Abstract
During fasting and vigorous exercise, a shift of brain cell energy substrate utilization from glucose to the ketone 3-hydroxybutyrate (3OHB) occurs. Studies have shown that 3OHB can protect neurons against excitotoxicity and oxidative stress, but the underlying mechanisms are unclear. Neurons maintained in the presence of 3OHB exhibited increased oxygen consumption and ATP production, and an elevated NAD+/NADH ratio. We found that 3OHB metabolism increases mitochondrial respiration which drives changes in expression of brain derived neurotrophic factor (BDNF) in cultured cerebral cortical neurons. The mechanism by which 3OHB induces Bdnf gene expression involves generation of reactive oxygen species, activation of the transcription factor NF-κB and activity of the histone acetyltransferase p300/EP300. Because BDNF plays important roles in synaptic plasticity and neuronal stress resistance, our findings suggest cellular signaling mechanisms by which 3OHB may mediate adaptive responses of neurons to fasting, exercise and ketogenic diets.
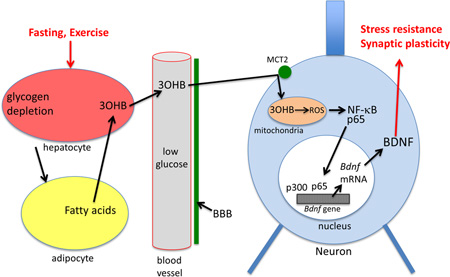
Graphical abstract
Introduction
Bioenergetic challenges such as vigorous exercise and intermittent fasting have been shown to enhance synaptic plasticity and neuronal stress resistance, and can protect neurons against dysfunction and degeneration in animal models of stroke, Alzheimer’s disease (AD), Huntington’s disease (HD) and Parkinson’s disease (PD) (for review see Mattson, 2012; Voss et al., 2013; Longo and Mattson, 2014; Neufer et al., 2015). Studies of responses of brain cells to exercise and intermittent fasting suggest that both types of energetic challenge up-regulate signaling pathways involved in cellular adaptations to stress and neuroplasticity. Examples include increased expression of genes encoding brain-derived neurotrophic factor (BDNF) (Marosi and Mattson, 2014), DNA repair enzymes (Yang et al., 2014), protein deacetylases SIRT1 and SIRT3 (Wang et al., 2013; Cheng et al., 2015) and antioxidant enzymes (Marosi et al., 2012).
Emerging evidence suggests that beneficial effects of exercise and fasting on the brain are mediated, at least in part, by signaling molecules released into the blood from peripheral organs. For example, levels of circulating insulin-like growth factor 1 (IGF1) increase in response to exercise, and IGF1 can enter the brain where it contributes to enhancement of cognitive function (Trejo et al., 2008). Recently, it was reported that a protein called irisin is released into the blood from muscle cells in response to exercise, and can then cross the blood-brain barrier and induce BDNF expression (Wrann et al., 2013). BDNF plays roles in the development, maintenance, and plasticity of the central and peripheral nervous systems (Chao, 2006). It promotes neurogenesis, enhances neurite outgrowth and synaptogenesis, and can prevent apoptosis (Mattson et al., 2004; Marosi and Mattson, 2014). Decrements in BDNF levels occur in vulnerable brain regions in several disorders including Alzheimer's disease (AD), Huntington's disease (HD) and major depression (Zuccato et al., 2009). Interventions that increase BDNF levels or activate TrkB have been shown to ameliorate clinical symptoms and underlying cellular and molecular neuropathologies in mouse models of AD, PD, HD, stroke and depression (Levivier et al., 1995; Gobbo et al., 2004; Spires et al., 2004; Duman et al., 2008; Devi et al., 2012).
Whether factors produced in the periphery mediate beneficial effects of fasting on neuroplasticity and neuronal stress resistance is unknown. A major physiological response to fasting and vigorous exercise is the mobilization of fatty acids from adipose cells and the hepatic production of ketone bodies from those fatty acids (Longo and Mattson, 2014). The major ketone, 3-hydroxybutyrate (3OHB), provides an energy source for neurons to sustain their function when glucose levels are reduced (Maalouf et al., 2009; Chowdhury et al., 2014). Ketogenic diets (McNally and Hartman, 2012) and fasting (Bruce-Keller et al., 1999) can protect hippocampal neurons against seizure-induced damage. Fasting and 3OHB can also counteract disease processes and improve functional outcome in animal models of Alzheimer’s and Parkinson’s diseases, stroke and traumatic brain injury (Duan and Mattson, 1999; Anson et al., 2003; Arumugam et al., 2010; Kashiwaya et al., 2013, Prins et al., 2014; Greco et al., 2015; Yin et al., 2015). Clinical data also suggest that ketone bodies can have a therapeutic benefit in neurodegenerative diseases such as AD and PD (Reger et al., 2004; Vanitallie et al., 2005).
In addition to being a source of acetyl coenzyme A for neuronal energy metabolism, recent findings suggest that 3OHB influences certain cellular signaling pathways. For example, 3OHB can inhibit or activate protein deacetylases (Newman and Verdin, 2014; Scheibye-Knudsen et al., 2014), and can inhibit mitochondrial membrane permeability transition pore opening (Kim et al., 2015). While multiple effects of energy restriction and exercise on neuroplasticity and neuroprotection have been linked to BDNF signaling (Marosi and Mattson, 2014), the mechanisms by which these bioenergetic challenges induce BDNF signaling and adaptive stress response pathways are unknown. Here we show that 3OHB changes neuronal bioenergetics by increasing mitochondrial respiration that results in increased BDNF expression.
Methods
Animals
All animal procedures were approved by the Animal Care and Use Committee of the National Institute on Aging Intramural Research Program. Male C57BL/6 mice (purchased from Jackson Laboratories, Bar Harbor, ME) were housed individually with (n = 8) or without (n = 8) running wheels beginning at 4 months of age. The mice were provided food and water ad libitum, and were maintained on a 12-h light/12-h dark cycle. After 6 weeks, mice in each group were euthanized, their brains were removed, flash-frozen, and stored at −80 °C. The BDNF protein levels in the brain were detected using a BDNF ELISA (Promega). Glucose and 3-hydroxybutyrate concentrations in the plasma were quantified using a Roche Cobas Fara II analyzer (Roche Diagnostic Systems; Montclair, NJ).
Cell culture and in vitro treatment
Cultures of cerebral cortical or hippocampal neurons were prepared from Sprague-Dawley rat embryos (male and female) at 18 days of gestation using methods similar to those described previously (Glazner et al., 2000). Dissociated cells were seeded into polyethyleneimine-coated plastic dishes or glass coverslips in MEM medium supplemented with 10% fetal bovine serum at a density of 80,000 cells/ cm2. After cells attached to the substrate, the medium was replaced with Neurobasal medium containing 5% B27 minus antioxidants, 1% GlutaMAX™ and 1% Anti-Anti (Gibco). Experiments were initiated on culture day 10. 3-D-hydroxybutyrate sodium salt and N-acetyl cysteine, cyclosporin A and sodium butyrate were obtained from Sigma. AR-C155858 was obtained from Tocris. SN-50 was purchased from Santa Cruz.
Western blotting and immunoprecipitation
Cells were lysed in RIPA cell lysis buffer containing protease inhibitors (Roche) and phosphatase inhibitors (Sigma). Lysates were sonicated for 1 min and centrifuged at 14, 000 × g for 10 min at 4 °C. Twenty micrograms of proteins were resolved in 4–10% NuPAGE Bis-Tris Mini gels (Invitrogen) and electrophoretically transferred to a nitrocellulose membrane (Invitrogen). The unspecific binding sites were blocked in blocking solution containing 5% milk or bovine serum albumin (BSA) for 1 h at room temperature. Then the membranes were incubated overnight in primary antibodies followed by incubation in secondary antibodies for 1 h at room temperature. The reaction products in the membranes were visualized using an enhanced chemiluminescence Western Blot Detection Kit (Thermo Scientific). For immunoprecipitation experiments, 500 µg of total protein lysates were incubated overnight at 4°C with anti-Flag agarose beads (Santa Cruz). The primary antibodies those against NF-κB p65 (Thermo Scientific); p300 (Abcam); SOD2 (Santa Cruz); total OXPHOS (Abcam) and β-actin (Sigma-Aldrich). The densities of protein bands were quantified using ImageJ software (National Institutes of Health, Bethesda, MD) and normalized to the β-actin band intensity for the same sample.
BDNF ELISA
The BDNF concentrations in the primary neuronal samples were determined by enzyme-linked immunosorbent assay (ELISA) (Promega). The assay was performed according to the manufacturer’s instructions.
