Abstract
The aim of the current work was to evaluate the ovarian follicle reserve and the ovarian transcriptome in Ames dwarf (df/df) mice. The results suggest a delayed ovarian aging in df/df mice compared to normal (N) mice. Although a high number of genes were differentially expressed during aging of N mice, only a small fraction of these changed with aging in df/df mice. These alterations involved more than 500 categorized biological processes. The majority of these biological processes, including inflammatory/immune responses, were up-regulated with aging in N mice, while old df/df mice were characterized by down-regulation of these same processes in comparison to age matched N mice. However, biological processes related to DNA damage and repairing were commonly down-regulated with aging in both genotypes. In conclusion, delayed ovarian aging in long-living df/df mice was associated with reduced expression of genes related to the inflammatory and immune responses.
Keywords: ovarian aging, GH, IGF, mRNA, transcriptome
Introduction
The ovarian reserve is defined as the quiescent pool of primordial follicles, oocytes surrounded by a single layer of flattened granulosa cells (Peters, 1969), which is established at mid-gestation in humans (Baker, 1963) and just after birth in mice (Peters, 1969). Oocytes enclosed in primordial follicles will remain quiescent until recruited to grow at some point of the female reproductive life (Hirshfield, 1994). This process of continuous depletion of the ovarian follicular reserve and progressive reduction in fertility observed as female mammals as they age is known as ovarian aging (te Velde et al., 1998). The depletion of the ovarian reserve in women marks the beginning of the menopause (Faddy et al., 1992), although long before fertility rates start to decline due to a reduced ovarian reserve (van Noord-Zaadstra et al., 1991). The primordial follicle reserve decreases about 10 times from the ages of 0.5 to 1.5 years in female mice (Kevenaar et al., 2006) and is already completely depleted in 2.5 year-old females (Słuczanowska-Głąbowska et al., 2013).
The activation of primordial follicles is a strictly regulated process coordinated by several paracrine and endocrine factors. One of the main pathways implicated is the phosphoinositide 3-kinase (PI3k)/protein kinase B (Akt1) signaling and its downstream effector Forkhead Box O3a (Foxo3a) (Castrillon et al., 2003; John et al., 2007; John et al., 2008). Foxo3a phosphorylation is associated with oocyte growth and the irreversible activation of primordial follicles. This event is coordinated by the surrounding granulosa cells producing kit ligand (KITL) in response to the activation of the mechanistic target of rapamycin complex 1 (mTORC1), which in turn activates the oocyte PI3k/Akt/Foxo3a pathway (Zhang et al., 2014). Despite these findings, it is still unknown which factors regulate the age at which somatic cells of the follicles begin their transformation and induce the oocyte to leave the resting pool (Zhang and Liu, 2015). Nevertheless, it seems clear that insulin-like growth factor I (IGF-I) signaling is involved in the initiation of follicle growth as demonstrated in growth hormone (GH) receptor disrupted mice treated with exogenous IGF-I (Slot et al., 2006) and caloric restricted rats with reduced circulating IGF-I levels (Li et al., 2011).
Ames dwarf mice (df/df) are considered an excellent model for the study of aging as they can live 30-65% longer than their normal littermates (Brown-Borg et al., 1996). The df/df mice have a defective Prop1 (Prophet of Pit1) gene, impairing anterior pituitary gland development and resulting in deficient secretion of GH, thyroid-stimulating hormone (TSH) and prolactin (Sornson et al., 1996). These mice are characterized by severely low circulating IGF-I and reduced adult body size (Chandrashekar and Bartke, 1993). Interestingly, treatment of df/df mice with exogenous GH from 2 to 6 wk of age can abolish positive effects on longevity and insulin sensitivity (Panici et al., 2010). Female df/df mice have normal ovarian cyclicity and can reproduce under hormonal stimulation, although they have delayed puberty (Bartke et al., 2001). We had previously demonstrated that Foxo3a phosphorylation is reduced in oocytes enclosed in primordial follicles from df/df mice, and that they have an aberrant expression of several genes in the PI3k/Akt/Foxo3a pathway (Schneider et al., 2014). Since reduced Foxo3a activation is suggested as the main regulatory mechanism of ovarian aging (Zhang et al., 2014), we hypothesize that it also leads to decreased activation of primordial follicles in df/df mice, although there is no published histological evidence of delayed ovarian aging in df/df mice to date.
Regulation of gene expression plays a key role in coordinating follicle development and aging within the ovary (Sharov et al., 2008). However, there is a very limited knowledge about the transcriptome of the aging ovary, since most knockout mouse models of important ovarian genes (e.g. PTEN and FoxO3) have complete ovarian failure early after puberty (Castrillon et al., 2003; John et al., 2008; Reddy et al., 2009) and do not allow the study of gene expression profiles in later ages. In addition, the use of newer techniques of next generation sequencing applied for RNA (RNA-Seq) has greatly improved the accuracy of transcriptomics studies in comparison to microarray (Matkovich et al., 2010) and very limited data regarding the aging ovary is available. Therefore the aim of the current work is 1) to evaluate the ovarian follicle reserve in aged df/df mice through histological sampling, and 2) to compare the transcriptomes of the aging ovaries in df/df and N mice.
Materials and Methods
Animals and tissue collection
Normal (N; n=14) and Ames dwarf mice (df/df; n=14) (all females) were bred and maintained under temperature- and light-controlled conditions (22 ± 2°C, 12 hour light/12 hour dark cycle) (Masternak et al., 2004). The animals were anesthetized and euthanized after overnight fasting and the pair of ovaries was collected. The ovarian collection for histological evaluation was performed at 12-13 mo of age (4 N and 4 df/df mice) and ovaries were immediately stored in 10% formaldehyde. The ovarian collection for RNA extraction was performed at 5-6 mo of age (young; 5 N and 5 df/df mice) and at 21-22 mo of age (old; 5 N and 5 df/df mice) and ovaries were immediately stored at −80° C. All animal procedures employed in our presented work were approved by and performed in accordance to the guidelines from the Laboratory Animal Care and Use Committee (LACUC) at the Southern Illinois University School of Medicine (Springfield, IL).
