Abstract
Patients surviving acute stages of sepsis often display impaired adaptive immune responses. Using the cecal ligation and puncture (CLP) model, we demonstrated that sepsis leads to substantial and long-lasting changes in the naïve CD8 T-cell repertoire affecting the capacity of the host to respond to new infections. However, the identity of CD8 T-cell extrinsic factor(s) and mechanism(s) that contribute to impaired CD8 T-cell responses after sepsis is currently unknown. Priming of naïve CD8 T-cells is critically dependent on the ability of dendritic cells (DCs) to provide Ag, co-stimulation, and inflammatory “signal 3” cytokines, therefore the sepsis-induced changes in the DC compartment might represent a contributing factor leading to diminished CD8 T-cell immunity in septic hosts. In a direct test of this hypothesis we show that in addition to numerical decline, sepsis leads to functional impairments in DCs diminishing their capacity to produce cytokines upon TLR stimulation in vitro or after infection in vivo. Importantly, we demonstrated a direct link between DC dysfunction and impairments in CD8 T-cell immunity after sepsis by directly targeting Ag to DCs. Finally, post-sepsis Flt3 ligand (Flt3L) treatment increased the number of DCs and improved DC function, including the ability to sense inflammation and produce IL-12 leading to improved primary CD8 T-cell responses to newly encountered antigens. Thus, sepsis-induced numerical and functional loss of DCs contributes to the observed defects in CD8 T-cell immunity, and therapeutic approaches designed to improve the status of the DC compartment after sepsis might facilitate the recovery of CD8 T-cell immunity.
Introduction
Sepsis is characterized as an injurious immune response resulting from an uncontrolled systemic infection. The global death toll of sepsis is estimated at 5.3 million individuals annually, yet even those surviving the initial septic insult suffer from long-term impairments and chronic immunosuppression characterized by increased susceptibility to new (secondary) infections and reactivation of latent viruses (1-6). Increased T cell apoptosis observed in human patients suggests that defects in T cell-mediated immunity can be an underlying cause, at least in part, for sepsis-induced general immunosuppression (7-9). Using the murine CLP model of sepsis induction we recently showed that sepsis leads to a numerical loss of naïve (Ag non-experienced) CD8 T cells and impairs primary CD8 T cell responses to acute and chronic infections (10-13). In addition, polymicrobial sepsis alters Ag-dependent and -independent memory CD8 T cell functions (i.e., provide protection to pathogen re-challenge or perform innate function such as capacity to produce IFN-γ in response to heterologous infections, respectively) (12, 13). While these observations demonstrated that sepsis leads to sustained impairments in naïve (primary) and memory (secondary) CD8 T cell responses, the contribution of the environment, in which CD8 T cells recognize and respond to their cognate Ag, to sepsis-induced immunosuppression is not well defined.
The optimal expansion of CD8 T cells following interaction with cognate Ag during an infection and/or vaccination is reliant on CD8 T cell extrinsic factors including Ag:MHC complex (signal 1), co-stimulatory ligands (signal 2), and signal 3 cytokines (e.g., IL-12 and type I IFNs) (14-17). Dendritic Cells (DCs) are professional antigen presenting cells (APCs) capable of providing CD8 T cells with Ag, co-stimulation, and signal 3 inflammatory cytokines critical for primary CD8 T cell expansion (18-20). Murine DCs are generally divided into two large subgroups: the plasmacytoid DCs (pDCs) and the conventional DCs (cDCs) (21). The pDCs, which express low to moderate CD11c levels, are found in lymphoid tissues as well as the blood. pDCs are especially important in viral infections where they recognize foreign nucleic acids, produce Type I IFN, and present viral Ag (22). Compared to pDCs, cDCs have an enhanced capability to process/present Ag and prime naïve T cell responses (22). The mouse spleen is comprised of three main cDC subtypes: CD4+ cDC (CD4+ CD8−), CD8+ cDC (CD4− CD8+), and DN cDC (CD4− CD8−) (21). CD4+ cDC, which make up the greatest percentage of cDC in the spleen, are located in the marginal zones of the spleen and efficiently activate CD4+ T cells (21, 23). CD8+ cDC are primarily located in the T cell zones of the spleen, express CD205, have the capacity for cross presentation and induction of CD8 T cell responses (21-23), and are potent producers of IL-12 (23). Therefore, the post-sepsis status of DCs, and their ability to provide the necessary signals for optimal priming of naïve CD8 T cells, could be an extrinsic factor contributing to the observed defect in primary CD8 T cell responses (18, 19, 24).
Sepsis leads to a loss of DCs in the spleen (25) and a reduction in myeloid and plasmacytoid DCs in the blood of septic patients (26). Moreover, DCs from septic patients have diminished HLA-DR expression and decreased capacity to produce pro-inflammatory cytokines in response to LPS stimulation (26). Importantly, low DC counts in patient blood correlates with increased sepsis severity (27), suggesting the DC compartment might play an important role during sepsis progression. The importance of DCs in sepsis has also been established in experimental models of sepsis, including the murine CLP model that closely mimics the disease course of septic patients (28-30). Studies using CD11c-diphtheria toxin (DT) receptor (DCKO) transgenic mice indicate that mice treated with DT to reduce DC numbers had increased sepsis severity that was partially recovered upon reconstituting the DC compartment with adoptively transferred DCs from non-septic hosts (31). In the CLP model, loss of DCs occurs in the spleen, peritoneum, bone marrow, and select lymph nodes of septic mice (29, 30, 32, 33). Bone marrow-derived DCs [BMDC] from septic mice showed increased IL-10 production and impaired Th1 CD4 T cell priming (33). Additional functional studies have established that DCs obtained from septic hosts have diminished capacity to produce IL-12 in vitro in response to TLR ligands (29, 30, 33-35). Decreased IL-12 and increased IL-10 production by DCs isolated from the lungs of septic mice has also been observed (35-37). Mechanistically, epigenetic changes in the IL-12 promoter corresponds with the diminished capacity of DCs from the septic host to produce sufficient amounts of IL-12 (34).
As these previous studies demonstrate a reduction in DC number and function following sepsis, restoration of both the number and function of DCs after sepsis could help restore immune function and provide therapeutic benefit. Indeed, the transfer of BMDC into septic mice reduces inflammation in the lungs, increases Th1 and decreases Th2 cytokine levels, and increases survival (36, 38). FMS-like tyrosine kinase 3 ligand (Flt3L), a hematopoietic growth factor capable of stimulating DC expansion, improves survival and protects against opportunistic infection when administered before and after burn wound infections in mice (39, 40). Furthermore, Flt3L administration to mice subjected to zymosan-induced peritonitis helped ameliorate the loss of pulmonary DCs and IL-12 production after zymosan administration while also diminishing pulmonary tissue damage and mortality (41).
The observed loss of DC numbers and diminished capacity to produce IL-12 post-sepsis provide support for a lesion in DCs contributing to CD8 T cell dysfunction. However, studies seeking to understand the status of DCs in the context of diminished primary CD8 T cell responses are lacking. Here, we establish the role of DCs in sepsis-induced CD8 T cell dysfunction by elucidating the contribution of the DC compartment in primary CD8 T cell responses to model pathogens. In addition, we show that therapeutic (e.g., post sepsis Flt3L treatment) approaches designed to increase DC number and function aid in reversing the sepsis-induced lesions in CD8 T cell immunity.
Materials and Methods
Ethics statement
All experimental procedures using mice were approved by the University of Iowa Animal Care and Use Committee under the ACURF protocol number 1312217. The experiments performed in this study were done under strict accordance to the Office of Laboratory Animal Welfare guidelines and the PHS Policy on Humane Care and Use of Laboratory Animals.
Mice and bacterial infections
C57BL/6 (B6; Thy1.2/1.2) and Swiss Webster mice were purchased from Jackson Laboratories (Bar Harbor, ME), housed under specific pathogen-free conditions and transferred to biosafety level 2 housing post-infection. Thy1.1/1.1 OT-I TCR-transgenic and Thy1.1/1.1 P14 TCR-transgenic (specific for LCMV-derived gp33 epitope) mice (TCR-Tg) were bred at the University of Iowa and housed under pathogen-free conditions. OVA257–expressing Listeria monocytogenes [LM-OVA, 103 CFU/mouse, i.v.] strains, virulent 10403s Listeria monocytogenes [LM, 105 CFU/mouse, i.v.] strains, and gp33-expressing attenuated Listeria monocytogenes [attLM, 106 CFU/mouse, i.v.] strains were grown and used as described (42). As a measure of bacterial clearance, CFUs of Listeria in the spleen and liver were determined on the indicated days post-infection by performing serial dilutions followed by inoculation of Streptomycin TSB Agar Plates (12, 42).