Quantitative reverse transcriptase PCR amplification
Total RNAs were isolated from lysates of cultured neurons using the RNAeasy purification kit (Qiagen). Isolated RNA was reverse-transcribed into cDNA by following the protocol from SuperScript III First-Strand Synthesis System (Invitrogen). Real-time polymerase chain reaction (PCR) was performed using a PTC-200 Thermal Cycler (Bio Rad) using 2× SYBR Green Master Mix (Applied Biosystems). Cycle parameters for all amplifications were as follows: 1 cycle of 95°C for 5 min, 40 cycles of 95°C for 30 sec, and 72°C for 1min, followed by melt-curve analysis (55°C+ for 10 sec × 80 cycles). The mRNA level of the targeted genes was normalized to the level of β-actin mRNA. Primers specific for the following mRNAs were used: mct2: sense, 5′- GGCCTTCGGTAGGATTAATAG-3′, antisense, 5′-ATGCCTGATGATAACACGACT-3′; bdnf: sense, 5′- CTGCGCCCATGAAAGAAG-3′, antisense, 5′-CCAGCAGCTCTTCGATCA-3′; β-actin sense, 5′- TCATGAAGTGTGACGTTGACATCCGTAAAG-3′, antisense, 5′-CCT AGAACGATTTCGGGTGCACGATGG AGG-3′,tfam, sense:5` GAAAGCACAAATCAAGAGGAG-3`, antisense: 5`-CTGCTTTTCATCATGAGACAG-3`, pgc1α, sense: 5`-GTGCAGCCAAGACTCTGTATGG-3`, antisense:5`GTCCAGGTCATTCACATCAAGTTC-3`, nrf1, sense: 5`-TTACTCTCTGCTGTGGCTGATGG-3`, antisense: 5`-CCTCTGATGCTTGCGTCGTCT-3`, nrf2, sense: 5`-TTCCTCTGCTGCCATTAGTCAGTC-3`, antisense: 3`-GCTCTTCCATTTCCGAGTCACTG-3`
Mitochondrial DNA relative copy number
DNA from primary cortical neurons was extracted using a QIAamp DNA blood extraction kit (QIAGEN). Mitochondrial DNA (mtDNA) copy number was measured using real time quantitative PCR. One primer pair specific for the mtDNA (12sR sense:5`CTCAAGACGCCTTCGCTAG `, antisense: 5`-CGTATGACCGCGGTGGCT-3`,) designed for relative quantification for mtDNA copy number. 20 ng of DNA was added to each reaction. Threshold cycle value differences were used to quantify mtDNA copy number relative to the actin gene sense: 5-GAAATCGTGCGTGACATTAAAG-3`, antisense: 5`-ATCGGAACCGCTCATTG-3`.
Measurement of metabolic activity
Oxygen consumption and glycolytic rate were measured using the Seahorse XF-96 instrument (Seahorse Biosciences, North Billerica). Neurons in 96 well Seahorse plates (40,000/well) were incubated in the treatment conditions for 1 and 24 hours and then the assay was performed in serum free unbuffered XF assay medium containing 1 mM pyruvate and 2 mM glutamax (pH 7.4). Respiration was measured in four consecutive 3-minute time periods. The basal respiration rate was measured during the first 3-minute period. Next, 1 µM oligomycin was added to evaluate the coupling efficiency. Maximal respiration was then initiated with 1 µM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP). Finally, 0.51 µM antimycin and rotenone were added and the last measurements were performed.
Luciferase assay
Cell transfections were performed on HEK293 cells that were maintained in DMEM supplemented with 10% fetal bovine serum. The cells were co-transfected with 2 µg Bdnf promoter promoter sequences to drive luciferase expression and 2 µg pRL-TK vector expressing Renilla luciferase (Promega) using Fugene 6 (Roche) according to the manufacturer’s instructions. Forty-eight h after transfection, cells were incubated in the presence of experimental treatments for 6 h. Luciferase activity was quantified using a Dual-Luciferase-Reporter System (Promega). Luciferase activity in each sample was normalized to the internal control renilla luciferase activity. Luminescence was expressed in an arbitrary scale as relative light units (RLU).
ROS and NAD+/NADH measurements
Cellular ROS were measured in neurons loaded with the fluorescent probe 2, 7-dichlorofluorescein diacetate (DCF) and dihydrorhodamine 123 (DHR123) using methods described previously (Wang and Joseph, 1999). In brief, DCF and DHR123 were added to cultures in a final concentration of 5 µM and then incubated at 37 °C for 20 minutes. After washing the cultures with PBS, the fluorescent intensity was measured by microplate-reader. The excitation and emission wavelengths for DCF were 488 and 510 nm, for DHR:500 and 536 nm. The NAD+/NADH ratio was measured using NAD+/NADH-Glo™ bioluminescent assay using the provided protocol (Promega). The assay uses NAD Cycling Enzyme that converts NAD+ to NADH. In the presence of NADH, a reductase enzymatically reduces a proluciferin reductase substrate to luciferin. The amount of light produced is proportional to the amount of NAD+ and NADH in a sample. Briefly the neurons were washed with PBS and the pellets were collected for the separate measurements of NAD+ and NADH. The neurons were homogenized in PBS and were split into two samples: One sample is treated with acid (0.4N HCl) to quantify NAD+, and the other is treated with base to quantify NADH (0.5M Trizma®base). The oxidized form (NAD+) is selectively destroyed by heating in basic solution, while the reduced form (NADH) is not stable in acidic solution. Thus, luminescence from acid-treated samples is proportional to the amount of NAD+, the luminescence from base-treated samples is proportional to the amount of NADH.
Histone acetyltransferase (HAT) activity
The HAT activity was measured using the colorimetric HAT activity assay kit (Abcam) according to the manufacturer’s instructions. Briefly, neuronal lysates were incubated with HAT substrates and NADH-generating enzyme in HAT assay buffer with the presence of and absence of 8mM 3OHB for 1 hr at 37°C. Absorbance was determined at 450 nm in an ELISA plate reader, with active nuclear extract used as a positive control and standard. The HAT activity was normalized to the protein content of the cell lysates.
ATP content
The ATP content in the neurons was measured using Molecular Probes® ATP Determination Kit bioluminescence according to the manufacturer’s instructions. The assay is based on luciferases requirement for ATP in producing light. The assay was performed on a 96-well plate, the number of neurons was 40,000 per well.
Cell viability assay
Cell survival was monitored 24 h after 100 µM kainic acid (KA) treatment by the determination MTS reduction. After 22 h of KA the neurons were incubated with MTS for 1 h. Formazan absorbance was measured spectrophotometrically at 570 nm. Cell viability is expressed as percentage of cell viability relative to the untreated control.
Chromatin immunoprecipitation
ChIP assays were performed using a Pierce Magnetic ChIP Kit according to the manufacturer’s instructions. Briefly, rat primary neurons were treated with 3OHB for 6 h followed by fixing in 1% formaldehyde and quenching with glycine. The cells were pelleted, lysed and subjected to MNase digestion. The lysates were sonicated to shear lengths of 200–1000 base pair DNA fragments using 15 sets of 10 sec pulses of sonication. The length of the fragments was verified by running in a 1% agarose gel. Ten percent of the digested chromatin was saved as total input sample and the remainder was incubated overnight under rotation at 4 °C with 5 µg of anti-p300 or normal IgG followed by incubation with ChIP Grade Protein A/G Magnetic Beads for 2 h at 4°C with mixing. Beads were then washed and incubated at 65°C for 30 minutes. A total of 20 mg/ml Proteinase K was added to all IP and total input samples and incubated for 1.5 h at 65°C. Then the DNA was purified on DNA Clean-UpColumns. DNA pellets were re-suspended in 50 µl of sterile water. The purified DNA was used directly as a template for PCR. The following exon specific BDNF primers were used in the qPCR reaction: exon I, sense, 5′- TGAGAGCTTGGCTTACACCG -3′, and antisense, 5′- GATGACTAGGCGAGAGGCAC -3′; exon II, sense, 5′- CTGCGTGGAACAAACTTGGG -3′, and antisense, 5′- TTAACCCCCTTGCGGATGTC -3′; Exon III, sense, 5′- CGGTGTCGCCCTTAAAAAGC -3′, and antisense, 5′- ACCCAGTATACCAACCCGGA -3′; exon IV, sense, 5′- GCGCGGAATTCTGATTCTGG -3′, and antisense, 5′- CTGCCTTGACGTGAGCTGTC -3′. The qRT-PCR was performed using SYBR GREEN MasterMix (Applied Biosystems). Cycling parameters for all amplifications were as follows: 1 cycle of 95°C for 15 min, 40 cycles of 95°C for 15 sec, and 65°C for 1min, followed by melt-curve analysis (55°C+ for 10 sec × 80 cycles). Data were normalized to input and nonspecific IgG, and fold increase versus control was calculated.
Plasma chemistry
Glucose and 3-hydroxybutyrate concentrations were quantified in serum using a Roche Cobas Fara II analyzer (Roche Diagnostic Systems; Montclair, NJ).
Statistical analyses
Data are presented as mean ± SEM. Statistical analyses were performed using one or two-way ANOVA for comparisons among the different treatment groups at different time points, and Tukey post hoc tests were performed for pairwise comparisons among treatment groups. For comparisons involving only two groups, Student's t-test was performed. Significant differences of p<0.05 are identified with an asterisk (*p < 0.05, **p < 0.01, ***p < 0.005). All experiments were repeated at least 3 times; fold changes to the untreated control were calculated and subjected to data analysis.
