Summary
Even though hyperthermia is a promising treatment for cancer, the relationship between specific temperatures and clinical benefits of and predictors of sensitivity of cancer to hyperthermia are poorly understood. Ovarian and uterine tumors have diverse hyperthermia sensitivities. Integrative analyses of the specific gene signatures in and the differences in response to hyperthermia between hyperthermia-sensitive and -resistant cancer cells identified CTGF as a key regulator of sensitivity. CTGF silencing sensitized resistant cells to hyperthermia. CTGF siRNA treatment also sensitized resistant cancers to localized hyperthermia induced by copper sulfide nanoparticles and near-infrared laser in orthotopic ovarian cancer models. CTGF silencing aggravated energy stress induced by hyperthermia and enhanced apoptosis of hyperthermia resistant cancers.
Keywords: Hyperthermia, ovarian cancer, CTGF, DOPC-liposome, thermosensitivity, copper sulfide nanoparticle
Introduction
Therapeutic hyperthermia is based on the premise of raising the temperature of tumor tissue to 40–43°C. It has been used for treatment of ovarian and other cancers. The rationale for this therapy is based on the direct-killing effects of temperatures above 41–42°C (Wust et al., 2002). Hyperthermia is also applied as an adjunctive therapy with various established cancer treatments, such as radiotherapy and chemotherapy (Moyer and Delman, 2008; Nagata et al., 1997; Palazzi et al., 2010). Some studies have suggested that hyperthermia activates the immune system against tumor cells by increasing the release of heat shock proteins (HSPs) associated with tumor-specific antigens from heat-stressed or dying tumor cells that are phagocytized by antigen-presenting cells (APCs) (Binder et al., 2000).
As interest in hyperthermic treatment of cancer has increased, significant progress has been made in developing strategies to heat tumors via local, regional, and whole-body hyperthermia (van der Zee, 2002). Different types of energy has been used, including microwaves and radio waves (Gazelle et al., 2000; Seki et al., 1999), magnetic heating (Lee et al., 2011; Rodriguez-Luccioni et al., 2011), and ultrasound (Jolesz and Hynynen, 2002). Regional hyperthermia (e.g., hyperthermic intraperitoneal chemotherapy [HIPEC] heated to 42°C), theoretically enables direct contact between tumor cells and the chemotherapeutic agent to control residual microscopic disease (Jinny Ha, 2012).
Even though hyperthermia is a promising therapeutic approach, multiple obstacles remain to be cleared. One of the major issues is that the tumor temperatures that must be reached for obtaining clinical efficacy are largely undefined (Wust et al., 2002). In the present study, we carried out a series of experiments to determine the molecular mechanisms of tumor response to hyperthermia. Moreover, we utilized nanoparticle-based approaches to silence key pathways to enhance tumor response to hyperthermia.
Results
Diverse of Hyperthermia Sensitivity in Ovarian and Uterine Cancer Cell Lines
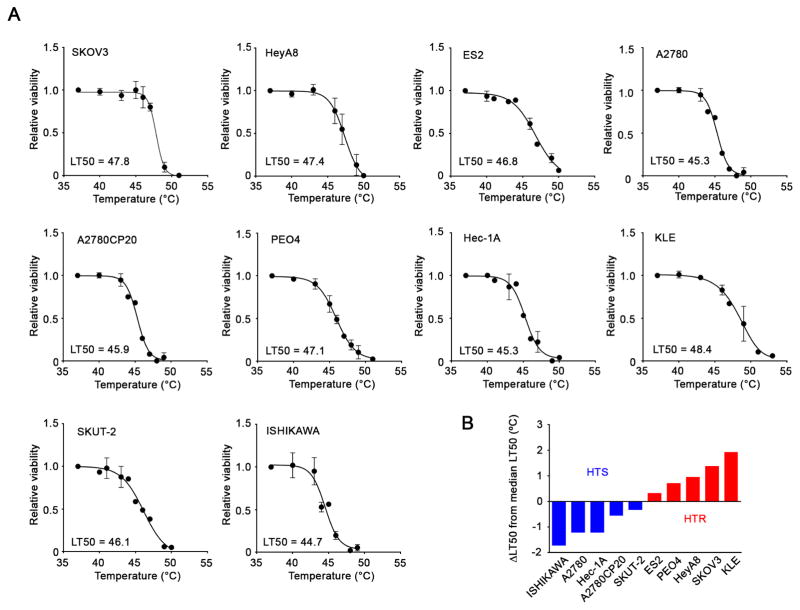
We first monitored the temperature transition in tumors during HIPEC in high grade serous ovarian cancer patients (Figure S1 and Table S1). Even though the perfusion temperature at the entrance was maintained at 42.5°C, the temperature in most of the tumors was about 40°C and the potential clinical benefit of these lower temperatures is unclear. We sought to determine the temperatures at which various cancer cells lose viability and to identify potential molecular regulators of sensitivity to hyperthermia. To address these questions, we tested hyperthermia sensitivity in a range of ovarian (A2780, A2780CP20, SKOV3, ES2, HeyA8 and PEO4) and uterine (Hec-1A, SKUT-2, KLE and ISHIKAWA) cancer cells. We treated each cell line with hyperthermia for 1 hr, equivalent to the duration of HIPEC, using an incubator at indicated temperatures and assessed cell viability (Figure 1A). We defined the temperature that produced 50% cell death as the median lethal temperature 50 (LT50) using curve fitting. Based on the calculation of LT50s, the difference between the lowest and highest LT50 was about 4°C, suggesting that the cells showed substantial variability in sensitivity to hyperthermia. The median LT50 in the tested cells was 46.5°C. We classified cells with LT50s above the median, SKOV3, HeyA8, ES2, PEO4, and KLE, as hyperthermia-resistant (HTR) cells, and below the median, A2780, A2780CP20, Hec-1A, ISHIKAWA, and SKUT-2, as hyperthermia-sensitive cells (HTS), respectively (Figure 1B). Since hyperthermia can increase blood flow, we also examined whether endothelial cells are affected by hyperthermia. For these experiments, we used RF24, endothelial cells (Pecot et al., 2013). The LT50 of RF24 cells was approximately 45.5°C, thus these cells were classified as HTS cells (Figure S2A). Hyperthermia treatment also enhanced sensitivity of HTS A2780 and HTR HeyA8 cells to cisplatin (Figure S2B).
