Abstract
Extended-hours hemodialysis offers substantially longer treatment time compared to conventional hemodialysis schedules and is associated with improved fluid and electrolyte control and favorable cardiac remodeling. However, whether extended-hours hemodialysis improves survival remains unclear. Therefore, we determined the association between extended-hours compared to conventional hemodialysis and the risk of all-cause mortality in a nationally representative cohort of patients initiating maintenance dialysis in the United States from 2007 to 2011. Survival analyses using causal inference modeling with marginal structural models were performed to compare mortality risk among 1,206 individuals undergoing thrice weekly extended-hours hemodialysis or 111,707 patients receiving conventional hemodialysis treatments. The average treatment time per session for extended-hours hemodialysis was 399 minutes compared to 211 minutes for conventional therapy. The crude mortality rate with extended-hours hemodialysis was 6.4 deaths per 100 patient-years compared with 14.7 deaths per 100 patient-years for conventional hemodialysis. In the primary analysis, patients treated with extended-hours hemodialysis had a 33% lower adjusted risk of death compared to those who were treated with a conventional regimen (95% confidence interval: 7% to 51%). Additional analyses accounting for analytical assumptions regarding exposure and outcome, facility-level confounders, and prior modality history were similar. Thus, in this large nationally representative cohort, treatment with extended-hours hemodialysis was associated with a lower risk for mortality compared to treatment with conventional in-center therapy. Adequately powered randomized clinical trials comparing extended-hours to conventional hemodialysis are required to confirm these findings.
Keywords: end-stage renal disease, maintenance dialysis, in-center hemodialysis, nocturnal hemodialysis, extended-hours hemodialysis, mortality risk
Introduction
Although the past decade has witnessed a modest improvement in survival for patients undergoing maintenance dialysis in the United States, mortality continues to be unacceptably high, approaching 20 percent per year.1 While early observational studies suggested that a higher delivered dose of dialysis may be associated with improved clinical outcomes, a benefit of increasing the dialysis dose above currently accepted standards has not been confirmed by randomized controlled clinical trial results.2–4 This has prompted a search for other modifiable dialysis parameters, including dialysis modality and treatment time, in order to improve long-term clinical outcomes. Consistent with this emphasis, the Institute of Medicine in the United States has identified comparative effectiveness of dialysis therapies as the only kidney disease-related topic among the top 100 national priorities for comparative effectiveness research.5
Numerous observational studies over the past two decades have demonstrated that shorter treatment times with conventional hemodialysis are associated with higher mortality.6–10 Recently, an increasing number of patients are being treated with extended-hours hemodialysis consisting of substantially longer treatment times, which has been associated in observational studies with lower hospitalization rates and improvements in metabolic parameters, left ventricular mass and hypertension.11–14 However, there are limited data on the association of extended-hours hemodialysis with patient survival, as prior studies have been small or single-center investigations, or have not addressed the multiple time-varying and facility-level factors which can cause confounding.15–19
Randomized controlled trials remain the gold standard for comparative effectiveness research. However, trials that have sought to randomly assign patients to one of two different dialysis modalities have encountered substantial challenges in enrolling the target number of patients.20–22 These challenges likely reflect that most patients are not willing to leave the selection of dialysis modality to random assignment if the therapies have substantial and widely differing effects on lifestyle, schedule, and weekly commitment to dialysis-related treatment.22 Additionally, no contemporary randomized controlled trial has sought to test the effect of extended hemodialysis treatment time independent of increased treatment frequency. Observational studies using contemporary causal inference modeling such as marginal structural models utilize robust statistical tools that address time-varying exposures and confounding, and thus represent an important alternative method for investigating the comparative effectiveness of dialysis modalities.23 In this study, we used marginal structural modelling to address the hypothesis that extended-hours hemodialysis is associated with lower risk for all-cause death compared to conventional hemodialysis.
Results
Study Cohort
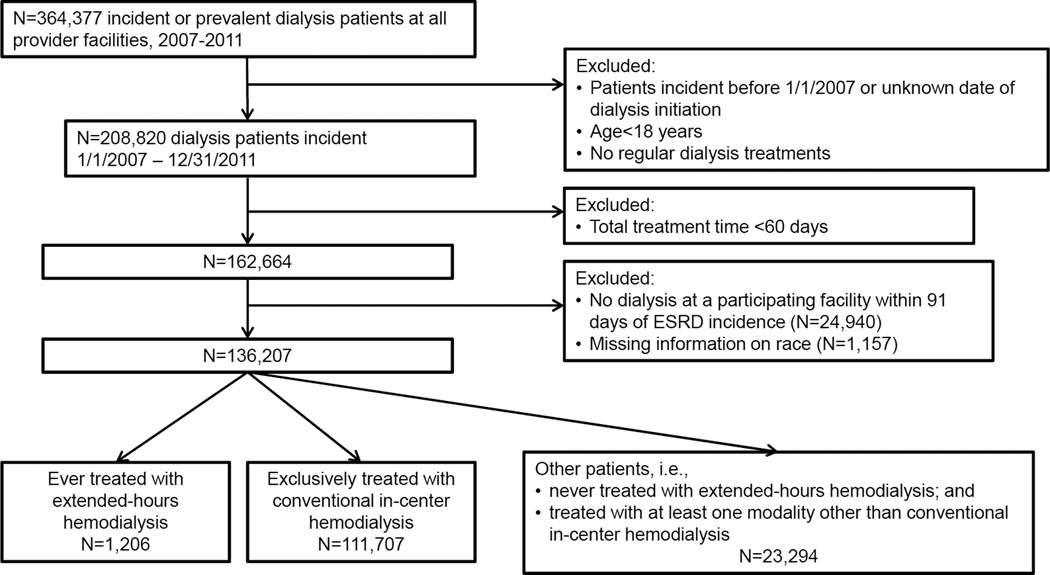
The study sample comprised 136,207 individuals with end-stage renal disease who initiated maintenance dialysis from 2007 to 2011 treated in dialysis facilities operated by a large US dialysis provider (Figure 1). Compared to individuals treated exclusively with conventional hemodialysis (n=111,707), patients categorized as treated with extended-hours hemodialysis for one or more 91-day periods (n=1,206) were younger, more likely to be male, black, have diabetes or comorbid cardiovascular disease, have primary insurance other than Medicare or Medicaid, and to live in the western region of the US (Table 1). Other patients, who were never treated with extended-hours hemodialysis and were treated with at least one modality other than conventional hemodialysis, differed from both extended-hours and exclusively conventional hemodialysis patients (Table 1). However, in the first 91-day period of dialysis, laboratory and treatment parameters were similar among patients ever treated with extended-hours hemodialysis, patients exclusively treated with conventional hemodialysis, and other patients (Table 1).
