Abstract
Background
To better understand a potential association of elevated C-reactive protein (CRP) with progression of chronic kidney disease (CKD), we examined the relationship of CRP with the development of end-stage renal disease (ESRD) in the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT).
Study Design
Post-hoc analysis of a randomized controlled trial.
Setting & Participants
4,038 patients with type 2 diabetes, CKD and anemia in the TREAT study.
Predictor
Baseline serum concentrations of CRP.
Outcomes
The primary outcome was development of ESRD; secondary outcomes included doubling of serum creatinine a composite of ESRD/serum creatinine doubling, and a composite of death or ESRD.
Measurements
We fit unadjusted and adjusted Cox regression models to test the association of baseline CRP with time to development of the outcomes of interest
Results
Mean age of participants was 67 years, 43% were male and 64% were white. Approximately half of the patients (48%) had a CRP concentration of >3.0 mg/L; 668 patients developed ESRD, while 1270 developed the composite outcome of death or ESRD. Compared to patients with baseline CRP ≤3.0 mg/L, those with moderate-markedly elevated levels of CRP (≥6.9 mg/L; 24% of patients) had a higher adjusted risk of ESRD (HR, 1.32; 95% CI, 1.07–1.63) and the composite outcome of death or ESRD (HR, 1.41; 95% CI, 1.21–1.64). Although nonsignificant, similar trends were noted in competing risk models.
Limitations
Results may not be generalizable to non-diabetic CKD or diabetic CKD in the absence of anemia.
Conclusions
Elevated concentrations of baseline CRP are common in type 2 diabetic patients with anemia and CKD, and are associated with the future development of ESRD and the composite of death or ESRD.
Keywords: C-Reactive Protein (CRP), end-stage renal disease (ESRD), risk factor, biomarker, inflammation, disease progression, kidney function trajectory, serum creatinine, mortality, Type 2 Diabetes Mellitus (T2DM), Chronic Kidney Disease (CKD), Anemia
Chronic kidney disease (CKD) affects approximately 11.5% of the general population1 and 40% of those with self-reported diabetes mellitus in the United States.2 Diabetes is a major risk factor for the progression of CKD and is the top cause of end-stage renal disease (ESRD) in the United States, accounting for approximately 50,000 new cases in 2012 alone.2
Elevated concentration of C-reactive protein (CRP), a biomarker associated with the presence of inflammation, is known to be associated with the development of future cardiovascular (CV) events in patients with3,4 and without5,6 a prior history of CV disease, and in patients with CKD.7,8 It is widely recognized that CKD is a risk factor for CV disease, with the majority of patients with diabetes and CKD ultimately dying from CV causes.9 In light of these relationships, it has been postulated that chronic inflammation may be a common etiological factor for the progression of both conditions. However, to date, evidence supporting an association of CRP with kidney function decline (as measured by changes in serum creatinine or estimated glomerular filtration rate [eGFR]) is conflicting, with some studies reporting the presence of a significant association,10–14 while others have not.15–17
The Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT)18 provided an opportunity to perform an examination of the association of baseline CRP with the time to the adjudicated outcomes of 1) ESRD and 2) the composite of death or ESRD in patients with type 2 diabetes (T2DM), CKD and anemia. We hypothesized that individuals with higher baseline CRP concentrations would be at greater risk for development of ESRD, and death or ESRD.
Methods
Study Design and Population
The design and original results of TREAT (trial registration: ClinicalTrials.gov; study number: NCT00093015) have been published.18,19 Briefly, TREAT was a prospective, double-blind, randomized controlled trial of darbepoetin alfa versus placebo for the treatment of anemia in 4,038 patients with T2DM, eGFR of 20–60 mL/min/1.73m2 according to the 4-variable MDRD (Modification of Diet in Renal Disease) Study equation, hemoglobin level <11.0 g/dL, and transferrin saturation >15%. Notable exclusion criteria included a recent (within 12 weeks) CV event, grand mal seizure, major surgery, or prior use of an erythropoiesis-stimulating agent (ESA), uncontrolled hypertension, known human immunodeficiency virus infection, current use of intravenous antibiotics, chemotherapy or radiotherapy, malignancy (except basal cell or squamous cell carcinoma of the skin), active bleeding, hematologic diseases, pregnancy, or kidney transplant recipients. All patients gave written informed consent for participation in the primary trial and the serum samples used in this analysis (Partners IRB 2005P000170).
Exposures and Outcomes
The primary exposure of interest was the baseline serum concentration of CRP. All baseline samples were stored locally by individual sites at −20°C before shipment on dry ice for long-term storage at −70°C in a central tissue repository. The CRP was measured using an immuno-turbidimetric assay. This was a standard-sensitivity assay with a lower limit of detection of 3 mg/L. Therefore for the purposes of these analyses, CRP was categorized as normal (≤3 mg/L), mildly elevated (>3.0 to <6.9 mg/L) and moderate-markedly elevated (≥6.9 mg/L). The latter two categories were dichotomized at the median for CRP concentrations >3mg/L.
The primary outcome of interest was the time from randomization to development of ESRD, defined as the initiation of renal replacement therapy (RRT; sustained for at least 30 days), initiation of RRT with death within 30 days, a physician recommendation to initiate RRT with documented patient refusal, or receipt of a kidney transplant. The secondary outcomes were the time to development of 1) doubling of serum creatinine; 2) the composite of ESRD or doubling of serum creatinine; 3) the composite outcome of ESRD or death from any cause; 4) composite CV outcome of death from any cause, nonfatal myocardial infarction (MI), stroke, heart failure or hospitalization for myocardial ischemia; and 5) death from any cause.18 The CV components of the composite end point, ESRD, and death were adjudicated by a clinical end points committee blinded to the treatment assignment. Sensitivity analyses were also performed to examine the association of categories of baseline CRP with 1) the change in eGFR from randomization to the development of ESRD or study exit; and 2) the difference in last measured eGFR for those individuals who developed ESRD. Change in eGFR was calculated as a linear slope in mL/min/1.73 m2 per year by plotting a line of best fit for all available eGFR values (3,544 individuals had at least two creatinine measurements from which the eGFR slope could be calculated; mean number of measurements per patient, 5.1). In exploratory analyses, the association of baseline CRP with ESRD was determined for the sub-group of patients with CRP concentrations > 3.0 mg/L.
