Graphical abstract
Keywords: Portal hypertension, Hepatic venous pressure gradient, Magnetic resonance imaging, Longitudinal T1 relaxation time
Abstract
Background & Aims
Hepatic venous pressure gradient (HVPG) measurement is currently the only validated technique to accurately evaluate changes in portal pressure. In this study, we evaluate the use of non-contrast quantitative magnetic resonance imaging (MRI) as a surrogate measure of portal pressure.
Methods
Thirty patients undergoing HVPG measurement were prospectively recruited. MR parameters of longitudinal relaxation time (T1), perfusion of the liver and spleen (by arterial spin labelling), and blood flow in the portal, splanchnic and collateral circulation (by phase contrast MRI) were assessed. We estimated the liver stiffness measurement (LSM) and enhanced liver fibrosis (ELF) score. The correlation of all non-invasive parameters with HVPG was evaluated.
Results
The mean (range) HVPG of the patients was 9.8 (1–22) mmHg, and 14 patients (48%) had clinically significant portal hypertension (CSPH, HVPG ⩾10 mmHg). Liver T1 relaxation time, splenic artery and superior mesenteric artery velocity correlated significantly with HVPG. Using multiple linear regression, liver T1 and splenic artery velocity remained as the two parameters in the multivariate model significantly associated with HVPG (R = 0.90, p <0.001). This correlation was maintained in patients with CSPH (R = 0.85, p <0.001). A validation cohort (n = 10) showed this linear model provided a good prediction of HVPG. LSM and ELF score correlated significantly with HVPG in the whole population but the correlation was absent in CSPH.
Conclusions
MR parameters related to both hepatic architecture and splanchnic haemodynamics correlate significantly with HVPG. This proposed model, confirmed in a validation cohort, could replace the invasive HVPG measurement.
Lay summary
In patients with cirrhosis, the development and progression of portal hypertension is related to worse outcomes. However, the standard technique of assessing portal pressure is invasive and not widely used in clinical practice. Here, we have studied the use of non-invasive MRI in evaluating portal pressure. The MRI measures of liver architecture and blood flow in the splenic artery correlated well with portal pressure. Therefore, this non-invasive method can potentially be used to assess portal pressure in clinical trials and monitoring treatment in practice.
Introduction
The majority of complications in patients with cirrhosis result from the development and progression of portal hypertension characterised by increased intrahepatic resistance and progressive splanchnic vasodilation. Distortion of hepatic architecture resulting from fibrogenesis and nodule formation results in ‘static’ hepatic vascular resistance, whilst a ‘dynamic’ component results from the active contraction of myofibroblasts and increased hepatic vascular tone [1]. The rise of portal pressure is perpetuated by the excessive release of endogenous vasodilators resulting in splanchnic vasodilation and increased portal blood flow.
Hepatic venous pressure gradient (HVPG) measurement [2] is the only validated technique to accurately evaluate changes in portal pressure. An HVPG threshold of 10 mmHg is termed clinically significant portal hypertension (CSPH) as it predicts the risk of formation of oesophageal varices [3], clinical decompensation [4] and development of hepatocellular carcinoma [5]. An HVPG >12 mmHg is associated with the risk of variceal bleeding [6] and an HVPG >16 mmHg correlates with increased mortality [7], [8], whilst in acute variceal bleeding an HVPG ⩾20 mmHg is an independent prognostic marker [9]. However, HVPG measurements are invasive and available only in specialised hepatology units, precluding its use in routine clinical practice. Thus, the development of non-invasive markers of portal pressure is highly desirable.
Liver stiffness measurement (LSM) as assessed with transient elastography (TE) has been suggested as an alternative measurement to HVPG. LSM is thought to reflect hepatic fibrosis and the resulting intrahepatic resistance. A significant correlation of LSM with HVPG has been demonstrated at an HVPG <10 mmHg, but no statistical significance at an HVPG >12 mmHg [10]. This has led to the suggestion that LSM can identify clinically significant or severe portal hypertension, but is not a good marker of its subsequent progression. This is likely due to extrahepatic factors, such as splanchnic vasodilation and a hyperdynamic circulation, that perpetuate the rise in portal pressure but do not affect the liver tissue stiffness [11]. TE has also been used to measure spleen stiffness which is able to identify the presence of varices and a linear model of spleen and liver stiffness predicting HVPG with a high accuracy [12]. However, there are significant technical challenges related to spleen size and an upper detection limit for tissue stiffness that limit the applicability of this technique. Magnetic resonance elastography (MRE) has the theoretical advantage over TE of assessing liver and splenic stiffness across a larger tissue area. In 36 patients with cirrhosis, MRE-measured loss modulus of the liver and spleen correlated well with HVPG (R = 0.44, p = 0.02, and R = 0.57, p = 0.002, respectively) [13]. However, the accessibility of this technique due to hardware availability and cost, and the feasibility of MRE in some patients, can limit its clinical translation.
The ratio of liver to spleen volume as measured by computed tomography has also been shown to predict HVPG, however this measure has the disadvantage of requiring ionising radiation [14]. Using Doppler ultrasound, changes in hepatic and splanchnic flow in portal hypertension have been studied, but results have been inconsistent [15], limiting wider use of this technique [16]. To date, all of these imaging modalities have investigated individual pathophysiological components of portal hypertension.
Recent advances in magnetic resonance imaging (MRI) have made it possible to measure multiple parameters associated with structural [17], blood flow [18] and perfusion [19] changes in the liver in a single scan session. Further, since MRI is non-invasive, repeated assessments are feasible and acceptable. The aim of this current study is to develop quantitative MRI as a surrogate of portal pressure. The MRI parameters of interest relate to the size, architecture and perfusion of the liver and spleen, and changes in portal and splanchnic blood flow. Specifically, we aim to study the correlation of these MRI variables with HVPG.
Materials and methods
Study population
Consecutive patients undergoing HVPG measurement for clinical indications at Nottingham University Hospitals NHS Trust and Derby Teaching Hospitals NHS Foundation Trust between April 2013 and June 2016 were prospectively screened and included in the study, providing a broad range of HVPG values. We excluded patients with hepatocellular carcinoma, portal or hepatic vein thrombosis, absolute contraindications for MR, abdominal/waist circumference larger than 112 cm (due to MR scanner bore constraints), age <18 years and pregnancy.