Figure 1. Construction of the study cohort.
Table 1.
Patient characteristics at the start of the first 91-day dialysis treatment period after initiation of dialysis, by modality, and total proportion of variables with missing information.
| Ever treated with extended-hours hemodialysis (N=1206) |
Exclusively treated with conventional hemodialysis (N=111,707) |
Others (N=23,294) |
% Missing | |
|---|---|---|---|---|
| Age, years | 0 | |||
| 18–24 | 3 | 1 | 2 | |
| 25–44 | 28 | 11 | 18 | |
| 45–59 | 42 | 27 | 30 | |
| 60–74 | 24 | 36 | 33 | |
| ≥75 | 3 | 24 | 16 | |
| Race, % | 0 | |||
| Asian | 3 | 3 | 4 | |
| Black | 37 | 31 | 21 | |
| White | 47 | 47 | 58 | |
| Hispanic | 10 | 15 | 13 | |
| Other | 3 | 4 | 3 | |
| Male, % | 70 | 57 | 56 | 0 |
| Primary Health Insurance,% | 0 | |||
| Medicare | 35 | 53 | 46 | |
| Medicaid | 7 | 7 | 5 | |
| Other insurance | 58 | 40 | 48 | |
| Geographic Location | 0 | |||
| Northeast, % | 9 | 13 | 12 | |
| Midwest, % | 30 | 18 | 19 | |
| West, % | 40 | 25 | 41 | |
| South, % | 21 | 44 | 28 | |
| Year of Incidence | 0 | |||
| 2007 | 25 | 20 | 20 | |
| 2008 | 24 | 20 | 21 | |
| 2009 | 24 | 21 | 22 | |
| 2010 | 18 | 21 | 22 | |
| 2011 | 9 | 18 | 15 | |
| Co-existing illnesses, % | 0 | |||
| Atherosclerotic Heart Disease |
21 | 14 | 18 | |
| Congestive Heart Failure | 55 | 37 | 30 | |
| Diabetes | 68 | 58 | 63 | |
| Other Cardiovascular | 19 | 15 | 16 | |
| Access Type, %a | 6.5 | |||
| Arteriovenous fistula or graft |
24 | 20 | 11 | |
| Central venous catheter | 73 | 80 | 46 | |
| Peritoneal dialysis catheter |
3 | 0 | 42 | |
| Treatment parametersa | ||||
| Body mass index | 32.5±9.5 | 28.2±7.4 | 28.3±7.2 | 8.7 |
| Pre-dialysis systolic blood pressure, mm Hg |
152±18 | 147±19 | 148±19 | 5.9 |
| Weekday inter-dialytic weight gain, % |
2.2 [1.5,3.0] | 2.3 [1.5,3.2] | 0.02 [0.013, 0.029] | 9.3 |
| Weekend inter-dialytic weight gain, % |
2.8 [2.0,4.1] | 3.1 [2.1, 4.2] | 0.027 [0.017, 0.038] | 8.9 |
| Laboratory Variables | ||||
| Serum albumin, g/dL | 3.6±0.5 | 3.5±0.5 | 3.6±0.5 | 1.6 |
| Alkaline phosphatase, u/L* |
87 [69, 111] | 87 [69, 115] | 82 [65, 106] | 1.8 |
| Serum calcium, mg/dL | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 | 1.5 |
| Serum ferritin, ng/mL | 234 [138, 373] | 282 [164, 484] | 237 [133, 413] | 2.8 |
| Hemoglobin, g/dL* | 11.1±1.2 | 11.1±1.2 | 11.3±1.2 | 1.3 |
| Serum iron saturation, % | 21 [17,25] | 22 [17, 27] | 23 [18, 29] | 2.1 |
| Parathyroid hormone, pg/mL |
378 [240, 569] | 314 [197, 486] | 294 [183, 470] | 2.0 |
| Serum phosphorous, mg/dL |
5.3±1.2 | 4.9±1.2 | 4.9±1.1 | 1.5 |
| spKt/V urea | 1.4 [1.2, 1.8] | 1.6 [1.4, 1.8] | 1.4 [1.2, 1.7] | 8.2 |
| Parenteral medications | ||||
| Cumulative iron, mg/month |
1062 [525,1600] | 1000 [400, 1400] | 400 [0, 1050] | 0 |
| Erythropoietin Dose, median units/week |
26,950 [16,500, 36,300] |
24,900 [13,200, 33,000] |
17,600 [9,000, 29,700] |
0 |
Summary statistics are mean ± SD, median and interquartile range, or proportions (%).
P>0.05 for the difference between extended-hours and conventional hemodialysis groups using ANOVA, Kruskal-Wallis test, or Chi-squared test. P<0.05 for all other comparisons.
Among patients ever-treated with extended hours hemodialysis, 67% were treated with conventional hemodialysis in the first 91-day period. Values for the period prior to extended-hours hemodialysis are available in Supplementary Table S1.
Patients who initiated extended-hours hemodialysis following one or more 91-day periods of conventional hemodialysis had higher serum alkaline phosphatase, ferritin, parathyroid hormone, spKt/V, and lower serum phosphorous, cumulative iron dose (prescribed over each 91-day period), and median erythropoietin dose during treatment with extended-hours hemodialysis compared to values during treatment with conventional hemodialysis prior to transfer (Supplemental Table S1).
