Abstract
The neurogenic heartbeat of certain invertebrates has long been studied both as a way of understanding how automatic functions are regulated and for how neuronal networks generate the inherent rhythmic activity that controls and coordinates this vital function. This review focuses on the heartbeat of decapod crustaceans and hirudinid leeches, which remain important experimental systems for the exploration of central pattern generator networks, their properties, network and cellular mechanisms, modulation, and how animal-to-animal variation in neuronal and network properties are managed to produce functional output.
Invertebrates, owing to their small numbers of central neurons all of which are uniquely identifiable, have served as important experimental systems for the exploration of neuronal networks, their properties, network and cellular mechanisms, modulation, and how animal to animal variation in neuronal and network properties are managed to produce functional output. Automatic functions like neurogenic heartbeat have been particularly attractive for study because of their ongoing nature and the robustness of fictive neuronal activity in isolated nervous system preparations. Here we focus on two examples that are being actively pursued by experimental and computational approaches.
Crustacean heartbeat
In decapod crustaceans, the heart is neurogenic and receives innervation from a cardiac ganglion containing 9 neurons, five of which (Large Cells) are motor neurons. Heartbeat is driven by rhythmic bursts of impulses (burst period 1–5 s) in these motor neuron, which produce depolarizing EPSPs on heart muscle cells. The comparative anatomy and physiology of crustacean cardiac ganglia and their innervation of the heart have been comprehensively and lovingly reviewed in [1] and a small fraction only of this work is highlighted here. The cardiac ganglion is in essence a central pattern generator that produces fictive heartbeat in the Large Cells in isolation (Fig. 1). There are 4 Small Cells, interneurons that are strongly electrically coupled, that pace activity in the ganglion by producing coherent bursts of action potentials that provide direct excitatory synaptic inputs to drive activity in the Large Cells (motor neurons). These motor neurons are themselves strongly electrically coupled and produce synchronous bursts to drive heart muscle. Both Small and Large Cells produce plateau-like regenerative potentials, called Driver Potentials, which support burst formation. Driver Potentials are triggered in the Large Cells by the rhythmic excitatory drive from the Small Cells; in Small Cells, Driver Potential are driven by a spontaneous slowly depolarizing pacemaker potential. Voltage-clamp analyses reveal that Driver Potentials result from the interplay of a Ca current (ICa) and a slow K current (IK) and to a lesser extent a Ca activated K current (IKCa); the source of the slowly depolarizing pacemaker potential remains controversial.
Figure 1.
Cardiac ganglion of the Jonah crab, Cancer borealis. Left: schematic of the ganglion showing the positions of the somata of the 5 Large Cells (LCs, motor neurons) and the 4 Small Cells (SCs, interneurons). Right: Typical electrical activity of an isolated cardiac ganglion. Top trace is an intracellular recording from two Large Cells (identified by color), showing their synchronous synaptic drive, driver potentials and spiking activity, and the bottom trace is an extracellular recording from a ganglionic trunk showing Small Cell activity (smaller spikes two different units clearly visible) and synchronous Large Cell activity (large spikes). Figure kindly provided by David J. Schulz.
More recent work has occurred on two fronts: on the identification and role of the large number of neuropeptide modulators that are active both in the ganglion and at neuromuscular junctions to enhance cardiac output and on how homeostatic mechanisms regulate membrane conductances to ensure uniformity in ganglionic output across the life of an animal and across animals.
The neuropeptide work [2–4], in the American Lobster, illustrates the complex nature of neuromodulation when operating on multiple components of the multilayered neuromuscular system: interneurons (SCs) and motor neurons (LCs), the neuromuscular junctions (NMJs) and muscles, and importantly feedback systems. Both SCs and LCs have stretch sensitive dendrites on the heart, which feedback positively to decrease cycle period [5], and both SCs and LCs are modulated by NO released by heart muscle during contraction, which slows their burst period [6]. Two related peptides GYSDRNYLRFamide (GYS) and SGRNFLRFamide (SGRN) were studied in detail [3]. They act similarly at low concentration (10−10 to 10−9 M) on both the ganglion and the neuromuscular system; they speed burst period, enhance stretch feedback, and increase contraction amplitude by acting either on the NMJs or the muscle, albeit by slightly different mechanisms. At higher concentration (10−8 M) they continue to increase contraction amplitude, but GYS increases cycle period and LC burst duration, and SGRN has no effect on cycle period. These differences are attributable to how the two peptides influence stretch and NO mediated feedback; GYS enhances NO feedback while SGRN enhances stretch feedback.