Thirty-four patients were enrolled for the derivation cohort. Four patients were excluded from the final analysis; three patients did not complete the MR scanning protocol due to claustrophobia, one patient had liver histology compatible with non-cirrhotic portal hypertension. MRI and LSM with TE were performed on the same day and within 6 weeks of the HVPG measurement. Patients received no therapeutic interventions between the HVPG measurement and MRI session.
The study protocol was approved by Staffordshire Research Ethics Committee (Ref 12/WM/0288). Patients gave written informed consent in accordance with the principles of the Declaration of Helsinki (revision of Edinburgh 2000).
HVPG measurement
HVPG measurements were carried out by interventional radiologists. HVPG was measured according to established standards [2] following an overnight fast. Under ultrasonographic guidance, the right internal jugular vein was cannulated and a 9-French vascular sheath placed by the modified Seldinger technique. A 6-French compliant balloon-tipped catheter (Berenstein occlusion catheter, Boston Scientific, UK) was guided into the right hepatic vein for the measurement of wedged and free hepatic venous pressures as recommended [8]. All measurements were obtained in triplicate and recorded via a pre-calibrated Philips IntelliVue MP50 patient monitor (Philips Healthcare, UK). HVPG was calculated from the difference between wedged hepatic pressure and free hepatic pressure, and the mean of triplicate measurements computed.
Liver stiffness measurement (LSM)
LSM was performed prior to the MRI scan, following an overnight fast, using FibroScan® (Echosens, Paris, France) by experienced operators [20]. Due to technical reasons, LSM values were not available on 2 patients, and measurements on 6 patients were unreliable (median LSM >7.1 kPa and interquartile range/median ratio >0.30).
Enhanced liver fibrosis (ELF) score
Blood samples were obtained prior to the MRI scan session. Serum samples were analysed for levels of tissue inhibitor of matrix metalloproteinase-1 (TIMP1), hyaluronic acid (HA) and aminoterminal peptide of procollagen III (P3NP) at an independent reference laboratory (iQur Limited, London, UK). The ELF score was calculated using an established algorithm [21].
MR data acquisition
All patients were scanned following an overnight fast on a 1.5 Tesla scanner (Achieva, Philips Medical Systems) with body transmit coil and 16-channel SENSE torso receive coil. All MR measures were acquired in a 1 h scan session.
Liver and spleen volume
Multi-slice balance turbo field echo (bTFE) localiser images were initially acquired in three orthogonal directions to locate the anatomy of organs and blood vessels of interest, and to estimate liver and spleen volume.
Longitudinal relaxation time (T1) of liver and spleen
A modified respiratory triggered inversion recovery sequence with spin-echo echo planar imaging (SE-EPI) readout (3 × 3 × 8 mm3 voxel size, 4 mm slice gap (33%), 96 × 96 image matrix, SENSE factor 2, echo time 27 ms) and fat suppression [17] was acquired to estimate the tissue longitudinal relaxation time (T1) in the liver from 13 inversion times (100–1200 ms in 100 ms steps and 1500 ms). Three sagittal SE-EPI slices were acquired through the right lobe of the liver with minimal temporal slice spacing (65 ms) in approximately 2 min, dependent on the patients’ respiratory rate.
In addition, T1 maps of the liver and spleen were acquired using a modified respiratory triggered inversion recovery sequence with a balanced steady state free precession (bSSFP, also termed bFFE (balanced fast field echo)) readout (echo/repetition time = 1.75/3.5 ms, flip angle (FA) 60°, linear k-space acquisition, SENSE 2, resolution 3 × 3 × 8 mm3). These maps were primarily collected to yield voxel wise T1 values for the quantification of perfusion measures (see tissue perfusion section), but also provided an alternative T1 measure from a bSSFP readout scheme as used by others for liver T1 mapping [22]. This readout scheme results in an apparent recovery time (T1∗), shorter than the actual longitudinal recovery time T1 due to the influence of T2/T2∗ [23]. For coverage of the liver and spleen, 5 coronal-oblique bFFE slices were collected at 9 inversion times (100–900 ms in 100 ms steps) with minimal temporal slice spacing (144 ms) in both ascend and descend slice acquisition order, thus increasing the dynamic range of inversion time values to (100–1500 ms), with data collected in 3 min.
Splanchnic and portal flow measurements
Phase contrast (PC)-MRI was used to quantify the velocity and cross-sectional area of the portal vein and hepatic artery (hepatic inflow), and the right, middle, left hepatic veins (hepatic outflow), as well as the splenic artery (SA) and superior mesenteric artery (SMA) (flow in splanchnic circulation) and azygous vein (collateral flow). Blood flow in each vessel was measured using a vectorcardiogram (VCG) gated 2D PC-MR on a single slice perpendicular to each targeted vessel of interest (echo/repetition time = 4.2/7.5 ms, FA 25°, field of view 280 × 146 mm2, reconstructed resolution 1.5 × 1.5 × 6 mm3, SENSE 3, 2 averages). 15 phases were collected for vein measurements and 20 phases for the arteries across the cardiac cycle, with defined velocity encoding (VENC) for each vessel (portal/hepatic/azygous veins VENC = 50 cms−1, hepatic/splenic arteries VENC = 100 cms−1, and SMA VENC = 140 cms−1). If aliasing occurred, the VENC was increased and the measure repeated. A flow measurement in each vessel was obtained in triplicate and the mean calculated. Each measurement was acquired during a 15–20 s breath-hold, dependent on the subjects’ heart rate.
