Abstract
Often distinct elements serve similar functions within a network. However, it is unclear whether this network degeneracy is beneficial, or merely a reflection of tighter regulation of overall network performance relative to individual neuronal properties. We review circumstances where data strongly suggest that degeneracy is beneficial in that it makes network function more robust. Importantly, network degeneracy is likely to have functional consequences that are not widely appreciated. This is likely to be true when network activity is configured by modulators with persistent actions, and the history of network activity potentially impacts subsequent functioning. Data suggest that degeneracy in this context may be important for the creation of latent memories, and for state-dependent task switching.
Introduction
Biological ‘degeneracy’ is defined as the ability of elements that are structurally distinct to fulfill the same function, e.g., some amino acids are specified by more than one nucleotide combination [1]. This review focuses on degeneracy at a different level, i.e., at the level of a neural network. Neural networks are characterized by a set of parameters that describe the intrinsic properties of the component neurons, and the synapses that they make [2]. Degeneracy is observed when more than one set of circuit parameters produces the same (or very similar) output.
Network degeneracy has been described in a number of systems leading to speculation as to why it is observed. It could be beneficial for an organism. Alternatively it could simply reflect the need for tight regulation of network performance without a similar need to restrict specific circuit parameters. We discuss research that strongly argues that network degeneracy makes circuit function more robust. Further, we suggest that network degeneracy can have other functional consequences. We focus on situations in which network activity is configured by persistent effects of neuromodulators, and mechanisms utilized to pattern one bout of activity impact subsequent network activation.
Variability in membrane and synaptic currents
The potential for degeneracy in network function was strikingly illustrated in a computational study that simulated more than 20 million versions of a relatively simple, triphasic motor program generated by the crustacean stomatogastric nervous system [3]. This study clearly demonstrated that virtually indistinguishable activity patterns could arise from widely disparate sets of circuit parameters.
To determine whether degeneracy is observed in biological systems, investigators have measured variability in neuronal properties in networks that reliably generate stereotypic activity. To a large extent this has been achieved in invertebrate preparations with well-characterized neurons that can be reliably identified as unique individuals. These studies have recently been reviewed in detail [4–6]* and report remarkable variability in both membrane and synaptic currents. For example, in the crustacean stomatogastric nervous system, ion channel expression was quantified using voltage clamp techniques to measure currents, followed by single cell PCR to measure mRNA expression [7]. Both conductances and mRNA levels varied by more than threefold, yet neurons had almost superimposable patterns of activity [7]. Similar variability has been reported in the strength of synaptic connections between neurons that control leech heartbeat [4,8,9].
Stabilizing effects of conductance correlations
Interestingly, however, a number of studies have demonstrated that this variability can be constrained in that certain circuit parameters co-vary. Namely, the expression levels of different ion channels can be linearly correlated with one another [10–12]. For example, Schulz et al. [10] measured mRNA expression of the ion channels in each cell type in the crab stomatogastric ganglion (STG) and observed correlations in most neurons, which could be pairwise, three-way or even four-way.
Experimental data has suggested that coordinated ion channel expression may play a role in stabilizing neural activity. For example, an early study in lobster STG neurons demonstrated that increases in the IA current produced relatively little change in activity, presumably as a consequence of an accompanying increase in Ih [13,14]. Further, experiments using the dynamic clamp technique to manipulate conductances in crab neurons have shown that effects on network activity induced by manipulation of a single current can be compensated for by simultaneous manipulation of other currents (as long as preexisting linear relationships are maintained) [15]. In some cases computational studies have reached similar conclusions [16–19]. Taken together this research suggests that degeneracy can lead to robustness in network output in that differences (or changes) in the expression of one conductance can be compensated for by differences (or changes) in another conductance (or conductances).
