Abstract
Blood pressure is a modifiable risk for cardiovascular disease (CVD). Among hemodialysis patients, there is a U-shaped association between blood pressure and risk of death. However, few studies have examined the association between blood pressure and CVD in patients with stage 4 and 5 chronic kidney disease. Here we studied 1,795 Chronic Renal Insufficiency Cohort (CRIC) Study participants with estimated glomerular filtration rate under 30 ml/min/1.73 m2 and not on dialysis. The association of systolic (SBP), diastolic (DBP) and pulse pressure with risk of physician-adjudicated atherosclerotic CVD (stroke, myocardial infarction or peripheral arterial disease) and heart failure were tested using Cox regression adjusted for demographics, comorbidity and medications. There was a significant association with higher SBP (adjusted hazard ratio 2.04 [95% confidence interval: 1.46, 2.84]) for SBP over 140 vs under 120 mmHg, higher DBP (2.52 [1.54, 4.11]) for DBP over 90 vs under 80 mmHg and higher pulse pressure (2.67 [1.82, 3.92]) for pulse pressure over 68 vs under 51 mmHg with atherosclerotic CVD. For heart failure, there was a significant association with higher pulse pressure only (1.42 [1.05, 1.92]) for pulse pressure over 68 vs under 51 mmHg, but not for SBP or DBP. Thus, among participants with stage 4 and 5 chronic kidney disease, there was an independent association between higher SBP, DBP and pulse pressure with risk of atherosclerotic CVD, while only higher pulse pressure was independently associated with greater risk of heart failure. Further trials are needed to determine whether aggressive reduction of blood pressure reduces the risk of CVD events in patients with stage 4 and 5 chronic kidney disease.
Keywords: Blood pressure, cardiovascular disease, chronic kidney disease
INTRODUCTION
Cardiovascular disease is the leading cause of morbidity and mortality among patients with chronic kidney disease (CKD), with higher event rates at progressively lower levels of glomerular filtration rate.1 Hypertension is very common in CKD2, 3 and is a well-established modifiable risk factor for cardiovascular disease in the general population. A recent landmark clinical trial, the SPRINT trial, demonstrated that targeting a systolic blood pressure less than 120 mm Hg resulted in lower rates of cardiovascular disease (CVD) and death in the general population and in the subgroup with mild to moderate CKD (which comprised 20% of trial population).4
However, few SPRINT participants had advanced CKD (e.g. stage 4 or 5, not requiring dialysis).5 And few other studies of CKD patients have focused on those with stage 4 and 5 CKD, defined as estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2. 6–9 There is concern that the relationship of blood pressure with clinical outcomes may differ as kidney disease progresses. Much of the uncertainty arises because among patients with stage 5D or end-stage renal disease (ESRD) receiving maintenance hemodialysis, studies have consistently shown a U-shaped association between level of blood pressure measured in the dialysis unit and mortality- with the nadir of the “U” being a range of blood pressure that would normally prompt treatment.10–16 In addition, an analysis from the Homocysteine in Kidney and ESRD study (HOST) study of patients with creatinine clearance <30 ml/min (not on dialysis) noted that systolic blood pressure (SBP) was not associated with risk of atherosclerotic cardiovascular disease events (ASCVD)17 which differs from what is observed in the non-CKD general population.18 We previously did not find an association between SBP and risk of all-cause death in patients with eGFR <30 ml/min/1.73 m2 in a prospective, observational study of CKD, the Chronic Renal Insufficiency Cohort (CRIC) Study.10 However, we did not examine CVD outcomes and we did not evaluate other blood pressure components. A greater understanding of the relationship between level of blood pressure and specific subtypes of CVD in persons with stage 4 and 5 CKD may inform management of this high-risk population and lead the way for further clinical trials.
Thus, we examined here the association of blood pressure components (SBP, DBP and PP) with risk of atherosclerotic CVD (ASCVD) and heart failure (HF) events among participants of the CRIC Study with stage 4 and 5 CKD.
RESULTS
Study population
A total of 1,795 CRIC participants had an eGFR either at study entry or during follow-up of <30 ml/min/1.72 m2, 46% were women and 46% were black. At the qualifying CRIC study visit, their mean age was 60 years. Mean eGFR was 26 ml/min/1.73 m2, mean BMI 32.5 kg/m2 and median urine protein-to-creatinine-ratio was 689 mg/gm. Mean SBP was 131 (24) mm Hg, mean DBP 69 (13) mm Hg and mean PP 62 (20) mm Hg. Those with higher SBP were more likely to be black, have lower eGFR, higher proteinuria, more likely to have diabetes and were more likely to be taking more BP medications (Table 1).
Table 1.