Tissue perfusion
A multiphase flow alternating inversion recovery arterial spin labelling (ASL) sequence [24] using a bFFE readout (echo/repetition time = 1.75/3.5 ms, FA 45°, linear k-space acquisition, SENSE 2, resolution 3 × 3 × 8 mm3) was used to quantify tissue perfusion in the liver and spleen. Data were collected with patients breathing freely by introducing a respiratory trigger delay of 200 ms prior to ASL labelling. Labelling was followed by a multiphase Look-Locker sampling scheme with an initial delay of 100 ms and subsequent readout spacing of 371 ms with 6 readout phases collected. Liver ASL data was acquired for a sagittal slice through the right lobe of the liver (50 ASL label/control pairs), whilst spleen data was acquired for a coronal-oblique slice through the spleen (30 ASL label/control pairs). In addition, equilibrium base magnetisation (M0) images were acquired for each slice orientation as well as a T1 map (see Longitudinal relaxation time (T1) of liver and spleen section) to allow quantification of perfusion.
MR data analysis
The investigators analysing the MR data were blind to the HVPG measurements.
Liver and spleen volume
Analyze® software (Mayo Clinic) was used to draw a region of interest around the liver and spleen within each bTFE image slice. Total liver and spleen organ volume was calculated from the sum of the volume measures across all slices.
Longitudinal relaxation time (T1) of liver and spleen
Inversion recovery data were fit to S(t) = M0∗abs (1–2exp(−t/T1)) to generate T1 and M0 maps for the SE-EPI data, and estimate apparent T1 relaxation time (T1∗) for the bFFE data. Binary organ masks were formed from the M0 image by manual segmentation. Histogram analysis was used to assess the distribution of relaxation time values within the liver and spleen. For the liver and spleen in each subject, and for each readout, a histogram of voxel values was fit to a Gaussian function and the peak (distribution mode) used to represent the T1 or T1∗ tissue relaxation time. This method provides an automated method to eliminate voxels containing blood in vessels [17]. In addition, the full-width-half-maximum (FWHM) of the Gaussian function was calculated to reflect the degree of heterogeneity of relaxation time values. All subjects were confirmed to have liver tissue T2∗ >22.6 ms [17].
Splanchnic and portal flow measurements
PC-MR data were analysed using Qflow software (Philips Medical System). For each vessel, a region of interest was drawn manually around the vessel lumen on each phase contrast image, with contour detection used. The mean signal intensity within each region of interest reflects flow velocity in the vessel of interest (cm/s) for each cardiac phase, and the mean velocity across the cardiac cycle was computed. The cross-sectional area of each vessel lumen was multiplied by the mean velocity, to compute mean blood flow (ml/s) in each vessel. From triplicate measures, the mean and coefficient of variation (CoV) in all flow measures was estimated.
Liver tissue perfusion
Individual perfusion-weighted difference images (control–label) were calculated for each of the 6 ASL readout phases. These were inspected for motion (excluding control/label pairs with movement of >1 voxel) and averaged to create a single perfusion-weighted (ΔM) map for each phase. Mean values of ΔM, the base equilibrium magnetisation M0 and T1 were used in an iterative model [19] to calculate tissue perfusion (ml/100 g/min) and tissue arrival time of the label (ms), assuming a T1 of blood at 1.5 T of 1.36 s.
Statistical analysis
Statistical analysis was performed using SPSS software version 21(IBM©). Quantitative variables were expressed as mean ± standard deviation (SD), and qualitative variables as absolute and relative frequencies. Shapiro-Wilk test was used to test the normality of the data. HVPG was used as a continuous parameter, and correlations between variables and HVPG were computed using Pearson’s or Spearman Rho correlation coefficient (R) as guided by the normal distribution of the data. MR measures that significantly correlated with HVPG in the univariate analyses were included in a multivariate linear regression analysis. In all analyses, p <0.05 was considered statistically significant. Due to the exploratory nature of this study, no adjustments were made for multiple comparisons.
Results
Patient characteristics
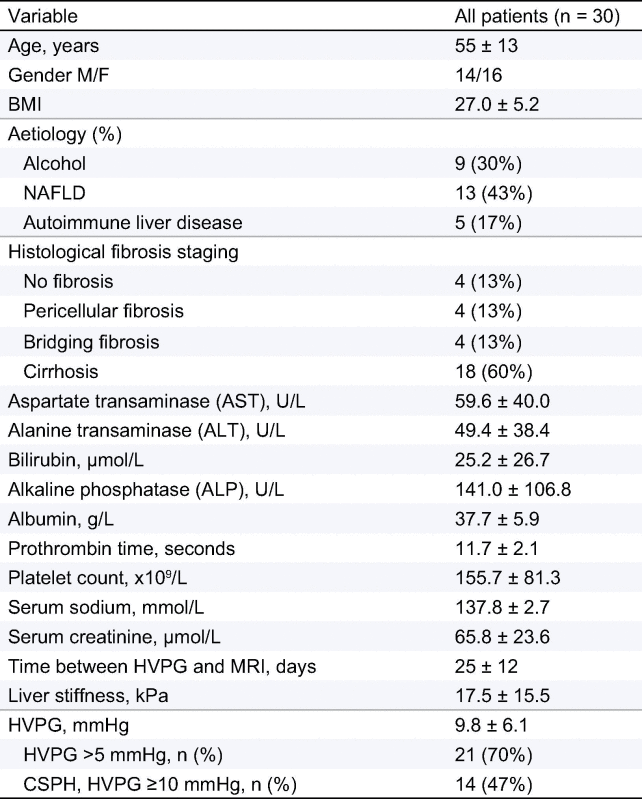
All major clinical and biochemical parameters of the initial patient group are presented in Table 1. Eighteen patients (60%) had histological evidence of cirrhosis and 4 patients (13%) had advanced fibrosis. In those with cirrhosis, 14 patients underwent oesophagogastroduodenoscopy (OGD) and oesophageal varices were present in five. In the whole population, nine patients (30%) had no portal hypertension (HVPG ⩽5 mmHg), 21 patients (70%) had portal hypertension of which 14 patients (47%) had clinically significant portal hypertension (HVPG >10 mmHg).
Table 1.