Statistical Analyses
Continuous variables were examined graphically and recorded as means ± standard deviations for normally distributed data, or medians (with interquartile ranges [IQRs]) for non-normally distributed data. Categorical variables were examined by frequency distribution and recorded as proportions. Tests for trend across categories of CRP were conducted using linear regression, Cuzick's non-parametric trend test and the Chi-square test for trend for continuous normal, continuous non-normal, and categorical data, respectively.
The relationship between categories of CRP with time to the events of interest was examined by proportional hazards regression. Initially an unadjusted model (Model 1) was fit. Subsequently, a multivariable adjusted model (Model 2) was fit; this model included terms for potential confounding variables that were measured at baseline20,21: age, gender, race, eGFR, log-transformed urine protein-creatinine ratio, history of acute kidney injury, duration of T2DM at baseline, HbA1c, retinopathy, insulin use, body mass index, hemoglobin, serum albumin, coronary artery disease (angina, MI, coronary artery bypass graft, percutaneous coronary intervention), cerebrovascular disease (including transient ischemic attack and carotid artery disease), peripheral arterial disease (including peripheral artery stenosis and aortic aneurysm repair), heart failure, systolic blood pressure, low-density lipoprotein (LDL) cholesterol, statin therapy, angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) therapy, smoking status (current, former, never), serum ferritin, transferrin saturation, iron therapy and randomized treatment assignment. The proportionality assumption was assessed on the basis of Schoenfeld residual testing. Additional models were created that included the corresponding predictor-time interaction variables in situations where violation of the proportional hazards assumption was evident. Additionally, a Cox regression spline model was fit to examine the continuous association of baseline CRP with the development of ESRD. For the purposes of this analysis, the 2,112 individuals with baseline CRP ≤3.0 mg/L were assumed to have a CRP=3.0 mg/L, which was taken as the reference value. Subsequently, as mortality may preclude the development of ESRD or doubling of serum creatinine, a multivariable adjusted competing risk model was fit according to the method of Fine and Gray22 in order to estimate the cumulative incidence function for ESRD, doubling of serum creatinine, and the composite of ESRD/doubling of serum creatinine, where death was considered as the competing risk.
For the sensitivity analyses, unadjusted and adjusted linear regression models were fit to estimate the differences in the slope of eGFR according to baseline CRP categories. For those who went on to develop ESRD, trend tests were used to determine the association of the last measured eGFR with increasing categories of baseline CRP.
Nominal 2-sided p values of <0.05 were considered statistically significant. All analyses were performed using STATA 13.0MP (StataCorp LP, College Station, Tex., USA).
Results
Baseline Characteristics
The primary cohort consisted of 4,038 individuals (57% women) with a median age of 68 years.18 Just over half of individuals (52.3%) had a baseline CRP concentration at or below the lower limit of detection (≤3.0 mg/L). Of the remainder, 953 individuals (23.6%) had a mildly elevated CRP (>3.0 to <6.9 mg/L) and 973 (24.1%) had a moderate-markedly elevated level (≥6.9 mg/L).
Individuals in the higher CRP categories were more likely to be younger and female; to have a history of acute kidney injury, peripheral arterial disease and heart failure; to be a current or former smoker; and to have higher LDL cholesterol and ferritin concentrations. In addition, those in the higher CRP categories were also more likely to use insulin and have a higher BMI and higher glycated hemoglobin, but to have lower hemoglobin and transferrin saturations, and were less likely to have retinopathy or to be taking a statin or an ACE inhibitor or ARB. Although serum creatinine concentration was statistically significantly higher (and eGFR lower) in the patients in the higher CRP categories, the absolute differences were very small and unlikely to be of clinical importance. No significant trend was noted in relation to baseline proteinuria (Table 1).
Table 1.