Characteristics of the CRIC study population with advanced CKD (defined as eGFR < 30 ml/min/1.73 m2) by category of systolic blood pressure (mm Hg) (N=1795)
| Characteristic | All (N=1795) | <120 N=627 |
120–140 N=601 |
>140 N=567 |
p value |
|---|---|---|---|---|---|
| Age, mean (SD), years | 59.9 (11.3) | 59.4 (11.9) | 59.8 (11.5) | 60.5 (10.1) | 0.2499 |
| Female (%) | 45.8 | 43.7 | 44.9 | 49.0 | 0.1587 |
| Race/ethnicity (%) | <.0001 | ||||
| Non-Hispanic White | 33.8 | 46.3 | 34.9 | 18.7 | |
| Non-Hispanic Black | 46.1 | 39.2 | 45.1 | 54.9 | |
| Hispanic | 16.7 | 11.0 | 16.3 | 23.5 | |
| Other | 3.4 | 3.5 | 3.7 | 3.0 | |
| Systolic blood pressure, mmHg | 131 (24) | 107 (10) | 129 (6) | 159 (16) | <.0001 |
| Diastolic blood pressure, mmHg | 69 (13) | 62 (10) | 70 (12) | 77 (14) | <.0001 |
| Pulse pressure, mmHg | 62 (20) | 46 (12) | 60 (13) | 82 (18) | <.0001 |
| Body Mass Index, kg/m2 | 32.5 (8.2) | 32.0 (8.0) | 33.2 (8.2) | 32.4 (8.3) | 0.0397 |
| Estimated Glomerular Filtration Rate, ml/min/1.73m2 | 26.2 (5.2) | 26.8 (5.0) | 26.3 (5.1) | 25.5 (5.4) | <.0001 |
| Proteinuria, median (IQR)mg/24 hour | 689 (163, 2480) | 263 (87, 946) | 699 (166, 2321) | 1953 (488, 4385) | <.0001 |
| Hypertension (%) | 95.0 | 90.0 | 95.5 | 100.0 | <.0001 |
| Number of blood pressure medication classes | 3.2 (1.5) | 3.0 (1.5) | 3.3 (1.4) | 3.3 (1.5) | 0.0002 |
| Diabetes (%) | 61.4 | 52.0 | 61.6 | 71.8 | <.0001 |
| Current Smoker (%) | 14.8 | 14.7 | 14.5 | 15.2 | 0.9431 |
| Prevalent atherosclerotic CVD (%) | 46.3 | 45.8 | 45.6 | 47.6 | 0.7452 |
| Prevalent heart failure (%) | 17.5 | 16.6 | 16.3 | 19.8 | 0.2288 |
| Statins (%) | 63.6 | 64.0 | 61.4 | 65.6 | 0.3530 |
| B-blockers (%) | 60.3 | 54.9 | 60.1 | 66.5 | 0.0002 |
| Calcium channel blockers (%) | 51.5 | 41.8 | 56.2 | 57.1 | <.0001 |
| ACE inhibitors/ARBS (%) | 71.1 | 77.8 | 71.6 | 63.1 | <.0001 |
| Diuretics (%) | 71.3 | 67.5 | 73.7 | 72.8 | 0.0213 |
| Alpha blockers and antagonists (%) | 29.6 | 26.8 | 31.1 | 31.2 | 0.1395 |
Association of blood pressure components with ASCVD and HF
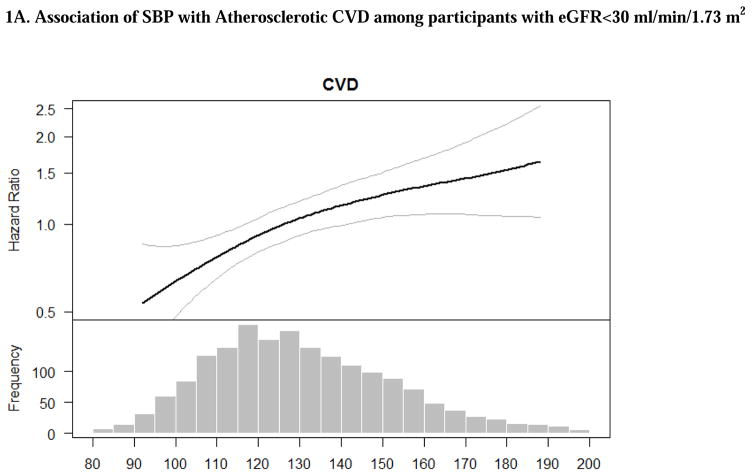
Overall, crude rates of ASCVD and HF events were greater with higher SBP and PP (Table 2). The associations of SBP with ASCVD and HF remained significant after adjustment for demographic characteristics. After additional adjustment for other cardiovascular risk factors, prevalent CVD and medication use, the association of higher SBP with ASCVD, but not HF, remained significant. Participants with progressively higher SBP had progressively higher adjusted rate of ASCVD (Table 2) compared to those with SBP <120 mmHg. The association between SBP and risk of ASCVD appeared linear based on multivariable splines (Figures 1A).
Table 2.
Association of blood pressure components and cardiovascular events among CRIC participants with eGFR<30 ml/min/1.73 m2 (N=1795)
| Risk of Atherosclerotic Disease (Defined as Stroke, Myocardial Infarction and Peripheral Arterial Disease) | Risk of heart failure | |||||||
