Abstract
Liposomes have been successfully employed in the clinic for several drugs to improve pharmacokinetics and reduce side effects. Furthermore, liposomes can be functionalized with antibodies for targeted delivery to cells that express the antigen. While the monoclonal antibody trastuzumab has been employed in the therapy of HER2-positive breast cancer, the resistance developed during treatment has been widely reported. Drugs, such as rapamycin, a lipophilic mTOR inhibitor, could be used in combination with trastuzumab for improved therapeutic response. In this study, we aimed to develop rapamycin-loaded liposomes and immunoliposomes with trastuzumab, characterize them and evaluate their in vitro cytotoxicity. Results showed that the SPC:Chol:DSPE-PEG formulation prepared at 1:10 drug to lipid ratio presented high encapsulation efficiency, appropriate particle size, low polydispersity, negative zeta potential and colloidal stability. Within the liposomes, rapamycin exhibited intermolecular interactions with lipids and underwent crystallinity reduction, indicated by FTIR and DSC. Rapamycin-loaded immunoliposomes were prepared with high trastuzumab functionalization efficiency, antibody stability and similar particle size, polydispersity and rapamycin encapsulation obtained with non-targeted liposomes. Cytotoxicity studies showed that the HER2-positive SK-BR-3 cell line was sensitive to trastuzumab, either as free drug or in the context of immunoliposomes, and is more sensitive to rapamycin than the triple negative MDA-MB-231 cells. For MDA-MB-231, the liposomal rapamycin was more cytotoxic than the free drug. Furthermore, the immunoliposomes showed potent cytotoxicity against SK-BR-3 cells. Finally, rapamycin and trastuzumab exhibited in vitro synergistic effect, particularly through immunoliposomes.
Keywords: Drug delivery, liposomes, immunoliposomes, breast cancer, rapamycin, trastuzumab
INTRODUCTION
Breast cancer is the leading type of cancer and the second most lethal cancer affecting women worldwide [1]. Treatment of breast cancer is complex and is influenced by the pathology of the tumor, and this disease is very heterogeneous. It typically involves partial or full mastectomy, as well as the removal of adjacent lymph nodes. Furthermore, radiotherapy and chemotherapy are employed, including cytotoxic and hormonal treatment, among others [2]. In this context, the presence of breast cancer tumor markers has been investigated in recent years and the identified markers are the estrogen receptor (ER), progesterone receptor (PR) and the human epidermal growth factor receptor 2 (HER2). The combination of these markers allows for classification of breast cancers into different groups, ER+ (ER+/HER2−), HER2− (ER−/HER2−), HER2+ (ER−/HER2+), triple negative (ER−/PR−/HER2−) and triple positive (ER+/PR+/HER2+) [3].
The HER2 receptor has an intracellular tyrosine kinase domain and an extracellular ligand binding domain, and is involved in important stages of growth and cell differentiation. As a key gene of cell survival, HER2 overexpression can lead to malignant progression, which is associated with unfavorable prognosis of breast, ovary, gastric, prostate cancers, among others [4]. HER2 overexpression is present in approximately 25% of all breast cancers and usually is associated with more aggressive disease and endocrine therapy resistance [5]. Studies showed that the inhibition of HER2 expression induced significant apoptosis in breast cancer cells and therefore HER2 became a target [6, 7]. A major advance in breast cancer treatment was the development of the humanized monoclonal antibody against the HER2 protein, named trastuzumab (Herceptin®), approved in 1998 by the FDA for treatment of HER2 positive breast cancer [8]. In metastatic breast cancer, trastuzumab is approved in combination with paclitaxel, with consequent improved efficacy and survival [9].
The mammalian target of rapamycin (mTOR) pathway plays a key role in regulating the translation of mRNA, protein synthesis, glucose metabolism, lipid synthesis and is involved in the malignant progression [10]. Consequently, the antitumor properties of the macrolide antibiotic rapamycin (also known as sirolimus), a lipophilic mTOR inhibitor used as immunosuppressant, have been extensively studied as an option for breast cancer treatment and promising results have been found, showing better patient prognosis [10, 11]. Clinical studies have demonstrated that rapamycin derivatives can overcome trastuzumab resistance [10]. Phase I and II clinical trials demonstrated that everolimus, a rapamycin derivative, combined with paclitaxel and trastuzumab, was well tolerated and presented promising results in patients with positive HER2 breast cancer. This combination is currently being investigated in a phase III clinical trial, BOLERO-1 [12, 13].
In the context of cancer treatment, nanostructured drug delivery systems have gained momentum in the research of new anticancer drugs. These systems can reduce drug side effects, increase blood circulation time, allowing for reduced dosage because of improved pharmacokinetics, and facilitate administration, assuring better patient compliance. At the tumor target, the delivery system can be passively accumulated by the enhanced permeability and retention (EPR) effect [14]. Several nanostructured delivery systems have been studied for cancer treatment, such as lipid systems, including liposomes and solid lipid nanoparticles, polymeric systems, such as polymeric nanoparticles and micelles, dendrimers and inorganic systems [15]. Among these systems, liposomes, phospholipid vesicular systems, are regarded as safe, effective, and are commercially available with a variety of hydrophilic and lipophilic drugs [16]. In this context, liposomes of different characteristics, particularly neutral and cationic, have been developed to systemically deliver drugs to tumors with high degree of success, in vitro and in vivo [17].
Monoclonal antibodies have recently been widely used for cancer treatment; however these macromolecules undergo rapid clearance in tumors. For instance, as an approach to circumvent this drawback, Wen et al., 2013 [18] developed a self-assembling injectable system to retain IgG antibodies in mammary tumors. Imunoliposomes, generated by the conjugation of monoclonal antibodies onto the surface of liposomes, are another approach for enhanced delivery using therapeutic antibodies. These ligands are specific for overexpressed receptors on cancer cells, allowing for targeted drug delivery, with consequent selective delivery of drugs to cancer cells. Therefore, this approach enables the reduction of side effects [19, 20]. Among these ligands, the antibody anti-HER2 is relevant for targeted delivery systems to breast cancer [4]. PEGylated immunoliposomes containing paclitaxel, functionalized with the antibody anti-HER2 were developed by Yang and collaborators [21]. In this study, it was shown efficient intracellular delivery through receptor-mediated endocytosis. Additionally, the authors showed that HER2-targeted immunoliposomes are promising for breast cancer treatment.
Previously, our research group developed co-loaded paclitaxel/rapamycin liposomes for breast cancer treatment, with promising in vitro and in vivo results Herein, we address the encapsulation of rapamycin in immunoliposomes functionalized with trastuzumab for HER2-positive breast cancer cells delivery, which, to our knowledge, has never been studied before [22]. Therefore, the purpose of this work is the development of rapamycin liposomes and rapamycin-loaded immunoliposomes targeted with trastuzumab. Herein, we report on the development, characterization and the in vitro efficacy evaluation of formulations against breast cancer.
