Abstract
Members of the nucleotide-binding domain and leucine-rich repeat containing (NLR) family and the pyrin and HIN-domain (PYHIN) family can form multiprotein complexes termed “inflammasomes”. The biochemical function of inflammasomes is to activate caspase-1, which leads to the maturation of interleukin 1β (IL-1β) and IL-18 and induction of pyroptosis, a form of cell death. Unlike other inflammasomes, the NLRP3 inflammasome can be activated by diverse stimuli. The importance of the NLRP3 inflammasome in immunity and human diseases has been well documented, but the mechanism and regulation of NLRP3 inflammasome activation remains unclear. In this review we summarize current understanding of the mechanism and regulation of NLRP3 inflammasome activation, as well as recent advances in the non-canonical and alternative inflammasome pathways.
Keywords: NLRP3 inflammasome, K+ efflux, Non-canonical inflammasome, Alternative inflammasome, Nek7
The NLRP3 inflammasome: A critical component of innate immunity and pathological contributor to human diseases
Inflammasomes are a group of cytosolic protein complexes that are formed to mediate host immune responses to microbial infection and cellular damage [1]. Assembly of an inflammasome triggers proteolytic cleavage of dormant procaspase-1 into active caspase-1, which converts the cytokine precursors pro-IL-1β and pro-IL-18 into mature and biologically active IL-1β and IL-18, respectively [2, 3]. Mature IL-1β is a potent proinflammatory mediator in many immune reactions, including the recruitment of innate immune cells to the site of infection and modulation of adaptive immune cells, whereas mature IL-18 is important for the production of interferon-γ and potentiation of cytolytic activity of natural killer cells and T cells [4]. Active caspase-1 also induces a proinflammatory form of cell death, known as pyroptosis [5]. Inflammasome formation requires a pattern recognition receptor (PRR) as the sensor, in most cases the adaptor ASC (apoptosis-associated spec-like protein containing a CARD), and the cysteine protease caspase-1. To date, five PRRs (NLRP1, NLRP3, NLRC4, Pyrin and AIM2) have been shown to form inflammasomes [6, 7]. Emerging evidence indicates that several other members of the NLR family and the PYHIN family, including NLRP6, NLRP7, NLRP12 and IFI16, can also form inflammasomes, but their composition remains obscure [7].
Among these inflammasomes, the NLRP3 inflammasome has been under intensive investigation given its possible involvement in several human diseases. NLRP3 belongs to the NLR protein family, which includes 22 members in human and at least 34 members in mouse [8]. Most NLRs have a tripartite structure defined as the following: an amino-terminal caspase-recruitment domain (CARD), pyrin domain, acidic transactivating domain or baculovirus inhibitor repeat that mediates downstream protein-protein interaction; a centrally located nucleotide-binding-and-oligomerization domain that mediates self-oligomerization; and carboxy-terminal leucine-rich repeats (LRRs) that are thought to be involved in recognition of stimuli [9]. During microbial infections, the NLRP3 inflammasome promotes host immune defense against infectious microbes, such as Influenza A virus, Staphylococcus aureus and Candida albicans [1]. However, dysregulated activation of the NLRP3 inflammasome has been implicated in the pathogenesis of inherited and acquired inflammatory diseases [10, 11].
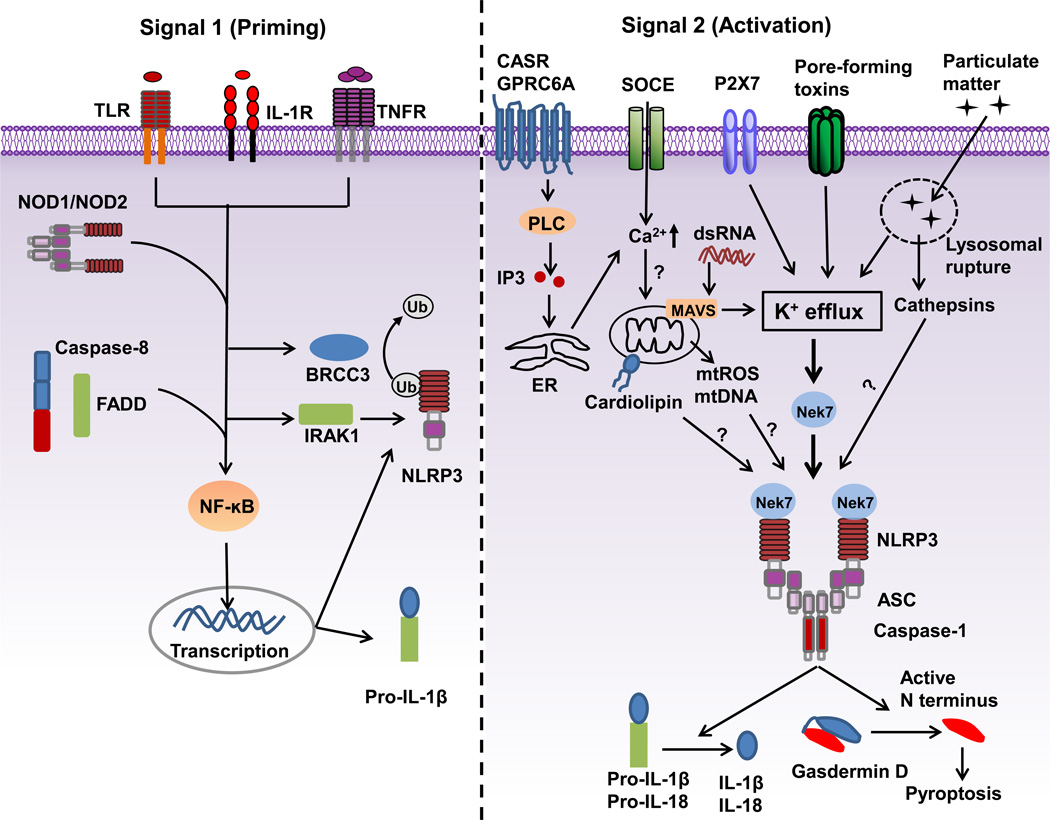