Figure 1. Variability in hyperthermia sensitivity of ovarian and uterine cancer cell lines.
(A) Ovarian and uterine cancer cells were treated with hyperthermia for 1 hr at the indicated temperatures and then incubated at 37°C. Cell viability was determined using an MTT assay at 24 hr. The value in each graph represents the LT50 that produced 50% cell death. (B) Difference in each LT50 from the median LT50 (46.5°C) in the tested cells. The cells having LT50s above or below and below the median LT50 were classified as HTR and HTS, respectively.
See also Figures S1 and S2
Specific Gene Signatures in HTR Cells as Determined using Comprehensive Gene Expression Analysis
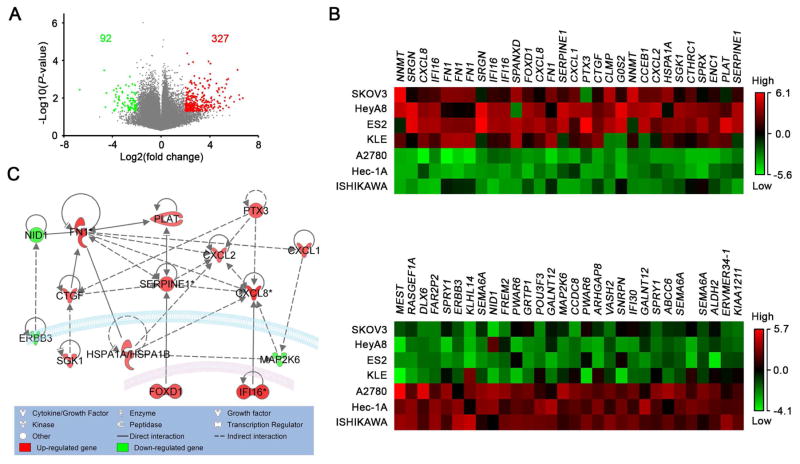
To identify potential molecular regulators of sensitivity of cancer cells to hyperthermia, we compared the gene expression profiles for HTS and HTR cells using the Cancer Cell Line Encyclopedia (CCLE) database. Specifically, we compared the gene expression profiles of HTS (A2780, ISHIKAWA, and Hec-1A), and HTR (SKOV3, HeyA8, ES2, and KLE) cell lines. We identified differentially expressed genes between HTS and HTR lines setting the cut-off at P-value < 0.05 and fold change > 2. This identified 327 upregulated genes and 92 downregulated genes in HTR cells (Figure 2A and Table S2). Heat maps of the expression of the top 30 upregulated and downregulated genes in HTR cells are shown in Figure 2B. Enriched Gene Ontology (GO) annotations for biological processes for these top 30 genes were related to wound healing, cell motion, and cell adhesion (Figure S3A). We then examined these 30 genes using Ingenuity pathway Analysis (IPA) to identify molecules that can possibly interact with each other in HTR cells. We found that 15 genes were potentially connected with each other in HTR cells (Figure 2C). We further analyzed the expression of the 15 genes in 10 cell lines using quantitative reverse transcription (qRT)-polymerase chain reaction (PCR) (Figure S3B). The baseline expression of these genes was generally correlated with increased gene expression in HTR cells in the CCLE database. Among the 15 genes, the expression of FN1, CXCL1, and IL8 was highly increased in HTR compared with HTS cells.
Figure 2. Specific gene signatures in HTR cells.
(A) Volcano plot of upregulated and downregulated genes in HTR cells compared with those in HTS cells from the CCLE database. The fold change in gene expression and P-values for HTR cells versus HTS cells are represented in the horizontal and vertical axes, respectively. Filtering according to a fold change greater than 2, and P-value less than 0.05, identified 327 upregulated 92 downregulated genes. (B) Heat maps of the expression of the top 30 upregulated and downregulated genes in HTR cells. (C) Network map of the top 30 upregulated and downregulated genes created using IPA. Red and green indicate upregulation and downregulation, respectively with darker shades indicating greater changes in expression. The lines indicate binding of two gene products, and lines terminating with arrows indicate one gene product acting on another gene product.
See also Figure S3
Determination of the Difference in Response of Ovarian Cancer Cells to Hyperthermia Using Quantitative Proteomics
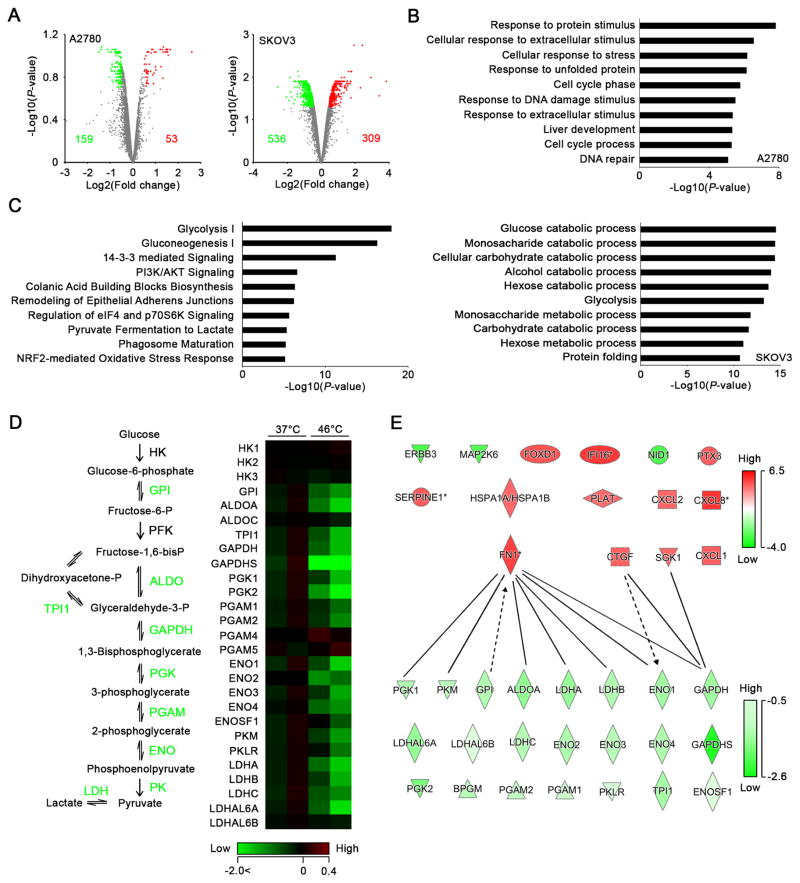
To examine the mechanisms by which HTR cells survive under hyperthermia, we first compared global changes in gene expression between HTS A2780 and HTR SKOV3 cells after hyperthermia using gene microarrays. Most genes that were significantly upregulated after hyperthermia were common between A2780 and SKOV3 cells (Figures S3C and D and Table S3). These results suggested that hyperthermia sensitivity was either not dependent on changes in gene expression or that there are changes in protein abundance in response to hyperthermia. To test this hypothesis, we subjected A2780 and SKOV3 cells treated with hyperthermia to isobaric Tandem Mass Tags (TMT) labeling and offline two-dimensional liquid chromatography-tandem mass spectrometry (2D-LC-MS/MS) to comprehensively assess changes in protein expression induced by hyperthermia. We treated the cells with hyperthermia at 46°C for 1 hr and harvested protein after incubation at 37°C for 4 hr. We identified 53 upregulated and 159 downregulated proteins in A2780 cells, and 309 upregulated and 536 