MATERIAL AND METHODS
Materials
Soy phosphatidylcholine (SPC), cholesterol (Chol), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] (DSPE-PEG), 1,2-dioleoyl-sn-Glycero-3-Phosphoethanolamine (DOPE), dipalmitoylphosphatidylcholine (DDPC), and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) were supplied by Avanti polar lipids (Alabaster, AL, USA). Oleic acid (OA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Traut’s reagent were obtained from Sigma Aldrich Co. (St. Louis, MO, USA). 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N[maleimide(polyethylene glycol)-2000] (ammonium salt) (DSPE-PEG-Mal) was obtained from LaySan Bio (Arab, AL, USA). Rapamycin, BCA™ protein assay and solvents (chloroform, acetonitrile and DMSO) were obtained from Fisher (Pittsburgh, PA, USA). Trastuzumab (Herceptin®) was kind gift from Ohio State University clinical pharmacy. Precision Plus Protein Dual Color Standards, ready gel Tris-HCL precast gels, Laemmli sample buffer 4X, 2-mercaptoethanol and Tris/ Glycine/ SDS buffer 10X were supplied by Biorad (Hercules, CA, USA). Sepharose CL-4B and Disposable PD-10 desalting columns were purchased from GE Healthcare (Pittsburgh, PA, USA). Antibiotic/antimycotic solutions, fetal bovine serum (FBS), RPMI-1640 medium and McCoy’s 5A medium were obtained from Gibco (Grand Island, NY) MDA-MB-231 (ATCC®HTB-26tm) and SK-BR-3 (ATCC®HTB-30™ were cultivated according to the American Type Culture Collection (ATCC) instructions.
Development of Formulations Encapsulating Rapamycin
Liposomes with SPC and Chol, with or without DSPE-PEG, and liposomes containing DOPE and OA, DPPC, or DSPC were prepared with the employment the classical hydration of the thin lipid film method described by Bangham et al. [23]. The drug to lipid ratio influence was evaluated. Lipids and drug were solubilized in chloroform, which was rotary evaporated for 30 min at 65°C. Subsequently, the lipid film was submitted to hydration with the employment of phosphate buffer (pH 7.4) for 30 min at 100 rpm. Finally, the liposomes underwent extrusion for 5 times in 0.2 μm membrane and then extrusion was repeated 3 times in 0.1 μm membrane. The liposomes were separated from unencapsulated drug by gel filtration chromatography in CL-4B column. Noteworthy, liposomes containing RAP have already been reported by our research group [22], but the development of these formulations, i.e the different lipids and ratios tested, is first shown herein.
Development of Rapamycin-loaded Immunoliposomes
The functionalization of liposomes with trastuzumab was done according to the work by Laginha et al. (2005), with modifications [24]. The reaction was schematically represented in Figure 01. Initially, liposomes containing rapamycin were prepared with SPC, Chol and DSPE-PEG-Mal, according to the procedure previously described. The trastuzumab antibody was diluted in phosphate buffer pH 8.0 containing EDTA (5mM) and the pH of the solution was adjusted to 8.0 with NaOH (0.1 N). Traut’s reagent was added to the trastuzumab solution (1 mg/mL) at the ratio 40:1. This solution was incubated for 1h at 37°C for thiolation. The separation of the thiolated antibody from excess Traut’s reagent was performed in PD-10 column gel filtration, eluting with PBS-EDTA pH 8.0 buffer. Then, the trastuzumab concentration was determined using the BCA assay, the fractions containing the antibody (fractions 4-6) were combined and added to the previously prepared liposome for the reaction. The mixture was incubated overnight and, finally, the immunoliposomes were purified in CL-4B column.
Fig. (1).
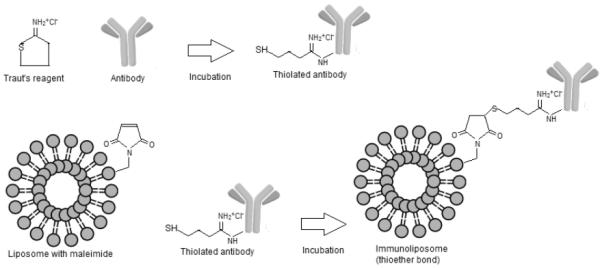
Schematic representation of the immunoliposome reaction.
Physicochemical Characterization of Formulations
Evaluation of Encapsulation Efficiency of Rapamycin in Liposomes
For the encapsulation efficiency analyses, the loaded liposome was separated from the unloaded drug by gel filtration chromatography. The total liposome (prior to separation from unencapsulated drug) and the liposome without free drug were solubilized using DMSO as solvent. Subsequently, the samples were dissolved with acetonitrile under sonication. Finally, the samples were filtered and then analyzed by spectrophotometry at 277 nm for the quantification [22]. Experiments were done in triplicate. The method was validated according to the ICH (Harmonized Tripartite Guidelines) [25]. The encapsulation efficiency (EE) was calculated as follows:
EE (%) = (drug in the formulation fraction (μg)) / (drug in the total fraction(μg)) × 100
Particle Size and Polydispersity
The particle size and polydispersity index of formulations were determined at 25°C, through dynamic light scattering, using the Zetasizer 3000 HSa (Malvern Instruments).
Zeta Potential
Surface charge of dispersions was measured as zeta potential Analyses were carried out using Zetasizer 3000 HSa equipment.
Fourier Infrared Spectroscopy (FTIR)
FTIR analyses were done using a Shimadzu IRPrestige-21 instrument in room temperature. The lyophilized samples were previously ground and mixed with potassium bromide. Scans were done from 4000 to 400 cm−1.
Differential Scanning Calorimetry (DSC)
DSC measurements were conducted using a Perkin Elmer, 8500 instrument. The lyophilized liposomes were placed in aluminum pans and heated from 15°C to 250°C at a rate of 10°C/min. Calibration was done with indium and n-octadecane.
Stability
For this evaluation, liposomes were analyzed for encapsulation efficiency, particle size and polydispersity index for 30 days of storage at 4°C.
Evaluation of Antibody Conjugation Efficiency in Immunoliposomes
Trastuzumab conjugation efficiency to liposomes was determined by BCA. Briefly, the working reagent was prepared through the mixture of reagents A and B, at the ratio 50:1. On 96-well microplates, 25 μL of each albumin standard (20 – 2000 μg/mL) or 25 μL of sample were added. On each well, 200 μL of working reagent were added and the microplate was homogenized for 30 seconds and incubated at 37°C for 30 min. After cooling, the absorbance was read at 562 nm in a microplate reader. The conjugation efficiency was calculated as the percentage ratio of antibody quantified in the immunoliposomes by the total antibody employed for conjugation.
Antibody Integrity Evaluation by SDS-PAGE Electrophoresis
The antibody integrity in immunoliposomes was investigated by polyacrylamide gel electrophoresis (SDS-PAGE) [21]. Samples were first incubated with 2-mercaptoethanol, a reducing agent, and Laemmli sample buffer. Then, samples were incubated at 60°C for 10 min. A Mini-PROTEAN electrophoresis chamber (Bio-Rad) SDS-Page gel 4-20% and Tris/glycine/SDS running buffer were employed. The electrophoresis was carried out at 175 V for 30 min. Precision Plus Dual Color Protein was employed as a protein ladder. After staining with Coomassie blue, gels were destained with deionized water and then photographed.