Quantification of ovarian follicles and histological analyses
For the histological analysis, ovaries (n=8, 12 mo old mice) were removed from formaldehyde and embedded in paraffin (Paraplast, Leica Biosystems, Richmond, IL, USA). One ovary of the pair was serially sectioned at 5 μm using a semi-automated rotary microtome (RM2245, Leica Biosystems, San Diego, CA, USA). Sampling started at the beginning of the visible area of the ovarian surface until the end of the structure, and every 6th section was selected and placed on a standard histological slide for staining and counting (adapted from Myers et al. (2004)). The slides were dried at 56°C for 24 h, stained with hematoxylin-eosin, and mounted with coverslips and synthetic resin (Sigma Chemical Company®, St. Louis, MO, USA). Images of the ovarian sections were captured with a digital camera coupled to a microscope (Nikon Eclipse E200, Nikon Corporation, Japan) using the 10 and 40X objectives, assisted by the software Moticam 5.0 (Motic, Hong Kong, China).
Only follicles containing an oocyte with clearly visible nucleus were counted in each slide. Follicle classification was based on Myers et al. (2004). Follicles were classified as primordial (oocyte surrounded by a single layer of flattened granulosa cells), in transition (oocyte surrounded by a layer of flattened granulosa cells and at least one cuboid granulosa cell), primary (oocyte surrounded by a single layer of cuboidal granulosa cells), secondary (oocyte surrounded by two or more layers of cuboid granulosa cells without a visible antrum) and tertiary (follicles with a clearly defined antral space and a with a layer of granulosa cells around the oocyte). To estimate actual follicle quantity, the number of follicles in each category was multiplied by six to account for the section sampling and by two to account for the fact that only one ovary of the pair was used. The diameter of the oocyte nucleus, oocyte and follicle for each category was measured for N and df/df mice (n=3/follicle type/mice).
RNA Sequencing analyses
The pair of ovaries from young (6 mo, n=5 per group) and old (22 mo, n=4 df/df and 5 N) was removed from the −80° C and homogenized with QIAzol (Qiagen, Valencia, CA, USA) using 0.5 mm zirconium oxide beads in the Bullet Blender 24 (Next Advance, Averill Park, NY, USA). Total RNA was extracted using a commercial column purification system (miRNeasy Mini Kit, Qiagen) and on-column DNase treatment (RNase-free DNase Set, Qiagen) following manufacturer's instructions. The quantity and quality of RNA samples was determined using BioAnalyzer and RNA Nano Lab Chip Kit (Agilent Technologies, Santa Clara, CA, USA). All samples had RNA Integrity Numbers higher than 7.0 and proceeded to further analysis (n=19).
Transcriptomic profile of individual samples was performed using RNA-sequencing as previously described (Matkovich and Dorn, 2015) using 1 μg of total RNA per sample as an initial input. Briefly, Poly A RNA enrichment was performed using Dynabeads mRNA Purification Kit (Life Technologies, Carlsbad, CA, USA) and mRNA fragmentation using a fragmentation buffer (40 mM Tris acetate, 100 mM K acetate, 30 mM Mg acetate, pH 8.2; 2 min 30 s at 95 C). First strand cDNA was generated using Superscript III 1st Strand Synthesis System (Life Technologies) and the second strand was generated using DNA Polymerase I (New England Biolabs, Ipswich, MA, USA), E. Coli DNA Ligase (New England Biolabs) and RNAse H (Life Technologies). Finally, libraries were prepared using End-It DNA End Repair Kit (Epicentre, Madison, WI, USA) and Klenow Fragment (3′→5′ exo-) (New England Biolabs) for the ligation of Illumina sequencing adapters (Illumina-Index-AdapterA and 5’-phosphorylated Illumina-Index-AdapterB) using the LigaFast™ Rapid DNA Ligation System (Promega, Madison, WI, USA). Fragments between 150-300 bp were recovered after 2% agarose gel electrophoresis in order to remove adapter dimers by size selection (QIAquick Gel Extraction Kit, Qiagen). Twenty different indexes were added to individual libraries using Phusion DNA polymerase (New England Biolabs) during 16 cycles of amplification. After that, samples were quantified (Nanodrop, Thermo Scientific, Wilmington, DE, USA), combined in two mixtures, and submitted to sequencing on two flowcell lanes (10 samples/lane) on a HiSeq 2500 instrument (Illumina Inc., San Diego, CA, USA) at Washington University GTAC.
The mapping of sequencing reads to the mouse transcriptome (Illumina iGenomes annotation for UCSC mm10, http://support.illumina.com/sequencing/sequencing_software/igenome.html) was performed using Tophat 2.1.0.0 and Bowtie 2.2.6 as its underlying alignment algorithm (Kim et al., 2013). mRNA abundance was generated using Cufflinks 2.1.1 (Trapnell et al., 2010) and is presented as FPKM (Fragments Per Kb of exon per Million reads mapped to mRNAs). The number of reads aligned to each known transcript/mRNA and its corresponding gene was generated using HTSeq 0.6.1 (Anders et al., 2015). Genes with an average less than 1/100,000th of the aligned reads in at least one group were eliminated from differential expression analyses (15,770 transcripts detected out of 23,457 transcripts in the mm10 database). Those mRNAs defined as significantly up and down-regulated (FDR < 0.02 and FC > 1.50, or FDR < 0.02 and FC < 0.75) were further analyzed for enrichment of gene ontology (GO) terms (biological processes, molecular function and cellular component) and pathway analysis using the Gage and Pathview packages in R (Luo et al., 2009; Luo and Brouwer, 2013), considering P values lower than 0.05 as significant.
All RNA-Seq data are available at the Gene Expression Omnibus (GEO) at NCBI under accession number GSE84078.
Statistical analyses
The results are presented as mean ± standard error of the mean (SEM). All statistical analyses for ovarian morphology were performed using Graphpad Prism 6 (Graphpad Software Inc., La Jolla, CA, USA). A t-test was performed to observe differences in the number of follicles in each stage and oocyte, nucleus and follicle diameter between N and df/df mice. A P value lower than 0.05 was considered statistically significant.
Statistical analyses of differentially expressed mRNAs was performed using the software R (3.2.2) and the Bioconductor package DESeq (1.2.0) (Anders and Huber, 2010) using the HTSeq output count. Read counts were normalized for library depth, and pairwise comparisons between genotypes and ages (age within each genotype and genotype within each age), measuring fold change, uncorrected P-values from the negative binomial distribution, and adjusted P values (false discovery rate; FDR) were obtained. Principal components analysis (PCA) was also performed using R to observe sample distribution in a two dimensional plot and eliminate outliers. Genes with a FDR < 0.02 and FC > 1.50 were considered up-regulated; and FDR < 0.02 and FC < 0.75 were considered down-regulated.