|---|---|---|---|---|---|---|---|---|
| All participants (n=1795) | N events/Incidence rate (per 100 py) | Unadjusted HR (95% CI) |
Adjusted for age, sex and race HR (95% CI) |
Adjusted for patient characteristics* HR (95% CI) |
N events/Incidence rate (per 100 py) | Unadjusted HR (95% CI) |
Adjusted for age, sex and race HR (95% CI) |
Adjusted for patient characteristics* HR (95% CI) |
| Systolic blood pressure (SBP), mm Hg | ||||||||
| SBP<120 (N=627) | 79/2.52 | ref | Ref | ref | 113/3.71 | ref | ref | ref |
| SBP 120–140 (N=601) | 112/3.98 | 1.58 (1.18 – 2.10)** | 1.57 (1.18–2.10) ** | 1.51 (1.09 – 2.09) ** | 156/5.85 | 1.57 (1.23 – 1.99) ** | 1.50 (1.17 – 1.91) ** | 1.22 (0.93 – 1.60) |
| SBP >140 (N=567) | 129/4.99 | 1.97 (1.49 –2.60) ** | 2.10 (1.57–2.80) ** | 2.04 (1.46 – 2.84) ** | 163/6.86 | 1.81 (1.43 – 2.31) ** | 1.70 (1.33 – 2.18) ** | 1.28 (0.97 – 1.70) |
| Diastolic blood pressure (DBP), mm Hg | ||||||||
| DBP<80 (N=1400) | 249/3.76 | ref | Ref | ref | 338/5.39 | ref | ref | ref |
| DBP 80–90 (N=295) | 47/3.29 | 0.88 (0.64 – 1.20) | 1.10 (0.80– 1.52) | 1.30 (0.90 – 1.88) | 71/5.31 | 0.99 (0.77 – 1.28) | 1.13 (0.86 – 1.47) | 1.24 (0.91 – 1.69) |
| DBP >90 (N=98) | 23/4.73 | 1.26 (0.82 – 1.92) | 1.73 (1.10 – 2.71) ** | 2.52 (1.54 – 4.11) ** | 21/4.51 | 0.84 (0.54 – 1.31) | 1.04 (0.66 – 1.64) | 1.19 (0.70 – 2.03) |
| Pulse pressure (PP), mm Hg | ||||||||
| PP, tertile 1 (15–51) (N=600) | 55/1.76 | ref | Ref | ref | 94/3.13 | ref | Ref | Ref |
| PP, tertile 2 (51–69) (N=599) | 119/4.19 | 2.37 (1.72 – 3.26) ** | 2.27 (1.64–3.15) ** | 2.06 (1.42 – 2.99) ** | 148/5.39 | 1.70 (1.32 – 2.21) ** | 1.60 (1.23 – 2.08) ** | 1.18 (0.89 – 1.58) ** |
| PP, tertile 3 (69–153) (N=594) | 145/5.64 | 3.16 (2.32 – 4.32) ** | 3.09 (2.23–4.28) ** | 2.67 (1.82 – 3.92) ** | 188/8.07 | 2.51 (1.96 – 3.21) ** | 2.23 (1.71 – 2.89) ** | 1.42 (1.05 – 1.92) ** |
patient characteristics include: age, gender, race/ethnicity, clinical site, tobacco use, BMI, diabetes, urine proteinuria, statin use, number of BP medication classes and prevalent CVD (defined as stroke, MI, PAD or HF)
p-value <0.05
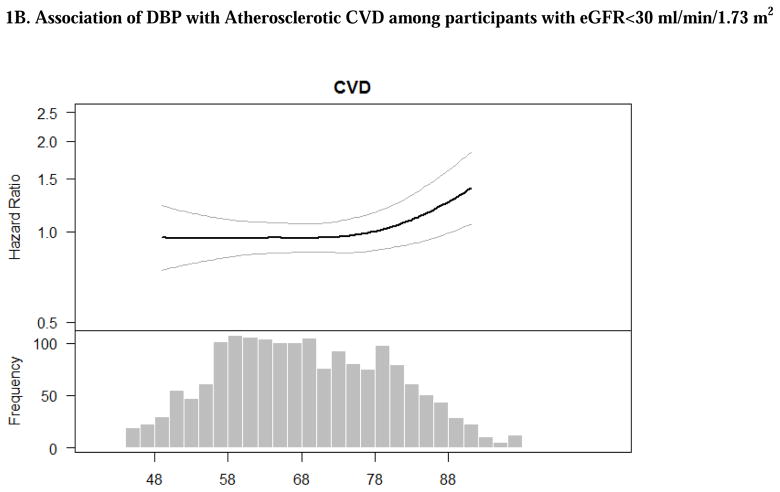
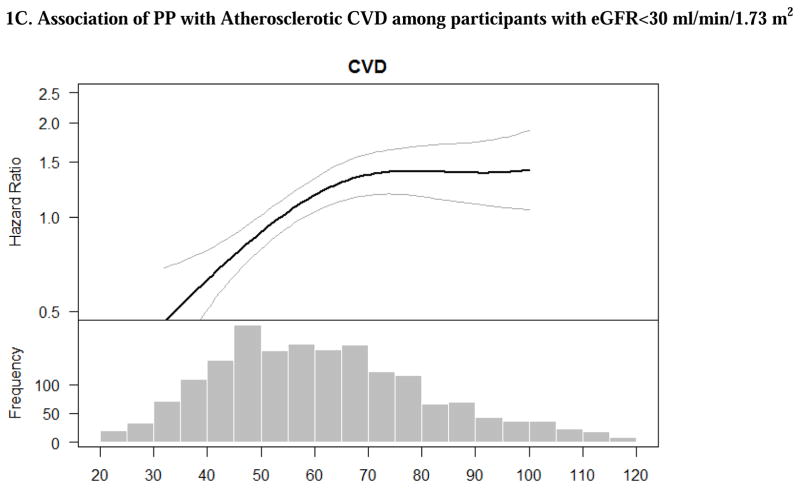
Figure 1. Adjusted associations of blood pressure components with atherosclerotic cardiovascular disease (stroke, myocardial infarction and peripheral arterial disease) among participants with eGFR<30 ml/min/1.73 m2. The smooth spline estimates the hazard ratio of atherosclerotic cardiovascular disease among CRIC participants with eGFR<30 ml/min/1.73 m2 according to (A) systolic blood pressure; (B) diastolic blood pressure and (C) pulse pressure.
All analyses are adjusted for age, gender, race/ethnicity, clinical site, tobacco use, BMI, diabetes, urine proteinuria, statin use, number of BP medication classes and prevalent CVD. Dotted lines represent 95% confidence intervals. Below each spline is the histogram of the distribution of the blood pressure component to indicate the range of the majority of the data.