Cytotoxicity Study in MDA-MB-231 and SK-BR-3 Cell Lines
The formulations and the control groups were evaluated for cytotoxicity in MDA-MB231 (HER2−) and SK-BR-3 (HER2+) cell lines cultivated, respectively, in RPMI-1640 and McCoy's media supplemented with 10% FBS and 1% antibiotic/antimycotic solution. The cytotoxicity was evaluated through the MTT assay, previously described, and done with minor modifications [26, 27]. Briefly, cells were trypsinized after reaching 80% confluence and then were seeded (10.000 cells/well) onto 96-well flat-bottom tissue-culture plates and incubated in 5% CO2 incubator (or without CO2 for MDA-MB-231) for 24h for seeding. After this period, the complete culture medium was removed, the wells were washed with PBS pH 7.4, the diluted formulations/control groups were added and the plates were kept at 37°C in the incubator for 120h. Then, the wells were washed with PBS and incomplete fresh medium and MTT solution (2,5 mg mL−1 ) were added, followed by incubation for 4h. Finally, the medium with MTT was discarded and DMSO was added. Microplates were shaken and absorbance was read at 570 nm using a microplate reader. The IC50 values (i.e., concentration resulting in 50% growth inhibition) of rapamycin or trastuzumab were graphically calculated [28].
Combination Index Evaluation
The combination index (CI) of rapamycin and trastuzumab was investigated [29]. The CI was calculated using the equation below:
Where, (D)1 and (D)2 are the doses of drug 1 and drug 2 required for the IC50 effect when combined, and (Dx)1 and (Dx)2 represent the doses of drug 1 and drug 2 necessary to produce individual IC50. For the interpretation, CI<1 represents synergistic effect, CI>1 represents antagonism and CI equivalent to 1 represents additive effect [30].
RESULTS AND DISCUSSION
Rapamycin-loading studies were initiated with the determination of the best drug to lipid ratio, in order to achieve nanometric particle size and high loading efficiency (Figure 02). The results showed that the ratios 1:10 and 1:15 of drug/SPC resulted in small particle size and higher values of rapamycin encapsulation efficiency of close to 90%. These observations are in agreement with Haeri et al. (2011) [31], who concluded that the increase of the lipid ratio (DSPC) to rapamycin positively influenced rapamycin encapsulation. In that occasion, the authors employed the remote film loading method, which involves the preparation of a drug film and the addition of empty liposomes, as an alternative for the class thin film hydration method by extrusion, which resulted in low drug encapsulation efficiency. The authors argued that this shortcoming can be attributed to liposome retention in the extrusion membrane, because DSPC is a saturated and rigid lipid, unlike SPC, more flexible, employed herein.
Fig. (2).
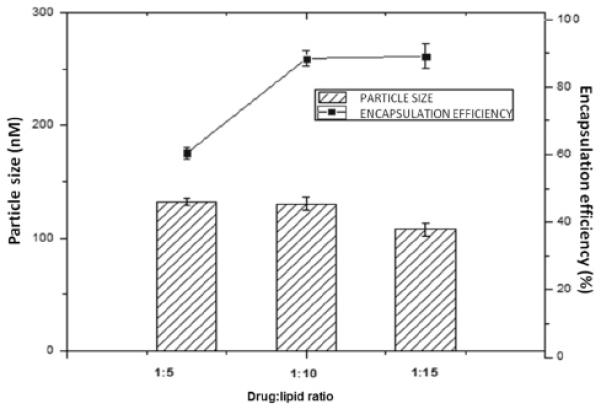
Influence of drug to lipid ratio on particle size and encapsulation efficiency.
Additionally, other lipids with different carbon chain lengths and polarities were tested, such as DSPC, DPPC or DOPE:OA, shown in Table 01, however the values of particle size of these formulations were larger. It should be considered that particle size, usually smaller than 200 nm, influences the pharmacokinetics of the drug. It is well known that nanoparticles are passively accumulated at the tumor site by the enhanced permeability and retention (EPR) effect [14]. Furthermore, other lipids rather than SPC resulted in decreased values of encapsulation efficiency, which is a major drawback. Therefore, it can be concluded that employing the classic method for liposome preparation, the lipid SPC resulted in better values of particle size, polydispersity index and encapsulation efficiency. SPC forms less rigid lipid bilayer, in which the lipophilic drug can partition, resulting in increased encapsulation efficiency. In addition, SPC may have better miscibility with the hydrophobic drug [32]. Regarding the zeta potential, the formulations exhibited negative values, due to the negative charge of lipids, particularly with DOPE:OA, which had a zeta potential of −48,73mV.
Table 1.
Physicochemical characterization of rapamycin and paclitaxel/rapamycin-loaded liposomes.
| Formulation | Encapsulation Efficiency (%) | Particle Size (nm) | Polydispersity | Zeta potential (mv) |
|---|---|---|---|---|
| DPPC:RAP (10:1) | 40.74 ± 7.45 | 145.91 ± 1.84 | 0.26 ± 0.05 | −14.30±1.95 |
| DSPC:RAP (10:1) | 44,23±5.98 | 202.65 ± 10.15 | 0.76 ± 0.05 | −14.77 ± 1.06 |
| DOPE:AO:RAP (10:2.5:1) | 45.32 ± 3.98 | 167.10 ± 1.84 | 1.16 ± 0.01 | −48.73 ± 2.14 |
| SPC:RAP (10:1) | 88.42 ± 4.92 | 130.15 ± 1.20 | 0.32 ± 0.02 | −35.96 ± 2.60 |
| SPC:Chol:RAP (10:1:1) | 48.42 ± 5.67 | 158.60 ± 10.93 | 0.36 ± 0.09 | −27.83 ± 1.25 |
| SPC:Chol:RAP (10:2:1) | 66.67 ± 6,76 | 142.15 ± 6.43 | 0.24 ± 0.04 | −27.50 ± 0.78 |
| SPC:Chol :RAP(10:3:1) | 62.67 ± 9,34 | 135.25 ± 10.45 | 0.41 ± 0.01 | −26.23 ± 0.70 |
| SPC:Chol:DSPE-PEG2000:RAP (10:2:0.5:1) | 59.58 ± 4.97 | 133.84 ± 8.35 | 0.26 ± 0.01 | −3.51 ± 0.24 |
Because SPC was shown to be more suitable to encapsulate rapamycin, this lipid was chosen for the addition of Chol in different ratios. The addition of Chol resulted in reduction of rapamycin encapsulation, particularly when 10 mol% was added. Chol has been already demonstrated to compete with paclitaxel, another lipophilic drug, for encapsulation. Furthermore, due to Chol rigidity, liposomal flexibility is compromised, which could influence drug encapsulation [33]. The formulations prepared with SPC:Chol at the ratio of 10:2 and 10:3 presented encapsulation efficiency at about 60%, however slightly higher for the formulation prepared with Chol 20mol%, which was the concentration selected for further studies. Haeri and collaborators reported as 25 mol% the maximum percentage of Chol that can be used to prepare RAP-loaded liposomes, because in higher amount Chol can lead to aggregation [31]. Although Chol has been reported to compete with hydrophobic drugs for encapsulation, such as paclitaxel, it must be taken into consideration that Chol is also important for liposomal stability [34]. Therefore, the formulations prepared with 10:2 and 10:3 (SPC:Chol) might have benefited to some extent from the improved stability of Chol and thus presented higher rapamycin encapsulation efficiency compared to the formulation prepared with 10:1(SPC:Chol). Furthermore, Chol influence in the encapsulation efficiency of molecules is rather complex and not straightforward, considering its influence in membrane organization, dynamics and function. In this context, Chol has been shown to either increase or decrease drug encapsulation efficiency, making it difficult to generalize Chol influence [16]. Particle size and polydispersity index values were close to the formulation without Chol and zeta potential remained negative.