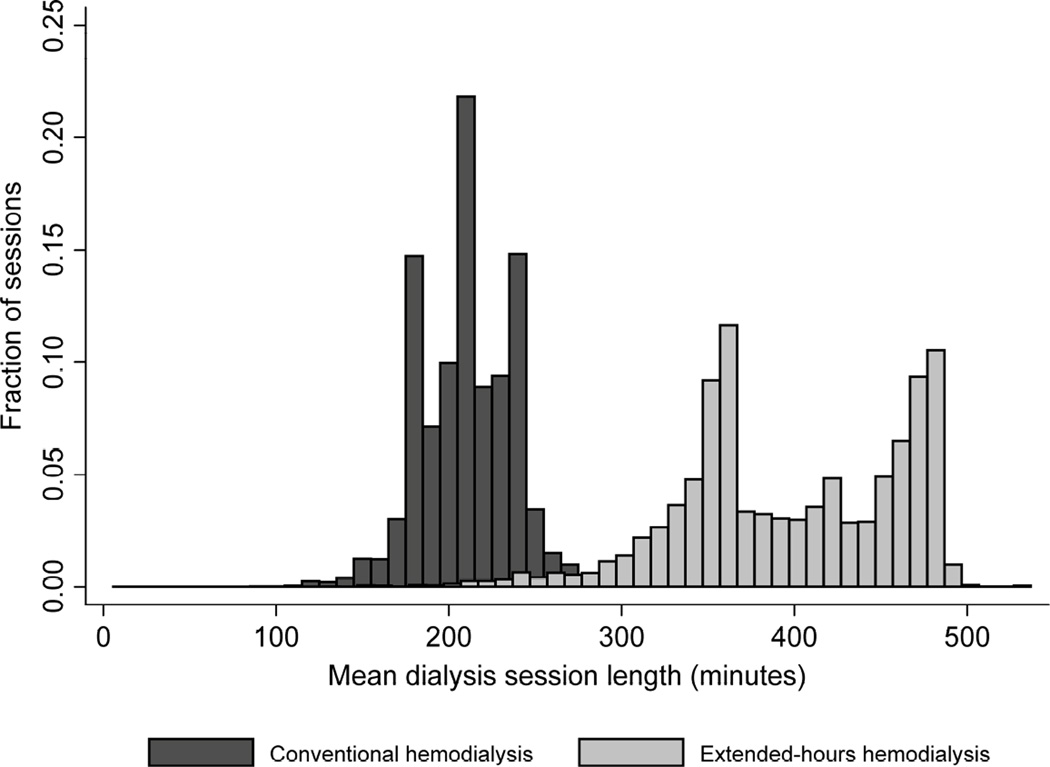
The average delivered treatment time per session with extended-hours hemodialysis was 399 ± 64 minutes compared to 211 ± 27 minutes with conventional hemodialysis (intra-patient coefficient of variation 10.8% and 6.8%, respectively) (Figure 1). Treatment frequency was similar among patients treated with extended-hours hemodialysis (2.8 treatments per week, interquartile range [IQR 2.4, 2.9]) and conventional hemodialysis (2.9 treatments per week, [IQR 2.7, 2.9]). Among extended-hours hemodialysis patients, extended-hours hemodialysis was the initial dialysis modality for 353 patients (29%); 823 (67.5%) started dialysis with conventional hemodialysis, 37 (3%) started with peritoneal dialysis, and 6 (0.5%) initiated with home hemodialysis or in-center hemodialysis less than 3 times per week. Overall, median time from initiation of dialysis to start of treatment with extended-hours hemodialysis was 6.7 months (IQR 1.0, 19.2). The median duration between initiation of hemodialysis and transfer to another modality, censoring, or death was 7.6 months (IQR 2.3, 17.6) for conventional hemodialysis and 7.2 months (IQR 3.4, 15.1) for extended-hours hemodialysis. Of patients treated with extended-hours hemodialysis, 535 (44%) transferred to another dialysis modality for one or more 91 day periods. Of these patients, none died and 78 were censored (66 due to end of follow-up) within 91 days of transfer from extended-hours hemodialysis. Of patients treated with conventional hemodialysis, 10% later transferred to another modality.
Extended-hours Hemodialysis and All-Cause Mortality
In total, 82 patients died during a 91-day period in which they were receiving extended-hours hemodialysis, compared to 29,778 deaths during periods of conventional hemodialysis. Crude mortality rates were 6.4 and 14.7 deaths per 100 patient-years for extended-hours and conventional hemodialysis, respectively (Table 2). Adjusted for treatment history and time-varying laboratory and treatment parameters using marginal structural models, as well as for case-mix factors, patients treated with extended-hours hemodialysis had a 33% lower adjusted risk of death compared to those treated with conventional hemodialysis (95% confidence interval [95% CI] 7% to 51% lower) (Table 2).
Table 2.
Risks for all-cause mortality comparing extended-hours hemodialysis to conventional hemodialysis, by time period for dialysis modality death attribution
| Death Attribution | Hemodialysis Modality |
Number of deaths |
Patient-years of follow-up |
Mortality ratea |
Hazard Ratiob (95% CI) |
|---|---|---|---|---|---|
| Current dialysis modality at time of deathc |
Extended-hours | 82 | 1,279 | 6.4 | 0.67 (0.49, 0.93) |
| Conventional | 29,796 | 203,046 | 14.7 | ||
| Dialysis modality of 91-day period prior to deathd |
Extended-hours | 82 | 1,279 | 6.4 | 0.68 (0.49, 0.93) |
| Conventional | 29,692 | 203,028 | 14.6 | ||
| Extended-hours, for all events after extended-hours initiation |
Extended-hours | 126 | 1,865 | 6.8 | 0.62 (0.47, 0.81) |
| Conventional | 29,761 | 202,582 | 14.7 |
Abbreviations: CI, confidence interval.
Mortality rate per 100 person-years
Hazard ratio from marginal structural Cox model comparing extended-hours hemodialysis to conventional hemodialysis, reference group: conventional hemodialysis, adjusted for age, sex, race, year of incidence, insurance, CHF, diabetes, ASHD, and other cardiovascular comorbidities.
