Abstract
Caveolin-1 is the predominant structural protein of caveolae, a subset of (lipid) membrane rafts that compartmentalize cell signaling. Caveolin-1 binds most G protein-coupled receptors and their signaling partners, thereby enhancing interactions among signaling cascade components and the relative activation of specific G protein-coupled pathways. This study reveals that chronic opioid exposure of μ-opioid receptor (MOR) expressing Chinese Hamster ovary cells (MOR-CHO) and chronic in vivo morphine exposure of rat spinal cord augmented recruitment of multiple components of MOR-adenylyl cyclase (AC) stimulatory signaling by caveolin-1. Strikingly, in MOR-CHO and spinal cord, blocking the caveolin-1 scaffolding domain substantially attenuated the chronic morphine-induced increased interaction of caveolin-1 with MOR, Gsα, protein phosphatase 2A (PP2A) and AC. Chronic morphine treatment also increased interactions among the above signaling proteins, thus enabling sufentanil to stimulate (rather than inhibit) cAMP production within lipid membrane micro-domains. The latter finding underscores the functionality of augmented interactions among MOR, Gsα, PP2A and AC. In the aggregate, our data strongly suggest that augmented caveolin-1 scaffolding undergirds the ability of chronic opioids to recruit an ancillary signaling pathway by acting as an organizing template for MOR-Gsα-AC signaling and delimiting the membrane compartment(s) in which it occurs. Since caveolin-1 binds a wide spectrum of signaling molecules, altered caveolin-1 scaffolding following chronic opioid treatment is likely to pertain to most, if not all, MOR signaling partners. The chronic morphine-induced trigger that augments caveolin-1 scaffolding could represent a seminal perturbation that initiates the wide spectrum of adaptations thought to contribute to opioid tolerance and dependence.
Keywords: mu-opioid receptor, opioid tolerance, opioids, caveolin-1, caveolae
Graphical Abstract
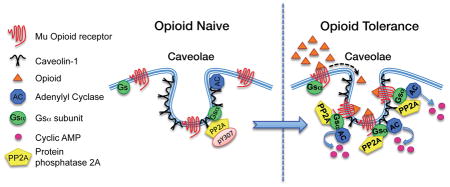
Chronic opioid treatment can lead to opioid tolerance and dependence, which confounds the use of opioids to treat chronic pain. Knowledge about underlying molecular adaptations still need to be increased to develop tolerance-mitigating treatments. Caveolin-1 is the predominant structural protein of caveolae, a subset of (lipid) membrane rafts that compartmentalize cell signaling. Chronic exposure to opioids augments recruitment by Caveolin-1 of mu-opioid receptors and proteins critical to the signaling cascade that activates adenylyl cyclase. This in turn enhances interaction among these signaling proteins, which is causally associated with the chronic morphine-induced shift from inhibitory to stimulatory mu-opioid receptor adenylyl cyclase signaling.
Introduction
We have identified an interrelated cluster of post-opioid receptor adaptations to chronic morphine that qualitatively alters the nature of the mu opioid receptor (MOR) signaling. These adaptations have convergent outcomes: they shift MOR signaling from predominantly inhibitory to stimulatory adenylyl cyclase (AC) signaling. The qualitative change in MOR signaling results from two sets of adaptations, those that augment stimulatory MOR signaling via Gβγ (Chakrabarti and Gintzler, 2003; Chakrabarti et al., 2005b; Chakrabarti et al., 1998; Rivera and Gintzler, 1998) and those that augment functional coupling of MOR to Gs (Chakrabarti et al., 2010; Chakrabarti and Gintzler, 2007; Chakrabarti et al., 2012; Chakrabarti et al., 2005a). The cellular events, their subcellular localization and regulation of these adaptations remain unknown.
One clue to a triggering stimuli for and the cellular loci in which augmented MOR Gs signaling occurs is provided by the finding that Gsα dephosphorylation increases its association with MOR (Chakrabarti and Gintzler, 2007). Since Gsα is a neutral protein (isoelectric point=5.49), its phosphorylation state is inversely related to hydrophobicity i.e., decreasing Gsα phosphorylation would increase its lipid solubility (Polyansky and Zagrovic, 2012). Thus, the inverse relationship between Gsα phosphorylation and its association with MOR suggests that MOR Gs signaling predominantly occurs in lipid-rich membrane compartments, aka lipid rafts. This inference was substantiated by the demonstration that acute activation of MOR stimulates the translocation of both Gsα and AC into lipid-rich (Triton-insoluble) membrane microdomains (Chakrabarti et al., 2010). Notably, lesser-phosphorylated Gsα, which preferentially interacts with MOR, is concentrated in these microdomains.
Caveolae are a subset of membrane (lipid) rafts that have a high concentration of sterol- and sphingolipids. The predominant structural protein of caveolae, caveolin-1 (Cav-1), is not only involved in maintaining the characteristic flask shape of caveolae, but is also a key regulator of cell signaling (as well as many other cellular processes). Caveolin-1 binds a plethora of signaling proteins e.g., heterotrimeric G proteins, G protein-coupled receptors (GPCRs), G protein–regulated effectors, Src family tyrosine kinases [see (Okamoto et al., 1998; Patel et al., 2008) for review]. In this fashion, Cav-1 acts as a scaffolding protein, recruiting and concentrating signaling molecules within caveolae and/or Cav-1 scaffolds (flat oligomerized Cav-1 microdomains at the plasma membrane that do not form caveolae). By enhancing their spatial juxtaposition, caveolin-1 can facilitate interactions among components of signaling cascades and thus influence the relative activation of specific G protein-coupled pathways (Carman et al., 1999; Razani et al., 1999; Schubert et al., 2002; Woodman et al., 2002).
Accordingly, the current study tested the hypothesis that the scaffolding functionality of the Cav-1 present in lipid rafts, orchestrates the amplification of the MOR-Gs-AC stimulatory signaling that emerges after chronic morphine treatment. Results reveal a striking ability of chronic morphine to not only enhance interactions of Cav-1 with multiple interrelated signaling molecules relevant to MOR AC stimulatory signaling but to also increase functional interactions among them within defined lipid-rich membrane domains, thereby facilitating the recruitment of an ancillary MOR signaling pathway. Implications of generalized relevance of altered Cav-1 scaffolding to the myriad of signaling adaptations, in addition to MOR Gs signaling, that have been associated with opioid tolerance and dependence is discussed.
Materials and methods
Cell Culture
Chinese Hamster Ovary (CHO) cells stably transfected with MOR (MOR-CHO) (Chakrabarti et al., 2005a,b) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing high glucose and L-glutamine (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Atlanta, GA), 100 units/ml penicillin/streptomycin and 100 μg/ml G-418 (Geneticin, Mediatech). All experiments were compliant with institutional policy.
Chronic Opioid treatment of cells in culture
Cells were plated (10 X106 cells/150 mm dishes) and grown at 37°C in a humidified atmosphere of 90% air/10% CO2. Two days later, at 90 to 95% confluence, cells were chronically treated with vehicle or morphine, oxycodone, hydrocodone, or sufentanil (1 μM, 48 h). Media used for chronic treatment was replenished every 24 h. Cells were also treated for 5 min with sufentanil (1 μM) to differentiate between acute effects that persisted vs. consequences of chronic exposure.
Chronic administration of morphine
Male rats (250–300 g, Charles River, Kingston, NY) were implanted with a permanent indwelling cannula inserted into the lumbar spinal cord subarachnoid space under sodium pentobarbital anesthesia (40 mg/kg, i.p.; Abbott Laboratories) as described previously and routinely performed in this laboratory (Liu et al., 2011). Five days following cannula implantation, rats were intrathecally injected (via the cannula), once daily for five days, with either morphine (15 μg), or morphine concomitant with the caveolin-1 scaffolding domain competing peptide rendered cell permeable (CSD, 10 μg; Millipore Corporation, Bedford, MA, USA). This regimen of intrathecal morphine, administration is sufficient to produce profound analgesic tolerance at the time of killing (Powell et al., 2000). On day 6, animals were sacrificed by decapitation in strict accordance with institutional guidelines. Lumbar spinal tissue was expelled by injecting ice-cold phosphate buffered saline into the rostral end of the spinal cord and immediately processed. Experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of SUNY Downstate Medical Center.
