA), 4-ABP, ionomycin, DMSO, ethoxyresorufin, 3-methylcholanthrene (3-MC), actinomycin D (Act D), cycloheximide (CHX), 2-methyl-2H-pyrazole-3-carboxylic acid (CH-223191) and α-Naphtoflavone (α-NF) were from Sigma Aldrich (St. Louis, MO, USA). MeIQx, AαC, and PhIP were purchased from Toronto Research Chemicals (Toronto, ON, Canada). FITC- and PE-conjugated antibodies were obtained from BD Biosciences (San Jose, CA, USA). N-(2′-deoxyguanosin-8-yl)-4-aminobiphenyl (dG-C8-4-ABP), N-(2′-deoxyguanosin-8-yl)-2-amino-9H-pyrido[2,3-b]indole (dG-C8-AαC), N-(2′-deoxyguanosin-8-yl)-2-amino-1-methyl-6-phenylmidazo[4,5-b]pyridine (dG-C8-PhIP), N-(2′-deoxyguanosin-8-yl)-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (dG-C8-MeIQx) and labelled dG-C8-[2H3C]-MeIQx, [13C10]-dG-C8-4-ABP [13C10]-dG-C8-AαC, and [13C10]-dG-C8-PhIP were synthesized as previously described [Bessette et al. 2009].
Cells isolation, cultures and treatment with HAA or 4-ABP
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient centrifugation from blood buffy coats of healthy donors, provided by the Etablissement Francais du Sang (EFS). The research protocol was conducted under French legal guidelines. After separation of monocytes by 1 h adhesion step, non-adherent PBMCs were activated by PMA (20 ng/ml) and Iono (1 μM). Cells were cultured in RPMI medium (Gibco, Life technologies, Carlsbad, CA, USA) supplemented with 10% decomplemented fetal calf serum (Life technologies), penicillin (100 IU/ml), and streptomycin (100 μg/ml). After activation (72 h), two-thirds of the medium was replaced with fresh medium and the carcinogen (4- ABP (10 μM), HAA (10 μM) or 3-MC (1 μM) was added to the medium.. All the treatment and control conditions contained DMSO (0.1% v/v) final concentration. For AhR inhibition experiments, after 72 h of activation, cells were pretreated with CH-223191 or α-NF for 1 h, followed by treatment with HAA 24 h with or without inhibitors. At the end of each experiment, the cells were centrifuged at 2000 rpm for 5 min at 4 °C and stored at −80 °C for further analysis. CD3+ T cells were purified from PBMCs. The cell purification was performed by negative selection using CD3 T cell Kit (Life Technologies, Carlsbad, CA, USA). The CD3+ T cells were activated with Dynabeads T-expander beads coated with anti-CD3 and anti-CD28 antibodies (Life Technologies) at a 1:1 bead/cell ratio.
Flow cytometry immunolabeling
Cells were incubated with PBS supplemented with 5% fetal calf serum for 30 min to avoid non-specific Ab binding. After, the cells were centrifuged at 2000 rpm for 5 min. The cells were washed with PBS, and the cell pellets were re-suspended in 50 μl of PBS and incubated with specific FITC or PE conjugated mAb (CD25, CD69, CD40L, HLA-DR or CTLA-4) or the appropriate isotypic controls (PE or FITC). After 30 min, the cells were washed and analyzed by flow cytometry using a FC500 flow cytometer (Beckman Coulter, Villepinte, France).
Enzyme activity assays
Ethoxyresorufin O-deethylation (EROD) associated with CYP1 activity was measured in cultured T lymphocytes as described by Burke and Mayer [Burke et al. 1983]. Briefly, the cells were centrifuged at 2000 rpm for 2 min and washed twice with phosphate-buffered saline at 37 °C. Subsequently, salicylamide (1.5 mM) was added to block phase II-conjugation enzymes. 7-Ethoxyresorufin (3 μM) was added 1 minute later, and the fluorescence of the resorufin generated by the oxidation of ethoxyresorufin was measured every 2 min for 30 min at 37 °C. The reaction rates were linear with time and proportional to protein concentration. Cellular protein content was estimated with the Bradford procedure. The activities were expressed as pmol/min/mg protein.
Total RNA isolation and real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using the TRIzol method (Invitrogen, Life technologies, Carlsbad, CA, USA) using 3×106 cells. Total RNA (1 μg) was reverse-transcribed into cDNA using RT Applied Biosystems Kit (Foster City, CA). Real-time quantitative PCR was performed by fluorescent SYBR Green methodology using SYBR Green PCR Master Mix (Applied Biosystems, Life technologies, Carlsbad, CA, USA) and the ABI Prism 7000 detector (Applied Biosystems). The 18S ribosomal RNA subunit was used as the internal reference for all analyses. The sequences of human CYP1A1 and CYP1B1 primers were the following: CYP1A1 forward primer, 5′-AAAGATCCAGGAGGAGGAGTTAGACACA-3′; CYP1A1 reverse primer, 5′-GAGGTCTGTCAGAAAGCCGG-3′. CYP1B1 forward primer, 5′-TGGCTGCTCCTCCTCTTC-3′; CYP1B1 reverse primer, 5′-GGCTGGTCACCCATACAA-3′.
DNA isolation and enzymatic digestion for DNA adduct measurements
Cell pellets (3 × 106) were homogenized in 400 μl of TE buffer (50 mM Tris-HCl containing 10 mM EDTA and 10 mM β-mercaptoethanol, pH 8.0) and incubated with RNase T1 (318.75 U) and RNase A (20 μg) for 30 min at 37 °C. After, proteinase K (200 μg) and SDS (0.05% final concentration) were added and the homogenate was incubated for 1 h at 37 °C. The DNA was processed with the phenol/chloroform method and precipitated with ethanol. DNA was re-suspended in 200 μl of sterile water and quantified with a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific). Each DNA sample (3 μg) was spiked with isotopically labeled internal standards at a level of 1 adduct per 107 bases. DNA from the separately treated 4-ABP- and HAA-treated T lymphocytes samples were then pooled. DNA digestion was performed in 5 mM Bis-Tris-HCl buffer (pH 7.1) as previously described [Nauwelaers et al. 2011]. Briefly, DNA (3 μg) was incubated with DNase I and Nuclease P1 at 37 °C for 3.5 h, followed by incubation with alkaline phosphatase and phosphodiesterase at 37 °C for 18 h. Thereafter, samples were evaporated to dryness by vacuum centrifugation. The DNA digest was re-suspended in 30 μl of 1:1 water/DMSO and sonicated for 5 min. Samples were then centrifuged for 5 min at 22000 g, and the supernatants were transferred to LC vials.