Death attributed to the dialysis modality for the 91-day period in which the death occurred.
Death attributed to the dialysis modality of the 91-day period prior to the 91-day period in which death occurred.
Attributing deaths to the dialysis modality 90 days prior to death did not meaningfully change the risk estimate (Table 2). An extreme approach—attributing all deaths following initiation of extended-hours hemodialysis to extended-hours hemodialysis, regardless of the actual modality at the time of death—increased the number of deaths attributed to extended-hours hemodialysis to 126, but the risk ratio between extended-hours and conventional hemodialysis did not change substantially (HR 0.62 [0.47 to 0.81]. (Table 2).
Starting follow-up from the 91st day following start of dialysis, further adjustment for vascular access type, or restricting the cohort to patients for whom extended-hours dialysis treatment was most likely to be available did not meaningfully change hazard ratio estimates (Table 3). Additionally, results of analyses among a restricted cohort that excluded patients who never initiated extended-hours hemodialysis, but to whom extended-hours hemodialysis was available, were similar (Table 3). Results from a matched analysis in which each patient ever-treated with extended-hour hemodialysis was matched with up to 20 other patients who had the same dialysis modality treatment history prior to initiation of extended-hours hemodialysis, were not substantially different (Table 3). Finally, in interaction analyses, no evidence of effect modification by age, sex, or race was found (P>0.2 for each).
Table 3.
Results of analysis with alternative assumptions: Risks for all-cause mortality comparing extended-hours hemodialysis to conventional hemodialysis.
| Analysis | Extended-hours Hemodialysis |
Conventional Hemodialysis |
Hazard Ratioa |
95% CI | ||
|---|---|---|---|---|---|---|
| Deaths | Patient-years | Deaths | Patient-years | |||
| Beginning analysis 91 days after dialysis initiation |
82 | 1,202 | 29,654 | 176,508 | 0.67 | (0.47, 0.94) |
| Including only patients with access to extended- hours hemodialysisb |
82 | 1,279 | 11,643 | 76,980 | 0.68 | (0.50, 0.93) |
| Excluding patients who never initiated extended- hours hemodialysis, but to whom it was available |
82 | 1,279 | 18,656 | 122,334 | 0.67 | (0.49, 0.91) |
| Additional adjustment for vascular access |
82 | 1,279 | 29,796 | 203, 046 | 0.69 | (0.50, 0.95) |
| Matched cohortc | 73 | 1470 | 2,041 | 17,717 | 0.58 | (0.43, 0.79) |
Abbreviations: CI, confidence interval
Hazard ratio for all-cause mortality comparing extended-hours with conventional hemodialysis, adjusted for age, sex, race, year of incidence, insurance, CHF, diabetes, ASHD, and other cardiovascular comorbidities..
A patient was identified as having access to extend-hours dialysis if (1) s/he was treated with extended-hours dialysis at any time, or (2) s/he was treated at a facility that had treated another patient who was him/herself treated with extended hours dialysis at any time (at any facility).
Extended-hours hemodialysis patients were matched with up to 20 other patients with the same modality for each 91-day period prior to initiating extended-hours hemodialysis and the same age category, sex, race, year of dialysis incidence, underlying renal disease, Charlson comorbidity index, and geographic region. All patients were drawn from those to whom extended-hours hemodialysis was available (footnote 2).
Discussion
Using contemporary causal inference methods in this large study of incident dialysis patients, we found that treatment with three times-weekly extended-hours hemodialysis was associated with 34% lower risk for death compared with conventional hemodialysis. Our results were robust, with consistent treatment effect estimates in multiple sensitivity analyses accounting for temporal assumptions regarding exposure and outcome, facility-level confounders related to the availability of extended-hours hemodialysis to patients, and prior modality history. To our knowledge, this is the largest study of extended-hours hemodialysis reported.
The past decade has seen a resurgent interest in exploring the benefit of more frequent and/or longer dialysis treatments. Culleton et al.24 randomly assigned 52 patients in Canada to three times-weekly in-center or home hemodialysis versus six times-weekly nocturnal hemodialysis, and found that the latter therapy resulted in a decrease in left ventricular mass and systolic blood pressure, and a reduction in serum phosphorus and parathyroid hormone levels. The Frequent Hemodialysis Network (FHN) nocturnal trial compared conventional three times-weekly hemodialysis performed at home with frequent home nocturnal hemodialysis performed six times-weekly for ≥6 hours per session, and showed that frequent nocturnal therapy resulted in lower blood pressure and reductions in serum phosphorus.21 Surprisingly, long-term follow-up of patients enrolled in the FHN nocturnal trial found an increased risk for death in the nocturnal dialysis group, although caution should be exercised in interpretation of these results given an unexpectedly low mortality rate observed in the conventional dialysis group and high rates of dialysis modality switches in this study.25 Of note, these two trials assessed the impact of increasing both hemodialysis treatment time and treatment frequency together and thus did not allow for assessment of the independent effect of changing treatment time. In the recently reported ACTIVE Dialysis Trial, extending hemodialysis hours to a target of ≥ 24 hours weekly compared to standard dialysis hours (12–15 hours weekly) resulted in reductions in serum phosphorus and blood pressure medication requirements.26 However, similar to the aforementioned FHN and Canadian trials of nocturnal hemodialysis, the intervention in ACTIVE was not designed to assess the independent impact of extending hemodialysis treatment times separate from changes in treatment frequency.27 In contrast, the Time to Reduce Mortality in End-Stage Renal Disease (TiME) trial is an ongoing pragmatic randomized clinical trial designed to test the hypothesis that three times-weekly hemodialysis with session durations of at least 4.25 hours improves mortality, hospitalization, and health-related quality-of-life compared to usual care consisting of treatments with mean duration of 3.5 hours.28 However, given this relatively small increase in treatment time for the intervention group compared to the marked differences in treatment time achieved with extended-hours hemodialysis, the results of the TiME trial will not directly address the impact of extended-hours hemodialysis on clinical outcomes.