Results
3OHB is utilized by cortical neurons that increases oxidative metabolism
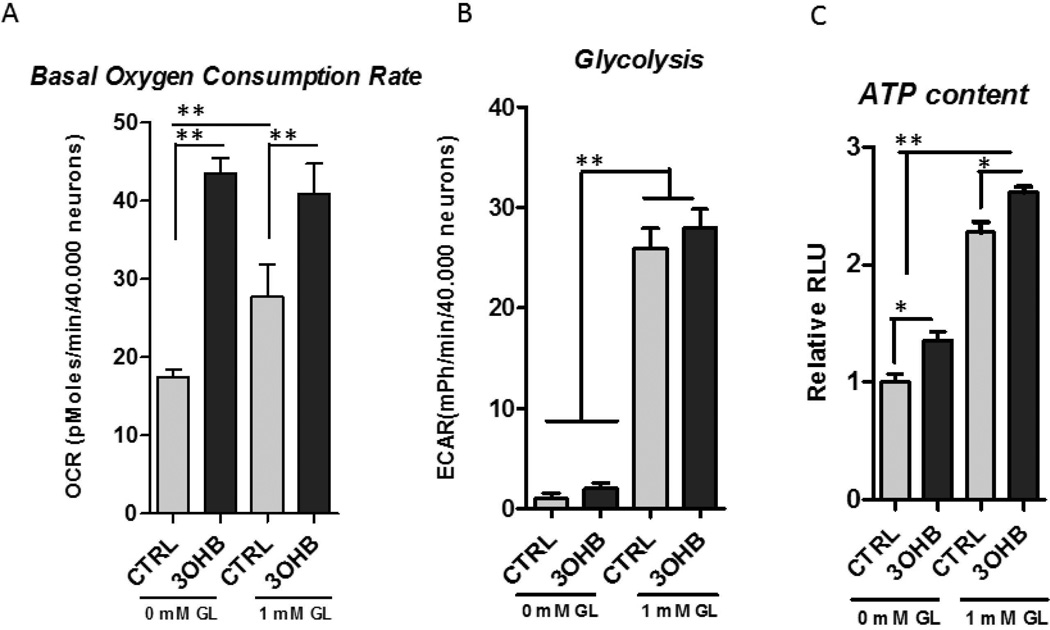
The primary cortical neurons were incubated in the presence and absence of glucose for 1 hour and the basal oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were measured with Seahorse XF-96 extracellular flux analyzer. Acute, 1hour incubation with 1mM 3OHB increased the basal oxygen consumption rate (Fig. 1A) and the ATP content (Fig. 1C) although it did not affect the glycolysis in the neurons (Fig. 1B) indicating that 3OHB provides an energy substrate that shifts the neuronal metabolism towards oxidative state.
Figure 1. 3OHB increases basal oxygen consumption and ATP production without altering glycolytic rate.
A. 3OHB (1 mM) elevates the basal oxygen consumption rate (OCR) in primary cortical neurons in the presence and absence of 1 mM glucose (**p<0.01). B. The extracellular acidification rates (ECAR) were not altered by 1 mM 3OHB. C. Acute, 1 hour incubation with 1 mM 3OHB increases cellular ATP content (*p<0.05).
3OHB induces metabolic rate by increasing functional changes in the mitochondria
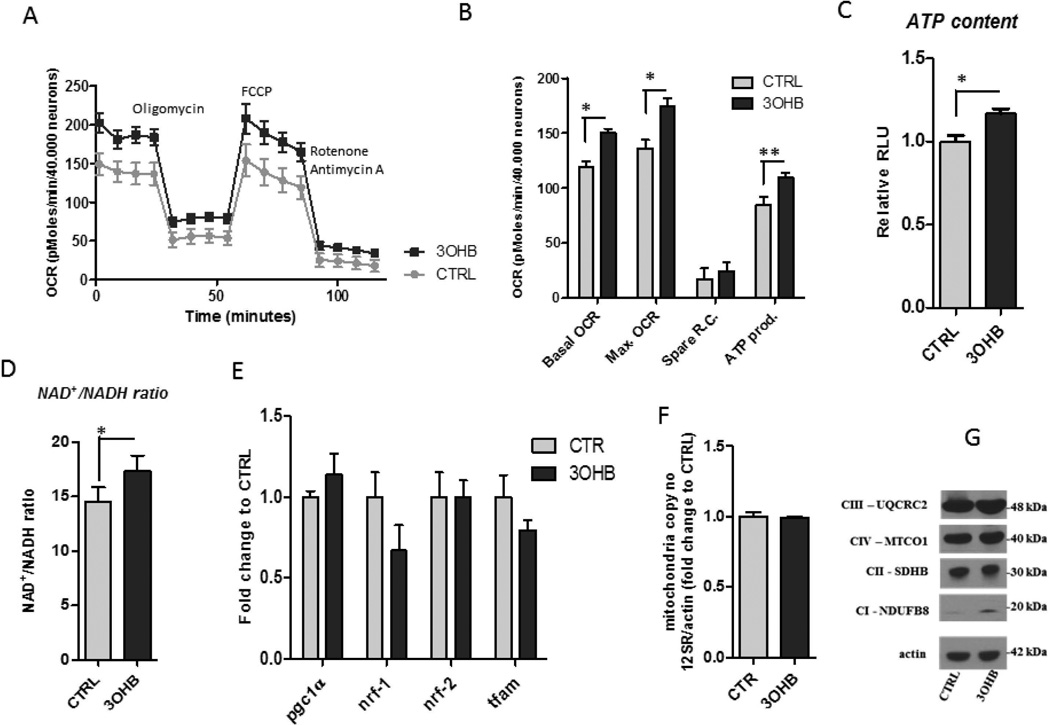
To further examine the effect of 3OHB on neuronal energy metabolism, we measured oxygen consumption in cortical neurons treated with 3OHB for 24 hours. After the pre-incubation in the treatment conditions, the metabolic assays were performed in serum free unbuffered XF assay medium which contained equal amounts of energy substrates in each condition (5 mM glucose, 1 mM pyruvate, and 2 mM glutamax). The basal oxygen consumption rate (OCR) normalized to neuronal numbers was elevated in the 3OHB treated neurons (Fig. 2A, B). The ATP production was estimated from the OCR rates following the 1 µM oligomycin treatment. ATP production was higher in the 3OHB treated neurons, which was confirmed by the direct measurement of cellular ATP content (Fig. 2C). The FCCP (2.5 mM) stimulated maximal OCR was significantly elevated with 3OHB treatment showing an increase in mitochondrial oxidative capacity (Fig. 2B). The stimulation of maximal respiration by 3OHB was absent in high glucose conditions (Fig. S1). The NAD+/NADH ratio was significantly increased in 3OHB-treated neurons (Fig. 2D), suggesting enhanced mitochondrial electron transport chain (ETC) activity. Next we investigated whether the enhanced metabolic rate of 3OHB-treated neurons is related to increased numbers of mitochondria or altered expression of the ETC proteins. We found that the total mtDNA content and the mRNA levels of transcription factors that regulate mitochondrial biogenesis remained unchanged after 3OHB treatment for 24 h (Fig. 2E, F), indicating that 3OHB treatment did not affect the number of mitochondria/neuron. 3OHB-treated neurons exhibited an increase in the level of the mitochondrial complex I protein NDUFB8, but not three other mitochondrial complex proteins, UQCRC2, MTCO1 and SDHB (Fig. 2G) further supporting no effect of 3OHB on mitochondrial numbers. NDUFB8 is an accessory subunit of the mitochondrial membrane respiratory chain NADH dehydrogenase (Complex I). The increase in levels of NDUFB8 in response to 3OHB may therefore lead to a higher NAD+ turnover rate (Fig. 2D) and an increase of ETC-mediated cellular respiration (Fig. 2B).
Figure 2. 3OHB stimulates mitochondrial respiration.
The neurons were pretreated with control medium containing 1 mM glucose (CTRL), or medium containing 8 mM 3OHB and 1 mM glucose for 24 hours, and then incubated with unbuffered Seahorse XF medium for 1 hour. A. Key parameters of mitochondrial function were determined by measuring the oxygen consumption rate (OCR) of the neurons in the presence of modulators of respiration that target components of the electron transport chain. The compounds (oligomycin, FCCP, and a mix of rotenone and antimycin A) were serially applied the neurons and the recorded OCRs were used to quantify relative levels of ATP production and maximal respiration, respectively. Spare respiratory capacity was calculated using these parameters and basal respiration. Basal and FCCP-induced oxygen consumption rates (OCR) were elevated in primary cortical neurons treated with 8 mM 3OHB for 24 hours compared to control cultures (CTRL). Representative graph shows the basal oxygen consumption rates (OCR) and the OCR values after oligomycin (1 µM), FCCP (1 µM) and rotenone plus antimycin A (0.5 µM) treatments measured using a Seahorse XF-96 instrument. p<0.05 versus CTRL. B. Values for basal OCR, maximal OCR, spare respiratory capacity (RC) and ATP production in cortical neurons that had been exposed for 24 h to vehicle (CTRL) or 8 mM 3OHB (n = 5 separate cultures). p<0.05, p<0.01, p<0.001 versus CTRL. C and D. Cortical neurons exhibited higher ATP levels (C) and NAD+/NADH ratio (D) after incubation with 8 mM 3OHB for 6 h compared to CTRL (n = 3 separate cultures). p<0.05. E. Results of quantitative PCR analysis reveal that 8 mM 3OHB treatment does not affect the expression of genes involved in the regulation of mitochondrial biogenesis (n = 3 separate cultures). F. Results of quantitative PCR analysis show that the mitochondrial DNA copy number is not affected by 3OHB (24 h treatment with 8 mM 3OHB) (n = 3 separate cultures). Mitochondrial DNA copy number was quantified as described in the Methods section. G. Immunoblots showing relative levels of the indicated mitochondrial proteins in cortical neurons that were treated with vehicle (CTRL) or 8 mM 3OHB for 24 hours.