DSPE-PEG 2000 can be added to the surface of liposomes for extended blood circulation times, because polyethylene glycol (PEG), a flexible hydrophilic polymer, reduces the nanoparticle uptake by the mononuclear phagocytic system (MPS). The consequence is the increased biological half-life and accumulation of liposomes in solid tumors via the EPR effect [35]. DSPE-PEG 2000 added to the SPC:Chol (10:2) liposome did not substantially change the physicochemical parameters of liposomes, however neutral zeta potential emerged. Noteworthy, the RAP-loaded liposome, based on a SPC:Chol:DSPE:PEG-2000 composition, has been reported previously [22], but the rationale for its development is shown herein for the first time.
FTIR analysis provides information on chemical bonding, functional groups and presence of intermolecular interactions. Changes in the spectrum confirm the interactions, which are visualized as new bands, bands disappearing, a shift or widening of the existing bands, which might modify their intensity [36]. It is known that the temperature can influence Raman peaks and widths, as discussed by Xu et al, 2010 [37]. Considering that FTIR and Raman are similar and complementary techniques, all the experiments were done at room temperature. In Figure 03, we present the FTIR spectra of rapamycin, blank liposome and rapamycin-encapsulated liposome. Bands attributed to rapamycin were at 1444, 1738 e 2934 cm−1, corresponding, respectively to the CH2 bond adjacent to the carbonyl, carbonyl bond axial deformation, and C-H aliphatic bond. These bands attributed to rapamycin were less intense in drug-loaded formulation, as previously reported by our research group [22]. Thus, the chemical structure of rapamycin appears not to be affected by the addition of the excipients and the process employed in the liposome preparation, similar to the observations by Devi and Gayatheri, in 2010, who studied paclitaxel-loaded polymeric nanoparticles [38]. Furthermore, it was noted a broadening and a slight change in the carbonyl peak in the rapamycin-loaded liposomes to a reduced wavelength, at 1717 cm−1, which could be attributed to hydrogen bonding of the carbonyl group with OH groups in the formulation or with residual H2O molecules in the more condensed bilayers at low temperatures [39].
Fig. (3).
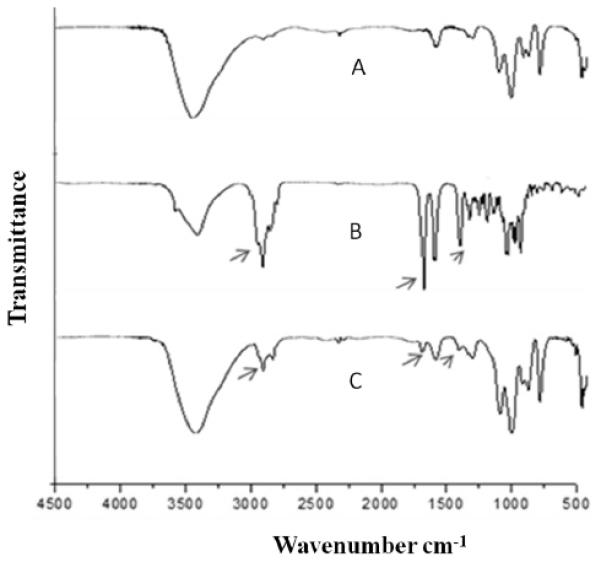
Infrared spectra of blank liposome (A) rapamycin (B), and rapamycin-loaded liposome (C). respectively. Peaks of interest were highlighted.
Thermoanalytical methods, such as DSC, have been employed to elucidate interactions between the drug and the nanocarrier [40, 41]. Herein, rapamycin presented two endothermic bands at 187 e 196°C, corresponding to the crystal fusion (Figure 04). The divided peak is probably related to two different crystalline forms, as discussed by Rouf et al, 2011 [42]. In the liposome containing rapamycin, the bands related to the crystalline drug melting shifted to more broadened bands at a higher temperature, around 225°C [22], which could indicate that rapamycin was converted from the crystalline to the amorphous form in the delivery system, similarly to the study by Yao et al., 2014, who concluded that lactoferrin was converted from the crystalline to the amorphous form when loaded in liposomes [43]. Amorphous drugs are more soluble and thus could present enhanced bioavailability [44].
Fig. (4).
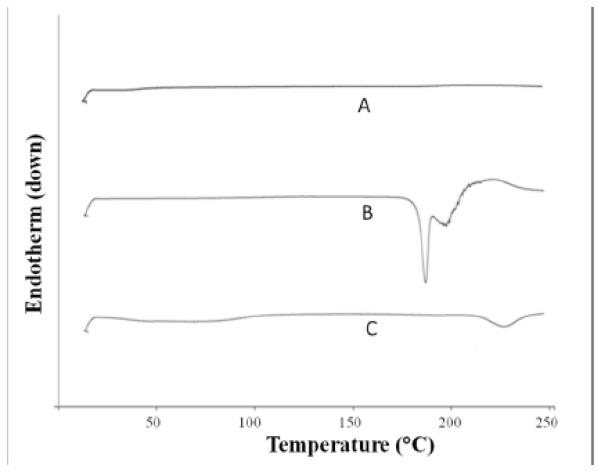
DSC curves obtained from blank liposome (A), rapamycin (B) and rapamycin-loaded liposome (C).
Finally, the colloidal stability of the PEGlyated liposome containing rapamycin was investigated (Figure 05). The particle size, polydispersity and encapsulation efficiency remained similar after 1 month at 4°C, which was regarded as an excellent stability of the formulations.
Fig. (5).
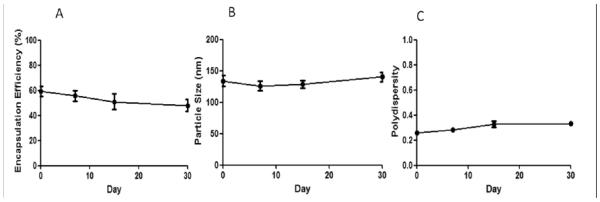
Colloidal stability of rapamycin-loaded liposomes at 4°C for 1 month through the evaluation of encapsulation efficiency (A), particle size (B) and polydispersity (C).