The NLRP3 inflammasome was initially shown to be activated by ATP and certain bacterial toxins [12]. Subsequently, a wide array of stimuli were identified to activate the NLRP3 inflammasome including multiple microbial products, endogenous molecules or particulate matter [13–15]. In subsequent studies, however, it became clear that most microbial stimuli, such as Toll-like receptor (TLR) ligands and muramyl dipeptide, do not directly activate the NLRP3 inflammasome, but they instead prime the NLRP3 inflammasome for activation [16]. Furthermore, NLRP3 activation induced by ATP, pore-forming toxins and particulate matter requires pretreatment of macrophages with microbial stimuli or cytokines [16–18]. Therefore, a two-signal model has been proposed for NLRP3 inflammasome activation in macrophages. In this model, the first signal (priming) is provided by microbial or endogenous molecules that induce NLRP3 and pro-IL-1β expression through activation of NF-κB; the second signal (activation) is triggered by ATP, pore-forming toxins, viral RNA and particulate matter (Figure 1).
Figure 1.
A two-signal model for NLRP3 inflammasome activation. Signal 1 (priming, left) is provided by microbial molecules or endogenous cytokines and leads to the upregulation of NLRP3 and pro-IL-1β through the activation of transcriptional factor NF-κB. Caspase-8 and FADD are involved in priming via the regulation of the NF-κB activation pathway. BRCC3 (a deubiquitinase) and IRAK1 regulate NLRP3 inflammasome activation independently of transcription. Signal 2 (activation, right) is provided by a plethora of stimuli, such as ATP, pore-forming toxins, viral RNA and particulate matter, and activates the NLRP3 inflammasome. A number of signaling events have been proposed as the key mechanism of NLRP3 activation. Most NLRP3 stimuli induce K+ efflux that is necessary and sufficient for NLRP3 activation. Ca2+ signaling is proposed to cause mitochondrial dysfunction and is implicated in NLRP3 inflammasome activation. Mitochondrial dysfunction-derived signals, such as reactive oxygen species (mtROS), oxidized mtDNA or externalization of phospholipid cardiolipin have also been suggested to mediate NLRP3 activation. Mitochondrial adaptor MAVS mediates NLRP3 activation induced by RNA viruses. Particulate matter activates NLRP3 through lysosomal rupture-induced K+ efflux and perhaps the release of cathepsins. Nek7 is an essential regulator of the NLRP3 inflammasome. TLR: Toll-like receptor; IL-1R: receptor for IL-1β; TNFR: receptor for tumor-necrosis factor; BRCC3: Lys-63-specific deubiquitinase BRCC36; FADD: FAS-associated death domain protein; IRAK1:Interleukin-1 receptor-associated kinase 1.
Priming the NLRP3 inflammasome: Beyond the induction of NLRP3 and pro-IL-1β
Macrophages, such as mouse bone marrow-derived macrophages, show no or minimal activation of the NLRP3 inflammasome when stimulated with NLRP3 activators, whereas pretreatment with microbial ligands strongly enhances NLRP3 inflammasome activation [16]. This pretreatment is defined as the priming step, which provides the first signal for NLRP3 inflammasome activation. Unlike ASC and caspase-1, the protein amounts of NLRP3 in resting macrophages are thought to be insufficient for NLRP3 activation. Expression of NLRP3 is induced by priming with microbial components such as TLR ligands or endogenous molecules, such as tumor necrosis factor and IL-β, through the activation of NF-κB [16, 18]. Recent studies have shown that FADD and caspase-8 also regulate the induction of NLRP3 expression during priming [19–21]. Consistent with this transcriptional role in the priming step, macrophages constitutively expressing high levels of NLRP3 via a retroviral vector show inflammasome activation after stimulation with NLRP3 activators in the absence of priming [16, 22]. Therefore, priming positively regulates the NLRP3 inflammasome through the induction of NLRP3 expression (Figure 1).