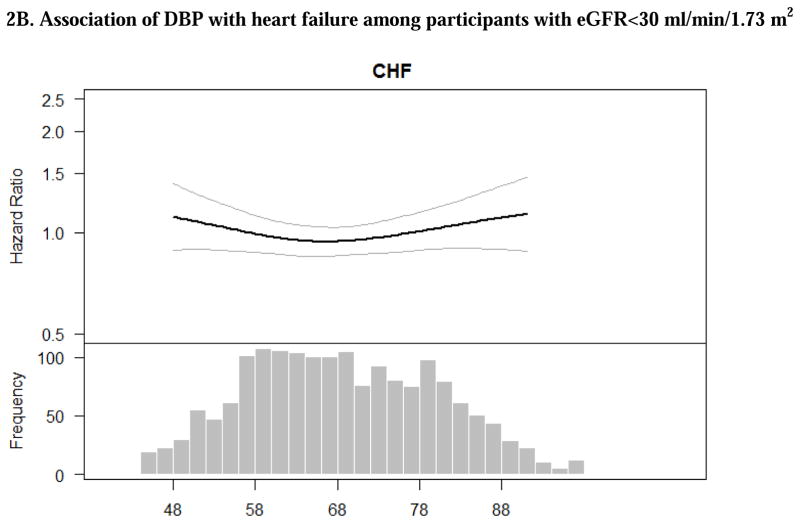
In unadjusted models, there was no significant association of DBP with either ASCVD or HF (Table 2). However, after adjustment for demographic characteristics and other cardiovascular risk factors, participants with DBP >90 mm Hg had a greater than twofold higher rate for ASCVD compared to those with DBP<80 (Table 2). Multivariable-adjusted spline was consistent in showing that risk was particularly pronounced among those with DBP >90 mm Hg (Figures 1B).
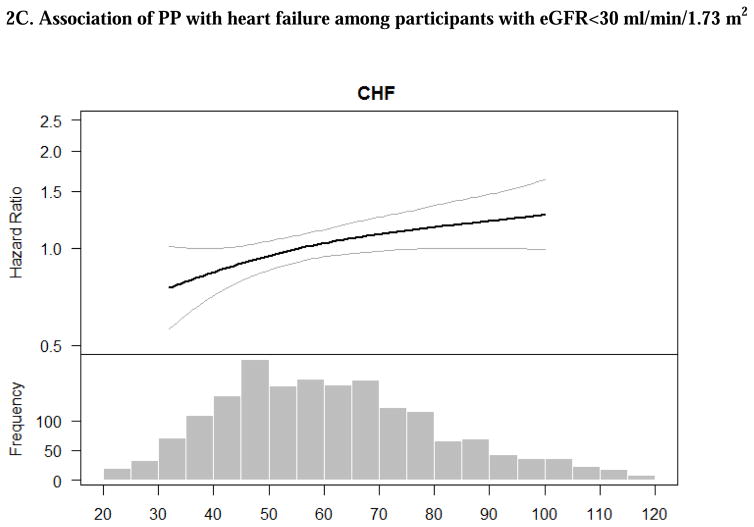
There were strong associations between higher PP with risk of ASCVD and HF in unadjusted models, which remained statistically significant after adjustment for demographic characteristics (Table 2). After further adjustment for additional participant characteristics, progressively higher PP was associated with greater risk of ASCVD and HF (Table 2). The association between PP and risk of ASCVD appeared to increase and plateau while the association of PP with HF appeared to linear based on multivariable splines (Figures 1C and 2C).
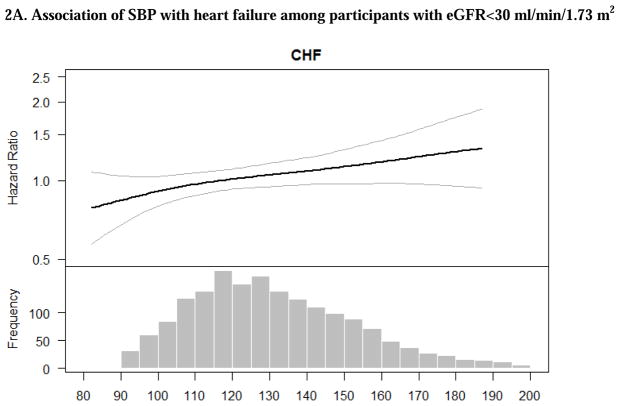
Figure 2. Adjusted associations of blood pressure components with heart failure among CRIC participants with eGFR<30 ml/min/1.73 m2. The smooth spline estimates the hazard ratio of heart failure among CRIC participants with eGFR<30 ml/min/1.73 m2 according to (A) systolic blood pressure; (B) diastolic blood pressure and (C) pulse pressure.
All analyses are adjusted for age, gender, race/ethnicity, clinical site, tobacco use, BMI, diabetes, urine proteinuria, statin use, number of BP medication classes. Dotted lines represent 95% confidence intervals. Below each spline is the histogram of the distribution of the blood pressure component to indicate the range of the majority of the data.
Secondary analysis: association of blood pressure components with incident ASCVD and HF
With exclusion of participants with prevalent ASCVD, the association of SBP, PP and DBP with incident ASCVD events was largely similar to the main analysis (Supplemental Table 1). When excluding participants with prevalent HF, there were statistically significant associations between both higher SBP and higher PP and higher rates of incident HF (Supplemental Table 1).
Secondary analysis: association of blood pressure components with risk of MI, CVA and PAD
Among the participants who had an ASCVD event, 164 were MIs, 67 were CVAs and 94 were PAD events. Of these adjudicated events, 5 participants had more than 1 event during a single admission (3 had MI +CVA and 2 had PAD +MI). Similar to the main analysis, higher SBP, DBP and PP were significantly associated with greater risk of MI. Similar associations were seen for the outcome of CVA, although some of these analyses were underpowered. Finally, only higher PP was significantly associated with greater risk of PAD, although power was limited for these analyses as well (Supplemental Table 2).