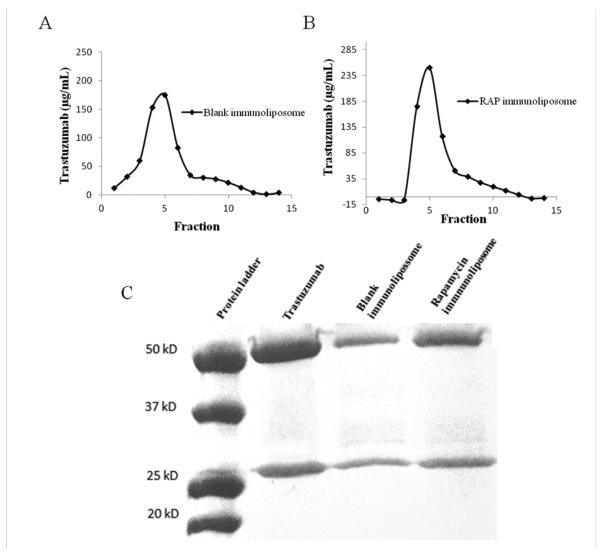
Liposomal drug delivery is considered suitable for cancer therapy, among other factors, because of improved safety profile of the encapsulated drug. In this context, several approaches have been reported to improve specificity and therapeutic efficacy of liposomal drug formulations, one of which is surface functionalization with antibodies to prepare immunoliposomes [45]. For the coupling of antibodies to liposomes, the antibody can be either post inserted in the liposome with micelles or can be directly linked to the liposome by covalent conjugation to groups present on the liposome surface [46]. Various covalent coupling methods have been reported for attaching antibodies at the PEG terminus using functionalized PEG-lipids with a chemically reactive end-group such as hydrazide, N-3-(pyridyldithio)proprionate, maleimide, succinyl, etc [47]. In this protocol the antibody must be derivatized in order to be linked to the lipid moieties. One of the most employed derivatization techniques, employed herein, uses the Traut’s reagent, which generates thiol groups in the antibody, allowing for the covalent bond through reaction with the maleimide in the DSPE-PEG-maleimide lipid [48, 49]. In the present work, the thiolated trastuzumab was successfully conjugated to DSPE-PEG-mal on the lipid bilayer surface via covalent bond. The functionalization efficiency corresponded to 62.78% and 80.49%, respectively for the blank immunoliposome and the rapamycin-loaded immunoliposome, evaluated through the quantification of protein in the immunoliposome fractions in relation to the total protein (Figure 06 and Table 02).
Fig. (6).
Chromatograms of blank immunoliposome (A) and rapamycin loaded immunoliposomes (B) eluted in CL-4B columns with PBS pH 7.4. Fractions from 4-6 are attributed to the immunoliposomes. C represents the trastuzumab integrity evaluation by SDS-PAGE electrophoresis under reducing conditions at 175V for 30 min.
Table 02.
Development of rapamycin-loaded immunoliposomes: functionalization efficiency, encapsulation efficiency, particle size, polydispersity and zeta potential.
| Formulation | Functionalization Efficiency (%) | Encapsulation Efficiency (%) | Particle Size (nm) | Polydispersity | Zeta potential (mv) |
|---|---|---|---|---|---|
| Blank Immunoliposome | 62.78 | * | 168.2 ± 12.6 | 0.376 ± 0.02 | −9.44 ± 0.33 |
| Immunoliposome RAP | 80.49 | 50.28 ± 3.54 | 138.2 ± 5.9 | 0.309 ± 0.03 | −10.50 ± 0.66 |
Trastuzumab integrity after liposome functionalization was confirmed by SDS-PAGE electrophoresis under reducing conditions (Figure 06). As expected, two distinct bands were visualized at 50 kDa and 25 kDa, corresponding, respectively, to the heavy and light chains of the antibody, which indicates that trastuzumab was incorporated intact to the liposome surface, similarly to the observations by Yang et al. (2007), who developed trastuzumab immunoliposomes containing paclitaxel [21].
Results in Table 02 also showed that the particle size, polydispersity and zeta potential remained approximately at 150 nm, 0.3 and −10 mV, respectively. Compared to the non-targeted liposomes, the particle size and polydispersity were slightly larger, while the zeta potential assumed a more negative charge, probably because of the charge imparted by the antibody. Very importantly, the encapsulation efficiency of rapamycin was maintained after the antibody conjugation. Thus, considering both the encapsulation and targeting efficiency values, the final doses of rapamycin and trastuzumab are, respectively, 5.77 mg and 3.93 mg per 100 mg of immunoliposomes.
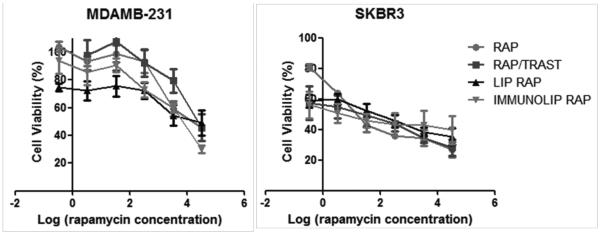
The cytotoxicity of rapamycin, trastuzumab and formulations were evaluated in MDA-MB-231, a HER2− cell line, and SK-BR-3, a HER2+ cell line (Figure 07 and Table 03). Interestingly, we observed that MDA-MB-231 cell line is much more resistant to either pure drug or liposomal rapamycin. Zheng et al. (2011) discussed that the human MDA-MB-231 cell line is resistant to rapamycin treatment in vitro because of some characteristics that contribute to its rapamycin-resistant molecular mechanism, including low activity of Akt, high level of phospholipase D (PLD), negative estrogen receptor, and low expression of HER2 [50]. SK-BR-3 higher sensitivity to rapamycin can be explained by HER2 amplification that activates the mTOR signaling pathway [51].
Fig. (7).
Cytotoxicity curves obtained with the MTT assay after 120h of incubation of rapamycin, rapamycin/trastuzumab, liposomes and immunoliposomes in MDA-MB-231 and SK-BR-3 cell lines.
Table 3.
IC50 values of drugs in solution, liposome or immunoliposomes.
| Group | IC50 (nM) | |
|---|---|---|
| MDAMB-231 | SKBR3 | |
| RAP | 38100.75 | 58.69 |
| TRAST | * | 0.46 |
| RAP/TRAST (IC50 RAP) | 39099.67 | 13.42 |
| RAP/TRAST (IC50 TRAST) | * | 0.028 |
| LIP RAP | 25511.78 | 53.09 |
| BLANK IMMNOLIP | * | 2.45 |
| IMMUNOLIP RAP (IC50 RAP) | 28122,16 | 7.56 |
| IMMUNOLIP RAP (IC50 TRAST) | * | 0.016 |
The rapamycin-loaded liposome had equivalent cytotoxicity of rapamycin free drug in SK-BR-3, whereas in MDA-MB-231 rapamycin-loaded liposome was more cytotoxic, which represents an advantage of rapamycin liposomal encapsulation and is in agreement with a previous report on rapamycin-loaded pH sensitive liposome [52]. However, other advantages of drug encapsulation besides improved cytotoxicity must be taken into consideration, such as the likely improved pharmacokinetics and decreased side effects. For instance, Doxil, the first liposome commercially available, composed by HSPC, Chol and DSPE-PEG, is considerably less toxic to OV-1063 cells than doxorubicin free drug. On that occasion, the authors concluded that lipids with higher temperature of phase transition, such as HSPC, released the drug more slowly and thus resulted in decreased cytotoxicity [53]. Despite showing less cytotoxicity, and also not presenting better clinical efficacy, as further demonstrated, Doxil is able to reduce acute cardiotoxicity better than the free drug.