Data suggest that, at least in the STG, the nervous system takes advantage of this potential to stabilize output during activity dependent homeostasis. For example Ransdell et al. [20] have shown that IA and IKCa currents are negatively correlated in crab neurons with a decrease in either current in an active network causing an increase in the other. This process is strikingly rapid, i.e., activity is stabilized over the course of ~ 60–90 minutes. In a more recent study Temporal et al. [21]** quantified multiple ion channel mRNAs from identified STG neurons and identified correlations. They then performed experiments in which they decoupled activity, synaptic connectivity, and neuromodulatory state, and concluded that observed correlations were activity dependent.
Computational work has also reached similar conclusions. Thus, Taylor et al. [22] generated large numbers of model STG neurons using random sets of conductance parameters without using a homeostatic tuning rule. They selected models that matched the electrophysiological properties of biological neurons, and found that conductance correlations were not observed. In contrast, O’Leary et al. [23]** and O’Leary et al. [24]** generated model neurons that did use homeostatic tuning rules and correlations were observed (also see [25]).
Degeneracy and robustness in behavior
Although a large body of work has demonstrated that degeneracy in ionic conductances can stabilize network activity, much less is known about how this impacts behavior. What is known is primarily a result of research conducted in C. elegans. For instance, C. elegans thermoregulate by modifying navigation, i.e., an animal that encounters a temperature higher than its cultivation temperature displays negative thermotaxis [26]. Cell ablation and rescue experiments have demonstrated that different combinations of thermosensory neurons are necessary and sufficient for negative thermotaxis under different conditions [27].
More recent work has demonstrated that there is also degeneracy in the C. elegans feeding circuitry [28]. C. elegans feed on bacteria via rhythmic contractions and relaxations of the pharynx (i.e., pharyngeal pumping) [29]. The pharynx is innervated by a well-mapped nervous system that can be optogenetically manipulated during behavior [30]. Experiments utilizing these techniques have demonstrated that more than one motor neuron is capable of triggering pumping [28].
In both cases it has been suggested that degeneracy makes C. elegans behavior more robust. For example, afferents that induce thermotaxis are activated in different temperature ranges [27]. This suggests that having multiple thermosensitive afferent types insures that negative thermotaxis can occur over a wider range of conditions. Consequently animals are better able to cope with changes in the external environment.
The invertebrate networks described above all mediate behaviors that might be expected to be robust because they are vital. Consistent with this, neuronal networks responsible for rhythmogenesis and central chemosensory processing in vertebrate respiratory systems are mediated by functionally similar but structurally distinct circuits [31]. This suggests that the consequences of network degeneracy at least for vital behaviors are widespread throughout the animal kingdom, although there does appears to be some controversy in the role of degeneracy in respiratory control (e.g., [32]).
Network degeneracy and experience dependent plasticity
In addition to permitting vital behavior under a variety of conditions, degeneracy in network function has been described in other contexts [33–38]. For example, surprising variability in network composition has been reported in large-scale voltage sensitive dye (VSD) imaging experiments monitoring the activity of neural networks mediating highly stereotypic behaviors in molluscs [35–38]**. Even for a behavior as stereotyped as escape swimming in Tritonia, the underlying circuitry exhibits both moment-to-moment and trial-to-trial variability [37].
A set of particularly intriguing recent experiments in Tritonia has demonstrated that the composition of the escape swim network also changes with the induction of short-term sensitization (i.e., during memory formation) [38]**. Although this is not surprising since there is a correlated change in behavior, some of the neurons that join the swim network when sensitization is induced remain after behavioral manifestations of learning are no longer apparent (Fig. 1). Other neurons that were part of the network prior to sensitization are not part of it afterwards. This suggests that the post-sensitization network is now in an altered state that reflects a latent ‘memory’ of previous events, i.e., there is a persistent representation of the prior experience.
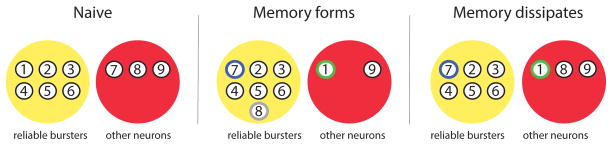
Figure 1.