Results
Ovarian morphology
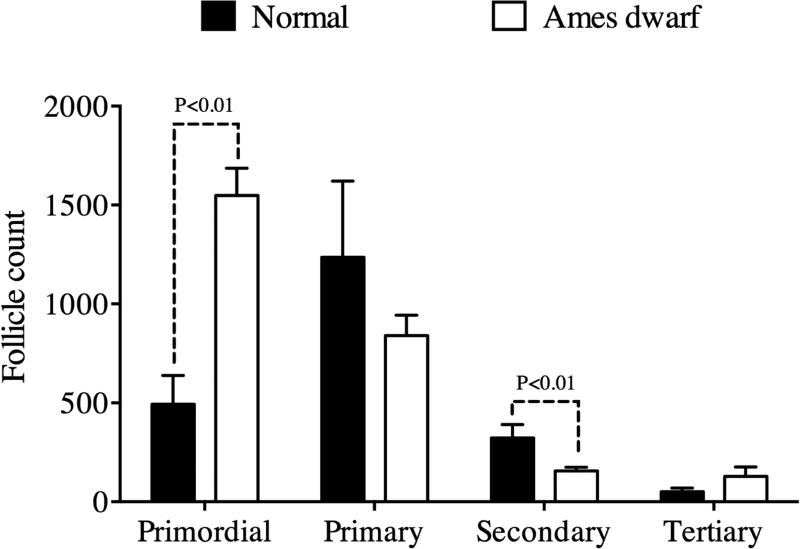
Twelve mo df/df mice had an increased ovarian reserve as indicated by a three-fold higher number of follicles in the primordial stage compared to N mice (P<0.01; Figure 1). The increased number of follicles in the primordial stage indicates a deficiency in the transition to the later stages of follicle development, confirmed by the reduced number of follicles in the secondary stage in df/df mice compared to N mice (P<0.01; Figure 1). The number of follicles in the tertiary stage (90.0 ± 27.6) and total follicles (2388.0 ± 287.7) was not different between df/df and N and mice (P>0.05). Regarding follicle and oocyte diameters, we only observed a smaller diameter for oocytes included in primordial follicles in df/df (5.5 ± 0.3 μm) compared to N mice (6.8 ± 0.4 μm, P = 0.02) (Table 1). Other histological parameters were not different between genotypes (Table 1 and Figure 2).
Figure 1.
Number of primordial, primary, secondary and tertiary follicles in the ovaries of 12 mo-old Ames dwarf (df/df) and Normal (N) mice. Differences at P<0.05 were considered as significant.
Table 1.
Diameter of nuclei, oocyte and follicle of primordial (n=12/group), primary (n=12/group), secondary (n=12/group) and tertiary (n=12/group) follicles in 12 mo Ames Dwarf and Normal mice.
| Category | Normal | Ames Dwarf | P value |
|---|---|---|---|
| Primordial follicle | |||
| Nucleus (μm) | 2.6 (±0.3) | 2.4 (±0.2) | 0.46 |
| Oocyte (μm) | 6.8 (±0.4) | 5.5 (±0.3) | 0.02 |
| Follicle (μm) | 8.8 (±0.2) | 7.9 (±0.5) | 0.29 |
| Primary follicle | |||
| Nucleus (μm) | 3.5 (±0.4) | 3.0 (±0.2) | 0.30 |
| Oocyte (μm) | 9.2 (±1.2) | 7.5 (±0.5) | 0.21 |
| Follicle (μm) | 14.0 (±1.5) | 11.4 (±0.6) | 0.11 |
| Secondary follicle | |||
| Nucleus (μm) | 6.2 (±0.7) | 5.8 (±0.5) | 0.70 |
| Oocyte (μm) | 19.1 (±1.4) | 22.7 (±1.2) | 0.17 |
| Follicle (μm) | 44.0 (±2.5) | 42.0 (±5.3) | 0.74 |
| Tertiary follicle | |||
| Nucleus (μm) | 9.6 (±0.8) | 8.8 (±0.5) | 0.07 |
| Oocyte (μm) | 107.4 (±20.0) | 102.6 (±8.3) | 0.82 |
| Follicle (μm) | 165.7 (±16.4) | 147.3 (±6.6) | 0.29 |
Data is presented as mean ± standard error of the mean
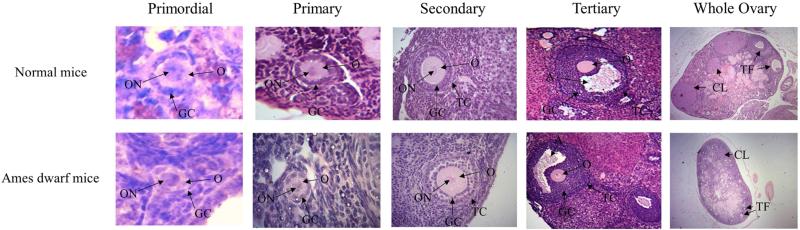
Figure 2.
Detailed histological images of each follicle stage (primordial, primary, secondary, and tertiary) and whole ovarian sections for Ames dwarf and Normal mice. The diameter of oocytes enclosed in primordial follicles was smaller in Ames dwarf than Normal mice. No further histological differences were observed between genotypes. O – oocyte, ON – oocyte nuclei, GC – granulosa cells, TC - theca cells, A – antrum, CL – corpus luteum, TF – tertiary follicle.