Ultraperformance liquid chromatography-electrospray ionization multistage scan mass spectrometry (UPLC-ESI-MS3) measurement of DNA adducts
The measurement of DNA adducts was performed by UPLC-ESI/MS3 with a NanoAcquity UPLC system (Waters Corp., New Milford, MA) equipped with a Waters Symmetry trap column (180 μm×20 mm, 5 μm particle size), a Michrom C18 AQ column (0.3 mm×150 mm, 3 μm particle size), and a Michrom Captive Spray source interfaced with a linear quadrupole ion trap mass spectrometer LTQ Velos (Thermo Fisher, San Jose, CA). The chromatography conditions and MS parameters were described previously (Nauwelaers et al. 2011). The protonated ions of the DNA adducts were monitored at the MS3 scan stage employing the following transitions: dG-C8-MeIQx (m/z 479.1 > 363.1 > 239.2, 318.4, 346.4) and dG-C8-[2H3C]-C8-MeIQx (m/z 482.1 > 366.1 > 242.2, 321.5, 349.5); dG-C8-AαC (m/z 449.1 > 333.1 > 209.2, 291.4, 316.4) and [13C10]-dG-C8-AαC (m/z 459.1 > 338.1 > 210.2, 295.5, 321.5); dG-C8-PhIP (m/z 490.1 > 374.1 > 250.2, 329.2, 357.2) and [13C10]-dG-C8-PhIP (m/z 400.1 > 379.1 > 251.2, 333.3, 362.2); and dG-C8-4-ABP (m/z 435.1 > 319.1 > 249.2, 277.2, 302.2) and [13C10]-dG-C8-4-ABP (m/z 445.1 > 324.1 >252.2, 281.2, 307.2). External calibration curves were constructed for quantification [Goodenough et al. 2007]. The limit of quantification was 3 adducts per 109 DNA bases [Gu et al. 2012].
Results
Activation of human T lymphocytes induces CYP1 activity
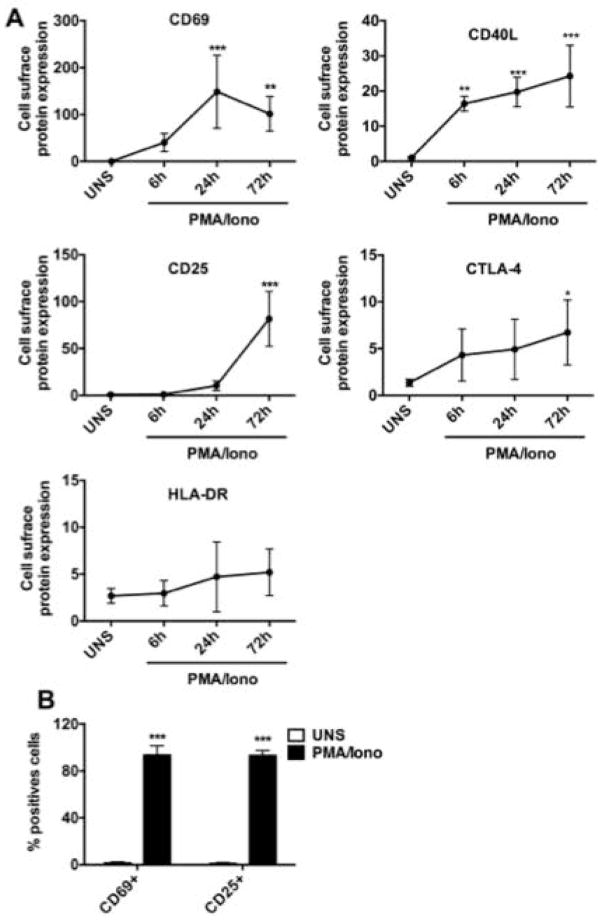
We first verified that PMA/Iono mainly activated T lymphocytes. As shown in Figure 2A, PMA/Iono rapidly induced CD69 and CD40L, which are cell surface proteins known to be early biomarkers of human T lymphocyte activation [Castle et al. 1993; Simms and Ellis 1996]. The CD69 expression reached a maximum after 24 h, and then decreased between 24 and 72 h. CD40L was also rapidly increased within 6 h of activation and remained highly expressed for the 72 h. In addition to these early markers, we also analyzed the expression of CD25 (IL-2Rα), CTLA-4 and HLA-DR, which are recognized as late-activation markers [Rea et al. 1999]. The cell surface expression level of CD25 is highly expressed at 72 h. Cell surface expression of CTLA-4 is detected in unstimulated cells at a very low level and increased gradually at 72 h in the presence of PMA/Iono. Both the early and late activation markers’ kinetics are similar to those observed with TCR/co-stimulation lymphocytes activation. Taken together, our data demonstrate that PMA/Iono, lead to an efficient activation of T lymphocytes. Figure 2B shows that after 72 h, more than 90% of cells are positive for the expression of CD25 and CD69, which demonstrates that the large majority of cells are activated human T lymphocytes.
Figure 2. Efficient activation of human T lymphocytes by PMA/Iono treatment.
(A) Kinetics of human T lymphocytes activation markers in PMA/Iono activated human T lymphocytes. Cells were either unstimulated (UNS) or activated with PMA/Iono for 6, 24, and 72 h. Cell surface expression of CD25, CD40L, CD69, HLA-DR and CTLA-4 was measured by flow cytometry. Data are expressed as a ratio relative to the mean fluorescence intensity of the unstimulated cells, arbitrarily set at 1. (B) Cells were either unstimulated (UNS) or activated with PMA/Iono for 72 h and cell surface expression of CD25 and CD69 was measured by flow cytometry. Data are representative of five independent experiments and shown as mean ± SD. (ANOVA followed by Dunnett’s multi-range test, * P<0.05; **P<0.01, ***P<0.005 versus UNS)
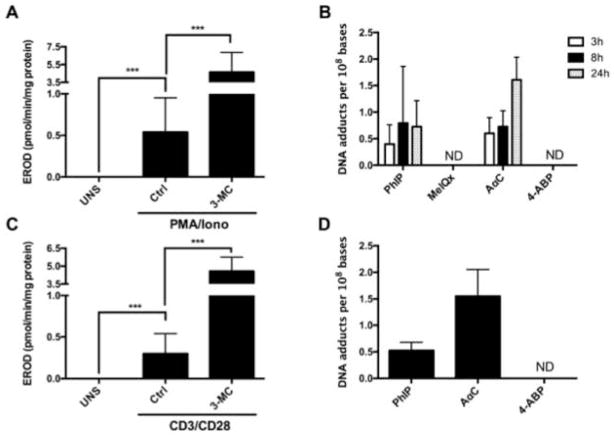
CYP1 family plays a crucial role in the bioactivation of procarcinogens, as this family is responsible for the bioactivation of more than 40% of known procarcinogens [Rendic and Guengerich 2012]. We examined the enzymatic activity of CYP1 in activated human T lymphocytes by measuring EROD activity in unstimulated and PMA/Iono activated human T lymphocytes. In unstimulated cells, CYP1 activity was not detectable (limit of detection: 0.2 pmol/min/mg protein). After 72 h of activation with PMA/Iono, EROD activity increased and reached an average activity of 0.54 ± 0.32 pmol/min/mg protein; however, the activity varied substantially among the individuals and ranged from 0.23 to 1.28 pmol/min/mg protein. Treatment of PMA/Iono activated lymphocytes with 3-MC, a well-known CYP1 inducer [Burke and Mayer 1983] resulted in statistically significant induction of EROD activity that was ~4 to 5 times higher from that of PMA/Iono activated lymphocytes (Figure 3A). This is the first report showing functional CYP1 activity in PMA/Iono activated lymphocytes.