Adoptive transfer of CD8 T cells
To generate a primary CD8 T cell response, 103 naïve Thy1.1/Thy1.1 OT-1 CD8 T cells or Thy1.1/Thy1.1 P14 CD8 T cells were obtained from the peripheral blood of young naïve TCR-Tg mice and transferred into Thy1.2 recipients 1 d before infection with 103 LM-OVA or 106 attLM-gp33, respectively.
Cell isolation/analysis
Before removal of lymphoid organs and tissues, samples of blood were obtained by retro-orbital puncture. For experiments enumerating DC subsets, spleens were treated with collagenase XI (125 U/mL) and DNAse I (10 μg/mL) and shaken at ~450 RPM for 10 min at 30° C. Single cell suspensions were then prepared and samples were again shaken at ~450 RPM for 10 min at 30° C. Appropriate volumes of EDTA were added to the samples for 5 min at room temperature to inhibit the action of collagenase. Samples were then spun down and re-suspended in fresh media. Single-cell suspensions from spleen and lymph nodes (LN) were washed before Ab staining.
Cecal ligation and puncture
Polymicrobial sepsis was induced by CLP (11, 12, 43). Briefly, mice were anesthetized, the abdomen was shaved, disinfected, and a midline incision was made. After identification of the cecum, the distal third was ligated with 4-0 silk sutures and punctured once using a 25-gauge needle to extrude a small amount of cecal content. The cecum was returned to the abdomen, the peritoneum was closed via continuous suture, and the skin was glued together using tissue adhesive (Vetbond; 3M, St. Paul, MN). Saline (1 ml) was administered s.c. following the procedure for resuscitation. Bupivacaine was given at the incision site, and flunixin meglumine was administered twice for postoperative analgesia. This level of injury was used to create a chronic septic state characterized by the loss of appetite and body weight, ruffled hair, shivering, diarrhea, and/or periorbital exudates and with 5–10% mortality rate. Sham-treated mice underwent the same procedure excluding CLP.
Abs, peptides, and intracellular stains
Flow cytometry data were acquired using a FACSCanto (BD Biosciences, San Diego, CA) and analyzed with FlowJo software (Tree Star, Ashland, OR). For enumerating DCs, an empty channel was utilized to eliminate autofluorescent contamination. To assess the expression of cell-surface proteins, mAb were incubated at 4°C for 30 min and fixed using Cytofix/Cytoperm Solution (BD Biosciences). Surface molecules were detected with the following mAb: CD8 (clone 53-6.7; eBioscience), Thy1.1 (HIS51; eBioscience), CD4 (clone GK1.5; eBioscience), CD19 (clone ID3; Tonbo Biosciences), B220 (clone RA3-6B2; eBioscience), CD11c (clone HL3; BD Biosciences), IA-IE (clone 2G9; BD Biosciences), CD40 (clone HMLIO-3; eBioscience), CD80 (clone 16-10AI; eBioscience), DEC-205 (clone NLDC-145; Biolegend), V∝2 TCR (clone B20.1; eBioscience), Vβ5.1,5.2 (clone MR9.4; BD Biosciences) and appropriate isotype controls. Endogenous OVA-specific CD8 T cells were quantified using Kb-OVA tetramers with Limit of Detection (L.O.D.) determined by calculating the background OVA-positive cells in a naïve, non-infected mouse without adoptively transferred OT-I cells.
Ex vivo intracellular stains
Mice infected with virulent Listeria were harvested 14 h after infection. After obtaining single cell suspensions from the spleen, cells were incubated for 5 h at 37°C in the presence of brefeldin A (Becon-Dickinson Biosciences) prior to ex vivo intracellular staining for IL-12/IL-23p40 (clone C15.6, Biolegend). Intracellular staining was performed after surface staining and fixation/permeabilization of the cell membrane using Cytofix/Cytoperm solution (BD Biosciences).
DC Enrichment and TLR stimulation
Single cell suspensions were produced from pooled groups of two spleens, filtered, and incubated with anti-CD11c mAb-conjugated magnetic microbeads (Clone N418; Miltenyi Biotech) for 15 min in the dark at 4°C according to manufacturer’s instructions. Cells were washed and resuspended in cold Automacs buffer and passed through an LS MACS separation column (Miltenyi Biotech). Columns were washed three times with 3 mL of cold Automacs buffer and the positive fraction collected. Enriched cells were plated at 0.4-1 × 106 cells/dish and stimulated for 7 h at 37°C with 1 μg/mL LPS (055:B5; Sigma-Aldrich) or 1 μg/mL CpG (ODN 1826; Integrated DNA Technologies). After 2 h of stimulation, 1 mg/mL of Brefeldin A (BD Biosciences) was added to each dish. After 7 h incubation, intracellular stains for IL-12/IL-23p40 (clone C15.6, Biolegend) and TNF-α (clone MPG-XT22, eBioscience) were performed after surface staining and fixation/permeabilization of the cell membrane using Cytofix/Cytoperm solution (BD Biosciences).
DEC-205 Ab production & administration
The anti-DEC-205 hybridoma (NLDC-145) was gifted by Deborah Palliser (Albert Einstein College of Medicine). The anti-DEC-205 mAb was purified with a protein G column prior to peptide conjugation. The peptides used for conjugation were synthesized with an extra cysteine on the c-terminus (Genscript). Sulfo-SMCC (Thermo Fisher Scientific) was used as a linker allowing for the conjugation between the cysteine on the peptide and the amine groups of the mAb. The conjugation was performed according to the Sulfo-SMCC manufacturer’s instructions. Conjugated DEC-205 mAb/Ag complex was diluted in sterile PBS to reach its desired concentration (10 μg/mouse) prior to i.v. administration. Immediately following the administration of DEC-205 mAb/Ag complex, CpG diluted in sterile PBS (50 μg/mouse) was administered i.p.
Serum ELISAs
IL-12 and IFN-γ levels (pg/mL) were detected from serum collected by retro-orbital bleed at 20 h post-infection using Mouse IL-12 Platinum ELISA (affymetrix, Cat. BMS616) and Mouse IFN-gamma Platinum ELISA (affymetrix Cat. BMS606). Tests were performed according to manufacturer’s instructions and absorbance values (450 nm) were measured and assessed using Gen5 software (BioTek).
Flt3L production and administration
Flt3L-Ig was purified from cell culture supernatants via Protein A affinity chromatography. 100ug of Flt3L-Ig was administered i.p. on days day 1-4 post CLP.
Statistical Analysis
Data were analyzed using Prism6 software (GraphPad) and two-tailed, unpaired Student t -test or one-way ANOVA with a confidence interval of >95% to determine significance (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and n.s. as no significance). Data generated as scatter plots and as bar graphs are presented as mean ± SEM
Results
Septic environment contributes to the impaired primary CD8 T cell expansion
Previous work from our lab has shown that vaccine- or infection-induced memory CD8 T cells present at the time of sepsis induction have diminished per-cell capacity to undergo proliferative expansion upon pathogen re-challenge or exert their innate functions and produce IFN-γ upon heterologous infection as compared to memory CD8 T cells from control (sham-treated) mice (12). Interestingly, this decrease in Ag-dependent and –independent functions also occurred in memory CD8 T cells that were adoptively transferred into CLP mice days after the surgery and resolution of the sepsis-induced hyper-inflammatory state. These data suggest the sepsis environment controls, at least in part, the ability of memory CD8 T cells to respond to cognate Ag stimulation and/or sense the inflammatory cues in the environment.
