Abstract
Sexuality as to its etymology presupposes the duality of sexes. Using quantitative neuroimaging meta-analyses, we demonstrate robust sex differences in the neural processing of sexual stimuli in thalamus, hypothalamus, and basal ganglia. In a narrative review, we show how these relate to the well-established sex differences on the behavioral level. More specifically, we describe the neural bases of known poor agreement between self-reported and genital measures of female sexual arousal, of previously proposed male proneness to affective sexual conditioning, as well as hints of unconscious activation of bonding mechanisms during sexual stimulation in women. In summary, our meta-analytic review demonstrates that neurofunctional sex differences during sexual stimulation can account for well-established sex differences in sexual behavior.
Keywords: activation likelihood estimation, ALE, fMRI, functional magnetic resonance imaging, meta-analysis, neuroimaging, PET, positron emission tomography, sex differences, sexual behavior
1. Introduction
In 1926, Freud noted that “the sexual life of adult women is a dark continent for psychology” (Freud, 1926). Since then, a large regiment of scientists have sought to shed light on human sexual behavior. But only recently have investigators been able to follow through on James’ notion that “a certain amount of brain-physiology must be presupposed or included in Psychology” (James, 1890), using modern neuroimaging techniques to explore the neural underpinnings of sexual stimulus processing. Remarkably, the results of these studies have been largely consistent with the Freudian concept of sexual drives (Freud, 1915; for an overview, cf. Stoléru, 2014). The drive concept involves a motor factor representing the urge of the so-called sexual instinct and satisfaction as the ultimate aim. It moreover includes an object “in regard to which or through which the instinct is able to achieve its aim”, which may be “a part of the subject’s own body”. Finally, it is characterized by the assumption of a somatic source, i.e., a somatic process in an organ or part of the body, which may be “of a chemical nature” (Freud, 1915).
Findings of two pioneering positron emission tomography (PET) investigations into cerebral activity during visual sexual stimulation have been taken as the basis for a “neurobehavioral model of sexual arousal” (Redouté et al., 2000; Stoléru et al., 1999). A later quantitative meta-analysis of functional imaging studies on sexual arousal corroborated the validity of the postulated model, therein referred to as “neurophenomenological model of sexual arousal” (Stoléru et al., 2012). On the basis of neuroimaging meta-analysis, four components were distinguished as links between psychological as well as physiological processes and neuroanatomy (Stoléru et al., 2012): 1) The cognitive component – comprised of appraisal of a potentially sexual stimulus, increased attention, and motor imagery – is believed to be mediated by the lateral orbitofrontal cortex (OFC), bilateral inferior temporal cortices, superior and inferior parietal lobules (SPL/IPL), premotor and supplementary motor areas (SMA), and the cerebellum. 2) The emotional component – i.e., pleasure associated with rising arousal and with the perception of specific bodily changes – presumably results from the interplay of the amygdala, posterior insula, and primary and secondary somatosensory cortices. 3) The motivational component – composed of goal-directed behavior including the perceived urge to express overt sexual behaviour – is assumed to arise from activity in the anterior cingulate cortex (ACC), claustrum, posterior parietal cortex, hypothalamus, substantia nigra, and ventral striatum. 4) These structures overlap with those of the autonomic and neuroendocrine component – including various physiological responses preparing for sexual behavior – which is thought to be mediated by ACC, anterior insula, putamen, and hypothalamus.
Although this model sine qua non simplifies the complex processes involved in human sexual behavior, its keystone elements – i.e. the delineation of a neural core circuit and neuroanatomical assignment of psychological and physiological components – have been corroborated by other meta-analyses of brain activity during sexual stimulation (Kühn and Gallinat, 2011; Poeppl et al., 2014). Given its complexity, however, the phenomenon of sexual excitement is very likely to originate from statistically dissociable neural networks responsible for specific aspects of sexual behavior (Georgiadis and Kringelbach, 2012). Accordingly, distinct brain networks underlying psychosexual (i.e., mental sexual) and physiosexual (i.e., physiological sexual) arousal have been delineated by recent quantitative meta-analyses (Poeppl et al., 2014). On the basis of these meta-analytic findings, a sequence of sexual stimulus-driven processing has been proposed (Poeppl et al., 2014): A potentially sexual stimulus is categorized through cognitive and memory-guided evaluation (lateral prefrontal cortex [LPFC], hippocampus), which induces attention to focus on the sexual target, so increasing top-down modulation of sensory processing (occipitotemporal cortex [OTC], superior parietal lobules [SPL]). These processes are triggered by relevance detection and affective evaluation (amygdala, thalamus). Based on initiated autonomic responses (hypothalamus), the resulting sexual urge (basal ganglia) finally ends in the awareness of sexual arousal (anterior insula). From this neural network of psychosexual arousal, a network of physiosexual arousal was distinguished (Poeppl et al., 2014): Autonomic and concomitant emotion regulation is reflected by activity in the subgenual ACC (sgACC), while the anterior middle cingulate cortex (aMCC) controls the initiation of (copulatory) behavior, i.e., action toward sexual urges. These in turn are represented primarily in the putamen and claustrum as well as in the anterior insular cortex, where awareness of both the rising sexual desire and the bodily reaction is engendered. To this end, the insular cortex also integrates, in a posterior-to-anterior sequence, somatosensory information from the operculum, which monitors the bodily changes during sexual arousal. Two regions that connect both neural networks with potentially dissociable functions were identified (Poeppl et al., 2014): The putamen might orchestrate the integration of sensorimotor information in the context of rising sexual desire, while the claustrum may be primarily responsible for crossmodal processing between and within the networks of sexual arousal. Finally, this meta-analysis demonstrated brain deactivations during sexual stimulation that suggested reduced metacognitive introspective and self-reflexive processing (temporoparietal junction [TPJ], hippocampus, retrosplenial cortex [RSC]) as well as a release of intrinsic inhibition of sexual arousal (inferior temporal sulcus [ITS], superior temporal gyrus [STG]).
In the broader context of the human sexual pleasure cycle, a comprehensive review of the pertinent brain imaging literature indicated that the functional neuroanatomy of sexual behavior is comparable to that involved in processing other rewarding stimuli (Georgiadis and Kringelbach, 2012). Moreover, this review concluded that sexual behavior can be organized into the phases of wanting, liking, and satiety, on the basis of differential brain network activity reflecting the stages of the human sexual response cycle (i.e., excitement, plateau, orgasm, resolution) (Masters and Johnson, 1966). The underlying neuroanatomical theory focuses on the OFC, which is assumed to receive input from somatosensory cortices and to provide a multimodal perceptual integration of sexual stimuli. The resulting potentially hedonic experience should be modulated by loops with other regions such as nucleus accumbens, ventral pallidum, hippocampus, amygdala, hypothalamus, as well as somatosensory and visceral input from the genitals (Georgiadis and Kringelbach, 2012).
The major drawback of these theories derived from neuroimaging results is that they are almost exclusively based on data from male subjects (Georgiadis and Kringelbach, 2012; Kühn and Gallinat, 2011; Poeppl et al., 2014; Stoléru et al., 2012). Therefore, the introduced models may only be accurate for men. Moreover, differences in the neural correlates of sexual stimulus processing between men and women seem inevitable: Where else should sex differences emerge if not in sexual behavior, given that sexuality (as to its etymology) presupposes the duality of sexes (Devereux, 1982)? In fact, initial results suggested that men and women differ in amygdala and hypothalamus response to visual sexual stimuli, even when reporting equivalent sexual arousal (Hamann et al., 2004). Yet, these findings were not consistent across studies, as a similar investigation could not locate differential effects in the amygdala (Karama et al., 2002). Furthermore, sex differences in hypothalamic activation disappeared when ratings of perceived sexual arousal were used as a covariate in the analysis (Karama et al., 2002). Two pioneering studies on sex differences in neural sexual stimulus processing thus yielded diverging results.
Since then, however, the number of studies investigating neural sexual stimulus processing in women and corresponding sex differences has remarkably increased. A narrative review of the relevant literature until 2010 suggested that “gender differences in brain responses to visual sexual stimuli may not apply to other sensory modalities” (Stoléru et al., 2012). Another comprehensive verbal review concluded that “there are no consistent and conclusive gender differences in visual sexual stimuli-related brain processing between men and women” (Georgiadis and Kringelbach, 2012). Mere reviews, however, run the risk of being subjective and lack the capability of quantitative assessment. For an objective assessment of interstudy concordance, automated meta-analyses that quantify the level of concordance and allow identification of brain regions associated with significant convergence in a testable manner are hence preferable. Activation likelihood estimation (ALE) as introduced to neuroimaging by Turkeltaub et al. (2002) and subsequently refined (Eickhoff et al., 2009, 2012; Laird et al., 2005; Turkeltaub et al., 2012) meets these demands and represents the most widely accepted approach for such quantitative integration of neuroimaging findings.
The following series of meta-analyses quantitatively reviews the relevant literature and delineates neural networks for sexual stimulus processing in women. In addition, it statistically and topographically assesses differences of these networks in comparison to men. It has been noted that reported sex differences in brain processing of visual sexual stimuli seem to be biased towards men, which may be attributed to a presumed greater role of visual stimuli in male sexual behavior (Georgiadis and Kringelbach, 2012; Laumann et al., 1994). Therefore, separate meta-analyses were performed focusing on visual sexual stimulation and on sexual stimulation irrespective of sensory modality. These analyses delineate the functional neuroanatomy of sexual processing in women and assess differences to men in order to spot neurobiobehavioral sex differences in neural sexual stimulus processing.
2. Methods
2.1. Study selection
We applied a similar search and selection strategy as a previous meta-analysis on the functional neuroanatomy of sexual stimulus processing in men (Poeppl et al., 2014). A stepwise procedure to identify the relevant experimental studies was used. First, we selected studies through a standard search in the PubMed (http://www.pubmed.gov) and ISI Web of Science (http://apps.isiknowledge.com) databases using the terms “sexual” or “erotic” in combination with “fMRI”, “functional MRI”, “functional magnetic resonance”, “PET”, “positron emission”, “ASL”, “arterial spin labeling”, “MEG”, “magnetoencephalography”, “neuroimaging”, or “imaging”. Second, further studies were found by means of the “related articles” function of the PubMed database and by tracing the references from review articles and the identified papers. Experiments were considered relevant when they were intended to sexually stimulate (sexual vs. control condition) heterosexual subjects from non-clinical populations, irrespective of the sensory modality. Only heterosexual subjects were considered to avoid potential confounding by differences in sexual orientation. Additional inclusion and exclusion criteria were as follows:
Only studies reporting results of whole-brain group analyses with coordinates referring to a standard reference space (Talairach-Tournoux or Montreal Neurological Institute [MNI]) were included. Reports including less than five subjects, results of region-of-interest analyses, and studies not reporting stereotaxic coordinates were excluded.
Only data from healthy subjects were included. Results from patients and data from conditions focusing on pharmalogical manipulation were excluded.
Since this study sought to globally delineate the female sexual brain response, experiments involving women during all phases of the menstrual cycle were included. Therefore, hormonal contraception was not considered an exclusion criterion.
Experiments involving men were only included either if (1) the analogous within-group experiment had also been performed in women, or if (2) the experiments comprised direct group (men vs. women) comparisons.
On the basis of these search criteria, 24 studies were found to be eligible for inclusion into the meta-analyses (cf., Tables 1–3). Sensory modalities included visual, tactile, and olfactory sexual stimulation. The employed putative pheromones and human sex-steroid-derived compounds (4,16-androstadien-3-one and oestra-1,3,5(10),16-tetraen-3-ol) have been shown to introduce sex-specific effects and to increase sexual arousal (Bensafi et al., 2004). Only functional magnetic resonance imaging (fMRI) and PET but no arterial spin labeling (ASL) or magnetoencephalography (MEG; which might considerably differ from fMRI and PET with regard to spatial uncertainty) studies fulfilled our search criteria. Conceptually, it is unproblematic to include both fMRI and PET techniques, because there should be no systematic bias. Although cluster sizes may be larger in PET than in fMRI, activation peaks should not systematically differ (Eickhoff et al., 2009; Feng et al., 2004; Nickerson et al., 2001; Xiong et al., 1998). Together, these studies reported 712 activation foci obtained from 53 individual within-group contrasts in men or women and 190 activation foci obtained from 26 between-group experiments (with a “study” referring to a paper, an “experiment” referring to an individual contrast reported in this paper). Only 29 deactivation foci obtained from 15 within-group experiments were reported, preventing any further analyses on deactivations. Differences in coordinate spaces (Talairach vs. MNI space) were accounted for by transforming coordinates reported in Talairach space into MNI coordinates using a linear transformation (Laird et al., 2010; Lancaster et al., 2007). There was no evidence of systematic bias with respect to age (cf., Supplementary Tables).
Table 1.