Combination therapy in cancer treatment is commonly used to target malignancies resistant to standard treatment, especially if the drugs have different mode of action [54]. The results evidenced that the combination of trastuzumab with rapamycin as free drugs or immunoliposome did not enhance their cytotoxic effect in MBA-MB231, confirming the expectations for a HER2− cell line. On the other hand in SK-BR-3, a HER2+ cell line, it was proven herein that the combination of trastuzumab with rapamycin in solution and in immunoliposomes increased the cytotoxicity of both rapamycin and trastuzumab (Figure 07 and Table 03). Although the immunoliposome cytotoxicity was not evaluated in normal cells, we expect that the toxicity would be decreased, considering that normal cells are growing slower than cancer cells and also normal cells do not present the HER2 receptor, necessary for enhanced uptake of the targeted liposome. For instance, Wang et al. 2010 showed enhanced binding and killing of target cells by drug-loaded liposomes functionalized with tumor-specific phage fusion coat protein [55]. As a consequence, when compared to breast cancer MCF-7 cells, the cytotoxicity was not significantly higher in NIH3T3 and C166 cells, respectively mouse fibroblasts and mouse yolk sac endothelial cells.
The CI determination of rapamycin and trastuzumab was done according to the method proposed by Chou and Talalay [29]. It should be noted that the presence of trastuzumab caused a potent enhancement of the cytotoxic effect of rapamycin in SK-BR-3 breast cancer cell line. The synergic effect was greater with drug combination in immunoliposomes than in solution, with CI values of 0.15 and 0.29, respectively, showing a potential advantage of the immunoliposome formulation to treat HER+ breast cancer.
The combination of rapamycin and trastuzumab is shown to be clinically relevant. Recently, a phase II study demonstrated that rapamycin administered daily with trastuzumab was well tolerated in patients. The authors suggested that mTOR inhibition may overcome resistance to trastuzumab in some HER2-positive tumors [56]. Other phase I and II clinical trials have demonstrated that everolimus, a rapamycin derivative, combined with paclitaxel and trastuzumab, was well tolerated and presented promising results in patients with HER2 positive breast cancer. Currently, this combination is being investigated in a phase III clinical trial, BOLERO-1 [12, 13].
5. CONCLUSION
Herein, it was shown that lower drug-to-lipid ratios enable better rapamycin encapsulation. Lipids with higher temperature of phase transition resulted in more polydisperse liposomes with larger particle size and lower rapamycin encapsulation. Liposome formulation composed of SPC:Chol:DSPE-PEG (10:2:0.5) prepared at 1:10 drug to lipid ratio had suitable physicochemical characterization parameters. Rapamycin-loaded immunoliposome was developed with high trastuzumab functionalization efficiency, high antibody stability after the functionalization process and similar particle size, polydispersity and rapamycin encapsulation obtained with the liposome. In vitro studies with MDA-MB-231 and SK-BR-3 breast cancer cell lines showed that SK-BR-3 is more sensitive to rapamycin and that liposomal rapamycin is more cytotoxic than rapamycin free drug to MDA-MB-231. Furthermore, the immunoliposomes showed potent cytotoxicity in SK-BR-3, a HER2+ cell line. Finally, rapamycin and trastuzumab exhibited in vitro synergistic effect in SK-BR-3 cells, particularly in the form of immunoliposomes.
ACKNOWLEDGEMENTS
We thank Dr. Yassuro Sugimoto for his assistance. The authors also thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for grants # 2012/10388-3, # 2012/21513-3 and #2013/05362-8.
Biography

Josimar O. Eloy

Robert J. Lee
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- [1].Chen Z, Wang Y, Warden C, Chen S. Cross-talk between ER and HER2 regulates c-MYC-mediated glutamine metabolism in aromatase inhibitor resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 2015;149:118–127. doi: 10.1016/j.jsbmb.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tanaka T, Decuzzi P, Cristofanilli M, Sakamoto JH, Tasciotti E, Robertson FM, Ferrari M. Nanotechnology for breast cancer therapy. Biomed. Microdevices. 2009;11:49–63. doi: 10.1007/s10544-008-9209-0. [DOI] [PubMed] [Google Scholar]
- [3].Bertos NR, Park M. Breast cancer - one term, many entities? J. Clin. Invest. 2011;121:3789–3796. doi: 10.1172/JCI57100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tai W, Mahat R, Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J. Control. Rel. 2010;146:264–275. doi: 10.1016/j.jconrel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Engel RH, Kaklamani VG. HER2-positive breast cancer: Current and future treatment strategies. Drugs. 2007;67:1329–1341. doi: 10.2165/00003495-200767090-00006. [DOI] [PubMed] [Google Scholar]
- [6].Faltus T, Yuan J, Zimmer B, Krämer A, Loibl S, Kaufmann M, Strebhardt K. Silencing of the HER2/neu gene by siRNA inhibits proliferation and induces apoptosis in HER2/neu-overexpressing breast cancer cells. Neoplasia. 2004;6:786–795. doi: 10.1593/neo.04313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang G, Ca i K.Q., Thompson-Lanza JA, Bast RC, Jr., Liu J. Inhibition of breast and ovarian tumor growth through multiple signaling pathways by using retrovirus-mediated small interfering RNA against Her-2/neu gene expression. J. Biol. Chem. 2004;279:4339–4345. doi: 10.1074/jbc.M311153200. [DOI] [PubMed] [Google Scholar]
- [8].Shawver LK, Slamon D, Ulrich D. Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell. 2002;2:117–123. doi: 10.1016/s1535-6108(02)00039-9. [DOI] [PubMed] [Google Scholar]
- [9].Chang HR. Trastuzumab-Based Neoadjuvant Therapy in Patients With HER2-Positive Breast Cancer. Cancer. 2010;116:2856–2867. doi: 10.1002/cncr.25120. [DOI] [PubMed] [Google Scholar]