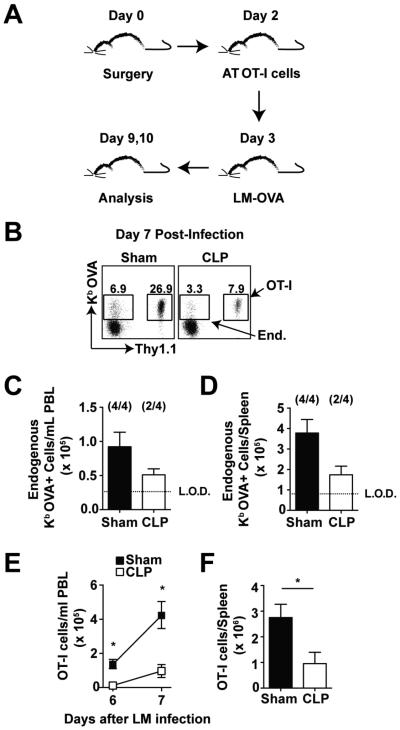
To formally prove that CD8 T cell extrinsic factors present early after sepsis-induction can also control primary CD8 T cell responses, naïve TCR transgenic OVA257-specific Thy 1.1 OT-I CD8 T cells were adoptively transferred into naïve Thy 1.2 C57BL/6 (B6) mice 2 days after sham or CLP surgery (Fig 1A). To examine the ability of ‘sensor’ OT-I CD8 T cells to respond to cognate Ag-stimulation, sham- and CLP-treated mice were infected with recombinant Listeria monocytogenes expressing OVA (LM-OVA; Fig 1A) 3 days after the sepsis-induction. As shown previously (44) the magnitude of the proliferative expansion of endogenous Kb OVA-specific CD8 T cells detected at the peak of the response (day 7 post LM-OVA) in the peripheral blood (PBL) and spleen was diminished in CLP mice compared to sham mice (2 out of 4 CLP mice had Kb OVA-specific CD8 T cells above the limit of detection; Fig 1B-D). Importantly, reduced number and decreased primary expansion was also observed with the adoptively transferred OT-I CD8 T cells (Fig 1B, E-F). Of note, differences in OT-I cell engraftment could account for the observed differences in LM-induced expansion observed in CLP compared to sham hosts. However, similar seeding of CFSE-labeled splenocytes (transferred 2 days post surgery) was detected in the spleens of both group of mice (data not shown) suggesting that the diminished primary CD8 T cell accumulation in CLP-treated hosts was not due to differences in the number of naïve CD8 T cell precursors present at the time of antigen encounter (infection). Thus, these data indicate that sepsis-induced changes in the environment contribute to sub-optimal expansion of naïve CD8 T cells upon Ag encounter in vivo, even when the naïve CD8 T cells were not exposed to the inflammatory cytokine milieu that characterizes early sepsis.
Figure 1. Septic environment contributes to the impaired primary CD8 T cell expansion.
(A) Experimental design. Naïve OVA257-specific T cell receptor transgenic (TCR-tg) OT-I CD8 T cells (Thy1.1; 103/mouse, i.v.) were adoptively transferred into B6 (Thy1.2) mice 2 d post sham or CLP surgery. All groups of recipient mice were challenged 1 d later with recombinant virulent LM expressing OVA257 (103 CFU/mouse, i.v.). (B) Representative dot plots show the frequency of tetramer positive (Kb–OVA) CD8 T cells (endogenous – Thy1.2 or TCR-Tg - Thy1.1) of CD8 T cells in PBL at day 7 post-infection. Naïve, non-infected mice were used to calculate L.O.D. for tetramer staining. (C) Total number of endogenous Kb–OVA+ cells in PBL on d 7 post-LM infection. Numbers inside parentheses indicate the number of mice that had detectable (above the LOD) Thy1.2 OVA257-specific CD8 T cell responses. (D) Total number of endogenous CD8 T cells isolated from the spleen on d 7 post-LM infection. Total number of OT-I OVA257-specific CD8 T cells isolated from the peripheral blood (E) at d 6 and 7 post-LM infection and the spleen (F) on d 7 post-LM infection. Data are presented as mean ± SEM of 3-4 mice per group and representative of two independent experiments. L.O.D. – represents the limit of detection as defined in naïve, non-infected B6 mice. *p ≤ 0.05 as determined by student T-test.
Sepsis leads to a reduction in number and change in relative composition of the DC compartment
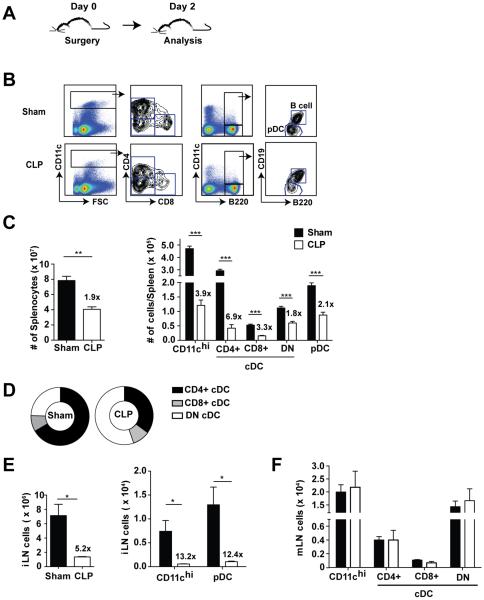
A reduction in DC number or a deficiency in the capacity of DCs to provide CD8 T cells with the necessary expansion cues could lead to suboptimal primary CD8 T cell expansion observed in septic mice. To explore how sepsis affects DC numbers, we performed sham or CLP surgery on naïve B6 mice followed by flow cytometric analysis of the spleen and lymph nodes (Fig 2A-B). Two days after the initial septic insult, there was close to a two-fold decline in total splenic cellularity (Fig 2C). Importantly, cDCs (CD11chi) and pDCs (CD11cmodB220+CD19−) were reduced four- and two-fold, respectively, in CLP-treated mice compared to their sham counterparts. Interestingly, the varying degrees of numerical loss in the cDC populations in the spleen led to a change in the relative composition of the DC compartment (Fig 2B-C) and cDCs from septic mice have an increased proportion of double-negative (CD11chiCD4−CD8−) DCs making them the major subset of the surviving cDCs (Fig 2D).
Figure 2. Reduced number of DCs early after sepsis induction.
(A) Experimental Design. Sham or CLP surgery was performed on naïve B6 mice and analysis in indicated organs was performed 2 d post-surgery. (B) Representative dot plots indicating gating strategy of conventional DC (cDC) and plasmacytoid DC (pDC) populations. (C) Cellularity and total numbers of DCs in the spleen. Fold difference in numbers of cells detected in sham and CLP groups of mice is shown. (D) Representation of cDC subsets in the spleen 2 d post sepsis-induction. Cellularity and DC numbers in the (E) distal and (F) proximal lymph nodes (inguinal – iLN and mesenteric- mLN, respectively) 2 d-post surgery. Data are presented as mean ± SEM of 3-5 mice per group and representative of three independent experiments. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 as determined by student T-test.
A decline in DC numbers was observed in the spleens early after sepsis induction suggesting that CLP leads to apoptosis of DCs. Alternatively, due to the sepsis-induced inflammation, DCs can follow inflammatory cues and be present in substantially higher numbers at the places proximal to the initial insult. To address these possibilities, DC numbers were determined in inguinal (iLN; non-reactive, distal) and mesenteric (mLN; reactive, proximal) lymph nodes 2 days after surgery. The total cellularity of the iLN was diminished five-fold and the number of cDC (CD11chi) and pDC recovered from iLN from CLP mice was greater than twelve-fold lower compared to sham mice (Fig 2E). However, no significant change was observed in the number of cDC and the subtype distribution in mLN that drain the gut mucosa and are located proximal to the site of the initial septic insult (Fig 2F), suggesting that sepsis did not change the distribution of DCs. Rather, these data suggest sepsis leads to significant decline in DC numbers at sites distal to the insult.
The previous experiments clearly demonstrate that sepsis leads to a decline in DC numbers; however, these experiments did not address the maturation status of the surviving DCs. Thus, the expression of co-stimulatory ligands CD40 and CD80 was assessed on splenic DCs 2 days after sham or CLP surgery (S1A Fig). As a positive control, LPS was administered in a separate group of naïve mice (45, 46). Interestingly, although cDCs from CLP mice had increased expression of CD40 and CD80 (as determined by gMFI) compared to sham controls, the increase in CD40 and CD80 expression was significantly lower than on DCs recovered from mice after LPS stimulation (S1B-C Fig). Thus, polymicrobial sepsis does not evoke full maturation of DCs in vivo suggesting that the ability to acquire and process new Ag is not impaired in sepsis-surviving DCs.