3OHB increases Bdnf gene and protein expression
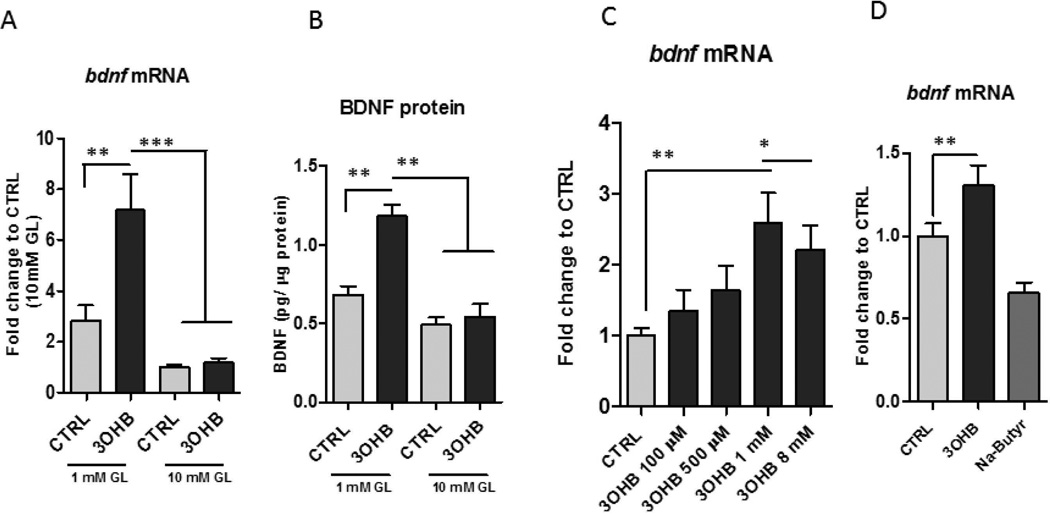
Primary neuronal cultures are typically maintained in medium containing a supraphysiological concentration of glucose (10–25 mM), whereas levels of extracellular glucose in the brain in vivo normally ranges from 0.2 to 2.5 mM in accord with changes in plasma glucose levels from hypoglycemia to hyperglycemic conditions (Silver et al., 1994). We found that when cortical and hippocampal neurons were incubated in medium containing 1 mM glucose and treated for 24 h with 0.1 – 8 mM 3OHB, a concentration range that includes 3OHB concentrations ranging from those occurring during non-fasting conditions (0.1 mM) to a concentration achieved during extended fasting (8 mM) (Owen et al., 1967), Bdnf mRNA levels were significantly increased by approximately 3-fold within 6 h (Fig. 3A, B, and S2). In contrast, Bdnf expression was unaffected by 3OHB in cortical neurons incubated in medium containing a high glucose concentration (10 mM). Similarly, there was a significant increase in the BDNF protein levels in the 3OHB treated neurons incubated in medium containing 1 mM glucose, but not in those incubated in medium containing 10 mM glucose (Fig. 3B). There were no significant differences in the Bdnf gene expression in neurons incubated in low compared to high glucose conditions. (Fig. 3A, B). In a concentration-response study, we found that when cortical neurons were incubated in medium containing 1 mM glucose and treated for 24 h with 0.1 – 8 mM 3OHB, low concentrations of 3OHB (0.1 and 0.5 mM) had no significant effect on Bdnf mRNA levels, whereas 1 mM 3OHB significantly increased Bdnf mRNA levels (Fig. 3C). Previous studies have shown that, similar to butyrate, 3OHB is an endogenous and specific inhibitor of class I histone deacetylases (Shimazu et al., 2013). We investigated whether sodium – butyrate (Na-butyrate) can similarly regulate the expression of Bdnf. No induction of Bdnf by Na-butyrate was found (Fig. 3 D), indicating that changes of Bdnf expression are not driven by the HDAC1 inhibitory properties of 3OHB.
Figure 3. 3OHB treatment increases BDNF expression in cerebral cortical neurons.
Cortical neurons were incubated in medium containing either a low (1 mM) or high (10 mM) concentration of glucose (GL) and were then exposed to 8 mM 3OHB or vehicle control (CTRL). Neurons were then harvested after either 6 hours of 3OHB treatment for measurement of Bdnf mRNA levels (A) or 24 hours for measurement of BDNF protein levels (B). Values are the mean and SEM of determinations made in 5 separate experiments. **p<0.01, ***p<0.001. C. Bdnf gene expression levels were increased by 3OHB in primary cortical neurons in a concentration-dependent manner in 1 mM glucose condition. ***p<0.001 D. No induction of Bdnf gene by Na-butyrate (8 mM) was detected in 1mM glucose condition.
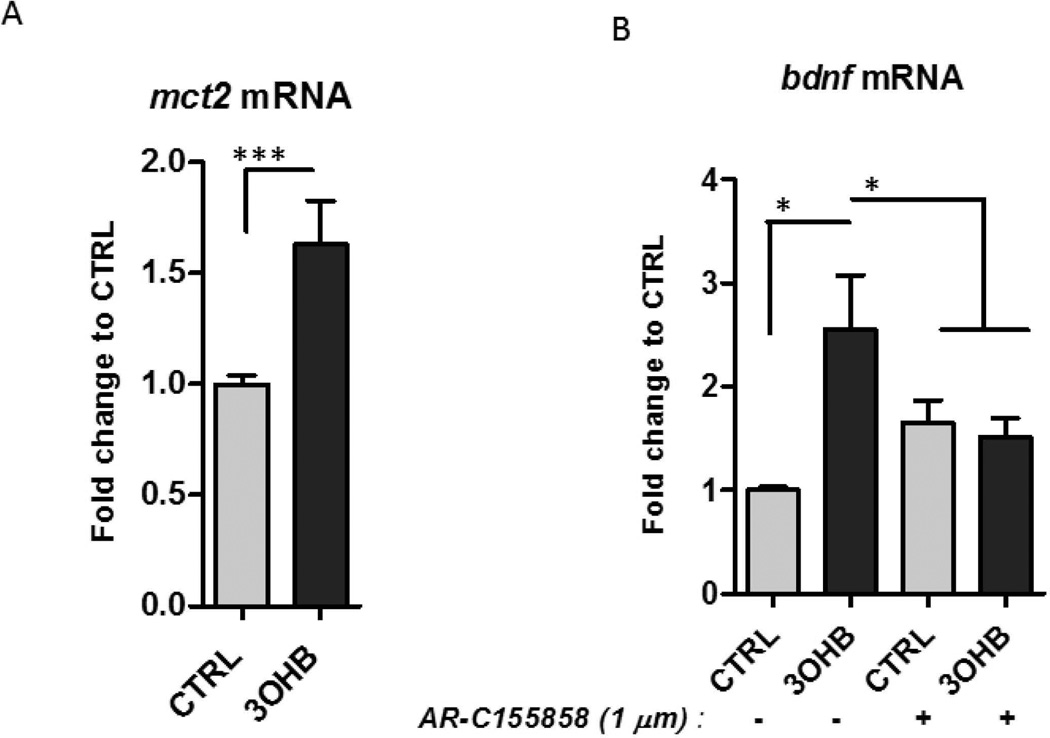
The monocarboxylic acid transporter 2 (MCT2) mediates the uptake of 3OHB into neurons (Martin et al., 2006; Chiry et al., 2008). We found that mct2 mRNA levels were increased significantly in response to 3OHB (Fig. 4A). Pre-incubation with an inhibitor of the monocarboxylate transporters (1 µM AR-C155858 (AR-C)) abolished the enhancement in Bdnf mRNA levels by 3OHB (Fig. 4B), consistent with a requirement for cellular uptake of 3OHB in the mechanism by which 3OHB induces BDNF expression.
Figure 4. 3OHB treatment increases BDNF expression in cerebral cortical neurons in a MCT2-dependent manner.
A. Cortical neurons were incubated in medium containing 1 mM glucose and were then exposed to 8 mM 3OHB for 6 hours. Levels of mct2 mRNA were quantified (n =5 separate cultures). ***p<0.001. B. Cortical neurons (in medium containing 1 mM glucose) were pre-incubated with or without the MCT2 inhibitor AR-C155858 (1 µM) for 1 hour. The neurons were then treated with 8 mM 3OHB or vehicle control for 6 hours and were then processed for measurement of Bdnf mRNA levels (n = 4 separate cultures). *p<0.05.