DISCUSSION
Among a large, diverse, well-characterized cohort of patients with eGFR was <30 ml/min/1.73 m2, we found that higher SBP, DBP and PP were associated with increased risk for ASCVD events. We also report a statistically significant association between higher SBP and PP for risk of incident HF events. These data are novel in that few studies have focused on the association between different blood pressure components and subtypes of CVD events in stage 4 and 5 CKD, which may inform the design of clinical trials to reduce the burden of CVD specifically in patients with advanced stages of CKD not on dialysis.
Our results should be interpreted in the context of the recently published SPRINT study, which randomized over 9,000 participants at high risk for CVD to SBP targets of either less than 120 mm Hg vs. less than 140 mm Hg.4 The primary composite outcome included fatal and non-fatal ASCVD, HF, stroke and other CVD. There was a 25% risk reduction in the primary outcome in participants randomized to the intensive target group. There was no statistically significant interaction by baseline CKD status. The results from this landmark trial have important clinical implications in the care of CKD patients. It should be noted that most of the SPRINT CKD participants had mild to moderate disease (mean eGFR 48 ml/min/1.73 m2),19,20, 21 so our study provides complementary information on those with more advanced CKD.
Our results are consistent with a contemporary meta-analysis of 19 clinical trials showing that participants in the intensive blood pressure lowering group (mean SBP of 133 mmHg) had lower risk of major CVD events, MI and stroke than participants in the less intensive blood pressure lowering group (mean SBP of 140 mmHg)..22 The majority of our study participants had diabetes mellitus. In the ACCORD trial of participants with type 2 diabetes there was no statistically significant difference in rates of CVD events between participants randomized to SBP<120 mmHg vs. <140 mmHg. However, the directions of association for the outcomes of interest were consistent with SPRINT and it is possible that ACCORD may be relatively underpowered.20, 21 Moreover, in contrast to SPRINT, ACCORD enrollees had preserved renal function (mean eGFR >90 ml/min/1.73 m2) so the ACCORD findings may be less relevant to our study population. Finally, another recently published meta-analysis of 40 clinical trials (100,354 participants) of participants with diabetes did report that reduction in SBP was significantly associated with lower risk of mortality, CVD events, coronary heart disease and stroke.23
A distinguishing feature of the current study is the focused on advanced CKD patients. Given the paucity of data from clinical trials in this population, these results—albeit observational in nature--offer some unique insight. In our study the association between SBP and CVD outcomes is linear in stage 4 and 5 CKD--i.e. not flat or associated with higher risk around the clinically important <120 mm Hg or <140 mmHg cutoffs as seen in the U-shape in studies of blood pressure and mortality in hemodialysis patients10–16---means it is possible that the benefits of more aggressive BP control may also extend also to patients with more advanced (not on dialysis) CKD; although clinical trials are needed to confirm this. Our findings contradict the notion (based on observational data) that the association of blood pressure with adverse clinical events in patients with CKD is “fundamentally different” from that of the general population.24
Previous studies of the associations of blood pressure with adverse outcomes in the setting of stage 4 and 5 CKD have been conflicting. Among participants with stage 4 and 5 CKD in a clinical trial to lower homocysteine (HOST), there was no association between SBP or DBP and risk for ASCVD events (or mortality).17 However, the HOST study enrolled participants who all had elevated homocysteine and were mainly older men; outcomes were ascertained differently; the event rate was higher; and there were significant differences in covariates adjusted for in that analysis vs. the current one (e.g., no adjustment for BP meds or proteinuria).25 Another study reported that SBP>133 mmHg was associated with lower risk of all-cause mortality, including participants with eGFR<30 ml/min/1.73 m2.26 A large study of U.S Veterans (N=39732 in subset with eGFR <30 ml/min/1.73m2) showed that compared with those with SBP <120 mmHg and SBP <80 mmHg, patients with pre-hypertension and stages 1 and 2 hypertension had about 30 percent lower mortality risk (25497 out of 39732 died in that subgroup during observation).27 However, important limitations of that study must be noted: first it relied on clinical blood pressure measurements so there is higher potential for detection bias compared with a research protocol driven BP ascertainment (since individuals who are more ill are more likely to have encounters with the healthcare system and have BP measured). Two, the findings were greatly attenuated when baseline BP (vs. time-updated BP) was used which suggested that time-updated BP associations may be biased by reverse causation (i.e. progressive disease process leading to death also led to lower and lower blood pressure over time).27 The conclusion from that study that the optimal SBP in patients with CKD seems to be 130–159mm Hg is not consistent with results from SPRINT and other large clinical trials of BP reduction.
We also found strong associations of elevated PP with ASCVD events. PP reflects stiffness of the large arteries or arteriosclerosis which tends to simultaneously increase SBP and lower DBP.28 PP has been shown to be strongly associated with CVD and death in the general population29, 30 and among patients on dialysis.31–34 Patients with CKD often have elevated PP due to arterial medial calcification among other processes. Among participants with advanced CKD enrolled in the HOST Study, PP was also associated with increased risk of atherosclerotic events.17 Although currently no anti-hypertensive medications reduce PP specifically, future investigations may focus on strategies that reduce arterial stiffness.
Our study confirmed the high rate of HF events among participants with stage 4 and 5 CKD. We observed a significant association of PP with risk of HF in the primary analysis. This is consistent with what has been reported in the general population.30 Among CRIC Study participants, elevated pulse wave velocity, also a measure of large artery stiffening similar to PP, was strongly associated with incident HF.35
While previous studies have shown an association between elevated SBP and risk of HF,30, 36 the association between SBP and HF events was modest and did not reach statistically significance in our primary analysis. Interestingly, a large meta-analysis of 19 clinical trials also noted that intensive BP reduction did not reduce rate of HF. When we excluded participants with prevalent HF, the association of SBP with incident HF was stronger and statistically significant (Table 2 vs. Supplemental Table 1). We do not have a definitive explanation for this discrepancy. One possible hypothesis is that once diagnosed with HF, CRIC participants (who have generally rather well controlled hypertension) underwent more aggressive treatment with anti-hypertensives, notably RAAS inhibitors and this blunted the association between SBP and subsequent HF events (which would include a mix of recurrent and incident HF events). Of course, other explanations may be possible. For example, decreased cardiac output in patients with heart failure with reduced ejection fraction (HFrEF) could have lower blood pressure.