The work on ion channel homeostasis [7–9] in the Jonah crab, Cancer borealis, is beginning to reveal homeostatic mechanisms and to uncover how ion channel expression levels that achieve similar activity still affect responses to perturbation. Using voltage-clamp measurements of ionic currents, molecular characterization and quantitative measurements of ionic channel mRNAs, various ionic currents have been identified in Large Cells [7] and shown to vary 2–4 fold across homologous neurons within the same ganglion as well as across animals [8,9]. These variable conductances make Large Cells susceptible to pharmacological perturbations so that these motor neurons become uncoordinated and show different firing patterns. Thus despite genetic identity within an animal and uniform and synchronous activity within the network, Large Cells show highly variable underlying maximal conductances that are tuned to achieve the same activity [9]. Remarkably, synaptically isolated Large Cells rapidly (30–90 minutes) compensate for changes in excitability induced by K+ channel blockers, by coordinately regulating two potassium currents (IA and IKCa) presumably to homeostatically maintain a target level of excitability [8]. Thus the crab cardiac ganglion is rapidly becoming a test bed for sophisticated analyses of homeostatic regulatory mechanism at the cellular and molecular level. Given the strong electrical coupling among Large Cells and the resultant potential for intercellular communication, the relative cellular independence of homeostatic regulatory mechanism revealed thus far seems all the more remarkable.
Leech heartbeat
The leech (Hirudo sp) heartbeat system (see [10] for a past review) offers the opportunity to study and understand an on-going rhythmic behavior from neuron biophysics, to neuronal networks of interneurons and motor neurons, to circulatory movements. Four decades of research have made the CPG that drives heartbeat one of the best characterized and most comprehensively modeled.
Bilateral tubular hearts, running the length of the animal, undergo coordinated rhythmic (period ~6–10s at 22°–23° C) constrictions to propel blood flow [11,12]. At any one time the left and right hearts coordinate their constrictions differently, with one showing a rear-to-front peristalsis and the other near synchrony, switching roles every ~40 beats (~300–400s) (Fig. 2). The difference in the coordination mode of the two hearts corresponds to the pattern of blood flow. Peristalsis generates high systolic pressure and propulsive blood flow forward and into main dorsal and ventral longitudinal vessels which feed the main capillary beds and the CNS respectively. Synchrony generates low systolic pressure and drives local peripheral blood flow in each segment. Switching coordination modes presumably balances local segmental blood flow over the time course of minutes.
Figure 2.
Heartbeat and heart motor neuron activity in a minimally dissected leech, Hirudo sp. Top: simultaneous extracellular (loose patch) recording from ipsilateral heart motor neurons in segments segments 8 and 12 (HE(R,8) and HE(R,12)) and tension recordings from the ipsilateral heart in the same segments. At the beginning of the record the right heart is in the peristaltic coordination mode; at the end of the record coordination mode of the heart has switched to synchronous. The switch occurs in the middle of the record and is accomplished in one beat cycle. Bottom: blow-ups of the shaded parts of the record at top to show how heart constriction is timed to heart motor neuron activity in each coordination mode. Diamonds mark the middle spike of each motor neuron burst and crosses the maximum rate of rise of heart tension (MRR). After [15].
The tubular heart walls encompass a mesh of spiral muscle fibers that are innervated in each midbody segment (3–18) by a segmental motor neuron [13,14]. These heart (HE) motor neurons, one on each side, reside in their respective segmental ganglia of the leech ventral nerve cord and project ipsilateral axons to innervate their segment (with some segmental overlap) of the heart tube. This innervation has been described in detail, but simplifying for clarity, each motor neurons provides excitatory junctional potentials that sum to trigger plateau-like muscle potentials that produce constrictions (Fig. 2). Constrictions are timed by motor neuron bursts, such that maximal tension is achieved after the middle spike of each burst when motor neuron firing frequency has reached a plateau (usually >10 Hz) [15]. The middle spike of each motor neuron burst is a useful indicator of heart muscle contraction and roughly corresponds to the maximum rate of rise (MRR) of the tension. Peristalsis is thus characterized by a phase lead of heart motor neuron firing (middle spike) and of heart muscle tension (MRR) rear-to-front between segments and synchrony by near simultaneity of these characteristics between segments. Switches in coordination mode are accomplished within a beat cycle – as shown in Figure 2, we see a switch from right peristaltic (left synchronous) to left peristaltic (right synchronous) – and are always reciprocal. Thus the leech heartbeat motor pattern is one that has complex dynamics on two temporal scales - beat timing and switch timing - that serve clear functional roles and provide function-based metrics for assessing temporal characteristic: how they are generated and how they vary within and across animals.
