Abstract
The orexin/hypocretin (ORX) system regulates motivation for natural rewards and drugs of abuse such as alcohol. ORX receptor antagonists, most commonly OX1R antagonists including SB-334867 (SB), decrease alcohol drinking, self-administration and reinstatement in both genetically-bred alcohol-preferring and outbred strains of rats. Importantly, levels of alcohol seeking and drinking in outbred rats are variable, as they are in humans. We have shown that OX1R antagonism selectively decreases homecage alcohol drinking in high-, but not low-alcohol-preferring rats. It is unknown, however, whether this effect is selective to homecage drinking or whether it also applies to alcohol seeking paradigms such as self-administration and reinstatement following extinction, in which motivation is high in the absence of alcohol. Here we trained Sprague Dawley rats to self-administer 20% ethanol paired with a light-tone cue on an FR3 regimen. Rats were then extinguished and subjected to cue-induced reinstatement. Rats were segregated into high- and low-ethanol-responding groups (HR and LR) based on self-administration levels. During self-administration and cue-induced reinstatement, rats were given SB or vehicle prior to ethanol seeking. In both conditions, OX1R antagonism decreased responding selectively in HR, but not LR rats. There were no non-specific effects of SB treatment on arousal or general behavior. These data indicate that ORX signaling at the OX1R receptor specifically regulates high levels of motivation for alcohol, even in the absence of direct alcohol reinforcement. This implicates the ORX system in the pathological motivation underlying alcohol abuse and alcoholism and demonstrates that the ORX1R may be an important target for treating alcohol abuse.
Keywords: alcoholism, reward, lateral hypothalamus, neuropeptide, motivation, individual differences
1. Introduction
Orexin (ORX, also known as hypocretin, HCRT) neurons are located in a limited region of the dorsal hypothalamus consisting of the lateral, perifornical, and dorsomedial hypothalamic areas (de Lecea et al., 1998; Peyron et al., 1998; Sakurai et al., 1998). These neurons project throughout the brain (Sakurai et al., 2005; Yoshida et al., 2006), and are thought to regulate a wide range of functions including arousal and reward motivation, among a number of others (Aston-Jones et al., 2010; Li et al., 2014; Mahler et al., 2014; Sakurai, 2007; Sakurai, 2014). ORX neurons produce two peptides, ORX-A and ORX-B (or HCRT-1 and HCRT-2) (de Lecea et al., 1998; Sakurai et al., 1998). These peptides differentially bind to two ORX receptors – the ORX-1 receptor (OX1R; HCRT1R) and the ORX-2 receptor (OX2R; HCRT2R) (de Lecea et al., 1998; Sakurai et al., 1998). The OX1R exhibits stronger selectivity for ORX-A, whereas the OX2R exhibits approximately equal selectivity for ORX-A and ORX-B (Sakurai et al., 1998). These receptors are distributed differentially across the brain (Marcus et al., 2001), and a number of studies have indicated that they likely play different roles in physiological function and behavior (Mahler et al., 2012; Mahler et al., 2014). Although not absolute, it has been hypothesized that whereas the OX2R is important in regulating the arousal-related functions associated with the ORX system, the OX1R plays a more important role in controlling the motivational functions of the ORX system (Mahler et al., 2012; Mahler et al., 2014).
Of particular importance, the OX1R has been widely associated with motivation for drugs of abuse, including alcohol (Brown et al., 2013a; Dayas et al., 2008; Jupp et al., 2011; Lawrence et al., 2006; Mahler et al., 2012; Martin-Fardon and Weiss, 2014; Moorman and Aston-Jones, 2009; Moorman et al., 2016). The OX1R-selective antagonist SB-334867 (SB) decreases seeking of multiple drugs of abuse (Bentzley and Aston-Jones, 2015; James et al., 2012; Mahler et al., 2012; Plaza-Zabala et al., 2012; Porter-Stransky et al., 2015). There is a particularly (though not exclusively) (Anderson et al., 2014; Barson et al., 2015; Brown et al., 2013b; Shoblock et al., 2011) strong relationship between the OX1R vs OX2R and alcohol seeking. SB decreased cue-induced reinstatement of alcohol seeking in alcohol-preferring rats (Jupp et al., 2011; Lawrence et al., 2006), decreased stress-induced reinstatement of alcohol seeking in Long-Evans rats (Richards et al., 2008), and decreased reinstatement of alcohol-seeking elicited by discriminative stimuli in Wistar rats (Martin-Fardon and Weiss, 2014). SB also decreased relapse to alcohol seeking/drinking after homecage deprivation in female alcohol-preferring rats, but only when alcohol was available (Dhaher et al., 2010). These effects are mediated, at least in part, by OXR1 signaling in the ventral tegmental area and prelimbic cortex, as SB infused into these regions independently decreased cue-induced ethanol seeking (Brown et al., 2016).
OX1R antagonism also decreases alcohol drinking. SB treatment decreased alcohol drinking in alcohol-preferring rats (Anderson et al., 2014) as well as in Sprague Dawley rats (Moorman and Aston-Jones, 2009) and C57BL/6J mice (Anderson et al., 2014; Olney et al., 2015). Antagonism of OX2R also has an effect on ethanol seeking in the presence of ethanol (Brown et al., 2013b; Shoblock et al., 2011), indicating potential differential contributions of ORX signaling at each receptor, possibly due to differential receptor distribution (Cluderay et al., 2002; Hervieu et al., 2001; Marcus et al., 2001; Trivedi et al., 1998)
We previously demonstrated that OX1R antagonism decreased two-bottle choice preference selectively in high-alcohol-preferring, and not in low-alcohol-preferring Sprague Dawley rats (Moorman and Aston-Jones, 2009). We also showed that the selective OX1R antagonist GSK1059865 decreased alcohol drinking preferentially in mice that had increased ethanol drinking following chronic intermittent access to ethanol (Lopez et al., 2016). These results align with findings in ethanol-preferring rats (Jupp et al., 2011; Lawrence et al., 2006), and extend those results to show that the effect of OX1R antagonism on alcohol drinking is maximally efficacious for individuals with high motivation to drink alcohol, compared to those with low motivation. These findings are also consistent with Fos activation of ORX neurons, in which strength of such activation typically correlates with alcohol seeking (Hamlin et al., 2007; Moorman et al., 2016). Taken together, these results indicate that OX1R treatment may be particularly important in individuals prone to alcohol abuse or addiction. However, the selective effect of OX1R antagonism has not yet been demonstrated on alcohol seeking in operant self-administration contexts or reinstatement paradigms, which model human alcohol seeking and relapse (Shaham et al., 2003). In the present study, we investigated the effects of OX1R antagonism on alcohol self-administration and cue-induced reinstatement in Sprague Dawley rats that exhibited high or low levels of alcohol seeking behavior. Intriguingly, we found that rats segregated into low- vs. high-responders as response demands increased (at the transition to FR3 seeking) and that this segregation was consistent over time. When treated with SB, high-responding animals exhibited decreases in ethanol self-administration and cue-induced reinstatement whereas low-responding animals were not affected. These results demonstrate a strong connection between alcohol seeking and ORX system, particularly the OX1R, and indicate that this system may be fundamentally involved in alcohol use disorders.