Figure 3. Formation of HAA derived DNA adducts and CYP1 activity in activated human T lymphocytes.
(A) CYP1 activity in PMA/Iono activated human T lymphocytes.. After 72 h of activation with PMA/Iono, the cells were treated with 0.1% DMSO (Ctrl) or with 1 μM of 3-MC for 24 h and CYP1 activity was measured. Data are expressed as the mean ± SD of eight independent experiments. (Student’s t-test, * P<0.05; **P<0.01, ***P<0.005). ND: not detectable (B) Kinetics of 4-ABP and HAA DNA adduct formation in PMA/Iono activated human T lymphocytes. After 72 h of activation with PMA/Iono, the cells were treated with PhIP, MeIQx, AαC or 4-ABP (10 μM) for 3, 8 or 24 h, and the DNA adducts were measured by UPLC/MS3. Data are representative of five donors; the DNA adduct level was measured in duplicate for each donor. Data are expressed as Mean ± SD. (C) CYP1 activity in CD3/CD28 activated human T lymphocytes. CD3+ T cells were either unstimulated (UNS) or activated with anti-CD3 and anti-CD28 Antibodies for 72 h and CYP1 activity was measured after 24 h of treatment with 0.1% DMSO (Ctrl) or 3-MC (1 μM). Data are expressed as the mean ± SD of four independent experiments. (Student’s t-test, * P<0.05; **P<0.01, ***P<0.005). (D) Levels of DNA adducts derived from 4-ABP, AαC and PhIP in CD3/CD28 activated human T lymphocytes. CD3+ T cells were activated with anti-CD3 and anti-CD28 antibody for 72 h, and the level of DNA adducts were measured after 24 h of treatment with PhIP, AαC or 4-ABP (10 μM). DNA adducts levels were measured in duplicate for each donor and are expressed as the mean ± SD. The data are representative of four independent donors. ND: not detectable
HAA form DNA adducts in activated human T lymphocytes
Based on functional CYP1 activity data, we investigated the bioactivation of PhIP, MeIQx, AαC, and 4-ABP in PMA/Iono activated human T lymphocytes, by measurement of DNA adducts after 3, 8 and 24 h of cell treatment with procarcinogens (10 μM).. As illustrated in Figure 3B, DNA adducts were detected after treatment with AαC and PhIP, whereas DNA adducts were not detected with MeIQx or 4-ABP. The limit of quantification was 3 adducts per 109 DNA bases, when 1 μg of DNA was assayed on-column. The amount of AαC adducts increased over the 24 hour time period and adduct levels exceeded those formed with PhIP. A representative UPLC-ESI/MS3 chromatogram and product ion spectra of DNA adduct are shown in supplementary data (Supplementary Figure S-1).
Interestingly, we show that treatment with anti-CD3/CD28 antibodies allowing human T lymphocytes activation through TCR leads to not only the expression of functional CYP1 activity, but also the formation of DNA adducts at similar levels to those observed in PMA/Iono activated T human lymphocytes (Figure 3C and 3D). Thus, bypassing TCR and CD28 receptors by PMA/Iono treatment did not influence ability of activated human T lymphocytes to biotransform HAA to reactive intermediates. Therefore, the activation of lymphocytes with PMA/Iono was performed for the remaining experiments of the present study.
AαC induces mRNA expression and CYP1A activity in human T lymphocytes
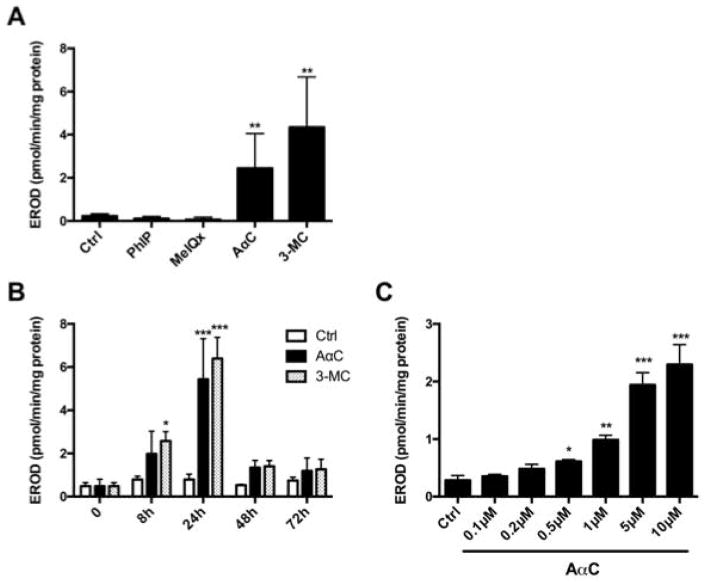
Several carcinogens are known to serve as both substrates and inducers of CYPs [Shimada et al. 2002]. As AαC formed high levels of DNA adducts, we investigated its potential to induce CYP1 activity in PMA/Iono activated human T lymphocytes. Following 24 h of treatment with AαC (10 μM), there was a significant induction of EROD activity, signifying that AαC significantly induces CYP1 activity. In contrast, PhIP and MeIQx had no effect on CYP1 activity. The level of CYP1 induced by AαC is comparable to that observed with the potent CYP1 inducer 3-MC (Figure 4A). Similar to 3-MC, AαC induced CYP1 within 8 h of treatment and the level of induction reached a maximum at 24 h, and then rapidly decreased within the ensuing 24 h of treatment (Figure 4B). AαC significantly induced CYP1 activity in a dose-dependent manner in lymphocytes treated with AαC at concentrations above 0.5 μM (Figure 4C).
Figure 4. Modulation of CYP1A activities in activated human T lymphocytes.
