Abstract
Objectives
To describe the variation in approaches to surgical and antibiotic treatment for first CSF shunt infection and adherence to Infectious Diseases Society of America (IDSA) guidelines.
Study design
We conducted a prospective cohort study of children undergoing treatment for first CSF infection at 7 HCRN hospitals from April 2008 through December 2012. Univariate analyses were performed to describe the study population.
Results
151 children underwent treatment for first CSF shunt-related infection. Most children had undergone initial CSF shunt placement before the age of 6 months (n=98, 65%). Median time to infection after shunt surgery was 28 days [interquartile range (IQR) 15–52 days]. Surgical management was most often shunt removal with interim external ventricular drain placement, followed by new shunt insertion (n=122, 81%). Median time from first negative CSF culture to final surgical procedure was 14 days (IQR 10–21 days). Median duration of IV antibiotic use duration was 19 days (IQR 12–28 days). For 84 infections addressed by IDSA guidelines, 7 (8%) met guidelines and 61 (73%) had longer duration of IV antibiotic use than recommended.
Conclusions
Surgical treatment for infection frequently adheres to IDSA guidelines of shunt removal with external ventricular drain placement followed by new shunt insertion. However, duration of IV antibiotic use in CSF shunt infection treatment was consistently longer than recommended by the 2004 IDSA guidelines.
Keywords: cerebrospinal, shunt, infection, treatment, antibiotic
Although life-saving and the mainstay of hydrocephalus treatment, (1) CSF shunts can cause new and chronic surgical and medical problems for children with hydrocephalus. Mechanical malfunction is frequent with 40% of shunts requiring surgical revision within 2 years.(2) With every surgery, the risk of CSF shunt infection increases.(3–5) CSF shunt infection rates range from 0 to 35% per surgery.(3, 6–16) CSF shunt re-infection is frequent and rates range from 12 to 26%.(4, 17, 18)
Even though numerous review articles have been written,(1, 19–22) no organization in the United States or elsewhere has published an official guideline for management of CSF shunt infection. Rather, embedded within 2004 guidelines for management of bacterial meningitis, the Infectious Disease Society of America (IDSA) provided guidelines for both surgical and antibiotic decisions in the treatment of CSF shunt infection.(23)
Surgical decisions in the treatment of CSF shunt infection include either shunt removal with external ventricular drain (EVD) placement followed by new shunt insertion, or shunt externalization followed by shunt replacement, once the CSF is sterile. Although the benefit of each approach in preventing CSF shunt re-infection remains unclear,(18–22, 24–26) the growing consensus within the neurosurgical community has been to remove the shunt at the time of the first infection surgery.(17, 27) The 2004 IDSA guideline reflects this growing consensus, suggesting shunt removal with EVD placement followed by new shunt insertion with an A-II ranking of evidence (i.e., good evidence from ≥1 well-designed clinical trial(s) without randomization, from cohort or case-controlled studies, etc.).(23)
Antibiotic decisions in the treatment of CSF shunt infection include the choice and duration of empiric and targeted intravenous (IV) antibiotics.(28–30) Here again, evidence is limited as no randomized controlled clinical trial has been conducted, prior studies being retrospective and limited in size.(22) The 2004 IDSA guidelines for duration of IV antibiotics were provided with a B-II ranking of evidence (i.e. moderate evidence from ≥1 well-designed clinical trial(s) without randomization, from cohort or case-controlled studies, etc.). (23) Reported duration of IV antibiotic use varies widely — ranging from 4 to 47 days in one study(17) — and depends, in part, on the surgical approach used, (4, 31) pathogen involved, (23, 30, 32) and persistence of the pathogen.
Identification of optimal surgical and antibiotic treatment for CSF shunt infections has the potential to improve outcomes, but before a prospective interventional trial can be designed, current practices need to be better understood. The Hydrocephalus Clinical Research Network (HCRN) provides a unique opportunity to understand current practices for treatment of CSF shunt infection following creation of the IDSA guidelines. We aimed to describe the variation in approaches to surgical and antibiotic treatment for first CSF shunt infection, and rate of adherence to IDSA guidelines for CSF shunt infection treatment in a prospective cohort of children with CSF shunt placement.
Methods
The HCRN is a collaboration of nine pediatric neurosurgical centers across North America, with seven participating in this study: Children’s Hospital of Alabama, Children’s Hospital of Pittsburgh, Hospital for Sick Children, Primary Children’s Medical Center, Seattle Children’s Hospital, Texas Children’s Hospital, and St. Louis Children’s Hospital. HCRN registry data use was approved by the HCRN and the Institutional Review Boards at the University of Utah and Seattle Children’s Hospital.
Within the HCRN registry, data from each neurosurgical admission for each child admitted is collected contemporaneously. Data collection began in April 2008 and, for this study, ended on December 31, 2012, except for children at the Hospital for Sick Children who were followed until December 31, 2011. The final cohort included children whose initial CSF shunt placement, interval CSF shunt revision(s), and first CSF shunt infection were recorded in the HCRN registry during the study period.
The HCRN consensus definition was used for first CSF shunt infection: (33–35) (a) microbiological determination of bacteria present in a culture or Gram stain of CSF, wound swab, and/or pseudocyst fluid, or (b) shunt erosion (visible hardware), or (c) abdominal pseudocyst (without positive culture); or for children with ventriculoatrial shunts, (d) presence of bacteria in a blood culture. The first CSF sample for diagnosis of infection usually was obtained from needle aspiration of the shunt reservoir under sterile conditions and before initiation of antibiotic therapy. To ensure that all infections were identified, all neurosurgical admissions involving two CSF shunt surgeries and ≥48 hours of IV antibiotic treatment were reviewed by TS and local HCRN staff to confirm that infection criteria were not met.