2. Results
Rats were trained to respond in an operant task for ethanol. Animals were first trained on an FR1 schedule for 13 days before being moved to FR2 (3 days) then FR3 (5 days) schedules. Following self-administration training, animals were divided into two groups – high responders (HR) and low responders (LR) – based on a median split of active lever response data on the final day of FR3 training. We then performed a number of analyses to compare HR versus LR rats in terms of home cage drinking behavior and self-administration behavior.
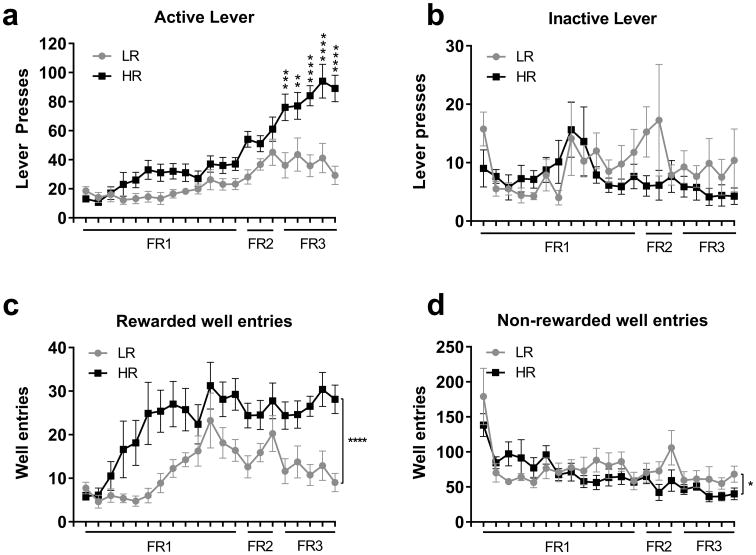
Analysis of self-administration data revealed very different patterns of ethanol self-administration behavior between HR and LR animals. There was a significant ‘group’ × ‘session’ interaction (F20,294=3.89, p<0.0001) with respect to the number of active lever responses animals made across self-administration training. Subsequent analyses revealed that HR rats tended to show higher levels of responding than LR rats across the extent of the self-administration training period, with significant differences arising during FR3 training (p<0.05 in all cases; Figure 1a). In contrast, HR and LR animals did not differ significantly in their inactive lever responding across all training sessions (‘group’ × ‘session’ interaction: F20,294=0.96, p>0.05; Fig 1b), indicating that differences between HR and LR animals were specific to goal-directed seeking behaviors. HR animals made significantly more rewarded well entries than LR animals across the entire training period (F1,294=99.18, p<0.0001; Fig 1c). LR animals made slightly more non-rewarded well entries than HR animals across all sessions (F1,294=4.32, p<0.05; Fig 1d), indicating that HR animals did not simply exhibit more general exploratory behavior than LR animals.
Figure 1. Comparison of alcohol self-administration behavior in HR vs LR rats.
(A) Although HR and LR animals showed similar patterns of active lever responding on FR1 and FR2 sessions, HR animals showed significantly greater active lever responses during FR3 sessions. (B) There were no differences between HR and LR animals in terms of inactive lever responses at any stage of self-administration training. (C) Similar to active lever responses, HR animals exhibited a significantly greater number of rewarded well entries on FR3 self-administration sessions. (D) HR and LR animals showed similar levels of non-rewarded well entries during self-administration training. ** p<0.01; *** p<0.001; **** p<0.0001.
We also measured amount of ethanol consumed approximately every other day during self-administration sessions, based on number of rewarded well-entries. Ethanol intake, measured in g/kg, increased over the course of FR1 (mean early: 0.27 +/- 0.03, mean late: 0.82 +/- 0.09). HR rats consumed more ethanol than LR rats in late FR1 sessions (HR: 1.12 +/- 0.11, LR: 0.59 +/- 0.10), but not early sessions (HR: 0.24 +/- 0.04, LR: 0.30 +/- 0.05). This increase over sessions was significant (F7,112=6.31, p<0.001), as was the difference between HR and LR rats (F1,112=26.60, p<0.001), although the interaction was not (F7,112=1.60, p>0.05), indicating that both groups increased consumption across FR1 sessions. This difference persisted through late FR2 (HR: 1.12 +/- 0.10, LR: 0.70 +/- 0.15) and FR3 sessions (HR: 1.02 +/- 0.10, LR: 0.30 +/- 0.07).