(A) Effect of HAA on CYP1 activity in activated human lymphocytes. CYP1 activity was measured by EROD activity in PMA/Iono activated human T lymphocytes after 24 h of treatment with 0.1% DMSO (Ctrl), HAA (10 μM) or 3-MC (1 μM). Data are expressed as the mean ± SD of seven independent experiments. (Student’s t-test, * P<0.05; **P<0.01, ***P<0.005 versus Ctrl). (B) Time-dependent effect of AαC on CYP1 activity in activated human T lymphocytes. CYP1 activity was measured by EROD activity in PMA/Iono activated human T lymphocytes after 0, 8, 24 and 72 h of treatment with 0.1% DMSO (Ctrl), AαC (10 μM) or 3-MC (1 μM). Data are expressed as the mean ± SD of four independent experiments (ANOVA followed by Tukey’s multiple comparison test, * P<0.05; **P<0.01, ***P<0.005 versus Ctrl). (C) Effect of different concentrations of AαC on CYP1 activity in activated human T lymphocytes. CYP1 activity was measured by EROD activity in PMA/Iono activated human T lymphocytes after 24 h of treatment with different concentrations of AαC (0.1 – 10 μM). Data are expressed as the mean ± SD of four independent experiments. (Student’s t-test, * P<0.05; **P<0.01, ***P<0.005 versus Ctrl).
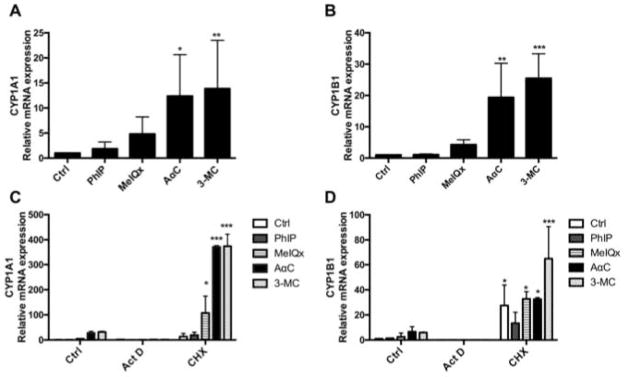
To determine whether AαC induced CYP1 activity is linked to an increase of mRNA expression, the levels of expression of CYP1A1 and CYP1B1 mRNA were measured by qPCR. As shown in Figure 5A and 5B, AαC significantly induced CYP1A1 and CYP1B1 mRNA levels, whereas MeIQx and PhIP did not. Indeed, CYP1A1 and CYP1B1 mRNA levels were 20 to 30-times higher after treatment of PMA/Iono activated human T lymphocytes with AαC for 24 h than those levels measured in control cells. This level of induction is comparable to that observed with 3-MC. In order to determine if AαC mediates the induction of CYP1A1 and CYP1B1 mRNA through a transcriptional mechanism, we blocked the transcription by Act D, a well-known transcription inhibitor [Perry and Kelley 1970]. Our results demonstrate that blockage of transcription by Act D completely suppressed the AαC-mediated induction of CYP1A1 and CYP1B1 mRNA levels. The treatment with CHX, a protein synthesis inhibitor [Obrig et al. 1971], leads to a super-induction of both CYP1A1 and CYP1B1 mRNA (Figure 5C and 5D). This finding is in agreement with previous reports showing that protein synthesis inhibition by CHX leads to a superinduction of CYP1A1 and CYP1B1 mRNA levels after TCDD treatment in various human tumor cell lines [Lusska et al. 1992]. In total, our data demonstrate that AαC regulates CYP1A1 and CYP1B1 activities through a transcriptional mechanism, which requires an active protein synthesis.
Figure 5. Regulation of CYP1A1 and CYP1B1 mRNA expression in activated human T lymphocytes by HAA.
Regulation of (A) CYP1A1 and (B) CYP1B1 mRNA expression by HAA in activated human T lymphocytes. PMA/Iono activated human T lymphocytes were treated for 24 h with 0.1% DMSO (Ctrl), HAA (10 μM) or 3-MC (1 μM) and CYP1A1 and CYP1B1 mRNA expression were analyzed by qRT-PCR. The levels of expression were normalized with 18s mRNA. Data are representative of five independent experiments and expressed as the mean ± SD. (ANOVA followed by Dunnett’s multi-range test, * P<0.05; **P<0.01, ***P<0.005 versus Ctrl). Effect of Actinomycin D (Act D) and Cycloheximide (CHX) on AαC induced (C) CYP1A1 and (D) CYP1B1 mRNA expression in activated human T lymphocytes. PMA/Iono activated human T lymphocytes were treated with PhiP, MeIQx and AαC (10 μM) or 3-MC (1 μM) for 16 h with or without Act D or CHX and the levels of CYP1A1 and CYP1B1 mRNA expression were analyzed by qRT-PCR. The data were normalized with 18s mRNA. Data are representative of three independent experiments and expressed as the mean ± SD. (ANOVA followed by Tukey’s multi-range test, * P<0.05; **P<0.01, ***P<0.005 versus Ctrl). Each treatment from Act D and CHX group has been compared to its corresponding treatment in the control group.
HAA derived DNA adduct formation is AhR dependent
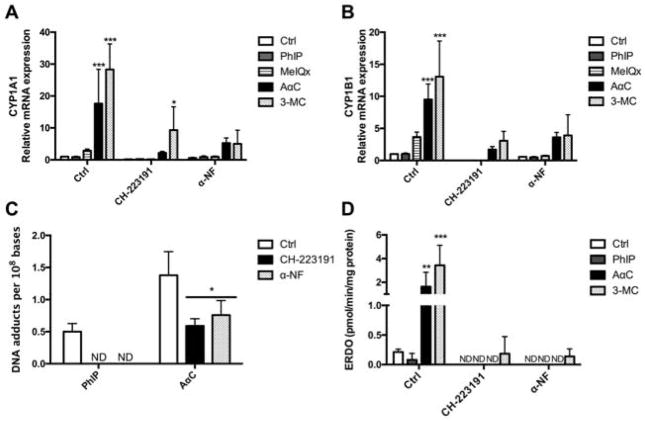
It is well established that the expression CYP1 family members is modulated through aryl hydrocarbon receptor (AhR) pathway [Nebert et al. 2004]. In order to determine whether AhR is involved in AαC-mediated CYP1 induction, we pre-treated cells with CH-223191 or α-Naphthoflavone (α-NF), two AhR inhibitors [Kim et al. 2006], prior to treatment with AαC, PhIP, MeIQx, or 3-MC. CH-223191 and α-NF are ligand-selective antagonists of AhR that exerts their antagonistic effect through direct competition for AhR binding [Merchant et al. 1993; Zhao et al. 2010]. Our results show that AαC-mediated increase in CYP1A1 and CYP1B1 mRNA expression was significantly reduced when cells were treated with either of these two AhR inhibitors (Figure 6A and 6B). We further evaluated the contribution of AhR pathway to the bioactivation of AαC and PhIP, by measuring DNA adduct formation in activated human T lymphocytes pre-treated or not with the two AhR inhibitors: CH-223191 and α-NF. As shown in Figure 6C, the inhibition of AhR leads to a significant decrease of the formation of DNA adducts derived from AαC and PhIP. These results are correlated with the significant inhibition of constitutive and induced EROD activity (Figure 6D). The findings reveal that AhR is involved in AαC-mediated increase of CYP1A1 and CYP1B1 mRNA expression and the ensuing HAA DNA adducts formed in activated human T lymphocytes.