Membrane Preparation
Cells or spinal tissues were washed thoroughly (twice, 15 ml each) with ice cold phosphate buffered saline (pH 7.3) and homogenized directly in 20 mM HEPES, pH 7.4, containing 10% sucrose, 5 mM EDTA, 1 mM EGTA, 2 mM Dithiothreitol [DTT], protease inhibitors, 1 mM Benzamidine, 0.2 mg/ml Bacitracin, 2 mg/l Aprotinin, 3.2 mg/l each of soybean trypsin inhibitor and Leupeptin, 20 mg/l each of N-tosyl-l-phenylalanine chloromethyl ketone, Na -p-tosyl-lysine chloromethyl ketone and phenylmethylsulfonyl fluoride (PMSF), and complete cocktail inhibitor tablet 1/50 ml. Homogenates were centrifuged at 1,000 x g, 4°C for 10 min. Supernatants obtained from the low speed spin were centrifuged at 105,000 g for 1h at 4°C. Membrane fractions obtained were re-suspended in the same HEPES buffer (pH 7.4) containing protease inhibitors without sucrose. Membranes were either stored at −80°C in aliquots or processed further. Naïve and chronic opioid-treated membranes were thawed once and incubated with 1% Triton x-100 (Triton; 30 min on ice). Sample preparations were centrifuged (105,000g for 30 min at 4°C) to separate the Triton-insoluble pellet from the Triton-soluble supernatant fraction. The pellet was washed again with 20 mM HEPES buffer (pH 7.4) and the Triton-insoluble pellet was solubilized (by agitation, 60 min at 4°C) with a mixture of detergents, 1% Octyl β-D-glucopyranoside, 0.4% Na-deoxycholate and 0.2% Na-dodecyl sulfate, in the same HEPES buffer containing protease inhibitors, 10% glycerol and 150 mM NaCl. After centrifugation (16,000 x g for 15 min at 4°C), clear supernatants were used for Protein Assay (Bradford).
Immunoprecipitation (IP) and Western analyses
For Cav-1 IP, purified mouse monoclonal anti-Cav-1 antibody (BD Biosciences; San Jose, CA; 2μl /100 μg protein) was used together with pre-washed Protein G-agarose (50 μl; Roche Molecular Biologicals, Indianapolis, IN) overnight at 4°C. For Gsα IP, rabbit polyclonal affinity purified anti-Gsα antibody generated against the C terminus of the Gsα subunit (aa 385–394; US Biologicals, Swampscott, MA) was used together with pre-washed Protein A-agarose (50 μl; Roche) overnight at 4°C. The beads were washed in 20 mM HEPES buffer (pH 7.4) containing 1 mM each of DTT and EDTA, 150 mM NaCl, 0.05% Octyl β-D-glucopyranoside and the same protease inhibitors as mentioned above. Immunoprecipitates were eluted by heating samples in 30 μl of sample buffer (15 min at 85°C). Samples separated on 4–12% gradient Bis-Tris gels (Invitrogen, Carlsbad, CA, USA) were electro-transferred onto nitrocellulose membranes and used for Western analyses.
Gsα and Caveolin-1 proteins were visualized using 1:5,000 dilution of a polyclonal rabbit anti-Gsα antibody raised against the C-terminus of Gsα (generously provided by Dr. J. Hildebrandt, Medical University of South Carolina) and a monoclonal mouse anti-caveolin-1 antibody (BD Biosciences, San Jose, CA), respectively. AC was visualized using a 1:1,000 dilution of the monoclonal anti-AC antibody, BBC4 (generated against the carboxyl terminus common to most AC isoforms by Dr. T. Pfeuffer and generously provided by Dr. Mollner, Heinrich Heine University, Düsseldorf, Germany; (Mollner and Pfeuffer, 1988). MOR was visualized using a 1:12,000 dilution of a polyclonal rabbit anti-MOR antibody generated against the C-terminal 50 aa of MOR (Chalecka-Franaszek et al., 2000), (generously provided by Dr. Thomas Cote, Uniformed Services University of the Health Sciences, Bethesda, Maryland). The secondary antibody utilized was either a peroxidase-labeled donkey anti-rabbit or sheep anti-mouse antibody (Amersham/GE Healthcare, Piscataway, NJ).
Antibody-substrate complex was visualized using a Supersignal West Dura Chemiluminescence detection kit (Pierce, Rockford, IL). Specificity of MOR, Gsα, PP2A and AC Western signals had been previously demonstrated (Chakrabarti et al., 2005a; b; Chakrabarti et al., 1998) and was therefore not repeated in the current study. Opioid naïve MOR-CHO (control) were processed, electrophoresed and blotted in parallel with either MOR-CHO treated acutely (5 min with 1 μM morphine or sufentanil), or MOR-CHO treated chronically with morphine, oxycodone, hydrocodone or sufentanil (1 μM; 48h). Similarly, spinal cord membranes obtained from opioid naïve and chronic morphine treated rats were processed, electrophoresed and blotted in parallel. Westerns of control and experimental samples were exposed concomitantly to a GBox (charge-coupled device camera; Syngene, Frederick, MD). Intensity of signal was quantified using Syngene software.
Lipid Raft Isolation by Sucrose Density Gradient Centrifugation
Low-density membrane raft fractions were isolated as described previously (Bourova et al., 2003) using sucrose density gradient centrifugation. MOR-CHO cells were washed in ice-cold phosphate-buffered saline (pH 7.3), harvested and homogenized in 50 mM Tris buffer (pH 7.4) containing 3 mM MgCl2, 1 mM EDTA, 1 mM PMSF and complete cocktail protease inhibitor tablet (1/50ml). Exactly 2 mL of the resulting homogenate was mixed with 2 mL of ice-cold 80% w/v sucrose in 50 mM Tris buffer (pH 7.4), transferred into a centrifuge tube for a Beckman ultracentrifuge SW 41 rotor and overlaid very slowly with 35%, 30%, 25%, 20% 15%, 10% (1 mL each) and 5% w/v (1.5 mL) sucrose in 50 mM Tris buffer (pH 7.4). Great care was devoted to prevent mixing of the layers. Following centrifugation at 188,000 g for 20 h, density gradient fractions (first 0.5 ml followed by eleven aliquots of 1 ml fractions) were collected from the meniscus using an Auto Densi-Flow density gradient fractionator (Labconco, Kansas city, MO). Aliquots were snap frozen and stored at −80°C until further use.
Adenylyl Cyclase Assay
AC activity was determined from low density membrane fractions (combination of 3,4,5 and 6 fractions) and was measured by the synthesis of [32P]cAMP from [α-32P]ATP (PerkinElmer, Waltham, MA,USA). Assays were initiated by the addition of 100 μl of reaction mixture to 100 μl of membrane fractions (5 μg protein). The assay mix contained: 50 mM HEPES buffer, pH 8.0, containing 10 mM MgCl2, 20 mM creatine phosphate, 10 units/sample creatine phosphokinase, 0.1 μM ATP, 10 μM GTP, 20 mM NaCl, 1 mM DTT, 1 mM EGTA, 0.125 μM rolipram (cAMP specific phosphodiesterase inhibitor), 0.1% bovine serum albumin, and 1 μCi [α-32P]ATP. Assays were run in the absence or presence of the opioid agonist sufentanil (1 μM). Reactions (30°C, 15 min) were terminated on ice and by the addition of 20 μl of 2.2 N HCl. Samples were then incubated at 95°C for exactly 4 min. [32P]cAMP generated in samples was then separated by neutral alumina column chromatography as described previously (Alvarez and Daniels, 1990). [3H]cAMP (0.005 uCi), added to each sample before fractionation, was used as an internal standard to correct for column recovery of the [32P]cAMP. Radioisotopes were quantified using liquid scintillation spectroscopy (Beckman LS 6500, Beckman, CA).