Simplified version of Figure 7 from Hill et al. [38] showing how the Tritonia escape swim network is altered as the memory for short-term sensitization forms and then dissipates. Left, before sensitization the network consists of neurons that are reliable bursters (RBs) (yellow) and other neurons, e.g., neurons that are unreliable bursters (red). Middle, in the sensitized state some neurons leave the RB pool (green), but more join it (blue and gray), resulting in a net increase in pool size. Right, as sensitization dissipates the RB pool returns to the naïve size, but with an altered neural composition (i.e., the blue neuron is now a RB and the green neuron is not).
A potential mechanism mediating the formation of this latent memory is the activation of modulatory serotonergic neurons [38]**. Modulators pattern network activity in many species, and although their effects are not always long lasting, they often persist. This suggests that effects of neuromodulation on subsequent network activation may not be uncommon. Further, modulators can use divergent mechanisms to generate a single pattern of activity [34]*. When this occurs the network changes that persist after network activity has been patterned by one modulator may differ from those induced by a second modulator. These sorts of differences in the pre-existing state have the potential to impact subsequent network function and suggest that degeneracy in the modulatory repertoire within a network may provide different contexts for experience dependent plasticity.
The consequences of network degeneracy for shifts between behavioral states has been nicely explored in the Aplysia feeding network, with several studies indicating that the pre-existing state can have important consequences for task switching. Thus, the feeding network of Aplysia generates two incompatible behaviors; ingestion and egestion [39–41]. In both behaviors the mouthparts are extended (“protracted”) and then retracted. For ingestion the mouthparts are open during protraction and closed during retraction. For egestion they are closed during protraction and open during retraction. However, when a single cycle of a motor program is triggered in a rested preparation neither ingestion, nor egestion is observed [42–46]. Instead motor programs are not well articulated, and are referred to as having ‘intermediate’ characteristics.
Well-defined egestive motor programs can be triggered in two ways. The egestive input to the feeding CPG, the esophageal nerve (EN) can be stimulated so that more than one cycle of motor activity is triggered [42,43,45]**. In this situation there are progressive changes in the firing patterns of motor neurons, and after a few cycles, activity becomes egestive. This has been referred to as ‘egestive repetition priming’. It is presumably mediated by cumulative effects of modulatory neuropeptides that are released by repeated EN stimulation [43,47]**.
A second method of inducing egestive activity involves a switch to EN stimulation after repeated stimulation of the ingestive input to the feeding CPG [48]**. A priori it might be expected that the induction of ingestive activity would either negatively impact the subsequent generation of egestive activity, or that it would have no effect. Surprisingly there is a ‘positive’ effect [48]**. Thus, if the EN is stimulated after ingestive repetition priming, fully egestive motor programs are immediately triggered (repeated EN stimulation is not necessary) [48,49]**. This has been referred to as ‘positive biasing’.
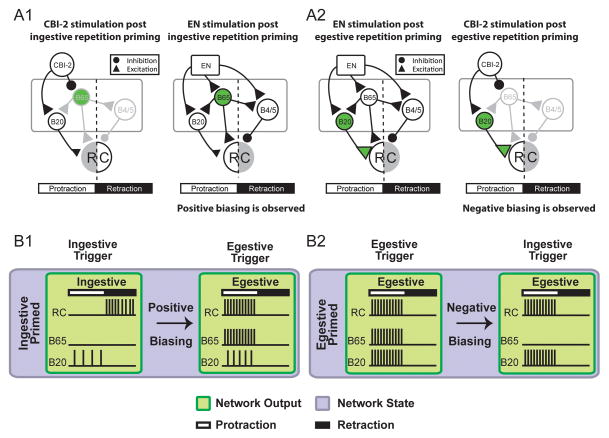
Data suggest that positive biasing and egestive repetition priming are mediated by two different circuit mechanisms. Positive biasing induces persistent increases in the excitability of the egestive interneuron B65 (Fig. 2A1left) [48,50]. In contrast, during egestive repetition priming there is a persistent increase in the excitability of a second egestive interneuron (B20) [51–53], and use dependent potentiation of synaptic transmission between B20 and follower motor neurons (Fig. 2A2left) [42,51]. Thus, these data indicate that there is degeneracy in the feeding circuitry in that egestive motor activity can be patterned via more than one set of circuit parameters.