Our study has several important implications. The first is that we did not find an alarming association between lower SBP and higher rates of adverse outcomes around the range of SBP 120–140 mmHg in patients with stage 4 and 5 CKD not on dialysis. This finding differs from epidemiology literature among stage 5D patients on dialysis 10–16 which has led to uncertainly about approach to treating BP in the absence of definitive clinical trial data in this study population. In our population of patients with eGFR<30 ml/min/1.73 m2, even modest increases in SBP >120 mm Hg (and certainly above 140 mm Hg) were associated with increased risk of ASCVD, which is consistent with the findings of the SPRINT trial (although the SPRINT enrollees had higher eGFR levels and our study is observational in design). The second implication of our findings is that in contrast to our previous study which reported no association between SBP and risk of mortality in the same study population,10 in the current study, we report a significant association between SBP and CVD events. Many prior studies of advanced CKD (e.g. stage 5D on dialysis) have focused on only on all-cause mortality as an outcome due to lack of reliable capture of other events. However other outcomes such as CVD are as important and should inform clinical decision making. And studies dedicated to understanding risk factors and treatment for CVD in the high-risk CKD population may be the foundation for future clinical trials.
Our study had several strengths. We studied a large, multi-center, well-characterized prospective cohort of patients with advanced CKD. Blood pressure measures were obtained in a standardized manner by trained staff to minimize bias in ascertainment.37 Our outcomes of interest were ascertained by rigorous standardized physician adjudication. We were able to capture comorbid conditions using research grade data. In contrast to previous studies,6–9 we focused on patients with advanced CKD (stage 4 or 5 not on dialysis) who were diverse in age and gender. Our study materially extends previous studies by evaluating associations of multiple blood pressure components with ASCVD in a well-characterized and diverse population of patients with advanced stages of CKD, a subgroup that is high risk for adverse outcomes but yet has been relatively understudied. Our separate examination of HF events is also novel as prior studies of the relation of blood pressure with risk of HF are limited in patients with CKD, either in that the focus has been on atherosclerotic CVD or HF has been included as part of a composite outcome.
We recognize some limitations as well. Our patient population was largely treated with anti-hypertensive medication and had fairly well-controlled blood pressure. This was an observational study and we are not able to make definitive conclusions about treatment of blood pressure and its affects on CVD outcomes since residual confounding is possible. For example, patients with higher blood pressure in our study were on more blood pressure medications and hence that group is enriched with those who have resistant hypertension which is itself a risk factor for adverse outcomes. As a study of research volunteers, it is possible that these results may not be fully generalizable to all CKD patients, particularly to untreated patients. We did not have 24-hour ambulatory or home BP measurements.
In conclusion, among patients with eGFR<30 ml/min/1.73 m2 and not on dialysis, we showed that higher SBP above 120 mmHg is associated stepwise with higher risk of ASCVD events and incident HF events. Following the lead of SPRINT and other trials, clinical trials to evaluate blood pressure treatment targets in patients with advanced CKD are needed.
METHODS
Study Population
The CRIC Study is a multi-center, prospective, observational cohort study which initially enrolled a total of 3939 men and women from 2003 to 2008. At study entry they were age 21 to 74 years with eGFR 20–70 ml/min/1.73m2 determined by the MDRD equation.3, 38–40 Exclusion criteria have been previously described and included New York Heart Association Class III or IV heart failure and severe liver disease.3, 38, 39 Study participants were followed annually in-person and with six-month interim telephone calls.
For the present analysis, we studied participants who had an eGFR<30 ml/min/1.73 m2 either at time of enrollment into the CRIC Study or any time thereafter (all before mid 2012) (N=1,795).
Predictors
We used the blood pressure value measured at the first CRIC study visit at which the participant’s eGFR was <30 ml/min/1.73 m2, the threshold for “advanced CKD” (stage 4 and 5). 5 Three seated resting blood pressure readings, obtained by study staff using a standardized method, were obtained and the mean value used.10
The main predictors were three blood pressure components (in mmHg) : SBP, DBP and PP (calculated from SBP minus DBP).
Outcomes
Outcomes were time to first HF event after eGFR was <30 ml/min/1.73 m2 and time to first ASCVD event (probable or definite myocardial infarction [MI], probable or definite ischemic stroke, or peripheral artery disease events) after eGFR was <30 ml/min/1.73 m2. Both outcomes were identified through mid 2012. We a priori separated out HF events and ASCVD events since their underlying pathophysiology and relationship with other vascular risk factors differs. Participants were censored at time of death or end of study period for this report. Deaths were identified from report from next of kin, retrieval of death certificates or obituaries, review of hospital records, and the Social Security Death Master File.