Statistical Analysis
One-way ANOVA was used to assess significance of differences of effects of chronic exposure to different opioids on the co-IP of targeted signaling proteins with Cav-1. ANOVA was also used to assess significance of effects of blocking the Cav-1 scaffolding domain on the chronic morphine-induced increase in Cav-1 co-IPs. Student’s t test was used to analyze differences in the co-IP of specific signaling proteins between opioid naïve and chronic opioid treated groups.
Results
Chronic opioids increase the association of MOR and Gsα with Cav-1
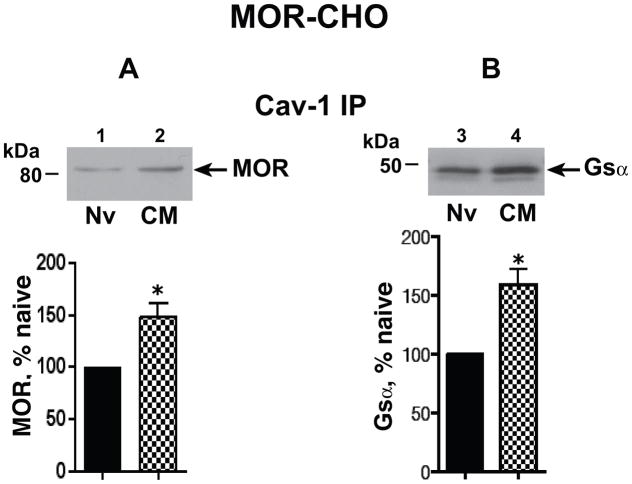
Since Cav-1 that is present in the Triton- insoluble membrane fraction is associated with caveolae/Cav-1 scaffolds, we used Triton insolubility as a partial purification step. We previously reported that both MOR (predominantly ≈80 kDa) and Gsα (predominantly ≈45 kDa) co-immunoprecipitated (co-IPed) with Cav-1. In this study, we now show that chronic morphine significantly increased the association of Cav-1 with both signaling proteins: MOR: 44.4±8.9%, n=4; Gsα: 58.6±13%, n=5; P<0.05 and p<0.01, respectively (Fig. 1). Notably, the predominant molecular mass of the MOR species (≈80 kDa) whose association with Cav-1 increased following chronic morphine is also the predominant MOR whose association with Gsα increases after analogous treatment with morphine (Chakrabarti et al., 2005a).
Figure 1.
Chronic morphine increased the association of MOR and Gsα with Cav-1. Membranes were obtained from opioid naïve (Nv) and chronic morphine (CM) treated (1 μM, 48 h) MOR-CHO. The Triton insoluble fraction was isolated, solubilized, and subjected to immunoprecipitation with anti-Cav-1 antibody as described in methods. Following SDS PAGE and electrotransfer of proteins, regions of the nitrocellulose membrane corresponding to MOR, Gsα, and Cav-1 were cut out and Western blotted with their corresponding antibodies. Quantification of Western signals corresponding to A: MOR (≈80 kDa) and B, Gsα (≈45 kDa) in Cav-1 ip was normalized to their corresponding directly IPed Cav-1 protein. The content of MOR or Gsα in the Cav-1 IP obtained from chronic morphine-treated MOR-CHO is expressed as a percent of their content in Cav-1 IP obtained from opioid naïve cells. Following chronic morphine, the association of MOR and Gsα with Cav-1 within caveolae/Cav-1 scaffolds is significantly increased. n=4–5; *indicates p<0.05.
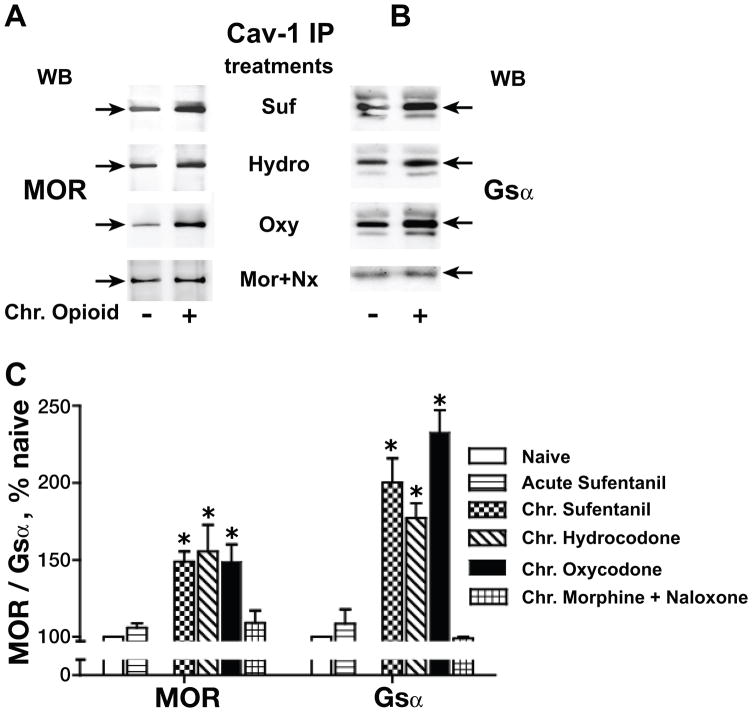
In order to ascertain if the ability of chronic morphine to augment Cav-1 recruitment of MOR and Gsα generalized to other clinically relevant opioids, we also determined the effect of chronic sufentanil, hydrocodone and oxycodone (1 μM each; 48h) on the interaction of Cav-1 with MOR and Gsα. Analysis of variance (one-way) revealed a significant difference of treatment effects among naïve and chronic opioid groups for MOR (F3,19 =22.78; p<0.0001) and for Gsα (F3,21 =55.68; p<0.0001). Fig. 2 illustrates that similar to the effect of chronic morphine treatment, chronic treatment with sufentanil, hydrocodone and oxycodone also augmented interactions of MOR (49±6.5%, 55±17.4%, 49±11%) and Gsα (100±15.7%, 77±9.6% 132±14.4%), (n=3–5; P<0.001 for all groups), respectively with Cav-1. This underscores that augmentation of Cav-1 scaffolding following chronic opioid treatment is not idiosyncratic to a particular MOR agonist.
Fig. 2.
Effects of chronic morphine on Cav-1 scaffolding generalize to sufentanil (SUF), hydrocodone (HYDRO) and oxycodone (OXY). The Triton insoluble membrane fraction was obtained from opioid naïve, acute (5 min) sufentanil-treated and chronic sufentanil-, hydrocodone- or oxycodone-treated (1 μM, 48 h) MOR-CHO, after which it was solubilized and subjected to immunoprecipitation with anti-Cav-1 antibody. MOR (A) and Gsα (B) were visualized and quantified as described for Fig. 1. All co-IPs were normalized to their corresponding directly IPed Cav-1 protein. The content of MOR or Gsα in the Cav-1 IP from opioid treated MOR-CHO is expressed as a percent of their content in Cav-1 IP obtained from opioid naïve cells (C). n=3–5; *indicates p<0.05. The ability of chronic morphine to augment Cav-1 scaffolding of MOR and Gsα generalized to sufentanil, oxycodone, and hydrocodone. Chronic, not acute, opioid exposure is a prerequisite for the observed augmented Cav-1 scaffolding, which is eliminated by concomitant exposure to naloxone (Nx).
Importantly, chronic treatment with morphine (1 μM) while in the presence (initiated 2 h prior to chronic morphine exposure) of the caveolin-1 scaffolding domain peptide [CSD, 5 μM; Millipore (aa 82–101 of Cav-1, rendered cell permeable)] (Bucci et al., 2000; Couet et al., 1997; Gratton et al., 2003; Sukumaran et al., 2002) failed to augment Cav-1 recruitment of MOR and Gsα (p<0.05 for both, n=3). In contrast, the scrambled CSD peptide (negative control) was without effect, i.e., in the presence of the CSD negative control peptide, the chronic morphine-induced increment in the co-IP of MOR and Gsα with Cav-1 persisted unabated. Additionally, the opioid antagonist naloxone (10 μM, added 30 min prior to and continued throughout chronic morphine treatment) blocked the increased recruitment of MOR (9±11%; p>0.05) and Gsα; (−2±1.4; p>0.05) by Cav-1 (Fig. 2). Furthermore, an acute (5 min) exposure to either morphine (data not shown) or sufentanil (1 μM for both) failed to augment Cav-1-MOR/Gsα interactions (Fig. 2C). Collectively, these data indicate that increased co-IP of MOR and Gsα with Cav-1 resulted from the augmented Cav-1 scaffolding, which was elicited by sustained activation of MOR, and not its acute stimulation.