Figure 2.
(A) Circuit diagrams showing connections between CPG inputs and egestive interneurons (primarily B20 and B65). Fast inhibitory connections are indicated by closed circles, and fast excitatory chemical connections are indicated by closed triangles. Neurons that are activated by input stimulation are black, those that are not activated are gray. The green shading indicates that repetition priming induced a persistent alteration in the circuit parameter. RC; radula closer motor neuron. When radula closing occurs during retraction (i.e., A1, left) activity is ingestive. In all other cases activity is egestive (radula closing occurs during protraction.) (A1) Positive biasing is observed when the egestive CPG input (the EN) is stimulated after ingestive repetition priming. At least in part, positive biasing is a consequence of the increase in B65 excitability that occurs during ingestive repetition priming (left). Although B65 is an egestive interneuron, the immediate induction of ingestive activity is not affected because CBI-2 directly inhibits B65 (left). However, when motor programs are subsequently triggered by EN stimulation the ‘latent’ increase in B65 excitability makes a contribution to the patterning of motor activity since B65 is directly excited by EN stimulation (right). (A2) Negative biasing is observed when the ingestive CPG input (CBI-2) is stimulated after egestive repetition priming. At least in part, data suggest that negative biasing is a consequence of the increase in B20 excitability and persistent potentiation of B20-B8 synaptic transmission that occurs during egestive repetitionp riming (left). B20 is an egestive interneuron that is excited by CBI-2 stimulation (right). (B) Schematic diagram indicating how degeneracy in feeding interneurons allows different network states (Blue) to achieve the same network output (green) with different consequences for subsequent output. (B1) Positive Biasing: Ingestive repetition priming causes an ingestive trigger to elicit an ingestive motor program with weak B20 firing during protraction and strong radula closer (RC) firing during retraction (black rectangle). A subsequent egestive trigger elicits a strongly egestive motor program due to a persistent enhancement of B65 excitability by neuromodulators released during ingestive priming. Negative Biasing: Egestive repetition priming causes an egestive trigger to elicit an egestive motor program with high firing rate from closure neurons (CN), B65 and B20 during protraction (white rectangle). A subsequent ingestive trigger also elicits an egestive motor program, despite B65 being repressed by the ingestive trigger. B) Positive Biasing: Ingestive repetition priming causes an ingestive trigger to elicit an ingestive motor program with weak B20 firing during protraction and strong CN firing during retraction (black rectangle). A subsequent egestive trigger elicits a strongly egestive motor program due to a persistent enhancement of B65 excitability by neuromodulators released during ingestive priming.
Data suggest that this difference will have consequences for task switching, i.e., the cessation of egestive activity and the initiation of ingestive activity. Thus, Proekt et al. [42] have demonstrated that after egestive repetition priming it is not possible to rapidly switch to ingestive activity. At least in part this is a consequence of the increased excitability of B20. B20 receives fast excitatory input from the ingestive input to the feeding CPG (Fig. 2A2right) [52]. As a result, persistent excitability increases that are induced during egestive priming impact the subsequent induction of ingestive motor programs (Fig. 2B2) [42,51].
In contrast, the egestive interneuron that mediates positive biasing (B65) is inhibited by ingestive input (Fig. 2A1right) [54]. Consequently, increases in its excitability that occur during positive biasing are not likely to negatively impact a subsequent return to ingestive activity (Fig. 2A1left, 2B1). Thus, B20 and B65 differ in that persistent modulator induced increases in the excitability in B20 impact the induction of both ingestive and egestive motor programs. In contrast, modulator induced increases in B65 excitability only impact the induction of egestive motor programs, i.e., they are manifested in a state-dependent manner. State dependent effects of modulators have been described in other contexts [55,56]** and are likely a widespread mechanism for regulating network dynamics. Thus, data in the feeding system of Aplysia suggest that the dynamics of task switching can be determined by the nature of the mechanisms that are used to pattern activity.