Studies on brain activations during sexual stimulation in women
| First Author | Year | Subjects | Menstrual Cycle | Subjective Stimulus Ratings | Imaging Method | Magnetic Field Strength | Experiment | Foci | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Activations | Deactivations | ||||||||
| Abler | 2013 | 12 | F | ✓+ | fMRI | 3.0 T | Erotic > Non-Erotic Videos (Follicular) | 25 | N/A |
| 12 | L | ✓+ | fMRI | 3.0 T | Erotic > Non-Erotic Videos (Luteal) | 21 | N/A | ||
| 12 | C | ✓+ | fMRI | 3.0 T | Erotic > Non-Erotic Videos (Contraceptives) | 25 | N/A | ||
| 12 | F | ✓+ | fMRI | 3.0 T | Erotic > Non-Erotic Pictures (Follicular) | 32 | N/A | ||
| 12 | L | ✓+ | fMRI | 3.0 T | Erotic > Non-Erotic Pictures (Luteal) | 22 | N/A | ||
| 12 | C | ✓+ | fMRI | 3.0 T | Erotic > Non-Erotic Pictures (Contraceptives) | 28 | N/A | ||
|
| |||||||||
| Arnow | 2009 | 20 | C/L (38/62%) | ✓ | fMRI | 3.0 T | Erotic > Sports Videos | 61 | 3 |
|
| |||||||||
| Berglund | 2006 | 12 | F/L | ✓ | PET | N/A | Male Pheromone > Air | 3 | N/A |
|
| |||||||||
| Berglund | 2008 | 12 | F/L | ✓ | PET | N/A | Male Pheromone > Air | 1 | N/A |
| 12 | F/L | ✓ | PET | N/A | Male > Female Pheromone | 4 | 3 | ||
|
| |||||||||
| Bianchi-Demicheli | 2011 | 15 | F | ✓ | fMRI | 3.0 T | Erotic > Non-Erotic Pictures | 12 | N/A |
|
| |||||||||
| Borga | 2014 | 20 | F (20% C) | ✓ | fMRI | 3.0 T | Sexual Penetration > Neutral Pictures | 12 | 0 |
| 20 | F (20% C) | ✓ | fMRI | 3.0 T | Sexual Penetration > Disgust Pictures | 5 | 1 | ||
|
| |||||||||
| Borgb | 2014 | 21 | F | ✓ | fMRI | 3.0 T | Sexual Penetration > Neutral Pictures | 16 | N/A |
|
| |||||||||
| Ciumas | 2009 | 13 | F/L | ✓ | PET | N/A | Male Pheromone > Air | 2 | N/A |
|
| |||||||||
| Georgiadis | 2006 | 12 | N/A | ✓ | PET | N/A | Clitoral Stimulation > Rest | 10 | 5 |
|
| |||||||||
| Gillath | 2012 | 20 | N/A | × | fMRI | 1.5 T | Sexual > Neutral Pictures (Supraliminal) | 24 | N/A |
| 20 | N/A | × | fMRI | 1.5 T | Sexual > Neutral Pictures (Subliminal) | 25 | N/A | ||
|
| |||||||||
| Hamann | 2014 | 13 | N/A | ✓ | fMRI | 3.0 T | Sexual Male > Female Pictures | 16 | 0 |
|
| |||||||||
| Karama | 2002 | 20 | F/L | ✓ | fMRI | 1.5 T | Erotic > Neutral Videos | 14 | N/A |
|
| |||||||||
| Klucken | 2009 | 20 | N/A | ✓ | fMRI | 1.5 T | Erotic > Neutral Pictures | 24 | N/A |
|
| |||||||||
| Safron | N/A* | 23 | N/A | ✓ | fMRI | 3 T | Erotic > Nature Videos | 26 | N/A |
| 22 | N/A | ✓ | fMRI | 3 T | Erotic Pictures > Fixation | 40 | N/A | ||
|
| |||||||||
| Savic | 2001 | 12 | F/L | ✓ | PET | N/A | Male Pheromone > Air | 4 | N/A |
| 12 | F/L | ✓ | PET | N/A | Male > Female Pheromone | 3 | 0 | ||
|
| |||||||||
| Savic | 2005 | 12 | F/L | ✓ | PET | N/A | Male Pheromone > Air | 1 | N/A |
| 12 | F/L | ✓ | PET | N/A | Male > Female Pheromone | 0 | 0 | ||
|
| |||||||||
| Savic | 2010 | 15 | F/L | ✓ | PET | N/A | Male Pheromone > Air | 1 | 3 |
|
| |||||||||
| Wehrum | 2013 | 50 | C/F/L (54/22/22%) | ✓ | fMRI | 1.5 T | Sexual > Neutral Pictures | 9 | N/A |
| 50 | C/F/L (54/22/22%) | ✓ | fMRI | 1.5 T | Sexual > Positive Pictures | 12 | N/A | ||
| 50 | C/F/L (54/22/22%) | ✓ | fMRI | 1.5 T | Sexual > Negative Pictures | 6 | N/A | ||
|
| |||||||||
| Woodard | 2013 | 6 | N/A | × | fMRI | 3.0 T | Sexually Explicit > Neutral Videos | 14 | N/A |
C, contraceptive; F, follicular; fMRI, functional magnetic resonance imaging; L, luteal; N/A, not available; PET, positron emission tomography.
Personal communication.
No differences between the three groups (in follicular/luteal stage or on contraceptives) with respect to sexual arousal ratings of stimuli.
Table 3.
Studies on sex differences in brain activations during sexual stimulation
| First Author | Year | Subjects | Menstrual Cycle | Subjective Stimulus Ratings | Imaging Method | Magnetic Field Strength | Experiment | Foci | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Male | Female | Men > Women | Women > Men | |||||||
| Georgiadis | 2009 | 11 | 12 | N/A | × | PET | N/A | Genital Stimulation > Rest | 4 | 7 |
|
| ||||||||||
| Gillath | 2012 | 19 | 20 | N/A | × | fMRI | 1.5 T | Sexual > Neutral Pictures (Supraliminal) | 0 | 0 |
| 19 | 20 | N/A | × | fMRI | 1.5 T | Sexual > Neutral Pictures (Subliminal) | 0 | 6 | ||
|
| ||||||||||
| Gizewski | 2006 | 22 | 22 | L | ✓ b | fMRI | 1.5 T | Erotic > Neutral Videos (Mid-Luteal) | 8 | 0 |
| 22 | 22 | F | ✓ c | fMRI | 1.5 T | Erotic > Neutral Videos (Menstrual) | 8 | 0 | ||
|
| ||||||||||
| Hamann | 2014 | 13 | 13 | N/A | ✓ b | fMRI | 3.0 T | Sexual > Neutral Pictures | 7 | 0 |
|
| ||||||||||
| Klucken | 2009 | 20 | 20 | N/A | × | fMRI | 1.5 T | Erotic > Neutral Pictures | 8 | 0 |
|
| ||||||||||
| Safron | N/A* | 25 | 23 | N/A | ✓ a | fMRI | 3 T | Erotic > Nature Videos | 24 | 0 |
| 26 | 22 | N/A | ✓ a | fMRI | 3 T | Erotic Pictures > Fixation | 71 | 0 | ||
|
| ||||||||||
| Sylva | 2013 | 12 | 11 | N/A | × | fMRI | 3 T | Sexual Pictures > Fixation | 40 | 2 |
|
| ||||||||||
| Wehrum | 2013 | 48 | 50 | C/F/L (54/22/22%) | ✓ c | fMRI | 1.5 T | Sexual > Neutral Pictures | 0 | 0 |
| 48 | 50 | C/F/L (54/22/22%) | ✓ c | fMRI | 1.5 T | Sexual > Positive Pictures | 1 | 0 | ||
| 48 | 50 | C/F/L (54/22/22%) | ✓ c | fMRI | 1.5 T | Sexual > Negative Pictures | 4 | 0 | ||
C, contraceptive; F, follicular; fMRI, functional magnetic resonance imaging; L, luteal; N/A, not available; PET, positron emission tomography.
Personal communication.
Comparison of women with men with respect to subjective ratings of the sexual stimulus is not available.
No significant difference between women and men with respect to subjective ratings of the sexual stimulus.
Significant difference between women and men with respect to subjective ratings of the sexual stimulus.
2.2. Meta-analytic conception
Convergence of reported activation coordinates was analyzed for the main effects of sexual stimulation in women, irrespective of stimulus modality (32 within-group experiments, 498 foci; cf., Table 1).
Furthermore, we assessed convergence of reported activation foci for commonalities and differences between men and women, irrespective of sexual stimulus modality (cf., Tables 2 and 3). To allow for well-balanced group comparisons, only experiments with analogous experimental design in men and women were fed into these analyses. Commonalities were studied on basis of the respective within-group contrasts (sexual vs. control condition; 214 foci from 21 experiments in men; 281 foci from 21 experiments in women). A double-track approach was applied with respect to group differences. First, we tested for differences in consistency of activations by comparing within-group contrasts of men and women (sexual vs. control condition; 214 foci from 21 experiments in men; 281 foci from 21 experiments in women; cf., Table 2). Second, consistent differences in activations were analyzed by meta-analyses over experiments reporting between-group differences (sexual vs. control condition; 175 foci from 13 experiments for men vs. women; cf., Table 3). Due to the negligible number of coordinates for greater activations in women compared to men (sexual vs. control condition; 15 foci from 13 experiments for women vs. men), no meaningful meta-analysis could be computed for this contrast.
Table 2.
Studies on brain activations during sexual stimulation in women and men
| First Author | Year | Subjects | Menstrual Cycle | Subjective Stimulus Ratings | Imaging Method | Magnetic Field Strength | Experiment | Foci | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Female | Male | Activations | Deactivations | |||||||
| Abler | 2013 | 12 | 0 | F | ✓a | fMRI | 3.0 T | Erotic > Non-Erotic Videos (Follicular) | 25 | N/A |
| Abler | 2011 | 0 | 18 | N/A | × | fMRI | 3.0 T | Erotic > Non-Erotic Videos | 26 | N/A |
|
| ||||||||||
| Abler | 2013 | 12 | 0 | F | ✓a | fMRI | 3.0 T | Erotic > Non-Erotic Pictures (Follicular) | 32 | N/A |
| Graf | 2013 | 0 | 18 | N/A | ✓a | fMRI | 3.0 T | Erotic > Non-Erotic Pictures | 33 | N/A |
|
| ||||||||||
| Berglund | 2006 | 12 | 0 | F/L | ✓b | PET | N/A | Male Pheromone > Air | 3 | N/A |
| 0 | 12 | N/A | ✓b | PET | N/A | Female Pheromone > Air | 3 | N/A | ||
|
| ||||||||||
| Berglund | 2008 | 12 | 0 | F/L | ✓b | PET | N/A | Male Pheromone > Air | 1 | N/A |
| 0 | 12 | N/A | ✓b | PET | N/A | Female Pheromone > Air | 3 | N/A | ||
|
| ||||||||||
| 12 | 0 | F/L | ✓b | PET | N/A | Male > Female Pheromone | 4 | 3 | ||
| 0 | 12 | N/A | ✓b | PET | N/A | Female > Male Pheromone | 4 | 1 | ||
|
| ||||||||||
| Ciumas | 2009 | 13 | 0 | F/L | ✓b | PET | N/A | Male Pheromone > Air | 2 | N/A |
| 0 | 13 | N/A | ✓b | PET | N/A | Female Pheromone > Air | 2 | N/A | ||
|
| ||||||||||
| Georgiadis | 2006 | 12 | 0 | N/A | ✓ | PET | N/A | Clitoral Stimulation > Rest | 10 | 5 |
| 2005 | 0 | 11 | N/A | × | PET | N/A | Penile Stimulation > Rest | 3 | 3 | |
|
| ||||||||||
| Gillath | 2012 | 20 | 0 | N/A | × | fMRI | 1.5 T | Sexual > Neutral Pictures (Supraliminal) | 24 | N/A |
| 0 | 19 | N/A | × | fMRI | 1.5 T | Sexual > Neutral Pictures (Supraliminal) | 19 | N/A | ||
|
| ||||||||||
| 20 | 0 | N/A | × | fMRI | 1.5 T | Sexual > Neutral Pictures (Subliminal) | 25 | N/A | ||
| 0 | 19 | N/A | × | fMRI | 1.5 T | Sexual > Neutral Pictures (Subliminal) | 0 | N/A | ||
|
| ||||||||||
| Hamann | 2014 | 13 | 0 | N/A | ✓b | fMRI | 3.0 T | Sexual Male > Female Pictures | 16 | 0 |
| 0 | 13 | N/A | ✓b | fMRI | 3.0 T | Sexual Female > Male Pictures | 5 | 0 | ||
|
| ||||||||||
| Karama | 2002 | 20 | 0 | F/L | ✓c | fMRI | 1.5 T | Erotic > Neutral Videos | 14 | N/A |
| 0 | 20 | N/A | ✓c | fMRI | 1.5 T | Erotic > Neutral Videos | 18 | N/A | ||
|
| ||||||||||
| Klucken | 2009 | 20 | 0 | N/A | ✓a | fMRI | 1.5 T | Erotic > Neutral Pictures | 24 | N/A |
| 0 | 20 | N/A | ✓a | fMRI | 1.5 T | Erotic > Neutral Pictures | 17 | 2 | ||
|
| ||||||||||
| Safron | N/A* | 23 | 0 | N/A | ✓a | fMRI | 3 T | Erotic > Nature Videos | 26 | N/A |
| 0 | 25 | N/A | ✓a | fMRI | 3 T | Erotic > Nature Videos | 15 | N/A | ||
|
| ||||||||||
| 22 | 0 | N/A | ✓a | fMRI | 3 T | Erotic Pictures > Fixation | 40 | N/A | ||
| 0 | 26 | N/A | ✓a | fMRI | 3 T | Erotic Pictures > Fixation | 28 | N/A | ||
|
| ||||||||||
| Savic | 2001 | 12 | 0 | F/L | ✓b | PET | N/A | Male Pheromone > Air | 4 | N/A |
| 0 | 12 | N/A | ✓b | PET | N/A | Female Pheromone > Air | 4 | N/A | ||
|
| ||||||||||
| 12 | 0 | F/L | ✓b | PET | N/A | Male > Female Pheromone | 3 | 0 | ||
| 0 | 12 | N/A | ✓b | PET | N/A | Female > Male Pheromone | 1 | 0 | ||
|
| ||||||||||
| Savic | 2005 | 12 | 0 | F/L | ✓b | PET | N/A | Male Pheromone > Air | 1 | N/A |
| 0 | 12 | N/A | ✓b | PET | N/A | Female Pheromone > Air | 1 | N/A | ||
|
| ||||||||||
| 12 | 0 | F/L | ✓b | PET | N/A | Male > Female Pheromone | 0 | 0 | ||
| 0 | 12 | N/A | ✓b | PET | N/A | Female > Male Pheromone | 0 | 0 | ||
|
| ||||||||||
| Wehrum | 2013 | 50 | 0 | C/F/L (54/22/22%) | ✓c | fMRI | 1.5 T | Sexual > Neutral Pictures | 9 | N/A |
| 0 | 48 | N/A | ✓c | fMRI | 1.5 T | Sexual > Neutral Pictures | 9 | N/A | ||
|
| ||||||||||
| 50 | 0 | C/F/L (54/22/22%) | ✓c | fMRI | 1.5 T | Sexual > Positive Pictures | 12 | N/A | ||
| 0 | 48 | N/A | ✓c | fMRI | 1.5 T | Sexual > Positive Pictures | 13 | N/A | ||
|
| ||||||||||
| 50 | 0 | C/F/L (54/22/22%) | ✓c | fMRI | 1.5 T | Sexual > Negative Pictures | 6 | N/A | ||
| 0 | 48 | N/A | ✓c | fMRI | 1.5 T | Sexual > Negative Pictures | 10 | N/A | ||
Matched experiment pairs are highlighted in the same color.