Clinical and laboratory features of the study population.
 |
ELF markers and LSM as predictors of HVPG
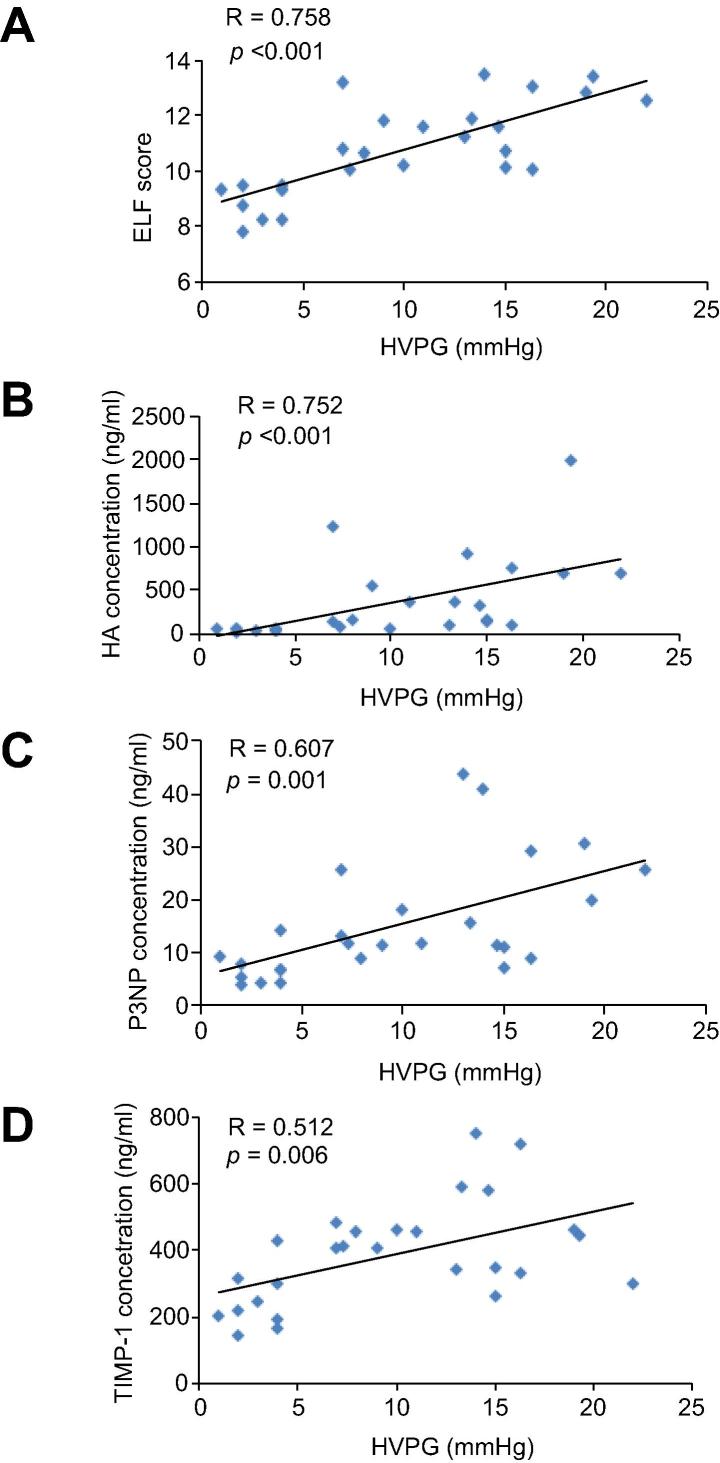
The ELF score correlated significantly with HVPG (Pearson R = 0.758, p <0.001). There was a significant correlation between each of the individual components of the ELF score with HVPG; HA (Spearman R = 0.752, p <0.001), P3NP (Spearman R = 0.607, p = 0.001) and TIMP1 (Pearson R = 0.512, p = 0.006) (Fig. 1). Valid LSM, as measured by TE, were available in 22 patients. LSM correlated significantly with HVPG (Spearman R = 0.791, p <0.001) (Fig.2A). However, for both ELF scores and LSM, there was no significant correlation in the subgroup of patients with portal hypertension and CSPH at HVPG >10 mmHg.
Fig. 1.
Correlation between HVPG and serum markers of liver fibrosis. (A) Enhanced Liver Fibrosis (ELF) score, (B) hyaluronic acid (HA), (C) aminoterminal peptide of procollagen III (P3NP) and (D) tissue inhibitor of matrix metalloproteinase 1 (TIMP-1) concentrations.
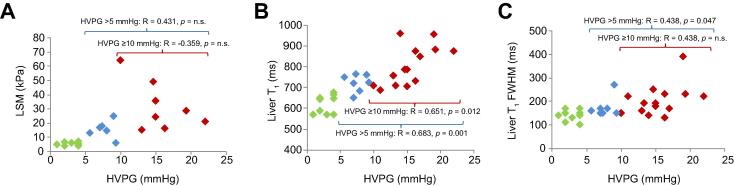
Fig. 2.
Correlation of HVPG with imaging markers of liver fibrosis. (A) liver stiffness measurement (LSM), (B) liver SE-EPI T1 relaxation time (ms) and (C) the full-width-half-maximum (FWHM) of liver SE-EPI T1 Gaussian distribution (ms).
Longitudinal relaxation time (T1) as a predictor of HVPG
Considering the whole patient group, there was a statistically significant positive correlation between HVPG and SE-EPI liver T1 relaxation time (Pearson R = 0.835, p <0.001; Predicted HVPG = 585 + 15∗(Liver SE-EPI T1)) (Fig.2B). This relationship was maintained in patients with portal hypertension with HVPG >5 mmHg (Pearson R = 0.683, p = 0.001) as well as CSPH with HVPG ⩾10 mmHg (Pearson R = 0.651, p = 0.012). The mean (± SD) number of voxels in the mask for liver T1 measurements was 3911(± 1463). The FWHM of the liver SE-EPI T1 Gaussian distribution showed a significant positive correlation with HVPG (Spearman R = 0.611, p <0.001) (Fig.2C), reflecting the increased heterogeneity in liver T1 with increased severity of portal hypertension.
