Abstract
Purpose of review
Clinical guidelines are not consistent regarding whether or how to utilize information on measures of chronic kidney disease (CKD) for predicting the risk of cardiovascular disease (CVD). This review summarizes recent literature regarding CVD prediction in the context of CKD.
Recent findings
Previous studies used different definitions of CKD measures and CVD outcomes and applied distinct statistical approaches. A recent individual-level meta-analysis from the CKD Prognosis Consortium is of value since it has uniformly investigated creatinine-based estimated glomerular filtration rate (eGFR) and albuminuria as CKD measures and applied the same statistical approach across 24 cohorts with >630,000 participants. In this meta-analysis, eGFR and albuminuria improve CVD risk prediction beyond traditional CVD risk factors, particularly for CVD mortality and heart failure. Albuminuria demonstrates more evident improvement than eGFR. Moreover, several recent studies have shown that other filtration markers, e.g., cystatin C and β2-microglobulin, and measures of atherosclerosis or cardiac damage (e.g., coronary artery calcium and cardiac troponins) can further improve CVD prediction in CKD population.
Summary
Future clinical guidelines may require updates regarding whether/how to incorporate CKD measures and other biomarkers in CVD prediction, depending on CVD outcomes of interest, target population, and availability of those measures/biomarkers in that population.
Keywords: estimated glomerular filtration rate, albuminuria, cardiovascular disease, risk prediction
Introduction
Chronic kidney disease (CKD) is recognized as an important public health issue.1 CKD affects 10%-15% of adults around the globe and increases the risk of various clinical adverse outcomes.1, 2 Cardiovascular disease (CVD) is one of the most important complications of CKD. Indeed, up to 50% of people with CKD die due to CVD even before most of them reach end-stage renal disease requiring renal replacement therapy.
Risk prediction is central for decision making for primary prevention of CVD such as statin or aspirin therapy.3, 4 However, there are several challenges in predicting CVD risk in persons with CKD. First of all, traditional risk factors have been shown to perform poorly in this clinical population.5 In addition, although numerous studies have demonstrated that persons with CKD are at high risk of CVD, interestingly, clinical guidelines are not consistent regarding how to utilize information on CKD measures for predicting CVD risk. This review summarizes current literature regarding CVD prediction with CKD measures as well as CVD prediction among those with CKD, with emphasis on recent studies.
Two key CKD measures for CVD prediction: representative individual studies
Two key measures of CKD, glomerular filtration rate (GFR) and albuminuria, have been consistently associated with high cardiovascular risk across a range of populations with different demographic, geographic, and clinical backgrounds.2, 6 However, the significant association does not necessarily indicate better prediction of CVD with CKD measures beyond traditional cardiovascular risk factors. Indeed, inconsistent results have been seen regarding whether CKD measures can improve CVD prediction beyond traditional risk factors (Table 1).7-13 These studies have used different definitions of CKD measures and CVD outcomes and have applied different statistical approaches, making it difficult to infer definite conclusions.
Table 1.
Representative individual studies exploring either or both of key CKD measures (GFR and albuminuria) for CVD prediction
| Lead author | Published year | Population | CKD measures | CVD outcomes | Authors’ conclusion about prediction improvement |
|---|---|---|---|---|---|
| Hallan7 | 2007 | General | eGFRcre & ACR | CVD mortality | Yes |
| Weiner8 | 2007 | General | eGFRcre | CHD | No |
| Clase9 | 2011 | High risk | eGFR & ACR | CVD composite (CVD death, MI, stroke, HF) | No |
| Ito10 | 2011 | General | Serum creatinine & cystatin C | CVD composite (CHD, stroke, HF, PAD) | No |
| Nerpin11 | 2011 | General | eGFR & ACR | CVD mortality | Yes |
| Chen12 | 2013 | CKD | eGFR & dipstick proteinuria | CVD composite (CHD, TIA, stroke, ventricular arrhythmia, HF) | Yes |
| Bittencourt13 | 2015 | High risk | Serum creatinine (dipstick proteinuria in subsample) | CVD composite (CVD death or non-fatal MI) | Yes |
ACR: urine albumin to creatinine ratio; CHD: coronary heart disease; CKD: chronic kidney disease; CV: cardiovascular; CVD: cardiovascular disease; eGFR: estimated glomerular filtration rate; eGFRcre: eGFR based on creatinine; HF: heart failure; MI: myocardial infarction; PAD: peripheral artery disease, TIA: transient ischemia attack.