The orexin-1 receptor antagonist SB-334867 attenuated ethanol self-administration behavior in HR but not LR rats
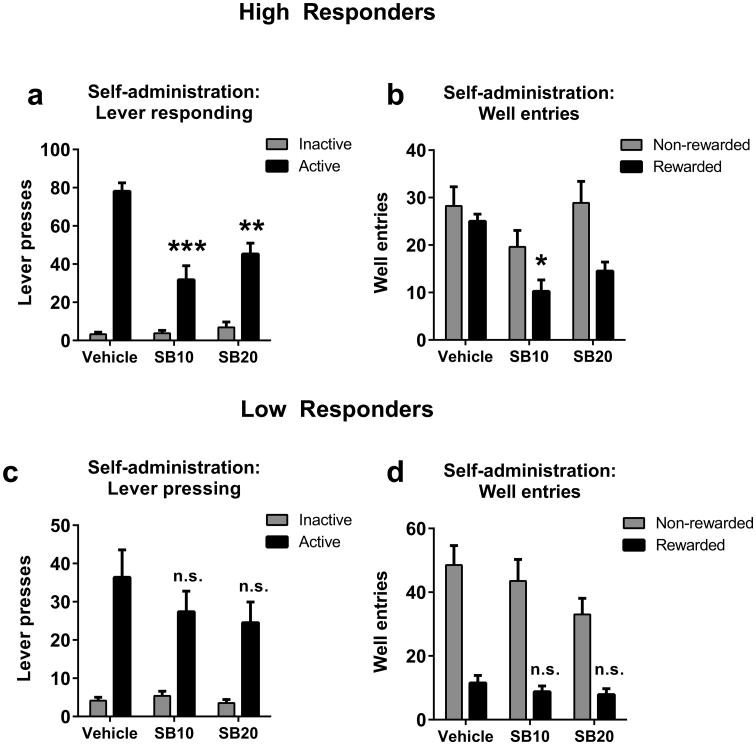
Next we examined whether OX1R signaling plays a differential role in regulating ethanol self-administration in HR versus LR rats. Rats were treated with SB-334867 (10 mg/kg, ip, SB10 or 20 mg/kg, ip, SB20) or vehicle 30 min prior to FR3 self-administration sessions (Fig. 2). We used a 2-factor repeated measures ANOVA to examine the effect of SB on responding on the active and inactive levers across the three treatment days. In HR animals, there was main effect of ‘treatment’ (F2,14=9.63, p<0.01) and ‘lever’ (F1,7=52.06, p<0.01), as well as a ‘treatment’ × ‘lever’ interaction (F2,14=19.59, p<0.001). Post-hoc tests revealed that both SB10 and SB20 significantly reduced responding on the active lever, relative to vehicle treatment (p<0.0001 and p<0.001, respectively; Figure 2a). There was no effect of SB on inactive lever responding (p>0.05; Figure 2a). A similar analysis examining the effect of SB on the number of well entries made revealed a significant main effect of ‘treatment’ (F2,14=4.20, p<0.05), and ‘well entry type’ (rewarded vs. non-rewarded; F1,7=10.27, p<0.05), but the ‘treatment’ × ‘well entry type’ interaction did not reach significance (p>0.05). Post-hoc analyses indicated that SB10 significantly reduced the number of rewarded well entries (p<0.05; Figure 2b). A similar trend was observed for SB20 that failed to reach significance (p>0.05). In contrast, there was no effect of SB on the number of non-rewarded well entries made (Figure 2b). In contrast, in LR rats there was a significant main effect of ‘lever’ (F1,7=13.75, p<0.01), but no main effect of ‘treatment’ (p>0.05) or ‘lever’ × ‘treatment’ interaction (p>0.05), indicating that SB treatment had no effect on responding on either lever in these animals (Figure 2c). Similarly, there was significant main effect of ‘well entry type’ (F1,7=20.93, p<0.001), but not ‘treatment’ (p>0.05) or a ‘well entry type’ × ‘treatment’ interaction (p>0.05), indicating that SB treatment did not affect either form of well entry in LR animals (Figure 2d). Ethanol intake measured in g/kg, based on number of rewarded well entries, was also selectively influenced by SB treatment in HR (veh: 0.86 +/- 0.07, SB10: 0.36 +/- 0.12, SB20: 0.50 +/- 0.09; F2,21=7.16, p<0.005) but not LR rats (veh: 0.38 +/- 0.12, SB10: 0.29 +/- 0.09, SB20: 0.28 +/- 0.09; F2,21=0.37, p>0. 05). These results indicate that SB treatment specifically attenuated ethanol-seeking behavior in HR animals.
Figure 2. SB-334867 attenuates alcohol self-administration behavior in HR, but not LR rats.
(A) In HR animals, SB10 and SB20 treatment significantly reduced active (but not inactive) lever responses during a 2-hr self-administration session. (B) HR animals also showed a significant reduction in the number of rewarded (but not non-rewarded) well entries following SB treatment. (C, D) In contrast to HR rats, self-administration behavior in LR rats was unaffected by SB treatment. *p<0.05; ** p<0.01; *** p<0.001; n.s. not significant.
SB-334867 attenuated cue-induced reinstatement of ethanol seeking in high-responding but not low-responding rats
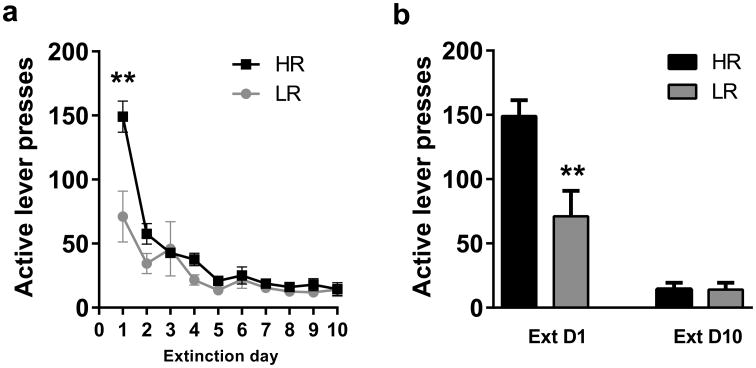
Lever pressing behavior was extinguished over a period of 10 days (Fig. 3). On the first day of extinction, animals in the HR group made a significantly greater number of responses on the active lever as compared to the LR group (t(14)=3.33, p<0.01; Figure 3a,b). On the final day of extinction, animals made an average of 14.19 (± 3.59) responses on the active lever and 2.89 (± 0.74) on the inactive lever. Lever responding on the final day of extinction did not differ between HR and LR groups (active lever: t(14)=0.05, p>0.05; inactive lever: t(14)=0.033, p>0.05; Figure 3a,b).