Sepsis diminishes DC capacity to produce IL-12 in vitro and in vivo
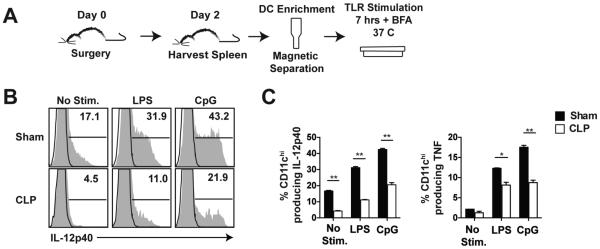
Our results thus far indicate that sepsis induces a numerical loss of DCs without significantly changing their maturation status. Next, we investigated the functionality of the surviving DCs, specifically their ability to produce inflammatory cytokines upon stimulation. To address this, an enriched CD11c+ population was obtained from sham and CLP hosts 2 days-post surgery. Following enrichment, cells were stimulated with TLR 4 or 9 ligands (LPS or CpG, respectively) and IL-12p40 and TNF production was assessed (Fig 3A). Unstimulated DCs from septic mice showed decreased production of IL-12p40 compared to sham mice, as well as decreased production when stimulated by LPS or CpG (Fig 3B-C). In addition to decreased IL-12p40 production, diminished TNF production was also observed (Fig 3C). Therefore, sepsis changes the ability of purified DCs to respond to TLR stimulation in vitro.
Figure 3. Sepsis diminishes DCs capacity to respond to TLR stimulation in vitro.
(A) Experimental Design. Enriched CD11c+ DCs from the spleens of sham and CLP mice were incubated in the presence or absence of TLR agonists (LPS and CpG) for 7 h at 37° C in the presence of Brefeldin A. (B) Representative histograms show the frequency of CD11chi cells producing IL-12p40 after in vitro incubation. (C) The percentage of CD11chi cells producing IL-12p40 and TNF upon TLR stimulation. Data are presented as mean ± SEM of 4 mice per group in pooled samples of two spleens. Data are representative of three similar and independent experiments. *p ≤ 0.05, **p ≤ 0.01 as determined by student T-test.
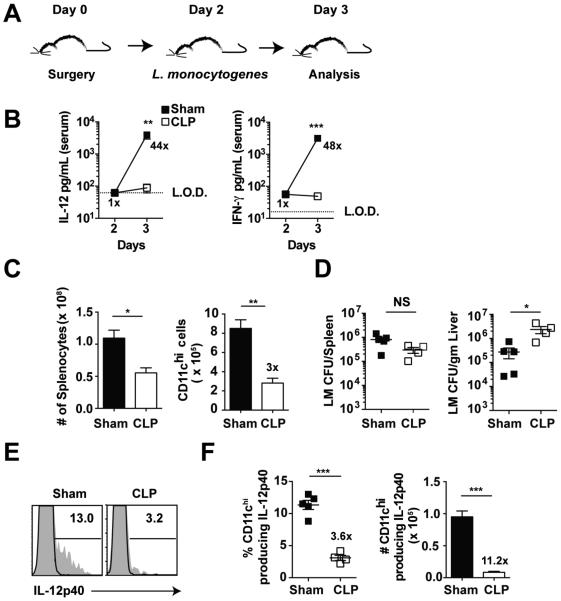
In vitro studies reveal important phenomena, yet they often fall short in accurately recapitulating the physiologic environment. To test the capacity of the host (in general) and DCs (in particular) to sense inflammation and provide important signal 3 cytokines (i.e. IL-12) in vivo, sham or CLP B6 mice were challenged with Listeria monocytogenes (LM) 2 days after surgery (Fig 4A). We and others have shown that LM has the capacity, in a dose dependent manner, to induce a vigorous early inflammatory response characterized by production of IL-12 and IFN-γ (15, 47, 48). Importantly, no difference in serum levels of IL-12 and IFN-γ was detected in sham or CLP mice at the time of LM infection (48 h post-surgery) indicating that the CLP-induced hyper-inflammatory phase had passed. One day after LM infection, sham mice showed a substantial increase (44-fold greater than CLP mice) in serum IL-12 concentration, while there was almost no increase in IL-12 production in the CLP-treated mice (Fig 4B). Similar results were obtained when examining the production of IFN-γ. Thus, these data strongly suggest that CLP-induced immunoparalysis is characterized by the inability of the host to produce pro-inflammatory cytokines upon secondary, unrelated infection.
Figure 4. Sepsis leads to an in vivo impairment of DCs to sense secondary bacterial infection and respond with IL-12 production.
(A) Experimental Design. Sham or CLP surgery was performed on naïve B6 (Thy 1.2) mice 2 d prior to infection with virulent L. monocytogenes. (10403s strain; 105 CFU/mouse, i.v.). (B) IL-12 and IFN-γ cytokine levels in serum before and 1 day post-infection. Fold difference in level of cytokine detected in Sham and CLP groups of mice is shown. Data are presented as mean ± SEM of 4 mice per group. L.O.D. – represents the limit of detection. (C) Splenic cellularity and DC numbers 1 d after LM infection. Fold difference in numbers of cells detected in sham and CLP groups of mice is shown. (D) LM titers in the spleen and liver 1 d post-infection. (E) Representative histogram showing the frequency of splenic CD11chi cells producing IL-12p40 1 day after LM infection. (F) Frequency and total numbers of splenic CD11chi cells producing IL-12p40 1 day after LM infection. Fold difference in frequency and number of cells producing cytokine in sham and CLP groups of mice is shown. Data are presented as mean ± SEM of 3-5 mice per group and representative of two independent experiments. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 as determined by student T-test.
To formally confirm the observed impairment in IL-12 production could be attributed to decreased IL-12 production by DCs in vivo, sham or CLP mice were infected with virulent LM two days after surgery and analysis was performed on day 3. Similar to our results from non-infected mice, septic mice had significantly reduced numbers of splenocytes and cDC as compared to sham controls (Fig 4C). Importantly, the bacterial LM burden in the spleen and liver was similar, if not increased in CLP group of mice, suggesting the inability of the CLP host to sense the infection and respond with production of inflammatory cytokines was not due to the decreased bacterial load in vivo (Fig 4D) (48). Direct ex vivo intracellular cytokine staining showed the frequency of cDCs producing IL-12p40 was significantly diminished in CLP mice (Fig 4E-F), suggesting sepsis changes the per-cell-capacity of DCs to respond to inflammatory cues in the environment. This 3.6-fold decrease in frequency of DCs producing IL-12p40 coupled with a 3-fold decrease in total number of cDCs recovered from the CLP mice led to an eleven-fold decrease in the number of IL-12p40 producing DCs compared to sham mice. Thus, diminished ability of DCs to produce signal 3 inflammatory cytokines in response to secondary infection suggests a potential role of the DC compartment in observed sub-optimal primary CD8 T cell responses after sepsis.
Reduced number and impaired functionality of dendritic cells early after sepsis induction in outbred hosts
The use of inbred mouse strains like B6 enables substantial advantages to the experimentalist (i.e., precise determination of pathogen-specific CD8 T cell responses); however, this genetically homogenous population does not reflect the true genetic diversity observed in the human population. To determine whether the loss of DCs following sepsis could be recapitulated in a genetically heterogeneous population, we performed sham or CLP surgery on outbred Swiss Webster mice (Fig 5A). Similar to results in inbred B6 mice, a decline in total cellularity, cDC numbers, and changes in the composition of remaining cDC populations in the spleens from outbred Swiss Webster mice was observed after the septic event (Fig 5B-C). Thus, the loss of cDC and their subtypes in genetically diverse outbred mice 48 h following sepsis indicates the sensitivity of DCs to sepsis is not limited to inbred mouse strains. Furthermore, in a manner similar to B6 mice, cDCs from outbred CLP mice two days after surgery showed increased expression of CD40 and CD80 (as determined by gMFI) as compared to outbred sham controls (SF1D-F). cDC from sham and CLP groups of outbred mice were also probed for their ability to produce IL-12 following LM infection (Fig 5D). Septic outbred mice showed a significant decline in both the number of cDC as well as the frequency of cDCs producing IL-12p40 compared to sham mice (Fig 5E-G). This decrease in both number and frequency lead to a significant decline of IL-12 producing cDC; a result that was similar to the observed impairment in B6 mice. Thus, IL-12 production from post-sepsis cDC following secondary infection is diminished in both inbred and genetically heterogeneous populations.
Figure 5. Reduced number and impaired functionality of DCs early after sepsis induction in outbred hosts.
(A) Experimental Design. Sham or CLP surgery was performed on outbred Swiss-Webster mice and analysis performed 2 d-post surgery. (B) Splenic cellularity and total number of DCs in the spleen. Fold difference in number of cells detected in Sham and CLP groups of mice is shown. (C) Representation of cDC subsets in the spleen 2 d post sepsis-induction. (D) Experimental design. Sham or CLP surgery was performed on outbred Swiss-Webster mice 2 d prior to infection with virulent L. monocytogenes (10403s strain; 105 CFU/mouse, i.v.). (E) DC numbers 1 d after LM infection. Fold difference in number of CD11chi cells detected in sham and CLP groups of mice is shown. (F) Frequency and (G) total number of splenic CD11chi cells producing IL-12p40 14 h after LM infection. Fold difference in frequency and number of cells producing cytokine in sham and CLP groups of mice is shown. Data are presented as mean ± SEM of 4-6 mice per group and are representative of two independent experiments.*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 as determined by student T-test.