Two key CKD measures for CVD prediction: representative clinical guidelines
Reflecting these conflicting data, clinical guidelines take considerably different positions as to whether or how to incorporate the two CKD measures for CVD prediction (Table 2). Specifically, the American Heart Association (AHA) and the American College of Cardiology (ACC) 2013 Guideline on the Assessment of Cardiovascular Risk14 does not take into account either GFR or albuminuria because “The contribution to risk assessment for a first ASCVD [atherosclerotic CVD] event using ApoB [apolipoprotein B], CKD, albuminuria, or cardiorespiratory fitness is uncertain at present.”14
Table 2.
Representative clinical guidelines and their position for incorporating CKD measures in CVD risk prediction
| AHA/ACC14 | European15 | KDIGO16 | |
|---|---|---|---|
| Published year | 2013 | 2012 | 2013 |
| Outcome(s) of interest | CHD (coronary death and non-fatal myocardial infarction) + stroke | CVD mortality | CHD (coronary death and non-fatal myocardial infarction) |
| CKD measures for risk classification | Not included due to uncertainty | very high (=10-year CVD mortality risk >10%): GFR <30, diabetes plus elevated albuminuria high (=10-year CVD mortality risk 5-10%): GFR 30-59 |
high risk: all persons with CKD aged ≥50 years and persons with CKD <50 years with predicted 10-year CHD risk >10% |
On the other hand, the 2012 European guidelines on CVD15 incorporate both GFR and albuminuria, with some prioritization on GFR over albuminuria. Specifically, the European guidelines consider individuals with GFR <30 ml/min/1.73m2 as very high risk (equivalent to 10-year predicted risk of CVD mortality ≥10%) and those with GFR 30-59 ml/min/1.73m2 as high risk (10-year CVD mortality risk ≥5% to <10%) (Table 1).15 Albuminuria is only discussed in the context of diabetes, and diabetic patients with albuminuria are categorized as very high risk.15 The 2013 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Lipid Management in CKD16 takes a more extreme approach and considers everyone aged ≥50 years with CKD (i.e., GFR <60 ml/min/1.73m2 and/or albuminuria ≥30 mg/day) as high CVD risk.16 For younger individuals with CKD, the KDIGO Guideline recommends estimating 10-year risk of coronary heart disease (i.e., coronary death or non-fatal myocardial infarction) and considering 10% or greater as high risk, a group that would benefit from statin therapy.16
A recent individual-level data meta-analysis for CKD measures improving CVD risk prediction
As aforementioned, previous studies have investigated different CKD measures and CVD outcomes and applied distinct statistical approaches. In this context, a recent individual-level data meta-analysis from the CKD Prognosis Consortium is of value since it has uniformly investigated estimated GFR (eGFR) based on creatinine and urine albumin-to-creatinine ratio (ACR) as CKD measures and applied the same statistical approach across 24 cohorts with >630,000 participants.17
This meta-analysis demonstrated that both eGFR and ACR were independently and significantly associated with four subtypes of CVD, i.e., CVD mortality, coronary heart disease, stroke, and heart failure.17 Of these, the two CKD measures were particularly strongly associated with CVD mortality and heart failure. Consistently, the addition of either or both of CKD measures more evidently improved the prediction (assessed by Harrell's c-statistic and net reclassification improvement) of CVD mortality and heart failure as compared to that of coronary disease and stroke (Figure 117). The improvement was more evident with ACR than with eGFR.
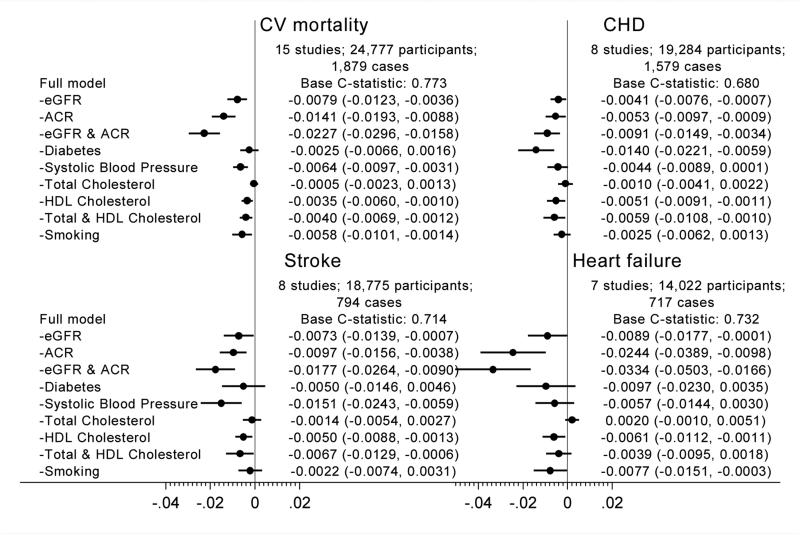
Figure 1.