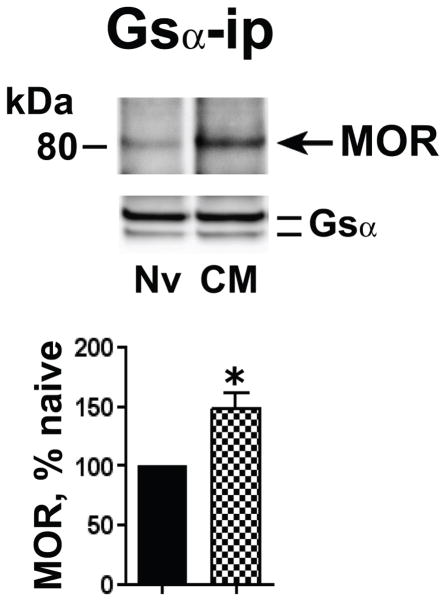
Chronic morphine augments the association of MOR with Gsα
It is possible that MOR and Gsα proteins each interact with different molecules of Cav-1. Thus, concomitant augmentation of Cav-1 with MOR and Gsα suggests, but does not necessarily indicate the augmented interaction between these signaling molecules. In order to investigate chronic morphine-induced augmented MOR association with Gsα, a prerequisite for increased MOR Gs signaling, we compared the magnitude of MOR that co-IPed with Gsα from the Triton-insoluble membrane fraction obtained from opioid naïve and chronic morphine-treated MOR-CHO. Fig. 3 illustrates that MOR and Gsα co-IP from the Triton-insoluble membrane fraction, as we previously reported using membranes from opioid naïve MOR-CHO (Chakrabarti et al., 2010). Notably, however, chronic morphine significantly elevated the presence of MOR in the Gsα IP (48±13%, n=4; p<0.01), suggesting that chronic morphine increased MOR Gs signaling inside lipid raft compartments.
Figure 3.
Chronic morphine augmented the association of MOR with Gsα. The Triton insoluble fraction obtained from membranes of opioid naïve (Nv) and chronic morphine (CM) treated MOR-CHO (1 μM, 48 h) was solubilized and subjected to immunoprecipitation using anti-Gsα antibodies. Co-IPed MOR was visualized by Western blotting. Quantification of band intensity was normalized to their corresponding directly IPed Gsα protein. The content of MOR in the Gsα IP obtained from chronic morphine-treated MOR-CHO is expressed as a percent of the MOR content of Gsα IP obtained from opioid naïve cells. Chronic morphine increased the association of MOR and Gsα within lipid raft compartments. n=4; *=p<0.01.
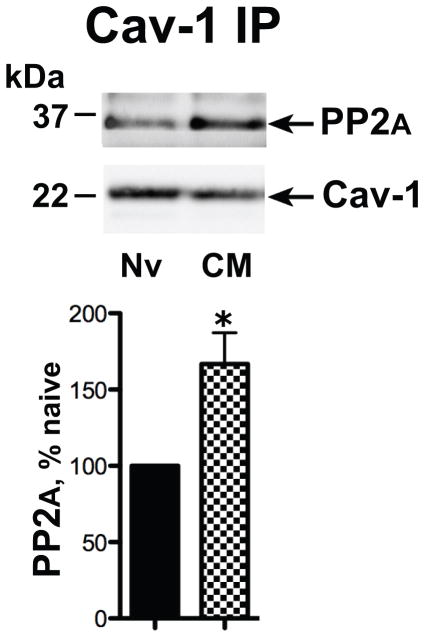
Chronic morphine augments the association of Cav-1 and PP2A
We previously reported that chronic morphine enhances dephosphorylation of Gsα by PP2A, thereby increasing the association of Gsα with MOR (Chakrabarti and Gintzler, 2007). Therefore, we hypothesized that chronic morphine would augment Cav-1-associated PP2A, in lipid rafts as a prelude to the observed increased MOR Gsα association. As an initial test of this hypothesis, we investigated if chronic morphine treatment increased the association of PP2A with Cav-1. In these experiments, we compared the magnitude of PP2A that co-IPed with Cav-1 from membrane fractions of opioid naïve and chronic morphine-treated MOR-CHO. Chronic morphine substantially increased PP2A association with Cav-1 (67±21%, n=3, p<0.05; Fig. 4).
Figure 4.
Chronic morphine augments the association of Cav-1 with PP2A. The Triton insoluble fraction obtained from membranes of opioid naïve (Nv) and chronic morphine (CM) treated MOR-CHO (1 μM, 48 h) was solubilized and subjected to immunoprecipitation using anti-Cav-1 antibodies. Co-IPed PP2A was visualized by Western blotting. Quantification of band intensity was normalized to their corresponding directly IPed Cav-1 protein. The content of PP2A in the Gsα IP obtained form chronic morphine-treated MOR-CHO is expressed as a percent of the Cav-1 content of Gsα IP obtained from opioid naïve cells. Chronic morphine increased association of PP2A and Cav-1 within caveolae/Cav-1 scaffolds. n=3; *=p<0.05.
Chronic morphine augments the association of activated PP2A with Gsα
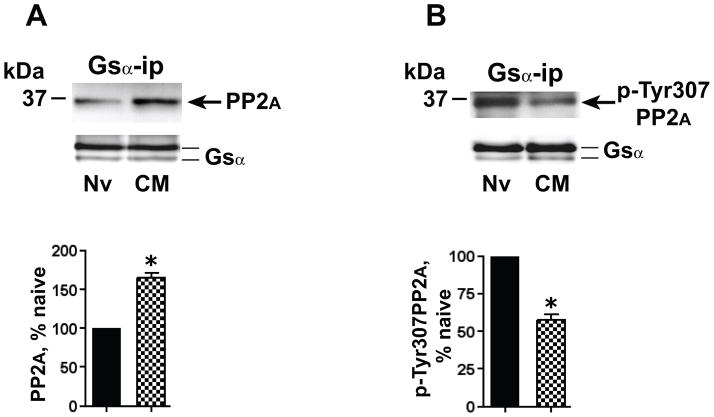
As was noted for MOR and Gsα, the concomitant augmentation of the association of both Gsα and PP2A with Cav-1 following chronic morphine, does not unambiguously establish the increased interaction between PP2A and Gsα. Accordingly, we directly assessed the interaction of PP2A with Gsα by quantifying the presence of PP2A in Gsα IP obtained from membrane fractions of opioid naïve vs. chronic morphine-treated MOR-CHO. Following chronic morphine, the magnitude of PP2A that co-IPed with Gsα from the Triton-insoluble membrane fraction increased by 65±6.6%, (p<0.05, n=5; Fig. 5A).
Figure 5.
Chronic morphine augments the association of activated (dephosphorylated) PP2A with Gsα. The Triton insoluble fraction of membranes of opioid naïve (Nv) and chronic morphine (CM) treated (1 μM, 48 h) MOR-CHO was solubilized and subjected to immunoprecipitation using anti-Gsα antibodies. A: Co-IPed PP2A was visualized by Western blotting using a pan anti-PP2A antibody. B: Gsα IP was obtained from an amount of Triton insoluble membrane protein identical to that used in panel A. The IP was Western blotted using an antibody specific for p-Tyr307PP2A. In both panels, quantification of band intensity was normalized by the amount of their corresponding directly IPed Gsα protein. The content of PP2A and p-Tyr307PP2A in the Gsα IP obtained from chronic morphine-treated MOR-CHO is expressed as a percent of their content in Gsα IP obtained from opioid naïve cells. Whereas the co-IP of PP2A with Gsα was augmented (≈65%) by chronic morphine, this treatment decreased by a similar magnitude (≈42%) the co-IP of p-Tyr307PP2A. In other words, the chronic morphine-induced increment in association of PP2A activity with Gsα is considerably greater than the increment in PP2A protein. n=5 for PP2A and n=3 for p-Tyr307PP2A; *=p<0.05.