Figure 3. HR rats exhibited greater levels of responding than LR rats on the first day of extinction.
(A) Active lever presses during 10 days of extinction in HR and LR rats. (B) HR rats responded significantly more than LR rats on extinction day 1. By the last day of extinction, active lever responses were equivalent between HR and LR groups. ** p<0.01.
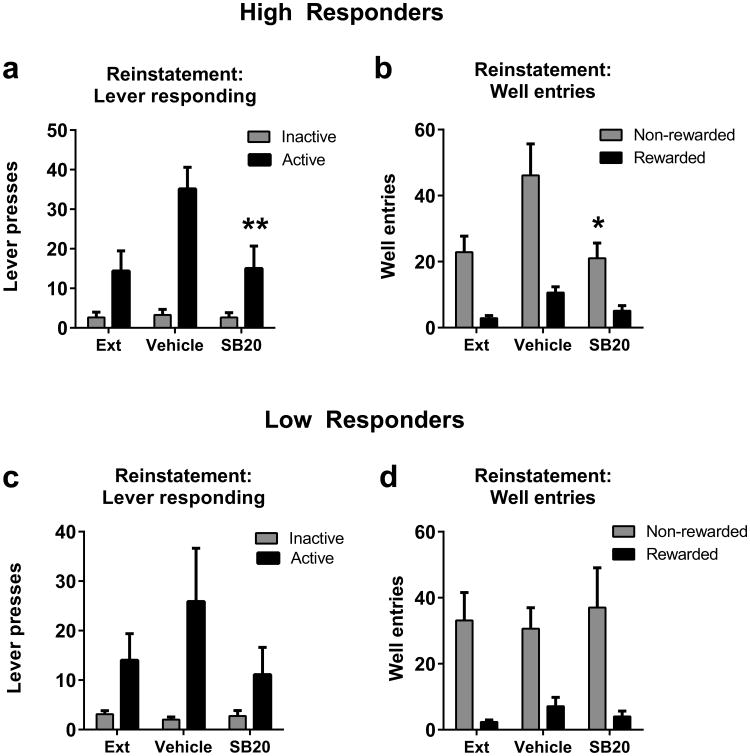
Next we examined the effect of systemic SB injections on cue-induced reinstatement behavior in HR versus LR rats (Fig. 4). We used a 2-factor repeated measures ANOVA to examine the effect of SB on responding on the active versus inactive lever during the two reinstatement tests. In HR animals, there was a significant main effect of ‘treatment’ (F1,7=8.41, p<0.05), and ‘lever’ (F1,7=20.13, p<0.01), and a significant ‘treatment’ × ‘lever’ interaction (F1,7=11.29, p<0.05). Post-hoc analyses showed that SB20 in HR rats significantly reduced reinstatement of responding on the active lever (p<0.01), but had no effect on inactive lever responding (p>0.05; Figure 4a). Similarly, when comparing the effects of SB on well entries in the HR group, there was a significant main effect of ‘treatment’ (F1,7=5.81, p<0.05) and ‘well entry type’ (F1,7=5.81, p<0.05), and a significant ‘treatment’ × ‘well entry type’ interaction (F1,7=18.44, p<0.01). Post-hoc analyses revealed that there was a non-significant trend towards an SB20-induced reduction in the number of ‘rewarded’ well entries made (p>0.05), and a significant SB20-induced reduction in the number of non-rewarded well entries made (p<0.01; Figure 4b).
Figure 4. SB-334867 attenuates cue-induced reinstatement of alcohol seeking behavior in HR but not LR.
rats. (A) HR animals showed a significant reinstatement of active lever responding during cue-induced reinstatement tests. This reinstatement was blocked by SB20 treatment. (B) Similarly, SB20 significantly attenuated the number of ‘rewarded’ well entries during cue-induced reinstatement tests and also reduced the number of non-rewarded well entries made. (C) SB20 treatment had no effect on the number of active or inactive lever responses made during reinstatement tests. (D) SB20 also did not affect the number of ‘rewarded’ or non-rewarded well entries made during testing. *p<0.05; ** p<0.01.
In contrast, in LR animals there was a significant main effect of ‘lever’ (F1,7=34.18, p<0.001), but no effect of ‘treatment’ (p<0.05) or ‘lever’ × ‘treatment’ interaction, indicating that SB had no effect on responding on either lever during reinstatement testing in these animals (Figure 4c). Similarly, with respect to well entries, there was a significant main effect of ‘well entry type’ (F1,7=24.22, p<0.001), but no effect of ‘treatment’ (p>0.05) or ‘well entry type’ × ‘treatment’ interaction (p>0.05), indicating that SB treatment had no effect on well entries made during reinstatement testing in LR animals (Figure 4d).
HR and LR rats did not differ in home cage ethanol intake
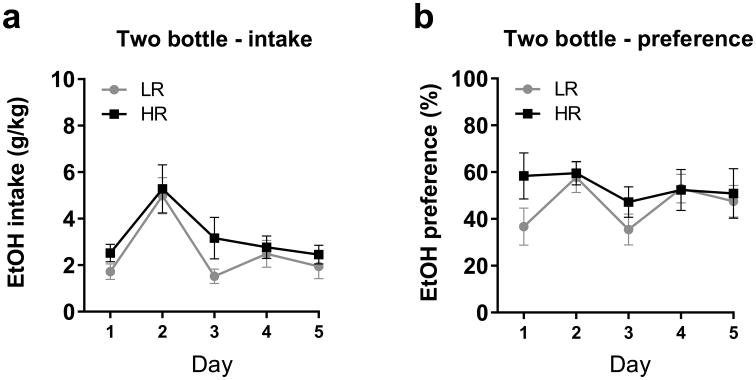
We were also interested in whether HR and LR animals exhibited differences in ethanol consumption when ethanol was freely available in the home cage. We therefore analyzed data from the initial homecage access period (prior to self-administration training), whereby rats were tested for ethanol preference (vs. water) in a 3-hour 2-bottle choice test for five days. Over the course of those tests, rats consumed on average 2.89g/kg (±0.57) of ethanol. There were no differences between HR and LR rats in terms of ethanol intake (F1,70=3.28, p>0.05; Figure 5A) or preference for ethanol (F1,70=2.47, p>0.05; Figure 5b) during this period. Similarly, operant ethanol seeking was not correlated with homecage ethanol drinking, as there was no relationship between responding for ethanol under the FR3 schedule and average ethanol intake (r=0.2883, p>0.05) or preference (r=0.2161, p>0.05) across the five days of testing in the two-bottle choice test.