Baseline characteristics considered included patient risk factors such as demographics, factors present prior to and at the time of initial CSF shunt placement, as well as intervening revision surgeries as previously described and shown in Table I.(34, 36) Complex chronic condition(s) (CCCs) were classified as described previously.(37) Of note, hospitals within the HCRN have instituted an infection prevention bundle used at the time of initial shunt placement and at revision(s), although adherence to all aspects is variable(33, 38) (Table IV; available at www.jpeds.com).
Table 1.
Patient and Treatment Characteristics for the Study Cohort (n=151)
|
|
||
|---|---|---|
| n | % | |
| Initial CSF shunt placement | ||
| Age at initial CSF shunt placement | ||
| 0–30 days | 45 | 30 |
| 1–6 months | 53 | 35 |
| 6–48 months | 29 | 19 |
| ≥48 months | 24 | 16 |
| Sex | ||
| Male | 86 | 57 |
| Weight at surgery (kg), median (IQR) | 4.1 (2.9, 10.3) | |
| Indication for shunt placement | ||
| Post-IVH due to prematurity | 46 | 31 |
| Congenital anomaly± | 23 | 15 |
| Myelomeningocele | 20 | 13 |
| Tumor (supratentorial, posterior fossa, midbrain) | 20 | 13 |
| Cyst (posterior fossa, intracranial) | 13 | 9 |
| Aqueductal stenosis | 11 | 7 |
| Post-head injury | 7 | 5 |
| Post-infectious | 6 | 4 |
| Other | 3 | 2 |
| Spontaneous ICH/IVH/SAH | 2 | 1 |
| Complex chronic conditions (CCCs) | ||
| None (exclusive of hydrocephalus) | 90 | 60 |
| 1 | 46 | 30 |
| ≥2 | 15 | 10 |
| Distal shunt location | ||
| Peritoneal | 149 | 99 |
| Atrial | 2 | 1 |
|
|
||
| CSF shunt revision(s) | ||
| Revisions before first infection | ||
| Zero | 106 | 70 |
| One | 27 | 18 |
| Two or more | 18 | 12 |
| Revisions before first infection, mean (SD) | 0.6 (1.4) | |
| Distal shunt location before first infection | ||
| Peritoneal | 147 | 97 |
| Atrial | 4 | 3 |
|
|
||
| First CSF shunt infection | ||
| Age at first CSF shunt infection | ||
| 0–30 days | 17 | 11 |
| 1–6 months | 58 | 38 |
| 6–48 months | 49 | 33 |
| ≥48 months | 27 | 18 |
| Time since previous CSF shunt surgery (days), median (IQR) | 28 (15, 52) | |
| LOS for infection treatment (days), median (IQR) | 23 (15, 56) | |
| Surgical approach to treatment | ||
| Full removal with EVD placement | 122 | 81 |
| Externalization | 9 | 6 |
| Failed externalization | 9 | 6 |
| Shunt removed without replacement | 6 | 4 |
| One or more components revised | 2 | 1 |
| Shunt left in place | 2 | 1 |
| Died during treatment | 1 | 1 |
| Time between infection surgeries* (days), median (IQR) | 16 (11, 26) | |
| Complications for 84 patients | ||
| Any medical complication | 14 | 17 |
| Any surgical complication | 24 | 29 |
| Shunt location after infection treatment | ||
| Peritoneal | 129 | 85 |
| Atrial | 11 | 7 |
| Pleural | 1 | 1 |
| Shunt removed/not applicable | 10 | 6 |
|
|
||
congenital includes communicating congenital, other congenital, encephalocele, craniosynostosis
calculated for 137 children with two infection surgeries
IVH intraventricular hemorrhage
ICH Intracranial hemorrhage
SAH subarachnoid hemorrhage
Table 4.
HCRN Infection Prevention Bundle Interventions
| Intervention33,38 | Dates |
|---|---|
| Prophylactic IV antibiotic use: cefazolin 30 mg/kg or, if allergic, vancomycin 15 mg/kg or clindamycin 10 mg/kg | Entire study period |
| Prophylactic IV antibiotic duration: one dose before incision and one dose postoperatively | Entire study period |
| Prophylactic IV antibiotic timing: one dose before incision | Entire study period |
| Type of skin cleanser: Chloraprep© | Entire study period |
| Double gloving by all participants | Entire study period |
| Ioban drape use | Entire study period |
| Prophylactic intrathecal antibiotic use: 1 mL of vancomycin at 10 mg/mL concentration mixed with 2 mL of gentamicin at 2 mg/mL concentration | Routine use stopped after 1/1/12 |
| Antibiotic impregnated shunt tubing: typically Bactiseal©, which includes infusions of clindamycin and rifampin in its silicone | Routine use started after 1/1/12 |
| Mandatory site preparation with hair clipping | Routine use stopped after 1/1/12 |
Factors considered during the treatment of first CSF shunt infection included chronologic age, complications after first shunt infection, and distal shunt location. Duration of time between infection surgeries was created to denote the time span between the first and final surgical approaches to infection treatment. Next we examined diagnostic and microbiological factors in CSF shunt infection.(35) We considered the specific infection criterion met; organism(s) recovered in CSF, blood, and wound culture(s); presence of bacteremia (defined as any organism recovered in blood culture, except for children with ventriculoatrial shunts diagnosed by blood culture); and, if available within 48 hours of infection diagnosis, results of the first CSF studies including Gram stain, white and red blood cell counts, glucose, and protein concentrations.
Among the CSF shunt infections diagnosed by CSF culture, we examined additional diagnostic and microbiological factors including:(35) duration of time from first positive culture to first infection surgery, duration of time from first persistently negative culture to final surgical procedure following infection, duration of positive CSF cultures, the presence of intermittent negative CSF cultures (defined as two or more positive CSF cultures with the same organism and at least one intervening negative culture), presence of secondary ventriculitis (recovery of a different organism from that recovered initially in CSF culture), and polymicrobial infection (defined as growth of more than one organism, including the same species, on the first culture from one source).