Figure 6. Effect of CH-223191 and α-Naphtoflavone (α-NF), two AhR antagonists on PhIP and AαC derived DNA adducts formation and CYP1 activity in activated human T lymphocytes.
Effect of CH-223191 and α-NF on AαC induced (A) CYP1A1 and (B) CYP1B1 mRNA expression in activated human T lymphocytes. PMA/Iono activated human T lymphocytes were treated for 1 hour with 0.1% DMSO (Ctrl), CH-223191 (5 μM) or α-NF (5 μM), followed by 24 h of treatment with 0.1% DMSO (Ctrl), HAA (10 μM) or 3-MC (1 μM). The levels of CYP1A1 and CYP1B1 mRNA expression were analyzed by qRT-PCR and normalized with 18s mRNA. Data are representative of three independent experiments and expressed as the mean ± SD. (ANOVA followed by Dunnett’s multirange test, * P<0.05; **P<0.01, ***P<0.005, versus Ctrl). Each treatment from CH-223191 and α-NF group has been compared to its corresponding treatment in the control group. (C) Effect of CH-223191 and α-NF on DNA adducts derived from AαC and PhIP formation in activated human T lymphocytes. PMA/Iono activated human T lymphocytes were pre-treated for 1 hour with 0.1 % DMSO (Ctrl), CH-223191 (5 μM) or α-NF (5 μM) followed by 24 h of treatment with 0.1% DMSO (Ctrl) or HAA (10 μM). The DNA adducts were measured by UPLC/MS3. DNA adducts were measured in duplicate for each donor and expressed as the mean ± SD. (ANOVA followed by Tukey’s multiple comparison test * P<0.05; **P<0.01, ***P<0.005, versus Ctrl). ND: not detectable. (D) Effect of CH-223191 and α-NF on CYP1 activity in activated human T lymphocytes. CYP1 activity was measured in PMA/Iono activated human T lymphocytes treated for 1 hour with 0.1 % DMSO (Ctrl), CH-223191 (5 μM) or α-NF (5 μM) followed by 24 h of treatment with 0.1% DMSO (Ctrl), PhIP (10 μM), AαC (10 μM) or 3-MC (1 μM). Data are representative of three independent experiments and expressed as the mean ± SD. (ANOVA followed by Tukey’s multiple comparison test, * P<0.05; **P<0.01, ***P<0.005 versus Ctrl). ND: not detectable.
Discussion
Certain HAA induce cancer in blood vessels, lymphoid tissue and the hematopoietic system, in addition to the liver, colon and prostate [Sugimura et al. 2004]. HAA require metabolic activation, mainly catalyzed by CYPs, to form DNA adducts which can induce mutations and lead to tumorigenesis. Thus, we proposed that lymphocytes that can express CYPs may be capable of bioactivating HAA. To investigate this hypothesis, we assessed the ability of activated human T lymphocytes in vitro to bioactivate HAA and form DNA adducts.
We have shown that the activation of human T lymphocytes results in the increase of both expression and activity of CYP1A1 and CYP1B1, two enzymes playing a critical role in the bioactivation of procarcinogens [Rendic and Guengerich 2012]. These results are in accordance with previous studies showing that CYP1A1 and CYP1B1 expression is dependent on the activation state of the lymphocytes [Siest et al. 2008]. Indeed, while only CYP1B1 mRNA is barely detectable in unstimulated cells, both CYP1A1 and CYP1B1 mRNA are induced in PMA/Iono activated human leukocyte, CD3/CD28 activated human T lymphocytes and phytohemaglutinin (PHA) stimulated human lymphocytes [Crawford et al. 1997; van Duursen et al. 2005; Prigent et al. 2014]. PHA treatment is not regarded as a physiologically relevant activation mechanism since this lectin is a non-specific mitogen that does not trigger TCR engagement and absolutely requires an accessory signal to fully activate human T lymphocytes [Ceuppens et al. 1988; Jungo et al. 2001]. In our study, we demonstrate for the first time, that human T lymphocytes activated by PMA/Iono or CD3/CD28; two different and independent mechanisms that mimic physiological activation, express detectable CYP1 activity as measured using a specific substrate the ethoxyresorufin.
Furthermore, we have demonstrated that DNA adducts are formed by AαC and PhIP in activated human T lymphocytes at levels reaching 10 to 20 adducts per 109 DNA bases for AαC and 4 to 6 adducts per 109 DNA bases for PhIP. These levels of DNA adducts are about 100 times lower than those formed in human hepatocytes treated with the same concentration of HAA [Nauwelaers et al. 2011]. The lower levels of DNA adducts in lymphocytes can be explained by the ~hundred-fold lower mRNA levels of CYPs in human T lymphocytes compared to the liver [Siest et al. 2008], as well as by the absence of CYP1A2 activity in activated human T lymphocytes (data not shown). Indeed, CYP1A2 is the predominant enzyme involved in the bioactivation of HAA in human hepatocytes [Langouet et al. 2001; Langouet et al. 2002] and also in vivo [Boobis et al. 1994]. Previous reports on the expression of CYP1A2 mRNA in human lymphocytes T are variable and range from non-detectable [Spatzenegger et al. 2000] to barely detectable [Haas et al. 2005]. The lack of CYP1A2 activity observed in our study suggests that even if CYP1A2 mRNA is expressed in activated human T lymphocytes, the level is too low to detect enzyme activity. In addition to CYP1A2, AαC can be metabolized at lower rates by recombinant human CYP1A1 and CYP2C10 expressed in E. coli. [Raza et al. 1996], while PhIP can be bioactivated by CYP1A1 and CYP1B1 [Shimada et al. 1996; Hammons et al. 1997]. There is no data reported in the literature on the CYP2C10 expression in human lymphocytes. Therefore, our data suggests that the expression of CYP1A1 and CYP1B1 (and potentially CYP2C10) could lead to the bioactivation and formation of AαC and PhIP-derived DNA adducts in activated human T lymphocytes. In contrast to AαC and PhIP, DNA adducts of MeIQx and 4-ABP were not detected in activated human T lymphocytes. The absence of DNA adducts can be explained by the lack of expression of CYP1A2 in those cells and by the fact that both MeIQx and 4-ABP are poor substrates for CYP1A1 and CYP1B [Shimada et al. 1996].