In addition to this transcriptional role, emerging evidence indicates that priming also regulates NLRP3 activation at the posttranscriptional level. This regulation was first revealed by the finding that priming macrophages for a short time (10 mins), which is not sufficient for the induction of NLRP3 expression, significantly enhances inflammasome activation [22, 23]. Deeper understanding of the signaling mechanism of priming was provided by experiments using mouse macrophages deficient in signaling molecules of the NF-κB pathway. The adaptor MyD88 and the downstream kinases IRAK1 and IRAK4 have been implicated in transcriptional regulation of NLRP3 activation by priming, whereas the adaptor TRIF and IRAK1 play a role in the posttranscriptional regulation of the priming step [16, 24, 25]. Furthermore, NLRP3 is deubiquitinated during priming [23, 26, 27]. BRCC3, a JAMM domain-containing Zn2+ metalloprotease, promotes NLRP3 deubiquitination during priming and regulates NLRP3 activation [26]. Therefore, the role of priming in NLRP3 activation is more complex than originally appreciated (Figure 1). Transcription-independent signaling of priming may further fine-tune NLRP3 inflammasome activation.
Activating the NLRP3 inflammasome: Integration of multiple cellular signaling events
Given the chemical and structural diversity of NLRP3-activating stimuli, it is unlikely that NLRP3 physically interacts with its activators. Instead, NLRP3 is likely to sense a common cellular signal induced in response to NLRP3 activators. The identity of this signal is currently under intensive debate. Several molecular and cellular events have been proposed as the trigger(s) for NLRP3 inflammasome activation, including K+ efflux, Ca2+ signaling, reactive oxygen species (ROS), mitochondrial dysfunction, and lysosomal rupture (Figure 1).
K+ efflux: a common step for NLRP3 inflammasome activation
K+ efflux has emerged as a common denominator in the activation of the NLRP3 inflammasome. This was first implicated from an earlier report that potassium ionophores, such as nigericin, trigger IL-1β maturation in LPS-stimulated murine macrophages [28]. The importance of K+ efflux in NLRP3 inflammasome activation has been further revealed by additional studies [29, 30]. These studies have shown that a high concentration of extracellular K+ blocks NLRP3 inflammasome activation, but not AIM2 or NLRC4 inflammasome activation [31, 32]. K+ efflux is induced in response to most or all NLRP3 stimuli, including ATP, nigericin and particulate matter, and lowering cytosolic K+ concentration is sufficient to activate the NLRP3 inflammasome [30]. Such a low concentration of K+ can also trigger NLRP3 inflammasome assembly in a cell-free system [31]. Therefore, the drop of intracellular K+ concentration has been proposed to be a common trigger for NLRP3 inflammasome activation [30]. In addition, K+ efflux is also required for NLRP3 activation in the non-canonical inflammasome pathway (see next section) [33, 34]. However, the mechanistic link between decreased levels of intracellular K+ and NLRP3 activation remains poorly understood. K+ efflux is thought to act at or upstream of NLRP3 activation. In support of this notion, K+ efflux precedes inflammasome activation [30]. Furthermore, activation of the NLRP3 inflammasome in macrophages harboring a NLRP3 activating mutation (NLRP3R258W) occurs in the absence of K+ efflux and is not affected by a high concentration of extracellular K+ [30, 35]. This suggests that the drop in intracellular K+ concentration might induce NLRP3 to undergo conformational changes that are already induced by the presence of NLRP3 activating mutations.
Ca2+ signaling and NLRP3 inflammasome activation
The involvement of Ca2+ signaling in NLRP3 inflammasome activation was suggested by studies showing that the Ca2+ chelator BAPTA-AM inhibits IL-1β secretion [36–38]. Several studies have supported a general requirement for Ca2+ signaling in NLRP3 activation [39–41]. NLRP3 stimuli including ATP, nigericin, and particulate matter induce Ca2+ mobilization during the process of NLRP3 inflammasome activation [40]. Importantly, inhibition of Ca2+ signaling blocks NLRP3 inflammasome activation, but has no effect on the activation of the AIM2 and NLRC4 inflammasomes [39, 40]. As a major intracellular Ca2+ reservoir, the endoplasmic reticulum (ER), appears to be critical for this process, because pharmacological inhibition or small hairpin RNA (shRNA) knockdown of inositol 1,4,5-triphosphate receptor (IP3R), a Ca2+ release channel on the ER, attenuates Ca2+ mobilization and NLRP3 activation [39]. Activation of IP3R is triggered by IP3, a product of phospholipase C (PLC)-mediated phosphatidylinositol 4,5-bisphosphate (PIP2) cleavage. Consistently, PLC inhibition blocks NLRP3 activation induced by multiple stimuli, whereas direct activation of PLC induces IL-1β secretion in the absence of any other exogenous stimuli [39]. How PLC is activated by NLRP3 stimuli remains unclear. Lee et al. reported that the Calcium-Sensing Receptor (CASR), a G protein-coupled receptor (GPCR), acts upstream of PLC to trigger Ca2+ mobilization and NLRP3 activation [39]. However, another study showed that CASR and a related family member GPRC6A are only required for extracellular Ca2+-induced NLRP3 activation, but not for ATP-induced NLRP3 activation [41]. Notably, the addition of Ca2+ to extracellular RPMI medium leads to the formation of particulate matter and K+ efflux, complicating the interpretation of these experiments [30]. The store-operated Ca2+ entry (SOCE) channel on the plasma membrane, which is often coupled to ER Ca2+ release, has been implicated in Ca2+ mobilization and NLRP3 activation [40]. How does Ca2+ signaling regulate NLRP3 inflammasome activation? Cytosolic Ca2+ may promote NLRP3 complex formation [39]. Alternatively, Ca2+ mobilization and mitochondrial Ca2+ overload may cause mitochondrial dysfunction, which may promote NLRP3 activation (see next subsection [40]. However, a recent study indicated that NLRP3 inflammasome activation is independent of Ca2+ signaling [42]. In this study, the authors compared the contribution of decreased cytosolic K+ concentrations versus increased cytosolic Ca2+ in NLRP3 activation and found that Ca2+ is largely dispensable for NLRP3 activation [42]. Surprisingly, this study also revealed that BAPTA blocks NLRP3 activation independently of its expected inhibitory effects on Ca2+ signaling [42]. Thus, the role of Ca2+ signaling in NLRP3 activation remains controversial.