Leech fictive heartbeat and the heartbeat central pattern generator
The isolated nerve cord of the leeches produces continuously a fictive motor program (Fig. 3a) that is startlingly similar to the motor program observed in nearly intact leeches, including the regular reciprocal switches in coordination mode [16,17]. Heart motor neurons fire in a rear-to-front progression (peristaltic coordination) on one side and a nearly synchronous pattern on the other (synchronous coordination) and coordination modes switch reciprocally abruptly within one beat cycle. Intersegmental phase differences (measured by the middle spikes of motor neuron bursts) in the fictive motor pattern characterize the peristaltic (substantial rear phase lead) and synchronous (near synchrony) coordination modes; these phase differences vary considerably in size across animals correspondingly as they do for the beat pattern of intact leeches measured with non-invasive video methods but remain characteristic of their coordination mode (Fig. 4b) [14].
Figure 3.
The heartbeat control system of the leeches: heart motor neurons and the heartbeat CPG. (a) Simultaneous ipsilateral extracellular recording of the four premotor interneurons of the core CPG and an intracellular recording of a heart motor neuron (HE(8)), which the interneurons all inhibit. The rhythmic inhibitory input sculpts out bursts in the heart motor neuron activity. (b) Bilateral circuit diagram including all the identified heart (HN) interneurons of the core CPG showing the inhibitory connections from the heart interneurons of the leech heartbeat CPG onto heart (HE) motor neurons in the first 12 midbody segmental ganglia. The ipsilateral HN(3) and HN(4) and the ipsilateral HN(6) and HN(7) premotor interneurons provide input to heart motor neurons [HE(3)–HE(12)]. (c) The segmental synaptic strength profiles of heart interneurons for the HE(8) and HE(12) motor neurons. In the upper panel, an HE(12) motor neuron was recorded in voltage clamp (holding potential -45 mV) simultaneously with extracellular recording from four ipsilateral premotor heart interneurons, HN(3), HN(4), HN(6), and HN(7) (middle spikes indicated by symbols), that provide its input. HN spikes from 11 bursts from each interneuron were used to generate the spike-triggered averages of IPSCs in the HE(12) motor neuron, and subsequently similar recordings were used to generate the spike-triggered averages of IPSCs for the HE(8) motor neuron in the same animal. Note the difference in the premotor input synaptic strength profiles between the two motor neurons. Upward arrows indicate the time of the triggering HN spike and the downward arrows indicate the peak of the averaged IPSC used to measure amplitude. Spike-triggered average IPSCs, left to right arise from the HN(3)–HN(7) premotor interneurons respectively. All recordings from isolated nerve cord preparations. Cell identity coded with color consistently across figures. Adapted from [18,19].
Figure 4.
(a) The synaptic strength illustrated in Figure 3, shows considerable animal-to-animal variation both in absolute terms and in the relative strengths of the four inputs to a given motor neuron. Iconic unilateral circuit diagram (above) identifies the neurons recorded for each graph and standard colors and symbols are used to indicate the strength of each HN input in each graph. Absolute strength in nS (upper graphs) or relative strength (lower graphs) is plotted vs. preparation ordered by the absolute or relative strength of the HN(3) input, respectively. (b) Complete analysis of input and output temporal patterns and synaptic strength patterns (spatial pattern) for 2 different preparations from a sample of 12 (included in (a)) in which simultaneous extracellular recordings (loose patch) were made of ipsilateral HN(3), HN(4), HN(6), and HN(7) premotor interneurons (inputs) and HE(8) and HE (12) motor neurons (outputs) in both peristaltic (p) and synchronous (p) coordination modes and then subsequently synaptic strength of each interneuron in the two motor neurons was measured in voltage clamp as in Figure 3c. Summary phase diagrams of the premotor interneurons (standard color code) and the HE(8) and HE(12) motor neurons in both the peristaltic (pink-outlined boxes) and synchronous (light blue-outlined boxes) coordination modes are shown. For both motor neurons and interneurons, the average duty cycle is indicated by the length of the bar: the left edge of each bar indicates the average phase of the first spike of the burst and the right edge indicates the average phase of the last spike of the burst. Average phase is indicated by a vertical line within the bar. Error bars indicate SDs. The strength pattern of the inputs is shown to the right of each composite phase diagram. Note the characteristic peristaltic and synchronous firing patterns in both the premotor interneurons and the motor neuron that yet demonstrate differences between the two preparations illustrated. Similarly the synaptic strength profiles are characteristic yet clearly different. Adapted from [19].