Surgical Management
The key surgical outcome variable was the surgical approach to treatment for CSF shunt infection.(39) Surgical approach was defined as either (a) full shunt removal with interim EVD placement followed by new shunt insertion once the CSF is sterile; (b) distal shunt externalization followed by shunt replacement; (c) distal shunt externalization followed by EVD placement followed by new shunt insertion (failed externalization); or more rarely, (d) shunt removal without replacement, (e) shunt revision, and (f) no surgical treatment.
Antibiotic Treatment
The key antibiotic treatment outcome variable was IV antibiotic use and duration, characterized following review by two authors (TDS and MPK) as (a) overall, defined by the start and end of any IV antibiotic; (b) concordant, defined by the start of IV antibiotic(s) to which the organism(s) isolated in culture was sensitive; (c) concordant and appropriate, defined by the start of concordant IV antibiotic at a dose appropriate for weight and with adequate central nervous system penetration; and (d) broad spectrum, defined by the start of IV antibiotic(s) that included gram-positive and gram-negative organisms. When not specifically provided, susceptibilities were anticipated when possible based on the organism grown (e.g., a methicillin susceptible Staphylococcus aureus isolate was presumed to be susceptible to cefazolin even if not tested directly). Drug levels were not available for review in determination of concordant and appropriate IV antibiotic use. Use of intrathecal antibiotics was handled as a dichotomous variable. Use of rifampin was considered synergistic and was not incorporated further into consideration of broad spectrum or concordant antibiotics as susceptibility data were not available.
Adherence to IDSA Guidelines
IDSA guidelines for management of CSF shunt infection include the surgical approach of full shunt removal with EVD placement followed by new shunt insertion once the CSF is sterile, and different antibiotic treatment durations according to the organism recovered, grouped as coagulase-negative Staphylococcus, Staphylococcus aureus, and gram-negative bacilli. When applicable, concurrent CSF cell counts and chemistry laboratory results, as well as subsequent CSF culture results also were reviewed. A normal CSF white blood cell count was considered ≤32 cells/mm3 in a child under one month of age and ≤10 cells/mm3 in a child older than one month.(40) Normal CSF glucose was ≥45 mg/dL and normal CSF protein was <200 mg/dL. Infections with secondary ventriculitis (defined above) were excluded from consideration of guideline adherence.
Statistical Analyses
The study population was described overall using frequency and proportion for binary and categorical variables; and mean and standard deviation (SD), or median and interquartile range (IQR), for continuous variables. Diagnostic characteristics and surgical and antibiotic treatment decisions, including antibiotic duration, were similarly described. Bivariate analyses of antibiotic treatment by center, organism, and surgical approach were conducted to assess systematic differences in surgical and antibiotic treatment decisions. All statistical analyses were performed using SAS (version 9.2, SAS Institute, Cary, NC).
Results
Of 3,131 children in the HCRN registry during the study period, 151 (4.8%) met inclusion criteria. Baseline patient and treatment factors are shown in Table I. Most children underwent initial CSF shunt placement under the age of 6 months (n=98, 65%), at a median age of 13 weeks (IQR 2, 73 weeks). Indication for CSF shunt placement was distributed between post-IVH due to prematurity (31%), congenital (15%), myelomeningocele (13%), tumor (13%), and other etiologies. Most children had zero CSF shunt revisions (n=106, 70%) prior to CSF shunt infection. The median time since previous CSF shunt surgery to infection was 28 days (IQR 15, 52 days). At the time of initial CSF shunt infection, the cohort had a median age of 27 weeks (IQR 12, 127 weeks).
Diagnostic characteristics of initial CSF shunt infection are provided in Table II. The majority were diagnosed by CSF culture (n=115, 76%). The initial organism recovered included coagulase-negative Staphylococcus (n=39, 34%), Staphylococcus aureus (n=36, 31%), and gram-negative bacilli (n=24, 21%).
Table 2.
Diagnostic Characteristics of Initial CSF Shunt Infections in the Study Cohort (n=151)
|
|
||
|---|---|---|
| n | % | |
|
|
||
| Diagnosis of first CSF shunt infection | ||
| CSF culture | 115 | 76 |
| Wound culture excluding CSF culture | 12 | 8 |
| Visible hardware only | 10 | 7 |
| Abdominal pseudocyst | 10 | 7 |
| Blood culture in VA shunt only | 3 | 2 |
| CSF Gram stain only | 1 | 0 |
| Organisms in 115 CSF cultures | ||
| Coagulase-negative Staphylococcus | 39 | 34 |
| Staphylococcus aureus | 36 | 31 |
| Enterococcus species | 3 | 2 |
| Gram-negative organism* | 24 | 20 |
| Other | 9 | 8 |
| More than one organism | 4 | 4 |
| Organisms in 12 wound cultures | ||
| Coagulase-negative Staphylococcus | 2 | 17 |
| Staphylococcus aureus | 1 | 8 |
| Other | 2 | 17 |
| More than one organism | 2 | 17 |
| Culture not obtained | 5 | 41 |
| Organisms in 3 blood cultures | ||
| Coagulase negative Staphylococcus | 1 | 33 |
| Staphylococcus aureus | 1 | 33 |
| Gram-negative organism | 1 | 33 |
| Bacteremia | 12 | 8 |
| Initial positive Gram stain | 69 | 46 |
| Initial white blood cell count†, median (IQR) | 48 (7, 273) | |
| Initial red blood cell count†, median (IQR) | 55 (4, 670) | |
| Initial glucose†, median (IQR) | 36 (25, 52) | |
| Initial protein†, median (IQR) | 110 (41, 447) | |
|
|
||
Gram-negative organisms included: Pseudomonas aeruginosa (n=6), Enterobacter cloacae (n=4), Escherichia coli (n=3), Klebsiella pneumoniae (n=3), Klebsiella oxytoca (n=2), Serratia marcescens (n=2), and one each of Enterobacter aerogenes, Haemophilus influenzae, Haemophilus parainfluenzae, Proteus mirabilis
CSF cell counts available for 145 children and chemistry laboratory test results available for 142 children
Surgical Management
The majority of initial CSF shunt infections were treated with full removal with EVD placement (n=122, 81%). The median time between first and final infection surgeries was 16 days (IQR 11, 26 days). The median time from first negative CSF culture to final surgery was 14 days (IQR 10, 21 days; Table III).