Mitochondrial dysfunction and ROS
The role of ROS and the mitochondria in NLRP3 inflammasome activation is a topic of long-standing debate [43, 44]. Cytosolic ROS produced by NAPDH oxidase were initially proposed to be the common signal responsible for NLRP3 inflammasome activation based on studies with chemical inhibitors [45]. However, human peripheral blood mononuclear cells and mouse macrophages lacking NAPDH oxidase activity show normal activation of the NLRP3 inflammasome [46, 47]. The implication of mitochondrial dysfunction in NLRP3 activation was originally based on chemical inhibitors in which the perturbation of mitochondria were found to promote activation of the NLRP3 inflammasome [48]. However, chemical inhibitors are prone to causing artifacts, especially at high concentrations. Indeed, at least one study showed that ROS inhibitors also affect the induction of NLRP3 at the priming stage when used at high concentrations [49]. Furthermore, although induced by most NLRP3 stimuli, mitochondrial perturbation and associated ROS production appear dispensable for NLRP3 activation [30]. In addition, oxidized mitochondrial DNA that can be released into the cytosol has been suggested to interact with and activate NLRP3 [50]. However, the release of mitochondrial DNA can occur downstream of NLRP3 activation [51]. Furthermore, cardiolipin, a mitochondria-specific phospholipid which is normally located in the inner membrane of mitochondria, can translocate to the outer membrane of mitochondria to bind the LRRs of NLRP3 [52]. However, additional studies are needed to support the role of cardiolipin in NLRP3 activation. The mitochondrial-associated adaptor MAVS has been also implicated in NLRP3 activation. However, the role of MAVS in NLRP3 inflammasome activation remains controversial [19, 53]. One study reported that MAVS interacts with NLRP3 and is required for NLRP3 inflammasome activation by soluble stimuli (e.g. ATP and nigericin), but not particulate matter (e.g. Alum and MSU) [53]. However, several studies showed a role for MAVS in NLRP3 activation induced by RNA viruses, but not non-viral stimuli (e.g. ATP and nigericin) [14, 19, 54, 55]. In conclusion, the exact role of the mitochondria in NLRP3 inflammasome activation remains to be determined.
Lysosomal leakage
Particulate matter activates the NLRP3 inflammasome through endocytosis and damages the lysosome membrane, resulting in the release of cathepsin B, a lysosomal cysteine protease of the papain family, into the cytosol [37]. A number of studies have shown that NLRP3 activation is inhibited by treatment with CA-074-Me, a chemical inhibitor for cathepsin B [47, 56, 57]. However, cathepsin B-deficient macrophages show a modest or no defect in NLRP3 activation induced by particulate matter [57]. Thus, inhibition of NLRP3 inflammasome activation by cathepsin B inhibitors may be due to an off-target effect or redundancy among members of the cathepsin family. Consistent with the latter, a recent study suggests that multiple cathepsins, which can be inhibited by CA-074-Me, promote NLRP3 priming and activation [58]. In addition to the cytosolic release of cathepsins, particulate matter also triggers K+ efflux that is dependent on phagocytosis and required for NLRP3 activation [30]. In accord with this notion, incubation of macrophages with the lysosomotropic agent Leu-Leu-O-Methyl ester alone induces rapid K+ efflux that precedes NLRP3 activation [30]. However, the mechanism linking particulate matter-induced lysosomal rupture to K+ efflux remains to be determined.
In conclusion, NLRP3 activators can trigger multiple cellular and molecular events including ion fluxes, mitochondrial dysfunction and lysosomal leakage. However, the role of these events in NLRP3 activation with the exception of K+ efflux remains unclear.