A set of segmental (HN) heart interneurons located in midbody ganglia 1–7 form the core heartbeat CPG, and the heart motor neurons are rhythmically inhibited by the premotor heart interneurons of this CPG (Fig. 3a), which are located in segments 3, 4, 6 and 7 [16,17]. These premotor interneurons form a stereotypical pattern of ipsilateral synaptic contacts with heart motor neurons in more caudal segments (Fig. 3b). These connections vary in strength among the segments; each heart interneurons has its own segmental profile of synaptic strength with general interneurons HN(3) and HN(4) dominating in more rostral segments and interneurons HN(6) and HN(7) dominating in more caudal segments (Fig. 3c) [18,19]. These synaptic strength profiles have been described in great detail using voltage clamp and do not change across switches in coordination mode within any given animal. Nevertheless they vary significantly among individuals both in absolute and more importantly in relative terms (Fig. 4a) [19].
The heart interneuron network (heartbeat CPG) itself produces a complex rhythmic pattern that mirrors the fictive motor pattern [17,20]. Premotor interneurons on one side fire in a rear-to-front progression that is associated with peristaltic coordination of the motor neurons and on the other with near synchrony (or a small front-to-rear progression) that is associated with synchronous coordination of the motor neurons (Fig. 4b). These phase differences (measured from the middle spikes of their bursts) vary in size across animals correspondingly as they do for the fictive motor pattern but remain characteristic of their coordination mode (Fig. 4b). These characteristic firing patterns of the premotor interneurons switch to produce the switches in coordination mode of the motor pattern.
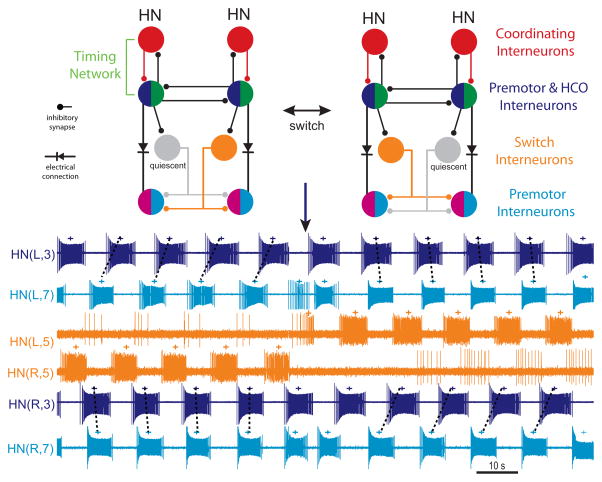
The heart interneurons form a network with a highly interconnected front kernel (HN(1) – HN(4) interneurons) characterized by reciprocal inhibition with feed forward inhibition and electrical excitation to the more reward interneurons (Fig. 5) [21]. The HN(5) interneurons serve to switch coordination modes within the network by interfacing between the front kernel with the HN(6) and HN(7) premotor interneurons. They are timed by rhythmic inhibition from the front kernel and make bilateral inhibitory connections to the HN(6) and HN(7) premotor interneurons. Repeated bilateral recording of HN(5) switch interneurons, both extra cellular (Fig. 5) and intracellular, show that the switch interneurons on the peristatic side of the network is quiescent while the one on the synchronous is robustly rhythmically active [21]. Switches in coordination mode are accomplished when the previously quiescent switch interneuron becomes suddenly active whereas the previously active switch interneuron becomes quiescent -within one beat cycle [22,23]. Modeling studies show that the balance of ipsilateral excitation from the HN(3) and HN(4) interneurons and inhibition from the active switch interneuron, which voltage clamp studies show is stronger ipsilaterally and in the HN(7) vs. the HN(6) interneuron, accounts for the characteristic peristaltic and synchronous phase differences among ipsilateral premotor interneurons [21,24].
Figure 5.
The leech heartbeat CPG and the role of the switch interneurons. Top: Circuit diagram of the identified heart interneurons of the core CPG showing their synaptic interconnections, inhibitory and electrical. The two possible states of the heartbeat CPG are illustrated one with the left switch interneuron quiescent and the right switch interneuron active (corresponding to left synchronous), and the other with the left switch interneuron active and the right switch interneuron quiescent (corresponding to left peristaltic). Heart interneurons that have similar input and output connections are lumped together for ease of presentation. Bottom: Simultaneous extracellular recordings of two bilateral pairs of premotor heart interneurons [HN(R/L,3) and HN(R/L,7)] and the bilateral pair of switch interneurons [HN(R/L,5)] during a switch in coordination mode from left synchronous to left peristaltic as indicated in the circuit diagrams above. Note the invariant activity of the HN(3) (HCO) interneurons and the shift in activity of the HN(7) premotor interneurons during the switch, which is accomplished in one beat cycle as the HN(5) interneurons move reciprocally between quiescent and active states. Symbols mark the middle spike of each interneuron burst to illustrate phase relationships. Cell identity coded with color consistently across figures. Adapted from [20,24].