downregulated proteins in SKOV3 cells (Figure 3A and Table S4). We analyzed the GO terms for biological processes for the differentially expressed proteins in A2780 and SKOV3 cells using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) and found that processes involved in glucose metabolism were specifically enriched in SKOV3 cells after hyperthermia (Figure 3B). We also found that glycolysis was the primary pathway affected by hyperthermia in SKOV3 cells according to canonical pathway analysis (Figure 3C). Expression of most of the proteins involved in the glycolysis pathway was downregulated in SKOV3 cells, suggesting that the glycolysis pathway was impaired after hyperthermia treatment (Figure 3D). Upon examining the interactions between the 15 molecules that are differentially expressed between HTS and HTR cells and the glycolysis-related molecules identified in the proteomics described here, we identified FN1, SGK1, CTGF, and CXCL1 to be the potential players regulating the glycolysis pathway in HTR cells (Figure 3E).
Figure 3. Determination of the difference in response to hyperthermia in A2780 and SKOV3 cells using quantitative proteomics.
(A) Volcano plots of differentially expressed proteins in HTS A2780 (left) and HTR SKOV3 (right) cells after hyperthermia identified using 2D-LC-MS/MS with TMT. Upregulated and downregulated proteins (fold change, >0.5; p < 0.2 for A2780, p < 0.05 for SKOV3) are highlighted in red and green, respectively. (B) Graphs of the top 10 enriched GO annotations for biological processes for differentially expressed proteins in A2780 (upper) and SKOV3 (lower) cells. (C) Graph of the top 10 canonical pathways in SKOV3 cells in response to hyperthermia as determined using IPA. (D) Left, schematic of the glycolysis pathway. Enzymes highlighted in green represent downregulated proteins in SKOV3 cells after hyperthermia. Right, heat map of differential expression patterns for proteins involved in the glycolysis pathway according to proteomic analysis. (E) Network map of 15 upregulated and downregulated genes in HTR cells that were identified in the CCLE database and glycolysis-involved proteins that were identified in SKOV3 cells using 2D-LC-MS/MS with TMT. Red and green indicate upregulation and downregulation, respectively with darker shades indicating greater changes in expression. The lines indicate binding of two gene products, and lines terminating with arrows indicate one gene product acting on another gene product.
See also Figure S3
Genes Responsible for Hyperthermia Resistance in Ovarian Cancer Cells
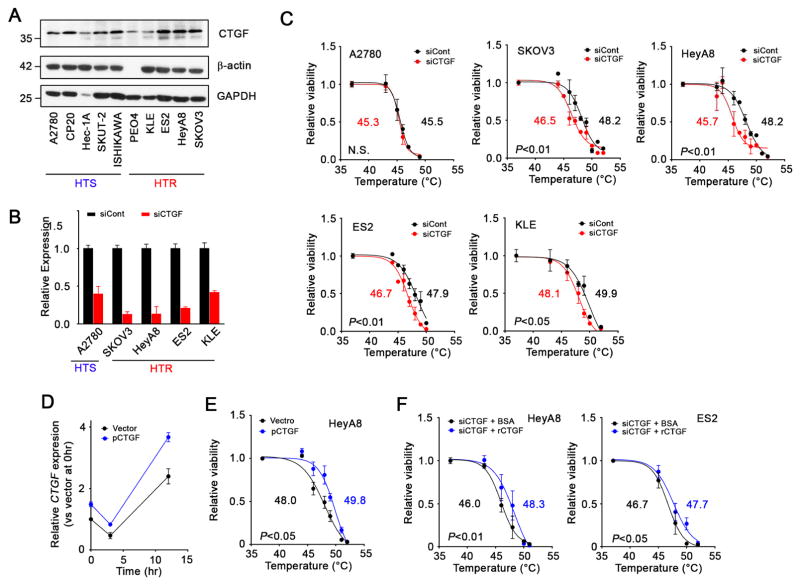
According to network mapping, FN1, SGK1, CTGF and CXCL1 were likely linked with glycolysis (Figure 3E). Because CXCL1 was likely downstream from the glycolysis pathway, we focused our effort on FN1, SGK1, and CTGF. We measured FN1, SGK1, and CTGF protein expression in 10 cell lines via Western blotting (Figures 4A and S4A). The expression was increased in HTR cells, which was generally consistent with the FN1, SGK1, and CTGF gene expression profiles of the 10 cell lines (Figure S3B). We then examined potential effects of silencing CTGF, FN1, or SGK1 on response to hyperthermia in HTR (SKOV3, HeyA8, ES2, and KLE) and HTS (A2780) cells using small interfering RNA (siRNA) (Figure 4B). Silencing of FN1 and SGK1 did not sensitize HTR or HTS cells to hyperthermia (Figures S4B–E). On the other hand, silencing of CTGF sensitized the HTR cells to hyperthermia with a decrease in the LT50 by 1.0–2.5°C (Figure 4C). Downregulation of CTGF expression had no effect on sensitization to hyperthermia in A2780 cells. No sensitization was observed when HTS RF24 endothelial cells were treated with siCTGF (Figures S2C and D). Transfection of a CTGF-expressing vector enhanced CTGF mRNA levels in HeyA8 cells, which made the cells tolerant of hyperthermia with about a 2°C increase in the LT50 (Figures 4D and 4E). Because CTGF is a secreted molecule, we treated HTR cells with recombinant human CTGF (rCTGF) in a medium to examine its functional effect. HTR HeyA8 and ES2 cells treated with CTGF siRNA (siCTGF) exhibited tolerance of hyperthermia with an increase of 2.3 and 1.0 °C of the LT50 following the addition of rCTGF (Figure 4F). Conversely, anti-CTGF antibody treatment sensitized SKOV3 cells to hyperthermia (Figure S2E).