RNA sequencing
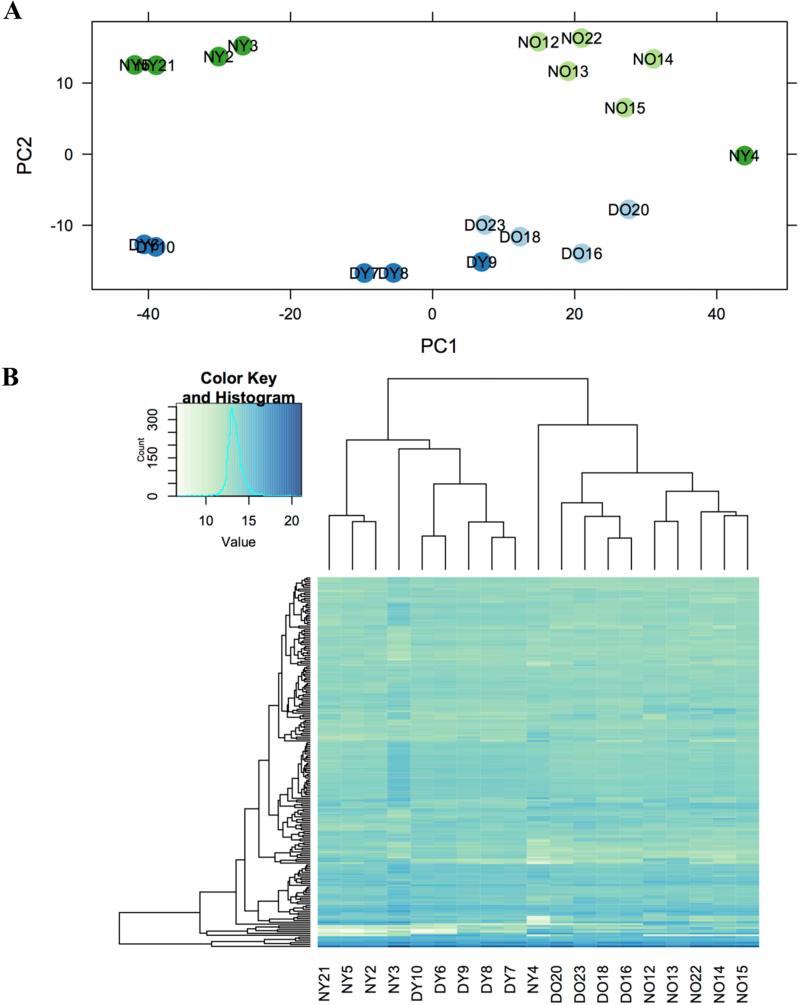
In average 26,577,010 reads per sample were obtained and of these 15,182,570 (55.3%) were aligned to the mouse genome. Using PCA of the 500 most variable genes (Figure 3A) and unsupervised hierarchical clustering for the 200 most expressed genes (Figure 3B), we can observe that samples grouped in similar pattern by genotype and age, indicating a low level of sample variability. Only one sample from a young N female mouse (Sample NY4) was removed from the study due to an anomalous profile.
Figure 3.
Principal components analysis of the 500 most variable ovarian expressed mRNAs Ames dwarf (n=9; Young – DY dark blue; Old – DO light blue) and Normal mice (n=10; Young – NY dark green; Old – NO light green) (Panel A). Unsupervised hierarchical clustering of expression levels for the top 200 most expressed genes in ovaries of df/df (n=9; Young – DY and Old – DO) and N mice (n=10; Young – NY and Old – NO) (Panel B).
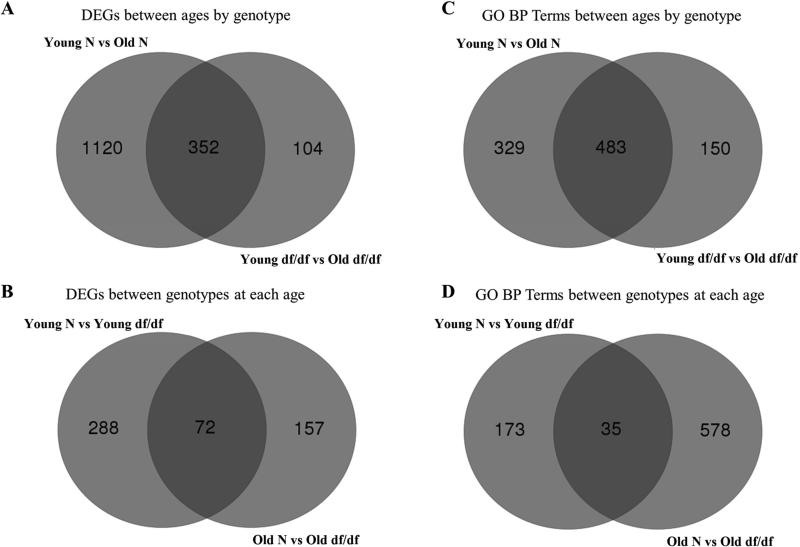
For analysis of differential gene expression (DEG) we considered the genes that were regulated with aging (22 vs 6 mo) within each genotype and also genes that changed between genotypes (df/df vs N) at each age. The highest number of DEGs was observed between old and young N mice (1,120 exclusively in N mice and 352 genes in common with df/df mice), while df/df mice had 104 genes exclusively regulated with aging (Figure 4A). Considering the differences between genotypes, there were 288 DEGs at 6 mo between df/df and N mice, 157 DEGs at 22 mo and 72 DEGs between df/df and N mice at both 6 and 22 mo of age (Figure 4B). The top 20 most differentially regulated genes (highest fold change) in each category are presented in Table 2 and a list with all differentially regulated genes is provided in Supplemental Table S1. Processes related to inflammatory response were highly regulated with aging in N mice and between old df/df and old N mice and a heatmap containing the top 50 DEG in this category is showed in Suppl. Figure 2.
Figure 4.
Number of differentially expressed genes (DEGs) between Ames dwarf (df/df) mice with aging (22 vs 6 mo; A), and between aged (22 mo old) and young (6 mo old) df/df and Normal (N) mice (B). Number of regulated gene ontology (GO) biological process (BP) terms between df/df mice with aging (22 vs 6 mo; C), and between aged (22 mo old) and young (6 mo old) df/df and N mice (D).
Table 2.
The top 20 most differentially regulated genes in each category.