Several modeling [25–27], and dynamic clamp [28] studies indicate that the coordination of the motor neurons by the premotor interneurons is realized by a combination of their characteristic peristaltic and synchronous firing patterns (intersegmental phase differences; rear-to-front vs. near synchrony) and the characteristic segmental synaptic profiles of the premotor interneurons onto the motor neurons. As a simplifying example consider that in peristaltic coordination the first firing HN(7) interneuron is strong in more rearward motor neurons and weaker up front, while the lagging HN(4) premotor interneuron is stronger up front and weaker rearward (Fig. 3c). This conjunction of temporal pattern in the interneurons and their segmental synaptic strength profiles causes the more rearward heart motor neuron to fire in advance of the more frontward. The temporal patterns determines the limits of what motor neuron phase difference can be achieved and the segmental synaptic strength profile determines what phase difference is realized within this limit. The animal-to-animal variability in the temporal pattern of premotor interneuron firing and in segmental synaptic profiles result in each animal arriving at a unique solution for peristaltic and synchronous coordination of heart motor neurons (Fig. 4) [19,28].
Beat rhythmicity and switch rhythmicity within the heartbeat CPG
The rhythmicity that underlies beat timing in the heart beat CPG arises in the HN(3) and HN(4) interneurons and percolates through the rest of the network. These interneurons (in addition to their premotor role detailed above) form strong reciprocal inhibitory connections with their bilateral homologs forming two classic half-center oscillators (HCOs) [21,29]. Numerous voltage clamp studies have detailed the ionic current that pace their activity, in addition to the fast inward and outward currents that produce spikes, they possess a non-inactivating K current [30], a persistent Na current that regulates general excitability [31] and most importantly a slowly-inactivating low-threshold Ca current (ICaS) [32] and a hyperpolarization activated inward current (Ih) [33]. ICaS drives the burst phase slowly inactivating to release, as spike rate wanes, the opposite inhibited neuron of the half-center oscillator, while the Ih activated by the hyperpolarization associated with inhibition, which also removes inactivation from ICaS), drives the inhibited neuron of the HCO toward escape and burst formation. This mixed escape-release model for rhythmicity in a half-center microcircuit has been repeatedly corroborated by modeling [29,34–36] and dynamic-clamp studies [37,38]. The endogenous peptide myomodulin speeds the burst rhythm of the two HCOs by up-modulating Ih and down modulating the current driven by the Na+/K+ pump [39]. Recent work indicates that the cycle-to-cycle dynamics of Na+/K+ pump current works in conjunction with Ih to regulate period of the HCOs [40].
The HN(1) and HN(2) interneurons serve as coordinating fibers that link the two HN(3) and HN(4) HCOs into a beat timing-network [21,41]. Inherent period differences between the two HCOs lead to the phase difference in activity observed ipsilaterally between them (Fig. 4c) [41–43]. Detailed modeling studies corroborate these findings [44,45].
The rhythmicity that underlies switch timing is not well understood. Switch interneurons do not interact synaptically, making a slow half-center oscillator comprising them highly unlikely [23]. Voltage-clamp studies show that switch interneurons in the quiescent state experience a tonic outward current that suppressing firing and that a switch between the quiescent and active state involves the termination of this current [22]. Artificially altering the state of a switch interneuron from quiescent to active or vice-versa with injected current or dynamic clamp does not influence the state of the opposite switch interneuron. Switch interneurons switch in isolated ganglia 5. Thus is seems likely that there exists a switch oscillator in ganglion 5 separate from the identified heartbeat CPG that engages the two switch interneuron with tonic inhibition alternately.
The leech heartbeat control system remains a vibrant test bed for ideas about the origins of rhythmicity in neuronal networks, intersegmental coordination, premotor control, the origins and importance of variability for neuronal network performance, and neuromodulation.
Highlights.
The cardiac ganglion reveals homeostatic mechanisms regulating electrical activity.
The leech heartbeat system can be studied at all levels from neurons to circulation.
In both systems individual variation is large, yet functional activity is maintained.