The activity of PP2A is regulated via its Tyr307 phosphorylation (pTyr307PP2A), which inhibits its phosphatase activity (Brautigan, 1995), i.e., dephosphorylated Tyr307PP2A represents the activated form of PP2A. In order to assess whether chronic morphine augments the association of Gsα with pTyr307PP2A (less activated) or dephosphorylated (more activated) PP2A, we determined the effect of chronic morphine on the phosphorylation state of the PP2A that co-IPed with Gsα. These experiments utilized an anti-pTyr307PP2A-specific antibody. Strikingly, following chronic morphine treatment, the co-IPed PP2A contained significantly less pTyr307PP2A immunoreactivity (42.3±4%; p<0.02, n=3; Fig. 5B). Thus, the observed ≈65% increment in total PP2A that co-IPs with Gsα following chronic morphine reflects an underestimate of the actual increase in PP2A activity that is associated with Gsα.
Chronic morphine increases the association of Gsα with AC
Adenylyl cyclase is a major target for signaling via Gsα. In order to assess whether AC was the transducer for augmented MOR Gsα coupling within lipid raft compartments following chronic morphine, we compared Gsα-AC association within Triton-insoluble membrane domains before and following chronic morphine. This was assessed by quantifying the content of AC in Gsα IP obtained from the Triton-insoluble membrane fraction of opioid naïve vs. chronic morphine-treated MOR-CHO. Following chronic morphine, the AC that co-IPed with Gsα increased by 75±8%, p<0.05, n=3; Fig. 6A).
Figure 6.
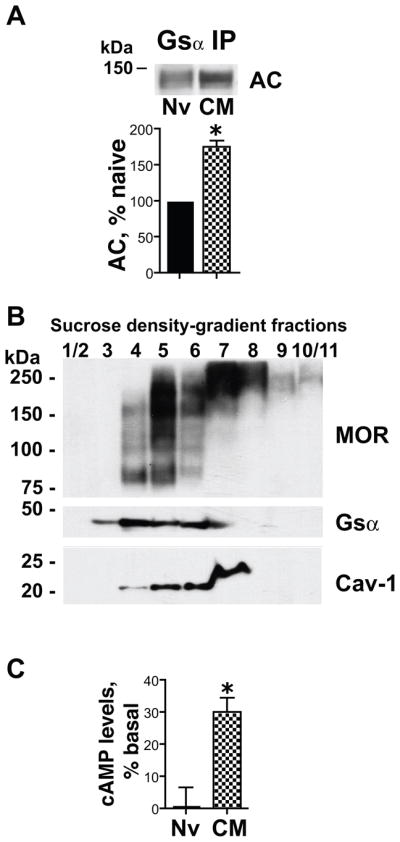
Chronic morphine increases Gsα adenylyl cyclase (AC) association and augments MOR stimulation of cAMP formation in caveolae/Cav-1 scaffold fractions. A: The Triton insoluble fraction of membranes of opioid naïve (Nv) and chronic morphine (CM) treated MOR-CHO (1 μM, 48 h) was solubilized and subjected to immunoprecipitation using anti-Gsα antibodies. Co-IPed AC was visualized by Western blotting using an anti-AC common monoclonal antibody. Co-IPed AC was normalized to its corresponding directly IPed Gsα protein. The content of AC in the Gsα IP obtained from chronic morphine-treated MOR-CHO is expressed as a percent of its content in Gsα IP obtained from opioid naïve cells. Chronic morphine increased by ≈75% the magnitude of the co-IP of AC with Gsα within lipid raft compartments. n=3; *=p<0.05. B: Triton-insoluble components of MOR-CHO membranes were fractionated using sucrose density gradient centrifugation as described in Methods. The presence of MOR, Gsα and Cav-1 across the sucrose gradient was determined by Western blotting. The majority of Cav-1 was contained within the 20% and 30% sucrose gradients (corresponding to fractions 3–6). C: AC activity was quantified by measuring the synthesis of [32P]cAMP from [α-32P]ATP) in membrane raft fractions contained in the 20% and 30% sucrose gradients, corresponding to fractions 3–6 (fraction 3 was included since, in addition to Gsα, longer exposure of Westerns revealed the presence of MOR and Cav1, albeit at lower levels than the other included fractions obtained from opioid naïve and chronic morphine-treated MOR-CHO). Sufentanil (1 μM) stimulated [32P]cAMP formation in membrane raft fractions obtained from chronic morphine-treated (30±4%, *=p<0.002, n=5) but not opioid naïve MOR-CHO. Findings indicate that chronic morphine induced the emergence of MOR-coupled stimulatory AC signaling within membrane (lipid) rafts.
Chronic morphine augments MOR stimulation of cAMP formation in caveolae/Cav-1 scaffold fraction
In order to determine whether or not the chronic morphine-induced augmented association of MOR/Gsα/AC was productive, we compared the ability of sufentanil to stimulate cAMP formation in the caveolae membrane fraction obtained from opioid naïve and chronic morphine-treated MOR-CHO. Since the dependent variable in these experiments was AC enzymatic activity, not AC immunoreactivity, caveolae were obtained using sucrose density gradient centrifugation (Bourova et al., 2003) in lieu of Triton insolubility, which would denature AC and thus diminish its activity.
Fig. 6B illustrates the distribution of MOR, Gsα and Cav-1 across the sucrose gradient. Most of the Cav-1 was present between 20% and 30% sucrose gradients (Fig. 6B), in agreement with previous findings (Bourova et al., 2003). These sucrose density fractions also contained MOR and Gsα immunoreactivity and were therefore used to validate the predicted emergence of sufentanil-stimulation of cAMP formation. The Cav-1 present in fraction 7 appears to have a slightly higher molecular mass (≈5 kDa) than that present in the earlier sucrose density fractions and the Cav-1 visualized in Western blots. Most likely, the larger molecular mass Cav-1 is an artifact and does not reflect either another caveolin isoform or a dimeric form of Cav-1. First, Cav-3 and Cav-2 are actually smaller than Cav-1. Second, although caveolins do form homo- and heterodimers, the molecular mass differential between the smaller and the slightly larger Cav-1 (≈5 kDa) is too small for it to be a dimeric form of Cav. Irrespective of the explanation for the ≈25 kDa Cav-1 in fractions 7, it does not confound data interpretation since the AC activity assay was performed using fractions 3–6, which contained Cav-1 of the predicted molecular mass.
AC activity was determined from caveolae/raft fractions isolated from MOR-CHO cells with and without chronic morphine treatments (Fig. 6C). As expected, sufentanil (1 μM) failed to stimulate cAMP formation in the caveolae/raft fraction obtained from opioid naïve MOR-CHO. Strikingly, however, in analogous fractions obtained from membranes of chronic morphine-treated MOR-CHO, the same concentration of sufentanil stimulated cAMP formation (30±4%, p<0.002, n=5; Fig 6C). These findings indicate that chronic morphine induces the emergence of MOR-coupled facilitatory AC signaling activity within caveolae.
Chronic morphine augments association of Gsα, PP2A and AC with Cav-1 in rat spinal cord
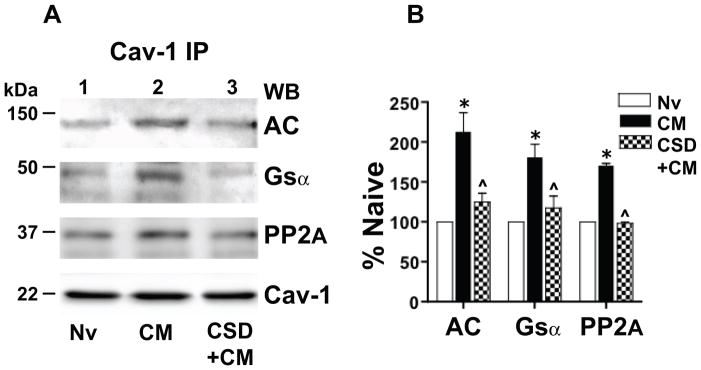
Validation of the inferred physiological relevance of current findings requires demonstration that chronic morphine induces altered Cav-1 scaffolding in the central nervous system as well as MOR-CHO. Accordingly, we tested the ability of chronic systemic morphine to increase Cav-1 recruitment of Gsα, PP2A and AC in rat spinal cord. One-way analysis of variance revealed a highly significant difference of treatment effects among naïve and chronic morphine treated groups (F3,17 =34.57; p<0.0001). A regimen of chronic morphine that produces spinal analgesic tolerance increased the association of AC (113±24%; p<0.001), Gsα (68±17%; p<0.001) and PP2A (70±2.2%; p<0.001) with Cav-1 present in Triton-insoluble membrane compartments of spinal tissue (n=3; Fig. 7A, lanes 2 vs. 1). These data indicate that increased Cav-1 scaffolding following persistent activation of MOR is not a consequence of MOR overexpression per se and furthermore is also manifest by complex integrated neuronal tissue following sustained in vivo exposure to morphine.