Figure 4. The effect of silencing of CTGF on sensitization of cancer cells to hyperthermia in vitro.
(A) Immunoblot of CTGF expression in tested ovarian and uterine cancer cell lines. (B) Graphs of the silencing of CTGF in tested cell lines by siRNA. Cells were transfected with siRNA at 40 nM. mRNA expression levels in the cells were evaluated 48 hr after transfection using qRT-PCR. (C) The effect of silencing of CTGF gene on sensitization of the cells to hyperthermia. Cells treated with siRNA for 48 hr were treated with hyperthermia at the indicated temperatures for 1 hr. Cells were further incubated at 37°C and their viability was determined using an MTT assay 24 hr after the initiation of hyperthermia. LT50s were calculated using a data set at least three times. (D) Time profile of CTGF mRNA expression in HeyA8 cells treated with CTGF expressing vector as determined using qRT-PCR. (E) The effect of CTGF expression on sensitization of cells to hyperthermia. HeyA8 cells that were treated with CTGF expressing vector or a control vector for 48hr were subjected to hyperthermia at the indicated temperature for 1 hr. Cell viability was determined 24 hr after the beginning of hyperthermic treatment. (F) The effect of recombinant CTGF (rCTGF) treatment on hyperthermia sensitivity. HeyA8 and ES2 cells were treated with rCTGF (200 ng/ml) 3 hr after the beginning of hyperthermic treatment. Cell viability was determined at 24 hr after the initiation of hyperthermia.
See also Figures S2 and S4
Sensitization of Tumors to Hyperthermia by CTGF Silencing In Vivo
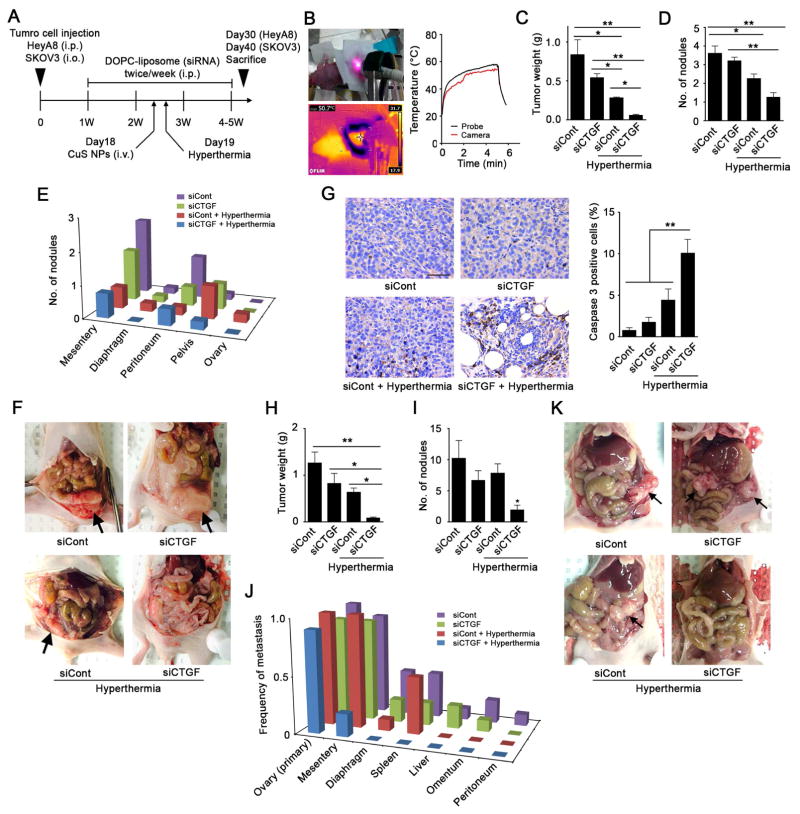
Next, we evaluated the effect of CTGF silencing on sensitization to hyperthermia and tumor growth in orthotopic models of ovarian cancer. First, in the HeyA8 model, we administered siCTGF into the peritoneal cavity twice weekly using the 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) nanoliposomal delivery platform (Figures 5A). This resulted in decreased CTGF expression in tumors (Figures S5A). The day before near-infrared (NIR) laser treatment, we intravenously injected CuS NPs modified with poly(ethylene glycol) (PEG-CuS NPs) into tumor-bearing mice (day 18). PEG-CuS NPs with about 10 nm in diameter (Figures S5D and E) circulate in the blood for long periods after systemic administration and passively accumulate in tumors via enhanced permeability and retention (EPR) effect (Zhou et al., 2010; Hatakeyama et al., 2011). We then treated tumors with local hyperthermia using an NIR laser at 980 nm for 5 min on day 19 because CuS NPs can convert the optical energy of an NIR laser to thermal energy (Figure S5F) (Zhou et al., 2015). As temperatures of 46–60°C are associated with reversible cellular damage in proportion to the exposure time (Vanagas et al., 2010; Wood et al., 2002), we maintained the tumor temperature at about 50°C (Figure 5B). We harvested HeyA8 tumors on day 30, and found that local hyperthermia decreased tumor weights and nodule numbers in control siRNA (siCont)- and siCTGF-treated mice (Figures 5C–5F). Furthermore, tumor growth and metastasis were markedly suppressed by the combination of CTGF silencing and local hyperthermia with no changes in body weight (Figures 5 and S5B). A significant increase in cleaved caspase 3 positive cells was observed after hyperthermic treatment in HeyA8 tumors treated with siCTGF (Figure 5G). No obvious decrease in the number of Ki67 positive tumor cells was observed in the siCTGF plus hyperthermia treatment group compared to the siControl group (Figure S5C). Next, we used the HTR SKOV3 tumors surgically implanted into the ovary of nude mice (n = 9–10 per group). The tumor weight and metastasis were significantly decreased by the combination of CTGF silencing and local hyperthermia (Figures 5H–K). These results demonstrated that CTGF silencing in vivo sensitized ovarian tumors to local hyperthermia treatment.