Table 3.
Treatment Decisions and Subsequent Diagnostic Characteristics for Infections with Organisms Recovered from CSF culture(s) in the Study Cohort (n=115)
|
|
|
| First CSF shunt infection | |
| Overall duration of intravenous (IV) antibiotic use (days), median (IQR) | 19 (12, 28) |
| Concordant IV antibiotic use, n (%) | 113 (98%) |
| Duration (days), median (IQR) | 16 (11, 24) |
| Concordant and appropriate IV antibiotic use, n (%) | 111 (97%) |
| Duration (days), median (IQR) | 14 (8, 20) |
| Broad spectrum IV antibiotic use, n (%) | 115 (100%) |
| Duration (days), median (IQR) | 5 (3, 14) |
| Use of IV antibiotics after shunt replacement, n (%) | 26 (23%) |
| Duration (days), median (IQR) | 2 (0, 6) |
| Use of outpatient antibiotics after shunt replacement, n (%) | 14 (12%) |
| Use of intrathecal antibiotics, n (%) | 68 (59%) |
| Use of rifampin, n (%) | 30 (26%) |
| Time from first positive culture to first infection surgery (hours), median (IQR) | 8 (0, 19) |
| Time from first negative culture to final surgical approach to infection (days), median (IQR) | 14 (10, 21) |
| Duration of positive CSF cultures in days, median (IQR) | 3 (1, 6) |
| Intermittent negative CSF cultures, n (%) | 18 (16%) |
| Secondary ventriculitis, n (%) | 15 (13%) |
| Polymicrobial CSF culture, n (%) | 15 (13%) |
| Culture positive from broth only, n (%) | 19 (17%)
|
Antibiotic Treatment
The median overall duration of IV antibiotic use for all CSF shunt infections was 17 days (IQR 11, 27 days; Figure 1; available at www.jpeds.com). Treatment decisions for the subset of 115 infections diagnosed by CSF culture are provided in Table III and Figure 2 (Figure 2; available at www.jpeds.com). The median duration of concordant and appropriate IV antibiotic use was 14 days (IQR 8, 20 days), with longer durations of overall and concordant IV antibiotic use observed. No differences were seen in duration of overall, concordant, and/or concordant and appropriate IV antibiotic use for infections diagnosed by CSF culture when stratified by organism, surgical approach, or hospital. (Data not shown) The median duration of broad spectrum IV antibiotic use, which likely represents empiric antibiotic coverage while awaiting culture results and CSF clearance, was 5 days (IQR 3, 14 days). The median duration of time from first negative culture to final surgery, which best represents days of therapy after CSF sterilization, was 14 days (IQR 10, 21 days). Fewer initial CSF shunt infections were treated with rifampin (n=30, 26%). Additional antibiotic use was observed in the hospital setting after shunt replacement (n= 26, 23%) and in the outpatient setting (n=14, 12%).
Figure 1 online.
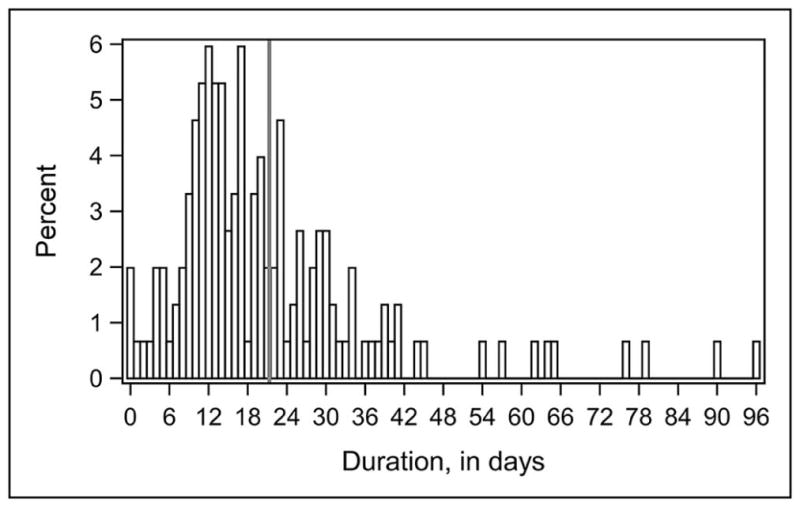
Distribution of overall duration of IV antibiotic use for all infection episodes (n=151)*
*vertical gray line denotes mean value
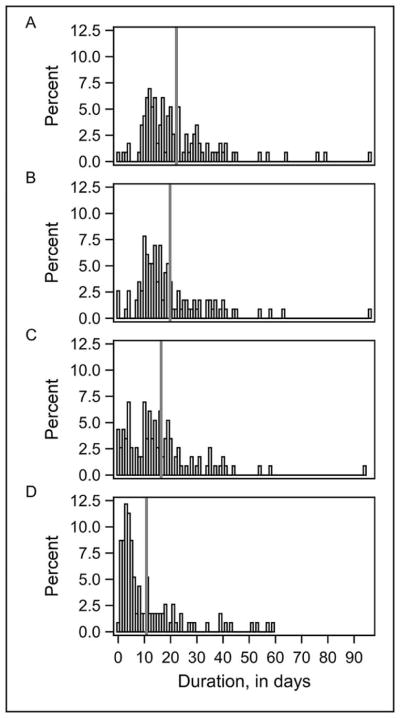
Figure 2 online.