Direct targeting of DCs using DEC-205 mAb conjugated to OVA reveals diminished expansion of adoptively transferred OT-I cells
The experiments discussed thus far have suggested that the numerical loss of DCs and their functional impairments in producing cytokines upon secondary pathogen exposure after sepsis are a T cell extrinsic factor directly contributing to sub-optimal CD8 T cell immunity; however, a direct link between the two has thus far not been provided. To definitively link the post-septic DC lesion with sub-optimal CD8 T cell expansion upon exposure to cognate Ag, we made use of an anti-DEC-205 mAb:peptide conjugate system (49). DEC-205 (CD205) is a surface receptor found on CD8+ cDCs which utilize it for receptor-mediated endocytosis of Ag prior to processing and presentation to T cells (50). To determine the extent that sepsis impacts CD205 expressing DCs, we first performed sham and CLP surgery and analyzed DCs from the spleen 2 days later (Fig 6A). As expected, sepsis leads to a significant decline in the number of CD11chi CD8+CD205+ DCs (Fig 6B). To directly probe the ability of the remaining CD205+ DCs to prime naïve Ag-specific CD8 T cells that were not initially influenced by the septic event, we adoptively transferred the same number of naïve OT-I CD8 T cells in sham or CLP groups of mice 2 days post-surgery. A day later, anti-DEC-205 mAb conjugated with the SIINFEKL peptide of the ovalbumin protein (DEC 205 mAb/Ag conjugates) in the presence of CpG was administered and total number of OT-I CD8 T cells was determined (Fig 6A). Interestingly, the CLP-treated mice had significantly diminished OT-I CD8 T cell expansion in the blood (days 6-7) and spleen (day 7) post-conjugate administration compared to sham mice (Fig 6C-E). Thus, direct targeting of DCs using DEC205 mAb/Ag conjugates reveals the role of DCs in sub-optimal priming of primary CD8 T cell responses after polymicrobial sepsis induction.
Figure 6. Sepsis leads to impaired CD8 T cell expansion after direct targeting of Ag to DCs.
(A) Experimental design. Naïve OVA257-specific TCR-tg OT-I CD8 T cells (Thy1.1; 103/mouse, i.v.) were adoptively transferred into B6 (Thy1.2) mice 2 d post sham or CLP surgery. All groups of recipient mice were administered anti-DEC-205 Ab/OVA conjugates (10 μg, i.v.) and CpG (50 μg, i.p.) one day after adoptive transfer of naïve OT-I CD8 T cells (d 3 post surgery). (B) Total number of CD8+DEC-205+ DCs in the spleen. Fold differences in number of cells detected in sham or CLP mice is shown. (C) Representative dot plots showing the frequency of tetramer positive (Kb–OVA) CD8 T cells (endogenous – Thy1.2 or TCR-Tg - Thy1.1) in PBL and spleen at d 7 post-infection. Total number of OT-I CD8 T cells isolated from the peripheral blood (D) on d 6 and 7 and the spleen (E) on d 7 after anti-DEC-205 Ab/OVA administration. Data are presented as mean ± SEM of 5-6 mice per group and representative of two independent experiments. *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001 as determined by student T-test.
Flt3L treatment following sepsis induction recovers the quantity and quality (function) of DCs
Thus far we have shown that sepsis leads to a significant reduction in number of DCs and functional impairment of the remaining DCs, evidenced by diminished IL-12 production. Together, these features contribute to the decreased priming of naïve CD8 T cells and reduced primary CD8 T cell expansion. Reversing the loss of DC number (as well as function) could prove beneficial in recovery of CD8 T cell expansion upon pathogen encounter and could contribute to a partial recovery in primary CD8 T cell responses.
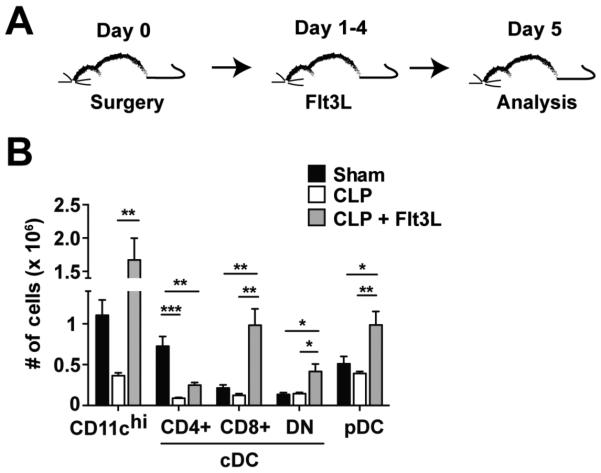
FMS-like tyrosine kinase 3 ligand (Flt3L) is a hematopoietic growth factor capable of increasing DC numbers and could aid in reversing the effects of the DC lesion in septic mice (51). Repeated treatments of Flt3L for consecutive days are needed to expand the DC pool. Our previous studies indicated that sepsis leads to a decline in DC numbers after sepsis induction. To determine how long the decline in DC numbers persists in CLP-treated mice we performed sham or CLP surgery and analyzed at seven days post sepsis induction (S2A Fig). Although the total cellularity of the spleen was not significantly different between sham or CLP mice, a significant reduction in cDCs, including the CD4+ and CD8+ cDC subsets, was still detected at day 7 post-surgery (S2B Fig). Also, CLP mice had diminished cellularity of the inguinal lymph nodes as well as significant declines in the number of cDC(CD11chi) and pDC (S2C Fig). Thus, the loss of DCs persists in the spleen and inguinal lymph nodes for at least 7 days following sepsis induction. We next examined the effect of Flt3L administration on septic mice, where Flt3L treatment began 1 day after CLP surgery and continued for four consecutive days prior to analysis (Fig 7A). Importantly, septic mice treated with Flt3L showed significantly increased numbers of CD11chi cells in the spleen, as well as significantly increased numbers of CD8+cDC, DN cDC, and pDC compared to untreated septic mice (Fig 7B). Thus, Flt3L treatment following sepsis induction leads to numerical recovery of DC populations in vivo.
Figure 7. Numerical recovery of DC compartment after Flt3L treatment of septic mice.
(A) Experimental Design. Sham or CLP surgery was performed on naïve B6 mice prior to Flt3L-Ig (100 μg i.p.) or PBS administration on d 1-4 following surgery and analysis on d 5. (B) Total number of DCs in the spleen 5 d-post surgery. Date are presented as mean ± SEM of 4 mice per group and representative of two independent experiments. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 as determined by one way ANOVA.
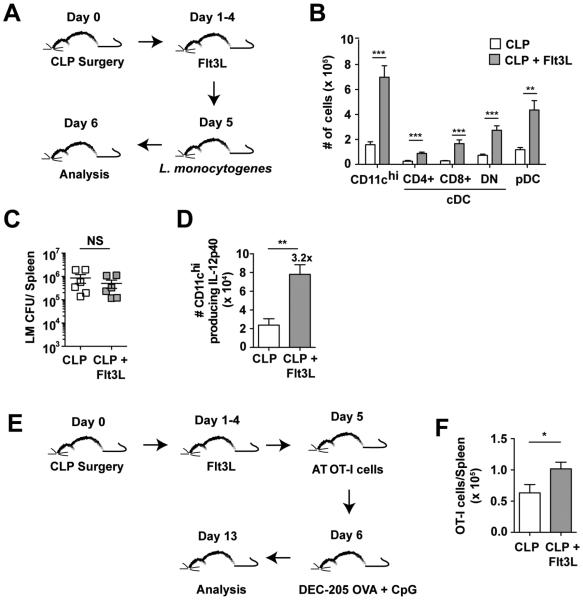
To determine whether the administration of Flt3L could aid in the reversal of DC functionality, septic mice were treated with Flt3L prior to infection with virulent LM on day 5 and direct ex-vivo analysis of IL-12p40 positive DCs was performed on day 6 post-surgery (Fig 8A). After LM infection, the number of CD11chi cells, cDC subsets (CD4+, CD8+, and DN), and pDCs was significantly increased in the spleen of Flt3L treated mice indicating that infection with LM did not diminish the DC expansion induced by Flt3L administration (Fig 8B). Similar to previously discussed experiments, bacterial burden after LM infection was similar (Fig 8C). Direct ex vivo intracellular cytokines staining revealed Flt3L treatment significantly (3.2-fold) increased numbers of IL-12p40 producing CD11chi DC cells compared to the PBS-treated CLP group (Fig 8D).