Non-canonical inflammasome pathway
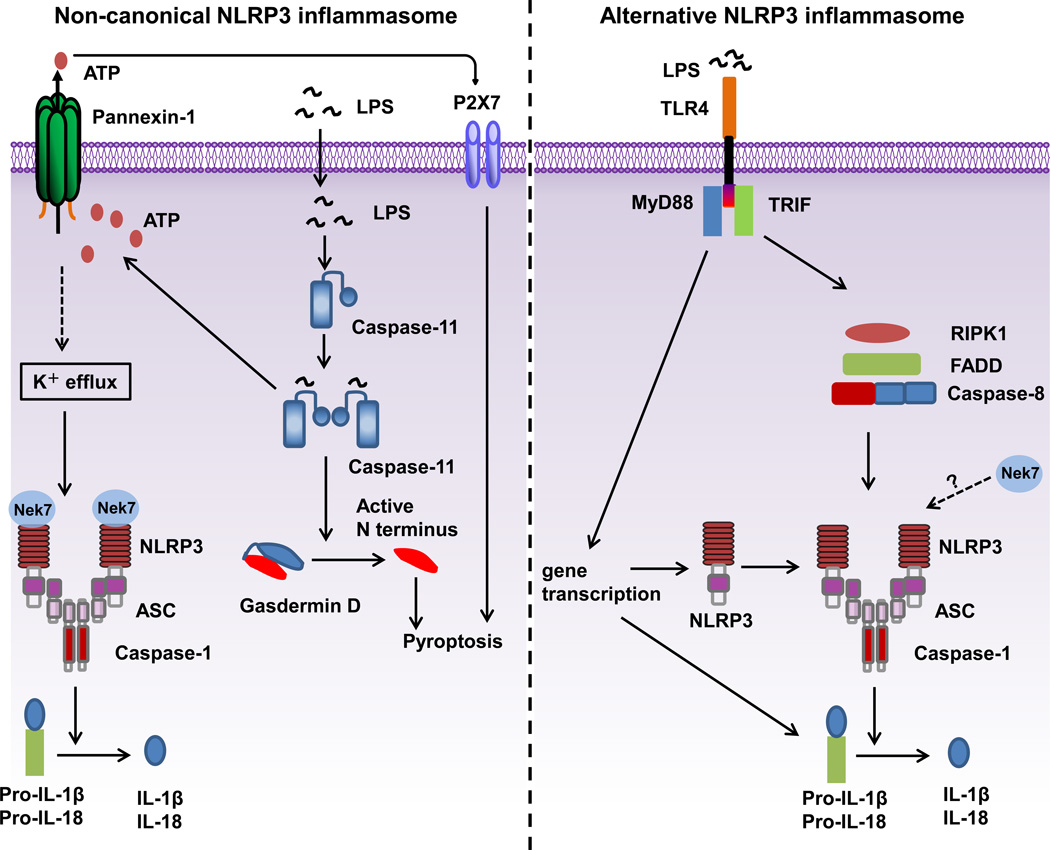
Most Gram-negative bacteria, such as Escherichia coli, Citrobacter rodentium, and Vibrio cholera, activate a caspase-11-mediated signaling pathway in mouse macrophages, resulting in maturation and release of IL-1β/IL-18, and pyroptosis (Figure 2) [59]. The release of IL-1β/IL-18 requires the NLRP3 inflammasome, whereas pyroptosis is independent of the NLRP3 inflammasome. In contrast to the NLRP3 inflammasome, this pathway is defined as a non-canonical inflammasome pathway because of the requirement of caspase-11 [59]. The signaling mechanism upstream of caspase-11 activation remains controversial. Several studies indicate that type I IFN signaling, activated downstream of the TLR4-TRIF signaling cascade, is required for the expression and activation of caspase-11 [60–62]. However, another study shows that type I IFN signaling is dispensable for the activation of this non-canonical inflammasome pathway [63]. Further studies are required to determine the role of type I IFN signaling in non-canonical inflammasome activation. This non-canonical inflammasome pathway is activated by Gram-negative, but not Gram-positive bacteria, suggesting a role for LPS in this pathway. Indeed, two studies have shown that cytosolic LPS, delivered by cholera toxin A or transfection, can activate this non-canonical inflammasome pathway [63, 64]. Furthermore, TLR4-deficient macrophages, which are primed with poly (I:C) for the induction of caspase-11, can activate this pathway, indicating that a dual recognition mechanism for LPS exists in host immune defense: TLR4 sensing the extracellular LPS and caspase-11 sensing cytosolic LPS [63, 64]. Inferred from the recognition mechanism of LPS by TLR4 on the cell surface, it was hypothesized that there exists an intracellular receptor which binds to LPS and engages caspase-11 directly or through another CARD-containing adaptor. Surprisingly, LPS directly binds to the CARD domains of caspase-11, human caspase 4 and caspase-5, but not other caspases [65]. This LPS binding can trigger the oligomerization and activation of caspase-11. It is unclear how LPS specifically binds to the CARD domain of caspase-11, human caspase 4 and caspase-5, but not other CARD domains of caspases. Structural studies of LPS-bound caspase-11, human caspase 4 or caspase-5 are needed to confirm these findings. How does activation of caspase-1 and caspase-11 cause pyroptosis? Recent progress has identified the protein gasdermin D (GSDMD) as the substrate of active caspase-11 and caspase-1 [66–68]. Active caspase-1 and caspase-11 enzymatically cleave GSDMD into two fragments (the N domain and C domain) after an aspartic residue at position 276 (human) or 275 (mouse). The GSDMD-N domain is responsible for pyroptosis induced by activated caspases. The GSDMD-N domain can form pores on lipid membranes and induce pyroptosis through cell membrane disruption [69–71]. Additionally, activated caspase-11 cleaves the cytosolic region of pannexin-1, a membrane channel for ATP, and thus induces the extracellular release of ATP, which engages the P2X7 receptor in an autocrine or paracrine manner to open P2X7 pores leading to pyroptosis [33].