Study participants were queried every 6 months during alternating in-person and telephone visits about whether they were hospitalized, reached ESRD, experienced a possible cardiovascular event, or underwent a selected set of diagnostic tests/procedures. International Classification of Diseases, Ninth Revision (ICD-9) discharge codes were obtained for all hospitalizations. When diagnostic or procedure codes indicative of a potential cardiovascular event were noted, medical records were retrieved for review by at least 2 physicians for possible events of HF, MI, and stroke. Trained study staff reviewed medical records classified with ICD-9 codes that suggest a peripheral artery disease event.41
HF events were determined based on clinical symptoms, radiographic evidence of pulmonary edema, physical examination of the heart and lungs, central venous hemodynamic monitoring data, and echocardiographic imaging in hospitalized patients based on the Framingham and ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) criteria.42, 43 Diagnosis of probable or definite MI was based on symptoms consistent with acute ischemia, cardiac biomarker levels, and electrocardiograms as recommended by a consensus statement on the universal definition of MI. Two neurologists reviewed all hospitalizations suggestive of stroke. Outcomes included both probable and definite ischemic stroke. The latter was determined based on autopsy findings or sudden onset of neurologic symptoms supported with computed tomography or magnetic resonance imaging demonstration of infarction in a territory where an injury or infarction would be expected to create those symptoms. The former was defined as sudden or rapid onset of 1 major or 2 minor neurologic signs or symptoms lasting for more than 24 hours or until the patient died with no evidence of hemorrhage or infarction on computed tomography or magnetic resonance imaging performed within 24 hours of the onset of symptoms. Ascertainment of peripheral artery disease was based on nurse-abstracted hospital records indicating that amputation, bypass procedure, angioplasty, or surgical/vascular procedure for abdominal aortic aneurysm or noncoronary arteries took place.41
In a sensitivity analysis, we excluded participants with prevalent ASCVD or HF. Prevalent disease was determined by: (1) self-report at cohort entry and updated through semi-annual self-report until eGFR < 30 ml/min/1.73 m2; or (2) CRIC study adjudicated interim ASCVD or HF event between cohort entry and progression to eGFR < 30 ml/min/1.73 m2.
Covariates
All covariates were obtained from the same study visit as the blood pressure measurement of interest. Covariates included: age, gender, race/ethnicity (Non-Hispanic white, Non-Hispanic black, Hispanic, other), tobacco use (current/former or never) and history of diabetes mellitus (diabetes). Body mass index (BMI) was calculated from height and weight recorded at the study visit (kg/m2). Urine protein was quantified by urine protein-to-creatinine ratio. Medication use, including blood pressure drugs, was confirmed by study coordinator’s review of containers with study participants.
Statistical methods
For the analysis, we examined each blood pressure component continuously and in categories. SBP and DBP categories were determined from clinically relevant cut-points and PP was examined in tertiles.
We calculated crude rates (per 100 patient years) of each CVD event type across categories of SBP, DBP and PP in the study population overall and in those without prevalent CVD. We then performed unadjusted and multivariable-adjusted Cox proportional hazard models of each blood pressure component (analyzed as three categories) and risk of ASCVD and risk of HF. We performed a series of nested models that sequentially adjusted for demographic characteristics only and then additionally for clinical site, tobacco use, BMI, diabetes, urine protein, statin use, number of blood pressure medication classes reported and prevalent CVD. These covariates were chosen a priori based on prior literature of CVD as well as biological rationale. We explored the functional form of the final multivariable-adjusted association between each blood pressure component and each outcome using adjusted penalized smoothing splines with N0.2 evenly spaced knots among the inner 99% distribution of the blood pressure component in Cox models to display the relation of each association without making assumptions about its shape.10
We performed two secondary analyses. In the first, we excluded participants with atherosclerotic CVD and HF to test the association of blood pressure components with incident atherosclerotic CVD and HF. In a second sensitivity analysis, we tested the association of blood pressure components with risk of MI, CVA and PAD individually (rather than as a composite outcome as in the main analysis).
All analyses were conducted using SAS 9.4 (Cary, NC) and R statistical package.
Supplementary Material
Supplemental Table 1. Association of blood pressure components and cardiovascular events among CRIC participants with eGFR<30 ml/min/1.73 m2, excluding those with prevalent cardiovascular disease
Supplemental Table 2. Association of blood pressure components and specific types of atherosclerotic cardiovascular events among CRIC participants with eGFR<30 ml/min/1.73 m2
Acknowledgments
SOURCES OF FUNDING
This work was supported by the following grants: K23 DK088865 (Bansal), R01 DK70939 (Hsu), K24 DK92291 (Hsu). Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). This work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003 and K01 DK092353 (Anderson), Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131.