In our study, patients treated with extended-hours hemodialysis had average treatment times that exceeded those of conventional hemodialysis by more than 3 hours. This substantial lengthening of the hemodialysis treatment is much greater than what is possible to achieve within the context of conventional in-center hemodialysis or more frequent hemodialysis, whether performed in-center or at home. There are a number of potential mechanisms by which such substantially longer hemodialysis treatments may lead to improved clinical outcomes, independent of any increase in dialysis frequency. Nocturnal extended-hours hemodialysis has been shown to enhance phosphorus removal and reduce arterial stiffness, potential mediators in the pathway between end stage renal disease and clinical cardiovascular events.7,29,30 Indeed, in our study, we found that for patients who switched from conventional in-center hemodialysis to extended-hours hemodialysis, serum phosphorus levels declined into the range associated with lower risk for death in prior observational studies.31 Prior randomized trials comparing extended-hours to standard length hemodialysis treatment have shown lower pre-dialysis serum phosphorus in patients assigned to extended-hours treatments, though such trials have also included increased dialysis frequency in the intensive therapy arm, preventing determination of the independent effect of dialysis duration.24,32 In addition to its impact on markers of mineral and bone disease, one of the major advantages of extended-hours hemodialysis is that longer treatment times facilitate a slower net fluid removal rate, which has been shown to be associated with lower systemic blood pressure and reduced cardiovascular morbidity and mortality.6,33 Prior randomized trials of increased dialysis frequency either with or without increased dialysis duration have demonstrated lower pre-dialysis systolic blood pressures among patient assigned to intensive dialysis therapies.32,34 Unfortunately, as with markers of mineral metabolism, there is a paucity of data from clinical trials on the impact of thrice-weekly extended-hours hemodialysis on clinical volume overload or blood pressure. Prior studies have demonstrated an association among conversion to extended-hours hemodialysis, regression of left ventricular mass, and improvement in myocardial mechanics and cardiomyocyte gene expression, which are all likely contributors to improved cardiovascular outcomes.35,36 One important benefit potentially favoring thrice-weekly extended-hours hemodialysis over other forms of dialysis intensification is avoidance of the need for more frequent use of the patient’s vascular access. In the FHN trials, patients undergoing more frequent hemodialysis were more likely to have complications related to vascular access compared to patients undergoing conventional hemodialysis.32,34 In contrast, there is no evidence that thrice-weekly extended-hours hemodialysis is associated with an increase in access-related complications compared to conventional therapy, although no data from adequately powered clinical trials are available.
We found a >30% reduction in the risk for death associated with extended-hours hemodialysis in our study. Due to the potential presence of residual confounding from unmeasured patient and treatment-related factors in an observational cohort study, the actual benefit of extended-hours hemodialysis may be smaller than this estimate. Lacson et al.17 have previously shown a 25% reduction in risk for death associated with conversion from conventional hemodialysis to nocturnal in-center hemodialysis in a propensity score-matched observational analysis. Long-term follow-up of patients from the FHN Daily Trial demonstrated a persistent 46% reduction in risk for death for patients randomized to frequent hemodialysis, even though the majority of such patients returned to conventional thrice-weekly therapy at the conclusion of the initial 12-month intervention period.37 Such results suggest that even short-term changes in the provision of dialysis therapy, such as treatment with extended-hours hemodialysis, have the potential to result in lasting benefit.
Our findings extend those of prior observational studies through a number of important strengths in study design and approach. First, our examination of the largest cohort of patients undergoing extended-hours hemodialysis maximizes statistical power and precision in estimates of treatment effect compared to prior studies.15–18 Second, we observed a large difference in the mean and overall distribution of delivered treatment times between patients undergoing conventional versus extended-hours hemodialysis, which enhances our ability to detect an association between treatment time and outcomes. Third, our study allows an examination of the association of longer dialysis treatment time with outcomes independent of increased treatment frequency by comparing in-center extended-hours versus conventional hemodialysis, both delivered three times weekly. Fourth, we used a contemporary marginal structural modelling approach to maximize our ability to account for time-varying exposures and confounders. Use of marginal structural models also directly accounts for between-group differences in time since initiation of dialysis, addressing the propensity of healthier patients to survive to transfer to extended-hours hemodialysis. Finally, we showed the consistency of our findings across many secondary and sensitivity analyses to ensure robustness of our treatment effect estimates.
Despite its strengths, our study also has limitations. Patients undergoing treatment with extended-hours hemodialysis are likely to differ in important ways from patients undergoing conventional hemodialysis, including attributes such as exercise capacity, cardiovascular function, social support structures, cognitive function, and motivation that are not captured in available data. Furthermore, we were not able to account in our analyses for the full range of patient coexisting illnesses which may differ in prevalence among patients undergoing extended-hours versus conventional hemodialysis and also impact mortality risk. Although we incorporated a large number of demographic and laboratory predictors into the development of the marginal structural models, it is likely that residual confounding by unmeasured characteristics remains, and such confounding may be partly responsible for the observed treatment effects. Although we were able to examine the influence of facility level practices in sensitivity analyses, we were unable to account for physician-level practices. Physicians providing extended-hours hemodialysis may differ systematically from those who do not with respect to care pathways and quality, and this may be a further source of residual confounding. Finally, over 40% of patients treated with extended-hours hemodialysis in our study subsequently transferred to another dialysis modality for at least one 91-day period. We did not find that transfers from extended-hours hemodialysis were followed by a high mortality rate on the subsequent modality, and indeed we observed that no patient who transferred from extended-hours hemodialysis died within 91-days of transfer. However, further studies are needed to investigate reasons and risk factors for transfers from extended-hours dialysis hemodialysis, as such attrition may pose a challenge to large-scale application of extended-hours dialysis therapies.