Figure 5. CTGF silencing-induced sensitization of HeyA8 tumors to hyperthermia by local thermal ablation with CuS NPs and an NIR laser in vivo.
(A) Our animal experiment schedule. Mice were injected intraperitoneally with luciferase-transfected HeyA8 cells or injected into ovary with luciferase-transfected SKOV3. SiRNA/DOPC-liposomes (5 μg of siRNA/mouse) were administered via intraperitoneal injection twice weekly beginning on day 7 after cell injection. On day 18, CuS NPs (8 OD/mouse) were injected intravenously into the mice and on day 19, tumors were treated with an NIR laser for 5 min. Tumors were collected from the mice on day 30 for HeyA8 model or day 40 for SKOV3 model. (B) Representative transition of the temperature of HeyA8 tumor. The temperature was monitored during local hyperthermic treatment using a thermal camera (red line) and fiber probe (black line). (C–E) Average tumor weights (C), numbers of tumor nodules (D), and numbers of distant metastatic nodules (E) of HeyA8 model on Day 30 (n = 4–5/group). (F) Representative pictures of HeyA8 tumor burdens in mice (arrows). (G) Immunohistochemistry assay of cleaved caspase-3 in tumor sections (scale bar = 50 μm). Left, representative images of cleaved caspase-3 staining in HeyA8 tumors. Right, bar graph represents ratio of cleaved caspase-3 positive cells per total cells per hpf, (n = 13–18 fields). (H–J) Average tumor weights (H), numbers of tumor nodules (I), and frequency of metastases to distant sites (J) of SKOV3 model on Day 40 (n = 9–10/group).) (K) Representative pictures of SKOV3 tumor burdens in mice (arrows).
See also Figure S5
Identification of the Mechanism by Which CTGF Causes Hyperthermia Resistance
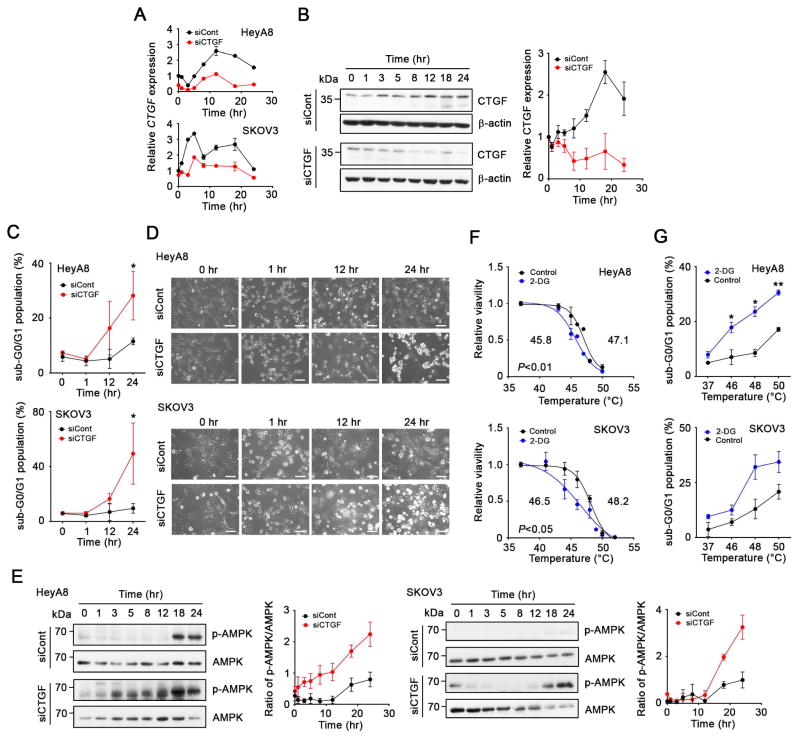
To further examine how CTGF silencing sensitized HTR cells to hyperthermia, we examined CTGF levels in HeyA8 and SKOV3 cells longitudinally. Since the expression of some housekeeping genes might be affected by hyperthermia, we confirmed that β-actin and 18S could be used as reference genes in hyperthermia experiment (Figure S6A–C). CTGF expression increased after hyperthermic treatment, and the increase of CTGF after hyperthermia was suppressed by siCTGF-based treatment (Figure 6A). CTGF protein expression also increased after hyperthermic treatment in HeyA8 cells, but the increase was blocked by siCTGF-based treatment over 24 hr (Figure 6B). Silencing of CTGF increased the population of sub-G0/G1 (apoptotic) cells at 24 after hyperthermia (Figure 6C) and induced cell death at 24 hr after hyperthermia treatment (Figure 6D). As shown in Figure 3D, quantitative proteomic analysis suggested that glycolysis was impaired in the HTR SKOV3 cells after hyperthermia treatment. Inhibition of glycolysis results in energy stress. AMP-activated protein kinase (AMPK) plays a key role in regulating cellular energy homeostasis under energy stress. AMPK is activated by phosphorylation when energy stress occurs (Hardie et al., 2012). We observed increased phosphorylated AMPK levels induced by hyperthermia and these levels were enhanced in HeyA8 and SKOV3 cells treated with siCTGF (Figure 6E). The increase was independent of LKB1 activity (Figure S6D). The sensitization to hyperthermia was also observed when SKOV3 cells were treated with metformin (Figure S2F), which can activate AMPK phosphorylation and induce energy stress (Lengyel, et al., 2015). It is possible that CTGF regulates glycolytic activity by modulating expression of glycolysis-related genes after hyperthermia. To confirm that inhibition of the glycolysis pathway affected sensitivity of HTR cells to hyperthermia, we treated HeyA8 and SKOV3 cells with a glycolysis inhibitor, 2-deoxy-D-glucose (2DG). This treatment sensitized HTR HeyA8 and SKOV3 cells to hyperthermia with a decrease in the LT50 by about 2°C (Figure 6F), and increased the population of sub-G0/G1 in HeyA8 and SKOV3 cells (Figure 6G).