Conclusions
Although a priori it cannot be assumed that degeneracy in network function creates a physiological advantage, data are emerging that suggest that in many cases it does. It is likely that it makes behavior more robust. Further, data suggest that it may be important for the induction of latent memories, and that it may create the potential for increased behavioral flexibility by impacting the dynamics of task switching.
Highlights.
More than one set of circuit parameters can produce the same network output.
Degeneracy is likely to make network function more robust.
Network degeneracy may enable the encoding of latent memories.
Network degeneracy may promote the ability of a network to task switch.
Acknowledgments
Supported by NIH grants NS066587, NS070583, and MH051393.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth C. Cropper, Email: elizabeth.cropper@gmail.com.
Andrew M. Dacks, Email: amdacks@mail.wvu.edu.
Klaudiusz R Weiss, Email: klaudiusz.weiss@gmail.com.
References
- 1.Tononi G, Sporns O, Edelman GM. Measures of degeneracy and redundancy in biological networks. Proc Natl Acad Sci U S A. 1999;96:3257–3262. doi: 10.1073/pnas.96.6.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Getting PA. Emerging principles governing the operation of neural networks. Annu Rev Neurosci. 1989;12:185–204. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- 3.Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–1352. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese RL, Norris BJ, Wenning A, Wright TM. Coping with variability in small neuronal networks. Integr Comp Biol. 2011;51:845–855. doi: 10.1093/icb/icr074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *5.Golowasch J. Ionic Current Variability and Functional Stability in the Nervous System. Bioscience. 2014;64:570–580. doi: 10.1093/biosci/biu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *6.Marder E, Goeritz ML, Otopalik AG. Robust circuit rhythms in small circuits arise from variable circuit components and mechanisms. Curr Opin Neurobiol. 2015;31:156–163. doi: 10.1016/j.conb.2014.10.012. These reviews (and Calabrese [4] thoroughly cover the complex literature that is only referred to here that indicates that there is a great deal of variability in circuit parameters even in networks that mediate highly stereotyped activity. Further, how this promotes robustness and functional stability is discussed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulz DJ, Goaillard JM, Marder E. Variable channel expression in identified single and electrically coupled neurons in different animals. Nat Neurosci. 2006;9:356–362. doi: 10.1038/nn1639. [DOI] [PubMed] [Google Scholar]
- 8.Norris BJ, Weaver AL, Wenning A, Garcia PS, Calabrese RL. A central pattern generator producing alternative outputs: pattern, strength, and dynamics of premotor synaptic input to leech heart motor neurons. J Neurophysiol. 2007;98:2992–3005. doi: 10.1152/jn.00877.2007. [DOI] [PubMed] [Google Scholar]
- 9.Roffman RC, Norris BJ, Calabrese RL. Animal-to-animal variability of connection strength in the leech heartbeat central pattern generator. J Neurophysiol. 2012;107:1681–1693. doi: 10.1152/jn.00903.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz DJ, Goaillard JM, Marder EE. Quantitative expression profiling of identified neurons reveals cell-specific constraints on highly variable levels of gene expression. Proc Natl Acad Sci U S A. 2007;104:13187–13191. doi: 10.1073/pnas.0705827104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khorkova O, Golowasch J. Neuromodulators, not activity, control coordinated expression of ionic currents. J Neurosci. 2007;27:8709–8718. doi: 10.1523/JNEUROSCI.1274-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobin AE, Cruz-Bermudez ND, Marder E, Schulz DJ. Correlations in ion channel mRNA in rhythmically active neurons. PLoS One. 2009;4:e6742. doi: 10.1371/journal.pone.0006742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacLean JN, Zhang Y, Johnson BR, Harris-Warrick RM. Activity-independent homeostasis in rhythmically active neurons. Neuron. 2003;37:109–120. doi: 10.1016/s0896-6273(02)01104-2. [DOI] [PubMed] [Google Scholar]