Figure 5. Comparison of homecage alcohol intake in HR vs LR rats.
(A) Ethanol intake during 3-hr 2-bottle choice tests was equivalent between HR and LR groups. (B) Ethanol preference was also similar between the two groups.
3. Discussion
We found that outbred Sprague Dawley rats exhibit substantial individual differences in self-administration of 20% ethanol during operant self-administration and cue-induced reinstatement of alcohol seeking. In previous work we showed that OX1R antagonism decreased homecage ethanol drinking selectively in high-ethanol-preferring animals (Lopez et al., 2016; Moorman and Aston-Jones, 2009), and that activation of specific populations of ORX neurons is correlated with alcohol preference and seeking (Moorman et al., 2016). Here, we extend these findings to demonstrate that SB-334867 treatment decreased both operant self-administration and cue-induced reinstatement of ethanol seeking after extinction selectively in high-, but not low-responding animals. These results indicate that the ORX system plays an important role in regulating highly motivated alcohol seeking, even when alcohol is not available.
We separated animals into high and low responders based on their stable performance after approximately 2 weeks of FR3 self-administration. Interestingly, although these two groups showed some differences in alcohol seeking under FR1 and FR2 schedules, they began to diverge dramatically at the onset of FR3 self-administration and this pattern persisted throughout FR3 training. These results indicate that one of the main determinants of individual differences in alcohol seeking in rats is the amount of effort or motivation required to acquire ethanol, and that individuals can be sorted based on their propensity to exert effort for ethanol reward. The fact that high levels of motivation were blunted by SB treatment argues that increased activation of the ORX system underlies the enhanced levels of seeking by highly-motivated individuals. We also observed that SB normalized cue-induced reinstatement of alcohol seeking specifically in HR animals. While low reinstatement behavior in LR animals may have precluded us from observing an effect of SB in these animals, this also likely reflects generally lower motivation for alcohol in LR animals. Together, these results strengthen the hypothesis that a major role of the ORX system is in regulating motivational activation (Mahler et al., 2014), in this case playing a particularly important role in elevated motivation for alcohol.
Importantly, the effects of ORX receptor antagonism were specific to ethanol seeking and did not generalize to non-selective behaviors. Thus, SB treatment had no effect on inactive lever pressing or non-rewarded well entries in either HR or LR rats during self-administration, and had no effect on inactive lever pressing in reinstatement. SB also had no effect on any measures in LR rats, strongly indicating its actions in HR rats are not simply due to a non-specific motor deficit. Moreover, LR animals tended to exhibit more non-rewarded well entries than HR animals during extinction, indicating that the reduced alcohol-seeking behavior observed in LR animals was not due to a general locomotor deficit in these animals. It is also interesting to note that the effect of SB on self-administration behavior was similar across both doses of SB (10 and 20mg/kg), indicating that lower doses of SB may be maximally efficacious in reducing alcohol seeking. Previous studies have reported effects of SB on drug seeking at both lower (1-5mg/kg; Hollander et al., 2008; Jupp et al., 2011) and higher (30mg/kg; Smith et al.) doses, however this may reflect differences in the reinforcer (alcohol, cocaine, nicotine) and/or the behavioral paradigm used in these studies.
We previously showed that high levels of homecage drinking, either innate or enhanced through chronic access to ethanol vapor, are reduced following OX1R antagonist treatment, indicating that this type of motivation is ORX-regulated (Lopez et al., 2016; Moorman and Aston-Jones, 2009). Intriguingly, there was minimal overlap between high drinkers in the homecage and high seekers in the operant environment. This finding indicates a distinction between an individual's preferred blood alcohol level when alcohol is freely available and their motivation to achieve and maintain these levels in an operant task. Thus, some individuals will consume large volumes of ethanol when it is freely available but will limit the amount of effort exerted to acquire it (as measured in the FR3 paradigm), whereas others exhibit strong preference and motivation in both contexts. Our results are in agreement with previous studies demonstrating a lack of correlation between the appetitive and consummatory aspects of ethanol use (Chappell and Weiner, 2008; Samson et al., 2001; Samson and Czachowski, 2003). Together, these results indicate that a thorough understanding of the neural substrates of alcohol use and abuse should consider multiple, potentially non-overlapping aspects of alcohol use and motivation. These include, but may not be limited to, preference for and willingness to consume freely-available alcohol as well as the amount of effort an individual will expend in order to acquire alcohol.
Previous studies have shown differential roles for neural systems in ethanol seeking, whereby serotonin 1B and dopamine D2 receptor signaling preferentially regulated appetitive aspects of ethanol (e.g., lever pressing), signaling through serotonin 1A and GABA(B) receptors regulated ethanol consumption, and mu, kappa, and delta opioid signaling influenced both, although a demonstrated complex interaction between receptor subtype and rat strain requires further study (Czachowski et al., 2001; Czachowski et al., 2002; Czachowski, 2005; Czachowski et al., 2006; Henderson-Redmond and Czachowski, 2014). Understanding these different types of motivation for alcohol may reveal subpopulations of alcohol use disorders, each with different neural substrates and different potential treatments. It is also important to note that some studies have demonstrated that, after approximately 2 months of homecage ethanol access, high-drinking animals show stronger operant ethanol seeking than do low-drinking animals (Spoelder et al., 2015). Therefore, it is possible that we may have observed a stronger relationship between homecage alcohol intake and self-administration in HR and LR rats had the homecage access period extended beyond two weeks.