| Category | Direction of change | Top 20 changed genes |
|---|---|---|
| Change with age (22 vs. 6 mo) | ||
| Normal mice | Up-regulated | Klk1b24, 1700012P22Rik, 1700018A04Rik, Zbbx, 4833428L15Rik, Klk1b21, Arhgap40, Cyp26a1, Uox, Cwh43, Pkp1, Agr2, A930001A20Rik, Rp1, Prss22, Gm609, 1700086L19Rik, Mogat1, Syt10, Slc34a2 |
| Down-regulated | C87977, Rfpl4, E330017A01Rik, Nlrp9b, Gm13084, Fbxw28, Wee2, Oog4, Mos, Nlrp4f, Tcl1b1, Amh, C87414, Pin1-ps1, Dppa3, Zp1, Klf17, Oas1e, Khdc1b, Obox5 | |
| Ames dwarf mice | Up-regulated | Prss29, Acsm3, Comp, Zpld1, Agr3, Msx3, Agr2, Mogat1, BC048546, Sectm1b, Ugt1a1, Cftr, Mmp8, Rasgrf1, Dhrs9, Pih1d2, Ovgp1, Cldn22, Cyp2b10, Stoml3 |
| Down-regulated | Pinlyp, Chga, Slc38a3, Otof, Slc30a3, Bfsp1, Khdc3, Nos2, Oas1d, AU015836, Zar1, Khdc1b, Dppa5a, Pou5f1, Gbx2, Padi6, E330034G19Rik, Fam78a, Amh, Zfp957 | |
| Change with genotype (Ames dwarf vs Normal mice) | ||
| 6 mo (young) | Up-regulated | Klk1b24, Cyp26a1, Klk1b21, Arhgap40, 1700086L19Rik, Npbwr1, Gm266, 1500015O10Rik, Insl3, Cwh43, Rp1, Ptgds, Prom2, Caly, Slc34a2, Cyp21a1, Ect2l, Iqub, Hsd17b3, Mlc1 |
| Down-regulated | Wnt10b, Onecut2, Plekhg4, Sfrp4, Ptgfr, Cyp4f18, Wisp2, Adcyap1, Sstr1, Abcb1b, Elfn1, 2010003K11Rik, Tmem200a, Atp1a3, Lrrc30, Tnc, Adh7, Cyp11a1, Tmem178, Gm13003 | |
| 22 mo (old) | Up-regulated | Hsd17b3, Ptgds, Elfn2, Cyp21a1, C7, C87977, Insl3, Fabp3, Serpina5, Svs5, Cntn6, Serpina3a, Serpina3c, Serpina3j, Itih2, Cdk15, Tcl1b1, Spink4, Wfikkn2, Nlgn1 |
| Down-regulated | Adcyap1, Wnt10b, Sfrp4, Onecut2, Wisp2, Abcb1b, Tnc, Atp1a3, Elfn1, Cyp11a1, Kcnab3, Tmem178, Ptgfr, Cma1, Ephx2, Sez6, Spp1, Stx11, Sgk1, Ctnna2 | |
The enriched Kegg pathways between df/df and N mice and with aging are represented in Supplemental Tables S2 and S3, respectively. There were 23 pathways enriched with aging exclusively in N mice, 8 exclusively in df/df mice and 26 pathways commonly enriched with aging in df/df and N mice. Regarding changes between genotypes, there were 16 pathways exclusively enriched in df/df and N mice at 6 mo old, 22 exclusively at 22 mo old, and only 3 in common at 6 and 22 mo old mice. We provided detailed diagrams for the transforming growth factor β (TGF-β) and oocyte meiosis pathways, which were downregulated with age in N mice (Suppl. Figures 2A and 3A), and up-regulated in old df/df compared to old N mice (Suppl. Figures 2B and 3B). On the other hand the toll-like receptor (TLR) pathway was up-regulated with age in N mice (Suppl. Figure 4A) and down-regulated in old df/df compared to old N mice (Suppl. Figure 4B). The main regulated Kegg pathways are summarized in Figure 5.
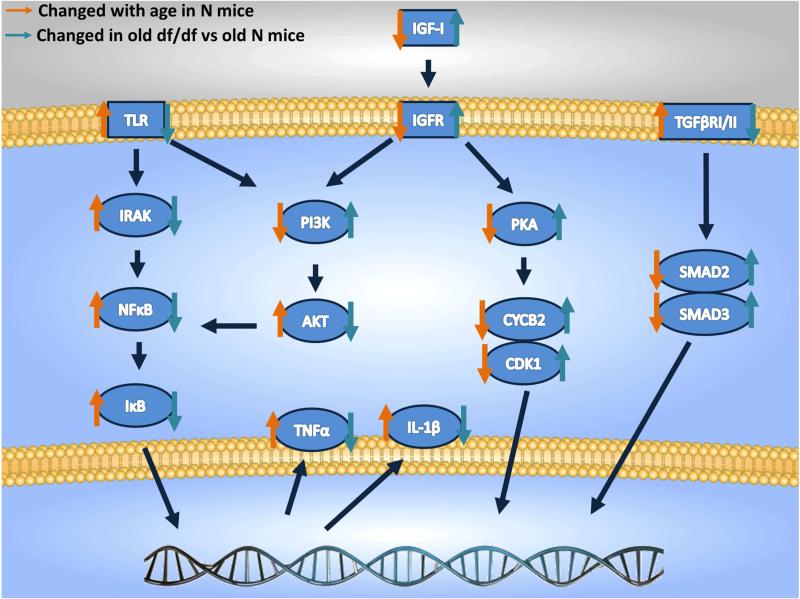
Figure 5.
Schematic representation of the regulated set of genes between old Normal (N) and young N mice (22 vs 6 mo) and between Ames dwarf (df/df) and N mice at old age (22 mo. IGF-I – insulin-like growth factor type I, IGFR – insulin-like growth factor receptor, TLR – toll like receptor, IRAK - IL-1 Receptor-Associated Kinases, NFκB – nuclear factor kappa B, IκB – inhibitor of κB, TNF – tumor necrosis factor alpha, IL-1β – interleukin 1 beta, PI3K - phosphatidylinositide 3-kinase, AKT – protein kinase B, PKA – protein kinase B, CYCB2 – cyclin B2, CDK1 - cyclin-dependent kinase 1, TGFβR – transforming growth factor beta receptor. Orange arrows indicate genes changed with age in Normal (N) mice and blue arrows indicate genes changed in old (22 mo) df/df compared to old N mice.
The number of enriched GO Terms for biological processes between ages and genotypes is presented in Figures 4C and 4D, respectively. The majority of processes that changed with age (22 vs 6 mo) were shared by N and df/df mice (483 GO Terms in common). On the other hand, there were more enriched GO terms between df/df and N mice at old age (578 terms) than at young age (173 terms). There were a low number of processes commonly regulated in df/df and N mice at both 6 and 22 mo of age (only 35 terms). The enriched GO Terms for biological processes are presented in Supplemental Tables S4 and S5 for genotype and age differences, respectively.