Figure 8. Functional recovery of DC compartment after Flt3L treatment of septic mice.
(A) Experimental Design. CLP surgery was performed on naïve B6 mice 5 d prior to infection with virulent L. monocytogenes (10403s strain; 1×105 CFU/mouse, i.v.). Flt3L-Ig (100 μg i.p.) was administered on d 1-4 following surgery and analysis was performed on d 6. (B) Splenic DC numbers 1 d after LM infection (d 6 post-surgery). (C) LM titers in the spleen 1 d after infection. (D) Total number of CD11chi cells producing IL-12p40 1 day post-infection. Fold difference in number of cells producing IL-12 in CLP and CLP+Flt3L treated groups of mice is shown. (E) Experimental Design. CLP surgery was performed on naïve B6 mice 5 d prior to adoptive transfer of naïve OT-I CD8 T cells (Thy1.1; 103/mouse, i.v.). Flt3L-treated CLP group was administered Flt3L-Ig (100 μg i.p.) on d 1-4 following surgery. All mice were administered DEC-205 OVA (10 μg, i.v.) and CpG (50 μg, i.p.) one day after adoptive transfer of naïve OT-I CD8 T cells (day 6 post surgery). (F) Total number of OT-I CD8 T cells isolated from the spleen 7 d after DEC-205 OVA administration (day 13 post surgery). Data are presented as mean ± SEM of 4-6 mice per group and representative of two independent experiments. * p≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 as determined by student T-test.
To further characterize whether Flt3L therapy post-sepsis could aid in the reversal of DC functionality and support recovery of primary CD8 T cell expansion, groups of CLP mice that received Flt3L or control treatments were adoptively transferred with physiological numbers of naïve OT-I or gp33-specific P14 TCR-tg CD8 T cells (103/mouse) one day before DEC-205 mAb/Ag + CpG administration (Fig 8E) or infection with attenuated LM expressing gp33 (S3A Fig), respectively. Importantly, Flt3L treatment significantly improved accumulation of primary effector CD8 T cells either after direct targeting of DCs with antigen (Fig 8F) or after infection with pathogen expressing the antigen (S3B,C Fig) suggesting that treatments aimed at recovery of the DC compartment have the capacity to lead to downstream recovery of primary CD8 T cell responses as well.
Once more, we used an outbred mouse model to verify that our results seen in a genetically homogenous population could be extrapolated to a genetically diverse one. In a similar experiment as in inbred B6 hosts, septic outbred mice were either injected with PBS or treated with Flt3L prior to LM infection (S4A Fig). Similar to inbred mice, septic outbred mice treated with Flt3L showed significantly increased numbers of cDC, cDC subsets (CD4+, CD8+, and DN), and pDCs as compared to untreated controls (S4B Fig). Ex vivo IL-12p40 staining revealed a significantly increased (8.6-fold) number of IL-12p40 producing CD11chi cells in Flt3L-treated septic mice as compared to untreated septic mice (S4C Fig).
In summary, these data demonstrate Flt3L administration to septic inbred or outbred mice leads to a significant recovery of DC number and function, including the ability to prime primary CD8 T cell expansion.
Discussion
Sepsis results from a systemic immune response to an infection that leads to an early hyper-inflammatory state followed by a state of immunosuppression (52). The early hyper-inflammatory state, characterized by increased production of both pro-inflammatory and anti-inflammatory cytokines, leads to increased levels of small molecules such as nitric oxide (NO) and leukotrienes that mediate physiological responses capable of producing massive hypotension, shock, and/or cardiovascular collapse that may lead to early septic deaths (53, 54). The transition from this hyper-inflammatory state to immunosuppression is evident in septic patients who show increased levels of lymphocyte apoptosis and often fall victim to secondary bacterial infections and reactivation of latent viruses such as Herpes Simplex Virus (HSV) or Cytomegalovirus (CMV) (3-5, 7, 55). Cecal ligation and puncture (CLP) is an experimental animal model of sepsis that mimics the course of sepsis in human patients, as it results in an initial hyper-inflammatory stage followed by a protracted stage of immunosuppression and is also characterized by apoptosis of lymphocytes and increased susceptibility to secondary infections (43, 56). Using the CLP model, we have reported a decrease in both the number and function of naïve and memory CD8 T cells (10, 12, 57), and rescuing primary and secondary CD8 T cell responses following sepsis could represent an important avenue for future treatment. In this study, we provide evidence of a T cell extrinsic factor, namely DCs, whose decline in both number and signal 3 cytokine production contributes to the observed impairments in primary CD8 T cell responses in septic mice. Additionally, we demonstrate the use of the DC mobilizing cytokine Flt3L to rescue the lesion of DC numbers in septic mice and contribute to the faster recovery of CD8 T cell immunity in general.
Sepsis-induced apoptosis of T and B cells, as well as loss of DCs, has been well characterized in human patients (7, 25, 55, 58). Furthermore, the apoptotic loss of DCs in the spleens of septic mice has been shown at 24-48 hours post-sepsis induction with significant losses occurring in the CD4+ and CD8+ cDC populations (28, 30). Consistent with these previous reports, the data presented herein show sepsis leads to a significant decline in the CD4+ and CD8+ cDC populations as well as the pDC population in the spleen two days post-sepsis. In contrast with previous investigations, we also demonstrated a significant decline in the DN cDC population; however, this difference is likely due to differences in CLP induction and the time point of analysis as differences in timing of analysis may accurately demonstrate the loss of a certain cell population while failing to capture the loss of a different cell population. In addition to timing, the location of analysis (spleen, lymph nodes, bone marrow, etc.) influences the variability observed between studies. Our results recapitulate previous studies demonstrating the loss of DCs following sepsis while providing additional data showing sepsis-induced reduction in DCs in outbred mice that more closely resemble the genetic heterogeneity found in humans – indicating that the loss of DCs following sepsis occurs regardless of host genetic background.
The numerical reduction in DCs following sepsis suggests a possible mechanism for impaired CD8 T cell immunity, as a decrease in number of DCs displaying cognate Ag could lead to decreased primary expansion of CD8 T cells. DCs in secondary lymphoid organs are a dynamic mixture of immature DCs, capable of vigorous Ag uptake but with minimal capacity to activate T cells, and mature DCs, which have a high capacity to generate a T cell response due to upregulation of co-stimulatory ligands but have diminished capacity to uptake Ag (59). DC expression of co-stimulatory ligands and their interactions with CD8 T cells (signal 2) are necessary for the expansion of Ag-specific CD8 T cells upon Ag recognition (15). Our results, like a previous study, demonstrate that sepsis leads to moderate up-regulation of co-stimulatory ligands in septic mice (30), but the increased expression of co-stimulatory ligands on DCs from septic mice is still substantially lower than that observed after LPS stimulation (45, 46). Preliminary data have also shown that CLP induction does not change the ability of enriched CD11c+ cells in vitro to acquire and process exogenous Ag. Thus, an altered maturation status of DCs does not control suboptimal priming of naïve CD8 T cells in vivo observed early after CLP-induced sepsis.
The primary expansion of Ag-specific CD8 T cells is predicated by the encounter of CD8 T cells with their cognate Ag in the presence of co-stimulation. Furthermore, pro-inflammatory cytokines, such as IL-12 and IFN-γ, influence the degree to which CD8 T cells expand, survive, and mediate effector function (15, 18-20, 60, 61). CD8 T cells exposed to signal 1 (Ag) and signal 2 (co-stimulation) in the presence of signal 3 (IL-12) have an increased ability to expand, survive, and kill compared to T cells exposed to signal 1 and 2 alone (18-20). Our study demonstrates that DCs from septic mice have diminished capacity to produce IL-12 when stimulated with TLR ligands in vitro, a result that has been previously described (30, 33, 34). In addition, using secondary infection with Listeria monocytogenes as an in vivo tool for probing the host’s capacity to produce pro-inflammatory cytokines, we have demonstrated that septic mice have a systemic loss in their capacity to produce the pro-inflammatory cytokines IL-12 and IFN-γ. This result is consistent with the paradigm of immunoparalysis in septic survivors and demonstrates an in vivo functional impairment in signal 3 production upon infection, linking the significant decline in DC number with the impairment in IL-12 production. Interestingly, a previous study has shown that the decline in IL-12 production from septic DCs corresponds with alterations in methylation patterns of IL-12p35 and IL-12p40 promoters suggestive of a repressive modification in chromatin structure associated with diminished gene expression. Furthermore, this study demonstrated that the impairment in IL-12 production from septic DCs, as well as their epigenetic changes, persists for up to six weeks after sepsis induction (34). Thus, enduring changes at the genomic level might lead to a long lasting impairment in cytokine production that contributes to persistent dysfunction in CD8 T cell immunity after sepsis. Future investigations that further address both the timing and downstream effects of these genomic level changes could be an intriguing area of future inquiry.