The ORX system may be a common factor in regulating these different types of motivation for alcohol. Here, SB decreased alcohol seeking selectively in high-responding individuals, both in the presence of ethanol during self-administration, and when only ethanol cues were presented during reinstatement. These results indicate that the ORX system is particularly involved in regulating high levels of motivation, whether in the context of free-access to ethanol, as in our previous studies (Lopez et al., 2016; Moorman and Aston-Jones, 2009), or in the context of active ethanol seeking, as shown here.
Our pharmacological results are supported by the observation that activation of specific populations of ORX neurons is correlated with preference for and seeking of a variety of rewards, including alcohol (Hamlin et al., 2008; Harris et al., 2005; Harris et al., 2007; Lasheras et al., 2015; Mahler et al., 2012; Moorman et al., 2016; Richardson and Aston-Jones, 2012). Further, a number of previous studies have shown that the ORX system is particularly involved in elevated motivation for drugs of abuse and natural rewards. OX1R antagonism does not decrease FR1 cocaine self-administration (Smith et al., 2009), but does decrease cocaine seeking in FR5 testing (Hollander et al., 2012), progressive ratio (Espana et al., 2010), and behavioral economic-type measures of enhanced motivation for cocaine (Bentzley and Aston-Jones, 2015; Espana et al., 2010). With respect to natural rewards, the ORX system has been shown to be more involved in the enhanced motivation for highly-palatable rewards, such as chocolate, as compared to lower motivation associated with less-preferable rewards such as rodent chow (Borgland et al., 2009). With respect to alcohol seeking, rats bred to express high levels of alcohol preference exhibit significant decreases in alcohol seeking following OX1R antagonism (Anderson et al., 2014; Jupp et al., 2011; Lawrence et al., 2006), as do individual high-drinking outbred animals (Moorman and Aston-Jones, 2009).
Previous studies have reported individual differences in alcohol preference in outbred rats (e.g., Momeni and Roman, 2014; Momeni et al., 2014; Pelloux et al., 2015; Sharko et al., 2013; Spoelder et al., 2015), though it is more common for studies to consider effects averaged across entire cohorts. The current results, combined with previous work from our lab (Lopez et al., 2016; Moorman and Aston-Jones, 2009), strongly indicate that these individual differences in ethanol motivation result, at least in part, from differential activation of the ORX system. We and others have emphasized that one of the major functions of the ORX system is in regulating strong drive states, including behaviors such as compulsive seeking of alcohol, other drugs, or other highly-motivating rewards such as high-fat foods (Alcaraz-Iborra and Cubero, 2015; Mahler et al., 2014; Sakurai, 2014; Thompson and Borgland, 2011). Understanding the contribution of the ORX system to highly-motivated alcohol seeking is of particular importance when considering alcohol abuse and addiction. Optimal treatments to control compulsive reward seeking would not completely eliminate normal motivation and drives. Instead, such treatments for alcohol abuse and alcoholism might preferentially reduce compulsive, unregulated motivation for alcohol. The present results, along with previous work from our lab and others described above, indicate that the ORX system may be an excellent target for reducing compulsive drive states while leaving more regulated and natural reward drives intact. Future research should also strive to understand whether specific ORX neuronal populations (e.g., lateral vs. medial ORX neurons (Harris and Aston-Jones, 2006; Moorman et al., 2016)) regulate different aspects of motivated behaviors. Specific populations of ORX neurons, defined by a number of factors (anatomical location, afferents, efferents, etc.) or their projection targets may be prime candidates for both understanding the contribution of ORX to reward seeking as well as for designing treatments for compulsive reward seeking and addictions.
4. Experimental Procedure
Subjects
Male Sprague-Dawley rats (n = 16; arrival weight 250-300g; Charles River, Wilmington, MA) were single-housed under a reversed 12-h light/dark cycle (lights off at 6 a.m.) and had ad libitum access to food and water. Animals were housed in a temperature- and humidity-controlled animal facility at MUSC (AAALAC-accredited). Operant and two-bottle choice tests were conducted during the dark/active phase of the light cycle. All procedures were approved by the Medical University of South Carolina's Institutional Animal Care and Use Committee and conducted according to specifications of the NIH as outlined in the Guide for the Care and Use of Laboratory Animals.
Procedures
Rats were trained to drink 20% ethanol (95% ethanol (AAPER, Shelbyville, KY) and filtered water) using intermittent access (IA) (Moorman and Aston-Jones, 2009; Simms et al., 2008; Wise, 1975). Rats received either 20% ethanol or water for 24 h on alternating days for 2 weeks. Ethanol was given in home cages with ad lib access to food and water. After IA access to develop ethanol drinking, animals were tested on five days of 3-hour two-bottle choice testing (20% ethanol and water). After choice testing, rats were trained on ethanol self-administration on a FR1 schedule. Training and testing occurred in sound-attenuated operant boxes with two levers and a reward well (Med-Associates). Active lever presses resulted in delivery of 0.1 ml of 20% ethanol to a reward well paired with a tone and light stimulus above the active lever. Presses on the inactive lever produced no outcome and were not recorded. Head entries into the ethanol-rewarded well were detected using an infrared beam break and were recorded. Entries were classified as rewarded (immediately following lever press) or non-rewarded (> 1 sec after lever press or during the intertrial interval). Intertrial intervals were 20 sec, during which time houselights were turned on and lever pressing produced no response but was recorded. FR1 training lasted for 13 days at which point animals were trained on FR2 (3 days) and finally, FR3 (15 days). Rats were then extinguished for 10 days: lever presses produced no ethanol, lights or tones but were recorded. Rats then received a series of 3 cue-induced reinstatement tests, in which active lever presses resulted in delivery of light-tone cues previously associated with ethanol, but no delivery of ethanol. Each test was separated by 6-7 days of extinction. During the 15 days of FR3 (days 10-12) animals received 0, 10, or 20 mg/kg doses (i.p.) of the OXR1 antagonist SB-334867 (SB), and on the first 2 days of cue-induced reinstatement animals received 0 or 20 mg/kg SB-334867. Injections of SB during FR3 and reinstatement testing were fully counterbalanced. On the final day of FR3 training before SB tests (day 9), all animals received vehicle injections (2ml; i.p.) so as to habituate animals to injection stress. SB-334867 (generously donated by National Institute on Drug Abuse) was suspended in 2% dimethylsulfoxide and 10% 2-hydroxypropyl-β-cyclodextrin in sterile water, and administered at a volume of 4 ml/kg (i.p.) 30 min prior to testing. SB test days were followed by additional self-administration or extinction sessions to minimize the impact on overall behavior. After a final (non-treated) reinstatement session, animals were perfused for immunohistochemistry. Results from the immunohistochemical studies are reported elsewhere (Moorman et al., 2016).