Acknowledgments
Supported by NIH NINDS 1 R01 NS085006
Footnotes
Conflict of Interest Statement:
Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Cooke IM. Reliable, responsive pacemaking and pattern generation with minimal cell numbers: the crustacean cardiac ganglion. Biol Bull. 2002;202:108–536. doi: 10.2307/1543649. [DOI] [PubMed] [Google Scholar]
- 2.Dickinson PS, Sreekrishnan A, Kwiatkowski MA, Christie AE. Distinct or shared actions of peptide family isoforms: I. Peptide-specific actions of pyrokinins in the lobster cardiac neuromuscular system. J Exp Biol. 2015;218:2892–2904. doi: 10.1242/jeb.124800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Dickinson PS, Calkins A, Stevens JS. Related neuropeptides use different balances of unitary mechanisms to modulate the cardiac neuromuscular system in the American lobster, Homarus americanus. J Neurophysiol. 2015;113:856–870. doi: 10.1152/jn.00585.2014. A careful dissection of how closely related peptides have different effects on the lobster cardiac system. They acts similarly on the neuromuscular system but differentially effect feedback systems and ganglion cells and have different effects according to concentration. Well illustrates how difficult it is to sort out how a modulator works at the systems level. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickinson ES, Johnson AS, Ellers O, Dickinson PS. Forces generated during stretch in the heart of the lobster Homarus americanus are anisotropic and are altered by neuromodulators. J Exp Biol. 2016;15:1187–1202. doi: 10.1242/jeb.135657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Crescioni K, Fort Timothy J, Stern E, Brezina V, Miller MW. Feedback from peripheral musculature to central pattern generator in the neurogenic heart of the crab Callinectes sapidus: role of mechanosensitive dendrites. J Neurophysiol. 2010;103:83–96. doi: 10.1152/jn.00561.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahadevan A, Lappe J, Rhyne RT, Cruz-Bermudez ND, Marder E, Goy MF. Nitric oxide inhibits the rate and strength of cardiac contractions in the lobster Homarus americanus by acting on the cardiac ganglion. J Neurosci. 2004;24:2813–2824. doi: 10.1523/JNEUROSCI.3779-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ransdell JL, Temporal S, West NL, Leyrer ML, Schulz DJ. Characterization of inward currents and channels underlying burst activity in motoneurons of crab cardiac ganglion. J Neurophysiol. 2013;110:42–54. doi: 10.1152/jn.00009.2013. [DOI] [PubMed] [Google Scholar]
- 8•.Ransdell JL, Nair SS, Schulz DJ. Rapid homeostatic plasticity of intrinsic excitability in a central pattern generator network stabilizes functional neural network output. J Neurosci. 2012;32:9649–9658. doi: 10.1523/JNEUROSCI.1945-12.2012. Impressive synergy of molecular and electrophysiological approaches to demonstrate rapid homeostatic regulation of excitability (30–90 minutes) in a rhythmically active neuronal network to pharmacological challenge. Presents a comprehensive hypothesis for plasticity of excitability from mRNA to network output whereby rapid compensation provides stabilization of cellular and network activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Ransdell JL, Nair SS, Schulz DJ. Neurons within the same network independently achieve conserved output by differentially balancing variable conductance magnitudes. J Neurosci. 2013;33:9950–9956. doi: 10.1523/JNEUROSCI.1095-13.2013. Uses electrophysiological techniques to demonstrate that neuron of the same type, in the same animal, and that have identical activity attain that status through different balances of inherent membrane conductances. A seminal work for understanding how uniform activity characteristics arise from variability in channels expression in an individual and the implication of that variation for responses to perturbation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristan WB, Jr, Calabrese RL, Friesen WO. Neuronal control of leech behavior. Prog Neurobiol. 2005;76:279–327. doi: 10.1016/j.pneurobio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Wenning A, Cymbalyuk GS, Calabrese RL. Heartbeat control in leeches. I. Constriction pattern and neural modulation of blood pressure in intact animals. J Neurophysiol. 2004;91:382–396. doi: 10.1152/jn.00526.2003. [DOI] [PubMed] [Google Scholar]
- 12.Wenning A, Meyer EP. Hemodynamics in the leech: blood flow in two hearts switching between two constriction patterns. J Exp Biol. 2007;210:2627–2636. doi: 10.1242/jeb.001644. [DOI] [PubMed] [Google Scholar]
- 13.Maranto AR, Calabrese RL. Neural control of the hearts in the leech, Hirudo medicinalis: I. Structure, innervation and electrical coupling of the hearts. J Comp Physiol A. 1984;154:367–380. [Google Scholar]
- 14.Maranto AR, Calabrese RL. Neural control of the hearts in the leech, Hirudo medicinalis: II. Myogenic activity and phasic control by heart motor neurons. J Comp Physiol A. 1984;154:389–391. [Google Scholar]