For infections diagnosed by CSF culture (n=115), distribution of (a) overall duration of IV antibiotic use, (b) duration of concordant IV antibiotic use, (c) duration of concordant and appropriate IV antibiotic use, and (d) duration of broad spectrum IV antibiotic use.*
*vertical gray line denotes mean value
Adherence to IDSA Guidelines for First CSF Shunt Infection
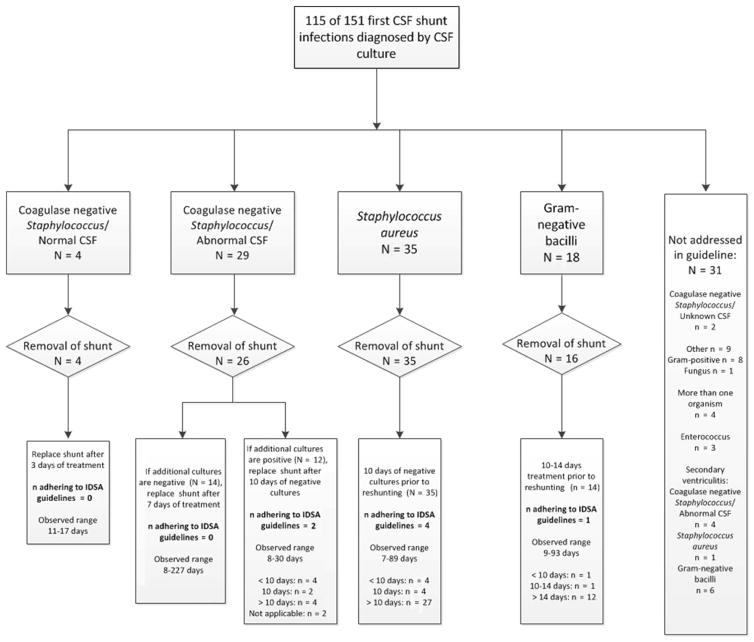
Adherence to IDSA guidelines for treatment of CSF shunt infection is provided in Figure 3. Among the 33 coagulase-negative Staphylococcus infections, 2 followed both surgical and antibiotic IDSA guidelines. Among the 31 non-adherent infections, 3 did not have shunt removal, 4 were treated for a shorter duration than recommended, and 22 were treated for a longer duration than recommended. Among the 35 Staphylococcus aureus infections, 4 followed both surgical and antibiotic IDSA guidelines. Among the 31 non-adherent infections, 4 were treated for a shorter duration than recommended, and 27 were treated for a longer duration than recommended. Among the 18 gram-negative bacillary infections, 1 followed both surgical and antibiotic IDSA guidelines. Among the 17 non-adherent infections, 2 did not have shunt removal, 1 was treated for a shorter duration than recommended, and 12 were treated for a longer duration than recommended. One patient died prior to shunt replacement. In all, for 84 infections addressed by IDSA guidelines, only 7 (8%) followed guidelines for both surgical and antibiotic treatment; 61 (73%) were treated for a longer duration of IV antibiotic use and 9 (11%) were treated for a shorter duration of IV antibiotic use than recommended.
Figure 3.
Adherence to IDSA guidelines*†
*rates were reported as n/N
†of two children with coagulase-negative Staphylococcus and abnormal CSF, one had no subsequent negative culture, and one did not have a shunt replaced; of two children with gram-negative bacilli, one did not have a shunt replaced and one died prior to shunt replacement
Discussion
In this prospective cohort of 151 children treated for initial CSF shunt infection at seven hospitals, the majority were surgically managed with full shunt removal and interim EVD placement, consistent with IDSA guidelines. The median overall duration of IV antibiotic use was 17 days, and for children diagnosed by positive CSF culture was 19 days. In all, for 84 infections addressed by IDSA guidelines, few adhered to IDSA guidelines for treatment in duration of IV antibiotic use and the majority were treated for a longer duration than recommended.
Key questions in the treatment of CSF shunt infection still include determination of the appropriate surgical management and appropriate duration of antibiotic therapy.(22, 24) Significant variation in antibiotic treatment decision-making in the treatment of CSF shunt infection has been demonstrated previously,(27, 41) and the 2004 IDSA guidelines suggested different treatment durations by pathogen in the absence of clear evidence based studies informing optimal treatment strategies.(23) Although this study demonstrates considerable variation and equipoise exists in the duration and selection of IV antibiotic treatment, it provides a necessary first step in the planning of a future trial by characterizing the range of IV antibiotic durations that could be tested in the setting of a controlled clinical trial. Additionally, the current study importantly suggests the feasibility of controlling and standardizing the surgical approach (shunt removal with interim EVD placement) to remove its potential confounding effects in a trial aimed at identifying an optimal antibiotic regimen. We believe future studies of the optimal duration of IV antibiotic treatment are critical to the creation of high quality evidence-based guidelines for management of CSF shunt infection.
Despite the paucity of evidence informing the IDSA guidelines, this study may suggest antibiotic overuse in the treatment of CSF shunt infections. Prolonged IV antibiotic treatment for CSF shunt infections could be associated with a number of potential patient harms. Antibiotic overuse is known to be associated with the selection of resistant organisms, potentially placing these patients at risk of future difficult-to-treat resistant infections.(42) Prolongation in EVD use introduces risk of secondary infection. Ongoing antibiotic exposure is associated with other individual harms such as C. difficile colitis and potential increased risks for obesity and autoimmune conditions such as inflammatory bowel disease and juvenile arthritis.(43–45) Lastly, children hospitalized for CSF shunt infection must usually remain hospitalized until their shunt is replaced, so each additional unnecessary day of antibiotics adds additional costs to their care for both the administered antibiotics and for lengthening the admission itself.