Difference in C-statistic for cardiovascular outcomes by adding kidney measure(s) to traditional models in the combined general population and high-risk cohorts.
The assessment of whether the addition of a biomarker can improve prediction, described above, is implicitly linked to a clinical question about whether we would benefit from newly assessing that biomarker. However, in CKD population, by definition, data on eGFR and albuminuria are likely to be readily available. In such a situation, a practical question is which data of CKD measures and traditional risk factors should be kept in consideration for CVD prediction. From this perspective, this meta-analysis has evaluated the drop in c-statistic by subtracting each predictor (or some combinations) in turn and demonstrated that the combination of both eGFR and ACR contributed to better risk discrimination more than any single traditional risk factors for almost all CVD subtypes (Figure 217).
Figure 2.
C-statistic difference for four cardiovascular outcomes by omitting kidney disease measures and traditional risk factors from a model with all risk factors in a CKD population
What would be key implications from this individual-level data meta-analysis to the representative clinical guidelines? The answer would depend on the CVD outcomes of interest, target population, and availability of CKD measures in that population. The AHA/ACC Guideline currently focuses on the risk of coronary heart disease and stroke, and for these two CVD outcomes, the addition of eGFR and ACR has only modestly improved the prediction. However, this AHA/ACC Guideline recognizes the importance of including heart failure as an outcome of interest. If such a modification occurs in the future, it is worth considering the addition of GFR and ACR. The European Guideline is based on the risk of CVD mortality and now takes into account both GFR and ACR. However, as mentioned above, this European Guideline prioritizes GFR over ACR in this context. Our data suggest the need for more emphasis on ACR for better classification of CVD mortality risk. For the KDIGO Lipid Guideline, this meta-analysis demonstrates that both GFR and ACR are useful to classify the risk of CVD in the CKD population. This KDIGO Lipid Guideline fundamentally focuses on statin therapy, but new treatment options of controlling cholesterol increasingly attract attention. Thus, refinement of the CVD risk within the CKD population may be needed. In such a situation, both GFR and ACR would be of value.
Other biomarkers for predicting CVD risk in individuals with CKD
The KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of CKD recommends evaluating general clinical risk (e.g., mortality and the risk of end-stage renal disease) with GFR and ACR. As described in the prior section, this approach will be generally useful for CVD risk prediction as well. However, in case finer risk classification is needed, there are several promising biomarkers as summarized below.
Filtration markers other than serum creatinine
The most studied alternative filtration marker is likely cystatin C. Several studies have consistently demonstrated that eGFR based on cystatin C predicts CVD risk better than eGFR based on serum creatinine.18, 19 Although eGFR based on both serum creatinine and cystatin C is shown to best estimate measured GFR, of interest, often eGFR based on cystatin C predicts clinical outcomes better than eGFR based on the combination of those two filtration markers. The contribution of non-GFR determinants of cystatin C such as inflammation to CVD prediction may account for this observation.18
β2-microglobulin (B2M) and β-trace protein (BTP) are other alternative filtration markers. Of these, a few studies have demonstrated particularly strong associations of B2M with CVD outcomes. For example, using data from the Chronic Renal Insufficiency Cohort, Foster et al. recently reported that in patients with CKD, only B2M, but not creatinine-based eGFR or BTP, was significantly associated with CVD events (myocardial infarction, heart failure, and stroke).20 Similarly, among cystatin C, B2M, and BTP, only B2M significantly improved the prediction of a composite of CVD outcomes beyond traditional risk factors among participants with CKD in the Atherosclerosis Risk in Communities Study.21 This strong association of B2M with CVD may reflect its property as a good filtration marker and/or a molecule damaging vasculature through amyloid formation.21, 22
Measures of subclinical atherosclerosis or cardiac damage/overload