C, contraceptive; F, follicular; fMRI, functional magnetic resonance imaging; L, luteal; N/A, not available; PET, positron emission tomography.
Personal communication.
Comparison of women with men with respect to subjective ratings of the sexual stimulus is not available.
No significant difference between women and men with respect to subjective ratings of the sexual stimulus.
Significant difference between women and men with respect to subjective ratings of the sexual stimulus.
It has been hypothesized that sex differences in the neural response to sexual stimuli may be specific to the visual modality (Stoléru et al., 2012). Therefore, the meta-analyses comparing both groups were additionally conducted with restriction to experiments using visual sexual stimuli. That is, we further assessed commonalities between men and women (sexual vs. control condition; 193 foci from 12 experiments in men; 253 foci from 12 experiments in women), meta-analytic differences using the same pool of experiments, and between-group contrasts in the original articles (171 foci from 12 experiments for men vs. women) with restriction to experiments using visual sexual stimuli (cf., Tables 2 and 3).
2.3. Activation likelihood estimation (ALE)
All meta-analyses were carried out using the revised ALE algorithm for coordinate-based meta-analysis of neuroimaging results (Eickhoff et al., 2012; Turkeltaub et al., 2012). This algorithm aims to identify areas with a convergence of reported coordinates across experiments that is higher than expected from a random spatial association. Reported foci are treated as centers of 3D Gaussian probability distributions capturing the spatial uncertainty associated with each focus (Eickhoff et al., 2009). Here, the between-subject variance is weighted by the number of participants per study, since larger sample sizes should provide more reliable approximations of the “true” activation effect and should therefore be modeled by “narrower” Gaussian distributions.
Subsequently, probabilities of all foci reported of a given experiment were combined for each voxel, yielding a modeled activation (MA) map (Turkeltaub et al., 2012). Voxelwise ALE scores (union across these MA maps) then quantified the convergence across experiments at each location in the brain. To distinguish “true” from random convergence, ALE scores were compared to an empirical null distribution reflecting a random spatial association among all MA maps. The resulting random-effects inference focuses on the above-chance convergence across studies rather than the clustering within a particular study (Eickhoff et al., 2009). This null hypothesis was derived by computing the distribution that would be obtained when sampling a voxel at random from each of the MA maps and taking the union of these values in the same manner as for the (spatially contingent) voxels in the original analysis (Eickhoff et al., 2012). The p value of a “true” ALE score was then given by the proportion of equal or higher values obtained under the null distribution. The resulting nonparametric p values were then assessed at a familywise error (FWE) corrected threshold of p < 0.05 on cluster level (cluster-forming threshold: p < 0.001 at voxel level) and transformed into z scores for display (Eickhoff et al., 2012).
2.4. Differences and conjunction analyses
Differences in consistency during sexual stimulation between men and women were tested by first performing separate ALE meta-analyses for both groups and computing the voxelwise difference between the ensuing ALE maps. The experiments contributing to either analysis were then pooled and randomly divided into two groups of the same size as the sets of contrasted experiments (Eickhoff et al., 2011). Voxelwise ALE scores for these two randomly assembled groups were subtracted from each other and recorded. Repeating this process 10,000 times yielded an empirical null distribution of ALE-score differences between the two conditions. Based on this permutation procedure, the map of true differences was then thresholded at a posterior probability of p > 0.95 for a true difference between the two samples. Surviving voxels were inclusively masked by the respective main effect, i.e., the significant effect of the ALE analysis for the minuend (Caspers et al., 2010; Eickhoff et al., 2011; Rottschy et al., 2012). In addition, a cluster extent threshold of k ≥ 10 voxels was applied to eliminate minor, presumably incidental findings. A conjunction analysis testing for convergence between the two different meta-analyses (men and women during sexual stimulation) employed inference by the minimum statistic, i.e., computing intersection of the thresholded z maps (Caspers et al., 2010).
2.5. Anatomical labeling
For macroanatomical labeling, the resulting brain regions were related to the probabilistic Harvard-Oxford atlas (Desikan et al., 2006) as provided by FSLView v3.1 (http://www.fmrib.ox.ac.uk/fsl/fslview/index.html). For microanatomical labeling, we capitalized on cytoarchitectonic maps of the human brain provided by the SPM Anatomy Toolbox (Eickhoff et al., 2005, 2006, 2007). Clusters were thus assigned to the most probable histologically defined area at the respective location. This probabilistic histology-based anatomical labeling is reported in each respective table. References to details regarding cytoarchitecture are given in the respective table notes.
3. Results
3.1. Functional neuroanatomy of sexual stimulation in women
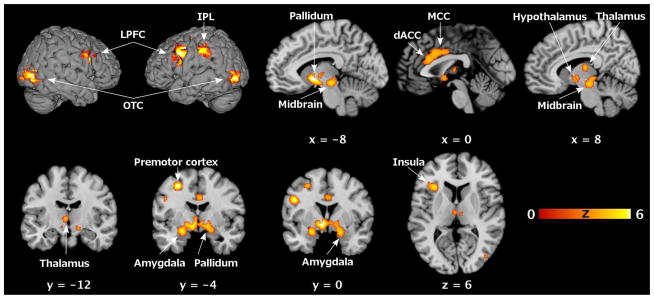
Across 32 within-group experiments (i.e., individual contrasts), convergent brain activations during sexual stimulation in women, irrespective of stimulus modality, were observed in a widespread network of cortical and subcortical brain areas (cf., Figure 1 and, also for histological assignment, Table 4). Corresponding to a majority of visual experiments (≈⅔) and in spite of the fact that virtually all of these experiments represented contrasts controlling for visual input, robust activity was found bilaterally in occipitotemporal visual association cortices. Also the LPFC exhibited bilaterally increased activity. Significant convergence of activation in the anterior insula, IPL, and premotor cortex, in contrast, was restricted to the left hemisphere. Furthermore, activations converged in two midline regions, more specifically the dorsal anterior and middle cingulate cortex (dACC/MCC). Finally, a large subcortical cluster of convergence comprised bilaterally the amygdala and pallidum as well as thalamus, hypothalamus, and midbrain.
Figure 1. Functional neuroanatomy of sexual stimulation in women.
Significant clusters where the ALE analysis revealed significant convergence of brain activations (p < 0.05, FWE corrected on cluster level) during sexual stimulation in healthy heterosexual women (cf. Table 4). Brain slices are shown at coordinates (x, y, z) in MNI space.
ALE, activation likelihood estimation; dACC, dorsal anterior cingulate cortex; FWE, familywise error; IPL, inferior parietal lobule; LPFC, lateral prefrontal cortex; MCC, middle cingulate cortex; MNI, Montreal Neurological Institute; OTC, occipitotemporal cortex.
Table 4.
Brain activations during sexual stimulation in women
| Macroanatomical Location | Cytoarchitectonic Location | Cluster Size in Voxels | MNI Coordinates | Z Score | ||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | ||||
| L Pallidum | 1081 | −8 | 2 | −6 | 7.14 | |
| L Amygdala | LB | −22 | −4 | −16 | 5.45 | |
| R Amygdala | SF | 20 | 0 | −18 | 4.75 | |
| R Midbrain | 8 | −22 | −14 | 4.74 | ||
| R Pallidum | 14 | −4 | −8 | 4.65 | ||
| R Hypothalamus | 8 | −4 | −4 | 4.27 | ||
| R Thalamus (Temporal) | 8 | −18 | 10 | 4.23 | ||
| R Midbrain | 6 | −28 | −6 | 4.10 | ||
| L Thalamus (Prefrontal) | −4 | −12 | 2 | 4.08 | ||
| R Occipitotemporal cortex | 516 | 48 | −68 | −4 | 7.45 | |
| L Lateral prefrontal cortex | Area 44 | 367 | −50 | 6 | 30 | 8.24 |
| Middle cingulate cortex | 357 | 2 | 12 | 30 | 4.50 | |
| Dorsal anterior cingulate cortex | 0 | 22 | 24 | 4.44 | ||
| R Lateral prefrontal cortex | Area 44 | 326 | 48 | 8 | 26 | 7.45 |
| L Occipitotemporal cortex | hOc5 | 282 | −48 | −72 | −6 | 6.13 |
| L Inferior parietal lobule | Area PFt | 148 | −58 | −26 | 34 | 4.71 |
| L Premotor cortex | 137 | −28 | −4 | 50 | 6.60 | |
| L Anterior insula | 132 | −32 | 22 | 6 | 5.43 | |
| L Midbrain | 123 | −8 | −24 | −12 | 5.10 | |
Convergent brain activations during sexual stimulation according to activation likelihood estimation (ALE) across 32 experiments in healthy heterosexual women (cf. Table 1).
FWE corrected on cluster level (p < 0.05) with a cluster forming threshold of p < 0.001 (uncorrected).
FWE, familywise error; L, left; MNI, Montreal Neurological Institute; R, right.
For detailed information on cytoarchitectonics and connectivity, see publications by Amunts (Area 44, LB, SF), Behrens (Thalamus-Prefrontal/-Temporal), Caspers (PFt), Malikovic (hOc5), and colleagues (Amunts et al., 2005, 1999; Behrens et al., 2003; Caspers et al., 2008, 2006; Malikovic et al., 2007).
3.2. Common functional neuroanatomy of sexual stimulation in women and men
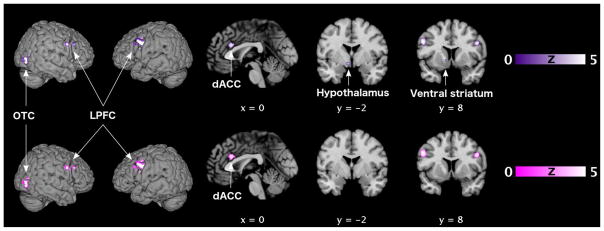
The conjunction analysis revealed a significant overlap between the meta-analyses on sexual stimulation in men and in women, irrespective of stimulus modality, in the right occipitotemporal cortex, bilaterally in the LPFC, dACC, left ventral striatum, and hypothalamus (cf., Figure 2 and Table 5).
Figure 2. Common functional neuroanatomy of sexual stimulation in women and men.
Locations of significant convergent brain activity (p < 0.05, FWE corrected on cluster level) in both healthy heterosexual women and men as revealed by conjunction (♀ ∩ ♂) analyses (cf. Tables 5/6). Upper row (violet): modality-independent convergence. Lower row (magenta): convergence for visual sexual stimulation.
Brain slices are shown at coordinates (x, y, z) in MNI space. dACC, dorsal anterior cingulate cortex; FWE, familywise error; LPFC, lateral prefrontal cortex; MNI, Montreal Neurological Institute; OTC, occipitotemporal cortex.