Footnotes
DISCLOSURES
None. None of the authors have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Peralta CA, Hicks LS, Chertow GM, et al. Control of hypertension in adults with chronic kidney disease in the United States. Hypertension. 2005;45:1119–1124. doi: 10.1161/01.HYP.0000164577.81087.70. [DOI] [PubMed] [Google Scholar]
- 3.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline Characteristics and Associations with Kidney Function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015 doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 6.Weiss JW, Johnson ES, Petrik A, et al. Systolic blood pressure and mortality among older community-dwelling adults with CKD. Am J Kidney Dis. 2010;56:1062–1071. doi: 10.1053/j.ajkd.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss JW, Peters D, Yang X, et al. Systolic BP and mortality in older adults with CKD. Clinical journal of the American Society of Nephrology : CJASN. 2015;10:1553–1559. doi: 10.2215/CJN.11391114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiner DE, Tighiouart H, Levey AS, et al. Lowest systolic blood pressure is associated with stroke in stages 3 to 4 chronic kidney disease. J Am Soc Nephrol. 2007;18:960–966. doi: 10.1681/ASN.2006080858. [DOI] [PubMed] [Google Scholar]
- 9.Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2015 doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 10.Bansal N, McCulloch CE, Rahman M, et al. Blood Pressure and Risk of All-Cause Mortality in Advanced Chronic Kidney Disease and Hemodialysis: The Chronic Renal Insufficiency Cohort Study. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.04334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal R. Blood pressure and mortality among hemodialysis patients. Hypertension. 2010;55:762–768. doi: 10.1161/HYPERTENSIONAHA.109.144899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalantar-Zadeh K, Block G, Humphreys MH, et al. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 13.Port FK, Hulbert-Shearon TE, Wolfe RA, et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33:507–517. doi: 10.1016/s0272-6386(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 14.Zager PG, Nikolic J, Brown RH, et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int. 1998;54:561–569. doi: 10.1046/j.1523-1755.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Lacson E, Jr, Lowrie EG, et al. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis. 2006;48:606–615. doi: 10.1053/j.ajkd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Robinson BM, Tong L, Zhang J, et al. Blood pressure levels and mortality risk among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2012;82:570–580. doi: 10.1038/ki.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palit S, Chonchol M, Cheung AK, et al. Association of BP with Death, Cardiovascular Events, and Progression to Chronic Dialysis in Patients with Advanced Kidney Disease. Clin J Am Soc Nephrol. 2015;10:934–940. doi: 10.2215/CJN.08620814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 19.Chertow GM, Beddhu S, Lewis JB, et al. Managing Hypertension in Patients with CKD: A Marathon, Not a SPRINT. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkovic V, Rodgers A. Redefining Blood-Pressure Targets--SPRINT Starts the Marathon. N Engl J Med. 2015;373:2175–2178. doi: 10.1056/NEJMe1513301. [DOI] [PubMed] [Google Scholar]
- 22.Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435–443. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 23.Emdin CA, Rahimi K, Neal B, et al. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. Jama. 2015;313:603–615. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 24.Kovesdy CP, Lu JL, Molnar MZ, et al. Observational Modeling of Strict vs Conventional Blood Pressure Control in Patients With Chronic Kidney Disease. JAMA internal medicine. 2014 doi: 10.1001/jamainternmed.2014.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinha AD, Agarwal R. BP Components in Advanced CKD and the Competing Risks of Death, ESRD, and Cardiovascular Events. Clin J Am Soc Nephrol. 2015;10:911–913. doi: 10.2215/CJN.04300415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, et al. Association of low blood pressure with increased mortality in patients with moderate to severe chronic kidney disease. Nephrol Dial Transplant. 2006;21:1257–1262. doi: 10.1093/ndt/gfk057. [DOI] [PubMed] [Google Scholar]
- 27.Kovesdy CP, Bleyer AJ, Molnar MZ, et al. Blood pressure and mortality in u.s. Veterans with chronic kidney disease: a cohort study. Ann Intern Med. 2013;159:233–242. doi: 10.7326/0003-4819-159-4-201308200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez-Fresnedo G, Rodrigo E, de Francisco AL, et al. Role of pulse pressure on cardiovascular risk in chronic kidney disease patients. J Am Soc Nephrol. 2006;17:S246–249. doi: 10.1681/ASN.2006080921. [DOI] [PubMed] [Google Scholar]
- 29.Benetos A, Thomas F, Joly L, et al. Pulse pressure amplification a mechanical biomarker of cardiovascular risk. J Am Coll Cardiol. 2010;55:1032–1037. doi: 10.1016/j.jacc.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 30.Haider AW, Larson MG, Franklin SS, et al. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–16. doi: 10.7326/0003-4819-138-1-200301070-00006. [DOI] [PubMed] [Google Scholar]
- 31.Blacher J, Guerin AP, Pannier B, et al. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 32.Fang W, Yang X, Bargman JM, et al. Association between pulse pressure and mortality in patients undergoing peritoneal dialysis. Perit Dial Int. 2009;29:163–170. [PubMed] [Google Scholar]
- 33.Klassen PS, Lowrie EG, Reddan DN, et al. Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA. 2002;287:1548–1555. doi: 10.1001/jama.287.12.1548. [DOI] [PubMed] [Google Scholar]
- 34.Tozawa M, Iseki K, Iseki C, et al. Pulse pressure and risk of total mortality and cardiovascular events in patients on chronic hemodialysis. Kidney Int. 2002;61:717–726. doi: 10.1046/j.1523-1755.2002.00173.x. [DOI] [PubMed] [Google Scholar]
- 35.Chirinos JA, Khan A, Bansal N, et al. Arterial stiffness, central pressures, and incident hospitalized heart failure in the chronic renal insufficiency cohort study. Circ Heart Fail. 2014;7:709–716. doi: 10.1161/CIRCHEARTFAILURE.113.001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iyer AS, Ahmed MI, Filippatos GS, et al. Uncontrolled hypertension and increased risk for incident heart failure in older adults with hypertension: findings from a propensity-matched prospective population study. Journal of the American Society of Hypertension : JASH. 2010;4:22–31. doi: 10.1016/j.jash.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman M, Griffin V, Kumar A, et al. A comparison of standardized versus “usual” blood pressure measurements in hemodialysis patients. Am J Kidney Dis. 2002;39:1226–1230. doi: 10.1053/ajkd.2002.33395. [DOI] [PubMed] [Google Scholar]
- 38.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 39.Fischer MJ, Go AS, Lora CM, et al. CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis. 2011;58:214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 41.Liu KD, Yang W, Go AS, et al. Urine neutrophil gelatinase-associated lipocalin and risk of cardiovascular disease and death in CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2015;65:267–274. doi: 10.1053/j.ajkd.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 43.Einhorn PT, Davis BR, Massie BM, et al. The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Heart Failure Validation Study: diagnosis and prognosis. Am Heart J. 2007;153:42–53. doi: 10.1016/j.ahj.2006.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Association of blood pressure components and cardiovascular events among CRIC participants with eGFR<30 ml/min/1.73 m2, excluding those with prevalent cardiovascular disease
Supplemental Table 2. Association of blood pressure components and specific types of atherosclerotic cardiovascular events among CRIC participants with eGFR<30 ml/min/1.73 m2