- [10].Vicier C, Dieci MV, Arnedos M, Delaloge S, Viens P, Andre F. Clinical development of mTOR inhibitors in breast cancer. Breast Cancer Res. 2014;16:1–9. doi: 10.1186/bcr3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Blanco E, Sangai T, Wu S, Hsiao A, Ruiz-Esparza GU, Gonzalez-Delgado CA, Cara FE, Granados-Principal S, Evans KW, Akcakanat A, Wang Y, Do KA, Meric-Bernstam F, Ferrari M. Colocalized delivery of rapamycin and paclitaxel to tumors enhances synergistic targeting of the PI2K/AKt/mTOR pathway. Mol Therapy. 2014;22:1310–1319. doi: 10.1038/mt.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Andre F, Campone M, O'Regan R, Manlius C, Massacesi C, Sahmoud T, Mukhopadhyay P, Soria JC, Naughton M, Hurvitz SA. Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J. Clin. Oncol. 2010;28:5510–5115. doi: 10.1200/JCO.2009.27.8549. [DOI] [PubMed] [Google Scholar]
- [13].Hurvitz SA, Dalenc F, Campone M, O'Regan RM, Tjan-Heijnen VC, Gligorov J, Llombart A, Jhangiani H, Mirshahidi HR, Tan-Chiu E, Miao S, El-Hashimy M, Lincy J, Taran T, Soria JC, Sahmoud T, André F. A phase 2 study of everolimus combined with trastuzumab and paclitaxel in patients with HER2-overexpressing advanced breast cancer that progressed during prior trastuzumab and taxane treatment. Breast Cancer Res. Ther. 2013;141:437–446. doi: 10.1007/s10549-013-2689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Maeda H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Rel. 2012;164:138–44. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- [15].Orive G, Hernández RM, Gascón AR, Pedraz JR. Micro and nano drug delivery systems in cancer therapy. Cancer ther. 2005;3:131–138. [Google Scholar]
- [16].Eloy JO, Souza MC, Petrilli R, Barcellos JPA, Lee RJ, Marchetti JM. Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids and Surfaces B: Biointerf. 2014;123:345–363. doi: 10.1016/j.colsurfb.2014.09.029. [DOI] [PubMed] [Google Scholar]
- [17].Wen Y, Meng WS. Recent In Vivo Evidences of Particle-Based Delivery of Small-Interfering RNA (siRNA) into Solid Tumors. J. PharmaceuticalInnovat. 2014;9(2):158–173. doi: 10.1007/s12247-014-9183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wen Y, Kolonich HR, Kruszevsky KM, Giannoukakis N, Gawalt ES, Meng WS. Retaining Antibodies in Tumors with Self-Assembling Injectable System. Mol. Pharmaceut. 2013;10:1035–1044. doi: 10.1021/mp300504z. [DOI] [PubMed] [Google Scholar]
- [19].Park JW, Kirpotin DB, Hong K, Shalaby R, Shao Y, Nielsen UB, Marks JD, Papahadjopoulos D, Benz CC. Tumor targeting using anti-her2 immunoliposomes. J. Control. Rel. 2001;74:95–113. doi: 10.1016/s0168-3659(01)00315-7. [DOI] [PubMed] [Google Scholar]
- [20].Hirsjarvi S, Passirani C, Benoit JP. Passive and active tumour targeting with nanocarriers. Curr. Drug Disc. Technol. 2011;8:188–196. doi: 10.2174/157016311796798991. [DOI] [PubMed] [Google Scholar]
- [21].Yang T, Choi MK, Cui FD, Kim JS, Chung SJ, Shim CK, Kim DD. Preparation and evaluation of paclitaxel-loaded PEGylated immunoliposome. J. Control. Rel. 2007;120:169–177. doi: 10.1016/j.jconrel.2007.05.011. [DOI] [PubMed] [Google Scholar]
- [22].Eloy JO, Petrilli R, Topan JF, Antonio HMR, Barcellos JPA, Chesca DL, Serafini LN, Tiezzi DG, Lee RJ, Marchetti JM. Co-loaded paclitaxel/rapamaycin liposomes: Development, characterization and in vitro and in vivo evaluation for breast cancer therapy. Colloids and Surfaces B: Biointerfaces. 2016;141:74–82. doi: 10.1016/j.colsurfb.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bangham M, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- [24].Laginha K, Mumbengegwi D, Allen T. Liposomes targeted via two different antibodies: Assay, B-cell binding and cytotoxicity. Biochimica etBiophysica Acta. 2005;1711:25–32. doi: 10.1016/j.bbamem.2005.02.007. [DOI] [PubMed] [Google Scholar]
- [25].ICH Harmonised Tripartite Guideline. Validation of analytical procedures: text and methodology Q2(R1); International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (ich); 2005. Current Step 4 version. [Google Scholar]
- [26].Zhang YP, Kong QH, Huang Y, Wang GL, Chang KJ. Inhibition of c-FLIP by RNAi enhances sensitivity of the human oesteogenic sarcoma cell line U2OS to TRAIL-induced apoptosis. Asi. Pacific J. Cancer Prevention. 2015;16(6):2251–2256. doi: 10.7314/apjcp.2015.16.6.2251. [DOI] [PubMed] [Google Scholar]
- [27].Schroeder BR, Ghare MI, Bhattacharya C, Paul R, Yu Z, Zaleski PA, Bozeman TC, Rishel MJ, Hecht SM. The Disaccharide Moiety of Bleomycin Facilitates Uptake by Cancer Cells. J. Am. Chem. Society. 2014;136:13641–13656. doi: 10.1021/ja507255g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sharma A, Sharma US, Straubinger RM. Paclitaxel-liposomes for intracavity therapy of intraperitoneal P388 leukemia. Cancer Lett. 1996;107:265–272. doi: 10.1016/0304-3835(96)04380-7. [DOI] [PubMed] [Google Scholar]
- [29].Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzy. Reg. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- [30].Jaafari A, Tilaoui M, Mouse HA, M’bark LA, Aboufatima R, Chait A, Lepoivre M, Zyad A. Comparative study of the antitumor effect of natural monoterpenes: Relationship to cell cycle analysis. Brazilian J. Pharmacog. 2012;22(3):5340–540. [Google Scholar]
- [31].Haeri A, Sadeghian S, Rabbani S, Anvari MS, Boroumand MA, Dadashzadeh S. Use of remote film loading methodology to entrap sirolimus into liposomes: preparation, characterization and in vivo efficacy for treatment of restenosis. Int. J. 2011;414:16–27. doi: 10.1016/j.ijpharm.2011.04.055. [DOI] [PubMed] [Google Scholar]
- [32].Desai TR, Wong JP, Hancock RE, Finlay WH. A novel approach to the pulmonary delivery of liposomes in dry powder form to eliminate the deleterious effects of milling. J. Pharmaceut. Sci. 2002;91:482–491. doi: 10.1002/jps.10021. [DOI] [PubMed] [Google Scholar]
- [33].Zhang JA, Anyarambhatla G, Ma L, Ugwu S, Xuan T, Sardone T, Ahmad I. Development and characterization of a novel Cremophor EL free liposome-based paclitaxel (LEP-ETU) formulation. Europ. J. Pharmaceut. Biopharmaceut. 2005;59:177–187. doi: 10.1016/j.ejpb.2004.06.009. [DOI] [PubMed] [Google Scholar]