Poor adherence to the IDSA guidelines in antibiotic treatment may be attributable to a lack of awareness in the neurosurgical community(46) because guidelines are embedded in guidelines for treatment of bacterial meningitis.(23) Pediatric neurosurgeons report standardized care processes to encourage adherence to IDSA guidelines at only two of the seven participating hospitals. We encourage ongoing efforts to disseminate these guidelines to the neurosurgical community. This can be achieved through collaboration between pediatric neurosurgeons and antibiotic stewardship programs to promote adherence to the shortest effective antibiotic durations advised. In addition, we encourage transparency about advised duration of treatment to promote the optimal timing of surgical procedures and to minimize the contribution of operating room scheduling barriers.
Our study has several limitations. The conduct of this study at HCRN centers that were adhering to an infection prevention bundle (33, 38) reduces generalizability of findings outside of the HCRN. However, the HCRN prevention bundle has been widely disseminated and implemented in the past 5 years. In addition, the multi-institutional nature of this study gives it greater generalizability than previous studies. Additional aspects of management of CSF shunt infection–including but not limited to the number, frequency, and method of CSF cultures obtained through EVDs, flushing of EVDs, and replacement of EVDs – is not standardized in the HCRN and was subject to variation within and between participating centers. Our definition of infection was developed by consensus within the HCRN and does not permit easy comparison with infection rates at non-HCRN sites or those used by hospital infection control groups. For neurosurgical procedures, the assumption is that patients return to the same center for care. Drug levels were not available for review in determination of concordant and appropriate IV antibiotic use.
Despite these limitations, this study demonstrates reasonable adherence to IDSA surgical guidelines to remove the shunt in treatment of CSF shunt infection but low adherence to IDSA antibiotic treatment guidelines for duration of IV antibiotic use. Nearly three-quarters of initial CSF shunt infections were treated for a longer duration than recommended. We encourage pediatric neurosurgeons to work with antibiotic stewardship programs to implement standardized care processes to promote adherence to IDSA guidelines. High-quality studies of the optimal duration of IV antibiotic treatment are critical to the creation of evidence-based guidelines for CSF shunt infection treatment.
Acknowledgments
Supported by private philanthropy, National Institute of Neurological Disorders and Stroke (NINDS; 1RC1NS068943-01), the Gerber Foundation (1692-3638), Patient-Centered Outcomes Research Institute (CER-1403-13857), and the Hydrocephalus Association. T.S., K.W., and N.G. were supported by the NINDS/Seattle Children’s Center for Clinical and Translational Research (K23NS062900) and the National Center for Research Resources, a component of the National Institutes of Health (ULI RR025014). D.L. was supported by NINDS, Patient Centered Outcomes Research Institute, the Hydrocephalus Association, Rudy Schulte, Medtronic, and Karl Storz. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the funding sponsors. The authors declare no conflicts of interest.
We thank the contributing children and families at all participating centers. We also thank Stephan John Nemeth, IV, Gabriel Finn Nemeth, and Daschel Simon Nemeth (family members of T.S.) for support and valuable feedback.
Abbreviations
- CSF
cerebrospinal fluid
- CCC
complex chronic conditions
- EVD
external ventricular drain
- HCRN
Hydrocephalus Clinical Research Network
- IDSA
Infectious Diseases of America
- IQR
interquartile range
- IV
intravenous
- SD
standard deviation
Appendix
Additional members of HCRN include:
Primary Children’s Hospital, University of Utah, Salt Lake City, Utah: Douglas L. Brockmeyer, MD; Robert Bollo, MD, MS; and coordinators Nicole Tattersall, RN, BSN and Tracey Habrock-Bach, BS.
Children’s Hospital of Alabama, University of Alabama at Birmingham, Birmingham, Alabama: Jeffrey P. Blount, MD; James M. Johnston, MD; Brandon Rocque, MD; and coordinators Anastasia Arynchyna, MPH and Amita Bey, MPH.
Hospital for Sick Children, University of Toronto, Toronto, Ontario: James M. Drake, BSE, MBBCh, MSc (HCRN investigator); Leslie Ackacpo-Satchivi, MD, PhD; Peter B. Dirks, MD, PhD; James T. Rutka, MD, PhD; Michael D. Taylor, MD, PhD; and coordinators Homa Ashrafpour, BSc and Lindsay O’Connor, MSc.
Texas Children’s Hospital, Baylor College of Medicine, Houston, Texas: Daniel J. Curry, MD; Robert C. Dauser, MD; Andrew H. Jea, MD; Sandi K. Lam, MD; and coordinators Sheila Martinez, BS and Sheila Ryan, JD, MPH.
Seattle Children’s Hospital, University of Washington, Seattle, Washington: Richard G. Ellenbogen, MD; Jeffrey G. Ojemann, MD, PhD; Amy Lee, MD; Anthony Avellino, MD, PhD; and coordinator Amy Anderson, BSN, RN.
Children’s Hospital of Pittsburgh, University of Pittsburgh, Pittsburgh, Pennsylvania: Ian F. Pollack, MD; Stephanie Greene, MD; Elizabeth C. Tyler-Kabara, MD, PhD; and coordinators Kimberly Diamond, BS, BA and Arlene Luther, BS, RN.
St. Louis Children’s Hospital, Washington University in St. Louis, Missouri: TS Park, MD; Matthew D. Smyth, MD; Jeffrey R. Leonard, MD; and coordinator Deanna Mercer.
Monroe Carell Jr. Children’s Hospital at Vanderbilt, Vanderbilt University Medical Center, Nashville, Tennessee: Robert P. Naftel, MD; Noel B. Tulipan, MD; and coordinator Stephen Gannon.
British Columbia Children’s Hospital, University of British Columbia, Vancouver, British Columbia: Douglas Cochrane, MD (HCRN investigator); Ash Singhal, MD, MSc; Paul Steinbok, MBBS; and coordinator Alexander Cheong, BS.