NF-κB mediates 3OHB-induced Bdnf gene transcription
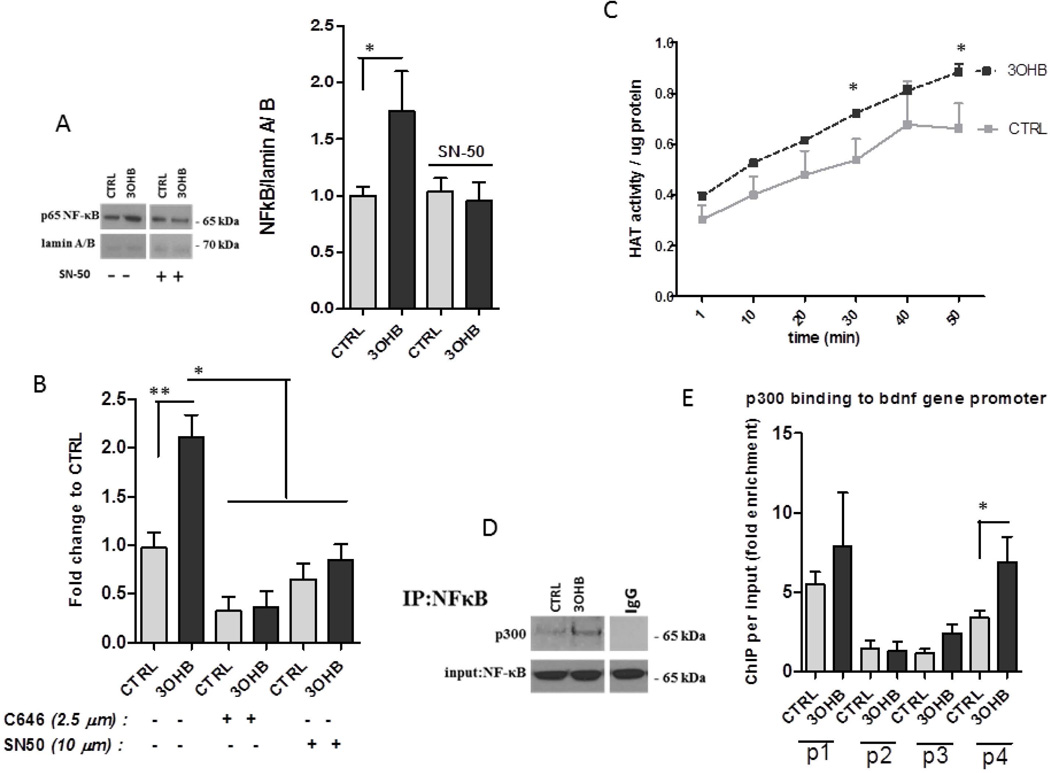
The rat Bdnf gene was reported to have four different promoters that regulate expression of distinct exons (Timmusk et al., 1993) that have been shown to be responsive to various stimuli including electrical activity, exercise, stress and calcium influx (Shieh and Ghosh, 1999; Sakata et al., 2010). To determine if how 3OHB induces Bdnf gene expression we transfected HEK293 cells with the luciferase gene driven by Bdnf promoter I, II, III or IV. We found that 3OHB induces expression from Bdnf promoter IV, but not from promoters I, II or III (Fig. S3). There was a slight increase in luciferase activity of promoter I as well so it is likely that the promoter I might also contribute to the effect of 3OHB on Bdnf gene expression. Two transcription factors that have been shown to induce Bdnf expression are cAMP response element-binding protein (CREB) and nuclear factor-κB (NF-κB) (Tao et al., 1998; Marini et al., 2004). We tested the phosphorylation of CREB and the nuclear translocation of NF-κB in response to 3OHB treatment. There was a non-significant trend towards increased CREB phosphorylation in 3OHB-treated neurons (K. Marosi, unpublished data). When neurons were treated with 3OHB for 6 h there was an increased amount of the NF-κB subunit p65 in the nucleus compared to vehicle-treated neurons (Fig. 5A). Treatment of neurons with the NF-κB inhibitor SN-50 (10 µM) completely blocked the ability of 3OHB to increase the level of nuclear p65 (Fig. 5A). Inhibition of NF-κB using SN-50 significantly suppressed 3OHB-induced Bdnf gene expression (Fig. 5B).
Figure 5. Evidence for the involvement of NF-κB and p300 in 3OHB-induced Bdnf gene transcription.
A. Cortical neurons were pretreated for 1 hour without or with the NF-κB inhibitor SN50, were then treated with vehicle (CTRL) or 8 mM 3OHB for 6 hours, and proteins in nuclei were isolated from the neurons and were subjected to immunoblot analysis with antibodies against the p65 subunit of NF-κB or the nuclear lamin A/B proteins. B. NF-κB inhibition using 10 µM SN-50 suppressed the induction of induction of Bdnf mRNA expression by 8 mM 3OHB in primary cortical neurons (n = 3 separate cultures). *p<0.05. C Global histone acetyltransferase activity (HAT) was measured in primary cortical neurons at the indicated time points after exposure to vehicle (CTRL) or 8 mM 3OHB (n = 3 separate cultures). *p<0.05. D. Cortical neurons were exposed to vehicle (CTRL) or 8 mM 3OHB for 6 hours and then the extracts were subjected to immunoprecipitation with an antibody against the p65 NF-κB protein and blotted with p300 antibody. Total NF-κB levels were used as the loading control. E. Chromatin immunoprecipitation performed on cortical neurons that had been exposed to vehicle (CTRL) or 8 mM 3OHB for 6 hours. Bdnf promoter targets (I, II, III and IV) were evaluated by quantitative PCR. Quantification of immunoprecipitated material relative to its input level is represented as the fold induction (n = 3 separate cultures). *p< 0.05. Graph B shows that inhibition of p300 blocks the effect of 3OHB on Bdnf expression Cortical neurons were pre-incubated with or without C646 (2.5 µM), a specific inhibitor of p300, for 1 hour and the neurons were then treated with 3OHB or vehicle (CTRL) for 6 h (n = 3 separate cultures). *p<0.05.
p300 interacts with NF-κB to regulate bdnf expression
It was reported that 3OHB can inhibit histone deacetylases (Shimazu et al., 2013) which might alter the dynamic balance between histone acetylation and deacetylation towards acetylation. Because histone acetylation can regulate gene transcription, we evaluated global histone acetyltransferase (HAT) activity in nuclear extracts from primary neurons that had been exposed to 3OHB for 1 h. Compared to neurons in control cultures, neurons exposed to 3OHB exhibited significantly elevated nuclear HAT activity (Fig. 5C). p300 is a histone acetyltransferase and transcriptional coactivator that can interact with NF-κB to promote neuronal survival (Culmsee et al., 2003; Greene and Chen, 2004). A co-immunoprecipitation assay revealed the binding of p300 to p65 in neurons treated with 3OHB for 6 h (Fig. 5D). We next tested the binding of p300 at promoters I – IV of the Bdnf gene. 3OHB significantly enhanced the recruitment of p 300 to the Bdnf promoter IV sequence (Fig. 5E). We then used a specific inhibitor of p300 (C646, 2.5 µM) to demonstrate a requirement for p300 in the stimulation of Bdnf gene expression by 3OHB (Fig 5B).