Figure 6. Mechanism by which CTGF regulates hyperthermia resistance.
(A) Time profile of CTGF mRNA expression in HeyA8 and SKOV3 cells treated with control siRNA (siCont) or siCTGF as determined using qRT-PCR. Cells treated with siCont of siCTGF were treated with hyperthermia at 46°C for 1 hr (0 to 1 hr), and further incubated at 37°C up to the time points. (B) Time profile of CTGF protein expression in HeyA8 cells treated with siCont or siCTGF. Left, immunoblot of CTGF and β-actin expression. Right, Bands intensity of CTGF and β-actin semiquantified using the Image-J software program (National Institutes of Health, Bethesda, Maryland). (C) Sub-G0/G1 (apoptotic) populations of HeyA8 and SKOV3 cells treated with siCont or siCTGF after hyperthermia at indicated time points. (D) Interference contrast images of HeyA8 and SKOV3 cells treated with siCont or siCTGF after hyperthermia. Scale bars; 100 μm. (E) Time profiles of phosphorylated AMPK (pAMPK) and AMPK expression in HeyA8 and SKOV3 cells treated with siCont or siCTGF. Left, Immunoblot of pAMPK and total AMPK expression. Right; Band intensity of pAMPK and AMPK semiquantified using the Image-J software program. (F) The effect of inhibition of glycolytic activity on the sensitivity of HTR HeyA8 and SKOV3 cells to hyperthermia. HeyA8 and SKOV3 cells treated with 2-DG (25 mM) were treated with hyperthermia at indicated temperatures and viability was determined at 24 hr after the initiation of hyperthermia. (G) Sub-G0/G1 populations of HeyA8 and SKOV3 cells treated with 2-DG (25 mM) were determined 24 hr after hyperthermic treatment at the indicated temperatures.
See also Figures S2 and S6
Discussion
Here, we identified molecular determinants of cancer cell sensitivity to hyperthermia. We characterized the role of CTGF as a key target for enhancing response to hyperthermia treatment. CTGF silencing inhibited the recovery of glycolytic activity after hyperthermia, which resulted in energy stress-induced cell death (Figure 7).
Figure 7. A proposed mechanism of CTGF-mediated hyperthermia resistance.
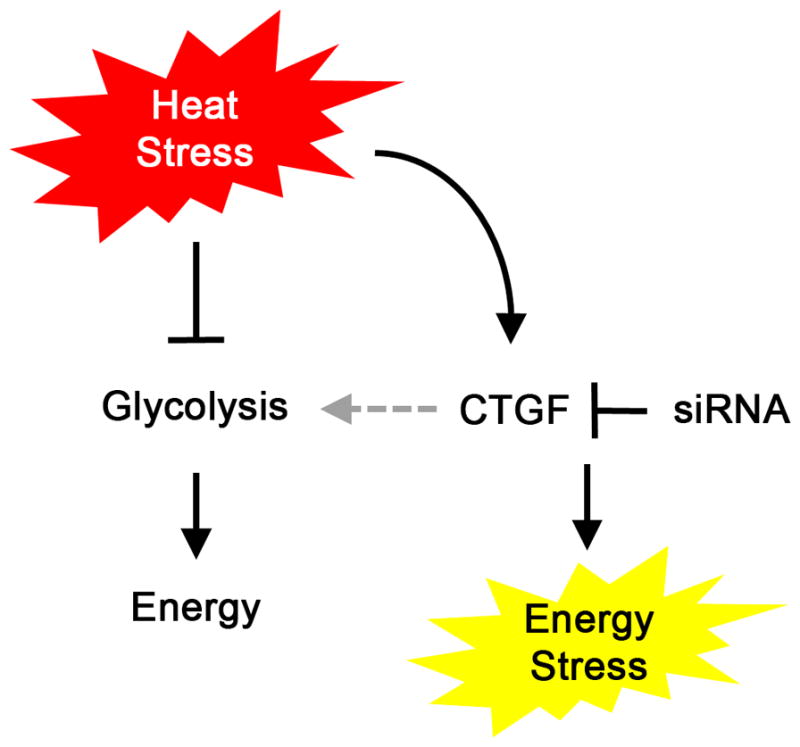
Hyperthermia inhibits glycolytic activity. On the other hand, CTGF is upregulated after hyperthermia. Upregulated CTGF may regulate glycolytic activity, which prevents HTR cells from undergoing energy stress and death. Silencing of CTGF presumably inhibits the activation of glycolytic activity in HTR cells after hyperthermia and induces energy stress and cell death.
The temperatures used in clinical hyperthermia treatment range from 40 to 45°C; typically, the temperature is 42.5°C in cases of regional hyperthermia, such as during HIPEC (Jinny Ha, 2012). However, most of the prior studies lacked any actual assessment of temperature in tumors. Moreover, given the gradient between the temperature of infusate and actual tumor-level temperatures, true hyperthermia is unlikely to have been achieved in such treatments. The clinical benefit of such a low temperature for cancer therapy is unclear. We discovered that cancer cells exhibited substantial diversity in their sensitivity to hyperthermia as determined according to the LT50.
We observed little difference in gene expression changes after hyperthermia between HTS vs. HTR cells despite substantial difference in protein expression. It is known that heat shock response where heat shock proteins are upregulated rapidly is highly conserved in all organisms for protection of cells from a wide range of harmful conditions, including heat shock (Jolly and Morimoto, 2000), which is consistent with our observation that enhanced gene expression induced by hyperthermic treatment occurred independently of hyperthermia sensitivity. While RNA molecules are typically only damaged above 85–90°C, thermal stability of protein represents a wide range (Bischof et al., 2005). These may account for wider effects on expression of protein than RNA by hyperthermia.
Our findings related to the role of CTGF in glycolysis-related genes are supported by its role in development. For example, downregulation of glycolysis-related genes such as Pgm1, Pgam1, and Eno1, and decrease in glycolytic activity and ATP levels have been reported in chondrocytes from Ctgf (Ccn2)-null mice (Maeda-Uematsu et al., 2014; Murase et al., 2015). It is likely that CTGF regulates expression of glycolysis-related genes after hyperthermia to prevent cancer cells from energy-stressed cell death. However, some studies have reported that energy stress downregulates the expression of CTGF via inactivation of YAP in the Hippo pathway in HEK293 cells (Mo et al., 2015; Wang et al., 2015). It is possible that such signaling may be context dependent since 14-3-3 signaling pathway was downregulated in the HTR SKOV3 cells after hyperthermia (Figures 3C and S3E).