Baseline Characteristics of Cohort According to Baseline CRP Categories
| Normal: ≤3.0 mg/L |
Mildly Elevated: >3.0–<6.9 mg/L |
Moderately/markedly Elevated: ≥6.9 mg/L |
Pa | |
|---|---|---|---|---|
| No. of Participants | 2,112 | 953 | 973 | |
| CRP | NA | 4.5 (3.7–5.4) | 13.3 (9.0–23.5) | |
| Age (y) | 67.8 ±10.7 | 67.1 ±10.6 | 66.5 ±10.6 | 0.001 |
| Male sex | 44.6 | 40.9 | 40.5 | 0.02 |
| Race | 0.2 | |||
| Black | 18.6 | 20.4 | 23.5 | |
| Hispanic | 16.3 | 11.0 | 9.1 | |
| Other | 4.0 | 2.1 | 1.0 | |
| White | 61.1 | 66.5 | 66.3 | |
| Creatinine (mg/dL) | 1.8 (1.5–2.3) | 1.9 (1.5–2.4) | 1.9 (1.5–2.4) | 0.04 |
| eGFR (mL/min/1.73m2) | 32 (25–40) | 31 (24–39) | 31 (24–41) | 0.05 |
| Urine PCR | 0.4 (0.1–1.8) | 0.5 (0.2–1.9) | 0.4 (0.1–1.9) | 0.1 |
| History of AKI | 7.7 | 10.9 | 13.2 | <0.001 |
| Duration of T2DM (y) | 15.5 (8.5–21.6) | 15.6 (8.6–21.9) | 15.0 (7.5–21.8) | 0.2 |
| HbA1c | 6.9 (6.2–7.8) | 7.1 (6.3–8.2) | 7.1 (6.3–8.1) | <0.001 |
| Neuropathy | 47.3 | 47.0 | 49.8 | 0.2 |
| Retinopathy | 50.5 | 46.1 | 40.1 | <0.001 |
| Insulin use | 44.4 | 55.1 | 54.2 | <0.001 |
| BMI (kg/m2) | 29.1 (25.7–33.5) | 31.2 (26.9–36.1) | 32.1 (27.4–38.5) | <0.001 |
| Hemoglobin (g/dL) | 10.4 ±1.0 | 10.4 ±1.0 | 10.3 ±1.0 | 0.01 |
| Albumin (g/dL) | 4.0 ±0.4 | 4.0 ±0.4 | 3.9 ±0.4 | <0.001 |
| CAD | 35.1 | 38.7 | 37.3 | 0.2 |
| Cerebrovascular disease | 17.4 | 16.5 | 19.2 | 0.3 |
| PAD | 18.1 | 20.4 | 23.3 | <0.001 |
| Heart Failure | 29.7 | 36.7 | 38.0 | <0.001 |
| SBP (mmHg) | 136.0 ±18.7 | 137.0 ±18.6 | 134.6 ±18.9 | 0.1 |
| LDL cholesterol (mg/dL) | 89 ±39 | 94 ±42 | 93 ±40 | 0.01 |
| Statin | 61.7 | 57.0 | 53.2 | <0.001 |
| ACEi or ARB | 82.1% | 77.4% | 77.2% | <0.001 |
| Smoking | 0.01 | |||
| Current | 4.5 | 4.7 | 6.5 | |
| Former | 38.0 | 38.7 | 40.7 | |
| Never | 57.5 | 56.6 | 52.8 | |
| Ferritin (µg/L) | 120 (60–240) | 133 (66–256) | 161 (90–299) | <0.001 |
| Transferrin Saturation (%) |
25.6±9.4 | 24.1±9.6 | 21.2±8.4 | <0.001 |
| Iron therapy | 42.9 | 42.0 | 48.8 | 0.01 |
| Treatment: Darbepoetin vs Placebo |
50.0 | 50.6 | 48.7 | 0.6 |
Note: Values for categorical variables are given as percentage; values for continuous variables, as mean ± standard deviation or median [interquartile range].
Abbreviations: AKI, acute kidney injury; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; PCR, protein-creatinine ratio; T2DM, type 2 diabetes mellitus; HbA1c, glycated hemoglobin; BMI, body mass index; CAD, coronary artery disease; PAD, peripheral arterial disease; SBP, systolic blood pressure; LDL, low-density lipoprotein; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
P values refer to a test for trend across increasing category of CRP concentration.
Associations of Baseline CRP With Various Outcomes
Association With Development of ESRD
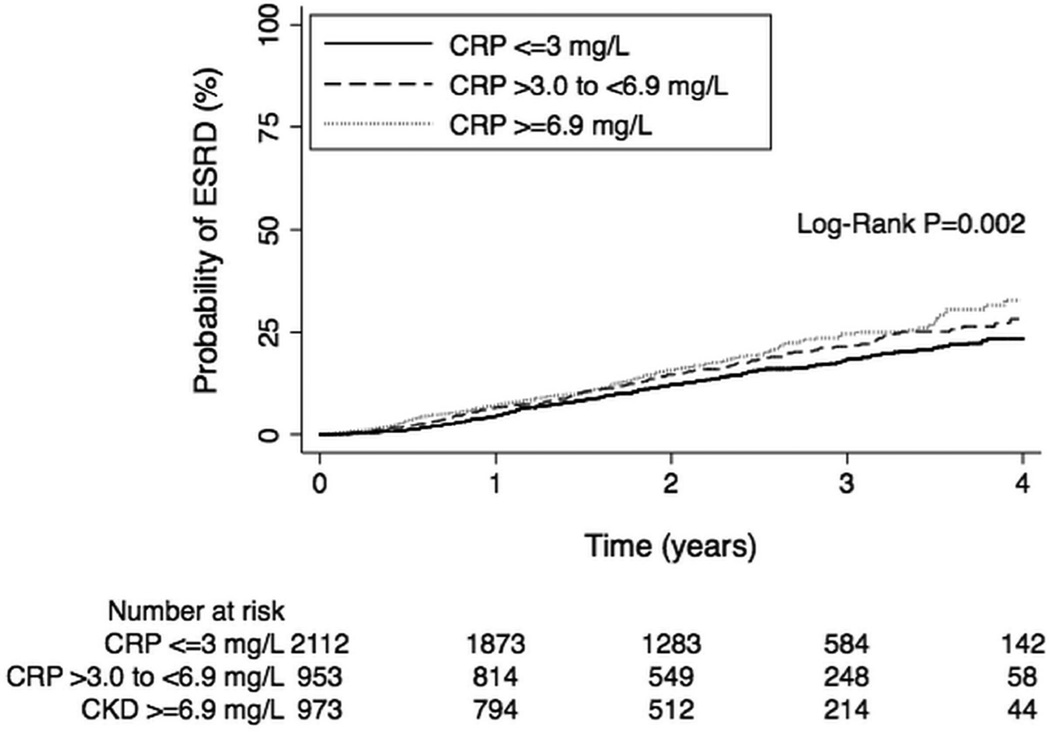
During a median follow-up time of 2.2 years, a total of 668 adjudicated ESRD events were recorded. In unadjusted analyses, compared with individuals with CRP ≤3.0 mg/L, those in the mildly elevated category had a 21% greater risk of developing ESRD (hazard ratio [HR], 1.21; 95% confidence interval [CI], 1.01–1.46; p=0.04), while those in the moderate-markedly elevated category had 37% greater risk (HR, 1.37; 95% CI, 1.14–1.64; p=0.001 [Figure 1]). In the fully adjusted analyses (Model 2), compared with individuals with CRP ≤3.0 mg/L, those in the moderate-markedly elevated category of baseline CRP had 32% greater risk of developing ESRD (HR, 1.32; 95% CI, 1.07–1.63; p=0.01; Tables 2 and 3). Effect estimates were essentially unchanged when time-varying coefficients were added to the model to address concerns of potential violation of the proportional hazards assumption (Table S1, available as online supplementary material). Using a multivariable competing risk model (with death as the competing outcome) gave similar findings, although the HR was somewhat attenuated (Table 2).