- 14.MacLean JN, Zhang Y, Goeritz ML, Casey R, Oliva R, Guckenheimer J, Harris-Warrick RM. Activity-independent coregulation of IA and Ih in rhythmically active neurons. J Neurophysiol. 2005;94:3601–3617. doi: 10.1152/jn.00281.2005. [DOI] [PubMed] [Google Scholar]
- 15.Zhao S, Golowasch J. Ionic current correlations underlie the global tuning of large numbers of neuronal activity attributes. J Neurosci. 2012;32:13380–13388. doi: 10.1523/JNEUROSCI.6500-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudson AE, Prinz AA. Conductance ratios and cellular identity. PLoS Comput Biol. 2010;6:e1000838. doi: 10.1371/journal.pcbi.1000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doloc-Mihu A, Calabrese RL. Identifying crucial parameter correlations maintaining bursting activity. PLoS Comput Biol. 2014;10:e1003678. doi: 10.1371/journal.pcbi.1003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamb DG, Calabrese RL. Correlated conductance parameters in leech heart motor neurons contribute to motor pattern formation. PLoS One. 2013;8:e79267. doi: 10.1371/journal.pone.0079267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golowasch J, Goldman MS, Abbott LF, Marder E. Failure of averaging in the construction of a conductance-based neuron model. J Neurophysiol. 2002;87:1129–1131. doi: 10.1152/jn.00412.2001. [DOI] [PubMed] [Google Scholar]
- 20.Ransdell JL, Nair SS, Schulz DJ. Rapid homeostatic plasticity of intrinsic excitability in a central pattern generator network stabilizes functional neural network output. J Neurosci. 2012;32:9649–9658. doi: 10.1523/JNEUROSCI.1945-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Temporal S, Lett KM, Schulz DJ. Activity-dependent feedback regulates correlated ion channel mRNA levels in single identified motor neurons. Curr Biol. 2014;24:1899–1904. doi: 10.1016/j.cub.2014.06.067. This study and Ransdell [20] present experimental data that indicate that conductance correlations are maintained via activity dependent mechanisms and tend to stabilize network activity. In general this research makes an important contribution to the idea that degeneracy can make network function robust. [DOI] [PubMed] [Google Scholar]
- 22.Taylor AL, Goaillard JM, Marder E. How multiple conductances determine electrophysiological properties in a multicompartment model. J Neurosci. 2009;29:5573–5586. doi: 10.1523/JNEUROSCI.4438-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **23.O’Leary T, Williams AH, Caplan JS, Marder E. Correlations in ion channel expression emerge from homeostatic tuning rules. Proc Natl Acad Sci U S A. 2013;110:E2645–2654. doi: 10.1073/pnas.1309966110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **24.O’Leary T, Williams AH, Franci A, Marder E. Cell types, network homeostasis, and pathological compensation from a biologically plausible ion channel expression model. Neuron. 2014;82:809–821. doi: 10.1016/j.neuron.2014.04.002. These theoretical and computational studies provide important insights into the nature of the mechanisms that couple conductance expression to neuronal activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calabrese RL. Channeling the Central Dogma. Neuron. 2014;82:725–727. doi: 10.1016/j.neuron.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedgecock EM, Russell RL. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1975;72:4061–4065. doi: 10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beverly M, Anbil S, Sengupta P. Degeneracy and neuromodulation among thermosensory neurons contribute to robust thermosensory behaviors in Caenorhabditis elegans. J Neurosci. 2011;31:11718–11727. doi: 10.1523/JNEUROSCI.1098-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trojanowski NF, Padovan-Merhar O, Raizen DM, Fang-Yen C. Neural and genetic degeneracy underlies Caenorhabditis elegans feeding behavior. J Neurophysiol. 