In conclusion, in a large observational cohort study, we demonstrated that treatment with extended-hours hemodialysis is associated with approximately one-third lower risk for death compared to conventional hemodialysis. Given the inherent limitations of observational studies in definitively determining causation, there is a thus a need for well-designed randomized clinical trials to test whether assignment of patients to treatment with extended-hours hemodialysis results in improvement in patient-centered clinical outcomes including not only mortality and hospitalization, but also patient autonomy, care burden and satisfaction, and health-related quality of life. To be successful and to ultimately impact clinical practice, future trials will need to be carefully designed to address barriers to patient enrollment given the substantial recruitment challenges observed in prior randomized studies testing the comparative effectiveness of dialysis modalities on patient outcomes.
Methods
Study Population and Data Source
The study cohort comprised all patients age 18 years or older who started maintenance dialysis in calendar years 2007 through 2011, and received treatment for at least 60 days at a facility operated by DaVita Inc. (N=162,664). Exclusion of patients with fewer than 60 days total dialysis treatment is common practice in analyses of dialysis cohorts.19 Patients without a dialysis treatment session at a participating facility within 91 days of ESRD incidence were excluded (N=24,940), as were patients missing information on race (N=1,517), resulting in a final cohort of 136,207 patients (Figure 2). All data were obtained from dialysis facility electronic medical records. All laboratory values were measured using standardized automated methods in a central laboratory (Deland, FL) within 24 hours of blood collection. Coexisting illnesses were coded as prevalent if they were recorded at any time during follow-up.
Figure 2. Distribution of mean treatment time per session over each 91-day period, for conventional hemodialysis (dark bars, 899,696 patient-periods) or extended-hours hemodialysis (light bars, 5,610 patient-periods).
For clarity one outlier with mean treatment time 693 minutes was omitted from the conventional-hours group. Three percent of values plotted were imputed with multiple imputation. Bin width is 10 minutes.
Modality was categorized as one of six: either conventional thrice-weekly hemodialysis, extended-hours hemodialysis, peritoneal dialysis, home hemodialysis, more frequent in-center hemodialysis (>3 times/week), or less frequent in-center hemodialysis (<3 times/week).19 Assignment to extended-hours hemodialysis for the present analyses was based on dialysis facility treatment records which identified patients as having received nocturnal in-center hemodialysis; these patients were treated overnight in-center three times-weekly while sleeping. To classify the dialysis modalities received by a patient over time, individual dialysis session records were analyzed for the date and modality of treatment administered. Subsequent to the first modality recorded, each patient was considered to have switched to a different modality if he or she was treated exclusively with that modality over a period of least 60 consecutive days, a rule commonly applied for examining comparative effectiveness of dialysis modalities.1 Hence, each patient’s record was divided into treatment intervals by modality, each interval after the first lasting at least 60 days unless the patient died or was censored.
Next, the follow-up period for each patient was divided into successive 91-day periods from the date of first dialysis; follow-up was available for up to 20 periods. The modality assigned for each 91-day period was that with which the patient was treated for >45 days in that period; however, for the 91-day period in which the patient was censored or died, the modality received for the longest period was assigned. Vascular or peritoneal access was assigned to each 91-day period in an analogous manner. Each 91-day period was assigned the mean of recorded laboratory and clinical measurements during the period. Facility was assigned as the facility at which the patient received the most dialysis sessions in a 91-day period.
All-cause mortality was defined by date of death during the follow-up period (January 1, 2007–December 31, 2011). Censoring reasons included kidney transplant, transfer to a facility operated by another dialysis provider, discontinuation of dialysis, and administrative end of follow-up. The Institutional Review Boards at the Los Angeles Biomedical Research Institute and the University of Washington approved the study as exempt from informed consent.
Statistical Analyses
For an overview of differences between patients according to dialysis modality, descriptive statistics were calculated for the first 91-day period of dialysis (baseline) for the mutually exclusive groups of patients ever treated with extended-hours hemodialysis (N=1,206), patients treated exclusively with conventional hemodialysis (N=111,707), and other patients (N=23,294).
The extent of missing data was examined at baseline and in subsequent 91-day periods. At baseline, the extent of missing data varied from approximately 9% for body weight measurements and 8% for single-pool urea Kt/V (spKt/V), to 1–2% for most serum and urinary measurements (e.g., albumin, serum alkaline phosphatase, serum calcium) (Table 1). Overall, approximately 25% of patients were missing information on one or more laboratory or clinical measurements for one or more 91-day period. Therefore, complete-case analysis would have deleted a substantial portion of patients; missing information for time-varying covariates was imputed using multiple imputation with ten repetitions. The multiple imputation model included a wide variety of laboratory and clinical measurements, case-mix variables, facility variables, dialysis modality, the estimated Nelson-Aalen cumulative hazard of mortality, an indicator of death or cause of censoring, and the total time for each patient under observation (see Detailed Statistical Methods available in Supplementary Appendix).38
For the primary analysis, the effect of treatment with extended-hours hemodialysis compared to conventional hemodialysis on all-cause mortality was assessed using marginal structural models, with dialysis modality treated as a time-varying exposure.23 Hazard ratio estimates from these models have the interpretation as the ratio of the mortality rates comparing cohorts treated with extended-hours versus conventional hemodialysis, and with the same prior treatment history and other covariates. A time-varying exposure approach was utilized to maximize accuracy of event attribution and minimize risk of immortal time bias (Detailed Statistical Methods available in Supplementary Appendix).39 Marginal structural models utilizing time-varying exposures have been shown to be valid even when the exposure of interest changes multiple times over follow-up.40,41
To construct the models, stabilized inverse probability of treatment weights (SIPTW) and stabilized inverse probability of censoring weights (SIPCW) were computed for each 91-day period to account for history of prior dialysis modality treatment and other potential time-varying confounders (see Supplemental Methods).23,42 The potential confounders used to separately estimate SIPTWs and SIPCWs included case-mix and demographic variables, dialysis modality from the immediately preceding 91-day period, a variety of time-varying laboratory and clinical measures, and facility-level variables. The final combined stabilized inverse probability weights were estimated by multiplying the SIPTW and SIPCW.