The apparent liver relaxation time (T1∗) measured from bFFE maps was also a predictor of HVPG. As expected, the bFFE readout T1∗ relaxation time was highly correlated with the SE-EPI T1 value (Pearson R = 0.890, p <0.001), but was lower than that of the true T1 measured using a SE-EPI readout, (Liver SE-EPI T1) = 141 + 0.92∗(Liver bFFE T1∗) (median Gaussian distribution values). A significant positive correlation of the bFFE T1∗ relaxation time with HVPG was found (Pearson R = 0.780, p <0.001), which was significant for HVPG >5 mmHg (Pearson R = 0.524, p = 0.018).
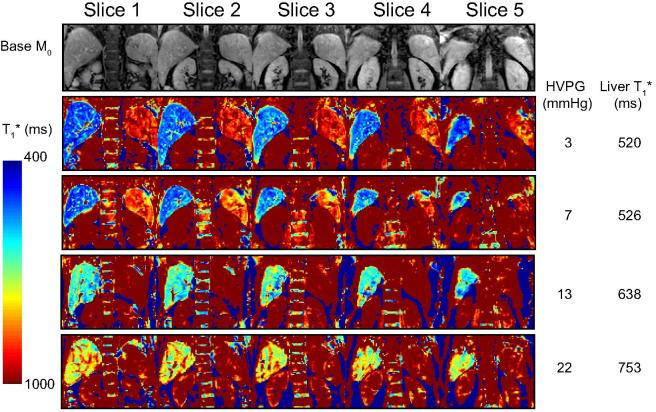
Spleen T1∗, estimated from the bFFE readout scheme, correlated with HVPG in the whole patient group (Pearson R = 0.40, p = 0.028) but this relationship was not significant in patients with portal hypertension and CSPH with HVPG ⩾10 mmHg. Fig. 3 illustrates example coronal bFFE T1∗ maps for patients with increasing HVPG measures.
Fig. 3.
Example of T1 relaxation maps. Top row shows example image quality of the coronal-oblique imaging slices for base equilibrium M0 data acquired from a patient with HVPG of 3 mmHg. Subsequent rows show example coronal-oblique bFFE T1∗ maps showing the liver and spleen, together with HVPG (mmHg) and mode of the liver T1∗ value (ms) across a range of HVPG from 3–22 mmHg, with columns showing each of the five slices collected for the T1∗ map.
Splanchnic and portal flow measures in predicting the HVPG
There was no significant relationship between inflow (portal vein, hepatic artery or total hepatic inflow) and outflow (right, middle and left or total hepatic veins) of the liver with HVPG (Table 2). Whilst in the splanchnic circulation, velocity of the blood flow in the SMA and SA correlated significantly with HVPG (Pearson R = 0.534, p = 0.002, R = 0.584, p = 0.003 respectively, Fig.4A-B). A significant positive correlation of SA velocity with HVPG was found for HVPG >5 mmHg (Pearson R = 0.555, p = 0.032), no significant correlation with SMA velocity was found for HVPG >5 mmHg or HVPG ⩾10 mmHg. No significant correlations were found between SMA or SA velocity at HVPG <10 mmHg, highlighting the haemodynamic changes associated with CSPH. In the azygous vein, velocity and flow correlated significantly with HVPG (Spearman R = 0.515, p = 0.004 and R = 0.656, p <0.001 respectively) (Fig.4C). In patients with CSPH, no MR flow parameters correlated significantly with HVPG. The within session CoV for PC-MR vessel measures are shown in Table 2.
Table 2.
Correlation coefficient and p value of portal, splanchnic and collateral circulation flow parameters as measured by phase contrast MR with HVPG, and the intra-session coefficient of variation (CoV) of flow measures.
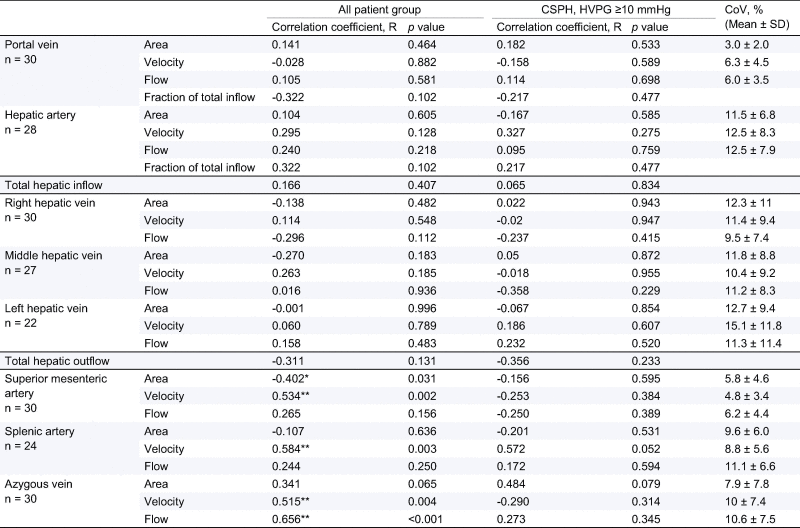 |
Fig. 4.
Correlation of HVPG with splanchnic and collateral flow. (A) superior mesenteric artery (SMA) velocity, (B) splenic artery (SA) velocity and (C) azygous vein (AZV) flow.
Tissue perfusion and relationship with HVPG
Valid liver perfusion measurements were obtained in 28 patients and spleen perfusion measurements in 26 patients. Liver tissue perfusion correlated positively with HVPG (Spearman R = 0.38, p = 0.046) and tissue arrival time negatively correlated with HVPG (Spearman R = −0.467, p = 0.021). However, this relationship was not present in patients with portal hypertension and CSPH. Spleen tissue perfusion was not related to HVPG.
Spleen and liver volume and their ratio to predict HVPG
Liver volume and spleen volume did not independently correlate with HVPG. The ratio of liver/spleen volume negatively correlated significantly with HVPG (Pearson R = −0.40, p = 0.028), but this relationship was absent in patients with portal hypertension and CSPH.