Addressing the DC lesion after sepsis may serve as an important therapeutic target for reversing some of the sepsis-induced deficits in immunity. To this end, we have demonstrated that the administration of Flt3L in both inbred and outbred septic hosts leads to a significant increase in DCs, as well as IL-12 producing DCs, as compared to untreated septic mice. Moreover, this increase in DCs and IL-12 producing DCs contributed to an increase in number of expanded CD8 T cells in Flt3L-treated mice. This important finding not only demonstrates the capacity of Flt3L to recover DC number and functionality after sepsis, but also reveals that targeted therapy of the DC lesion post-sepsis may allow reversal of lesions in the immune system, in our case CD8 T cell immunity, in which DCs play a vital role. Therapeutic use of Flt3L has been reported in experimental tumor models (62). Daily administration of Flt3L to mice rapidly increases the number of DCs in multiple tissues (51, 63). Importantly, this increase in DCs is maintained only as long as the Flt3L is given, and the organs that display the increased size and cellularity during Flt3L therapy return to normal within 7-10 d of stopping treatment. The potent hematopoietic effects of Flt3L in preclinical models led to its initial testing in clinical trials nearly 20 years ago (64-70). Despite being well tolerated and demonstrating biologic activity, clinical development of Flt3L was suspended. Clinical interest in Flt3L has been renewed, with a recent report examining the efficacy and safety of a recombinant human Flt3L (CDX-301) (71). Based on the long history of testing Flt3L in multiple immunological settings, it is tempting to speculate on the potential benefits of using Flt3L in sepsis patients based on the data presented herein.
In summary, we have shown that CLP-induced sepsis leads to numerical and functional decreases in DCs, which contribute to the sub-optimal CD8 T cell expansion upon Ag encounter. Furthermore, through the administration of the DC mobilizing cytokine Flt3L, we have established that the number of DCs may be recovered in septic mice leading to an increase in the number of IL-12 producing DCs as well as an increase in primary CD8 T cell expansion. These data highlight the fact that sepsis-induced immune suppression is a multicellular defect, where both T cell-intrinsic and –extrinsic numerical and functional deficiencies likely contribute to the increased susceptibility of sepsis survivors to secondary infections.
Supplementary Material
Acknowledgements
We thank Dr. Matthew Martin, Deepa Rai, Stacey Hartwig, and Lecia Epping for technical assistance and all members of our laboratories for helpful discussions.
Footnotes
Supported by National Institutes of Health Grants GM113961, AI119160, AI114543 (V.P.B.), and GM115462 (T.S.G.), T32AI997313 (D.I.K) and U.S. Department of Veterans Affairs Merit Review Award (T.S.G.)
References
- 1.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K, International Forum of Acute Care, Trialists Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 2.Donnelly JP, Hohmann SF, Wang HE. Unplanned Readmissions After Hospitalization for Severe Sepsis at Academic Medical Center-Affiliated Hospitals. Crit. Care Med. 2015;43:1916–1927. doi: 10.1097/CCM.0000000000001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat. Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang ML, Santo Hayes TK, Corey L, Boeckh M. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luyt CE, Combes A, Deback C, Aubriot-Lorton MH, Nieszkowska A, Trouillet JL, Capron F, Agut H, Gibert C, Chastre J. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am. J. Respir. Crit. Care Med. 2007;175:935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 6.Quartin AA, Schein RM, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063. [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Le Tulzo Y, Pangault C, Gacouin A, Guilloux V, Tribut O, Amiot L, Tattevin P, Thomas R, Fauchet R, Drenou B. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002;18:487–494. doi: 10.1097/00024382-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J. Immunol. 2005;174:5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 10.Condotta SA, Rai D, James BR, Griffith TS, Badovinac VP. Sustained and incomplete recovery of naive CD8+ T cell precursors after sepsis contributes to impaired CD8+ T cell responses to infection. J. Immunol. 2013;190:1991–2000. doi: 10.4049/jimmunol.1202379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Condotta SA, Khan SH, Rai D, Griffith TS, Badovinac VP. Polymicrobial Sepsis Increases Susceptibility to Chronic Viral Infection and Exacerbates CD8+ T Cell Exhaustion. J. Immunol. 2015;195:116–125. doi: 10.4049/jimmunol.1402473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duong S, Condotta SA, Rai D, Martin MD, Griffith TS, Badovinac VP. Polymicrobial sepsis alters antigen-dependent and -independent memory CD8 T cell functions. J. Immunol. 2014;192:3618–3625. doi: 10.4049/jimmunol.1303460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danahy DB, Strother RK, Badovinac VP, Griffith TS. Clinical and Experimental Sepsis Impairs CD8 T-Cell-Mediated Immunity. Crit. Rev. Immunol. 2016;36:57–74. doi: 10.1615/CritRevImmunol.2016017098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 2014;15:1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 17.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 18.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 19.Schmidt CS, Mescher MF. Adjuvant effect of IL-12: conversion of peptide antigen administration from tolerizing to immunizing for CD8+ T cells in vivo. J. Immunol. 1999;163:2561–2567. [PubMed] [Google Scholar]
- 20.Schmidt CS, Mescher MF. Peptide antigen priming of naive, but not memory, CD8 T cells requires a third signal that can be provided by IL-12. J. Immunol. 2002;168:5521–5529. doi: 10.4049/jimmunol.168.11.5521. [DOI] [PubMed] [Google Scholar]
- 21.Turley SJ, Fletcher AL, Elpek KG. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat. Rev. Immunol. 2010;10:813–825. doi: 10.1038/nri2886. [DOI] [PubMed] [Google Scholar]
- 22.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 24.Lanzavecchia A, Sallusto F. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat. Immunol. 2001;2:487–492. doi: 10.1038/88678. [DOI] [PubMed] [Google Scholar]
- 25.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J. Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 26.Poehlmann H, Schefold JC, Zuckermann-Becker H, Volk HD, Meisel C. Phenotype changes and impaired function of dendritic cell subsets in patients with sepsis: a prospective observational analysis. Crit. Care. 2009;13:R119. doi: 10.1186/cc7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guisset O, Dilhuydy MS, Thiebaut R, Lefevre J, Camou F, Sarrat A, Gabinski C, Moreau JF, Blanco P. Decrease in circulating dendritic cells predicts fatal outcome in septic shock. Intensive Care Med. 2007;33:148–152. doi: 10.1007/s00134-006-0436-7. [DOI] [PubMed] [Google Scholar]
- 28.Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, Hotchkiss RS. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J. Immunol. 2003;171:909–914. doi: 10.4049/jimmunol.171.2.909. [DOI] [PubMed] [Google Scholar]
- 29.Ding Y, Chung CS, Newton S, Chen Y, Carlton S, Albina JE, Ayala A. Polymicrobial sepsis induces divergent effects on splenic and peritoneal dendritic cell function in mice. Shock. 2004;22:137–144. doi: 10.1097/01.shk.0000131194.80038.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flohe SB, Agrawal H, Schmitz D, Gertz M, Flohe S, Schade FU. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J. Leuk. Biol. 2006;79:473–481. doi: 10.1189/jlb.0705413. [DOI] [PubMed] [Google Scholar]
- 31.Scumpia PO, McAuliffe PF, O'Malley KA, Ungaro R, Uchida T, Matsumoto T, Remick DG, Clare-Salzler MJ, Moldawer LL, Efron PA. CD11c+ dendritic cells are required for survival in murine polymicrobial sepsis. J. Immunol. 2005;175:3282–3286. doi: 10.4049/jimmunol.175.5.3282. [DOI] [PubMed] [Google Scholar]
- 32.Efron PA, Martins A, Minnich D, Tinsley K, Ungaro R, Bahjat FR, Hotchkiss R, Clare-Salzler M, Moldawer LL. Characterization of the systemic loss of dendritic cells in murine lymph nodes during polymicrobial sepsis. J. Immunol. 2004;173:3035–3043. doi: 10.4049/jimmunol.173.5.3035. [DOI] [PubMed] [Google Scholar]