Table 5.
Comparison of brain activations during sexual stimulation between women and men
| Analysis | Macroanatomical Location | Cytoarchitectonic Location | Cluster Size in Voxels | MNI Coordinates | Z Score | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| ♀ | R Occipitotemporal cortex | hOc5 / Area FG2 | 223 | 48 | −68 | −6 | 6.95 |
| L Lateral prefrontal cortex | Area 44 | 198 | −52 | 6 | 30 | 7.46 | |
| L Pallidum / Caudate / Hypothalamus | 173 | −6 | 2 | −6 | 6.66 | ||
| R Lateral prefrontal cortex | Area 44 | 123 | 50 | 8 | 26 | 5.27 | |
| L Premotor cortex | 122 | −30 | −4 | 50 | 6.16 | ||
| Dorsal anterior cingulate cortex | 110 | 0 | 22 | 22 | 4.67 | ||
| ♀ ∩ ♂ | R Occipitotemporal cortex | Area FG2 / hOc5 | 161 | 48 | −66 | −6 | 6.22 |
| L Lateral prefrontal cortex | Area 44 | 105 | −52 | 6 | 30 | 5.23 | |
| R Lateral prefrontal cortex | 67 | 48 | 8 | 28 | 4.93 | ||
| Dorsal anterior cingulate cortex | 61 | 0 | 22 | 24 | 4.64 | ||
| L Hypothalamus | 31 | −4 | −2 | −10 | 3.56 | ||
| L Ventral striatum | −6 | 2 | −4 | 3.54 | |||
| L Ventral striatum | −10 | 8 | −6 | 3.35 | |||
| ♀ > ♂ Differences in consistency | L Caudate | 15 | −4 | 6 | −10 | 1.79 | |
| L Pallidum | −12 | 2 | −8 | 1.79 | |||
| ♂ > ♀ Differences in consistency | Thalamus (Temporal) | 13 | 2 | −12 | 6 | 1.98 | |
| ♂ > ♀ Consistent differences | Thalamus (Temporal) | 255 | −2 | −16 | 12 | 5.28 | |
| Thalamus (Prefrontal) | −18 | −22 | 18 | 4.98 | |||
| Thalamus (Temporal) | 0 | −8 | 8 | 3.24 | |||
♀: Convergent brain activations during sexual stimulation according to ALE across 21 experiments in healthy heterosexual women, which equivalent experiments in heterosexual men exist for (cf. Table 2).
♀ ∩ ♂: Conjunction analysis of ALE maps across 21 equivalent experiments on brain activations during sexual stimulation in healthy heterosexual women and men, respectively (cf. Table 2).
♀ > ♂/♂ > ♀: Differences in consistency of brain activations during sexual stimulation between healthy heterosexual women and men according to subtraction analyses of ALE maps across 21 equivalent experiments, respectively (cf. Table 2).
♂ > ♀: Consistent differences in brain activations during sexual stimulation between women and men according to ALE across 13 experiments reporting direct group comparisons (cf. Table 3).
Significance threshold set to p > 0.95 posterior probability, cluster size k ≥ 10 voxels, for the subtraction analyses. For all other analyses, FWE correction on cluster level (p < 0.05) with a cluster forming threshold of p < 0.001 (uncorrected) was applied.
ALE, activation likelihood estimation; FWE, familywise error; L, left; MNI, Montreal Neurological Institute, R, right.
For detailed information on cytoarchitectonics and conncectivity, see publications by Amunts (Area 44), Behrnes (Thalamus-Prefrontal/-Temporal), Caspers (FG2), Malikovic (hOc5), and colleagues (Amunts et al., 1999; Behrens et al., 2003; Caspers et al., 2013; Malikovic et al., 2007).
When restricted to experiments employing visual sexual stimulation, the conjunction analysis showed significant overlap in all aforementioned cortical areas, i.e., right OTC, bilaterally in the LPFC, and in the dACC, but not in ventral striatum and hypothalamus (cf., Figure 2 and Table 6).
Table 6.
Comparison of brain activations during visual sexual stimulation between women and men
| Analysis | Macroanatomical Location | Cytoarchitectonic Location | Cluster Size in Voxels | MNI Coordinates | Z Score | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| ♀ | R Occipitotemporal cortex | hOc5 / Area FG2 | 235 | 48 | −68 | −6 | 7.03 |
| L Lateral prefrontal cortex | Area 44 | 206 | −52 | 6 | 30 | 7.55 | |
| R Lateral prefrontal cortex | Area 44 | 128 | 50 | 8 | 26 | 5.34 | |
| L Premotor cortex | 123 | −30 | −4 | 50 | 6.23 | ||
| Dorsal anterior cingulate cortex | 95 | 0 | 22 | 24 | 4.53 | ||
| L Occipitotemporal cortex | hOc5 | 90 | −46 | −70 | 0 | 4.24 | |
| ♀ ∩ ♂ | R Occipitotemporal cortex | Area FG2 / hOc5 | 143 | 48 | −66 | −6 | 5.52 |
| L Lateral prefrontal cortex | Area 44 | 109 | −52 | 6 | 30 | 5.28 | |
| R Lateral prefrontal cortex | 72 | 48 | 8 | 28 | 4.98 | ||
| Dorsal anterior cingulate cortex | 61 | 0 | 22 | 24 | 4.53 | ||
| ♀ > ♂ Differences in consistency | No significant results | ||||||
| ♂ > ♀ Differences in consistency | Mammillary bodies | 17 | 0 | −4 | −10 | 1.95 | |
| Hypothalamus | −2 | −2 | −14 | 1.66 | |||
| ♂ > ♀ Consistent differences | Thalamus (Temporal) | 262 | −2 | −16 | 12 | 5.30 | |
| Thalamus (Prefrontal) | −18 | −22 | 18 | 4.99 | |||
| Thalamus (Temporal) | 0 | −8 | 8 | 3.25 | |||
♀ : Convergent brain activations during visual sexual stimulation according to ALE across 12 experiments in healthy heterosexual women, which equivalent experiments in heterosexual men exist for (cf. Table 2).
♀ ∩ ♂ : Conjunction analysis of ALE maps across 12 equivalent experiments on brain activations during visual sexual stimulation in healthy heterosexual women and men, respectively (cf. Table 2).
♀ > ♂/♂ > ♀ : Differences in consistency of brain activations during visual sexual stimulation between healthy heterosexual women and men according to subtraction analyses of ALE maps across 12 equivalent experiments, respectively (cf. Table 2).
♂ > ♀ : Consistent differences in brain activations during visual sexual stimulation between women and men according to ALE across 12 experiments reporting direct group comparisons (cf. Table 3).
Significance threshold set to p > 0.95 posterior probability, cluster size k ≥ 10 voxels, for the subtraction analyses. For all other analyses, FWE correction on cluster level (p < 0.05) with a cluster forming threshold of p < 0.001 (uncorrected) was applied.
ALE, activation likelihood estimation; FWE, familywise error; L, left; MNI, Montreal Neurological Institute, R, right.
For detailed information on cytoarchitectonics and conncectivity, see publications by Amunts (Area 44), Behrens (Thalamus-Prefrontal/-Temporal), Caspers (FG2), Malikovic (hOc5), and colleagues (Amunts et al., 1999; Behrens et al., 2003; Caspers et al., 2013; Malikovic et al., 2007).
3.3. Differences in the functional neuroanatomy of sexual stimulation in women and men
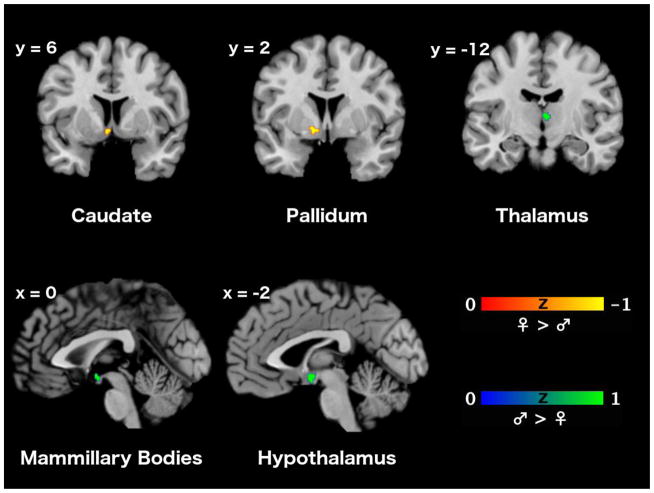
Subtraction analyses between brain activations elicited by sexual stimulation in men and those related to sexual stimulation in women, irrespective of stimulus modality, revealed significantly different strengths in convergent activity only in subcortical areas. While sexual stimulation in men was significantly stronger associated with activity in the mediodorsal thalamus, sexual stimulation in women was significantly more strongly associated with activity in the left caudate head and ventromedial pallidum (cf., Figure 3 and Table 5). The analogous analyses with restriction to experiments employing visual sexual stimuli revealed significantly stronger convergence in the hypothalamus and mammillary bodies for men, while no significantly stronger association with activity in any brain region could be found for women (cf., Figure 3 and Table 6).
Figure 3. Differences in consistency of the functional neuroanatomy of sexual stimulation between women and men.
Comparison of brain activity between healthy heterosexual women and men as revealed by subtraction (♂ > ♀, ♀ > ♂) analyses (cf. Tables 5/6). Significance threshold set to p > 0.95 posterior probability, cluster size k ≥ 10 voxels. Upper row: modality-independent differences. Lower row: differences for visual sexual stimulation. Brain slices are shown at coordinates (x, y, z) in MNI space.
MNI, Montreal Neurological Institute; OFC, orbitofrontal cortex.
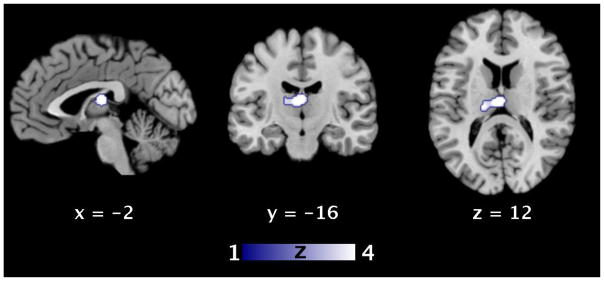
In a second approach, we assessed sex differences in brain activations during sexual stimulation on the basis of experiments reporting direct group comparisons. Here, our meta-analysis identified the mediodorsal thalamus as the region where men consistently exhibit stronger activity during sexual stimulation (cf., Figure 4 and Table 5). The analogous meta-analysis with restriction to visual experiments likewise demonstrated a significantly consistently stronger activity of the thalamus in men as compared to women (cf., Figure 4 and Table 6). The reverse analyses, i.e. with respect to stronger activity in women as compared to men, could not be performed due to a negligible number of foci.
Figure 4. Consistent differences in the functional neuroanatomy of sexual stimulation between women and men.
ALE analysis revealed significant convergence of differential brain activations (p < 0.05, FWE corrected on cluster level) during sexual stimulation in the thalamus in healthy heterosexual women and men. Modality-independent analysis and analysis restricted to visual sexual stimulation showed similar results (cf. Tables 5/6). Brain slices are shown at coordinates (x, y, z) in MNI space.
ALE, activation likelihood estimation; FWE, familywise error; MNI, Montreal Neurological Institute.
In summary, the meta-analyses of between-group contrasts indicated stronger activity of the thalamus during sexual stimulation in men, but no stronger activity of any brain region in women. The meta-analytic contrasts comparing within-group experiments also revealed significantly more consistent activity of the thalamus in men related to sexual stimulus processing. In turn, more consistent activity in women was detected in left caudate and pallidum. Finally, assessment of meta-analytic contrasts during visual sexual stimulation only demonstrated significantly more consistent activity of the mammillary bodies and hypothalamus in men, but not of any brain region in women.
4. Discussion
4.1. General comment
The present study assessed the functional neuroanatomy of sexual stimulation in women and the differences to that of men in order to elucidate neurobiobehavioral sex differences in neural sexual stimulus processing. ALE meta-analyses on sexual stimulation in women and men revealed statistically significant differences between the sexes that were restricted to subcortical regions. These differences are unlikely due to variations in hormone levels of women because hormone cycle-related changes in the female brain response to sexual stimuli have been shown exclusively for cortical regions (Abler et al., 2013; Gizewski et al., 2006; Zhu et al., 2010). In contrast, our meta-analyses demonstrated that corresponding networks of both sexes also share subcortical structures but mainly overlap within cortical regions. Furthermore, studies on the modulation of brain responses to sexual stimuli by female sexual hormones concluded that hormonal influences are weak and subjects’ sex exerts greater influence on neural activations patterns (Abler et al., 2013; Gizewski et al., 2006). In addition, there was a considerable variability in hormonal status of female subjects included in the meta-analyses (cf. Tables 1–3). Differences in hormonal status and their potential effects on neural activity are hence to be regarded as a non-systematic source of variance in the female subjects. It should thus be harder to identify consistent differences between men and women due to increased variance in the female group. That we (nonetheless) found robust sex differences in brain responses to sexual stimuli can – in summary – not be well explained by hormonal status. This conclusion based on data from human subjects seems to be in line with recent studies in animals suggesting that the estrous cycle in mice and rats also is not a major contributor to sex differences in behavior, physiology, and gene expression (Arnold et al., 2016; Becker et al., 2016; Itoh and Arnold, 2015; Prendergast et al., 2014). However, there is also evidence that female rodent sexual receptive behavior is tied to the estrous cycle (Clark et al., 2004; Nomoto and Lima, 2015; Zinck and Lima, 2013), although the latter in turn may be dependent on the presence of males (Féron and Gheusi, 2003).