Discussion
The present study evaluated for the first time the ovarian histological profile and transcriptome signature from df/df and N mice during aging. Although the expression of several genes changed with aging in N mice, only 31% of these genes also changed with aging in df/df mice, indicating that ovaries of df/df mice go through fewer gene expression changes as they age in comparison to N mice. As a result, we observed that several important processes related to inflammation/immune response up-regulated with age in N mice and were down-regulated in old df/df compared to N mice. This is compatible with our histological data, which indicate that the ovarian primordial follicle pool is almost three times larger in df/df than N mice, which strongly indicates preservation of a younger ovarian phenotype as these long-living df/df mice age. Indeed, the expression of the anti-Müllerian hormone (Amh) gene, an indicator of the ovarian reserve (Kevenaar et al., 2006), was about seven fold higher in the ovaries of old df/df compared to old N mice, although not different between young df/df and N mice (Suppl. Table 1). Similar results were observed for the expression of Zp1, Zp2 and Zp3 genes (Suppl. Table 1), which are exclusively expressed in oocytes and can also indicate the state of the follicle reserve (Epifano et al., 1995), further confirming a sharp decline in the oocyte reserve in N mice with aging, which was much less pronounced in df/df mice. We also observed a reduced primordial follicle oocyte diameter in df/df mice. The transition of follicles from the primordial to the primary stage is dependent on oocyte growth (Lintern-Moore and Moore, 1979). In this sense, it is possible that oocyte size can be an indicator of the rate of follicular depletion, since the FoxO3a and PTEN knockout mice have premature ovarian failure associated to an increased oocyte size in primordial follicles only (Castrillon et al., 2003; Reddy et al., 2008). Therefore, these histological and molecular findings further validate the df/df mice as a good model for ovarian aging, where the slower activation rate of primordial follicles resulted in fewer changes in gene expression as the mice age. One of the main characteristics of df/df mice is the severely reduced levels of serum IGF-I, therefore we also overlap the 72 DEGs between df/df and N mice at both ages with findings from in vitro IGF treated mice fibroblast cell lines (Dupont et al., 2001). However, no common target genes were identified, which can be due to the fact different cell types are being compared or also there are other characteristics unique to df/df mice, such as low insulin levels, decreased anti-inflammatory profile, adipose tissue cell composition and reduced oxidative stress (Bartke, 2005; Bartke, 2011; Masternak and Bartke, 2012), can also be responsible for its longevity phenotype and contribute to differential ovarian gene expression.
The top 150 enriched gene ontology terms for biological processes up-regulated with aging in N mice were all related to the inflammatory/immune response. Interestingly, the top 150 down-regulated terms between old df/df and old N mice are also related to the inflammatory/immune response, with 138 overlapping terms. This indicates that many processes differentially regulated between old df/df mice and old N mice originate from genes that are up-regulated with age in N mice but remain unchanged in df/df mice, as we observed 70% less DEGs with age in df/df compared to N mice. For example, we observed that most genes in the TLR signaling pathway were up-regulated with aging in N mice, although were down-regulated in old df/df compared to old N mice. Therefore, our results point to regulation of the inflammatory response as a central player in ovarian aging. The df/df, GHRKO and calorie restricted mice have all been extensively characterized as having a reduced pro-inflammatory profile, which may represent one of the major mechanisms promoting increased insulin sensitivity and extended longevity in these mice (Masternak and Bartke, 2012), therefore this was also expected to be observed in the ovary. Previous studies suggested that the reduction of the ovarian reserve is associated with an increased pro-inflammatory status of the ovarian surface (Smith and Xu, 2008). This increased inflammatory activity in the aging ovary is claimed as one of the main reasons for the higher incidence of ovarian epithelial cancer in postmenopausal women (Smith and Xu, 2008). In fact there is evidence that during ovarian aging in mice, along with follicular depletion, there is intense stromal remodeling associated with the activation of pro-inflammatory and anti-proliferative pathways (Zimon et al., 2006). Increased expression of inflammatory/immune response associated genes was also previously observed for the ovaries of aging mice, although calorie restriction did not affect the same pathways (Sharov et al., 2008), as observed in our current study for df/df mice, which are GH-deficient. On the other hand, mice subjected to high-fat diet have an accelerated depletion of the ovarian reserve, associated with an increased macrophage infiltration in the ovarian stroma (Skaznik-Wikiel et al., 2016). We observed that macrophage chemotaxis, macrophage activation and macrophage differentiation were also among the down-regulated biological processes in old df/df compared to old N mice. Therefore, these findings point to an association between the depletion of the ovarian reserve and an increased ovarian pro-inflammatory status, which were both attenuated in the ovaries of aging df/df mice. However, controversy remains regarding whether the increased follicular atresia with aging is eliciting a pro-inflammatory response in the ovaries or if the increased overall pro-inflammatory status with aging is accelerating the depletion of the ovarian follicular reserve. Recently, it was demonstrated that IL-1 knockout mice have delayed ovarian aging (Uri-Belapolsky et al., 2014), suggesting an active role for the inflammatory response in the process of follicle depletion. We also observed that IL-1 production was one of the down-regulated biological processes in old df/df compared to old N mice.
Most of the gene ontology terms down-regulated with aging in both N and df/df mice (237 matching terms) are related to biological processes such as DNA repair, DNA replication, response to DNA damage, chromatin organization, double-strand DNA break repair, spindle organization, and related processes. Maintenance of oocyte DNA integrity is essential for fertility preservation, and the extreme longevity of oocytes enclosed in primordial follicles renders them vulnerable to oxidative damage. It is well known that the age-related decline in reproductive performance in women is paralleled by a decrease in the ovarian reserve and an increase in chromosomally abnormal conceptions (Titus et al., 2013). Irradiation induced DNA damage in oocytes enclosed in primordial follicles leads to the activation of mitochondrial apoptotic pathways and the loss of the ovarian follicle reserve (Hanoux et al., 2007; Kerr et al., 2012). Therefore, it is proposed that the damaged oocyte DNA itself can be one of the causes for reduction in the size of the ovarian reserve with aging. In addition, impairment of DNA double strand break repair is associated with accelerated loss of ovarian follicular reserve (Titus et al., 2013). Expression of BRCA1, a key member of a family of DNA double strand break repair proteins, was reduced with aging in human oocytes (Titus et al., 2013). In our study we observed a three-fold reduction in BRCA1 gene expression with aging in N mice, although its expression did not change with aging in df/df mice (Suppl. Table 1). Therefore, it is suggested that maintenance of high levels of DNA protecting factors can be a key element for preserving the ovarian reserve for a longer period. We have previously demonstrated that the delayed ovarian aging in df/df mice is associated with an increased presence of the FoxO3a transcription factor in its non-phosphorylated form in the nucleus (Schneider et al., 2014), which is essential not only for maintaining the primordial follicles dormant (John et al., 2008) but also for increasing the protection of the cell against oxidative stress (Salih and Brunet, 2008). Additionally, mutations in the BRCA1 gene are also directly related to the development of ovarian carcinomas (Godlewski and Kapuscinska, 1996). There is no evidence that the GH/IGF-I axis can regulate BRCA1 gene expression, however BRCA1 can regulate IGF-I and IGF receptor gene expression and therefore, BRCA1 is an important regulator for tumor progression (Kang et al., 2012; Maor et al., 2000).