To overcome of the challenges with fluid-based hyperthermia, we used local thermal ablation with CuS NPs and an NIR laser. CuS NPs have smaller size than plasmonic gold based nanostructures, which give them better chances to extravasate into extravascular fluid space. In addition, the absorption properties of CuS NPs are not subject to change upon repetitive laser irradiation. Our approach for gene silencing with DOPC nanoliposomal delivery system can be ready translated into clinical settings (Rupaimoole et al., 2014; Wu et al., 2014) to sensitize cancers to hyperthermia, and can be used for other key targets. Such an approach could also be beneficial for cancer cells only exposed to sub-lethal thermal stress by HIPEC.
Tumor endothelial cell is also an important target for hyperthermia. Based on the LT50 of RF24 cells, endothelial cells were sensitive to hyperthermia. Thus, while our study focuses on the effect of hyperthermic treatment on tumor cells, it is possible that the decreases in tumor burden after hyperthermia treatment in vivo were due to the effects of both tumor and endothelial compartments.
In summary, we discovered that cancer cells have diverse hyperthermia sensitivity. CTGF is an important regulator of hyperthermia sensitivity and may represent an important target for further development.
Experimental Procedures
Our materials, HIPEC procedure, cell culture, the cell-cycle study, immunoblotting, RNA extraction, qRT-PCR, TMT Labeling and 2D-LC-MS/MS-Based Quantitative Proteomics, cDNA microarray analysis, synthesis and transmission electron microscopy (TEM) of CuS NPs, and immunohistochemistry are described in Supplemental Experimental Procedures.
Hyperthermic Treatment of Cancer Cells in Vitro
The details are described in Supplemental Experimental Procedures. Briefly, cells were treated with hyperthermia via heating cells in an incubator for 1 hr at indicated temperatures followed by incubation at 37°C in a regular cell culture incubator. At indicated time points, viability was determined by MTT assay. The LT50 that produced 50% cell death was calculated via curve fitting. Transfection of siRNA or pDNA, and treatment procedures are described in Supplemental Experimental Procedure.
CCLE Database Analysis
The expression data for HTR cells (SKOV3, HeyA8, ES2, and KLE) and HTS cells (A2780, Hec-1A, and ISHIKAWA) were downloaded from the CCLE website (https://www.broadinstitute.org/ccle/data). The details are available in Supplemental Experimental Procedure.
DOPC Liposome Preparation
DOPC liposomes encapsulating siRNA were prepared as described previously (Rupaimoole et al., 2014; Wu et al., 2014).
Synthesis of CuS NPs
CuS NPs were synthesized according to previously reported procedures (Zhou et al., 2015; Zhou et al., 2010). The details are available in Supplemental Experimental Procedure.
In Vivo Models
Orthotopic mouse models of ovarian cancer were developed as described previously (Rupaimoole et al., 2014; Wu et al., 2014) and the details are available in Supplemental Experimental Procedure. SiRNA-incorporated neutral DOPC liposomes were injected intraperitoneally at a dose of 5 μg siRNA/mouse twice weekly for about 3 weeks beginning 1 week after cell injection. Photothermial ablation using CuS NPs and an NIR laser was used for in vivo local hyperthermic treatment. The details are described in Supplemental Experimental Procedure.
Protein Expression Analysis Using TMT Labeling and 2D-LC-MS/MS-Based Quantitative Proteomics
The details in proteomic sample preparation, LC-MS/MS analysis and proteomics data analysis are available in Supplemental Experimental Procedures. All raw files were deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PRIDE: PXD005055 and 10.6019/PXD005055.
Supplementary Material
Acknowledgments
H.H. is supported by JSPS Postdoctoral Fellowships for Research Abroad, and Mochida Memorial Foundation for Medical and Pharmaceutical Research. S.Y.W. is supported by Ovarian Cancer Research Fund, Alliance, Foundation for Women’s Cancer, and CPRIT training grants (RP101502 and RP101489). P.L.D. is supported by Computational Cancer Biology Training Program from CPRIT (RP140113), and Caring Together, NY Ovarian Cancer Research Grant from Foundation for Women’s Cancer. Proteomic analysis was supported by the NCI CPTAC program (U24CA160019). This study was also supported, in part, by grants from the NIH (CA016672, P50 CA083639, P50 CA098258, U54 CA151668, UH3 TR000943), CPRIT (RP110595), the Ovarian Cancer Research Fund, Inc., the Blanton Davis Ovarian Cancer Research Program, the RGK Foundation, and the Frank McGraw Memorial Chair in Cancer Research to A.K.S.; and John S. Dunn Foundation to C. L.
Footnotes
Supplemental Information includes figures, tables, and Supplemental Experimental Procedures can be found with this article online.