Another factor potentially influencing (sex differences in) brain activity is subjective sexual arousal during sexual stimulation. It has to be noted that information on potential group differences in subjective sexual arousal was not available for 38% of matched within-group experiment pairs and 19% of the matched pairs reported significant sex differences in subjective ratings (pointing to greater subjective sexual arousal in men) (cf. Table 2). A similar pattern could be observed for experiments relying on direct between-group comparisons (54% not available, significant differences in 31%; cf. Table 3). Although ratings did not differ significantly in 43% of the experiment pairs (15% for direct between-group comparisons), it might yet be argued that sex differences in subjective sexual arousal account for the sex differences in brain response to (visual) sexual stimuli. This assertion depends on the unavailable data of subjective responses though. However, a seminal study on sex differences in brain responses to visual sexual stimuli reported that results were independent of subjective sexual arousal (Hamann et al., 2004). More specifically, sex differences in brain activity were not only observed when comparing men with women rating the experimental stimuli equally, but remained stable even when women reported greater subjective sexual arousal (Hamann et al., 2004). Remarkably, higher activity was located to hypothalamus and thalamus (as well as amygdala), i.e., particularly in regions that also emerged in our meta-analyses, although this study could not be included due to its statistical region-of-interest approach and did hence not contribute to the meta-analytic results. This agreement of both independent and complementary approaches (meta-analytic and region-of-interest taking into account subjective responses) speaks against sex differences in brain activation originating from (potential) sex differences in subjective sexual arousal in the meta-analyses.
Finally, sex differences localized to subcortical regions. Reliable detection of activity in subcortical regions during sexual processing by fMRI may be difficult due to limited spatial resolution and modest signal-to-noise ratios (Walter et al., 2008b). These limitations could represent a general issue resulting in lower sensitivity with respect to particularly small structures as the hypothalamus. That is, this potential low sensitivity could entail oversight of activations but does in contrast not point to sex differences in subcortical activations being false positives. Moreover, these limitations apply to experiments in both men and women because included experiments were matched with respect to design, paradigm, and imaging parameters (cf., Tables 2 and 3). Hence, differences in activation between men and women cannot be explained by these variables.
In summary, there is little evidence suggesting the observed sex differences in subcortical activations can be attributed to subjective (perceived sexual arousal), objective (hormonal status), or technical (experimental design and imaging parameters) factors. They rather seem to represent distinctive neural features associated with male or female processing of sexual stimuli as follows.
4.2. Neural network for sexual stimulus-driven processing in women
The meta-analysis of the female functional neuroanatomy of sexual stimulus-driven processing, based on within-group contrasts, demonstrates that sexual stimulation recruits a broad network of cortical and subcortical brain regions in heterosexual women. This female brain response to sexual stimuli is in agreement with results of previous meta-analyses of neural activity during visual sexual stimulation in men (Kühn and Gallinat, 2011; Poeppl et al., 2014; Stoléru et al., 2012). Convergence of activation foci in occipitotemporal visual cortices has been supposed to reflect attentional enhancement of visual processing that is triggered by the behavioral saliency of the sexual stimulus but is not specific to its sexual nature (Kastner et al., 1999; Poeppl et al., 2014). Accordingly, OTC activity has been related to cognitive attentional and appraisal mechanisms in this context, modulated by top-down signals of the IPL in terms of attentional control (Culham and Kanwisher, 2001; Kastner et al., 1999; Poeppl et al., 2014; Stoléru et al., 2012). Attentional modulation mediated by the IPL is likely to be triggered by the LPFC, which plays a pivotal role in encoding of category-based reward information and therefore presumably also sexual input (Freedman et al., 2001; Pan et al., 2008; Poeppl et al., 2014).
The consistent bilateral activation of the amygdala, as also here observed in the ALE analysis of within-group results in women during sexual stimulation, has been assigned to the emotional component of the neurophenomenological model of sexual arousal (Stoléru et al., 2012). More specifically, the amygdala may impel attentional modulation during sexual stimulation due to its critical function in social and emotional relevance detection irrespective of the modality of sensory input and due to its role as a coordinator of brain networks evaluating stimulus significance (Ball et al., 2007; Bzdok et al., 2011; Pessoa and Adolphs, 2010). This view is corroborated by evidence that amygdala activity reflects only a general emotional component during sexual stimulus processing but is not modulated by the stimulus’ specific sexual intensity (Walter et al., 2008a). In the same vein, convergent activation observed in the mediodorsal thalamus should cohere to a general feeling of pleasure during sexual stimulation, according to its correlation with subjective emotional involvement but not perceived sexual intensity (Walter et al., 2008a). In contrast, another diencephalic structure, the hypothalamus, may reflect more specific effects of sexual intensity since its activity correlates with subjective sexual valence (Karama et al., 2002; Walter et al., 2008a). Moreover, it is assumed to trigger autonomic responses to sexual stimuli (Ferretti et al., 2005).
Similarly, the basal ganglia, where we located convergent activity, have been shown to feature activity that is specific to sexual intensity (Walter et al., 2008a). In consideration of their involvement in the functional anatomy of urges (Jackson et al., 2011), the basal ganglia have been implicated in the regulation of sexual urge, i.e. desire (Karama et al., 2002; Redouté et al., 2000; Stoléru et al., 1999). The basal ganglia hold strong structural and functional connections with motor areas (Draganski et al., 2008; Postuma, 2006). Corticobasal ganglia loops are believed to mediate the perceived urge to express sexual behavior and therefore sexual motivation (Kühn and Gallinat, 2011; Stoléru, 2014; Tanaka et al., 2004). Sexual motivation in turn modulates excitability of motor cortices (Schecklmann et al., 2015), where we observed convergence of activation during sexual stimulation in women. This reflection of sexual motivation in motor cortex excitability provides evidence for motor preparation processes in sexual behavior in humans (Schecklmann et al., 2015; Stoléru, 2014). Convergent activity in the dACC and MCC should also contribute to sexual motivation given the connectivity of both structures with LPFC and premotor areas (Beckmann et al., 2009; Etkin et al., 2011). Accordingly, their important role in the regulation and elicitation of behavioral responses has been stressed (Etkin et al., 2011), which is in line with the involvement of the MCC (together with LPFC and premotor areas) in intentional initiation of behavior (Hoffstaedter et al., 2014). In the context of sexual behavior, the correlation of aMCC activity with penile erection suggests that the aMCC controls the initiation of (copulatory) behavior, i.e., action toward sexual urges (Poeppl et al., 2014). Activity of the anterior insular cortex is assumed to be particularly associated with the awareness of “urges for action” (Jackson et al., 2011), which certainly applies in a similar way to sexual desire. This notion is endorsed by the convergence of activation foci in the right (but not left) anterior insula, as right-sided insular activity may represent “aroused” and “sympathetic” feelings according to the asymmetric emotional processing of the insula (Craig, 2005).
In summary, our ALE meta-analysis in heterosexual women during sexual stimulation supports previous neurobehavioral models of sexual processing established for men (Poeppl et al., 2014; Stoléru et al., 2012) and extends them to female subjects. These models propose the recruitment of brain regions for cognitive evaluation (LPFC), top-down modulation of attention and sensory processing (IPL, occipitotemporal cortex), relevance detection and affective evaluation (amygdala, thalamus), as well as regions involved in the representation of urges (basal ganglia, dACC/aMCC, insular cortex) and in triggering autonomic responses (hypothalamus) and are derived from neuroimaging in heterosexual men. In this regard, the corresponding functional neuroanatomy in women is hence comparable to that in men (Poeppl et al., 2014; Stoléru et al., 2012).
4.3. Common (“unisex”) neural sexual networks in women and men
The conjunction analysis substantiated similarly consistent activity in the left OTC, the bilateral LPFC, the dACC, hypothalamus, and ventral striatum in men and women (cf., Table 5). Translated into the neurophenomenological model of sexual arousal (Stoléru et al., 2012), men and women thus in principle share cognitive (OTC, LPFC), motivational (dACC, hypothalamus, ventral striatum), and autonomic/endocrine (dACC, hypothalamus) components. More specifically, this overlap suggests that during sexual stimulation both sexes are similarly equipped with cognitive top-down control mechanisms over motivational systems according to known integration of motivation by the LPFC through interaction with the dACC (Kouneiher et al., 2009). Together with the OTC, hypothalamus and ventral striatum, these regions are constituents of a neural reward circuit, wherein activity in the OTC and hypothalamus seems to be specific to erotic rewards as revealed by previous meta-analyses (Sescousse et al., 2013). Moreover, activations in the two latter regions have been found to represent specific sexual intensity and were related to sexual intensity ratings in both sexes (Walter et al., 2008a).
In contrast, no common convergent activity of hypothalamus or ventral striatum could be demonstrated in the conjunction analysis restricted to visual sexual stimuli (cf., Table 6). Although this might be due to the comparably low number of included experiments relative to the modality-independent conjunction analysis, this finding points to sex differences in both regions during stimulation by visual sexual stimuli. With respect to the hypothalamus, such difference would meta-analytically confirm previous evidence for stronger response to visual sexual stimuli in men as compared to women from a hypothesis-driven region-of-interest analysis using fMRI (Hamann et al., 2004).
These findings also underline the involvement of higher cortical areas such as the extrastriate visual cortex in sexual arousal of both men and women. In rodents, by contrast, pheromonal signals which are processed via direct inputs from the olfactory bulbs to the medial amygdala and on to the hypothalamus (without cortical involvement) have been established as critical determinants of sexual motivation and/or mating performance (Sakuma, 2008). This may account for the absence of any significant cortical role in rodent sexual arousal in either sex. In contrast, sexual preference is exclusively controlled by subcortical regions in both rodents and humans (Balthazart, 2016; Poeppl et al., 2016; Sakuma, 2008).
The brain regions that consistently respond to sexual stimuli in men and women according to our meta-analyses might be considered as potential targets for manipulations in an effort to modulate sexual arousal. Such an approach could also specify the neural circuits that actually control sexual arousal as well as mating behavior in men and women. Moreover, brain stimulation protocols targeting the reported regions could be of therapeutic use for hyper- or hyposexual syndromes. In fact, deep brain stimulation of the hypothalamus has been discussed as an option to reduce sexual drive (Fuss et al., 2015). However, our results suggest that manipulation of superficial cortical areas such as the LPFC might be an alternative that can easily be implemented by non-invasive methods such as transcranial magnetic or direct-current stimulation.
4.4. Sex differences in neural processing of sexual stimuli
While no sex-specific differences within the hypothalamus could be ascertained by the modality-independent meta-analytic contrast (comparing within-group experiments), the analogous analysis with restriction to visual sexual stimuli showed sex-specific effects in this region. This result validates the absent activation overlap in the hypothalamus in the conjunction analysis with respect to visual sexual stimuli. The lack of sex-specific differences in the modality-independent analysis may be interpreted as indicative of a greater role of visual stimuli in male sexual behavior and moreover of responsiveness to a wider variety of sexual stimuli in women (Chivers et al., 2007; Georgiadis and Kringelbach, 2012; Laumann et al., 1994). The hypothalamus plays a pivotal role in human sexual behavior and is presumably in particular involved in the regulation of autonomic responses (Saper and Lowell, 2014). More precisely, the hypothalamus is believed to trigger the physiological aspects of sexual arousal, e.g. penile erection (Ferretti et al., 2005; Poeppl et al., 2014). At the same time, its activity has been shown to positively correlate with subjective specific sexual intensity of a visual sexual stimulus (Walter et al., 2008a). Yet, stronger hypothalamic activation in response to visual sexual stimuli was reported in men than in women, even when women reported greater subjective arousal (Hamann et al., 2004). This finding is corroborated by our meta-analysis and points to a stronger relationship between subjective and physiological sexual arousal in men than in women. In accordance with this interpretation, correlations between subjective and physiological sexual arousal, robustly present in men (Chivers et al., 2010), have been reported to be low or even non-significant in women (Laan et al., 1994; Vilarinho et al., 2014). Moreover, agreement of self-reported and genital measures of sexual arousal is in fact significantly greater in men than in women (Chivers et al., 2010; Suschinsky et al., 2009). This may be based on low impact of peripheral feedback from consciously detected genital arousal on subjective sexual arousal in women (Laan et al., 1995, 1994). Interestingly, even negative affect during visual sexual stimulation can be positively associated with genital response in women (Peterson and Janssen, 2007). This is in line with the remarkable finding that in women implicit negative and disgust-related associations pertaining to explicit visual sexual stimuli predict strong responses in regions implicated in visual sexual processing, but not the hypothalamus (Borg et al., 2014a). Taken together, it may be inferred that the hypothalamus acts more autonomously and dissociatedly from other sexual processing-regions during visual sexual stimulation in women than in men. Such sex-specific hypothalamic autarchy may rest upon sex differences in the morphology and connectivity of this structure (Byne, 1998; Hines, 2010; Ibanez et al., 2001; Kilpatrick et al., 2006; Lenz and McCarthy, 2010; Makris et al., 2013; Pérez et al., 1990; Sá and Madeira, 2005; Wang et al., 2014). Furthermore, given its neuroanatomical specificity relating to sexual orientation and functional relevance for encoding sexual preferences (Balthazart, 2016; Bao and Swaab, 2011; LeVay, 2011; Poeppl et al., 2016), the sex differences in hypothalamus activation during sexual stimulation might well be represent the neural correlate of behavioral findings pointing to a less distinct sexual orientation in women (Bailey, 2009).