Theoretically it seems reasonable that measures reflecting the disease process in the cardiovascular system predict clinical CVD events well. In this context, the AHA/ACC Cholesterol Guideline recommends the assessment of coronary artery calcium (CAC) or ankle brachial index (ABI) to refine CVD risk.4 Of these, a body of evidence demonstrates CAC as one of the strongest predictor of CVD in the general population.23 Despite some concerns about the clinical implications of CAC in patients with CKD due to their unique calcium-phosphate metabolism,24 our group recently confirmed the usefulness of CAC over two other measures of subclinical atherosclerosis, ABI and carotid intima-media thickness, for predicting CVD events in persons with CKD.25
Blood levels of cardiac troponins and natriuretic peptides are commonly used to diagnose myocardial infarction and heart failure, respectively, in clinical practice. A growing body of evidence suggests that these cardiac markers can also inform future risk of incident CVD events. Of interest, high-sensitivity cardiac troponin T levels are detected in the majority of the middle-aged and older general population and are shown to be more strongly associated with heart failure than coronary heart disease.26 There have been some concerns about how to interpret cardiac troponin T and natriuretic peptides in patients with CKD, as these markers may be elevated due to reduced kidney function but not necessarily cardiac damage or overload. Nonetheless, several studies, including ours, have demonstrated that both cardiac troponin T and natriuretic peptides improve the prediction of CVD events, particularly heart failure, beyond traditional risk factors even in the CKD population.21
Areas of future research
A risk-centered approach is currently incorporated for lipid management and aspirin therapy.3, 4 For blood pressure management, although not yet implemented in U.S. guidelines,27 a recent meta-analysis from the Blood Pressure Lowering Treatment Trialists’ Collaboration28 has demonstrated that such a risk-centered approach may also be advantageous for hypertension management. Since hypertension is prevalent among those with CKD, future studies would be warranted to determine whether predicted risk can guide blood pressure control in persons with CKD.
The AHA/ACC Guideline on the Assessment of Cardiovascular Risk14 emphasizes the importance of “risk discussion” between patients and physicians for shared decision making. Such a discussion would include the patient's predicted risk for CVD, potential benefits/harms of treatments, and patient preferences of preventive therapies.14 However, to our best knowledge, data on these aspects for patients with CKD are sparse.
Most previous studies have investigated the risk prediction of CVD mortality, coronary heart disease, stroke, and heart failure. However, there are some other CVD subtypes relevant to individuals with CKD such as sudden cardiac death, atrial fibrillation, and peripheral artery disease including ischemic limb amputation. Statin and/or aspirin therapy based on risk of CHD and stroke may modify the risk of these understudied CVD subtypes or their complications as well. However, since there are specific preventive and therapeutic options available for sudden cardiac death, atrial fibrillation, and peripheral artery disease, specific investigations for these CVD subtypes are needed.
Conclusions
Two key CKD measures, eGFR and albuminuria, improve CVD risk prediction beyond traditional risk factors, particularly for CVD mortality and heart failure. Overall, albuminuria demonstrates more evident improvement than eGFR based on serum creatinine. It seems reasonable to take into account these CKD measures for CVD prediction, particularly when the data have been already measure for some clinical indications (e.g., CKD patients). When the risk prediction should be further refined other biomarkers can be also useful, and measures reflecting pathophysiological process of CVD such as coronary artery calcium and high-sensitivity cardiac troponin T seem particularly promising. Future clinical guidelines may require updates regarding whether/how to incorporate CKD measures and other biomarkers in CVD prediction, depending on the CVD outcomes of interest, target population, and availability of those measures and biomarkers in that population.
KEY POINTS.
A recent international meta-analysis of individual-level data demonstrates that eGFR and albuminuria improve CVD risk prediction beyond traditional CVD risk factors, particularly for CVD mortality and heart failure.
This meta-analysis also shows that albuminuria contributes to better CVD risk prediction more than eGFR and most modifiable traditional risk factors.
Filtration markers other than serum creatinine, e.g., cystatin C and β2-microglobulin, lead to better CVD risk prediction beyond creatinine-based eGFR.
If the goal is to predict CVD risk, measures of CVD process, e.g., coronary artery calcium and cardiac troponins, are promising in the CKD population.
Acknowledgements
None
Financial support and sponsorship
Drs. Matsushita, Ballew, and Coresh were partly supported by the US National Institute of Health and US National Kidney Foundation.