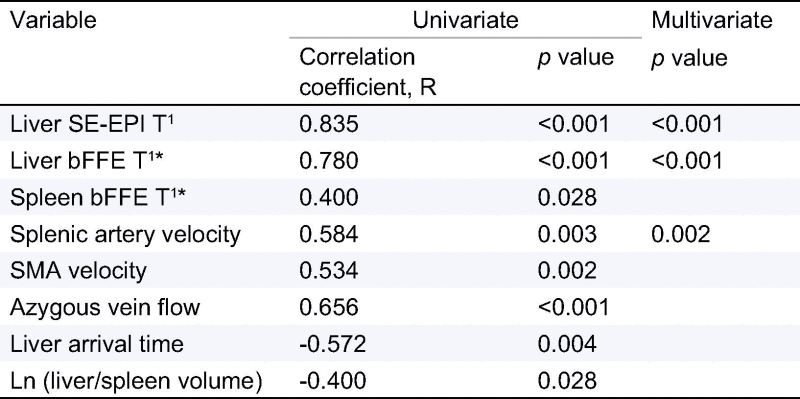
Predictive MR model of HVPG
Table 3 shows those MR parameters that correlated with HVPG in the univariate analysis. The best predictive model for HVPG (that provides the minimum sum-of-squares between measured and predicted HVPG) included liver SE-EPI T1 relaxation time and SA velocity:
Table 3.
Correlation coefficient and p value of MR variables included in univariate analysis.
 |
HVPG = −28 + 0.04∗(Liver SE-EPI T1) + 0.27∗(SA velocity) (Spearman R = 0.90, p <0.001),
This correlation was maintained in patients with CSPH (R = 0.85, p <0.001).
Validation cohort
Additionally, 10 patients were enrolled to the study as a validation cohort, which included 4 with non-alcoholic fatty liver disease, 4 with alcoholic liver disease, 1 each with primary biliary cholangitis and autoimmune hepatitis. Of these, 4 patients had portal hypertension, of which 2 had CSPH. In this cohort, there was a statistically significant positive correlation between HVPG and the SE-EPI T1 relaxation time of the liver (Pearson R = 0.83, p = 0.003). Fig. 5 illustrates a Bland-Altman plot showing predicted HVPG using liver SE-EPI T1 alone, and the combined model of liver SE-EPI T1 and the haemodynamic measure of SA velocity. The combined model can be seen to yield an improved estimation of HVPG, particularly in CSPH.
Fig. 5.
Predictive MRI model for HVPG. Bland-Altman plot showing the difference between measured and predicted HVPG against the measured HVPG using (A) liver SE-EPI T1 relaxation time alone, and (B) predictive MR model of liver SE-EPI T1 relaxation time and splenic artery velocity. Data shown for original (blue diamonds) and validation cohort (red squares), with mean difference (solid line) and ±1.96 standard deviations (broken line) shown.
Discussion
In liver cirrhosis, the disruption of sinusoidal architecture with progressive fibrogenesis and intrahepatic vasoconstriction leads to an increase in intrahepatic resistance resulting in the rise of portal pressure. This is further accentuated by splanchnic vasodilation and increased portal blood flow. In the present study we have demonstrated that a combination of non-invasive quantitative MR measures of liver SE-EPI T1 relaxation time and SA velocity can provide a non-invasive estimation of portal pressure. The combined model of structural and haemodynamic MR measures identified in this study provides the best predictor to accurately reflect the portal pressures through its full range from normal to CSPH (Fig. 5).
The relationship between the degree of hepatic fibrosis and portal pressure has been reported from studies comparing histological changes in liver biopsy with HVPG. For example, quantitative liver biopsy analysis with collagen proportionate area measurement correlated significantly with HVPG [25]. However, histological analyses are limited by the inherent sampling variability associated with liver biopsies [26], [27]. We have previously shown that liver T1 relaxation time is associated with the degree of fibrosis and inflammation in the liver [17]. This acquisition and analysis approach has been shown to be highly repeatable in healthy subjects [17], with a CoV between visits of 1.8%, and a low inter- and intra-observer variability with intra-class correlation coefficients of more than 0.99. Here, SE-EPI T1 data were acquired with fat suppression, removing the effect of fat on the calculated liver T1 value, which results from the water liver tissue compartment. In contrast, T1∗ data acquired with a bFFE readout is affected by the hepatic fat content in a manner dependent on the phase between the fat and water signal (as determined by field strength and repetition time) [28]. Further since our T1 measurement method is both respiratory triggered and multi-slice, it allows a large volume of the liver to be sampled [mean (± SD) of 3911 (± 1463) voxels covering 281 (± 106) cm3] in a reasonable imaging time without the need for breath-hold, making this imaging scheme ideal for patient studies. Previous studies using a modified look-locker inversion recovery (MOLLI) T1 mapping method with bFFE readout have shown a correlation with fibrosis [22]. However, this technique requires a breath-hold for each slice acquired, and it has been shown that hepatic fat content can be large enough to cause severe MOLLI T1 alterations [28]. The distribution of liver T1 values (Gaussian FWHM) was also shown to increase with the worsening portal hypertension, reflecting the increasing heterogeneity of T1 values across the liver volume. This emphasises the sampling variability associated with liver biopsy and potentially TE, and highlights the need for architectural changes to be studied across the whole liver. In the subgroup of patients with portal hypertension and CSPH, the correlation between liver SE-EPI T1 relaxation time and HVPG remained significant, demonstrating its applicability in assessing portal pressure in patients with severe portal hypertension. There was no corresponding correlation between LSM and HVPG over this higher HVPG range in patients with portal hypertension and CSPH. Our findings are similar to those of Vizutti and colleagues who also report that LSM did not correlate with HVPG >10 mmHg and it is likely that LSM does not reflect the extrahepatic haemodynamic changes in advanced portal hypertension. The correlation between the ELF score and HVPG has not been previously reported. However, similar to the LSM, the correlation was lost in patients with portal hypertension and CSPH. A previous study has demonstrated the correlation of MRE-measured liver loss modulus with HVPG (r = 0.44, p = 0.02) [13], a lower correlation than using T1 alone. It would be of interest to use T1 measures in conjunction with MRE-derived assessment of liver stiffness to assess the prediction of HVPG.