In the current study we demonstrated that df/df mice have an increased ovarian primordial follicle reserve compared to N mice, which is suggested to occur due to a lower rate of primordial follicle activation, since there was a reduction in the number of secondary growing follicles. One of the main pathways involved activation of primordial follicles and maintenance of the quiescent stage is the TGF-β signaling pathway (Knight and Glister, 2006). We observed that the KEGG pathways representing TGF-β signaling and resumption of oocyte meiosis were up-regulated in old df/df in comparison to N mice, although the same pathways were down-regulated with age in N mice. Therefore, this indicates a potential role for this pathway in the reduced rate of activation of primordial follicles observed in df/df mice, which is compatible to the overall lower activation of growth signaling pathways in other tissues observed in dwarf mice models for aging studies (Swindell, 2007). Moreover, we could observe the terms sexual reproduction, fertilization, gamete generation, oogenesis and germ cell development among the gene ontology terms for up-regulated biological processes in old df/df compared to old N mice. This further indicates that growth signaling pathways play a central role in the reduced rate of activation of the primordial reserve and cell proliferation and are a possibly reason for the delayed ovarian aging in this model.
In conclusion, the present study indicates delayed ovarian aging in long-living df/df mice in comparison to N mice, which was accompanied by a profound change in the ovarian transcriptome signature. Although there was a high number of DEGs with aging in N mice, only a small fraction of those changed with aging in df/df mice. We have shown that more than 500 different biological processes were down-regulated in the ovaries of old df/df in comparison to old N mice. Importantly, our study showed that the majority of the biological processes including inflammatory and immune responses were up-regulated with aging in N mice, and these were down-regulated in old df/df mice when compared to age matched littermates. This important observation confirms the delayed aging phenotype in these unique long living animals, showing beneficial regulation of longevity in the reproductive organs of female df/df mice.
Supplementary Material
Highlights.
The ovarian reserve is three times larger in Ames dwarf (df/df) mice than normal (N) mice.
More genes were differentially expressed during aging in N than df/df mice.
Inflammatory response genes were up-regulated with aging in N mice
Inflammatory response genes were down-regulated in old df mice compared to N mice.
DNA damage and repairing genes were down-regulated with aging in both genotypes.
Acknowledgments
This work was supported by Universidade Federal de Pelotas internal funds to AS; Washington University Department of Medicine internal funds to SJM; and National Institute on Aging of the National Institutes of Health (grants number R01AG032290, P01AG031736). This work was also supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
A.S., S.J.M., A.B. and M.M.M. contributed to study design, sample analysis and manuscript preparation. T.S., B.V., L.S., M.L. and P.G. contributed to sample analysis and manuscript revision. All authors reviewed and approved the manuscript.
Competing interests
The authors disclose no competing interests.
References
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–9. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TG. A Quantitative and Cytological Study of Germ Cells in Human Ovaries. Proc R Soc Lond B Biol Sci. 1963;158:417–33. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H, Mattison J, Kinney B, Hauck S, Wright C. Prolonged longevity of hypopituitary dwarf mice. Exp Gerontol. 2001;36:21–8. doi: 10.1016/s0531-5565(00)00205-9. [DOI] [PubMed] [Google Scholar]
- Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–23. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- Bartke A. Pleiotropic effects of growth hormone signaling in aging. Trends Endocrinol Metab. 2011;22:437–42. doi: 10.1016/j.tem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–8. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V, Bartke A. Induction of endogenous insulin-like growth factor-I secretion alters the hypothalamic-pituitary-testicular function in growth hormone-deficient adult dwarf mice. Biol Reprod. 1993;48:544–51. doi: 10.1095/biolreprod48.3.544. [DOI] [PubMed] [Google Scholar]
- Dupont J, Khan J, Qu BH, Metzler P, Helman L, LeRoith D. Insulin and IGF-1 induce different patterns of gene expression in mouse fibroblast NIH-3T3 cells: identification by cDNA microarray analysis. Endocrinology. 2001;142:4969–75. doi: 10.1210/endo.142.11.8476. [DOI] [PubMed] [Google Scholar]
- Epifano O, Liang LF, Familari M, Moos MC, Jr., Dean J. Coordinate expression of the three zona pellucida genes during mouse oogenesis. Development. 1995;121:1947–56. doi: 10.1242/dev.121.7.1947. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–6. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- Godlewski D, Kapuscinska M. BRCA1 and BRCA2 genes – new risk factors in hereditary forms of breast cancer and ovarian carcinoma. Reports of Practical Oncology and Radiotherapy. 1996;1:53–55. [Google Scholar]
- Hanoux V, Pairault C, Bakalska M, Habert R, Livera G. Caspase-2 involvement during ionizing radiation-induced oocyte death in the mouse ovary. Cell Death Differ. 2007;14:671–81. doi: 10.1038/sj.cdd.4402052. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Relationship between the supply of primordial follicles and the onset of follicular growth in rats. Biol Reprod. 1994;50:421–8. doi: 10.1095/biolreprod50.2.421. [DOI] [PubMed] [Google Scholar]
- John GB, Shirley LJ, Gallardo TD, Castrillon DH. Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction. 2007;133:855–63. doi: 10.1530/REP-06-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Yi YW, Kim HJ, Hong YB, Seong YS, Bae I. BRCA1 negatively regulates IGF-1 expression through an estrogen-responsive element-like site. Cell Death Dis. 2012;3:e336. doi: 10.1038/cddis.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, Bouillet P, Mills A, Scott CL, Findlay JK, Strasser A. DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa. Mol Cell. 2012;48:343–52. doi: 10.1016/j.molcel.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP, Themmen AP, Visser JA. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology. 2006;147:3228–34. doi: 10.1210/en.2005-1588. [DOI] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- Li L, Fu YC, Xu JJ, Chen XC, Lin XH, Luo LL. Caloric restriction promotes the reproductive capacity of female rats via modulating the level of insulin-like growth factor-1 (IGF-1) Gen Comp Endocrinol. 2011;174:232–7. doi: 10.1016/j.ygcen.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Lintern-Moore S, Moore GP. The initiation of follicle and oocyte growth in the mouse ovary. Biol Reprod. 1979;20:773–8. doi: 10.1095/biolreprod20.4.773. [DOI] [PubMed] [Google Scholar]