Data analysis
All statistical analyses were conducted using GraphPad Prism (Version 5.1). Animals were divided into HR versus LR groups based on a median split of the number of active lever responses made on of the final day of FR3 self-administration training. Differences in ethanol intake and preference between HR and LR rats during the five days of 3-hour two-bottle choice testing were compared using a day (day 1-5) × group (HR vs LR) mixed model ANOVA. Differences in the number of active/inactive lever responses and rewarded/non-rewarded well entries during self-administration between HR and LR animals were explored using a session (session 1-21) × group (HR vs LR) mixed model ANOVA and subsequent Tukey post-hoc comparisons. In HR and LR animals, the effect of SB on active/inactive lever responding during self-administration tests was assessed using separate 2-way repeated measures ANOVAs with ‘treatment’ (vehicle, SB10, SB20) and ‘lever type’ (active, inactive) as the variables. Similarly, the effect of SB on well entries made during self-administration tests was assessed using 2-way repeated measures ANOVAs with ‘treatment’ (vehicle, SB10, SB20) and ‘well entry type’ (rewarded well entries, non-rewarded well entries) as the variables. Rewarded (when cues and ethanol were available) or ‘Rewarded’ (during reinstatement, when cues but no ethanol were available) well entries refers to entries made during cue presentation after FR3 response, whereas non-rewarded well entries refers to all other entries. The effect of SB20 on lever pressing during reinstatement testing was analyzed separately for HR and LR animals using 2-way repeated measures ANOVA with ‘treatment’ (vehicle, SB20) and ‘lever type’ (active, inactive) as the variables. Similarly, the number of well entries made during reinstatement was assessed using 2-way repeated measures ANOVAs with ‘treatment’ (vehicle, SB20) and ‘well entry type’ (‘rewarded’ well entries, non-rewarded well entries) as the variables. We used Sidak's multiple comparisons tests to determine differences between treatment groups. An alpha value of 0.05 was adopted for all statistical tests.
Highlights.
Rats were segregated into low (LR) and high (HR) operant responders for 20% ethanol
Operant ethanol seeking was not correlated with homecage ethanol drinking
Orexin-1 receptor antagonism decreased both self-administration and reinstatement
The effects were selective for HR but not LR animals
Orexin signaling may underlie elevated motivation in alcohol abuse and addiction
Acknowledgments
Supported by PHS grants R21-DA032005, P50-DA015369, R37/R01-DA006214, R01-MH092868, P50-AA010761, UL1-RR029882, NHMRC CJ Martin Fellowship 1072706.
Footnotes
Contributions: DEM and GAJ designed experiments. DEM and EAK collected data. MHJ and DEM analyzed data and wrote the manuscript. DEM, MHJ, EAK, and GAJ edited the manuscript. All authors have approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David E. Moorman, Email: moorman@cns.umass.edu.
Morgan H. James, Email: morgan.james@rutgers.edu.
References
- Alcaraz-Iborra M, Cubero I. Do Orexins contribute to impulsivity-driven binge consumption of rewarding stimulus and transition to drug/food dependence? Pharmacology Biochemistry and Behavior. 2015;134:31–34. doi: 10.1016/j.pbb.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Becker HC, Adams BL, Jesudason CD, Rorick-Kehn LM. Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models. Front Neurosci. 2014;8:33. doi: 10.3389/fnins.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Ho HT, Leibowitz SF. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addiction Biology. 2015;20:469–481. doi: 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci. 2015;41:1149–56. doi: 10.1111/ejn.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–25. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Khoo SYS, Lawrence AJ. Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. International Journal of Neuropsychopharmacology. 2013a;16:2067–2079. doi: 10.1017/S1461145713000333. [DOI] [PubMed] [Google Scholar]
- Brown RM, Khoo SY, Lawrence AJ. Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int J Neuropsychopharmacol. 2013b;16:2067–79. doi: 10.1017/S1461145713000333. [DOI] [PubMed] [Google Scholar]
- Brown RM, Kim AK, Khoo SY, Kim JH, Jupp B, Lawrence AJ. Orexin-1 receptor signalling in the prelimbic cortex and ventral tegmental area regulates cue-induced reinstatement of ethanol-seeking in iP rats. Addict Biol. 2016;21:603–12. doi: 10.1111/adb.12251. [DOI] [PubMed] [Google Scholar]
- Chappell AM, Weiner JL. Relationship between ethanol's acute locomotor effects and ethanol self-administration in male Long-Evans rats. Alcohol Clin Exp Res. 2008;32:2088–99. doi: 10.1111/j.1530-0277.2008.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 2002;104:131–44. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. Effects of raclopride in the nucleus accumbens on ethanol seeking and consumption. Alcohol Clin Exp Res. 2001;25:1431–40. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Santini LA, Legg BH, Samson HH. Separate measures of ethanol seeking and drinking in the rat: effects of remoxipride. Alcohol. 2002;28:39–46. doi: 10.1016/s0741-8329(02)00236-7. [DOI] [PubMed] [Google Scholar]
- Czachowski CL. Manipulations of serotonin function in the nucleus accumbens core produce differential effects on ethanol and sucrose seeking and intake. Alcohol Clin Exp Res. 2005;29:1146–55. doi: 10.1097/01.alc.0000171944.50381.86. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Stansfield KH. Ethanol and sucrose seeking and consumption following repeated administration of the GABA(B) agonist baclofen in rats. Alcohol Clin Exp Res. 2006;30:812–8. doi: 10.1111/j.1530-0277.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–7. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher R, Hauser SR, Getachew B, Bell RL, McBride WJ, McKinzie DL, Rodd ZA. The Orexin-1 Receptor Antagonist SB-334867 Reduces Alcohol Relapse Drinking, but not Alcohol-Seeking, in Alcohol-Preferring (P) Rats Journal of Addiction Medicine. 2010;4:153–159. doi: 10.1097/ADM.0b013e3181bd893f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretinorexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–48. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–36. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–70. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–7. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson-Redmond A, Czachowski C. Effects of systemic opioid receptor ligands on ethanol-and sucrose seeking and drinking in alcohol-preferring (P) and Long Evans rats. Psychopharmacology (Berl) 2014;231:4309–21. doi: 10.1007/s00213-014-3571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–97. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Pham D, Fowler CD, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front Behav Neurosci. 2012;6:47. doi: 10.3389/fnbeh.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Yeoh JW, Graham BA, Dayas CV. Insights for developing pharmacological treatments for psychostimulant relapse targeting hypothalamic peptide systems. J Addict Res Ther. 2012 S4:008. [Google Scholar]