Figure 7.
Chronic morphine augments association of Gsα, protein phosphatase 2A (PP2A) and adenylyl cyclase (AC) with Cav-1 in rat spinal cord. Their association is substantially attenuated/eliminated by blocking the Cav-1 scaffolding domain. A: The Triton insoluble fraction obtained was obtained from lumbar spinal cord membranes of opioid naïve rats (Nv, Lane 1), i.t. chronic morphine (CM)-treated rats (lane 2), or rats treated chronically with i.t. morphine concomitant with 10 μg of the Cav-1 scaffolding domain peptide CSD (CSD+CM) (lane 3; see Methods). The Triton insoluble fraction was solubilized and subjected immunoprecipitation using anti-Cav-1 antibodies. Following SDS PAGE and electrotransfer of proteins, regions of the nitrocellulose membrane corresponding to AC, Gsα, PP2A and Cav-1 were cut out and Western blotted with their corresponding antibodies. Quantification of band intensity was normalized to the amount of their corresponding directly IPed Cav-1 protein. B: The content of Gsα, PP2A and AC in the Cav-1 IP obtained from spinal cord of the above treated rats is expressed as a percent of their content in Cav-1 IP obtained from spinal cord of opioid naïve rats. A regimen of chronic in vivo morphine that produces spinal analgesic tolerance increased the association of Gsα, PP2A and AC with Cav-1 within caveolae/Cav-1 scaffolds of rat spinal cord. This increment was essentially eliminated by concomitant treatment with CSD. n=3; *=p<0.001 for CM vs. Nv; ^= p<0.01 for CM+CSD vs. CM.
Blockade of the Cav-1 scaffolding domain eliminates the chronic morphine-induced increased association of Cav-1 with Gsα, PP2A and AC in spinal cord
We determined the effect of blocking the Cav-1 scaffolding domain on the chronic morphine-induced increased association of Gsα, PP2A and AC with Cav-1 to validate our inference from MOR-CHO that it resulted from augmented Cav-1 scaffolding. In these experiments, the Cav-1 scaffolding domain-blocking peptide CSD was applied intrathecally, once daily, 45 min prior to i.t. morphine, for five days. One-way ANOVA revealed a highly significant difference of treatment effects between the chronic morphine and CSD+chronic morphine treated groups (F3,17 =11.92; p<0.0004). As was observed in MOR-CHO, CSD (10 μg i.t.) essentially eliminated the chronic morphine-induced increment in the co-IP of Gsα, PP2A and AC with Cav-1 (Fig. 7A, lane 3 versus lane 2; p<0.01 for all comparisons; lane 3 vs. lane 1 was not significantly different for all co-IPed proteins). This underscores the importance of Cav-1 scaffolding to the observed effects of chronic morphine on Cav-1 interactions with targeted signaling proteins.
Discussion
Cav-1 has long been recognized to bind a wide spectrum of signaling molecules (see (Okamoto et al., 1998; Patel et al., 2008) for review). This ability enables Cav-1 to recruit many classes of signaling molecules and thereby facilitate their incorporation into signaling complexes. Despite the well-established ability of Cav-1 to spatially juxtapose signaling molecules, thereby facilitating their interactions and maximizing signal transduction efficiency, the role of Cav-1 in the development of chronic opioid-induced sequelae remains largely unknown.
The ability of chronic opioid exposure to alter Cav-1 recruitment of signaling molecules was investigated in the present study with respect to the ability of chronic morphine to augment stimulatory MOR Gs AC signaling (Chakrabarti et al., 2010; Chakrabarti and Gintzler, 2007; Chakrabarti et al., 2012; Chakrabarti et al., 2005a) within caveolae/Cav-1 scaffolds. In order to define the principal structural component of this lipid microdomain responsible for undergirding the tolerant-associated emergent MOR-Gsα-AC stimulatory signaling, we studied their association with Cav-1, the predominant structural component of caveolae. Specifically, we tested the hypothesis that chronic opioids would stimulate recruitment by Cav-1 of MOR, PP2A, Gsα and AC and thereby enhance their interactions in membrane microdomains.
Present findings demonstrate that chronic morphine: (1) enhanced Cav-1 recruitment of Triton-insoluble MOR, Gsα, activated PP2A and AC in MOR-CHO, as well as rat spinal cord, which was substantially attenuated by blocking the Cav-1 scaffolding domain. (2) augmented interactions among these signaling proteins in caveolae/Cav-1 scaffolds; (3) enabled the ability of sufentanil to stimulate cAMP production in lipid-rich membrane rafts. These findings underscore that the observed augmented interactions among MOR, Gsα, activated PP2A and AC are functional. Although our data also strongly suggest that augmented Cav-1 scaffolding undergirds the ability of chronic morphine to recruit an ancillary signaling pathway, we cannot eliminate the possibility that Cav-1 may be interacting with another protein that in turn binds to MOR and/or other related signaling molecules.
Several studies have reported that cholesterol depletion (via methyl-β-cyclodextrin) attenuates specific sequela of sustained MOR exposure (Levitt et al., 2009; Qiu et al., 2011). By suggesting the importance of lipid rafts/caveolae to chronic opioid-induced sequelae, these earlier studies are consonant with current findings, although they do not unambiguously indicate the importance of Cav-1 per se [see (Oner et al., 2010), which suggests that methyl-β-cyclodextrin may interfere with receptor signaling independent of its caveola-disrupting property]. To our knowledge, this report is the first demonstration that chronic exposure to opioids enhances recruitment by Cav-1 of multiple interrelated signaling molecules, thereby facilitating interactions among them and thus the recruitment of an ancillary MOR signaling pathway.
Several observations indicate the likely relevance of altered Cav-1 scaffolding to adaptations causally associated with opioid tolerance/dependence. (1) The ability of chronic oxycodone, hydrocodone and sufentanil to also augment Cav-1 recruitment of MOR and Gsα indicates that altered Cav-1 scaffolding is a common adaptation to chronic opioid exposure. (2) Naloxone blocked the chronic morphine-induced augmentation of Cav-1 scaffolding indicating that it was MOR-mediated. (3) Acute treatment of MOR-CHO was not sufficient to augment Cav-1 scaffolding, underscoring the prerequisite for chronic exposure. In contrast to the latter finding, we previously reported using spinal cord as well as MOR-CHO, that acute sufentanil produces a naloxone-reversible enhancement of the co-IP of Gsα and AC with Cav-1 (Chakrabarti et al., 2010). This discrepancy likely resulted from the temporal variation in sufentanil action in membranes of MOR-CHO or spinal cord (earlier report) vs. intact cells (current study), given the importance of cellular compartmentalization of signaling proteins and their subcellular relocation (which would be disrupted in a membrane preparation) to altered patterns of signaling generated by surface receptors (Kholodenko, 2006).
The present demonstration that chronic in vitro and in vivo opioid treatment of MOR-CHO and spinal cord, respectively, increases the recruitment (≥ 50%) by Cav-1 of components of stimulatory MOR AC signaling suggests but does not unequivocally establish that Cav-1 facilitates interactions among them. In order to directly test this inference, we compared interactions among MOR, Gsα, PP2A and AC before and following chronic morphine, by quantifying their co-IP from MOR-CHO. Co-IP analyses revealed that chronic morphine increased the association of MOR, PP2A and AC with Gsα. The current demonstration that sufentanil stimulates cAMP formation in the same membrane fraction obtained from chronic morphine-treated, but not opioid naïve MOR-CHO, underscores that the increment in co-IP of MOR and AC with Gsα reflects their increased functional interactions.