3OHB enhances mitochondrial oxidative metabolism that plays a role in the regulation of Bdnf gene expression
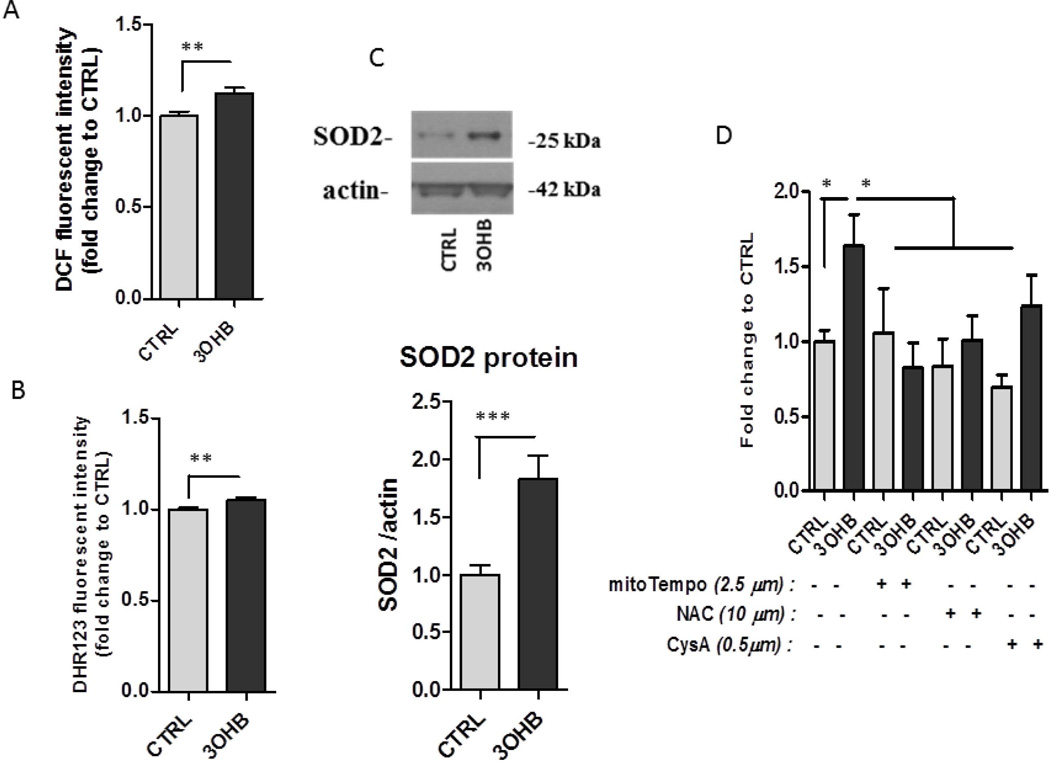
We next investigated the possible mechanism by which 3OHB triggers NF-κB activation and Bdnf gene transcription. Because metabolism of non-glycolytic substrates is accompanied by elevated levels of mitochondrial ROS (Forsberg et al., 1998), and because NF-κB and p300 are known to be redox sensitive transcription regulators (Li et al., 1999; Dansen et al., 2009; Chen et al., 2011), we hypothesized that 3OHB-induced Bdnf gene transcription is mediated by mitochondrial reactive oxygen species (ROS) production. We found that exposure of cortical neurons to 3OHB resulted in an increase in ROS levels that was evident within 6 h (Fig. 6A–B). Consistent with an adaptive redox response to 3OHB, levels of the antioxidant enzyme SOD2, was elevated in 3OHB-treated neurons (Fig. 6C). Next, we determined whether the suppression of ROS production can affect 3OHB-induced Bdnf expression. We incubated cells with or without 3OHB and an antioxidant for 6 h and then measured Bdnf promoter IV activity. The antioxidants included: the glutathione precursor N-acetylcysteine (NAC, 10 µM); the superoxide scavenger mitoTempo (5 µM); and the cyclosporin A (CysA 0.5µM) that prevents the opening of the mitochondria permeability transition pore in response to toxic insults. The antioxidants prevented the induction of Bdnf gene (Fig. 6D) although CysA did not have similar effect indicating that 3OHB does not cause alteration in the mitochondria permeability transition pore.
Figure 6. Evidence that 3OHB induces Bdnf expression by a mechanism involving mitochondrial reactive oxygen species.
A and B. Relative levels of ROS (DCF and DHR123 fluorescence) in cortical neurons incubated without (CTRL) or with 8 mM 3OHB for 6 hours. Values for 3OHB-treated cultures were expressed as fold of the value for CTRL cultures (n =4 separate cultures). *p<0.01. C. Immunoblots showing relative levels of the SOD2 protein in samples from cultured cortical neurons that had been exposed to 8 mM 3OHB for 24 hours. These blots are representative of results obtained in 3 experiments. D. Stimulation of Bdnf gene expression by 3OHB is blocked by mitochondrial antioxidants but not cyclosporin A (CysA). Cortical neurons were incubated in medium lacking or containing mitoTempo, N-acetylcistein and cyclosporin A and were then exposed for 6 hours to vehicle (CTRL) or 8 mM 3OHB. Neurons were then harvested and levels of Bdnf mRNA were quantified (n = 3 separate cultures). *p<0.05.
BDNF is involved in the protection of 3OHB by kainic acid-induced cell death
Next we investigated the functional role of the induction of BDNF by 3OHB. Neurons were pre-incubated with or without 3OHB for 6 hours in the presence or absence of TrkB inhibitor ANA-12 (2 µM). 3OHB treatment slightly increased the survivor of the neurons in the presence of kainic acid (Fig. S4). Although there was no difference in the neuronal survivor between the groups when the TrkB receptor was blocked indicating that BDNF plays a role in the neuroprotective effect of 3OHB.
Exercise decreases plasma glucose levels, increases 3OHB levels and induces BDNF in the brain
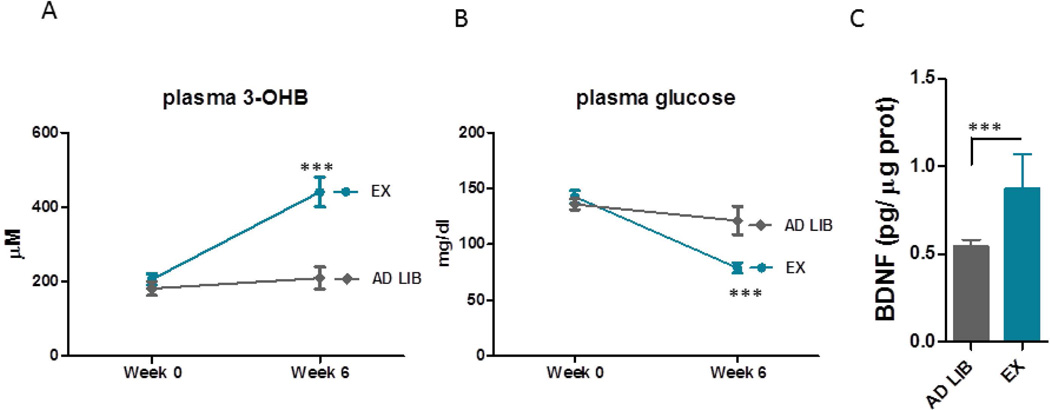
Aerobic exercise has been known to enhance brain BDNF levels. We investigated whether exercise induces changes the concentration of the circulating energy sources for the neurons that might be associated with BDNF production in the brain. We tested the levels of the main energy sources, glucose and 3OHB levels in the sedentary mice and in the mice subjected to 6 weeks of voluntary exercise. We also measured the BDNF levels in the hippocampus. We found that exercise decreased blood basal glucose levels and increased the 3OHB levels in the plasma (Fig. 7A, B) Hippocampal BDNF levels were also induced by aerobic exercise (Fig. 7C), which corresponds to previous data. In addition, we found a correlation between the circulating levels of 3OHB and the hippocampal BDNF levels suggesting that 3OHB might regulate BDNF levels in vivo (Fig. S5).
Figure 7. Voluntary exercise induces 3OHB levels in the plasma and BDNF expression in the brain.
A and B. Exercise decreased plasma basal glucose levels (A) and increased 3OHB levels (B). C. Hippocampal BDNF levels were elevated in mice subjected to aerobic exercise compared to the sedentary mice (C) p<0.001.
Discussion
Our findings suggest that 3OHB induces expression of the Bdnf gene and increases BDNF protein levels in cerebral cortical neurons via activation of the Bdnf gene promoter IV by a mechanism involving the transcription factor NF-κB and the histone acetyltransferase p300. Inhibition of MCT2 prevented 3OHB-induced Bdnf expression indicating a requirement for cellular uptake of 3OHB for stimulation of Bdnf expression. Cultured primary neurons are typically maintained in media containing concentrations of glucose (10 – 25 mM) that would be considered pathologically hyperglycemic (i.e., diabetic) in vivo (e.g., Brewer et al., 1993). We found that whereas 3OHB stimulated Bdnf expression in neurons maintained in a relatively low concentration of glucose (1 mM), it did not increase Bdnf expression in neurons maintained in a high concentration of glucose (10 mM). During prolonged fasting, plasma glucose concentrations are maintained low (3 – 5 mM) while 3OHB concentrations are elevated greatly (5 – 10 mM). However, under the latter conditions the extracellular glucose concentration in the brain is believed to be considerably lower that plasma glucose concentration (de Vries et al., 2003). Previous studies have shown that BDNF levels are reduced in the hippocampus in rodent models of type 2 diabetes including in leptin receptor mutant mice (Stranahan et al., 2009) and rats maintained on a high fat plus glucose diet (Stranahan et al., 2008). Conversely, intermittent fasting induces BDNF expression in the hippocampus, which may mediate beneficial effects of intermittent fasting on hippocampal neurogenesis, synaptic plasticity and neuroprotection (Lee et al., 2002; Arumugam et al., 2010). Our findings therefore suggest the possibility that 3OHB contributes to increased BDNF expression in response to fasting.
It was previously reported that NF-κB induces N-methyl-D-apartate receptor-mediated Bdnf gene transcription in cultured cerebellar granule cells (Lipsky et al., 2001; Marini et al., 2004). We found that 3OHB induces the nuclear translocation of the NF-κB p65 subunit and increases the interaction of the transcriptional coactivator p300 with p65 in cortical neurons. Consistent with our findings in neurons, it was recently reported that 3OHB increases NF-κB activation in calf hepatocytes (Shi et al., 2014). We found that cortical neurons treated with 3OHB exhibited enhanced recruitment of p300 to a Bdnf promoter IV sequence, and inhibitors of NF-κB and p300 blocked the ability of 3OHB to induce Bdnf gene expression. Increased mitochondrial ROS generation appears to mediate 3OHB-induced Bdnf expression because we found that agents that reduced mitochondrial superoxide levels prevented 3OHB-induced Bdnf promoter activity. Interestingly, we found that levels of two antioxidant enzymes (SOD2 and heme oxygenase 1) that are encoded by genes previously shown to be induced by NF-κB (Kiningham et al., 2001; Naidu et al., 2008) were increased in 3OHB-treated neurons. We found that 3OHB increased mitochondrial respiration in cortical neurons suggesting that upregulation of antioxidant defenses in response to 3OHB may represent an adaptive response to cope with elevated mitochondrial ROS generation.