- 15.Wenning A, Norris BJ, Doloc-Mihu A, Calabrese RL. Variation in motor output and motor performance in a centrally generated motor pattern. J Neurophysiol. 2014;112:95–109. doi: 10.1152/jn.00856.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenning A, Hill AA, Calabrese RL. Heartbeat control in leeches. II. Fictive motor pattern. J Neurophysiol. 2004;91:397–409. doi: 10.1152/jn.00528.2003. [DOI] [PubMed] [Google Scholar]
- 17.Norris BJ, Weaver AL, Wenning A, García PS, Calabrese RL. A central pattern generator producing alternative outputs: phase relations of leech heart motor neurons with respect to premotor synaptic input. J Neurophysiol. 2007;98:2983–2991. doi: 10.1152/jn.00407.2007. [DOI] [PubMed] [Google Scholar]
- 18.Norris BJ, Weaver AL, Wenning A, García PS, Calabrese RL. A central pattern generator producing alternative outputs: pattern, strength, and dynamics of premotor synaptic input to leech heart motor neurons. J Neurophysiol. 2007;98:2992–3005. doi: 10.1152/jn.00877.2007. [DOI] [PubMed] [Google Scholar]
- 19•.Norris BJ, Wenning A, Wright TM, Calabrese RL. Constancy and variability in the output of a central pattern generator. J Neurosci. 2011;31:4663–4674. doi: 10.1523/JNEUROSCI.5072-10.2011. Uses the leech heartbeat CPG to explore in detail variation in premotor activity pattern, motor activity pattern, and synaptic interactions between premotor interneurons and motor neurons in twelve individuals. Reaches the conclusion that motor patterns express characteristic functional activity that nevertheless varies enough across individuals, so that it is clear that individuals arrive a unique solutions for circuit output and synaptic interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norris BJ, Weaver AL, Morris LG, Wenning A, García PA, Calabrese RL. A central pattern generator producing alternative outputs: temporal pattern of premotor activity. J Neurophysiol. 2006;96:309–326. doi: 10.1152/jn.00011.2006. [DOI] [PubMed] [Google Scholar]
- 21•.Roffman RC, Norris BJ, Calabrese RL. Animal-to-animal variability of connection strength in the leech heartbeat central pattern generator. J Neurophysiol. 2012;107:1681–1693. doi: 10.1152/jn.00903.2011. Comprehensive exploration of animal-to-animal variation in synaptic strengths in a highly interconnected pattern generating neuronal network. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gramoll S, Schmidt J, Calabrese RL. Switching in the activity state of an interneuron that controls coordination of the hearts in the medicinal leech (Hirudo medicinalis) J Exp Biol. 1994;186:157–171. doi: 10.1242/jeb.186.1.157. [DOI] [PubMed] [Google Scholar]
- 23.Lu J, Gramoll S, Schmidt J, Calabrese RL. Motor pattern switching in the heartbeat pattern generator of the medicinal leech: membrane properties and lack of synaptic interaction in switch interneurons. J Comp Physiol A. 1999;184:311–324. doi: 10.1007/s003590050329. [DOI] [PubMed] [Google Scholar]
- 24.Weaver AL, Roffman RC, Norris BJ, Calabrese RL. A role for compromise: synaptic inhibition and electrical coupling interact to control phasing in the leech heartbeat CPG. Front Behav Neurosci. 2010;4 doi: 10.3389/fnbeh.2010.00038. pii: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García PS, Wright TM, Cunningham IR, Calabrese RL. Using a model to assess the role of the spatiotemporal pattern of inhibitory input and intrasegmental electrical coupling in the intersegmental and side-to-side coordination of motor neurons by the leech heartbeat central pattern generator. J Neurophysiol. 2008;100:1354–1371. doi: 10.1152/jn.90579.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright TM, Jr, Calabrese RL. Contribution of motoneuron intrinsic properties to fictive motor pattern generation. J Neurophysiol. 2011;106:538–553. doi: 10.1152/jn.00101.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb DG, Calabrese RL. Correlated conductance parameters in leech heart motor neurons contribute to motor pattern formation. PLoS One. 2013;8:e79267. doi: 10.1371/journal.pone.0079267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright TM, Jr, Calabrese RL. Patterns of presynaptic activity and synaptic strength interact to produce motor output. J Neurosci. 2011;31:17555–17571. doi: 10.1523/JNEUROSCI.4723-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill AA, Lu J, Masino MA, Olsen OH, Calabrese RL. A model of a segmental oscillator in the leech heartbeat neuronal network. J Comput Neurosci. 2001;10:281–302. doi: 10.1023/a:1011216131638. [DOI] [PubMed] [Google Scholar]
- 30.Simon TW, Opdyke CA, Calabrese RL. Modulatory effects of FMRF-NH2 on outward currents and oscillatory activity in heart interneurons of the medicinal leech. J Neurosci. 1992;12:525–537. doi: 10.1523/JNEUROSCI.12-02-00525.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opdyke CA, Calabrese RL. A persistent sodium current contributes to oscillatory activity in heart interneurons of the medicinal leech. J Comp Physiol A. 1994;175:781–789. doi: 10.1007/BF00191850. [DOI] [PubMed] [Google Scholar]