2014;112:951–961. doi: 10.1152/jn.00150.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trojanowski NF, Raizen DM, Fang-Yen C. Pharyngeal pumping in Caenorhabditis elegans depends on tonic and phasic signaling from the nervous system. Sci Rep. 2016;6:22940. doi: 10.1038/srep22940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trojanowski NF, Fang-Yen C. Simultaneous Optogenetic Stimulation of Individual Pharyngeal Neurons and Monitoring of Feeding Behavior in Intact C. elegans. Methods Mol Biol. 2015;1327:105–119. doi: 10.1007/978-1-4939-2842-2_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mellen NM. Degeneracy as a substrate for respiratory regulation. Respir Physiol Neurobiol. 2010;172:1–7. doi: 10.1016/j.resp.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones SE, Dutschmann M. Testing the hypothesis of neurodegeneracy in respiratory network function with a priori transected arterially perfused brainstem preparation of rat. J Neurophysiol. 2016 doi: 10.1152/jn.01073.2015. jn 0107302015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez GJ, O’Leary T, Marder E. Multiple mechanisms switch an electrically coupled, synaptically inhibited neuron between competing rhythmic oscillators. Neuron. 2013;77:845–858. doi: 10.1016/j.neuron.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Rodriguez JC, Blitz DM, Nusbaum MP. Convergent rhythm generation from divergent cellular mechanisms. J Neurosci. 2013;33:18047–18064. doi: 10.1523/JNEUROSCI.3217-13.2013. This paper demonstrates network degeneracy in the context of modulation in that it demonstrates that a single rhythm can be induced by two different modulatory inputs that act via different mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu JY, Tsau Y, Hopp HP, Cohen LB, Tang AC, Falk CX. Consistency in nervous systems: trial-to-trial and animal-to-animal variations in the responses to repeated applications of a sensory stimulus in Aplysia. J Neurosci. 1994;14:1366–1384. doi: 10.1523/JNEUROSCI.14-03-01366.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill ES, Bruno AM, Frost WN. Recent developments in VSD imaging of small neuronal networks. Learn Mem. 2014;21:499–505. doi: 10.1101/lm.035964.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill ES, Vasireddi SK, Bruno AM, Wang J, Frost WN. Variable neuronal participation in stereotypic motor programs. PLoS One. 2012;7:e40579. doi: 10.1371/journal.pone.0040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **38.Hill ES, Vasireddi SK, Wang J, Bruno AM, Frost WN. Memory Formation in Tritonia via Recruitment of Variably Committed Neurons. Curr Biol. 2015;25:2879–2888. doi: 10.1016/j.cub.2015.09.033. This study suggests an important new view of the potential significance of degeneracy in network function, i.e., that degenerate states may encode latent memories. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kupfermann I. Feeding Behavior in Aplysia - Simple System for Study of Motivation. Behavioral Biology. 1974;10:1–26. doi: 10.1016/s0091-6773(74)91644-7. [DOI] [PubMed] [Google Scholar]
- 40.Morton DW, Chiel HJ. The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J Comp Physiol A. 1993;173:519–536. doi: 10.1007/BF00197761. [DOI] [PubMed] [Google Scholar]
- 41.Morton DW, Chiel HJ. In vivo buccal nerve activity that distinguishes ingestion from rejection can be used to predict behavioral transitions in Aplysia. J Comp Physiol A. 1993;172:17–32. doi: 10.1007/BF00214712. [DOI] [PubMed] [Google Scholar]