Figure 2.
Mechanisms of activation for non-canonical and alternative NLRP3 inflammasomes. Non-canonical NLRP3 inflammasome activation (left side of the figure) is induced by Gram-negative bacteria. LPS is delivered into the cytosol through transfection or infection and activates caspase-11. Active caspase-11 triggers the opening of the pannexin-1 channel through cleavage, which induces the K+ efflux required for NLRP3 inflammasome activation and release of IL-1β. Caspase-11 activation also drives pyroptosis through the cleavage of gasdermin D. The gasdermin D N domain disrupts cellular functions by forming pores on the membrane. The activation of P2X7 by ATP released from the pannexin-1 channel also contributes to pyroptosis. The alternative NLRP3 inflammasome (right side of the figure) is activated in human monocytes in response to LPS. For this pathway, K+ efflux is dispensable, and molecules RIPK1, FADD and CASP8 are required, and no ASC speck formation or pyroptosis is induced.
Alternative NLRP3 inflammasome pathway
Human monocytes, unlike macrophages, are able to release mature IL-1β in response to TLR ligands alone, such as LPS [29, 72]. This may be achieved through microbial activation of the NLRP3 inflammasome through an autocrine mechanism. In this model, LPS induces the release of endogenous ATP from human monocytes, which in turn activates the P2X7 receptor to trigger NLRP3 inflammasome activation and IL-1β maturation. Mouse bone marrow-derived dendritic cells can also secrete mature IL-1β in response to TLR ligands alone, but in contrast to human monocytes this occurs through an ATP-P2X7 independent pathway [73]. Furthermore, in vivo experiments with P2X7-deficient mice indicate that P2X7 is dispensable for IL-1β secretion in an LPS-induced septic shock model [73]. However, the cell type(s) involved in the production of IL-1β in vivo remains unclear. An alternative NLRP3 inflammasome pathway is also activated in human monocytes in response to LPS (Figure 2) [74]. In this pathway, K+ efflux is not required, which contrasts with NLRP3 activation induced by ATP, pore-forming toxins and particulate matter. Thus, this pathway is defined as an alternative NLRP3 inflammasome pathway [74]. Mechanistically, molecules RIPK1, FADD and CASP8 act downstream of TLR4-TRIF signaling to activate NLRP3 after LPS treatment. Although both ASC and caspase-1 are required, there is no evidence for ASC speck formation or pyroptosis in this pathway.
Regulators of NLRP3 inflammasome activation
Several regulators of NLRP3 inflammasome activation including double-stranded RNA-dependent protein kinase (PKR), guanylate-binding protein 5 (GBP5) and Nek7 [75–79] have been reported.
PKR regulates the activation of all known inflammasomes, including NLRP1, NLRP3, NLRC4, and AIM2 [76]. Deletion or inhibition of PKR leads to reduced activation of caspase-1 and maturation of IL-1β and IL-18 in response to a wide array of stimuli. However, the role of PKR in inflammasome activation was not independently confirmed in a different study with macrophages from two different Pkr-deficient mice, including the mutant mice in the former study [80]. Therefore, further studies are required to clarify the role of PKR in NLRP3 inflammasome activation.
Similar to PKR, the role of GBP5 in NLRP3 inflammasome activation remains controversial. GBP5 was shown to promote NLRP3 inflammasome activation in response to ATP, nigericin, and bacteria, but not particulate matter [75]. In contrast, another study observed normal activation of the NLRP3 inflammasome in macrophages from an independently generated Gbp5-deficient mouse line [62]. It is not clear what accounts for the discrepancies in these studies.
Unlike PKR and GBP5, the critical role of Nek7 in NLRP3 inflammasome activation has been shown independently in three independent studies [77–79]. Nek7 belongs to the NIMA-related kinases family that regulates the mitotic progression and DNA damage response [81]. Nek7 deficiency in mice results in late lethality during embryogenesis and growth retardation, indicating a critical role of Nek7 in development and survival [82]. Nek7 is required for NLRP3 inflammasome activation induced by all NLRP3 stimuli tested including ATP, nigericin, monosodium urate crystals and Alum [77, 78]. In contrast, Nek7 is dispensable for the activation of NLRC4 or AIM2 inflammasomes [77–79]. Nek7 interacts with NLRP3 through the catalytic domain of Nek7 and the LRR domain of NLRP3, which is enhanced by NLRP3 stimulation but is independent of Nek7 kinase activity [77, 78]. Furthermore, the kinase activity of Nek7 is also not required for Nek7-mediated NLRP3 activation [77, 78]. Nek7 was shown to control NLRP3 oligomerization, ASC speck formation and caspase-1 activation downstream of potassium efflux [77]. Notably, both Nek6, a close paralog of Nek7, and Nek9, a known upstream regulator of Nek7 during mitosis, do not interact with NLRP3 and are also dispensable for NLRP3 inflammasome activation [77]. Furthermore, Nek7 is also required for NLRP3 activation in macrophages harboring a NLRP3 activating mutation (NLRP3R258W) [77]. The critical role of Nek7 in NLRP3 activation has been further demonstrated by three in vivo models, in which Nek7 deficiency results in reduced IL-1β secretion, attenuated recruitment of immune cells and decreased disease severity as compared to wild-type mice [77, 78]. Therefore, these results clearly reveal that Nek7 is a bona fide positive regulator of NLRP3 inflammasome activation. How does Nek7 regulate NLRP3 inflammasome activation? The Nek7/NLRP3 interaction requires K+ efflux as this interaction is abolished in the presence of a high extracellular concentration of K+. It is possible that a drop in intracellular K+ causes conformational changes in NLRP3, which allow the binding of Nek7 to NLRP3. In line with this hypothesis, a mutation in NLRP3 that allows K+ efflux-independent activation still requires Nek7 for inflammasome activation [77]. Understanding the signaling mechanism of Nek7 in response to NLRP3 activators will provide new insight into the molecular mechanism of NLRP3 inflammasome activation.