It has been reported that human lymphocytes express SULT enzymes activities [Hoelzl et al. 2008], which play a crucial role in the formation of HAA derived DNA adducts [Turesky and Le Marchand 2011]. Further studies are needed to identify the specific human CYPs, NAT and SULT enzymes responsible for AαC and PhIP bioactivation in lymphocytes.
The highest level of DNA adducts formed in activated human T lymphocytes treated with HAA or 4-ABP occurred with AαC. It is noteworthy that adduct formation of AαC in human hepatocytes was also greater than that observed with other HAA but comparable to adduct levels formed with 4-ABP [Nauwelaers et al. 2011; Nauwelaers et al. 2013]. The high level of DNA binding of AαC is in part attributed to the ability of AαC to induce CYP1 enzymes in human T lymphocytes. This is the first study to report an induction of CYP1 in human T lymphocytes by an HAA.
The induction of CYP1A1 and CYP1B1 by AαC occurs through a transcriptional mechanism that involves the AhR pathway. This regulation shows similarities to previous studies reported on the regulation of CYP1A1 and CYP1B1 by other carcinogens such as benzo(a)pyrene and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin) [Harrigan et al. 2004]. However, the regulation of CYP1A and CYP1B by AαC seems to be tissue specific, as no significant CYP1 induction occurred in primary human hepatocytes treated with AαC (unpublished data. S. Langouët). The inhibition of AhR pathway, leads to a total inhibition of CYP activity and a complete inhibition of PhIP derived DNA adducts formation demonstrating the critical role of CYP1A1 and CYP1B1 enzymes in PhIP bioactivation in activated human T lymphocytes. In contrast, the total inhibition of CYP1 activity leads to a significant decrease but not to a total inhibition of AαC-derived DNA adduct formation. This result implicates other CYP enzymes or other non-CYP enzymes in the bioactivation of AαC in activated human T lymphocytes. Indeed, AαC is bioactivated by oxidase other than the CYPs since AαC derived DNA adducts were detected in the liver of P-450 reductase null mice [Turesky et al. 2015].
In conclusion, our data demonstrate that AαC undergoes efficient bioactivation in activated human T lymphocytes to form DNA adducts, and that AαC induces CYP1A1 and CYP1B1 expression and activity through AhR pathway. The up-regulation of CYP1A1 and CYP1B1 by AαC is of utmost interest and highlights the potential of AαC to increase the capacity of activated human T lymphocytes to bioactivate other HAA or other procarcinogens. Potential CYP1 induction caused by AαC or other constituents in tobacco must be taken into account when evaluating the health risk of AαC or other pro-carcinogens.
Supplementary Material
Acknowledgments
This work was supported by Institut National de la Santé et de la Recherche Médicale (Inserm) (SL), la Ligue contre le Cancer du Grand Ouest (SL), PNREST Anses Cancer TMOI AVIESAN [2013/1/166] (SL), in part by the National Cancer Institute [R01 CA122320, R01 CA134700] (R.J.T) and in part by National Cancer Institute Cancer Center Support [CA077598] (R.J.T). MB was a recipient of Anses and UFR Pharmacie of University of Rennes 1 as well as travel fellowships from the Université Européenne de Bretagne (UEB).
Footnotes
Statement of Author Contribution
SL designed and conceptualized the study. MB performed most of the experiments with the help of LLH. RJT set-up, analyzed and interpreted all mass spectrometry data. MB prepared the first manuscript draft with the intellectual input of LV and GB. MB performed statistical analyses. RJT and SL finalized the manuscript. All authors read and approved the final manuscript.
References
- Bessette EE, Goodenough AK, Langouet S, Yasa I, Kozekov ID, Spivack SD, Turesky RJ. Screening for DNA adducts by data-dependent constant neutral loss-triple stage mass spectrometry with a linear quadrupole ion trap mass spectrometer. Anal Chem. 2009;81:809–819. doi: 10.1021/ac802096p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boobis AR, Lynch AM, Murray S, de la Torre R, Solans A, Farre M, Segura J, Gooderham NJ, Davies DS. CYP1A2-catalyzed conversion of dietary heterocyclic amines to their proximate carcinogens is their major route of metabolism in humans. Cancer Res. 1994;54:89–94. [PubMed] [Google Scholar]
- Burke MD, Mayer RT. Differential effects of phenobarbitone and 3-methylcholanthrene induction on the hepatic microsomal metabolism and cytochrome P-450-binding of phenoxazone and a homologous series of its n-alkyl ethers (alkoxyresorufins) Chem Biol Interact. 1983;45:243–258. doi: 10.1016/0009-2797(83)90072-8. [DOI] [PubMed] [Google Scholar]
- Burke MD, Murray GI, Lees GM. Fluorescence-microscopic measurement of intracellular cytochrome P-450 enzyme activity (ethoxyresorufin O-de-ethylation) in unfixed liver section. Biochem J. 1983;212:15–24. doi: 10.1042/bj2120015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle BE, Kishimoto K, Stearns C, Brown ML, Kehry MR. Regulation of expression of the ligand for CD40 on T helper lymphocytes. J Immunol. 1993;151:1777–1788. [PubMed] [Google Scholar]
- Ceuppens JL, Baroja ML, Lorre K, Van Damme J, Billiau A. Human T cell activation with phytohemagglutinin. The function of IL-6 as an accessory signal. J Immunol. 1988;141:3868–3874. [PubMed] [Google Scholar]
- Crawford RB, Holsapple MP, Kaminski NE. Leukocyte activation induces aryl hydrocarbon receptor up-regulation, DNA binding, and increased Cyp1a1 expression in the absence of exogenous ligand. Mol Pharmacol. 1997;52:921–927. doi: 10.1124/mol.52.6.921. [DOI] [PubMed] [Google Scholar]