- 42.Proekt A, Brezina V, Weiss KR. Dynamical basis of intentions and expectations in a simple neuronal network. Proc Natl Acad Sci U S A. 2004;101:9447–9452. doi: 10.1073/pnas.0402002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **43.Friedman AK, Weiss KR, Cropper EC. Specificity of repetition priming: the role of chemical coding. J Neurosci. 2015;35:6326–6334. doi: 10.1523/JNEUROSCI.4562-14.2015. This study and characterizes mechanisms that mediate repetition priming, a ubiquitous phenomenon that is not well understood at the cellular/molecular level. In general, it and [46] describe how a state change can be induced by cumulative effects of modulatory transmitters. Further, this study points to the potential importance of chemical coding in this context. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman AK, Weiss KR. Repetition priming of motoneuronal activity in a small motor network: intercellular and intracellular signaling. J Neurosci. 2010;30:8906–8919. doi: 10.1523/JNEUROSCI.1287-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman AK, Zhurov Y, Ludwar B, Weiss KR. Motor outputs in a multitasking network: relative contributions of inputs and experience-dependent network states. J Neurophysiol. 2009;102:3711–3727. doi: 10.1152/jn.00844.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dacks AM, Siniscalchi MJ, Weiss KR. Removal of default state-associated inhibition during repetition priming improves response articulation. J Neurosci. 2012;32:17740–17752. doi: 10.1523/JNEUROSCI.4137-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cropper EC, Friedman AK, Jing J, Perkins MH, Weiss KR. Neuromodulation as a mechanism for the induction of repetition priming. Curr Opin Neurobiol. 2014;29:33–38. doi: 10.1016/j.conb.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **48.Dacks AM, Weiss KR. Latent modulation: a basis for non-disruptive promotion of two incompatible behaviors by a single network state. J Neurosci. 2013;33:3786–3798. doi: 10.1523/JNEUROSCI.5371-12.2013. Although ‘latent’ modulation is likely to be present in many species and contexts relatively few studies have sought to determine how it impacts behavior. Results of this study indicate that it may have important consequences for task switching. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Proekt A, Wong J, Zhurov Y, Kozlova N, Weiss KR, Brezina V. Predicting adaptive behavior in the environment from central nervous system dynamics. PLoS One. 2008;3:e3678. doi: 10.1371/journal.pone.0003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Due MR, Jing J, Weiss KR. Dopaminergic contributions to modulatory functions of a dual-transmitter interneuron in Aplysia. Neurosci Lett. 2004;358:53–57. doi: 10.1016/j.neulet.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 51.Proekt A, Jing J, Weiss KR. Multiple contributions of an input-representing neuron to the dynamics of the aplysia feeding network. J Neurophysiol. 2007;97:3046–3056. doi: 10.1152/jn.01301.2006. [DOI] [PubMed] [Google Scholar]
- 52.Jing J, Weiss KR. Neural mechanisms of motor program switching in Aplysia. J Neurosci. 2001;21:7349–7362. doi: 10.1523/JNEUROSCI.21-18-07349.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jing J, Weiss KR. Interneuronal basis of the generation of related but distinct motor programs in Aplysia: implications for current neuronal models of vertebrate intralimb coordination. J Neurosci. 2002;22:6228–6238. doi: 10.1523/JNEUROSCI.22-14-06228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jing J, Weiss KR. Generation of variants of a motor act in a modular and hierarchical motor network. Curr Biol. 2005;15:1712–1721. doi: 10.1016/j.cub.2005.08.051. [DOI] [PubMed] [Google Scholar]
- **55.Gutierrez GJ, Marder E. Modulation of a Single Neuron Has State-Dependent Actions on Circuit Dynamics. eNeuro. 2014:1. doi: 10.1523/ENEURO.0009-14.2014. This computational study elegantly illustrate how effects of modulatory neurotransmitters can be state (i.e., context) dependent. Since modulators configure network activity in many species this work is likely to have important general implications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marder E, O’Leary T, Shruti S. Neuromodulation of circuits with variable parameters: single neurons and small circuits reveal principles of state-dependent and robust neuromodulation. Annu Rev Neurosci. 2014;37:329–346. doi: 10.1146/annurev-neuro-071013-013958. [DOI] [PubMed] [Google Scholar]