- 33.Pastille E, Didovic S, Brauckmann D, Rani M, Agrawal H, Schade FU, Zhang Y, Flohe SB. Modulation of dendritic cell differentiation in the bone marrow mediates sustained immunosuppression after polymicrobial sepsis. J. Immunol. 2011;186:977–986. doi: 10.4049/jimmunol.1001147. [DOI] [PubMed] [Google Scholar]
- 34.Wen H, Dou Y, Hogaboam CM, Kunkel SL. Epigenetic regulation of dendritic cell-derived interleukin-12 facilitates immunosuppression after a severe innate immune response. Blood. 2008;111:1797–1804. doi: 10.1182/blood-2007-08-106443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benjamim CF, Lundy SK, Lukacs NW, Hogaboam CM, Kunkel SL. Reversal of long-term sepsis-induced immunosuppression by dendritic cells. Blood. 2005;105:3588–3595. doi: 10.1182/blood-2004-08-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pene F, Zuber B, Courtine E, Rousseau C, Ouaaz F, Toubiana J, Tazi A, Mira JP, Chiche JD. Dendritic cells modulate lung response to Pseudomonas aeruginosa in a murine model of sepsis-induced immune dysfunction. J. Immunol. 2008;181:8513–8520. doi: 10.4049/jimmunol.181.12.8513. [DOI] [PubMed] [Google Scholar]
- 37.Wen H, Hogaboam CM, Gauldie J, Kunkel SL. Severe sepsis exacerbates cell-mediated immunity in the lung due to an altered dendritic cell cytokine profile. Am. J. Pathol. 2006;168:1940–1950. doi: 10.2353/ajpath.2006.051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang HW, Yang W, Gao L, Kang JR, Qin JJ, Liu YP, Lu JY. Adoptive transfer of bone marrow-derived dendritic cells decreases inhibitory and regulatory T-cell differentiation and improves survival in murine polymicrobial sepsis. Immunology. 2015;145:50–59. doi: 10.1111/imm.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toliver-Kinsky TE, Cui W, Murphey ED, Lin C, Sherwood ER. Enhancement of dendritic cell production by fms-like tyrosine kinase-3 ligand increases the resistance of mice to a burn wound infection. J. Immunol. 2005;174:404–410. doi: 10.4049/jimmunol.174.1.404. [DOI] [PubMed] [Google Scholar]
- 40.Bohannon J, Cui W, Cox R, Przkora R, Sherwood E, Toliver-Kinsky T. Prophylactic treatment with fms-like tyrosine kinase-3 ligand after burn injury enhances global immune responses to infection. J. Immunol. 2008;180:3038–3048. doi: 10.4049/jimmunol.180.5.3038. [DOI] [PubMed] [Google Scholar]
- 41.Wang HW, Yang W, Lu JY, Tian G, Li F, Wang XH, Kang JR, Yang Y. Treatment with Fms-like tyrosine kinase 3 ligand reverses lung dendritic cell immunoparalysis and ameliorates zymosan-induced secondary lung injury in mice. Clin. Exp. Immunol. 2012;170:156–166. doi: 10.1111/j.1365-2249.2012.04641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Badovinac VP, Harty JT. Adaptive immunity and enhanced CD8+ T cell response to Listeria monocytogenes in the absence of perforin and IFN-gamma. J. Immunol. 2000;164:6444–6452. doi: 10.4049/jimmunol.164.12.6444. [DOI] [PubMed] [Google Scholar]
- 43.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gurung P, Rai D, Condotta SA, Babcock JC, Badovinac VP, Griffith TS. Immune unresponsiveness to secondary heterologous bacterial infection after sepsis induction is TRAIL dependent. J. Immunol. 2011;187:2148–2154. doi: 10.4049/jimmunol.1101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, De Baetselier P, Urbain J, Leo O, Moser M. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 2001;167:5067–5076. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Auerbuch V, Brockstedt DG, Meyer-Morse N, O'Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 2004;200:527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin MD, Badovinac VP. Antigen-dependent and -independent contributions to primary memory CD8 T cell activation and protection following infection. Sci. Rep. 2015;5:18022. doi: 10.1038/srep18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 51.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dinarello CA. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest. 1997;112:321S–329S. doi: 10.1378/chest.112.6_supplement.321s. [DOI] [PubMed] [Google Scholar]
- 54.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 55.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr., Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 56.Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7:247–253. doi: 10.1097/00024382-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Condotta SA, Cabrera-Perez J, Badovinac VP, Griffith TS. T-cell-mediated immunity and the role of TRAIL in sepsis-induced immunosuppression. Crit. Rev. Immunol. 2013;33:23–40. doi: 10.1615/critrevimmunol.2013006721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat. Rev. Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 59.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 60.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 61.Whitmire JK, Tan JT, Whitton JL. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J. Exp. Med. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lynch DH, Andreasen A, Maraskovsky E, Whitmore J, Miller RE, Schuh JC. Flt3 ligand induces tumor regression and antitumor immune responses in vivo. Nat. Med. 1997;3:625–631. doi: 10.1038/nm0697-625. [DOI] [PubMed] [Google Scholar]
- 63.Lyman SD. Biology of flt3 ligand and receptor. Int. J. Hematol. 1995;62:63–73. doi: 10.1016/0925-5710(95)00389-a. [DOI] [PubMed] [Google Scholar]
- 64.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski CR, Hoek J, Caron D, Lebsack ME, McKenna HJ. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood. 2000;96:878–884. [PubMed] [Google Scholar]
- 65.Pulendran B, Banchereau J, Burkeholder S, Kraus E, Guinet E, Chalouni C, Caron D, Maliszewski C, Davoust J, Fay J, Palucka K. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J. Immunol. 2000;165:566–572. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 66.Freedman RS, Vadhan-Raj S, Butts C, Savary C, Melichar B, Verschraegen C, Kavanagh JJ, Hicks ME, Levy LB, Folloder JK, Garcia ME. Pilot study of Flt3 ligand comparing intraperitoneal with subcutaneous routes on hematologic and immunologic responses in patients with peritoneal carcinomatosis and mesotheliomas. Clin. Cancer Res. 2003;9:5228–5237. [PubMed] [Google Scholar]
- 67.Higano CS, Vogelzang NJ, Sosman JA, Feng A, Caron D, Small EJ. Safety and biological activity of repeated doses of recombinant human Flt3 ligand in patients with bone scan-negative hormone-refractory prostate cancer. Clin. Cancer Res. 2004;10:1219–1225. doi: 10.1158/1078-0432.ccr-1404-02. [DOI] [PubMed] [Google Scholar]
- 68.Marroquin CE, Westwood JA, Lapointe R, Mixon A, Wunderlich JR, Caron D, Rosenberg SA, Hwu P. Mobilization of dendritic cell precursors in patients with cancer by flt3 ligand allows the generation of higher yields of cultured dendritic cells. J. Immunother. 2002;25:278–288. doi: 10.1097/01.CJI.0000016307.48397.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morse MA, Nair S, Fernandez-Casal M, Deng Y, St Peter M, Williams R, Hobeika A, Mosca P, Clay T, Cumming RI, Fisher E, Clavien P, Proia AD, Niedzwiecki D, Caron D, Lyerly HK. Preoperative mobilization of circulating dendritic cells by Flt3 ligand administration to patients with metastatic colon cancer. J. Clin. Oncol. 2000;18:3883–3893. doi: 10.1200/JCO.2000.18.23.3883. [DOI] [PubMed] [Google Scholar]
- 70.Gasparetto C, Gasparetto M, Morse M, Rooney B, Vredenburgh JJ, Long GD, Rizzieri DA, Loftis J, Chao NJ, Smith C. Mobilization of dendritic cells from patients with breast cancer into peripheral blood stem cell leukapheresis samples using Flt-3-Ligand and G-CSF or GM-CSF. Cytokine. 2002;18:8–19. doi: 10.1006/cyto.2002.1009. [DOI] [PubMed] [Google Scholar]
- 71.Anandasabapathy N, Breton G, Hurley A, Caskey M, Trumpfheller C, Sarma P, Pring J, Pack M, Buckley N, Matei I, Lyden D, Green J, Hawthorne T, Marsh HC, Yellin M, Davis T, Keler T, Schlesinger SJ. Efficacy and safety of CDX-301, recombinant human Flt3L, at expanding dendritic cells and hematopoietic stem cells in healthy human volunteers. Bone Marrow Transplant. 2015;50:924–930. doi: 10.1038/bmt.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.