- Ferrucci LM, Sinha R, Huang WY, Berndt SI, Katki HA, Schoen RE, Hayes RB, Cross AJ. Meat consumption and the risk of incident distal colon and rectal adenoma. Br J Cancer. 2012;106:608–616. doi: 10.1038/bjc.2011.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough AK, Schut HA, Turesky RJ. Novel LC-ESI/MS/MS(n) method for the characterization and quantification of 2′-deoxyguanosine adducts of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by 2-D linear quadrupole ion trap mass spectrometry. Chem Res Toxicol. 2007;20:263–276. doi: 10.1021/tx0601713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu D, Turesky RJ, Tao Y, Langouet SA, Nauwelaers GC, Yuan JM, Yee D, Yu MC. DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and 4-aminobiphenyl are infrequently detected in human mammary tissue by liquid chromatography/tandem mass spectrometry. Carcinogenesis. 2012;33:124–130. doi: 10.1093/carcin/bgr252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas CE, Brazeau D, Cloen D, Booker BM, Frerichs V, Zaranek C, Frye RF, Kufel T. Cytochrome P450 mRNA expression in peripheral blood lymphocytes as a predictor of enzyme induction. Eur J Clin Pharmacol. 2005;61:583–593. doi: 10.1007/s00228-005-0971-0. [DOI] [PubMed] [Google Scholar]
- Hammons GJ, Milton D, Stepps K, Guengerich FP, Tukey RH, Kadlubar FF. Metabolism of carcinogenic heterocyclic and aromatic amines by recombinant human cytochrome P450 enzymes. Carcinogenesis. 1997;18:851–854. doi: 10.1093/carcin/18.4.851. [DOI] [PubMed] [Google Scholar]
- Harrigan JA, Vezina CM, McGarrigle BP, Ersing N, Box HC, Maccubbin AE, Olson JR. DNA adduct formation in precision-cut rat liver and lung slices exposed to benzo[a]pyrene. Toxicol Sci. 2004;77:307–314. doi: 10.1093/toxsci/kfh030. [DOI] [PubMed] [Google Scholar]
- Hoelzl C, Glatt H, Meinl W, Sontag G, Haidinger G, Kundi M, Simic T, Chakraborty A, Bichler J, Ferk F, Angelis K, Nersesyan A, Knasmuller S. Consumption of Brussels sprouts protects peripheral human lymphocytes against 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and oxidative DNA-damage: results of a controlled human intervention trial. Mol Nutr Food Res. 2008;52:330–341. doi: 10.1002/mnfr.200700406. [DOI] [PubMed] [Google Scholar]
- Hoffmann DHI. Letters to the editor, tobacco smoke components. Beiträge zur Tabakforschung International/Contributions to Tobacco Research. 1998:18. [Google Scholar]
- IARC. Some aromatic amines, organic dyes, and related exposures. IARC Monogr Eval Carcinog Risks Hum. 2010;99:1–658. [PMC free article] [PubMed] [Google Scholar]
- IARC. Chemical agents and related occupations. IARC Monogr Eval Carcinog Risks Hum. 2012;100:9–562. [PMC free article] [PubMed] [Google Scholar]
- Jagerstad M, Skog K, Grivas S, Olsson K. Formation of heterocyclic amines using model systems. Mutat Res. 1991;259:219–233. doi: 10.1016/0165-1218(91)90119-7. [DOI] [PubMed] [Google Scholar]
- John EM, Stern MC, Sinha R, Koo J. Meat consumption, cooking practices, meat mutagens, and risk of prostate cancer. Nutr Cancer. 2011;63:525–537. doi: 10.1080/01635581.2011.539311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephy PD, Batty SM, Boverhof DR. Recombinant human P450 forms 1A1, 1A2, and 1B1 catalyze the bioactivation of heterocyclic amine mutagens in Escherichia coli lacZ strains. Environ Mol Mutagen. 2001;38:12–18. doi: 10.1002/em.1045. [DOI] [PubMed] [Google Scholar]
- Jungo F, Dayer JM, Modoux C, Hyka N, Burger D. IFN-beta inhibits the ability of T lymphocytes to induce TNF-alpha and IL-1beta production in monocytes upon direct cell-cell contact. Cytokine. 2001;14:272–282. doi: 10.1006/cyto.2001.0884. [DOI] [PubMed] [Google Scholar]
- Kim D, Guengerich FP. Cytochrome P450 activation of arylamines and heterocyclic amines. Annu Rev Pharmacol Toxicol. 2005;45:27–49. doi: 10.1146/annurev.pharmtox.45.120403.100010. [DOI] [PubMed] [Google Scholar]
- Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS, Lee TG, Kang JK, Gasiewicz TA, Ryu SH, Suh PG. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol. 2006;69:1871–1878. doi: 10.1124/mol.105.021832. [DOI] [PubMed] [Google Scholar]
- Langouet S, Paehler A, Welti DH, Kerriguy N, Guillouzo A, Turesky RJ. Differential metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in rat and human hepatocytes. Carcinogenesis. 2002;23:115–122. doi: 10.1093/carcin/23.1.115. [DOI] [PubMed] [Google Scholar]
- Langouet S, Welti DH, Kerriguy N, Fay LB, Huynh-Ba T, Markovic J, Guengerich FP, Guillouzo A, Turesky RJ. Metabolism of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in human hepatocytes: 2-amino-3-methylimidazo[4,5-f]quinoxaline-8-carboxylic acid is a major detoxification pathway catalyzed by cytochrome P450 1A2. Chem Res Toxicol. 2001;14:211–221. doi: 10.1021/tx000176e. [DOI] [PubMed] [Google Scholar]
- Lusska A, Wu L, Whitlock JP., Jr Superinduction of CYP1A1 transcription by cycloheximide. Role of the DNA binding site for the liganded Ah receptor. J Biol Chem. 1992;267:15146–15151. [PubMed] [Google Scholar]
- Manabe S, Kurihara N, Wada O, Izumikawa S, Asakuno K, Morita M. Detection of a carcinogen, 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine, in airborne particles and diesel-exhaust particles. Environ Pollut. 1993;80:281–286. doi: 10.1016/0269-7491(93)90049-t. [DOI] [PubMed] [Google Scholar]
- Merchant M, Krishnan V, Safe S. Mechanism of action of alpha-naphthoflavone as an Ah receptor antagonist in MCF-7 human breast cancer cells. Toxicol Appl Pharmacol. 1993;120:179–185. doi: 10.1006/taap.1993.1101. [DOI] [PubMed] [Google Scholar]
- Murkovic M. Formation of heterocyclic aromatic amines in model systems. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802:3–10. doi: 10.1016/j.jchromb.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Nauwelaers G, Bellamri M, Fessard V, Turesky RJ, Langouet S. DNA adducts of the tobacco carcinogens 2-amino-9H-pyrido[2,3-b]indole and 4-aminobiphenyl are formed at environmental exposure levels and persist in human hepatocytes. Chem Res Toxicol. 2013;26:1367–1377. doi: 10.1021/tx4002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauwelaers G, Bessette EE, Gu D, Tang Y, Rageul J, Fessard V, Yuan JM, Yu MC, Langouet S, Turesky RJ. DNA adduct formation of 4-aminobiphenyl and heterocyclic aromatic amines in human hepatocytes. Chem Res Toxicol. 2011;24:913–925. doi: 10.1021/tx200091y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- Ni W, McNaughton L, LeMaster DM, Sinha R, Turesky RJ. Quantitation of 13 heterocyclic aromatic amines in cooked beef, pork, and chicken by liquid chromatography-electrospray ionization/tandem mass spectrometry. J Agric Food Chem. 2008;56:68–78. doi: 10.1021/jf072461a. [DOI] [PubMed] [Google Scholar]