Figure 1.
Kaplan-Meier failure probabilities of the association of baseline categories of CRP with risk of ESRD.
Table 2.
Association of Baseline CRP Category with ESRD, Doubling of Scr, Composite of ESRD or Doubling of Scr, Composite of ESRD or Death, CV Composite, and Death alone
| Model: Events/Total | HR (95% CI) | P for trend |
|
|---|---|---|---|
| CRP >3.0–<6.9 mg/L | CRP ≥6.9 mg/L | ||
| Development of ESRD | |||
| Unadjusted: 668/4,038 | 1.21 (1.01 – 1.46) | 1.37 (1.14 – 1.64) | 0.001 |
| Adjusted: 598/3,642 | 1.08 (0.88 – 1.33) | 1.32 (1.07 – 1.63) | 0.01 |
| Competing Risk: 598/3,642 | 1.01 (0.81–1.25) | 1.23 (0.99–1.53) | 0.09 |
| Doubling of Scr | |||
| Unadjusted: 428/3,654 | 0.96 (0.76 – 1.22) | 1.06 (0.84 – 1.35) | 0.7 |
| Adjusted: 367/3,305 | 0.90 (0.69 – 1.18) | 0.94 (0.71 – 1.25) | 0.6 |
| Competing Risk: 367/3,305 | 0.86 (0.65–1.14) | 0.93 (0.69–1.25) | 0.5 |
| Composite of ESRD/Scr Doubling | |||
| Unadjusted: 871/4,038 | 1.16 (0.99 – 1.37) | 1.28 (1.09 – 1.51) | 0.002 |
| Adjusted: 769/3,642 | 1.05 (0.87 – 1.26) | 1.17 (0.97 – 1.42) | 0.1 |
| Competing Risk: 769/3,642 | 0.99 (0.82–1.20) | 1.12 (0.92–1.36) | 0.3 |
| Death or ESRD | |||
| Unadjusted: n=1,270/4,038 | 1.21 (1.05 – 1.39) | 1.57 (1.38 – 1.79) | <0.001 |
| Adjusted: n=1,139/3,642 | 1.11 (0.96 – 1.29) | 1.41 (1.21 – 1.64) | <0.001 |
| CV Composite Outcome* | |||
| Unadjusted: n=1,234/4,038 | 1.26 (1.09 – 1.45) | 1.77 (1.55 – 2.02) | <0.001 |
| Adjusted: n=1,111/3,642) | 1.16 (0.99 – 1.35) | 1.55 (1.34 – 1.81) | <0.001 |
| Association With Death From Any Cause | |||
| Unadjusted: n=807/4,038 | 1.20 (1.00 – 1.43) | 1.85 (1.57 – 2.16) | <0.001 |
| Adjusted: n=734/3,642 | 1.15 (0.95 – 1.39) | 1.59 (1.32 – 1.91) | <0.001 |
Note: CRP ≤ 3.0 mg/L was reference category for all. The multivariable models were adjusted for age, gender, race, estimated glomerular filtration rate, log-transformed urine protein-creatinine ratio, history of acute kidney injury, duration of type 2 diabetes mellitus, glycated hemoglobin, retinopathy, insulin use, body mass index, hemoglobin, serum albumin, coronary artery disease, cerebrovascular disease, peripheral arterial disease, heart failure, systolic blood pressure, low-density lipoprotein cholesterol concentration, statin therapy, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy, smoking status, ferritin, transferrin saturation, iron therapy and randomized treatment assignment. Multivariable adjusted competing risk models were fit, with death as the competing outcome, for the end points of ESRD, doubling of Scr and the composite of ESRD/doubling of Scr.
CI, confidence intervak; CRP, C-reactive protein; CV, cardiovacular; ESRD, end-stage renal disease; HR, hazard ratio; Scr, serum creatinine
CV composite included death from any cause, nonfatal MI, stroke, heart failure or hospitalization for myocardial ischemia
Table 3.