The stoichiometry of Gsα phosphorylation is inversely proportional to its interaction with MOR; dephosphorylation of Gsα augments association with MOR (Chakrabarti and Gintzler, 2007). Although earlier reports (Chakrabarti and Gintzler, 2007) revealed that chronic morphine reduced phosphorylation of Gsα, via its increased association with PP2A, these earlier studies did not define the subcellular localization of these events and the role of Cav-1 scaffolding.
Chronic morphine not only increased the association of Cav-1 with Gsα, but also increased interactions between Cav-1 and PP2A. Moreover, the increment in PP2A association with Cav-1 was comprised of an increased proportion of activated (dephosphorylated) PP2A. Thus, the ≈65% increment in co-IP of PP2A with Cav-1 is an underestimate of the Cav-1-associated increment in PP2A activity. These findings strongly suggest that the chronic morphine-induced dephosphorylation of Gsα occurs within caveolae/Cav-1 scaffold membrane domains and that the Cav-1 contained therein orchestrates Gsα dephosphorylation, thereby facilitating MOR Gs coupling.
Multiple studies demonstrate that Gα subunits translocate to caveolae (Li et al., 1995; Murthy and Makhlouf, 2000), but the functional significance of this redistribution and association with Cav-1 remains controversial. One school of thought is that Cav-1 facilitates signal transduction by acting as a scaffolding molecule to organize signaling complexes within caveolae, where they are brought into proximity with downstream effectors (Bhatnagar et al., 2004). In this scenario, by spatially juxtaposing signaling molecules and effectors, Cav-1 would have a permissive effect on MOR Gs signaling. In striking contrast to this formulation, there is evidence that Cav-1 binding maintains Gsα proteins in an inactive GDP-bound state (Allen et al., 2009; Couet et al., 1997; Li et al., 1996; Li et al., 1995), thereby dampening GPCR signaling, i.e., translocation of signaling molecules into caveolae results in the termination of GPCR signaling, not their facilitation. This controversy underscores the complexity of interactions between G protein-coupled receptors/components of their signaling cascades and constituents of caveolae.
In the present study, the distinction between facilitating vs. terminating MOR-Gs-AC signaling by Cav-1 was investigated by determining the ability of the MOR agonist sufentanil to stimulate cAMP formation in the light membrane caveolae/lipid raft fraction obtained from opioid naïve vs. chronic morphine treated MOR-CHO. The ability of sufentanil to stimulate cAMP formation in the latter, but not the former, unambiguously indicates that lipid-rich membrane domains from opioid tolerant/dependent MOR-CHO provide a subcellular milieu supportive of MOR Gs AC stimulatory signaling. Since chronic morphine exposure increases the association of MOR, Gsα, activated PP2A and AC with Cav-1, it is likely that Cav-1 provides a supportive scaffolding matrix for the MOR Gs-coupled AC signaling that emerges following chronic treatment with opioids, an inference bolstered by (1) our finding that the chronic morphine-induced increment in Gsα that binds to Cav-1 contains increased levels of lesser-phosphorylated Gsα (Chakrabarti et al., 2010), which preferentially binds to MOR (Chakrabarti and Gintzler, 2007) and (2) down stream effectors of MOR Gsα AC signaling, e.g., protein kinase A catalytic and regulatory subunits (Vang et al., 2001) and A-kinase-anchoring proteins (Tasken and Aandahl, 2004), are concentrated in membrane rafts.
While Cav-1 is essential for the formation of caveolae, Cav-1 also forms (Triton-insoluble) scaffolds that are structurally and functionally distinct from caveolae (Lajoie et al., 2007; Zheng et al., 2011). This could be particularly relevant to the ability of Cav-1 to regulate signaling in cells lacking caveolae, such as neurons (Boscher and Nabi, 2012). Differential expression of caveolae-associated Cav-1 vs. Cav-1 scaffolds could be responsible for opposing regulation of signaling cascades that has been observed. Therefore, while the current study demonstrates the importance of Cav-1 to opioid tolerance-associated augmented MOR Gs AC signaling, the relative involvement of Cav-1 that is outside of (e.g., associated with Cav-1 scaffolds) vs. integral to caveolae remains to be defined.
The relative participation of caveolae-associated Cav-1 vs. Cav-1 scaffolds could be signaling pathway specific, which would explain, in part, the observation that regulatory consequences of Cav-1 are dependent on associated signaling partners, cellular context, and specific signaling pathways, e.g., Cav-1 suppresses the epidermal growth factor activation of ERK via the Grb2-Sos-Ras pathway (Park et al., 2000) whereas ERK activation of PI3 kinase pathway is facilitated by Cav-1 (Park and Han, 2009).
In summary, the opioid tolerance-associated shift in MOR-G protein-AC signaling from predominantly inhibitory to stimulatory (which would negate opioid perturbations believed to be prerequisite for opioid antinociception and thus result in opioid analgesic tolerance) is undergirded by increased interactions of Gsα with MOR, activated PP2A and AC within lipid-rich membrane microdomains (e.g., caveolae, Cav-1 scaffolds, etc). The augmented association of each of these signaling molecules with Cav-1 following chronic morphine suggests that this scaffolding protein recruits and orchestrates the enhancement of protein-protein interactions that underlie enhanced MOR-Gs-AC signaling following chronic morphine.
The chronic morphine-induced trigger that initiates the altered Cav-1 recruitment of MOR, Gsα, and related signaling partners remains unknown. Also, unknown is whether or not the ability of chronic morphine to alter Cav-1 scaffolding properties generalizes to other signaling partners of MOR. Knowledge of these considerations could help identify the seminal perturbation(s) produced by chronic opioids that result in the plethora of adaptations to chronic opioids that have thus far been elucidated.
Acknowledgments
Supported by NIDA grant DA027663 (to ARG).
All experiments were conducted in compliance with the ARRIVE guidelines.
Abbreviations
- MOR
Mu-opioid receptor
- AC
adenylyl cyclase
- Cav-1
Caveolin-1
- MOR-CHO
Chinese hamster ovary cells stably transfected with mu-opioid receptor
- PP2A
protein phosphatase 2A
- pTyr307PP2A
phosphotyrosine307 protein phosphatase
- co-IP
co-immunoprecipitate
Footnotes
conflict of interest discloser
The authors declare that they have no competing interests.