Finally, a Cox proportional hazards models weighted by the stabilized inverse probability weights was fit comparing hazard of mortality while treated with extended-hours dialysis to conventional hemodialysis (reference), adjusted for age, gender, race, type of insurance, diabetes, congestive heart failure, atherosclerotic heart disease, other cardiovascular illness, and year of dialysis incidence. Laboratory and other time-varying intermediate variables were not included as adjustment covariates in the final Cox model.
Several sensitivity analyses were performed to investigate modeling assumptions. First, to assess the potential for delayed effect of changes in dialysis modality, analyses were repeated by attributing death to the dialysis modality with which the patient was treated in the 91-day period prior to the period in which death occurred. Second, analyses were repeated in which any death following initiation of extended-hours dialysis was attributed to extended-hours dialysis, regardless of whether the patient had transferred from extended-hours dialysis. Third, to remove the immediate impact of dialysis initiation, analyses were conducted in which follow-up was started on the 91st day after initiation of dialysis. Fourth, to investigate the impact of additional patient-level and facility-level variables that could determine availability and initiation of extended-hours dialysis, analyses were performed in a restricted cohort comprising patients who were treated at a facility that had (1) treated any patient with extended-hours hemodialysis or (2) treated any patient who was treated with extended-hours dialysis at a different facility. Additionally, analyses were repeated in a second restricted cohort comprising patients who were ever treated with extended-hours hemodialysis and patients only treated with conventional hemodialysis who were treated at facilities where extended-hours HD was unavailable. Fifth, analyses were repeated on a matched cohort in which patients who were treated with extended-hours hemodialysis were matched with up to 20 patients with identical dialysis treatment modality history in each 91-day period prior to initiation of extended-hours dialysis (by the index patient), year of incidence, age, sex, race, underlying renal disease, Charlson index, and geographic region. The matched cohort analysis was also restricted to patients to whom extended-hours treatment was available as described above (Supplemental Methods and Figure S1).
Effect modification was investigated by first repeating the marginal structural Cox model analysis estimating the association of dialysis modality with mortality, within strata of age, sex, and race. Subsequently, to conduct statistical testing of the interaction, the analysis was repeated adding a multiplicative interaction term between dialysis modality and age, sex, or race to the marginal structural Cox model.23
All analyses followed the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.43 Further detail regarding statistical methods, including elimination of immortal time bias, is available in the Supplementary Material. Analyses were performed with Stata 13.1 (Stata Corp, College Station, TX, USA
Supplementary Material
Acknowledgments
Supported by grants from the NIH/NIDDK (R01DK095668 to Drs. Mehrotra and Kalantar-Zadeh, R21AG047306 to Drs. Molnar, Mehrotra and Kalantar-Zadeh, and T32DK007467 supporting Dr. Rivara). The authors extend their thanks to the many staff members and providers working in DaVita clinics and to DaVita Clinical Research®.
M.B.R. and S.V.A. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The funding bodies had no role in designing the study; in collecting, analyzing, or interpreting the data; or in preparing or writing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
None.
References
- 1.United States Renal Data System. 2014 USRDS annual data report. An overview of the epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2014. [Google Scholar]
- 2.Hakim RM, Breyer J, Ismail N, et al. Effects of dose of dialysis on morbidity and mortality. Am. J. Kidney Dis. 1994;23:661–669. doi: 10.1016/s0272-6386(12)70276-7. [DOI] [PubMed] [Google Scholar]
- 3.Miller JE, Kovesdy CP, Nissenson AR, et al. Association of Hemodialysis Treatment Time and Dose With Mortality and the Role of Race and Sex. Am. J. Kidney Dis. 2010;55:100–112. doi: 10.1053/j.ajkd.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N. Engl. J. Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 5.IOM (Institute of Medicine) Initial National Priorities for Comparative Effectiveness Research. Washington, DC: The National Academies Press; 2009. [Google Scholar]
- 6.Saran R, Bragg-Gresham JL, Levin NW, et al. Longer treatment time and slower ultrafiltration in hemodialysis: Associations with reduced mortality in the DOPPS. Kidney Int. 2006;69:1222–1228. doi: 10.1038/sj.ki.5000186. [DOI] [PubMed] [Google Scholar]
- 7.Tentori F, Zhang J, Li Y, et al. Longer dialysis session length is associated with better intermediate outcomes and survival among patients on in-center three times per week hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol. Dial. Transplant. 2012;27:4180–4188. doi: 10.1093/ndt/gfs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunelli SM, Chertow GM, Ankers ED, et al. Shorter dialysis times are associated with higher mortality among incident hemodialysis patients. Kidney Int. 2010;77:630–636. doi: 10.1038/ki.2009.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall MR, Byrne BG, Kerr PG, et al. Associations of hemodialysis dose and session length with mortality risk in Australian and New Zealand patients. Kidney Int. 2006;69:1229–1236. doi: 10.1038/sj.ki.5000188. [DOI] [PubMed] [Google Scholar]
- 10.Flythe JE, Curhan GC, Brunelli SM. Shorter length dialysis sessions are associated with increased mortality, independent of body weight. Kidney Int. 2013;83:104–113. doi: 10.1038/ki.2012.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan CT, Floras JS, Miller JA, et al. Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int. 2002;61:2235–2239. doi: 10.1046/j.1523-1755.2002.00362.x. [DOI] [PubMed] [Google Scholar]
- 12.Ok E, Duman S, Asci G, et al. Comparison of 4- and 8-h dialysis sessions in thrice-weekly in-centre haemodialysis A prospective, case-controlled study. Nephrol. Dial. Transplant. 2011;26:1287–1296. doi: 10.1093/ndt/gfq724. [DOI] [PubMed] [Google Scholar]