Unadjusted and Multivariable adjusted associations of Baseline Factors with Development of ESRD
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| CRP category | ||||
| ≤3.0 mg/L | 1.00 (reference) | - | 1.00 (reference) | - |
| >3.0 – <6.9 mg/L | 1.21 (1.01–1.46) | 0.04 | 1.08 (0.88–1.33) | 0.4 |
| ≥6.9 mg/L | 1.37 (1.14–1.64) | 0.001 | 1.32 (1.07–1.63) | 0.01 |
| Age, per 10 y older | 0.71 (0.67–0.77) | <0.001 | 0.91 (0.83–1.00) | 0.04 |
| Male sex, vs female | 1.75 (1.51–2.04) | <0.001 | 1.84 (1.53–2.20) | <0.001 |
| Race | ||||
| White | 1.00 (reference) | – | 1.00 (reference) | - |
| Black | 1.65 (1.38–1.97) | <0.001 | 1.44 (1.18–1.77) | <0.001 |
| Hispanic | 1.53 (1.23–1.90) | <0.001 | 0.87 (0.67–1.13) | 0.3 |
| Other | 1.13 (0.71–1.82) | 0.7 | 0.93 (0.57–1.53) | 0.8 |
| eGFR, per 10–mL/min/1.73 m2 increase |
0.40 (0.36–0.44) | <0.001 | 0.46 (0.41–0.50) | <0.001 |
| Log Urine PCR, per 1-unit greater | 2.04 (1.93–2.15) | <0.001 | 1.77 (1.63–1.92) | <0.001 |
| History of AKI, vs none | 1.63 (1.30–2.03) | <0.001 | 1.42 (1.12–1.81) | 0.004 |
| Duration of T2DM, per 12 mo longer |
1.01 (1.01–1.02) | 0.001 | 1.01 (1.00–1.02) | 0.03 |
| HbA1c, per 1% greater | 1.10 (1.05–1.15) | <0.001 | 0.98 (0.93–1.04) | 0.5 |
| Retinopathy, vs none | 1.83 (1.56–2.13) | <0.001 | 1.05 (0.87–1.26) | 0.6 |
| Insulin use, vs none | 1.55 (1.33–1.81) | <0.001 | 1.11 (0.92–1.33) | 0.3 |
| BMI, per10-kg/m2 greater | 0.85 (0.77–0.95) | 0.01 | 0.84 (0.73–0.95) | 0.01 |
| Hemoglobin, per 1-g/dL greater | 0.78 (0.73–0.84) | <0.001 | 0.95 (0.88–1.03) | 0.3 |
| Albumin, per 1-g/dL greater | 0.24 (0.21–0.27) | <0.001 | 0.58 (0.47–0.72) | <0.001 |
| CAD, vs none | 0.94 (0.80–1.10) | 0.4 | 1.10 (0.91–1.32) | 0.3 |
| CVD, vs none | 0.94 (0.77–1.16) | 0.6 | 1.13 (0.90–1.42) | 0.3 |
| PAD, vs none | 1.09 (0.90–1.32) | 0.4 | 1.00 (0.81–1.25) | 0.9 |
| Heart failure, vs none | 1.33 (1.13–1.55) | <0.001 | 1.46 (1.22–1.75) | <0.001 |
| SBP, per 10–mm Hg greater | 1.16 (1.12–1.21) | <0.001 | 1.01 (0.96–1.05) | 0.7 |
| Statin, vs none | 0.85 (0.73–1.00) | 0.04 | 0.94 (0.79–1.12) | 0.5 |
| ACEi/ARB, vs none | 0.86 (0.71–1.03) | 0.1 | 0.97 (0.79–1.19) | 0.8 |
| LDL cholesterol, per 10-mg/dL greater |
1.06 (1.04–1.08) | <0.001 | 1.01 (0.99–1.02) | 0.6 |
| Smoking | ||||
| Current | 1.00 (reference) | – | 1.00 (reference) | - |
| Former | 0.64 (0.48–0.86) | 0.004 | 0.84 (0.60–1.18) | 0.3 |
| Never | 0.56 (0.41–0.75) | <0.001 | 0.83 (0.59–1.15) | 0.3 |
| Ferritin, per 100-µg/L greater | 1.04 (1.03–1.06) | <0.001 | 1.01 (0.99–1.03) | 0.3 |
| Iron Saturation, per 1% greater | 1.02 (1.01–1.02) | <0.001 | 1.00 (1.00–1.01) | 0.3 |
| Iron therapy, vs none | 1.08 (0.93–1.26) | 0.3 | 1.07 (0.91–1.26) | 0.4 |
| Darbepoetin, vs placebo | 1.01 (0.87–1.18) | 0.9 | 1.08 (0.92–1.27) | 0.4 |
Note: The multivariable models were adjusted for age, gender, race, eGFR, log-transformed urine PCR, history of acute kidney injury, duration of T2DM, HbA1c, retinopathy, insulin use, BMI, hemoglobin, serum albumin, coronary artery disease, cerebrovascular disease, peripheral arterial disease, heart failure, systolic blood pressure, low-density lipoprotein cholesterol concentration, statin therapy, ACEi or ARB therapy, smoking status, ferritin, transferrin saturation, iron therapy and randomized treatment assignment.
Abbreviations: AKI, acute kidney injury; CI, confidence interval; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; PCR, protein-creatinine ratio; T2DM, type 2 diabetes mellitus; HbA1c, glycated hemoglobin; HR, hazard ratio; BMI, body mass index; CAD, coronary artery disease; PAD, peripheral arterial disease; SBP, systolic blood pressure; LDL, low-density lipoprotein; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
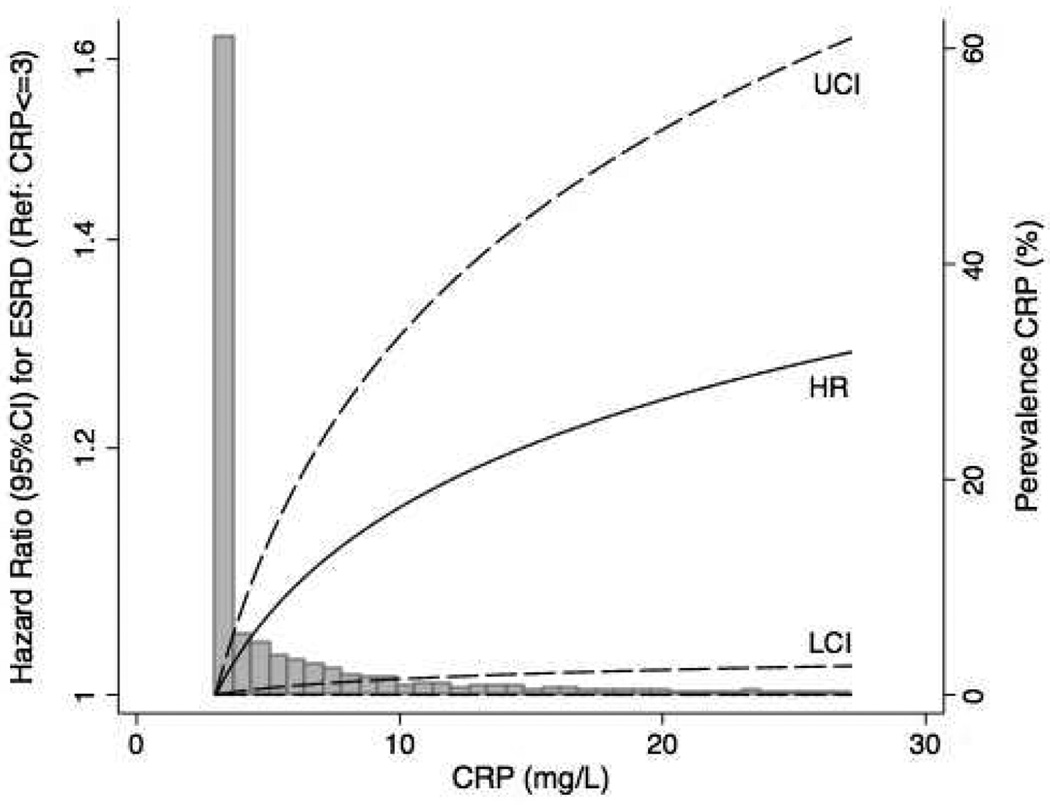
Similar trends were noted when sub-group analyses of individuals with baseline CRP >3.0 mg/L (n=1,926) were performed (Table S2). When CRP was examined as a continuous variable there was evidence for a monotonic association of higher CRP with increasing risk of ESRD (Figure 2).