The mammillary bodies, canonically considered part of the hypothalamus, have received considerably less attention with respect to the central processing of sexual stimuli but featured another maximum difference of convergence between sexes in our analysis. This difference reinforces the importance of the mammillary nuclei in male (but not female) sexual behavior, which could be based on sex differences in local distribution of androgen receptors in this region, irrespective of sexual orientation (Fernández-Guasti et al., 2000; Kruijver et al., 2001; Swaab et al., 2001). While it is possible to distinguish the local maximum of convergent activation in the mammillary bodies from that in the (more anterior) hypothalamus, the limited resolution of fMRI and PET prohibits the allocation to distinct subnuclei. The more consistent activation of the hypothalamus in men mainly comprised its anterior part and might hence relate to the (medial) preoptic region rather than the ventromedial nucleus. Such specificity in the neural correlates of human sexual behavior would confirm animal studies suggesting that the preoptic region is essential to male sexual behavior, while the ventromedial nucleus of the hypothalamus is more associated with female sexual behavior (Aou et al., 1988; Oomura et al., 1983; Rand and Crews, 1994). More specifically, animal literature described the effects of forebrain lesions as well as forebrain steroid hormone implants on the expression of mating in male and female rodents and monitored mating-induced immediate-early gene expression in the forebrains of male and female rodents. These animal studies point to the medial preoptic area (male) and the ventromedial nucleus (female) subdivisions of the hypothalamus as critical segments in the circuitry controlling male- and female-typical sexual arousal, respectively, that is shown in response to pheromonal, visual, and auditory stimuli from opposite-sex conspecifics (Alekseyenko et al., 2007; Crews et al., 1993; Melo et al., 2008; Nyby et al., 1992; Robarts and Baum, 2007; Tetel et al., 1994).
It has to be noted that the sex differences in behavioral response to sexual stimuli discussed above were without exception reported in studies employing visual sexual stimuli. They may thus be regarded as either cause for or consequence of sex differences in attending to different aspects of the same visual sexual stimuli, which in turn has been interpreted as pre-existing cognitive biases possibly contributing to sex differences in neural, subjective, and physiological arousal (Rupp and Wallen, 2007). In fact, women consider olfactory (in comparison with visual, auditory, and tactile) information the most important variable for their sexual responsivity, while this seems not true for men (Herz and Cahill, 1997). This behavioral difference may be explained by sexual dimorphism in the human olfactory bulb that contains more neurons and glial cells in women than in men (Oliveira-Pinto et al., 2014). Since the hypothalamus is activated during olfactorily induced sexual arousal also in men (Huh et al., 2008), the lack of sex differences in hypothalamic activity in our modality-independent meta-analysis is coherent, given that a considerable portion of the included experiments employed olfactory stimulation.
In contrast to the relative underactivation of the hypothalamus in women, we observed more consistent activity in a left-hemispheric cluster comprising the caudate head and the ventromedial pallidum. Recent topographical models of basal ganglia functions based on quantitative neuroimaging meta-analyses have provided convincing evidence for motor, cognitive, affective, and somatosensory subdivisions of the basal ganglia (Arsalidou et al., 2013). Here, the left medial pallidum was associated with emotion, the left caudate head in turn with reward (Arsalidou et al., 2013). Moreover, meta-analytic connectivity modeling (MACM) with behavioral filtering localized cognition- and emotion-related networks to the caudate head (Robinson et al., 2012). Notably, both caudate nucleus and ventral pallidum have also been described as critical mediators of rodents’ pair bonding, implicating emotional and social attachment, regulated by oxytocin and the opioid system (Burkett et al., 2011; Young and Wang, 2004). These findings from animal research are corroborated by the association of romantic love as a model for mammalian mate choice with activity of the left caudate head and ventral pallidum (Acevedo et al., 2012; Aron et al., 2005; Bartels and Zeki, 2000; Fisher et al., 2006). Hence, sexual stimulation seems to activate key regions for emotional attachment and pair bonding more consistently in women than in men. This functional difference may be based on sexual dimorphism in pallidum and caudate nucleus; gray matter volume of the latter has also been found to be negatively associated with X-chromosomes (Lentini et al., 2012; Rijpkema et al., 2012). Neither region is implicated in conscious emotion regulation (Frank et al., 2014; Kohn et al., 2014). Hence, emotion-related mechanisms associated with differential recruitment of left caudate head and medial pallidum during sexual stimulation according to our meta-analysis should operate unconsciously in women.
The striatum and particularly the medial globus pallidus project to the thalamus (Alexander and Crutcher, 1990; Draganski et al., 2008; Postuma, 2006), where we most robustly observed stronger and more consistent activity during sexual stimulation in men than in women. It was commonly believed that medial pallidal neurons first and foremost send inhibitory GABAergic projections to thalamic motor nuclei such as the ventral anterior and lateral anterior nuclear complex (Alexander and Crutcher, 1990). More recent research, however, segregated motor, limbic, and cognitive basal ganglia-thalamostriatal loops (Smith et al., 2004). Moreover, inhibitory GABAergic neurons of the ventromedial pallidum project to the mediodorsal thalamus (Root et al., 2015). We found less consistent and reduced activity in this very thalamic subregion and concomitantly more consistent activity in the ventromedial pallidum in women as compared to men. It thus seems likely that in women (but not men) activity of the thalamus is inhibited under influence of the medial pallidum during sexual stimulation. Yet, the direction of interdependence of these subcortical regions exhibiting sex-specific activity during sexual processing remains unclear due to the lack of information on their effective connectivity.
In the context of sexual arousal, it has been proposed that activation in the mediodorsal thalamus is associated only with a general emotional component (Walter et al., 2008a). In line with this notion, the mediodorsal nucleus is considered a part of the “limbic thalamus” that signals relevant information in a neural circuit encompassing the laterobasal amygdala and the prefrontal cortex (Vertes et al., 2015; Wolff et al., 2015). However, the mediodorsal thalamus has also been implicated in cognition including memory processes and is considered a higher order thalamic relay nucleus important for learning due to its extensive excitatory cortico-thalamo-cortical connections with the prefrontal cortex (Mitchell, 2015). In concordance with this coincidence of cognitive and affective signaling in the mediodorsal thalamus, appetitive conditioning has been associated with hemodynamic responses in the thalamus (Klucken et al., 2013). In fact, activity of the thalamus during conditioning of sexual arousal occurred in men but not women (Klucken et al., 2009). The consistently stronger activation of the thalamus in men according to our meta-analyses might thus reflect proposed relative proneness to sexual conditioning in men as compared to women (Klucken et al., 2009; Letourneau and O’Donohue, 1997). Interestingly, while there is generally also support for unconscious sexual conditionability in women, available evidence seems to relate more robustly to genital rather than emotional-subjective responses (Both et al., 2008).
4.5. Conclusions
The current meta-analysis of brain activity in healthy heterosexual women during sexual stimulation demonstrated involvement of brain regions for cognitive evaluation (LPFC), top-down modulation of attention and sensory processing (IPL, OTC), relevance detection and affective evaluation (amygdala, thalamus), as well as regions implicated in the representation of urges (basal ganglia, dACC/aMCC, insular cortex) and in triggering autonomic responses (hypothalamus). The functional neuroanatomy of sexual stimulation in women is thus in general very comparable to that in men. This consensus is most evident in the right OTC, dACC, and bilateral prefrontal cortex, pointing to similar cognitive processing of sexual stimuli in both sexes. However, less consistent activation of the hypothalamus in women may indicate less relevance of peripheral feedback from consciously detected genital arousal on subjective sexual arousal in women and represent the neurobiological basis of known poor agreement between self-reported and genital measures of female sexual arousal in women. More consistent activation of the mediodorsal thalamus in men suggests differential affective learning processes during sexual stimulation including previously proposed male proneness to affective sexual conditioning. In contrast, more consistent recruitment of the caudate head and ventromedial pallidum in the female brain, two key regions mediating emotional and social attachment, may imply unconscious activation of bonding mechanisms during sexual stimulation in women. Neurofunctional sex differences during sexual stimulation can thus account for well-established sex differences on the behavioral level.
Supplementary Material
Highlights.
Similar activity of occipitotemporal, dorsal anterior cingulate, and lateral prefrontal cortex in both sexes
Less consistent activation of hypothalamus and mammillary bodies in women
Higher and more consistent activation of the thalamus in men
More consistent recruitment of caudate head and ventromedial pallidum in women
Neurofunctional sex differences complement the well-established behavioral sex differences
Acknowledgments
This study was in part supported by the Deutsche Forschungsgemeinschaft (DFG; BZ 2/2-1, BZ 2/3-1, EI 816/4-1, EI 816/6-1, LA 3071/3-1), the National Institute of Mental Health (R01-MH074457), the Helmholtz Portfolio Theme “Supercomputing and Modeling for the Human Brain” and the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 604102 (Human Brain Project). We also acknowledge support by the German National Merit Foundation (D.B.).