- [34].Kirby C, Clarke J, Gregoriadis G. Effect of the Cholesterol Content of Small Unilamellar Liposomes on their Stability in vivo and in vitro. Biochem. J. 1980;186:591–598. doi: 10.1042/bj1860591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yang T, Cui FD, Choi MK, Cho JW, Chung SJ, Shim CK, Kim DD. Enhanced solubility and stability of PEGylated liposomal paclitaxel: In vivo and In vitro evaluation. Int. J. Pharmaceut. 2007;338:317–326. doi: 10.1016/j.ijpharm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- [36].Emani S, Valizadeh H, Islambulchilar Z, Zakeri-Milani P. Development and Physicochemical Characterization of Sirolimus Solid Dispersions Prepared by Solvent Evaporation Method. Adv. Pharmaceut. Bulletin. 2014;4(4):369–374. doi: 10.5681/apb.2014.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xu H, Alur S, Wang Y, Cheng A-J, Kang K, Sharma Y, Park M, Ahyi C, Williams J, Gu C, Hanser A, Paskova T, Preble EA, Evans KR, Zhow Y. In situ Raman Analysis of a Bulk GaN-Based Schottky Rectifier Under Operation. J. Elect. Mat. 2010;39(10):2237–2242. [Google Scholar]
- [38].Devi TSR, Gayathri S. FTIR and FT-RAMAN spectral analysis of paclitaxel drugs. Int. J. Pharmaceut. Sci. Res. 2010;2:106–110. [Google Scholar]
- [39].Popova AV, Hincha DK. Intermolecular Interactions in Dry and Rehydrated Pure and Mixed Bilayers of Phosphatidylcholine and Digalactosyldiacylglycerol: A Fourier Transform Infrared Spectroscopy Study. Biophys. J. 2003;85:1682–1690. doi: 10.1016/S0006-3495(03)74598-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mainardes RM, Gremião MPD, Evangelista RC. Thermoanalytical studies of praziquantel-loaded PLGA nanoparticles. Brazilian J. Pharmaceut. Sci. 2006;42(4):523–530. [Google Scholar]
- [41].Kumar VD, Verma PRP, Singh SK. Development and evaluation of biodegradable polymeric nanoparticles for the effective delivery of quercetin using a quality by design approach LWT. Food Science and Technol. 2015;61:330–338. [Google Scholar]
- [42].Rouf MA, Vural I, Billensoy E, Hincal A, Erol DD. Rapamycin-cyclodextrin complexation: improved solubility and dissolution rate. J. Inclusion Phenomena and Macrocyclic Chem. 2011;70:167–175. [Google Scholar]
- [43].Yao X, Bunt C, Cornish J, Quek S-Y, Wen J. Preparation, Optimization and Characterization of Bovine Lactoferrin-loaded Liposomes and Solid Lipid Particles Modified by Hydrophilic Polymers Using Factorial Design. Chem. Biol. Drug Des. 2014;85:560–575. doi: 10.1111/cbdd.12269. [DOI] [PubMed] [Google Scholar]
- [44].Eloy JO, Saraiva J, Albuquerque S, Marchetti JM. Solid dispersion of ursolic acid in Gelucire 50/13: a strategy to enhance drug release and trypanocidal activity. AAPS Pharm.Sci.Tech. 2012;13(4):1436–1445. doi: 10.1208/s12249-012-9868-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Limasale YDP, Tezcaner A, Ozen A, Keskin D, Banerjee S. Epidermal growth factor receptor-targeted immunoliposomes for delivery of celecoxib to cancer cells. Int. J. Pharmaceut. 2015;479:364–373. doi: 10.1016/j.ijpharm.2015.01.016. [DOI] [PubMed] [Google Scholar]
- [46].Pan X, Wu G, Yang W, Barth RF, Tjarks W, Lee RJ. Synthesis of cetuximab-immunoliposomes via cholesterol-based membrane anchor for targeted delivery of a neutron capture therapy (NCT) agent to glioma cells. Bioconjugate Chem. 2007;18:101–108. doi: 10.1021/bc060174r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schnyder A, Krähenbühl S, Török M, Drewe J, Huwyler J. Targeting of skeletal muscle in vitro using biotinylated immunoliposomes. Biochem. J. 2004;377:61–67. doi: 10.1042/BJ20031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proceeding of the National Academy of Sciences of the United States of America. 1996;93(24):14164–14169. doi: 10.1073/pnas.93.24.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Barrajón-Catalán E, Menéndez-Gutiérrez MP, Falcó A, Saceda M, Catania A, Micol V. Immunoliposomes: A Multipurpose Strategy in Breast Cancer Targeted Therapy. Breast Cancer: Current and Alternative Therapeutic Modalities. 2011:437–452. ISBN 978-953-307-776-5. [Google Scholar]
- [50].Zeng Q, Yang Z, Gao YJ, Yuan H, Cui K, Shi Y, Wang H, Huang X, Wong ST, Wang Y, Kesari S, Ji RR, Xu X. Treating triple-negative breast cancer by a combination of rapamycin and cyclophosphamide: An in vivo bioluminescence imaging study. Europ. J. Cancer. 2010;46:1132–1143. doi: 10.1016/j.ejca.2010.01.014. [DOI] [PubMed] [Google Scholar]
- [51].Miller TW, Forbes JT, Shah C, Wyatt SK, Manning HC, Olivares MG, Sanchez V, Dugger TC, de Matos Granja N, Narasanna A, Cook RS, Kennedy JP, Lindsley CW, Arteaga CL. Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2-overexpressing cancer cells. Clin. Cancer Res. 2009;15:7266–7276. doi: 10.1158/1078-0432.CCR-09-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ghanbarzadeh S, Arami S, Pourmoazzen Z, Khorrami A. Improvement of the antiproliferative effect of Rapamycin on tumor cell lines by poly (monomethylitaconate)-based pH-sensitive, plasma stable liposomes. Colloids and Surfaces B: Biointerfaces. 2014;115:323–330. doi: 10.1016/j.colsurfb.2013.12.024. [DOI] [PubMed] [Google Scholar]
- [53].Horowitz AT, Barenholz Y, Gabizon AA. In vitro cytotoxicity of liposome-encapsulated doxorubicin: Dependence on liposome composition and drug release. Biochimica et Biophisica acta. 1992;U09:203–209. doi: 10.1016/0005-2736(92)90084-y. [DOI] [PubMed] [Google Scholar]
- [54].He X, Wang Y, Zhu J, Orloff M, Eng C. Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling. Cancer Lett. 2011;301:168–176. doi: 10.1016/j.canlet.2010.11.012. [DOI] [PubMed] [Google Scholar]
- [55].Wang T, D’Souza GG, Bedi D, Fagbohun OA, Potturi LP, Papahadjopoulos-Sternberg B, Petrenko VA, Torchilin VP. Enhanced binding and killing of target tumor cells by drug-loaded liposomes modified with tumor-specific phage fusion coat protein. Nanomedicine. 2010;5(4):563–574. doi: 10.2217/nnm.10.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Acevedo-Gadea C, Hatzis C, Chung G, Fishback N, Lezon-Geyda K, Zelterman D, DiGiovanna MP, Harris L, Abu-Khalaf MM. Sirolimus and trastuzumab combination therapy for HER2-positive metastatic breast cancer after progression on prior trastuzumab therapy. Breast Cancer Res. Treat. 2015;150:157–167. doi: 10.1007/s10549-015-3292-8. [DOI] [PubMed] [Google Scholar]