Figure 2. Spline analysis of the association of CRP with ESRD.
Hazard ratios (HR; continuous line) and 95% Upper and Lower Confidence Intervals (UCI and LCI; dashed lines) for the association of CRP as a continuous variable with the risk of ESRD. For the purposes of this analysis, the 2,112 individuals with baseline CRP ≤3.0 mg/L were assumed to have a CRP=3 mg/L, which was taken as the reference value (large bar at the extreme left of the x-axis). Effect estimates were adjusted for age, gender, race, estimated GFR, log-transformed urine protein-creatinine ratio, history of acute kidney injury, duration of T2DM, HbA1c, retinopathy, insulin use, body mass index, hemoglobin, serum albumin, coronary artery disease, cerebrovascular disease, peripheral arterial disease, heart failure, systolic blood pressure, low-density lipoprotein concentration, statin therapy, ACE inhibitor or ARB therapy, smoking status, ferritin, transferrin saturation, iron therapy and randomized treatment assignment. Estimates are presented for CRP values between up to the 95th percentile of recorded values.
Association With Serum Creatinine Doubling and Composite of ESRD or Serum Creatinine Doubling
During a median follow-up of 2.1 years, the end point of doubling of serum creatinine occurred in 428 individuals. There was no significant association of elevated categories of baseline CRP with the risk of development of doubling of serum creatinine, or with the development of the composite outcome of ESRD or doubling of serum creatinine (Table 2). Effect estimates were essentially unchanged when time-varying coefficients were added to the model to address concerns of potential violation of the proportional hazards assumption (Table S1). Using a multivariable competing risk model (with death as the competing outcome) higher baseline CRP was not associated with either doubling of serum creatinine or the composite of ESRD/doubling of serum creatinine (Table 2).”
Association With Development of Composite of Death or ESRD, CV Composite Outcome, and Death From Any Cause
During a median follow-up period of 2.3 years, a total of 1,270 adjudicated composite events of death or ESRD were recorded. In unadjusted models, compared with CRP ≤3.0 mg/L, there was a 21% (HR, 1.21; 95% CI, 1.05–1.39; P=0.01) and 57% (HR, 1.57; 95% CI, 1.38–1.79; P<0.001) greater risk, respectively, of the combined end point with increasing categories of baseline CRP (Tables 2 and 3). In the fully adjusted model (Model 2), the effects estimates were attenuated, but remained significant for the moderate-markedly elevated CRP category (HR, 1.41; 95% CI, 1.21–1.64; p<0.001). Similar patterns of association were noted for the CV composite end point and all-cause death (Table 2).
Association With Change in eGFR
The association of baseline categories of CRP with the change in eGFR (mL/min/1.73 m2 per year) from randomization to development of ESRD or study exit was then assessed. The median change in eGFR in the whole cohort was −1.8 (95% CI, −5.9 to 1.4) mL/min/1.73m2 per year. In unadjusted analyses, compared with individuals with normal baseline CRP (≤3.0 mg/L), there was no evidence for a significant difference in the change in eGFR in either the mildly elevated (difference of −0.3 [95% CI, −1.1 to 0.5] mL/min/1.73 m2 per year; P=0.5) or moderate-markedly elevated (difference of 0.4 [95% CI, −0.4 to 1.2] mL/min/1.73 m2 per year; P=0.3) CRP categories. Similar findings were noted in the adjusted models: difference of −0.2 (95% CI, −1.0 to 0.5) mL/min/1.73 m2 per year (P=0.6) for the mildly elevated CRP category and a difference of 0.5 (95% CI, −0.3 to 1.3) mL/min/1.73 m2 per year (P=0.3) for the moderate-markedly elevated category, compared with individuals with a normal baseline CRP (≤3.0 mg/L; Table S3).
For those who ultimately went on to develop ESRD, the median last eGFR value was lower in patients with lower CRP measurements: 14.9 (IQR,11.6–20.9) mL/min/1.73 m2 for those with CRP ≤3.0 mg/L; 16.8 (IQR, 12.7–23.3) mL/min/1.73 m2 for those with CRP >3.0 to <6.9 mg/L; and 17.1 (IQR, 12.6–22.6) mL/min/1.73 m2 for those with CRP ≥6.9 mg/L (P for trend=0.02). The median time that these measurements were collected prior to the declaration of ESRD was 88 days for those with CRP ≤3.0 mg/L;106 days, CRP >3.0 to <6.9 mg/L; and 96 days, CRP ≥6.9 mg/L (P for trend=0.9).