Conflicts of interest: none
References
- Allen JA, Yu JZ, Dave RH, Bhatnagar A, Roth BL, Rasenick MM. Caveolin-1 and lipid microdomains regulate Gs trafficking and attenuate Gs/adenylyl cyclase signaling. Mol Pharmacol. 2009;76:1082–1093. doi: 10.1124/mol.109.060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez R, Daniels DV. A single column method for the assay of adenylate cyclase. Anal Biochem. 1990;187:98–103. doi: 10.1016/0003-2697(90)90423-7. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J Biol Chem. 2004;279:34614–34623. doi: 10.1074/jbc.M404673200. [DOI] [PubMed] [Google Scholar]
- Boscher C, Nabi IR. Caveolin-1: role in cell signaling. Advances in experimental medicine and biology. 2012;729:29–50. doi: 10.1007/978-1-4614-1222-9_3. [DOI] [PubMed] [Google Scholar]
- Bourova L, Kostrnova A, Hejnova L, Moravcova Z, Moon HE, Novotny J, Milligan G, Svoboda P. delta-Opioid receptors exhibit high efficiency when activating trimeric G proteins in membrane domains. J Neurochem. 2003;85:34–49. doi: 10.1046/j.1471-4159.2003.01667.x. [DOI] [PubMed] [Google Scholar]
- Brautigan DL. Flicking the switches: phosphorylation of serine/threonine protein phosphatases. Seminars in cancer biology. 1995;6:211–217. doi: 10.1006/scbi.1995.0028. [DOI] [PubMed] [Google Scholar]
- Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nature medicine. 2000;6:1362–1367. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- Carman CV, Lisanti MP, Benovic JL. Regulation of G protein-coupled receptor kinases by caveolin. J Biol Chem. 1999;274:8858–8864. doi: 10.1074/jbc.274.13.8858. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Chang A, Gintzler AR. Subcellular localization of mu-opioid receptor G(s) signaling. J Pharmacol Exp Ther. 2010;333:193–200. doi: 10.1124/jpet.109.165142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Gintzler AR. Phosphorylation of Gb is augmented by chronic morphine and enhances Gbg stimulation of adenylyl cyclase activity. Mol Brain Res. 2003;119:144–151. doi: 10.1016/j.molbrainres.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Gintzler AR. Phosphorylation of Gs{alpha} influences its association with the mu-opioid receptor and is modulated by chronic morphine. Mol Pharmacol. 2007;72:753–760. doi: 10.1124/mol.107.036145. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Liu NJ, Zadina JE, Sharma T, Gintzler A. Pleiotropic opioid regulation of spinal endomorphin 2 release and its adaptations to opioid withdrawal are sexually dimorphic. J Pharmacol Exp Ther. 2012;340:56–63. doi: 10.1124/jpet.111.186874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Regec A, Gintzler AR. Biochemical demonstration of mu-opioid receptor association with Gsalpha: enhancement following morphine exposure. Mol Brain Res. 2005a;135:217–224. doi: 10.1016/j.molbrainres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Regec A, Gintzler AR. Chronic morphine acts via a protein kinase Cγ-Gβ-adenylyl cyclase complex to augment phosphorylation of Gβ and Gβγ stimulatory adenylyl cyclase signaling. Mol Brain Res. 2005b;138:94–103. doi: 10.1016/j.molbrainres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Wang L, Tang W-J, Gintzler AR. Chronic morphine augments adenylyl cyclase phosphorylation: relevance to altered signaling during tolerance/dependence. Mol Pharmacol. 1998;54:949–953. doi: 10.1124/mol.54.6.949. [DOI] [PubMed] [Google Scholar]
- Chalecka-Franaszek E, Weems HB, Crowder AT, Cox BM, Cote TE. Immunoprecipitation of high-affinity, guanine nucleotide-sensitive, solubilized mu-opioid receptors from rat brain: coimmunoprecipitation of the G proteins G(alpha o), G(alpha i1), and G(alpha i3) J Neurochem. 2000;74:1068–1078. doi: 10.1046/j.1471-4159.2000.0741068.x. [DOI] [PubMed] [Google Scholar]
- Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- Gratton JP, Lin MI, Yu J, Weiss ED, Jiang ZL, Fairchild TA, Iwakiri Y, Groszmann R, Claffey KP, Cheng YC, Sessa WC. Selective inhibition of tumor microvascular permeability by cavtratin blocks tumor progression in mice. Cancer cell. 2003;4:31–39. doi: 10.1016/s1535-6108(03)00168-5. [DOI] [PubMed] [Google Scholar]
- Kholodenko BN. Cell-signalling dynamics in time and space. Nature reviews Molecular cell biology. 2006;7:165–176. doi: 10.1038/nrm1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie P, Partridge EA, Guay G, Goetz JG, Pawling J, Lagana A, Joshi B, Dennis JW, Nabi IR. Plasma membrane domain organization regulates EGFR signaling in tumor cells. J Cell Biol. 2007;179:341–356. doi: 10.1083/jcb.200611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt ES, Clark MJ, Jenkins PM, Martens JR, Traynor JR. Differential effect of membrane cholesterol removal on mu- and delta-opioid receptors: a parallel comparison of acute and chronic signaling to adenylyl cyclase. J Biol Chem. 2009;284:22108–22122. doi: 10.1074/jbc.M109.030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Couet J, Lisanti MP. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996;271:29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995;270:15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- Liu NJ, Chakrabarti S, Schnell S, Wessendorf M, Gintzler AR. Spinal Synthesis of Estrogen and Concomitant Signaling by Membrane Estrogen Receptors Regulate Spinal {kappa}- and {micro}-Opioid Receptor Heterodimerization and Female-Specific Spinal Morphine Antinociception. J Neurosci. 2011;31:11836–11845. doi: 10.1523/JNEUROSCI.1901-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollner S, Pfeuffer T. Two different adenylyl cyclases in brain distinguished by monoclonal antibodies. EurJBiochem. 1988;171(1–2):265–271. doi: 10.1111/j.1432-1033.1988.tb13785.x. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. Heterologous desensitization mediated by G protein-specific binding to caveolin. J Biol Chem. 2000;275:30211–30219. doi: 10.1074/jbc.M002194200. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Oner SS, Kaya AI, Onaran HO, Ozcan G, Ugur O. beta2-Adrenoceptor, Gs and adenylate cyclase coupling in purified detergent-resistant, low density membrane fractions. Eur J Pharmacol. 2010;630:42–52. doi: 10.1016/j.ejphar.2009.12.035. [DOI] [PubMed] [Google Scholar]
- Park JH, Han HJ. Caveolin-1 plays important role in EGF-induced migration and proliferation of mouse embryonic stem cells: involvement of PI3K/Akt and ERK. Am J Physiol Cell Physiol. 2009;297:C935–944. doi: 10.1152/ajpcell.00121.2009. [DOI] [PubMed] [Google Scholar]
- Park WY, Park JS, Cho KA, Kim DI, Ko YG, Seo JS, Park SC. Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J Biol Chem. 2000;275:20847–20852. doi: 10.1074/jbc.M908162199. [DOI] [PubMed] [Google Scholar]
- Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyansky AA, Zagrovic B. Protein Electrostatic Properties Predefining the Level of Surface Hydrophobicity Change upon Phosphorylation. The journal of physical chemistry letters. 2012;3:973–976. doi: 10.1021/jz300103p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KJ, Ma W, Sutak M, Doods H, Quirion R, Jhamandas K. Blockade and reversal of spinal morphine tolerance by peptide and non-peptide calcitonin gene-related peptide receptor antagonists. Br J Pharmacol. 2000;131:875–884. doi: 10.1038/sj.bjp.0703655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Wang Y, Law PY, Chen HZ, Loh HH. Cholesterol regulates micro-opioid receptor-induced beta-arrestin 2 translocation to membrane lipid rafts. Mol Pharmacol. 2011;80:210–218. doi: 10.1124/mol.110.070870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Rubin CS, Lisanti MP. Regulation of cAMP-mediated signal transduction via interaction of caveolins with the catalytic subunit of protein kinase A. J Biol Chem. 1999;274:26353–26360. doi: 10.1074/jbc.274.37.26353. [DOI] [PubMed] [Google Scholar]
- Rivera M, Gintzler AR. Differential effect of chronic morphine on mRNA encoding adenylyl cyclase isoforms: relevance to physiological sequela of tolerance/dependence. Mol Brain Research. 1998;54:165–169. doi: 10.1016/s0169-328x(97)00303-3. [DOI] [PubMed] [Google Scholar]
- Schubert W, Frank PG, Woodman SE, Hyogo H, Cohen DE, Chow CW, Lisanti MP. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- Sukumaran SK, Quon MJ, Prasadarao NV. Escherichia coli K1 internalization via caveolae requires caveolin-1 and protein kinase Calpha interaction in human brain microvascular endothelial cells. J Biol Chem. 2002;277:50716–50724. doi: 10.1074/jbc.M208830200. [DOI] [PubMed] [Google Scholar]
- Tasken K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2004;84:137–167. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- Vang T, Torgersen KM, Sundvold V, Saxena M, Levy FO, Skalhegg BS, Hansson V, Mustelin T, Tasken K. Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor. The Journal of experimental medicine. 2001;193:497–507. doi: 10.1084/jem.193.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, Tang B, Jelicks LA, Kitsis RN, Christ GJ, Factor SM, Tanowitz HB, Lisanti MP. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- Zheng YZ, Boscher C, Inder KL, Fairbank M, Loo D, Hill MM, Nabi IR, Foster LJ. Differential impact of caveolae and caveolin-1 scaffolds on the membrane raft proteome. Molecular & cellular proteomics : MCP. 2011;10:M110. doi: 10.1074/mcp.M110.007146. 007146. [DOI] [PMC free article] [PubMed] [Google Scholar]