HCRN Data Coordinating Center, Department of Pediatrics, University of Utah, Salt Lake City, Utah: Volker Freimann, BA, Nichol Nunn, BS, MBA and Jeff Yearley, BA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kestle JR. Pediatric hydrocephalus: current management. Neurol Clin. 2003;21:883–95. vii. doi: 10.1016/s0733-8619(03)00016-1. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni AV, Riva-Cambrin J, Butler J, Browd SR, Drake JM, Holubkov R, et al. Outcomes of CSF shunting in children: comparison of Hydrocephalus Clinical Research Network cohort with historical controls: clinical article. J Neurosurg Pediatr. 2013;12:334–8. doi: 10.3171/2013.7.PEDS12637. [DOI] [PubMed] [Google Scholar]
- 3.McGirt MJ, Zaas A, Fuchs HE, George TM, Kaye K, Sexton DJ. Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2003;36:858–62. doi: 10.1086/368191. [DOI] [PubMed] [Google Scholar]
- 4.Vinchon M, Dhellemmes P. Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2006;22:692–7. doi: 10.1007/s00381-005-0037-8. [DOI] [PubMed] [Google Scholar]
- 5.Albright AL, Pollack IF, Adelson PD, Solot JJ. Outcome data and analysis in pediatric neurosurgery. Neurosurgery. 1999;45:101–6. doi: 10.1097/00006123-199907000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Piatt JH, Jr, Carlson CV. A search for determinants of cerebrospinal fluid shunt survival: retrospective analysis of a 14-year institutional experience. Pediatr Neurosurg. 1993;19:233–41. doi: 10.1159/000120738. discussion 42. [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: a prospective study of risk factors. Journal of neurosurgery. 2001;94:195–201. doi: 10.3171/jns.2001.94.2.0195. [DOI] [PubMed] [Google Scholar]
- 8.Odio C, McCracken GH, Jr, Nelson JD. CSF shunt infections in pediatrics. A seven-year experience. Am J Dis Child. 1984;138:1103–8. doi: 10.1001/archpedi.1984.02140500009004. [DOI] [PubMed] [Google Scholar]
- 9.McGirt MJ, Leveque JC, Wellons JC, 3rd, Villavicencio AT, Hopkins JS, Fuchs HE, et al. Cerebrospinal fluid shunt survival and etiology of failures: a seven-year institutional experience. Pediatr Neurosurg. 2002;36:248–55. doi: 10.1159/000058428. [DOI] [PubMed] [Google Scholar]
- 10.Griebel R, Khan M, Tan L. CSF shunt complications: an analysis of contributory factors. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1985;1:77–80. doi: 10.1007/BF00706686. [DOI] [PubMed] [Google Scholar]
- 11.Younger JJ, Simmons JC, Barrett FF. Operative related infection rates for ventriculoperitoneal shunt procedures in a children’s hospital. Infect Control. 1987;8:67–70. doi: 10.1017/s0195941700067102. [DOI] [PubMed] [Google Scholar]
- 12.Ronan A, Hogg GG, Klug GL. Cerebrospinal fluid shunt infections in children. Pediatr Infect Dis J. 1995;14:782–6. doi: 10.1097/00006454-199509000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Schoenbaum SC, Gardner P, Shillito J. Infections of cerebrospinal fluid shunts: epidemiology, clinical manifestations, and therapy. J Infect Dis. 1975;131:543–52. doi: 10.1093/infdis/131.5.543. [DOI] [PubMed] [Google Scholar]
- 14.Shurtleff DB, Stuntz JT, Hayden PW. Experience with 1201 cerebrospinal fluid shunt procedures. Pediatr Neurosci. 1985;12:49–57. doi: 10.1159/000120218. [DOI] [PubMed] [Google Scholar]
- 15.Cochrane DD, Kestle J. Ventricular shunting for hydrocephalus in children: patients, procedures, surgeons and institutions in English Canada, 1989–2001. Eur J Pediatr Surg. 2002;12:S6–11. doi: 10.1055/s-2002-36864. [DOI] [PubMed] [Google Scholar]
- 16.Quigley MR, Reigel DH, Kortyna R. Cerebrospinal fluid shunt infections. Report of 41 cases and a critical review of the literature. Pediatr Neurosci. 1989;15:111–20. [PubMed] [Google Scholar]
- 17.Kestle JR, Garton HJ, Whitehead WE, Drake JM, Kulkarni AV, Cochrane DD, et al. Management of shunt infections: a multicenter pilot study. Journal of neurosurgery. 2006;105:177–81. doi: 10.3171/ped.2006.105.3.177. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni AV, Rabin D, Lamberti-Pasculli M, Drake JM. Repeat cerebrospinal fluid shunt infection in children. Pediatr Neurosurg. 2001;35:66–71. doi: 10.1159/000050393. [DOI] [PubMed] [Google Scholar]
- 19.Gardner P, Leipzig T, Phillips P. Infections of central nervous system shunts. Med Clin North Am. 1985;69:297–314. [PubMed] [Google Scholar]
- 20.Gardner P, Leipzig TJ, Sadigh M. Infections of mechanical cerebrospinal fluid shunts. Curr Clin Top Infect Dis. 1988;9:185–214. [PubMed] [Google Scholar]
- 21.Kanev PM, Sheehan JM. Reflections on shunt infection. Pediatr Neurosurg. 2003;39:285–90. doi: 10.1159/000075255. [DOI] [PubMed] [Google Scholar]
- 22.Tamber MS, Klimo P, Jr, Mazzola CA, Flannery AM. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 8: Management of cerebrospinal fluid shunt infection. J Neurosurg Pediatr. 2014;14:60–71. doi: 10.3171/2014.7.PEDS14328. [DOI] [PubMed] [Google Scholar]
- 23.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39:1267–84. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 24.Williams MA, McAllister JP, Walker ML, Kranz DA, Bergsneider M, Del Bigio MR, et al. Priorities for hydrocephalus research: report from a National Institutes of Health-sponsored workshop. Journal of neurosurgery. 2007;5(Suppl):345–57. doi: 10.3171/PED-07/11/345. Pediatrics. [DOI] [PubMed] [Google Scholar]