Discussion
In this post-hoc analysis of the TREAT study we found that higher baseline concentrations of CRP were associated with greater risk of the adjudicated outcomes of ESRD and the composite of death or ESRD, but not with changes in eGFR or doubling of serum creatinine.
C-reactive protein (molecular weight, ~23 kDa) is a member of the pentraxin protein family, which is intimately involved in the activity of the innate immune system.23 It is primarily produced by hepatocytes, and its synthesis can be markedly up-regulated as part of the acute phase response.24 Although cause and effect has not been proven, prior epidemiological studies have demonstrated a clear association of higher CRP concentrations with the future development of MI and ischemic stroke.5,6,25 The association of higher levels of CRP with greater risk of CV disease also appears to be consistent in patients with a history of CKD.7,8
The presence of inflammation has been proposed as a common etiological pathway in the pathogenesis and progression of both CV and CKD. However, to date, the prior evidence linking CRP with progressive decline in kidney function is conflicting, with some studies reporting no association,7,15–17 while others have reported a positive association.11,14 Importantly, the ascertainment of the kidney outcome appears to play an important role in the heterogeneity of prior reports. For example, Fried et al. reported a significant association of baseline CRP with greater decline in eGFR (using the 4-variable MDRD Study equation) in 4,620 community-based individuals aged <65 years in the CV Health Study (25% had diabetes).10 However, an analysis of the same study by Keller et al. failed to find such an association when decline in eGFR was assessed using cystatin C.12 In another report, Hiramoto et al. found discrepant results in their analyses of 4,966 participants of the Multi-Ethnic Study of Atherosclerosis (MESA): baseline CRP was significantly associated with decline in eGFR using the CKD-EPI cystatin C equation, but not when using CKD-EPI creatinine equation.13 Indeed, some have questioned the accuracy of estimating equations for GFR in patients with T2DM,26 raising further concerns regarding the use of changes in such parameters over time.
Our analyses were performed in individuals with the triad of T2DM, anemia and CKD. We did not find a significant association of baseline CRP with changes in eGFR (calculated using serum creatinine) or doubling of serum creatinine during the study period, but we did report an association with development of the clinically important adjudicated end point of ESRD. While we recognize that the use of a linear slope to describe progression of CKD in a group of patients does not accurately reflect individual rates and patterns of progressive kidney function decline, it is also noteworthy that the last available eGFR prior to the development of ESRD was higher across increasing CRP categories.
As higher baseline CRP was not associated with progression of CKD as measured by a decline in eGFR (but was associated with progression to clinical ESRD), a tentative hypothesis might be that higher CRP identifies individuals with a greater burden of comorbidities who may be more likely to require earlier initiation of RRT. Alternatively, higher CRP may identify individuals with a greater burden of comorbid conditions leading to overestimation of measured GFR due to lower creatinine generation.27 Our finding that the risk of developing the CV composite outcome during the trial was higher in those with higher baseline CRP provides supportive evidence for both possibilities.
It is worth noting that moderately/markedly elevated CRP is associated with significantly greater risk of death and with a greater risk of ESRD. In the competing risk model, the association of elevated CRP with ESRD becomes attenuated and of borderline significance. However, in this model death precludes progression to ESRD, and so we may interpret this constellation of findings to indicate that, among those who have not died, higher CRP is a risk factor for ESRD. This interpretation is supported by the finding that higher CRP is associated with a greater risk of developing the composite end point of ESRD or death.
The major strengths of our study are the large number of independently adjudicated clinical end points and detailed data collection that occurred in the setting of a double-blind randomized controlled trial. However, there are limitations. The first relates to the use of standard sensitivity measurements of CRP with a lower limit of measurement of 3.0 mg/L, thereby restricting our ability to examine CRP as a continuous variable. On the other hand, despite the lack of high-sensitivity measurements, 48% of these patients with T2DM, CKD and anemia had baseline CRP measurements >3.0 mg/L, which highlights the prevalence of underlying inflammation in this important patient population. Another limitation is that only one baseline measurement of CRP was available. However, the stability of CRP in individual patients has been demonstrated previously (albeit in non-CKD patients),28 while the clinical relevance of our findings remains applicable, such that those with moderate-markedly elevated CRP measurements (≥6.9 mg/L) were significantly more likely to develop ESRD. That the direction of association was similar in the analyses of the composite outcome of ESRD or death, the CV composite outcome and the outcome of death from any cause provides additional reassurance of the clinical relevance of our findings. In the setting of a secondary analysis of a randomized trial, several potential confounding variables had to be considered in the model building process. In this regard it is possible that residual confounding based on variables not considered, or due to incomplete adjustment of those that were considered, still exists. This post-hoc study was not powered to assess the influence of anti-inflammatory therapies or other factors that could potentially modify the relationship between CRP and ESRD. Finally, the sample in this study consisted of patients with T2DM, CKD and anemia in the setting of a randomized controlled trial, limiting the generalizability to individuals without this comorbid disease pattern.
In conclusion, we found that higher baseline CRP was associated with a greater risk of developing ESRD and the composite of ESRD or death in patients with the triad of T2DM, CKD and anemia. When reviewing a patient with these comorbidities, the presence of an elevated CRP may prompt the clinician to explore for potentially modifiable sources of inflammation, such as infection. Whether interventions that lower CRP will result in a reduced risk of ESRD is unknown, but may provide opportunities for future research.
Supplementary Material
Acknowledgments
Support: Dr. Mc Causland is supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK102511.