The authors would like to express their gratitude to Birgit Abler (University of Ulm), Tim Klucken (University of Giessen), and Martin Walter (University of Magdeburg) for providing additional information on their publications. The authors thank Sylvia Dorner-Mitschke and Ines Poeppl for assistance in data keying.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abler B, Kumpfmüller D, Grön G, Walter M, Stingl J, Seeringer A. Neural correlates of erotic stimulation under different levels of female sexual hormones. PLoS One. 2013;8:e54447. doi: 10.1371/journal.pone.0054447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abler B, Seeringer A, Hartmann A, Grön G, Metzger C, Walter M, Stingl J. Neural correlates of antidepressant-related sexual dysfunction: A placebo-controlled fMRI study on healthy males under subchronic paroxetine and bupropion. Neuropsychopharmacology. 2011;36:1837–47. doi: 10.1038/npp.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo BP, Aron A, Fisher HE, Brown LL. Neural correlates of long-term intense romantic love. Soc Cogn Affect Neurosci. 2012;7:145–59. doi: 10.1093/scan/nsq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends Neurosci. 1990;13:266–71. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alekseyenko OV, Waters P, Zhou H, Baum MJ. Bilateral damage to the sexually dimorphic medial preoptic area/anterior hypothalamus of male ferrets causes a female-typical preference for and a hypothalamic Fos response to male body odors. Physiol Behav. 2007;90:438–49. doi: 10.1016/j.physbeh.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–52. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K. Broca’s region revisited: Cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–41. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Aou S, Oomura Y, Yoshimatsu H. Neuron activity of the ventromedial hypothalamus and the medial preoptic area of the female monkey during sexual behavior. Brain Res. 1988;455:65–71. doi: 10.1016/0006-8993(88)90115-1. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Reue K, Eghbali M, Vilain E, Chen X, Ghahramani N, Itoh Y, Li J, Link JC, Ngun T, Williams-Burris SM. The importance of having two X chromosomes. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150113. doi: 10.1098/rstb.2015.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnow BA, Millheiser L, Garrett A, Lake Polan M, Glover GH, Hill KR, Lightbody A, Watson C, Banner L, Smart T, Buchanan T, Desmond JE. Women with hypoactive sexual desire disorder compared to normal females: A functional magnetic resonance imaging study. Neuroscience. 2009;158:484–502. doi: 10.1016/j.neuroscience.2008.09.044. [DOI] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–37. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Arsalidou M, Duerden EG, Taylor MJ. The centre of the brain: Topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum Brain Mapp. 2013;34:3031–54. doi: 10.1002/hbm.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JM. What is sexual orientation and do women have one? Nebr Symp Motiv. 2009;54:43–63. doi: 10.1007/978-0-387-09556-1_3. [DOI] [PubMed] [Google Scholar]
- Ball T, Rahm B, Eickhoff SB, Schulze-Bonhage A, Speck O, Mutschler I. Response properties of human amygdala subregions: Evidence based on functional MRI combined with probabilistic anatomical maps. PLoS One. 2007;2:e307. doi: 10.1371/journal.pone.0000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J. Sex differences in partner preferences in humans and animals. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150118. doi: 10.1098/rstb.2015.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao AM, Swaab DF. Sexual differentiation of the human brain: Relation to gender identity, sexual orientation and neuropsychiatric disorders. Front Neuroendocrinol. 2011;32:214–26. doi: 10.1016/j.yfrne.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829–34. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: A meta-analysis of neuroscience studies. Biol Sex Differ. 2016;7:34. doi: 10.1186/s13293-016-0087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–90. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–7. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Brown WM, Khan R, Levenson B, Sobel N. Sniffing human sex-steroid derived compounds modulates mood, memory and autonomic nervous system function in specific behavioral contexts. Behav Brain Res. 2004;152:11–22. doi: 10.1016/j.bbr.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Berglund H, Lindstrom P, Dhejne-Helmy C, Savic I. Male-to-female transsexuals show sex-atypical hypothalamus activation when smelling odorous steroids. Cereb Cortex. 2008;18:1900–8. doi: 10.1093/cercor/bhm216. [DOI] [PubMed] [Google Scholar]
- Berglund H, Lindström P, Savic I. Brain response to putative pheromones in lesbian women. Proc Natl Acad Sci U S A. 2006;103:8269–74. doi: 10.1073/pnas.0600331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi-Demicheli F, Cojan Y, Waber L, Recordon N, Vuilleumier P, Ortigue S. Neural bases of hypoactive sexual desire disorder in women: An event-related fMRI study. J Sex Med. 2011;8:2546–59. doi: 10.1111/j.1743-6109.2011.02376.x. [DOI] [PubMed] [Google Scholar]
- Borg C, de Jong PJ, Georgiadis JR. Subcortical BOLD responses during visual sexual stimulation vary as a function of implicit porn associations in women. Soc Cogn Affect Neurosci. 2014a;9:158–66. doi: 10.1093/scan/nss117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg C, Georgiadis JR, Renken RJ, Spoelstra SK, Weijmar Schultz W, de Jong PJ. Brain processing of visual stimuli representing sexual penetration versus core and animal-reminder disgust in women with lifelong vaginismus. PLoS One. 2014b;9:e84882. doi: 10.1371/journal.pone.0084882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both S, Spiering M, Laan E, Belcome S, van den Heuvel B, Everaerd W. Unconscious classical conditioning of sexual arousal: Evidence for the conditioning of female genital arousal to subliminally presented sexual stimuli. J Sex Med. 2008;5:100–9. doi: 10.1111/j.1743-6109.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, Young LJ. Activation of μ-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology. 2011;36:2200–10. doi: 10.1038/npp.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byne W. The medial preoptic and anterior hypothalamic regions of the rhesus monkey: Cytoarchitectonic comparison with the human and evidence for sexual dimorphism. Brain Res. 1998;793:346–50. doi: 10.1016/s0006-8993(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Caspers S, Kurth F, Habel U, Zilles K, Laird A, Eickhoff SB. ALE meta-analysis on facial judgments of trustworthiness and attractiveness. Brain Struct Funct. 2011;215:209–23. doi: 10.1007/s00429-010-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers J, Zilles K, Eickhoff SB, Schleicher A, Mohlberg H, Amunts K. Cytoarchitectonical analysis and probabilistic mapping of two extrastriate areas of the human posterior fusiform gyrus. Brain Struct Funct. 2013;218:511–26. doi: 10.1007/s00429-012-0411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct. 2008;212:481–95. doi: 10.1007/s00429-008-0195-z. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: Cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33:430–48. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–67. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivers ML, Seto MC, Blanchard R. Gender and sexual orientation differences in sexual response to sexual activities versus gender of actors in sexual films. J Pers Soc Psychol. 2007;93:1108–21. doi: 10.1037/0022-3514.93.6.1108. [DOI] [PubMed] [Google Scholar]
- Chivers ML, Seto MC, Lalumière ML, Laan E, Grimbos T. Agreement of self-reported and genital measures of sexual arousal in men and women: A meta-analysis. Arch Sex Behav. 2010;39:5–56. doi: 10.1007/s10508-009-9556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciumas C, Hirschberg AL, Savic I. High fetal testosterone and sexually dimorphic cerebral networks in females. Cereb Cortex. 2009;19:1167–74. doi: 10.1093/cercor/bhn160. [DOI] [PubMed] [Google Scholar]
- Clark AS, Kelton MC, Guarraci FA, Clyons EQ. Hormonal status and test condition, but not sexual experience, modulate partner preference in female rats. Horm Behav. 2004;45:314–23. doi: 10.1016/j.yhbeh.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: A neuroanatomical basis? Trends Cogn Sci. 2005;9:566–71. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Crews D, Robker R, Mendonça M. Seasonal fluctuations in brain nuclei in the red-sided garter snake and their hormonal control. J Neurosci. 13:5356–64. doi: 10.1523/JNEUROSCI.13-12-05356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol. 2001;11:157–63. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devereux G. Femme et Mythe. Fammarion, Paris: 1982. [Google Scholar]
- Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJM, Deichmann R, Ashburner J, Frackowiak RSJ. Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci. 2008;28:7143–52. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–61. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage. 2011;57:938–49. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32:570–82. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–26. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–21. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng CM, Narayana S, Lancaster JL, Jerabek PA, Arnow TL, Zhu F, Tan LH, Fox PT, Gao JH. CBF changes during brain activation: fMRI vs. PET Neuroimage. 2004;22:443–6. doi: 10.1016/j.neuroimage.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol. 2000;425:422–35. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Féron C, Gheusi G. Social regulation of reproduction in the female mound-builder mouse (Mus spicilegus) Physiol Behav. 2003;78:717–22. doi: 10.1016/s0031-9384(03)00044-1. [DOI] [PubMed] [Google Scholar]
- Ferretti A, Caulo M, Del Gratta C, Di Matteo R, Merla A, Montorsi F, Pizzella V, Pompa P, Rigatti P, Rossini PM, Salonia A, Tartaro A, Romani GL. Dynamics of male sexual arousal: Distinct components of brain activation revealed by fMRI. Neuroimage. 2005;26:1086–96. doi: 10.1016/j.neuroimage.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Fisher HE, Aron A, Brown LL. Romantic love: A mammalian brain system for mate choice. Philos Trans R Soc B Biol Sci. 2006;361:2173–86. doi: 10.1098/rstb.2006.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Hussein AA, Smart LM, Sabatinelli D. Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev. 2014;45:202–11. doi: 10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–6. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- Freud S. Triebe und Triebschicksale. Int Zeitschrift für Psychoanal. 1915;3:84–100. [Google Scholar]
- Freud S. Die Frage der Laienanalyse. Internationaler Psychoanalytischer Verlag; Leipzig, Wien, and Zürich: 1926. [Google Scholar]
- Fuss J, Auer MK, Biedermann SV, Briken P, Hacke W. Deep brain stimulation to reduce sexual drive. J Psychiatry Neurosci. 2015;40:429–31. doi: 10.1503/jpn.150003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadis JR, Holstege G. Human brain activation during sexual stimulation of the penis. J Comp Neurol. 2005;493:33–8. doi: 10.1002/cne.20735. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR, Kortekaas R, Kuipers R, Nieuwenburg A, Pruim J, Reinders AATS, Holstege G. Regional cerebral blood flow changes associated with clitorally induced orgasm in healthy women. Eur J Neurosci. 2006;24:3305–16. doi: 10.1111/j.1460-9568.2006.05206.x. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR, Kringelbach ML. The human sexual response cycle: Brain imaging evidence linking sex to other pleasures. Prog Neurobiol. 2012;98:49–81. doi: 10.1016/j.pneurobio.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR, Reinders AATS, Paans AMJ, Renken R, Kortekaas R. Men versus women on sexual brain function: Prominent differences during tactile genital stimulation, but not during orgasm. Hum Brain Mapp. 2009;30:3089–101. doi: 10.1002/hbm.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillath O, Canterberry M. Neural correlates of exposure to subliminal and supraliminal sexual cues. Soc Cogn Affect Neurosci. 2012;7:924–36. doi: 10.1093/scan/nsr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizewski ER, Krause E, Karama S, Baars A, Senf W, Forsting M. There are differences in cerebral activation between females in distinct menstrual phases during viewing of erotic stimuli: A fMRI study. Exp Brain Res. 2006;174:101–8. doi: 10.1007/s00221-006-0429-3. [DOI] [PubMed] [Google Scholar]
- Graf H, Abler B, Hartmann A, Metzger CD, Walter M. Modulation of attention network activation under antidepressant agents in healthy subjects. Int J Neuropsychopharmacol. 2013;16:1219–30. doi: 10.1017/S1461145712001368. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci. 2004;7:411–6. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Hamann S, Stevens J, Vick JH, Bryk K, Quigley CA, Berenbaum SA, Wallen K. Brain responses to sexual images in 46,XY women with complete androgen insensitivity syndrome are female-typical. Horm Behav. 2014;66:724–30. doi: 10.1016/j.yhbeh.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Herz RS, Cahill ED. Differential use of sensory information in sexual behavior as a function of gender. Hum Nat. 1997;8:275–86. doi: 10.1007/BF02912495. [DOI] [PubMed] [Google Scholar]
- Hines M. Sex-related variation in human behavior and the brain. Trends Cogn Sci. 2010;14:448–56. doi: 10.1016/j.tics.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, Fox PT, Eickhoff SB. The role of anterior midcingulate cortex in cognitive motor control. Hum Brain Mapp. 2014;35:2741–53. doi: 10.1002/hbm.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh J, Park K, Hwang IS, Jung S, II, Kim HJ, Chung TW, Jeong GW. Brain activation areas of sexual arousal with olfactory stimulation in men: A preliminary study using functional MRI. J Sex Med. 2008;5:619–25. doi: 10.1111/j.1743-6109.2007.00717.x. [DOI] [PubMed] [Google Scholar]
- Ibanez MA, Gu G, Simerly RB. Target-dependent sexual differentiation of a limbic-hypothalamic neural pathway. J Neurosci. 2001;21:5652–9. doi: 10.1523/JNEUROSCI.21-15-05652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Arnold AP. Are females more variable than males in gene expression? Meta-analysis of microarray datasets. Biol Sex Differ. 2015;6:18. doi: 10.1186/s13293-015-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SR, Parkinson A, Kim SY, Schüermann M, Eickhoff SB. On the functional anatomy of the urge-for-action. Cogn Neurosci. 2011;2:227–243. doi: 10.1080/17588928.2011.604717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. The principles of psychology. Henry Holt and Company; New York: 1890. [Google Scholar]
- Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, Joubert S, Beauregard M. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–61. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30:452–61. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Klucken T, Schweckendiek J, Merz CJ, Tabbert K, Walter B, Kagerer S, Vaitl D, Stark R. Neural activations of the acquisition of conditioned sexual arousal: Effects of contingency awareness and sex. J Sex Med. 2009;6:3071–85. doi: 10.1111/j.1743-6109.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- Klucken T, Wehrum S, Schweckendiek J, Merz CJ, Hennig J, Vaitl D, Stark R. The 5-HTTLPR polymorphism is associated with altered hemodynamic responses during appetitive conditioning. Hum Brain Mapp. 2013;34:2549–60. doi: 10.1002/hbm.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation–An ALE meta-analysis and MACM analysis. Neuroimage. 2014;87:345–55. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouneiher F, Charron S, Koechlin E. Motivation and cognitive control in the human prefrontal cortex. Nat Neurosci. 2009;12:939–45. doi: 10.1038/nn.2321. [DOI] [PubMed] [Google Scholar]
- Kruijver FP, Fernández-Guasti A, Fodor M, Kraan EM, Swaab DF. Sex differences in androgen receptors of the human mamillary bodies are related to endocrine status rather than to sexual orientation or transsexuality. J Clin Endocrinol Metab. 2001;86:818–27. doi: 10.1210/jcem.86.2.7258. [DOI] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. A quantitative meta-analysis on cue-induced male sexual arousal. J Sex Med. 2011;8:2269–75. doi: 10.1111/j.1743-6109.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- Laan E, Everaerd W, van Bellen G, Hanewald G. Women’s sexual and emotional responses to male- and female-produced erotica. Arch Sex Behav. 1994;23:153–69. doi: 10.1007/BF01542096. [DOI] [PubMed] [Google Scholar]
- Laan E, Everaerd W, van der Velde J, Geer JH. Determinants of subjective experience of sexual arousal in women: Feedback from genital arousal and erotic stimulus content. Psychophysiology. 1995;32:444–51. doi: 10.1111/j.1469-8986.1995.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–64. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Robinson JL, McMillan KM, Tordesillas-Gutiérrez D, Moran ST, Gonzales SM, Ray KL, Franklin C, Glahn DC, Fox PT, Lancaster JL. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: Validation of the Lancaster transform. Neuroimage. 2010;51:677–83. doi: 10.1016/j.neuroimage.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann EO, Gagnon JH, Michael RT, Michaels S. The Social Organization of Sexuality. The University of Chicago Press; Chicago: 1994. [Google Scholar]
- Lentini E, Kasahara M, Arver S, Savic I. Sex differences in the human brain and the impact of sex chromosomes and sex hormones. Cereb Cortex. 2012;23:2322–36. doi: 10.1093/cercor/bhs222. [DOI] [PubMed] [Google Scholar]
- Lenz KM, McCarthy MM. Organized for sex – Steroid hormones and the developing hypothalamus. Eur J Neurosci. 2010;32:2096–104. doi: 10.1111/j.1460-9568.2010.07511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau EJ, O’Donohue W. Classical conditioning of female sexual arousal. Arch Sex Behav. 1997;26:63–78. doi: 10.1023/a:1024573420228. [DOI] [PubMed] [Google Scholar]
- LeVay S. From mice to men: Biological factors in the development of sexuality. Front Neuroendocrinol. 2011;21:110–3. doi: 10.1016/j.yfrne.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Makris N, Swaab DF, van der Kouwe A, Abbs B, Boriel D, Handa RJ, Tobet S, Goldstein JM. Volumetric parcellation methodology of the human hypothalamus in neuroimaging: Normative data and sex differences. Neuroimage. 2013;69:1–10. doi: 10.1016/j.neuroimage.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malikovic A, Amunts K, Schleicher A, Mohlberg H, Eickhoff SB, Wilms M, Palomero-Gallagher N, Armstrong E, Zilles K. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: A probabilistic, stereotaxic map of area hOc5. Cereb Cortex. 2007;17:562–74. doi: 10.1093/cercor/bhj181. [DOI] [PubMed] [Google Scholar]
- Masters WH, Johnson V. Human sexual response. Little, Brown, and Company; Boston: 1966. [Google Scholar]
- Melo AI, Chirino R, Jiménez A, Cuamatzi E, Beyer C, González-Mariscal G. Effect of forebrain implants of testosterone or estradiol on scent-marking and sexual behavior in male and female rabbits. Horm Behav. 2008;54:676–83. doi: 10.1016/j.yhbeh.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Mitchell AS. The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neurosci Biobehav Rev. 2015;54:76–88. doi: 10.1016/j.neubiorev.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Nickerson LD, Martin CC, Lancaster JL, Gao JH, Fox PT. A tool for comparison of PET and fMRI methods: Calculation of the uncertainty in the location of an activation site in a PET image. Neuroimage. 2001;14:194–201. doi: 10.1006/nimg.2000.0732. [DOI] [PubMed] [Google Scholar]
- Nomoto K, Lima SQ. Enhanced male-evoked responses in the ventromedial hypothalamus of sexually receptive female mice. Curr Biol. 2015;25:589–94. doi: 10.1016/j.cub.2014.12.048. [DOI] [PubMed] [Google Scholar]
- Nyby J, Matochik JA, Barfield RJ. Intracranial androgenic and estrogenic stimulation of male-typical behaviors in house mice (Mus domesticus) Horm Behav. 1992;26:24–45. doi: 10.1016/0018-506x(92)90029-u. [DOI] [PubMed] [Google Scholar]
- Oliveira-Pinto AV, Santos RM, Coutinho RA, Oliveira LM, Santos GB, Alho ATL, Leite REP, Farfel JM, Suemoto CK, Grinberg LT, Pasqualucci CA, Jacob-Filho W, Lent R. Sexual dimorphism in the human olfactory bulb: Females have nore neurons and glial cells than males. PLoS One. 2014;9:e111733. doi: 10.1371/journal.pone.0111733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomura H, Yoshimatsu H, Aou S. Medial preoptic and hypothalamic neuronal activity during sexual behavior of the male monkey. Brain Res. 1983;266:340–3. doi: 10.1016/0006-8993(83)90666-2. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Aou S, Koyama Y, Fujita I, Yoshimatsu H. Central control of sexual behavior. Brain Res Bull. 1988;20:863–70. doi: 10.1016/0361-9230(88)90103-7. [DOI] [PubMed] [Google Scholar]
- Pan X, Sawa K, Tsuda I, Tsukada M, Sakagami M. Reward prediction based on stimulus categorization in primate lateral prefrontal cortex. Nat Neurosci. 2008;11:703–12. doi: 10.1038/nn.2128. [DOI] [PubMed] [Google Scholar]
- Pérez J, Naftolin F, García Segura LM. Sexual differentiation of synaptic connectivity and neuronal plasma membrane in the arcuate nucleus of the rat hypothalamus. Brain Res. 1990;527:116–22. doi: 10.1016/0006-8993(90)91068-r. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: From a “low road” to “many roads” of evaluating biological significance. Nat Rev Neurosci. 2010;11:773–83. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson ZD, Janssen E. Ambivalent affect and sexual response: the impact of co-occurring positive and negative emotions on subjective and physiological sexual responses to erotic stimuli. Arch Sex Behav. 2007;36:793–807. doi: 10.1007/s10508-006-9145-0. [DOI] [PubMed] [Google Scholar]
- Poeppl TB, Langguth B, Laird AR, Eickhoff SB. The functional neuroanatomy of male psychosexual and physiosexual arousal: A quantitative meta-analysis. Hum Brain Mapp. 2014;35:1404–21. doi: 10.1002/hbm.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppl TB, Langguth B, Rupprecht R, Laird AR, Eickhoff SB. A neural circuit encoding sexual preference in humans. Neurosci Biobehav Rev. 2016;68:530–6. doi: 10.1016/j.neubiorev.2016.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–21. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Rand MS, Crews D. The bisexual brain: Sex behavior differences and sex differences in parthenogenetic and sexual lizards. Brain Res. 1994;663:163–7. doi: 10.1016/0006-8993(94)90474-x. [DOI] [PubMed] [Google Scholar]
- Redouté J, Stoléru S, Grégoire MC, Costes N, Cinotti L, Lavenne F, Le Bars D, Forest MG, Pujol JF. Brain processing of visual sexual stimuli in human males. Hum Brain Mapp. 2000;11:162–77. doi: 10.1002/1097-0193(200011)11:3<162::AID-HBM30>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpkema M, Everaerd D, van der Pol C, Franke B, Tendolkar I, Fernández G. Normal sexual dimorphism in the human basal ganglia. Hum Brain Mapp. 2012;33:1246–52. doi: 10.1002/hbm.21283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robarts DW, Baum MJ. Ventromedial hypothalamic nucleus lesions disrupt olfactory mate recognition and receptivity in female ferrets. Horm Behav. 2007;51:104–13. doi: 10.1016/j.yhbch.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, Fox PM, Uecker A, Friehs G, Young KA, Griffin JL, Lovallo WR, Fox PT. The functional connectivity of the human caudate: An application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage. 2012;60:117–29. doi: 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Melendez RI, Zaborszky L, Napier TC. The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol. 2015;130:29–70. doi: 10.1016/j.pneurobio.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: A coordinate-based meta-analysis. Neuroimage. 2012;60:830–46. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp HA, Wallen K. Sex differences in viewing sexual stimuli: An eye-tracking study in men and women. Horm Behav. 2007;51:524–33. doi: 10.1016/j.yhbeh.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Sá SI, Madeira MD. Estrogen modulates the sexually dimorphic synaptic connectivity of the ventromedial nucleus. J Comp Neurol. 2005;484:68–79. doi: 10.1002/cne.20451. [DOI] [PubMed] [Google Scholar]
- Sakuma Y. Neural substrates for sexual preference and motivation in the female and male rat. Ann N Y Acad Sci. 2008;1129:55–60. doi: 10.1196/annals.1417.009. [DOI] [PubMed] [Google Scholar]
- Saper CB, Lowell BB. The hypothalamus. Curr Biol. 2014;24:R1111–6. doi: 10.1016/j.cub.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Savic I, Berglund H. Androstenol–A steroid derived odor activates the hypothalamus in women. PLoS One. 2010;5:e8651. doi: 10.1371/journal.pone.0008651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I, Berglund H, Gulyas B, Roland P. Smelling of odorous sex hormone-like compounds causes sex-differentiated hypothalamic activations in humans. Neuron. 2001;31:661–8. doi: 10.1016/s0896-6273(01)00390-7. [DOI] [PubMed] [Google Scholar]
- Savic I, Berglund H, Lindstrom P. Brain response to putative pheromones in homosexual men. Proc Natl Acad Sci. 2005;102:7356–61. doi: 10.1073/pnas.0407998102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecklmann M, Engelhardt K, Konzok J, Rupprecht R, Greenlee MW, Mokros A, Langguth B, Poeppl TB. Sexual motivation is reflected by stimulus-dependent motor cortex excitability. Soc Cogn Affect Neurosci. 2015;10:1061–5. doi: 10.1093/scan/nsu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Caldú X, Segura B, Dreher JC. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37:681–96. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: A highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–7. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Stoléru S. Reading the Freudian theory of sexual drives from a functional neuroimaging perspective. Front Hum Neurosci. 2014;8:157. doi: 10.3389/fnhum.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoléru S, Fonteille V, Cornélis C, Joyal C, Moulier V. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: A review and meta-analysis. Neurosci Biobehav Rev. 2012;36:1481–509. doi: 10.1016/j.neubiorev.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Stoléru S, Grégoire MC, Gérard D, Decety J, Lafarge E, Cinotti L, Lavenne F, Le Bars D, Vernet-Maury E, Rada H, Collet C, Mazoyer B, Forest MG, Magnin F, Spira A, Comar D. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav. 1999;28:1–21. doi: 10.1023/a:1018733420467. [DOI] [PubMed] [Google Scholar]
- Suschinsky KD, Lalumière ML, Chivers ML. Sex differences in patterns of genital sexual arousal: Measurement artifacts or true phenomena? Arch Sex Behav. 2009;38:559–73. doi: 10.1007/s10508-008-9339-8. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Chung WC, Kruijver FP, Hofman Ma, Ishunina Ta. Structural and functional sex differences in the human hypothalamus. Horm Behav. 2001;40:93–98. doi: 10.1006/hbeh.2001.1682. [DOI] [PubMed] [Google Scholar]
- Sylva D, Safron A, Rosenthal AM, Reber PJ, Parrish TB, Bailey JM. Neural correlates of sexual arousal in heterosexual and homosexual women and men. Horm Behav. 2013;64:673–84. doi: 10.1016/j.yhbeh.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004;7:887–93. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- Tetel MJ, Getzinger MJ, Blaustein JD. Estradiol and progesterone influence the response of ventromedial hypothalamic neurons to tactile stimuli associated with female reproduction. Brain Res. 1994;646:267–72. doi: 10.1016/0006-8993(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Linley SB, Hoover WB. Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev. 2015;54:89–107. doi: 10.1016/j.neubiorev.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarinho S, Laja P, Carvalho J, Quinta-Gomes AL, Oliveira C, Janssen E, Nobre PJ. Affective and cognitive determinants of women’s sexual response to erotica. J Sex Med. 2014;11:2671–8. doi: 10.1111/jsm.12667. [DOI] [PubMed] [Google Scholar]
- Walter M, Bermpohl F, Mouras H, Schiltz K, Tempelmann C, Rotte M, Heinze HJ, Bogerts B, Northoff G. Distinguishing specific sexual and general emotional effects in fMRI-subcortical and cortical arousal during erotic picture viewing. Neuroimage. 2008a;40:1482–94. doi: 10.1016/j.neuroimage.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Walter M, Stadler J, Tempelmann C, Speck O, Northoff G. High resolution fMRI of subcortical regions during visual erotic stimulation at 7 T. Magn Reson Mater Physics, Biol Med. 2008b;21:103–111. doi: 10.1007/s10334-007-0103-1. [DOI] [PubMed] [Google Scholar]
- Wang G, Erpelding N, Davis KD. Sex differences in connectivity of the subgenual anterior cingulate cortex. Pain. 2014;155:755–63. doi: 10.1016/j.pain.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Wehrum S, Klucken T, Kagerer S, Walter B, Hermann A, Vaitl D, Stark R. Gender commonalities and differences in the neural processing of visual sexual stimuli. J Sex Med. 2013;10:1328–42. doi: 10.1111/jsm.12096. [DOI] [PubMed] [Google Scholar]
- Wolff M, Alcaraz F, Marchand AR, Coutureau E. Functional heterogeneity of the limbic thalamus: From hippocampal to cortical functions. Neurosci Biobehav Rev. 2015;54:120–30. doi: 10.1016/j.neubiorev.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Woodard TL, Nowak NT, Balon R, Tancer M, Diamond MP. Brain activation patterns in women with acquired hypoactive sexual desire disorder and women with normal sexual function: A cross-sectional pilot study. Fertil Steril. 2013;100:1068–76. doi: 10.1016/j.fertnstert.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Rao S, Gao JH, Woldorff M, Fox PT. Evaluation of hemispheric dominance for language using functional MRI: A comparison with positron emission tomography. Hum Brain Mapp. 1998;6:42–58. doi: 10.1002/(SICI)1097-0193(1998)6:1<42::AID-HBM4>3.0.CO;2-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang X, Parkinson C, Cai C, Gao S, Hu P. Brain activation evoked by erotic films varies with different menstrual phases: An fMRI study. Behav Brain Res. 2010;206:279–85. doi: 10.1016/j.bbr.2009.09.027. [DOI] [PubMed] [Google Scholar]
- Zinck L, Lima SQ. Mate choice in Mus musculus is relative and dependent on the estrous state. PLoS One. 2013;8:e66064. doi: 10.1371/journal.pone.0066064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.