Author Contributions
H.H. and A.K.S. conceived the project and designed the experiments. H.H. S.Y.W., Y.A.L., S.P., W.W., Q.H., K.A.C., F.S., Y.H., T.H., T.M., N.J., P.L.D. and L.S.M. carried out or participated in vitro and in vivo experiments. W.W., Q.H. and C.L. designed and prepared CuS NPs for in vivo hyperthermic treatment. T.L., S.N., V.A.P., A.A.S., A.K.S. and K.D.R. performed proteomic analyses. C.R.-A. and G.L.-B. prepared liposomes for in vivo studies. J.S. and J.-S.L helped with cDNA array analysis. M.P. and A.F. obtained the clinical data. H.H. wrote the manuscript. S.Y.W., M.T.-L., C.L. and A.K.S. participated in manuscript preparation. All authors edited and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- Bischof JC, He X. Thermal stability of proteins. Ann N Y Acad Sci. 2005;1066:12–33. doi: 10.1196/annals.1363.003. [DOI] [PubMed] [Google Scholar]
- de Winter P, Leoni P, Abraham D. Connective tissue growth factor: structure-function relationships of a mosaic, multifunctional protein. Growth Factors. 2008;26:80–91. doi: 10.1080/08977190802025602. [DOI] [PubMed] [Google Scholar]
- Lengyel E, Litchfield LM, Mitra AK, Nieman KM, Mukherjee A, Zhang Y, Johnson A, Bradaric M, Lee W, Romero IL. Metformin inhibits ovarian cancer growth and increases sensitivity to paclitaxel in mouse models. Am J Obstet Gynecol. 2015;212:479e–1e10. doi: 10.1016/j.ajog.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633–646. doi: 10.1148/radiology.217.3.r00dc26633. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama H, Akita H, Harashima H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Adv Drug Deliv Rev. 2011;63:152–160. doi: 10.1016/j.addr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Jinny Ha MW. Current Indications, Techniques and Results of Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Intra-Abdominal Malignancies. Surgery: Curr Res. 2012;02 [Google Scholar]
- Jolesz FA, Hynynen K. Magnetic resonance image-guided focused ultrasound surgery. Cancer J. 2002;8(Suppl 1):S100–112. [PubMed] [Google Scholar]
- Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cacner Inst. 2000;92:1564–1572. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- Lee JH, Jang JT, Choi JS, Moon SH, Noh SH, Kim JW, Kim JG, Kim IS, Park KI, Cheon J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat Nanotechnol. 2011;6:418–422. doi: 10.1038/nnano.2011.95. [DOI] [PubMed] [Google Scholar]
- Lim T, Lee I, Kim J, Kang WK. Synergistic Effect of Simvastatin Plus Radiation in Gastric Cancer and Colorectal Cancer: Implications of BIRC5 and Connective Tissue Growth Factor. Int J Radiat Oncol Biol Phys. 2015;93:316–325. doi: 10.1016/j.ijrobp.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Maeda-Uematsu A, Kubota S, Kawaki H, Kawata K, Miyake Y, Hattori T, Nishida T, Moritani N, Lyons KM, Iida S, Takigawa M. CCN2 as a novel molecule supporting energy metabolism of chondrocytes. J Cell Biochem. 2014;115:854–865. doi: 10.1002/jcb.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer HR, Delman KA. The role of hyperthermia in optimizing tumor response to regional therapy. Int J Hyperthermia. 2008;24:251–261. doi: 10.1080/02656730701772480. [DOI] [PubMed] [Google Scholar]
- Murase Y, Hattori T, Aoyama E, Nishida T, Maeda-Uematsu A, Kawaki H, Lyons KM, Sasaki A, Takigawa M, Kubota S. Role of CCN2 in Amino Acid Metabolism of Chondrocytes. J Cell Biochem. 2016;117:927–937. doi: 10.1002/jcb.25377. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Hiraoka M, Nishimura Y, Masunaga S, Mitumori M, Okuno Y, Fujishiro M, Kanamori S, Horii N, Akuta K, et al. Clinical results of radiofrequency hyperthermia for malignant liver tumors. Int J Radiat Oncol Biol Phys. 1997;38:359–365. doi: 10.1016/s0360-3016(96)00625-6. [DOI] [PubMed] [Google Scholar]
- Palazzi M, Maluta S, Dall’Oglio S, Romano M. The role of hyperthermia in the battle against cancer. Tumori. 2010;96:902–910. [PubMed] [Google Scholar]
- Pecot CV, Rupaimoole R, Yang D, Akbani R, Ivan C, Lu C, Wu S, Han HD, Shah MY, Rodriguez-Aguayo C, et al. Tumour angiogenesis regulation by the miR-200 family. Nat Commun. 2013;4:2427. doi: 10.1038/ncomms3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Luccioni HL, Latorre-Esteves M, Mendez-Vega J, Soto O, Rodriguez AR, Rinaldi C, Torres-Lugo M. Enhanced reduction in cell viability by hyperthermia induced by magnetic nanoparticles. Int J Nanomedicine. 2011;6:373–380. doi: 10.2147/IJN.S14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupaimoole R, Wu SY, Pradeep S, Ivan C, Pecot CV, Gharpure KM, Nagaraja AS, Armaiz-Pena GN, McGuire M, Zand B, et al. Hypoxia-mediated downregulation of miRNA biogenesis promotes tumour progression. Nat Commun. 2014;5:5202. doi: 10.1038/ncomms6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Wakabayashi M, Nakagawa T, Imamura M, Tamai T, Nishimura A, Yamashiki N, Inoue K. Percutaneous microwave coagulation therapy for solitary metastatic liver tumors from colorectal cancer: a pilot clinical study. Am J Gastroenterol. 1999;94:322–327. doi: 10.1111/j.1572-0241.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Huang CY, Su HL, Tang CH. CCN2 enhances resistance to cisplatin-mediating cell apoptosis in human osteosarcoma. PLoS One. 2014;9:e90159. doi: 10.1371/journal.pone.0090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13:1173–1184. doi: 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]
- Vanagas T, Gulbinas A, Pundzius J, Barauskas G. Radiofrequency ablation of liver tumors (I): biological background. Medicina (Kaunas) 2010;46:13–17. [PubMed] [Google Scholar]
- Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood BJ, Ramkaransingh JR, Fojo T, Walther MM, Libutti SK. Percutaneous tumor ablation with radiofrequency. Cancer. 2002;94:443–451. doi: 10.1002/cncr.10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SY, Yang X, Gharpure KM, Hatakeyama H, Egli M, McGuire MH, Nagaraja AS, Miyake TM, Rupaimoole R, Pecot CV, et al. 2′-OMe-phosphorodithioate-modified siRNAs show increased loading into the RISC complex and enhanced anti-tumour activity. Nat Commun. 2014;5:3459. doi: 10.1038/ncomms4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3:487–497. doi: 10.1016/s1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- Zhou M, Chen Y, Adachi M, Wen X, Erwin B, Mawlawi O, Lai SY, Li C. Single agent nanoparticle for radiotherapy and radio-photothermal therapy in anaplastic thyroid cancer. Biomaterials. 2015;57:41–49. doi: 10.1016/j.biomaterials.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP, Tian M, Liang D, Li C. A chelator-free multifunctional [64Cu]CuS nanoparticle platform for simultaneous micro-PET/CT imaging and photothermal ablation therapy. J Am Chem Soc. 2010;132:15351–15358. doi: 10.1021/ja106855m. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.