We found that neurons treated with BDNF exhibited an increased mitochondrial respiration rate, and that a BDNF blocking antibody prevented 3OHB-induced increases in mitochondrial respiration. It was previously reported that BDNF can increase mitochondrial respiratory coupling in rat brain mitochondria (Markham et al., 2004) and increases respiratory coupling efficiency in mouse brain synaptosomes (Markham et al., 2012). It has also been shown that when cultured rat cortical neurons are treated with 3OHB their respiratory capacity increases and they are better able to sustain mitochondrial function when exposed to high levels of glutamate (Laird et al., 2013). Our findings suggest the possibility that the improvement of neuronal the neuroprotective actions of 3OHB are mediated, at least in part, by BDNF signaling. Numerous studies have shown that fasting is neuroprotective in animal models relevant to AD, PD and HD (Duan and Mattson, 1999; Duan et al., 2003; Halagappa et al., 2007; Griffioen et al., 2013), as well as stroke (Arumugam et al., 2010) and traumatic brain injury (Davis et al., 2008). If and to what extent 3OHB contributes to such neuroprotection remains to be established. However, the ability of ketogenic diets (Gasior et al., 2006; Maalouf et al., 2009) and 3OHB supplementation (Kashiwaya et al., 2013) to protect neurons and improve functional outcome in animal models of neurodegenerative conditions is consistent with an important role for 3OHB in the neuroprotective effects of fasting.
Evolutionary considerations and experimental findings suggest that cognitive function is bolstered by vigorous physical activity and food scarcity/fasting (Mattson, 2015). The ability to outsmart one’s competitors in the battle for limited food resources has been fundamental to the evolution of the brains of most species including humans. The integration of signaling pathways by which the brain and other organ systems respond adaptively to evolutionarily fundamental bioenergetics challenges are undoubtedly complex involving activation of CNS neural circuits, neuroendocrine pathways and signals from peripheral organs to the brain. Emerging evidence suggests that BDNF is key mediator of adaptive responses of the brain and peripheral organ systems to bioenergetic challenges (Marosi et al., 2014). Our findings suggest a role for 3OHB in up-regulation of BDNF signaling in brain cells in response to exercise and fasting. We found that voluntary exercise increases plasma 3OHB levels and that there is a significant positive correlation between the concentration of circulating 3OHB and levels of BDNF in the hippocampus. Consistent with a role for 3OHB-induced BDNF expression in the neuroprotective effects of fasting and exercise in vivo, it was reported that fasting engages TrkB signaling, promotes neuroplasticity and improves behavioral recovery after spinal cord injury (Plunet et al., 2008). For example, BDNF signaling in the CNS regulates appetite (Kernie et al., 2000), enhances peripheral insulin sensitivity (Nakagawa et al., 2000) and enhances parasympathetic regulation of heart rate (Wan et al., 2014). Because BDNF expression in multiple brain regions is increased in response to energetic challenges that elevate 3OHB levels, our finding that 3OHB acts directly on neurons to stimulate BDNF expression suggests potential roles for 3OHB in up-regulating BDNF expression under such conditions and could, by this mechanism, contribute to the beneficial effects of fasting and vigorous exercise on cognitive performance, and to improved peripheral energy metabolism and cardiovascular fitness (Wan et al., 2003; van Praag et al., 2014).
Supplementary Material
Acknowledgments
This research was supported, in its entirety, by the Intramural Research Program of the National Institute on Aging.
List of abbreviations
- 3OHB
3-hydroxybutyrate
- AD
Alzheimer’s disease
- BDNF
brain derived neurotrophic factor
- CREB
cAMP response element-binding protein
- CSA
cyclosporin A
- ECAR
extracellular acidification rate
- ETC
electron transport chain
- HAT
histone acetyltransferase
- HD
Huntington’s disease
- KA
kainic acid
- MCT2
monocarboxylic acid transporter 2
- NAC
N-acetylcysteine
- NAD+
nicotinamide adenine dinucleotide oxidized
- NADH
nicotinamide adenine dinucleotide reduced
- NF-kB
nuclear factor kappa B
- OCR
oxygen consumption rate
- PD
Parkinson’s disease
- ROS
reactive oxygen species
- SIRT1
sirtuin 1
- SIRT3
sirtuin 3
- SOD2
superoxide dismutase 2
Footnotes
None of the authors have any conflicts of interest to declare.
References
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Burkhalter J, Fiumelli H, Allaman I, Chatton JY, Martin JL. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J Neurosci. 2003;23:8212–8220. doi: 10.1523/JNEUROSCI.23-23-08212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophin signalling in health and disease. Clin. Sci. (Lond.) 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK, Kharkwal GB, Saleem T, Mooney D, Yull FE, Blackwell TS, Hamblin MR. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One. 2011;6(7):e22453. doi: 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiry O, Fishbein WN, Merezhinskaya N, Clarke S, Galuske R, Magistretti PJ, Pellerin L. Distribution of the monocarboxylate transporter MCT2 in human cerebral cortex: an immunohistochemical study. Brain Res. 2008;1226:61–69. doi: 10.1016/j.brainres.2008.06.025. [DOI] [PubMed] [Google Scholar]
- Chowdhury GM, Jiang L, Rothman DL, Behar KL. The contribution of ketone bodies to basal and activity-dependent neuronal oxidation in vivo. J Cereb Blood Flow Metab. 2014;34:1233–1242. doi: 10.1038/jcbfm.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Siewe J, Junker V, Retiounskaia M, Schwarz S, Camandola S, El-Metainy S, Behnke H, Mattson MP, Krieglstein J. Reciprocal inhibition of p53 and nuclear factor-kappaB transcriptional activities determines cell survival or death in neurons. J Neurosci. 2003;23:8586–8595. doi: 10.1523/JNEUROSCI.23-24-08586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansen TB, Smits LM, van Triest MH, de Keizer PL, van Leenen D, Koerkamp MG, Szypowska A, Meppelink A, Brenkman AB, Yodoi J, Holstege FC, Burgering BM. Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat Chem Biol. 2009;5:664–672. doi: 10.1038/nchembio.194. [DOI] [PubMed] [Google Scholar]
- Davis LM, Pauly JR, Readnower RD, Rho JM, Sullivan PG. Fasting is neuroprotective following traumatic brain injury. J Neurosci Res. 2008;86:1812–1822. doi: 10.1002/jnr.21628. [DOI] [PubMed] [Google Scholar]
- de Vries MG, Arseneau LM, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes. 2003;52:2767–2773. doi: 10.2337/diabetes.52.11.2767. [DOI] [PubMed] [Google Scholar]
- Devi L, Ohno M. 7,8-Dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2012;37:434–444. doi: 10.1038/npp.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci U S A. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg H, Eriksson UJ, Melefors O, Welsh N. Beta-hydroxybutyrate increases reactive oxygen species in late but not in early postimplantation embryonic cells in vitro. Diabetes. 1998;47:255–262. doi: 10.2337/diab.47.2.255. [DOI] [PubMed] [Google Scholar]
- Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17:431–439. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazner GW, Chan SL, Lu C, Mattson MP. Caspase-mediated degradation of AMPA receptor subunits: a mechanism for preventing excitotoxic necrosis and ensuring apoptosis. J Neurosci. 2000;20:3641–3649. doi: 10.1523/JNEUROSCI.20-10-03641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbo OL, O’Mara SM. Impact of enriched-environment housing on brain-derived neurotrophic factor and on cognitive performance after a transient global ischemia. Behav Brain Res. 2004;152:231–241. doi: 10.1016/j.bbr.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Greco T, Glenn TC, Hovda DA, Prins ML. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J Cereb Blood Flow Metab. 2015 Oct 13; doi: 10.1177/0271678X15610584. pii: 0271678X15610584. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene WC, Chen LF. Regulation of NF-kappaB action by reversible acetylation. Novartis Found Symp. 2004;259:208–217. [PubMed] [Google Scholar]
- Griffioen KJ, Rothman SM, Ladenheim B, Wan R, Vranis N, Hutchison E, Okun E, Cadet JL, Mattson MP. Dietary energy intake modifies brainstem autonomic dysfunction caused by mutant α-synuclein. Neurobiol Aging. 2013;34:928–935. doi: 10.1016/j.neurobiolaging.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutsaeva DR, Carraway MS, Suliman HB, Demchenko IT, Shitara H, Yonekawa H, Piantadosi CA. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci. 2008;28:2015–2024. doi: 10.1523/JNEUROSCI.5654-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, Okun E, Clarke K, Mattson MP, Veech RL. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer's disease. Neurobiol Aging. 2013;34:1530–1539. doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim do Y, Simeone KA, Simeone TA, Pandya JD, Wilke JC, Ahn Y, Geddes JW, Sullivan PG, Rho JM. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol. 2015;78:77–87. doi: 10.1002/ana.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiningham KK, Xu Y, Daosukho C, Popova B, St Clair DK. Nuclear factor kappaB-dependent mechanisms coordinate the synergistic effect of PMA and cytokines on the induction of superoxide dismutase 2. Biochem J. 2001;353:147–156. [PMC free article] [PubMed] [Google Scholar]