- Obrig TG, Culp WJ, McKeehan WL, Hardesty B. The mechanism by which cycloheximide and related glutarimide antibiotics inhibit peptide synthesis on reticulocyte ribosomes. J Biol Chem. 1971;246:174–181. [PubMed] [Google Scholar]
- Patrianakos C, Hoffmann D. Chemical Studies on Tobacco-Smoke. 64. Analysis of Aromatic-Amines in Cigarette-Smoke. Journal of Analytical Toxicology. 1979;3:150–154. [Google Scholar]
- Perry RP, Kelley DE. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol. 1970;76:127–139. doi: 10.1002/jcp.1040760202. [DOI] [PubMed] [Google Scholar]
- Prigent L, Robineau M, Jouneau S, Morzadec C, Louarn L, Vernhet L, Fardel O, Sparfel L. The aryl hydrocarbon receptor is functionally upregulated early in the course of human T-cell activation. Eur J Immunol. 2014;44:1330–1340. doi: 10.1002/eji.201343920. [DOI] [PubMed] [Google Scholar]
- Raza H, King RS, Squires RB, Guengerich FP, Miller DW, Freeman JP, Lang NP, Kadlubar FF. Metabolism of 2-amino-alpha-carboline. A food-borne heterocyclic amine mutagen and carcinogen by human and rodent liver microsomes and by human cytochrome P4501A2. Drug Metab Dispos. 1996;24:395–400. [PubMed] [Google Scholar]
- Rea IM, McNerlan SE, Alexander HD. CD69, CD25, and HLA-DR activation antigen expression on CD3+ lymphocytes and relationship to serum TNF-alpha, IFN-gamma, and sIL-2R levels in aging. Exp Gerontol. 1999;34:79–93. doi: 10.1016/s0531-5565(98)00058-8. [DOI] [PubMed] [Google Scholar]
- Rendic S, Guengerich FP. Contributions of human enzymes in carcinogen metabolism. Chem Res Toxicol. 2012;25:1316–1383. doi: 10.1021/tx300132k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res. 1996;56:2979–2984. [PubMed] [Google Scholar]
- Shimada T, Inoue K, Suzuki Y, Kawai T, Azuma E, Nakajima T, Shindo M, Kurose K, Sugie A, Yamagishi Y, Fujii-Kuriyama Y, Hashimoto M. Arylhydrocarbon receptor-dependent induction of liver and lung cytochromes P450 1A1, 1A2, and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in genetically engineered C57BL/6J mice. Carcinogenesis. 2002;23:1199–1207. doi: 10.1093/carcin/23.7.1199. [DOI] [PubMed] [Google Scholar]
- Shimada T, Murayama N, Yamazaki H, Tanaka K, Takenaka S, Komori M, Kim D, Guengerich FP. Metabolic activation of polycyclic aromatic hydrocarbons and aryl and heterocyclic amines by human cytochromes P450 2A13 and 2A6. Chem Res Toxicol. 2013;26:529–537. doi: 10.1021/tx3004906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siest G, Jeannesson E, Marteau JB, Samara A, Marie B, Pfister M, Visvikis-Siest S. Transcription factor and drug-metabolizing enzyme gene expression in lymphocytes from healthy human subjects. Drug Metab Dispos. 2008;36:182–189. doi: 10.1124/dmd.107.017228. [DOI] [PubMed] [Google Scholar]
- Simms PE, Ellis TM. Utility of flow cytometric detection of CD69 expression as a rapid method for determining poly- and oligoclonal lymphocyte activation. Clin Diagn Lab Immunol. 1996;3:301–304. doi: 10.1128/cdli.3.3.301-304.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatzenegger M, Horsmans Y, Verbeeck RK. CYP1A1 but not CYP1A2 proteins are expressed in human lymphocytes. Pharmacol Toxicol. 2000;86:242–244. doi: 10.1034/j.1600-0773.2000.d01-42.x. [DOI] [PubMed] [Google Scholar]
- Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickett A, Kwan YL. T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods. 2003;275:251–255. doi: 10.1016/s0022-1759(03)00010-3. [DOI] [PubMed] [Google Scholar]
- Truneh A, Albert F, Golstein P, Schmitt-Verhulst AM. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985;313:318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Turesky RJ. Heterocyclic aromatic amine metabolism, DNA adduct formation, mutagenesis, and carcinogenesis. Drug Metab Rev. 2002;34:625–650. doi: 10.1081/dmr-120005665. [DOI] [PubMed] [Google Scholar]
- Turesky RJ, Konorev D, Fan X, Tang Y, Yao L, Ding X, Xie F, Zhu Y, Zhang QY. Effect of cytochrome P450 reductase deficiency on 2-amino-9H-pyrido[2,3-b]indole metabolism and DNA adduct formation in liver and extrahepatic tissues of mice. Chem Res Toxicol. 2015;28:2400–2410. doi: 10.1021/acs.chemrestox.5b00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesky RJ, Le Marchand L. Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: lessons learned from aromatic amines. Chem Res Toxicol. 2011;24:1169–1214. doi: 10.1021/tx200135s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesky RJ, Vouros P. Formation and analysis of heterocyclic aromatic amine-DNA adducts in vitro and in vivo. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802:155–166. doi: 10.1016/j.jchromb.2003.10.053. [DOI] [PubMed] [Google Scholar]
- van Duursen MB, Sanderson JT, van den Berg M. Cytochrome P450 1A1 and 1B1 in human blood lymphocytes are not suitable as biomarkers of exposure to dioxin-like compounds: polymorphisms and interindividual variation in expression and inducibility. Toxicol Sci. 2005;85:703–712. doi: 10.1093/toxsci/kfi089. [DOI] [PubMed] [Google Scholar]
- Yang L, Dong J, Jiang S, Shi W, Xu X, Huang H, You X, Liu H. Red and processed meat consumption increases risk for non-hodgkin lymphoma: a PRISMA-compliant meta-analysis of observational studies. Medicine (Baltimore) 2015;94:e1729. doi: 10.1097/MD.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha Q, Qian NX, Moldoveanu SC. Analysis of polycyclic aromatic hydrocarbons in the particulate phase of cigarette smoke using a gas chromatographic-high-resolution mass spectrometric technique. J Chromatogr Sci. 2002;40:403–408. doi: 10.1093/chromsci/40.7.403. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ashley DL, Watson CH. Quantitative analysis of six heterocyclic aromatic amines in mainstream cigarette smoke condensate using isotope dilution liquid chromatography-electrospray ionization tandem mass spectrometry. Nicotine Tob Res. 2011;13:120–126. doi: 10.1093/ntr/ntq219. [DOI] [PubMed] [Google Scholar]
- Zhao B, Degroot DE, Hayashi A, He G, Denison MS. CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicol Sci. 2010;117:393–403. doi: 10.1093/toxsci/kfq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Lee SA. Well-done meat intake, heterocyclic amine exposure, and cancer risk. Nutr Cancer. 2009;61:437–446. doi: 10.1080/01635580802710741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.