We show a significant correlation between blood flow velocity in the splanchnic circulation, in SMA and SA with HVPG, which is likely to represent the hyperdynamic state in portal hypertension. Previous Doppler ultrasound studies have reported increased flow in the SMA and SA in patients with cirrhosis [29] but no direct comparisons with HVPG have been made. Doppler ultrasound has also been widely used to assess the changes in portal and splanchnic blood flow in liver disease. However, the reproducibility of Doppler ultrasound has been questionable with high intra- and inter-observer variation [16], [30]. PC-MR is a non-invasive flow measurement technique without intravenous contrast, whereby the phase shift of flowing blood is proportional to the velocity. Yzet and colleagues reported that PC-MR was a more reliable measure of hepatic blood flow compared to Doppler ultrasound with lower variability and higher reproducibility [18]. In this study, we have shown that within session CoV of the velocity measurement of SMA and SA by PC-MR is less than 10%, in agreement with a previous study [31].
It is an interesting observation that HVPG can potentially be assessed non-invasively using a simple linear model of MRI parameters of liver SE-EPI T1 relaxation time and SA velocity. Fig. 5 highlights that this linear model provides good prediction of HVPG across the span of HVPG values from normal to CSPH, better than SE-EPI liver T1 relaxation time (or SA velocity) alone. The scan time required to collect the data for this model (Liver T1 and triplicate SA data) is 5–10 min, dependent on breathing rate of the patient, with PC-MR data being planned whilst the respiratory triggered T1 sequence is acquired.
Various non-invasive markers of HVPG, including LSM, have been reported as being accurate as a binary predictor of the presence or absence of CSPH [32]. However, we believe that the MR measures of hepatic architecture and splanchnic haemodynamics do have the advantage of being able to accurately estimate HVPG values on a continuous scale as identification of the progression of portal hypertension beyond the threshold of CSPH (HVPG ⩾10 mmHg), and this has prognostic implications in patients with cirrhosis [7], [9]. We could potentially utilise this MR model to monitor the HVPG response in portal hypertensive patients. For example, MRE has been used for the first time in a recent clinical trial [33], and this proposed algorithm could now be used in future trials in cirrhosis patients to potentially demonstrate and assess diagnostic test characteristics, for example to assess beta-blocker therapy for lowering of HVPG (HVPG to <12 mmHg or reduction of 20% from baseline).
Here, we have included all patients who were undergoing HVPG measurements for clinical suspicion of portal hypertension in our study population. Although patients without cirrhosis and portal hypertension were included in the study, this reflects the use of HVPG and the potential for non-invasive alternatives in clinical practice. Moreover, the patients included ranged from those with normal portal pressures to severe portal hypertension which enabled the MR measures to be evaluated over a wide range of HVPG values.
In conclusion, in a well characterised patient population, we have shown that a combination of quantitative MR measures of liver T1 and SA velocity correlate significantly with HVPG; this was replicated in our second cohort. If these results are confirmed by external validation, this non-invasive model including both architectural (liver T1 relaxation time) and haemodynamic (SA velocity) measures could be used as a surrogate measure of HVPG in clinical trials of portal hypertension as well as monitoring treatment in clinical practice.
Financial support
The authors acknowledge the financial support from NIHR Nottingham Digestive Diseases Biomedical Research Unit, Nottingham University Hospitals NHS Trust and University of Nottingham, and CORE charity. This paper presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Authors’ contributions
NP (Acquisition, analysis and interpretation of data, statistical analysis, drafting of the manuscript); EC (Acquisition, analysis and interpretation of data, critical revision of the manuscript); SF (Study concept and design, interpretation of data, drafting and critical revision of the manuscript); CB, (Acquisition and analysis of data), RS, RON, GR, ST, HW, RS, PT (Acquisition of data); AA, ING, GPA (Study concept and design, critical revision of the manuscript).
See Editorial, pages 1079–1080
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jhep.2016.07.021.
Supplementary data
References
- 1.Reynaert H., Thompson M.G., Thomas T., Geerts A. Hepatic stellate cells: role in microcirculation and pathophysiology of portal hypertension. Gut. 2002;50:571–581. doi: 10.1136/gut.50.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groszmann R.J., Wongcharatrawee S. The hepatic venous pressure gradient: Anything worth doing should be done right. Hepatology. 2004;39:280–282. doi: 10.1002/hep.20062. [DOI] [PubMed] [Google Scholar]
- 3.Groszmann R.J., Garcia-Tsao G., Bosch J., Grace N.D., Burroughs A.K., Planas R. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–2261. doi: 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- 4.Ripoll C., Groszmann R., Garcia-Tsao G., Grace N., Burroughs A., Planas R. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Ripoll C., Groszmann R.J., Garcia-Tsao G., Bosch J., Grace N., Burroughs A. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923–928. doi: 10.1016/j.jhep.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garciatsao G., Groszmann R.J., Fisher R.L., Conn H.O., Atterbury C.E., Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985;5:419–424. doi: 10.1002/hep.1840050313. [DOI] [PubMed] [Google Scholar]
- 7.Merkel C., Bolognesi M., Bellon S., Zuin R., Noventa F., Finucci G. Prognostic usefulness of hepatic vein catheterization in patients with cirrhosis and esophageal-varices. Gastroenterology. 1992;102:973–979. doi: 10.1016/0016-5085(92)90185-2. [DOI] [PubMed] [Google Scholar]