- 25.Morissette I, Gourdeau M, Francoeur J. CSF shunt infections: a fifteen-year experience with emphasis on management and outcome. Can J Neurol Sci. 1993;20:118–22. doi: 10.1017/s0317167100047661. [DOI] [PubMed] [Google Scholar]
- 26.Venes JL. Infections of CSF shunt and intracranial pressure monitoring devices. Infect Dis Clin North Am. 1989;3:289–99. [PubMed] [Google Scholar]
- 27.Whitehead WE, Kestle JR. The treatment of cerebrospinal fluid shunt infections. Results from a practice survey of the American Society of Pediatric Neurosurgeons. Pediatr Neurosurg. 2001;35:205–10. doi: 10.1159/000050422. [DOI] [PubMed] [Google Scholar]
- 28.Walters BC, Hoffman HJ, Hendrick EB, Humphreys RP. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. Journal of neurosurgery. 1984;60:1014–21. doi: 10.3171/jns.1984.60.5.1014. [DOI] [PubMed] [Google Scholar]
- 29.Sells CJ, Shurtleff DB, Loeser JD. Gram-negative cerebrospinal fluid shunt-associated infections. Pediatrics. 1977;59:614–8. [PubMed] [Google Scholar]
- 30.Fan-Havard P, Nahata MC. Treatment and prevention of infections of cerebrospinal fluid shunts. Clin Pharm. 1987;6:866–80. [PubMed] [Google Scholar]
- 31.Nelson JD. Cerebrospinal fluid shunt infections. Pediatr Infect Dis. 1984;3:S30–2. doi: 10.1097/00006454-198405001-00011. [DOI] [PubMed] [Google Scholar]
- 32.Younger JJ, Christensen GD, Bartley DL, Simmons JC, Barrett FF. Coagulase-negative staphylococci isolated from cerebrospinal fluid shunts: importance of slime production, species identification, and shunt removal to clinical outcome. J Infect Dis. 1987;156:548–54. doi: 10.1093/infdis/156.4.548. [DOI] [PubMed] [Google Scholar]
- 33.Kestle JR, Riva-Cambrin J, Wellons JC, 3rd, Kulkarni AV, Whitehead WE, Walker ML, et al. A standardized protocol to reduce cerebrospinal fluid shunt infection: the Hydrocephalus Clinical Research Network Quality Improvement Initiative. J Neurosurg Pediatr. 2011;8:22–9. doi: 10.3171/2011.4.PEDS10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon TD, Whitlock KB, Riva-Cambrin J, Kestle JR, Rosenfeld M, Dean JM, et al. Revision surgeries are associated with significant increased risk of subsequent cerebrospinal fluid shunt infection. Pediatr Infect Dis J. 2012;31:551–6. doi: 10.1097/INF.0b013e31824da5bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuan TJ, Thorell EA, Hamblett NM, Kestle JR, Rosenfeld M, Simon TD. Treatment and microbiology of repeated cerebrospinal fluid shunt infections in children. Pediatr Infect Dis J. 2011;30:731–5. doi: 10.1097/INF.0b013e318218ac0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon TD, Whitlock KB, Riva-Cambrin J, Kestle JR, Rosenfeld M, Dean JM, et al. Association of intraventricular hemorrhage secondary to prematurity with cerebrospinal fluid shunt surgery in the first year following initial shunt placement. J Neurosurg Pediatr. 2012;9:54–63. doi: 10.3171/2011.10.PEDS11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon TD, Riva-Cambrin J, Srivastava R, Bratton SL, Dean JM, Kestle JR. Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J Neurosurg Pediatrics. 2008;1:131–7. doi: 10.3171/PED/2008/1/2/131. [DOI] [PubMed] [Google Scholar]
- 38.Kestle JR, Holubkov R, Douglas Cochrane D, Kulkarni AV, Limbrick DD, Jr, Luerssen TG, et al. A new Hydrocephalus Clinical Research Network protocol to reduce cerebrospinal fluid shunt infection. J Neurosurg Pediatr. 2015:1–6. doi: 10.3171/2015.8.PEDS15253. [DOI] [PubMed] [Google Scholar]
- 39.Simon TD, Hall M, Dean JM, Kestle JR, Riva-Cambrin J. Reinfection following initial cerebrospinal fluid shunt infection. J Neurosurg Pediatr. 2010;6:277–85. doi: 10.3171/2010.5.PEDS09457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallach J. Interpretation of Diagnostic Tests. 8. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 307. [Google Scholar]
- 41.Li V, Dias MS. The results of a practice survey on the management of patients with shunted hydrocephalus. Pediatr Neurosurg. 1999;30:288–95. doi: 10.1159/000028813. [DOI] [PubMed] [Google Scholar]
- 42.Marchaim D, Chopra T, Bhargava A, Bogan C, Dhar S, Hayakawa K, et al. Recent exposure to antimicrobials and carbapenem-resistant Enterobacteriaceae: the role of antimicrobial stewardship. Infection control and hospital epidemiology. 2012;33:817–30. doi: 10.1086/666642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA pediatrics. 2014;168:1063–9. doi: 10.1001/jamapediatrics.2014.1539. [DOI] [PubMed] [Google Scholar]
- 44.Horton DB, Scott FI, Haynes K, Putt ME, Rose CD, Lewis JD, et al. Antibiotic Exposure and Juvenile Idiopathic Arthritis: A Case-Control Study. Pediatrics. 2015;136:e333–43. doi: 10.1542/peds.2015-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics. 2012;130:e794–803. doi: 10.1542/peds.2011-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA : the journal of the American Medical Association. 1999;282:1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]