Footnotes
Conflicts of interest:
None
References
- 1.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Kottgen A, Levey AS, Levin A. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–69. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 2.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibbins-Domingo K. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation StatementAspirin Use for the Primary Prevention of CVD and CRC. Annals of Internal Medicine. 2016 doi: 10.7326/M16-0577. N/A:N/A-N/A. [DOI] [PubMed] [Google Scholar]
- 4.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr., Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 5.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. 2007;50:217–24. doi: 10.1016/j.jacc.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 6.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, Levey AS, de Jong PE, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–52. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 7.Hallan S, Astor B, Romundstad S, Aasarod K, Kvenild K, Coresh J. Association of Kidney Function and Albuminuria With Cardiovascular Mortality in Older vs Younger Individuals: The HUNT II Study. Arch Intern Med. 2007;167:2490–2496. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 8.Weiner DE, Tighiouart H, Griffith JL, Elsayed E, Levey AS, Salem DN, Sarnak MJ. Kidney disease, Framingham risk scores, and cardiac and mortality outcomes. Am J Med. 2007;120:552, e1–8. doi: 10.1016/j.amjmed.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 9.Clase CM, Gao P, Tobe SW, McQueen MJ, Grosshennig A, Teo KK, Yusuf S, Mann JF. Estimated glomerular filtration rate and albuminuria as predictors of outcomes in patients with high cardiovascular risk: a cohort study. Ann Intern Med. 2011;154:310–8. doi: 10.7326/0003-4819-154-5-201103010-00005. [DOI] [PubMed] [Google Scholar]
- 10.Ito H, Pacold IV, Durazo-Arvizu R, Liu K, Shilipak MG, Goff DC, Jr., Tracy RP, Kramer H. The effect of including cystatin C or creatinine in a cardiovascular risk model for asymptomatic individuals: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2011;174:949–57. doi: 10.1093/aje/kwr185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nerpin E, Ingelsson E, Riserus U, Sundstrom J, Larsson A, Jobs E, Jobs M, Hallan S, Zethelius B, Berglund L, Basu S, Arnlov J. The combined contribution of albuminuria and glomerular filtration rate to the prediction of cardiovascular mortality in elderly men. Nephrol Dial Transplant. 2011;26:2820–7. doi: 10.1093/ndt/gfq848. [DOI] [PubMed] [Google Scholar]
- 12.Chen SC, Su HM, Tsai YC, Huang JC, Chang JM, Hwang SJ, Chen HC. Framingham risk score with cardiovascular events in chronic kidney disease. PLoS One. 2013;8:e60008. doi: 10.1371/journal.pone.0060008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Bittencourt MS, Hulten EA, Ghoshhajra B, Abbara S, Murthy VL, Divakaran S, Nasir K, Gowdak LH, Riella LV, Chiumiento M, Hoffmann U, Di Carli MF, Blankstein R. Incremental prognostic value of kidney function decline over coronary artery disease for cardiovascular event prediction after coronary computed tomography. Kidney Int. 2015;88:152–9. doi: 10.1038/ki.2014.426. [This is one of the most recent single studies exploring the additional value of reduced kidney function for predicting CVD risk among those without a clinical history of CVD. This study found that reduced kidney function and the presence of coronary artery detected by coronary computed tomography angiography synergestically improve CVD prediction. Using data in a subsample, this study also reports potentail value of proteinuria.] [DOI] [PubMed] [Google Scholar]
- 14.Goff DC, Jr., Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Sr., Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson J, Schwartz JS, Shero ST, Smith SC, Jr., Sorlie P, Stone NJ, Wilson PW. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte Op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F, Cooney MT, Bax J, Baumgartner H, Ceconi C, Dean V, Fagard R, Funck-Brentano C, Hasdai D, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Aboyans V, Ezquerra EA, Baigent C, Brotons C, Burell G, Ceriello A, De Sutter J, Deckers J, Del Prato S, Diener HC, Fitzsimons D, Fras Z, Hambrecht R, Jankowski P, Keil U, Kirby M, Larsen ML, Mancia G, Manolis AJ, McMurray J, Pajak A, Parkhomenko A, Rallidis L, Rigo F, Rocha E, Ruilope LM, van der Velde E, Vanuzzo D, Viigimaa M, Volpe M, Wiklund O, Wolpert C. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) * Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). European heart journal. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney International Supplements. 2013;3:259–305. doi: 10.1016/j.kisu.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GW, Muntner P, Roderick P, Sairenchi T, Schottker B, Shankar A, Shlipak M, Tonelli M, Townend J, van Zuilen A, Yamagishi K, Yamashita K, Gansevoort R, Sarnak M, Warnock DG, Woodward M, Arnlov J. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. The lancet Diabetes & endocrinology. 2015;3:514–25. doi: 10.1016/S2213-8587(15)00040-6. [This is probably the most comprehensive study specifically evaluating the additional value of two CKD measures, eGFR and albuminuria, in improving CVD risk prediction. This collaborative meta-analysis of individual level data demonstrates that both CKD measures improve cardiovascular risk prediction, particularly for cardiovascular mortality and heart failure. Comparing the two CKD measures, the prediction improvement is greater with albuminuria than eGFR.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RT. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–43. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waheed S, Matsushita K, Sang Y, Hoogeveen R, Ballantyne C, Coresh J, Astor BC. Combined association of albuminuria and cystatin C-based estimated GFR with mortality, coronary heart disease, and heart failure outcomes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2012;60:207–16. doi: 10.1053/j.ajkd.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Foster MC, Coresh J, Hsu CY, Xie D, Levey AS, Nelson RG, Eckfeldt JH, Vasan RS, Kimmel PL, Schelling J, Simonson M, Sondheimer JH, Anderson AH, Akkina S, Feldman HI, Kusek JW, Ojo AO, Inker LA, Consortium CKDB and the CSI Serum beta-Trace Protein and beta-Microglobulin as Predictors of ESRD, Mortality, and Cardiovascular Disease in Adults With CKD in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2016;68:68–76. doi: 10.1053/j.ajkd.2016.01.015. [Using data from a US representative cohort of CKD patients, this study reports that levels of B2M and BTP may contribute additional risk information beyond creatinine-based eGFR. In terms of CVD risk, B2M was most strongly associated compared to BTP.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsushita K, Sang Y, Ballew SH, Astor BC, Hoogeveen RC, Solomon SD, Ballantyne CM, Woodward M, Coresh J. Cardiac and kidney markers for cardiovascular prediction in individuals with chronic kidney disease: the Atherosclerosis Risk in Communities study. Arterioscler Thromb Vasc Biol. 2014;34:1770–7. doi: 10.1161/ATVBAHA.114.303465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liabeuf S, Lenglet A, Desjardins L, Neirynck N, Glorieux G, Lemke H-D, Vanholder R, Diouf M, Choukroun G, Massy ZA. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82:1297–1303. doi: 10.1038/ki.2012.301. [DOI] [PubMed] [Google Scholar]
- 23.Yeboah J, Young R, McClelland RL, Delaney JC, Polonsky TS, Dawood FZ, Blaha MJ, Miedema MD, Sibley CT, Carr JJ, Burke GL, Goff DC, Jr., Psaty BM, Greenland P, Herrington DM. Utility of Nontraditional Risk Markers in Atherosclerotic Cardiovascular Disease Risk Assessment. J Am Coll Cardiol. 2016;67:139–47. doi: 10.1016/j.jacc.2015.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharples EJ, Pereira D, Summers S, Cunningham J, Rubens M, Goldsmith D, Yaqoob MM. Coronary artery calcification measured with electron-beam computerized tomography correlates poorly with coronary artery angiography in dialysis patients. American Journal of Kidney Diseases. 2004;43:313–319. doi: 10.1053/j.ajkd.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 25*.Matsushita K, Sang Y, Ballew SH, Shlipak M, Katz R, Rosas SE, Peralta CA, Woodward M, Kramer HJ, Jacobs DR, Sarnak MJ, Coresh J. Subclinical atherosclerosis measures for cardiovascular prediction in CKD. J Am Soc Nephrol. 2015;26:439–47. doi: 10.1681/ASN.2014020173. [This study compares three representative measures of subclinical atherosclerosis, CAC, ABI, and carotid intima-media thickness, for improving CVD risk prediciton beyond traditional risk factors in those with and without CKD. In this context, among those with CKD, CAC outperforms the other two.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr., Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr., Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 28.Blood Pressure Lowering Treatment Trialists C. Sundstrom J, Arima H, Woodward M, Jackson R, Karmali K, Lloyd-Jones D, Baigent C, Emberson J, Rahimi K, MacMahon S, Patel A, Perkovic V, Turnbull F, Neal B. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591–8. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]