- 8.Silva-Junior G., Baiges A., Turon F., Torres F., Hernandez-Gea V., Bosch J. The prognostic value of HVPG in patients with cirrhosis is highly dependent on the accuracy of the technique. Hepatology. 2015;62:1584–1592. doi: 10.1002/hep.28031. [DOI] [PubMed] [Google Scholar]
- 9.Moitinho E., Escorsell N., Bandi J.C., Salmeron J.M., Garcia-Pagan J.C., Rodis J. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology. 1999;117:626–631. doi: 10.1016/s0016-5085(99)70455-5. [DOI] [PubMed] [Google Scholar]
- 10.Vizzutti F., Arena U., Romanelli R.G., Rega L., Foschi M., Colagrande S. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290–1297. doi: 10.1002/hep.21665. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Pagan J.C., Gracia-Sancho J., Bosch J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol. 2012;57:458–461. doi: 10.1016/j.jhep.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Colecchia A., Montrone L., Scaioli E., Bacchi-Reggiani M.L., Colli A., Casazza G. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646–654. doi: 10.1053/j.gastro.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 13.Ronot M., Lambert S., Elkrief L., Doblas S., Rautou P.E., Castera L. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur Radiol. 2014;24:1394–1402. doi: 10.1007/s00330-014-3124-y. [DOI] [PubMed] [Google Scholar]
- 14.Iranmanesh P., Vazquez O., Terraz S., Majno P., Spahr L., Poncet A. Accurate computed tomography-based portal pressure assessment in patients with hepatocellular carcinoma. J Hepatol. 2014;60:969–974. doi: 10.1016/j.jhep.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Baik S.K. Haemodynamic evaluation by Doppler ultrasonography in patients with portal hypertension: a review. Liver Int. 2010;30:1403–1413. doi: 10.1111/j.1478-3231.2010.02326.x. [DOI] [PubMed] [Google Scholar]
- 16.Sacerdoti D., Gaiani S., Buonamico P., Merkel C., Zoli M., Bolondi L. Interobserver and interequipment variability of hepatic, splenic, and renal arterial Doppler resistance indices in normal subjects and patients with cirrhosis. J Hepatol. 1997;27:986–992. doi: 10.1016/s0168-8278(97)80141-9. [DOI] [PubMed] [Google Scholar]
- 17.Hoad C.L., Palaniyappan N., Kaye P., Chernova Y., James M.W., Costigan C. A study of T1 relaxation time as a measure of liver fibrosis and the influence of confounding histological factors. NMR Biomed. 2015;28:706–714. doi: 10.1002/nbm.3299. [DOI] [PubMed] [Google Scholar]
- 18.Yzet T., Bouzerar R., Allart J.D., Demuynck F., Legallais C., Robert B. Hepatic vascular flow measurements by phase contrast MRI and doppler echography: a comparative and reproducibility study. J Magn Reson Imaging. 2010;31:579–588. doi: 10.1002/jmri.22079. [DOI] [PubMed] [Google Scholar]
- 19.Francis S.T., Bowtell R., Gowland P.A. Modeling and optimization of Look-Locker spin labeling for measuring perfusion and transit time changes in activation studies taking into account arterial blood volume. Magn Reson Med. 2008;59:316–325. doi: 10.1002/mrm.21442. [DOI] [PubMed] [Google Scholar]
- 20.McCorry R.B., Palaniyappan N., Chivinge A., Kaye P., James M.W., Aithal G.P. Development and evaluation of a nurse-led transient elastography service for the staging of hepatic fibrosis in patients with suspected chronic liver disease. QJM. 2012;105:749–754. doi: 10.1093/qjmed/hcs043. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg W.M.C., Voelker M., Thiel R., Becka M., Burt A., Schuppan D. Serum markers detect the presence of liver fibrosis: A cohort study. Gastroenterology. 2004;127:1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee R., Pavlides M., Tunnicliffe E.M., Piechnik S.K., Sarania N., Philips R. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014;60:69–77. doi: 10.1016/j.jhep.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt P., Griswold M.A., Jakob P.M., Kotas M., Gulani V., Flentje M. Inversion recovery TrueFISP: Quantification of T-1, T-2 and spin density. Magn Reson Med. 2004;51:661. doi: 10.1002/mrm.20058. 698–698. [DOI] [PubMed] [Google Scholar]
- 24.Liss P., Cox E.F., Eckerbom P., Francis S.T. Imaging of intrarenal haemodynamics and oxygen metabolism. Clin Exp Pharmacol Physiol. 2013;40:158–167. doi: 10.1111/1440-1681.12042. [DOI] [PubMed] [Google Scholar]
- 25.Calvaruso V., Burroughs A.K., Standish R., Manousou P., Grillo F., Leandro G. Computer-assisted image analysis of liver collagen: relationship to ishak scoring and hepatic venous pressure gradient. Hepatology. 2009;49:1236–1244. doi: 10.1002/hep.22745. [DOI] [PubMed] [Google Scholar]
- 26.Ratziu V., Charlotte F., Heurtier A., Gombert S., Giral P., Bruckert E. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 27.Bedossa P., Dargere D., Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Mozes F.E., Tunnicliffe E.M., Pavlides M., Robson M.D. Influence of fat on liver T1 measurements using modified Look-Locker inversion recovery (MOLLI) methods at 3T. J Magn Reson Imaging. 2016;44:105–111. doi: 10.1002/jmri.25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwiebel W.J., Mountford R.A., Halliwell M.J., Wells P.N.T. Splanchnic blood-flow in patients with cirrhosis and portal-hypertension – Investigation with duplex-doppler us. Radiology. 1995;194:807–812. doi: 10.1148/radiology.194.3.7862983. [DOI] [PubMed] [Google Scholar]
- 30.Sabba C., Weltin G.G., Cicchetti D.V., Ferraioli G., Taylor K.J., Nakamura T. Observer variability in echo-Doppler measurements of portal flow in cirrhotic patients and normal volunteers. Gastroenterology. 1990;98:1603–1611. doi: 10.1016/0016-5085(90)91097-p. [DOI] [PubMed] [Google Scholar]
- 31.Cox E.F., Smith J.K., Chowdhury A.H., Lobo D.N., Francis S.T., Simpson J. Temporal assessment of pancreatic blood flow and perfusion following secretin stimulation using noninvasive MRI. J Magn Reson Imaging. 2015;42:1233–1240. doi: 10.1002/jmri.24889. [DOI] [PubMed] [Google Scholar]
- 32.Berzigotti A., Seijo S., Arena U., Abraldes J.G., Vizzutti F., Garcia-Pagan J.C. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144:102–111. doi: 10.1053/j.gastro.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Loomba R., Sirlin C.B., Ang B., Bettencourt R., Jain R., Salotti J. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial) Hepatology. 2015;61:1239–1250. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.