- Luo W, Friedman MS, Shedden K, Hankenson KD, Woolf PJ. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics. 2009;10:161. doi: 10.1186/1471-2105-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Brouwer C. Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics. 2013;29:1830–1. doi: 10.1093/bioinformatics/btt285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maor SB, Abramovitch S, Erdos MR, Brody LC, Werner H. BRCA1 suppresses insulin-like growth factor-I receptor promoter activity: potential interaction between BRCA1 and Sp1. Mol Genet Metab. 2000;69:130–6. doi: 10.1006/mgme.1999.2958. [DOI] [PubMed] [Google Scholar]
- Masternak MM, Al-Regaiey K, Bonkowski MS, Panici J, Sun L, Wang J, Przybylski GK, Bartke A. Divergent effects of caloric restriction on gene expression in normal and long-lived mice. J Gerontol A Biol Sci Med Sci. 2004;59:784–8. doi: 10.1093/gerona/59.8.b784. [DOI] [PubMed] [Google Scholar]
- Masternak MM, Bartke A. Growth hormone, inflammation and aging. Pathobiol Aging Age Relat Dis. 2012;2 doi: 10.3402/pba.v2i0.17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkovich SJ, Zhang Y, Van Booven DJ, Dorn GW., 2nd Deep mRNA sequencing for in vivo functional analysis of cardiac transcriptional regulators: application to Galphaq. Circ Res. 2010;106:1459–67. doi: 10.1161/CIRCRESAHA.110.217513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkovich SJ, Dorn GW., 2nd Deep sequencing of cardiac microRNA-mRNA interactomes in clinical and experimental cardiomyopathy. Methods Mol Biol. 2015;1299:27–49. doi: 10.1007/978-1-4939-2572-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127:569–80. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24:5073–9. doi: 10.1096/fj.10-163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H. The development of the mouse ovary from birth to maturity. Acta Endocrinol (Copenh) 1969;62:98–116. doi: 10.1530/acta.0.0620098. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–3. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Reddy P, Adhikari D, Zheng W, Liang S, Hamalainen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, Liu K. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet. 2009;18:2813–24. doi: 10.1093/hmg/ddp217. [DOI] [PubMed] [Google Scholar]
- Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–36. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Zhi X, Moreira F, Lucia T, Jr., Mondadori RG, Masternak MM. Primordial follicle activation in the ovary of Ames dwarf mice. J Ovarian Res. 2014;7:120. doi: 10.1186/s13048-014-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Falco G, Piao Y, Poosala S, Becker KG, Zonderman AB, Longo DL, Schlessinger D, Ko M. Effects of aging and calorie restriction on the global gene expression profiles of mouse testis and ovary. BMC Biol. 2008;6:24. doi: 10.1186/1741-7007-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaznik-Wikiel ME, Swindle DC, Allshouse AA, Polotsky AJ, McManaman JL. High-Fat Diet Causes Subfertility and Compromised Ovarian Function Independent of Obesity in Mice. Biol Reprod. 2016 doi: 10.1095/biolreprod.115.137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot KA, Kastelijn J, Bachelot A, Kelly PA, Binart N, Teerds KJ. Reduced recruitment and survival of primordial and growing follicles in GH receptor-deficient mice. Reproduction. 2006;131:525–32. doi: 10.1530/rep.1.00946. [DOI] [PubMed] [Google Scholar]
- Słuczanowska-Głąbowska S, Laszczy Ska M, Piotrowska K, W GB, Rumianowski B, Masternak M, Arum O, Kucia M, Kopchick JJ, Bartke A, Ratajczak MZ. The effect of calorie restriction on the presence of apoptotic ovarian cells in normal wild type mice and low-plasma-IGF-1 Laron dwarf mice. J Ovarian Res. 2013;6:67. doi: 10.1186/1757-2215-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ER, Xu XX. Ovarian ageing, follicle depletion, and cancer: a hypothesis for the aetiology of epithelial ovarian cancer involving follicle depletion. Lancet Oncol. 2008;9:1108–11. doi: 10.1016/S1470-2045(08)70281-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O'Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–33. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- Swindell WR. Gene expression profiling of long-lived dwarf mice: longevity-associated genes and relationships with diet, gender and aging. BMC Genomics. 2007;8:353. doi: 10.1186/1471-2164-8-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde ER, Scheffer GJ, Dorland M, Broekmans FJ, Fauser BC. Developmental and endocrine aspects of normal ovarian aging. Mol Cell Endocrinol. 1998;145:67–73. doi: 10.1016/s0303-7207(98)00171-3. [DOI] [PubMed] [Google Scholar]
- Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S, Oktay K. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5:172ra21. doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNASeq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–5. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uri-Belapolsky S, Shaish A, Eliyahu E, Grossman H, Levi M, Chuderland D, Ninio-Many L, Hasky N, Shashar D, Almog T, Kandel-Kfir M, Harats D, Shalgi R, Kamari Y. Interleukin-1 deficiency prolongs ovarian lifespan in mice. Proc Natl Acad Sci U S A. 2014;111:12492–7. doi: 10.1073/pnas.1323955111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noord-Zaadstra BM, Looman CW, Alsbach H, Habbema JD, te Velde ER, Karbaat J. Delaying childbearing: effect of age on fecundity and outcome of pregnancy. BMJ. 1991;302:1361–5. doi: 10.1136/bmj.302.6789.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Risal S, Gorre N, Busayavalasa K, Li X, Shen Y, Bosbach B, Brannstrom M, Liu K. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 2014;24:2501–8. doi: 10.1016/j.cub.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Human Reproduction Update. 2015:dmv037. doi: 10.1093/humupd/dmv037. [DOI] [PubMed] [Google Scholar]
- Zimon A, Erat A, Von Wald T, Bissell B, Koulova A, Choi CH, Bachvarov D, Reindollar RH, Usheva A. Genes invoked in the ovarian transition to menopause. Nucleic Acids Res. 2006;34:3279–87. doi: 10.1093/nar/gkl387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.