Financial Disclosure: TREAT was funded by Amgen. This analysis was conducted independently by the authors and used the data set held at the Brigham & Women’s Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions: Research idea and study design: FMC, BC, MAP; data acquisition: EAB, K-UE, RK, ASL, JJVM, PP, GR, AKS, SDS, RDT, MAP; data analysis/interpretation: FMC, BC, MAP; statistical analysis: FMC, BC; supervision or mentorship: MAP. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. FMC, BC and MAP take responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplementary Material
Table S1: Association of baseline CRP category with ESRD, Scr doubling, composite of ESRD or Scr doubling, composite of ESRD or death, CV composite, and death alone.
Table S2: Association of baseline CRP tertiles with ESRD in patients with baseline CRP above lower limit of detection. Table S3: Difference in eGFR slope according to baseline CRP category.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Supplementary Material Descriptive Text for Online Delivery
Supplementary Table S1 (PDF). Association of baseline CRP category with ESRD, Scr doubling, composite of ESRD or Scr doubling, composite of ESRD or death, CV composite, and death alone.
Supplementary Table S2 (PDF). Association of baseline CRP tertiles with ESRD in patients with baseline CRP above lower limit of detection.
Supplementary Table S3 (PDF). Difference in eGFR slope according to baseline CRP category.
References
- 1.Levey AS, Stevens LA, Schmid CH, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saran R, Li Y, Robinson B, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2015;65(6 Suppl 1):A7. doi: 10.1053/j.ajkd.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet. 1997;349(9050):462–466. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Rifai N, Pfeffer MA, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98(9):839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 7.Menon V, Wang X, Greene T, et al. Relationship between C-reactive protein, albumin, and cardiovascular disease in patients with chronic kidney disease. American Journal of Kidney Diseases. 2003;42(1):44–52. doi: 10.1016/s0272-6386(03)00407-4. [DOI] [PubMed] [Google Scholar]
- 8.Busch M, Franke S, Müller A, et al. Potential cardiovascular risk factors in chronic kidney disease: AGEs, total homocysteine and metabolites, and the C-reactive protein. Kidney International. 2004;66(1):338–347. doi: 10.1111/j.1523-1755.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- 9.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16(2):489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 10.Fried L, Solomon C, Shlipak M, et al. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol. 2004;15(12):3184–3191. doi: 10.1097/01.ASN.0000146422.45434.35. [DOI] [PubMed] [Google Scholar]
- 11.Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G. Biomarkers of inflammation and progression of chronic kidney disease. Kidney International. 2005;68(1):237–245. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 12.Keller C, Katz R, Sarnak MJ, et al. Inflammatory biomarkers and decline in kidney function in the elderly: the Cardiovascular Health Study. Nephrol Dial Transplant. 2010;25(1):119–124. doi: 10.1093/ndt/gfp429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiramoto JS, Katz R, Peralta CA, et al. Inflammation and coagulation markers and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2012;60(2):225–232. doi: 10.1053/j.ajkd.2012.02.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kugler E, Cohen E, Goldberg E, et al. C reactive protein and long-term risk for chronic kidney disease: a historical prospective study. J Nephrol. 2015;28(3):321–327. doi: 10.1007/s40620-014-0116-6. [DOI] [PubMed] [Google Scholar]
- 15.Ortega O, Rodriguez I, Gallar P, et al. Significance of high C-reactive protein levels in pre-dialysis patients. Nephrol Dial Transplant. 2002;17(6):1105–1109. doi: 10.1093/ndt/17.6.1105. [DOI] [PubMed] [Google Scholar]
- 16.Sarnak MJ, Poindexter A, Wang S-R, et al. Serum C-reactive protein and leptin as predictors of kidney disease progression in the Modification of Diet in Renal Disease Study. Kidney International. 2002;62(6):2208–2215. doi: 10.1046/j.1523-1755.2002.00677.x. [DOI] [PubMed] [Google Scholar]
- 17.Shankar A, Sun L, Klein BEK, et al. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney International. 2011;80(11):1231–1238. doi: 10.1038/ki.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeffer MA, Burdmann EA, Chen C-Y, et al. A Trial of Darbepoetin Alfa in Type 2 Diabetes and Chronic Kidney Disease. N Engl J Med. 2009;361(21):2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer MA, Burdmann EA, Chen C-Y, et al. Baseline characteristics in the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT) Am J Kidney Dis. 2009;54(1):59–69. doi: 10.1053/j.ajkd.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 20.McMurray JJV, Uno H, Jarolim P, et al. Predictors of fatal and nonfatal cardiovascular events in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia: an analysis of the Trial to Reduce cardiovascular Events with Aranesp (darbepoetin-alfa) Therapy (TREAT) Am Heart J. 2011;162(4):748.e3–755.e3. doi: 10.1016/j.ahj.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Desai AS, Toto R, Jarolim P, et al. Association between cardiac biomarkers and the development of ESRD in patients with type 2 diabetes mellitus, anemia, and CKD. Am J Kidney Dis. 2011;58(5):717–728. doi: 10.1053/j.ajkd.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American statistical …. 1999 [Google Scholar]
- 23.Osmand AP, Friedenson B, Gewurz H, Painter RH, Hofmann T, Shelton E. Characterization of C-reactive protein and the complement subcomponent C1t as homologous proteins displaying cyclic pentameric symmetry (pentraxins) Proc Natl Acad Sci USA. 1977;74(2):739–743. doi: 10.1073/pnas.74.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006;119(2):166.e17–166.e28. doi: 10.1016/j.amjmed.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 26.Gaspari F, Ruggenenti P, Porrini E, et al. The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney International. 2013;84(1):164–173. doi: 10.1038/ki.2013.47. [DOI] [PubMed] [Google Scholar]
- 27.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function-- measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 28.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43(1):52–58. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.