- 32.Angstadt JD, Calabrese RL. Calcium currents and graded synaptic transmission between heart interneurons of the leech. J Neurosci. 1991;11:746–759. doi: 10.1523/JNEUROSCI.11-03-00746.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angstadt JD, Calabrese RL. A hyperpolarization-activated inward current in heart interneurons of the medicinal leech. J Neurosci. 1989;9:2846–2857. doi: 10.1523/JNEUROSCI.09-08-02846.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doloc-Mihu A, Calabrese RL. A database of computational models of a half-center oscillator for analyzing how neuronal parameters influence network activity. J Biol Phys. 2011;37:263–283. doi: 10.1007/s10867-011-9215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doloc-Mihu A, Calabrese RL. Identifying crucial parameter correlations maintaining bursting activity. PLoS Comput Biol. 2014;10:e1003678. doi: 10.1371/journal.pcbi.1003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Doloc-Mihu A, Calabrese RL. Analysis of family structures reveals robustness or sensitivity of bursting activity to parameter variations in a half-center oscillator (HCO) model. eNeuro. 2016 doi: 10.1523/ENEURO.0015-16.2016. eN-NWR-0015-16 eN-NWR-0015-16R1. Introduces the family - all members vary only by the one parameter that defines the family – as a means of analyzing robustness and sensitivity in large databases of model instances and applies this method to a database of heart interneuron HCO model instances. It then explores the trade-off between robustness of activity type and desirable change in activity characteristics when intrinsic conductances are altered and identified the hyperpolarization-activated (h) current as an ideal target for modulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorensen M, DeWeerth S, Cymbalyuk G, Calabrese RL. Using a hybrid neural system to reveal regulation of neuronal network activity by an intrinsic current. J Neurosci. 2004;24:5427–5438. doi: 10.1523/JNEUROSCI.4449-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olypher A, Cymbalyuk G, Calabrese RL. Hybrid systems analysis of the control of burst duration by low-voltage-activated calcium current in leech heart interneurons. J Neurophysiol. 2006;96:2857–2867. doi: 10.1152/jn.00582.2006. [DOI] [PubMed] [Google Scholar]
- 39.Tobin AE, Calabrese RL. Myomodulin increases Ih and inhibits the NA/K pump to modulate bursting in leech heart interneurons. J Neurophysiol. 2005;94:3938–3950. doi: 10.1152/jn.00340.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Kueh D, Barnett WH, Cymbalyuk GS, Calabrese RL. Na+/K+ pump interacts with the h-current to control bursting activity in central pattern generator neurons of leeches. eLife. 2016 doi: 10.7554/eLife.19322. 01–07-2016-RA-eLife-19322R1. This paper uses electrophysiology on leech heart interneurons and a computational model of a heartbeat half-center oscillator to explore the role of the Na+/K+ pump current in ongoing rhythmic bursting. Monensin, a Na+/H+ antiporter, was used to stimulate the pump and strophanthidin or K+-free saline was used to inhibit the pump. Stimulating the pump decreased the burst period of these neurons significantly, an effect that was prevented or reversed when the h-current was blocked by Cs+. Burst period also decreased when the pump was inhibited. These results in conjunction with the model indicate that the that the cycle-to-cycle dynamics of Na+/K+ pump current works with Ih to regulate period of the half-center oscillators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masino MA, Calabrese RL. Phase relationships between segmentally organized oscillators in the leech heartbeat pattern generating network. J Neurophysiol. 2002;87:1572–1585. doi: 10.1152/jn.00336.2001. [DOI] [PubMed] [Google Scholar]
- 42.Masino MA, Calabrese RL. Period differences between segmental oscillators produce intersegmental phase differences in the leech heartbeat timing network. J Neurophysiol. 2002;87:1603–1615. doi: 10.1152/jn.00338.2001. [DOI] [PubMed] [Google Scholar]
- 43.Masino MA, Calabrese RL. A functional asymmetry in the Leech Heartbeat Timing Network is revealed by driving the network across various cycle periods. J Neurosci. 2002;22:4418–4427. doi: 10.1523/JNEUROSCI.22-11-04418.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill AA, Masino MA, Calabrese RL. Model of intersegmental coordination in the leech heartbeat neuronal network. J Neurophysiol. 2002;87:1586–1602. doi: 10.1152/jn.00337.2001. [DOI] [PubMed] [Google Scholar]
- 45.Jezzini SH, Hill AA, Kuzyk P, Calabrese RL. Detailed model of intersegmental coordination in the timing network of the leech heartbeat central pattern generator. J Neurophysiol. 2004;91:958–977. doi: 10.1152/jn.00656.2003. [DOI] [PubMed] [Google Scholar]