- Jupp B, Krivdic B, Krstew E, Lawrence AJ. The orexin(1) receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res. 2011;1391:54–9. doi: 10.1016/j.brainres.2011.03.045. [DOI] [PubMed] [Google Scholar]
- Lasheras MC, Laorden ML, Milanes MV, Nunez C. Corticotropin-releasing factor 1 receptor mediates the activity of the reward system evoked by morphine-induced conditioned place preference. Neuropharmacology. 2015;95:168–80. doi: 10.1016/j.neuropharm.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–9. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hu Z, de Lecea L. The hypocretins/orexins: integrators of multiple physiological functions. Br J Pharmacol. 2014;171:332–50. doi: 10.1111/bph.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Moorman DE, Aston-Jones G, Becker HC. The highly selective orexin/hypocretin 1 receptor antagonist GSK1059865 potently reduces ethanol drinking in ethanol dependent mice. Brain Res. 2016 doi: 10.1016/j.brainres.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17:1298–303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F. N-(2-methyl-6-benzoxazolyl)-N′-1,5-naphthyridin-4-yl urea (SB334867), a hypocretin receptor-1 antagonist, preferentially prevents ethanol seeking: comparison with natural reward seeking. Addict Biol. 2014;19:233–6. doi: 10.1111/j.1369-1600.2012.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momeni S, Roman E. Subgroup-dependent effects of voluntary alcohol intake on behavioral profiles in outbred Wistar rats. Behav Brain Res. 2014;275:288–96. doi: 10.1016/j.bbr.2014.08.058. [DOI] [PubMed] [Google Scholar]
- Momeni S, Sharif M, Agren G, Roman E. Individual differences in risk-related behaviors and voluntary alcohol intake in outbred Wistar rats. Behav Pharmacol. 2014;25:206–15. doi: 10.1097/FBP.0000000000000036. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol. 2009;43:379–86. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, Kilroy EA, Aston-Jones G. Orexin/hypocretin neuron activation is correlated with alcohol seeking and preference in a topographically specific manner. Eur J Neurosci. 2016 doi: 10.1111/ejn.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE. Binge-like consumption of ethanol and other salient reinforcers is blocked by orexin-1 receptor inhibition and leads to a reduction of hypothalamic orexin immunoreactivity. Alcohol Clin Exp Res. 2015;39:21–9. doi: 10.1111/acer.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Costentin J, Duterte-Boucher D. Differential involvement of anxiety and novelty preference levels on oral ethanol consumption in rats. Psychopharmacology (Berl) 2015;232:2711–21. doi: 10.1007/s00213-015-3910-5. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Maldonado R, Berrendero F. The hypocretin/orexin system: implications for drug reward and relapse. Mol Neurobiol. 2012;45:424–39. doi: 10.1007/s12035-012-8255-z. [DOI] [PubMed] [Google Scholar]
- Porter-Stransky KA, Bentzley BS, Aston-Jones G. Individual differences in orexin-I receptor modulation of motivation for the opioid remifentanil. Addict Biol. 2015 doi: 10.1111/adb.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–17. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson KA, Aston-Jones G. Lateral hypothalamic orexin/hypocretin neurons that project to ventral tegmental area are differentially activated with morphine preference. J Neurosci. 2012;32:3809–17. doi: 10.1523/JNEUROSCI.3917-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–81. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The role of orexin in motivated behaviours. Nat Rev Neurosci. 2014;15:719–31. doi: 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A, Czachowski C, Sharpe A. Measuring ethanol-seeking behavior: the effect of using repeated extinction trials. Alcohol. 2001;24:205–9. doi: 10.1016/s0741-8329(01)00157-4. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. Int Rev Neurobiol. 2003;54:107–43. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sharko AC, Kaigler KF, Fadel JR, Wilson MA. Individual differences in voluntary ethanol consumption lead to differential activation of the central amygdala in rats: relationship to the anxiolytic and stimulant effects of low dose ethanol. Alcohol Clin Exp Res. 2013;37(1):E172–80. doi: 10.1111/j.1530-0277.2012.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, Palmer J, Bonaventure P, Carruthers NI, Lovenberg TW, Boggs J, Galici R. Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology (Berl) 2011;215:191–203. doi: 10.1007/s00213-010-2127-x. [DOI] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–23. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, See R, Aston-Jones G. Orexin / hypocretin signaling at the OX1 receptor regulates cue-elicited cocaine-seeking. Europ J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelder M, Hesseling P, Baars AM, Lozeman-van 't Klooster JG, Rotte MD, Vanderschuren LJ, Lesscher HM. Individual Variation in Alcohol Intake Predicts Reinforcement, Motivation and Compulsive Alcohol Use in Rats. Alcohol Clin Exp Res. 2015;39:2427–37. doi: 10.1111/acer.12891. [DOI] [PubMed] [Google Scholar]
- Thompson JL, Borgland SL. A role for hypocretin/orexin in motivation. Behav Brain Res. 2011;217:446–53. doi: 10.1016/j.bbr.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–5. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Wise RA. Maximization of ethanol intake in the rat. Adv Exp Med Biol. 1975;59:279–94. doi: 10.1007/978-1-4757-0632-1_19. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–61. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]