Concluding remarks
In the past decade, intense efforts have been put into the investigation of the mechanism of NLRP3 inflammasome activation. However, a unified mechanism has not yet emerged. Recent progress has been made to reveal a critical role for K+ efflux in NLRP3 activation while the contribution of Ca2+ signaling, ROS and mitochondrial dysfunction remains controversial. Importantly, the identification of Nek7 as an essential regulator of the NLRP3 inflammasome, as well as the discoveries of a non-canonical inflammasome and an alternative NLRP3 inflammasome pathway, represent major advances in the inflammasome field. Clearly, much more work is needed to understand the mechanism by which Nek7 regulates NLRP3 inflammasome activation and how diverse cell signaling events are integrated to activate the NLRP3 inflammasome (See Outstanding Questions).
Outstanding Questions.
Which are the convergent signaling events for NLRP3 inflammasome activation?
Does NLRP3 recognize an endogenous molecule released downstream or in parallel to K+ efflux in response to NLRP3 stimuli?
What is the composition and structural organization of the NLRP3 inflammasome and the NLRP3-NEK7 complex?
Are changes in intracellular K+ levels sensed directly by NLRP3 or by another factor upstream of NLRP3?
Which are the proximal signaling events required for NLRP3 activation in the non-canonical and alternative inflammasome pathways?
Does Nek7 regulate NLRP3 inflammasome activation by directly promoting NLRP3 oligomerization or recruiting other regulators?
Does NLRP3 also need other NLRs for activation, similar to that seen in the NLRC4 inflammasome which needs NAIPs as upstream sensors?
Trends.
The NLRP3 inflammasome is an essential mediator of host immune responses through the activation of caspase-1 and IL-1β/IL-18.
The NLRP3 inflammasome is thought to sense the disturbance of cellular homeostasis rather than directly recognizing a common motif present in its activators, and multiple cellular signals have been proposed for triggering its activation, including K+ efflux, Ca2+ signaling, mitochondrial dysfunction and lysosomal rupture.
Non-canonical and alternative inflammasome pathways have been recently identified to activate the NLRP3 inflammasome.
Nek7 has emerged as an essential regulator of NLRP3 inflammasome activation.
Acknowledgments
The work on inflammasomes in the Núñez laboratory is funded by NIH grants R01AI063331 and R01DK091191. Y. H. was supported by NIH training grant T32HL007517.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Franchi L, et al. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinon F, et al. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem. 2002;277:29874–29880. doi: 10.1074/jbc.M203915200. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annual Review of Immunology. 2009:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 5.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 6.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ting JP, et al. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchi L, et al. Function of Nod-like receptors in microbial recognition and host defense. Immunological Reviews. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 11.Strowig T, et al. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 12.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 13.Sha W, et al. Human NLRP3 inflammasome senses multiple types of bacterial RNAs. Proc Natl Acad Sci U S A. 2014;111:16059–16064. doi: 10.1073/pnas.1412487111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franchi L, et al. Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. J Immunol. 2014;193:4214–4222. doi: 10.4049/jimmunol.1400582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowling JK, O'Neill LA. Biochemical regulation of the inflammasome. Critical reviews in biochemistry and molecular biology. 2012;47:424–443. doi: 10.3109/10409238.2012.694844. [DOI] [PubMed] [Google Scholar]
- 16.Bauernfeind FG, et al. Cutting Edge: NF-kappa B Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. Journal of Immunology. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harder J, et al. Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-kappa B activation but proceeds independently of TLR signaling and P2X7 receptor. J Immunol. 2009;183:5823–5829. doi: 10.4049/jimmunol.0900444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franchi L, et al. Cutting Edge: TNF-alpha Mediates Sensitization to ATP and Silica via the NLRP3 Inflammasome in the Absence of Microbial Stimulation. Journal of Immunology. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allam R, et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 2014;15:982–990. doi: 10.15252/embr.201438463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurung P, et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemmers B, et al. Essential role for caspase-8 in Toll-like receptors and NFkappaB signaling. J Biol Chem. 2007;282:7416–7423. doi: 10.1074/jbc.M606721200. [DOI] [PubMed] [Google Scholar]
- 22.Schroder K, et al. Acute lipopolysaccharide priming boosts inflammasome activation independently of inflammasome sensor induction. Immunobiology. 2012;217:1325–1329. doi: 10.1016/j.imbio.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Juliana C, et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes-Alnemri T, et al. Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J Immunol. 2013;191:3995–3999. doi: 10.4049/jimmunol.1301681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin KM, et al. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc Natl Acad Sci U S A. 2014;111:775–780. doi: 10.1073/pnas.1320294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Py BF, et al. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49:331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Castejon G, et al. Deubiquitinases regulate the activity of caspase-1 and interleukin-1beta secretion via assembly of the inflammasome. J Biol Chem. 2013;288:2721–2733. doi: 10.1074/jbc.M112.422238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 29.Piccini A, et al. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1 beta and IL-18 secretion in an autocrine way. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munoz-Planillo R, et al. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrilli V, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death and Differentiation. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 32.Franchi L, et al. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. Journal of Biological Chemistry. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 33.Yang D, et al. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity. 2015;43:923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruhl S, Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol. 2015;45:2927–2936. doi: 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- 35.Meng G, et al. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30:860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldmeyer L, et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Current biology : CB. 2007;17:1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 37.Chu J, et al. Cholesterol-dependent cytolysins induce rapid release of mature IL-1beta from murine macrophages in a NLRP3 inflammasome and cathepsin B-dependent manner. J Leukoc Biol. 2009;86:1227–1238. doi: 10.1189/jlb.0309164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brough D, et al. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J Immunol. 2003;170:3029–3036. doi: 10.4049/jimmunol.170.6.3029. [DOI] [PubMed] [Google Scholar]
- 39.Lee GS, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murakami T, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2012;109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossol M, et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun. 2012;3:1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsnelson MA, et al. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J Immunol. 2015;194:3937–3952. doi: 10.4049/jimmunol.1402658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawlor KE, Vince JE. Ambiguities in NLRP3 inflammasome regulation: is there a role for mitochondria? Biochimica et biophysica acta. 2014;1840:1433–1440. doi: 10.1016/j.bbagen.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 44.Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. 2015;265:35–52. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Bruggen R, et al. Human NLRP3 inflammasome activation is Nox1-4 independent. Blood. 2010;115:5398–5400. doi: 10.1182/blood-2009-10-250803. [DOI] [PubMed] [Google Scholar]
- 47.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nature Immunology. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou RB, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 49.Bauernfeind F, et al. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimada K, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakahira K, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iyer SS, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Subramanian N, et al. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153:348–361. doi: 10.1016/j.cell.2013.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park S, et al. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J Immunol. 2013;191:4358–4366. doi: 10.4049/jimmunol.1301170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ermler ME, et al. Rift Valley fever virus infection induces activation of the NLRP3 inflammasome. Virology. 2014;449:174–180. doi: 10.1016/j.virol.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halle A, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nature Immunology. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dostert C, et al. Malarial Hemozoin Is a Nalp3 Inflammasome Activating Danger Signal. Plos One. 2009;4 doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orlowski GM, et al. Multiple Cathepsins Promote Pro-IL-1beta Synthesis and NLRP3-Mediated IL-1beta Activation. J Immunol. 2015;195:1685–1697. doi: 10.4049/jimmunol.1500509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 60.Rathinam VA, et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gurung P, et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-beta (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J Biol Chem. 2012;287:34474–34483. doi: 10.1074/jbc.M112.401406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meunier E, et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509:366–370. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- 63.Kayagaki N, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 64.Hagar JA, et al. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi J, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 66.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 67.Kayagaki N, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 68.He WT, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ding J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016 doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 70.Liu X, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aglietti RA, et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A. 2016;113:7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Netea MG, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1 beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He Y, et al. TLR agonists stimulate Nlrp3-dependent IL-1beta production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol. 2013;190:334–339. doi: 10.4049/jimmunol.1202737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gaidt MM, et al. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity. 2016;44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 75.Shenoy AR, et al. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science. 2012;336:481–485. doi: 10.1126/science.1217141. [DOI] [PubMed] [Google Scholar]
- 76.Lu B, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He Y, et al. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–357. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi H, et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17:250–258. doi: 10.1038/ni.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmid-Burgk JL, et al. A Genome-wide CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) Screen Identifies NEK7 as an Essential Component of NLRP3 Inflammasome Activation. J Biol Chem. 2016;291:103–109. doi: 10.1074/jbc.C115.700492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He Y, et al. The protein kinase PKR is critical for LPS-induced iNOS production but dispensable for inflammasome activation in macrophages. Eur J Immunol. 2013;43:1147–1152. doi: 10.1002/eji.201243187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fry AM, et al. Cell cycle regulation by the NEK family of protein kinases. Journal of cell science. 2012;125:4423–4433. doi: 10.1242/jcs.111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salem H, et al. Nek7 kinase targeting leads to early mortality, cytokinesis disturbance and polyploidy. Oncogene. 2010;29:4046–4057. doi: 10.1038/onc.2010.162. [DOI] [PubMed] [Google Scholar]