- 13.Bergman A, Fenton SSA, Richardson RMA, et al. Reduction in cardiovascular related hospitalization with nocturnal home hemodialysis. Clin. Nephrol. 2008;69:33–39. doi: 10.5414/cnp69033. [DOI] [PubMed] [Google Scholar]
- 14.Powell JR, Oluwaseun O, Woo YM, et al. Ten Years Experience of In-Center Thrice Weekly Long Overnight Hemodialysis. Clin. J. Am. Soc. Nephrol. 2009;4:1097–1101. doi: 10.2215/CJN.06651208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansen KL, Zhang R, Huang Y, et al. Survival and hospitalization among patients using nocturnal and short daily compared to conventional hemodialysis: a USRDS study. Kidney Int. 2009;76:984–990. doi: 10.1038/ki.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacson E, Wang W, Lester K, et al. Outcomes Associated with In-Center Nocturnal Hemodialysis from a Large Multicenter Program. Clin. J. Am. Soc. Nephrol. 2010;5:220–226. doi: 10.2215/CJN.06070809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacson E, Xu J, Suri RS, et al. Survival with Three-Times Weekly In-Center Nocturnal Versus Conventional Hemodialysis. J. Am. Soc. Nephrol. 2012;23:687–695. doi: 10.1681/ASN.2011070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nesrallah GE, Lindsay RM, Cuerden MS, et al. Intensive Hemodialysis Associates with Improved Survival Compared with Conventional Hemodialysis. J. Am. Soc. Nephrol. 2012;23:696–705. doi: 10.1681/ASN.2011070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, et al. Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol. Dial. Transplant. 2015;30:1208–1217. doi: 10.1093/ndt/gfv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korevaar JC, Feith G, Dekker FW, et al. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: A randomized controlled trial. Kidney Int. 2003;64:2222–2228. doi: 10.1046/j.1523-1755.2003.00321.x. [DOI] [PubMed] [Google Scholar]
- 21.Rocco MV, Lockridge RS, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80:1080–1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sergeyeva O, Gorodetskaya I, Ramos R, et al. Challenges to enrollment and randomization of the Frequent Hemodialysis Network (FHN) Daily Trial. J. Nephrol. 2012;25:302–309. doi: 10.5301/jn.5000160. [DOI] [PubMed] [Google Scholar]
- 23.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Culleton BF, Walsh M, Klarenbach SW, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: A randomized controlled trial. JAMA. 2007;298:1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 25.Rocco MV, Daugirdas JT, Greene T, et al. Long-term Effects of Frequent Nocturnal Hemodialysis on Mortality: The Frequent Hemodialysis Network (FHN) Nocturnal Trial. Am. J. Kidney Dis. doi: 10.1053/j.ajkd.2015.02.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jardine MJ, Zuo L, Gray NA, et al. Impact of Extended Weekly Hemodialysis Hours on Quality of Life and Clinical Outcomes: The ACTIVE Dialysis Multinational Trial. J. Am. Soc. Nephrol. 2014;25:B2. [Google Scholar]
- 27.Jardine MJ, Zuo LI, Gray NA, et al. Design and participant baseline characteristics of “A Clinical Trial of IntensiVE Dialysis”: the ACTIVE Dialysis Study. Nephrol. Carlton Vic. 2015;20:257–265. doi: 10.1111/nep.12385. [DOI] [PubMed] [Google Scholar]
- 28.National Institute of Diabetes and Digestive and Kidney Diseases, University of Pennsylvania. ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US); 2000. A Cluster-randomized, Pragmatic Trial of Hemodialysis Session Duration (TiME) [Google Scholar]
- 29.Mucsi I, Hercz G, Uldall R, et al. Control of serum phosphate without any phosphate binders in patients treated with nocturnal hemodialysis. Kidney Int. 1998;53:1399–1404. doi: 10.1046/j.1523-1755.1998.00875.x. [DOI] [PubMed] [Google Scholar]
- 30.Walsh M, Culleton B, Tonelli M, et al. A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int. 2005;67:1500–1508. doi: 10.1111/j.1523-1755.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 31.Rivara MB, Ravel V, Kalantar-Zadeh K, et al. Uncorrected and Albumin-Corrected Calcium, Phosphorus, and Mortality in Patients Undergoing Maintenance Dialysis. J. Am. Soc. Nephrol. 2015;26:1671–1681. doi: 10.1681/ASN.2014050472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rocco MV, Lockridge RS, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80:1080–1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011;79:250–257. doi: 10.1038/ki.2010.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.FHN Trial Group. Chertow GM, Levin NW, et al. In-center hemodialysis six times per week versus three times per week. N. Engl. J. Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wald R, Yan AT, Perl J, et al. Regression of left ventricular mass following conversion from conventional hemodialysis to thrice weekly in-centre nocturnal hemodialysis. BMC Nephrol. 2012;13:3. doi: 10.1186/1471-2369-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan CT, Arab S, Carasso S, et al. Impact of frequent nocturnal hemodialysis on myocardial mechanics and cardiomyocyte gene expression. Circ. Cardiovasc. Imaging. 2012;5:474–480. doi: 10.1161/CIRCIMAGING.111.971606. [DOI] [PubMed] [Google Scholar]
- 37.Chertow GM, Levin NW, Beck GJ, et al. Long-Term Effects of Frequent In–Center Hemodialysis. J. Am. Soc. Nephrol. 2015 doi: 10.1681/ASN.2015040426. ASN.2015040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummings P. Missing data and multiple imputation. JAMA Pediatr. 2013;167:656–661. doi: 10.1001/jamapediatrics.2013.1329. [DOI] [PubMed] [Google Scholar]
- 39.Suissa S. Immortal time bias in pharmaco-epidemiology. Am. J. Epidemiol. 2008;167:492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 40.Karim ME, Gustafson P, Petkau J, et al. Marginal Structural Cox Models for Estimating the Association Between β-Interferon Exposure and Disease Progression in a Multiple Sclerosis Cohort. Am. J. Epidemiol. 2014;180:160–171. doi: 10.1093/aje/kwu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook NR, Cole SR, Hennekens CH. Use of a marginal structural model to determine the effect of aspirin on cardiovascular mortality in the Physicians’ Health Study. Am. J. Epidemiol. 2002;155:1045–1053. doi: 10.1093/aje/155.11.1045. [DOI] [PubMed] [Google Scholar]
- 42.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiol. Camb. Mass. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. The Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.