- Laird MD, Clerc P, Polster BM, Fiskum G. Augmentation of normal and glutamate-impaired neuronal respiratory capacity by exogenous alternative biofuels. Transl Stroke Res. 2013;4:643–651. doi: 10.1007/s12975-013-0275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Levivier M, Przedborski S, Bencsics C, Kang UJ. Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson's disease. J. Neurosci. 1995;15:7810–7820. doi: 10.1523/JNEUROSCI.15-12-07810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YP, Atkins CM, Sweatt JD, Reid MB. Mitochondria mediate tumor necrosis factor-alpha/NF-kappaB signaling in skeletal muscle myotubes. Antioxid Redox Signal. 1999;1:97–104. doi: 10.1089/ars.1999.1.1-97. [DOI] [PubMed] [Google Scholar]
- Lipsky RH, Xu K, Zhu D, Kelly C, Terhakopian A, Novelli A, Marini AM. Nuclear factor kappaB is a critical determinant in N-methyl-D-aspartate receptor-mediated neuroprotection. J Neurochem. 2001;78:254–264. doi: 10.1046/j.1471-4159.2001.00386.x. [DOI] [PubMed] [Google Scholar]
- Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini AM, Jiang X, Wu X, Tian F, Zhu D, Okagaki P, Lipsky RH. Role of brain-derived neurotrophic factor and NF-kappaB in neuronal plasticity and survival: From genes to phenotype. Restor Neurol Neurosci. 2004;22:121–130. [PubMed] [Google Scholar]
- Markham A, Cameron I, Franklin P, Spedding M. BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur J Neurosci. 2004;20:1189–1196. doi: 10.1111/j.1460-9568.2004.03578.x. [DOI] [PubMed] [Google Scholar]
- Markham A, Cameron I, Bains R, Franklin P, Kiss JP, Schwendimann L, Gressens P, Spedding M. Brain-derived neurotrophic factor-mediated effects on mitochondrial respiratory coupling and neuroprotection share the same molecular signalling pathways. Eur J Neurosci. 2012;35:366–374. doi: 10.1111/j.1460-9568.2011.07965.x. [DOI] [PubMed] [Google Scholar]
- Marosi K, Bori Z, Hart N, Sárga L, Koltai E, Radák Z, Nyakas C. Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience. 2012;226:21–28. doi: 10.1016/j.neuroscience.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PM, Gopal E, Ananth S, Zhuang L, Itagaki S, Prasad BM, Smith SB, Prasad PD, Ganapathy V. Identity of SMCT1 (SLC5A8) as a neuron-specific Na+-coupled transporter for active uptake of L-lactate and ketone bodies in the brain. J Neurochem. 2006;98:279–288. doi: 10.1111/j.1471-4159.2006.03878.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Wan Z, Guo Z. Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroRx. 2004;1:111–116. doi: 10.1602/neurorx.1.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing Res Rev. 2015;20:37–45. doi: 10.1016/j.arr.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally MA, Hartman AL. Ketone bodies in epilepsy. J Neurochem. 2012;121:28–35. doi: 10.1111/j.1471-4159.2012.07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu S, Wijayanti N, Santoso S, Kietzmann T, Immenschuh S. An atypical NF-kappa B-regulated pathway mediates phorbol ester-dependent heme oxygenase-1 gene activation in monocytes. J Immunol. 2008;181:4113–4123. doi: 10.4049/jimmunol.181.6.4113. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Tsuchida A, Itakura Y, Nonomura T, Ono M, Hirota F, Inoue T, Nakayama C, Taiji M, Noguchi H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49:436–444. doi: 10.2337/diabetes.49.3.436. [DOI] [PubMed] [Google Scholar]
- Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, Booth FW, Kohrt WM, Gerszten RE, Mattson MP, Hepple RT, Kraus WE, Reid MB, Bodine SC, Jakicic JM, Fleg JL, Williams JP, Joseph L, Evans M, Maruvada P, Rodgers M, Roary M, Boyce AT, Drugan JK, Koenig JI, Ingraham RH, Krotoski D, Garcia-Cazarin M, McGowan JA, Laughlin MR. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015;22:4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014;25:42–52. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF. Brain metabolism during fasting. JCI. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ML, Matsumoto JH. The collective therapeutic potential of cerebral ketone metabolism in traumatic brain injury. J Lipid Res. 2014;55:2450–2457. doi: 10.1194/jlr.R046706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, Hyde K, Chapman D, Craft S. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25:311–314. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- Robinet C, Pellerin L. Brain-derived neurotrophic factor enhances the expression of the monocarboxylate transporter 2 through translational activation in mouse cultured cortical neurons. J Cereb Blood Flow Metab. 2010;30:286–298. doi: 10.1038/jcbfm.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata K, Jin L, Jha S. Lack of promoter IV-driven BDNF transcription results in depression-like behavior. Genes Brain Behav. 2010;9:712–721. doi: 10.1111/j.1601-183X.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- Scheibye-Knudsen M, Mitchell SJ, Fang EF, Iyama T, Ward T, Wang J, Dunn CA, Singh N, Veith S, Hasan-Olive MM, Mangerich A, Wilson MA, Mattson MP, Bergersen LH, Cogger VC, Warren A, Le Couteur DG, Moaddel R, Wilson DM, 3rd, Croteau DL, de Cabo R, Bohr VA. A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab. 2014;20:840–855. doi: 10.1016/j.cmet.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma J, Johnston MV, Hossain MA. Sex differences in mitochondrial biogenesis determine neuronal death and survival in response to oxygen glucose deprivation and reoxygenation. BMC Neurosci. 2014;15:9. doi: 10.1186/1471-2202-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Li X, Li D, Li Y, Song Y, Deng Q, Wang J, Zhang Y, Ding H, Yin L, Zhang Y, Wang Z, Li X, Liu G. β-Hydroxybutyrate activates the NF-κB signaling pathway to promote the expression of pro-inflammatory factors in calf hepatocytes. Cell Physiol Biochem. 2014;33:920–932. doi: 10.1159/000358664. [DOI] [PubMed] [Google Scholar]
- Shieh PB, Ghosh A. Molecular mechanisms underlying activity-dependent regulation of BDNF expression. J Neurobiol. 1999;41:127–134. [PubMed] [Google Scholar]
- Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV, Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: Continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Grote HE, Varshney NK, Cordery PM, van Dellen A, Blakemore C, Hannan AJ. Environmental enrichment rescues protein deficits in a mouse model of Huntington’s disease, indicating a possible disease mechanism. J Neurosci. 2004;24:2270–2276. doi: 10.1523/JNEUROSCI.1658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JL, Murphy EA, McClellan JL, Carmichael MD, Davis JM. Exercise training increases mitochondrial biogenesis in the brain. J Appl Physiol. 2011;111:1066–1071. doi: 10.1152/japplphysiol.00343.2011. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1108. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martín MV, Torres-Alemán I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Vanitallie TB, Nonas C, Di Rocco A, Boyar K, Hyams K, Heymsfield SB. Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology. 2005;64:728–730. doi: 10.1212/01.WNL.0000152046.11390.45. [DOI] [PubMed] [Google Scholar]
- van Praag H, Fleshner M, Schwartz MW, Mattson MP. Exercise, energy intake, glucose homeostasis, and the brain. J Neurosci. 2014;34:15139–15149. doi: 10.1523/JNEUROSCI.2814-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J Nutr. 2003;133:1921–1929. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- Wan R, Weigand LA, Bateman R, Griffioen K, Mendelowitz D, Mattson MP. Evidence that BDNF regulates heart rate by a mechanism involving increased brainstem parasympathetic neuron excitability. J Neurochem. 2014;129:573–580. doi: 10.1111/jnc.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Wang R, Li JJ, Diao S, Kwak YD, Liu L, Zhi L, Büeler H, Bhat NR, Williams RW, Park EA, Liao FF. Metabolic stress modulates Alzheimer's β-secretase gene transcription via SIRT1-PPARγ-PGC-1 in neurons. Cell Metab. 2013;17:685–694. doi: 10.1016/j.cmet.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Lin YT, Chuang PC, Bohr VA, Mattson MP. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromolecular Med. 2014;16:161–174. doi: 10.1007/s12017-013-8270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Han P, Tang Z, Liu Q, Shi J. Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. J Cereb Blood Flow Metab. 2015;35:1783–1789. doi: 10.1038/jcbfm.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.