Abstract
We sought to estimate the prevalence of autism spectrum disorder (ASD) in children born extremely preterm relative to the 1.5% risk of ASD in the general U.S. population. 889 of 966 (92%) 10-year-old children from the Extremely Low Gestational Age Newborn birth cohort, delivered at 23–27 weeks gestation in 2002–2004, participated. 26 participants were not assessed for ASD because their severe sensory or motor impairment prevented a valid assessment of ASD. In the remaining sample, 61 children met criteria for ASD, resulting in a prevalence of 7.1% (approximately 7 in 100 children). ASD risk decreased with increasing gestational age, from 15.0% for 23–24 weeks, 6.5% for 25–26 weeks, to 3.4% for 27 weeks gestational age. The association we found between children's ASD risk and lower gestational age was independent of IQ. Among children diagnosed with ASD, 40% had intellectual disability. The male-to-female ratio of children with ASD was approximately 2 to 1, lower than in the general population in which ASD is estimated to be 3 to 4 times more common in males. ASD prevalence in the ELGAN cohort was 4 times higher than in the general population, and was strongly associated with gestational age, underscoring the need for enhanced ASD screening of children born preterm, and suggesting that some risk factors associated with preterm birth may also play a causal role in ASD.
Keywords: Diagnosis, Epidemiology – descriptive, Intellectual disability, Pre- and perinatal risk factors, Prevalence, Sex differences
Scientific Abstract
We sought to estimate the prevalence of autism spectrum disorder (ASD) in children born extremely preterm relative to the U.S. population risk of 1.5% (CDC, 2014) using the best-available diagnostic procedures and minimizing confounding with other neurodevelopmental impairments. 889 of 966 (92%) 10-year-old children from the Extremely Low Gestational Age Newborn birth cohort, delivered at 23–27 weeks gestation in 2002–2004, participated. Children meeting ASD screening criteria on the Social Communication Questionnaire were evaluated with the Autism Diagnostic Interview–Revised (ADI-R). Those meeting ADI-R criteria were assessed with the Autism Diagnostic Observation Schedule-2 (ADOS-2). A positive ADOS-2 score was the criterion for ASD. 26 participants were not assessed for ASD because of severe sensory or motor impairment. In the remaining sample, 61 children met criteria for ASD, resulting in a prevalence of 7.1% (95% CI=5.5–9.0). ASD risk decreased with increasing gestational age, from 15.0% (95% CI=10.0–21.2) for 23–24 weeks, 6.5% (95% CI=4.2–9.4) for 25–26 weeks, to 3.4% (95% CI=1.6–6.1) for 27 weeks gestational age, and this association was independent of IQ. Among children with ASD, 40% had intellectual disability. The male-to-female ratio of children with ASD was 2.1:1 (95% CI=1.2:1–3.5:1), lower than in the general population (4:1). ASD prevalence in the ELGAN cohort was 4 times higher than in the general population, and was strongly associated with gestational age, underscoring the need for enhanced ASD screening of children born preterm, and suggesting that some risk factors associated with preterm birth may also play a role in the etiology of autism.
INTRODUCTION
Autism spectrum disorder (ASD) is characterized by impairments in reciprocal social interaction and communication and by restrictive interests and repetitive behaviors (APA, 2013). According to the most recent estimates from the Centers for Disease Control, ASD occurs in approximately 1.5% of individuals in the U.S., four times more often in males than females, and about 30% of children diagnosed with ASD also have intellectual disability (CDC, 2014).
Although ASD is highly heritable (Ronald & Hoekstra, 2011; Sandin, Kolevzon, Levine, Hultman, & Reichenberg, 2013), environmental factors appear to contribute to ASD susceptibility (Hallmayer et al., 2011; Sandin et al., 2013). Preterm birth and environmental factors mediating prematurity are associated with increased risk of ASD (Abel et al., 2013; Lampi et al., 2012; Larsson et al., 2005; Losh, Esserman, Anckarsater, Sullivan, & Lichtenstein, 2012; Maimburg & Vaeth, 2006; Moore, Kneitel, Walker, Gilbert, & Xing, 2012; Schendel & Bhasin, 2008). Among children born preterm, ASD risk has been reported to increase with each decreasing week of gestation (Kuzniewicz et al., 2014).
To date, four prospective studies have used standardized screening or diagnostic instruments to examine ASD in school-age cohorts of preterm children (Hack et al., 2009; Johnson et al., 2010; Pinto-Martin et al., 2011; Treyvaud et al., 2013), yielding prevalence estimates from 3–8%. In the three of these studies that reported characteristics specifically associated with ASD (Hack et al., 2009; Johnson et al., 2010; Pinto-Martin et al., 2011), an average of 60% (39–75%) of cases had intellectual disability (ID), and a large proportion also had severe neuromotor or neurosensory impairment. According to DSM-5, a diagnosis of ASD requires that autism-related symptoms are not better accounted for by global (i.e., non-autism-specific) neurodevelopmental disability, and the impairment in social-communicative ability is greater than would be expected based on cognitive ability (APA, 2013). This raises the question of whether the heightened occurrence of ASD reported among children born extremely preterm might in a significant number of cases be more accurately attributable to global neurodevelopmental disability.
In the present study, we examined the frequency of ASD in the Extremely Low Gestational Age Newborn (ELGAN) cohort of children, born in the U.S. in 2002–2004 at 23 to 27 weeks gestational age. We used gold-standard autism diagnostic measures, including in-depth parent interviews and direct behavioral observation to assess ASD. We excluded children with severe neurodevelopmental impairment, for whom a diagnosis of ASD would not be valid based on DSM-5 criteria requiring disproportionate impairment in social-communicative abilities. We assessed the association between ASD outcomes at age 10 years and gestational age at birth. We also evaluated whether the frequency of intellectual disability and the male:female sex ratio among ELGANs with ASD differed appreciably from estimates for the general ASD population reported by the CDCP.
METHODS
Participants
The ELGAN Study is a multicenter observational study of the risk of structural and functional neurologic disorders in extremely preterm infants. During the years 2002–2004, women delivering before 28 weeks gestation at 14 sites in 11 cities in 5 states (Connecticut, Illinois, Massachusetts, Michigan, North Carolina) were asked to enroll in the study. 1249 mothers of 1506 infants consented to participate. Of the 1198 children who survived to 10 years of age, 966 were actively recruited for follow-up (because of the availability of data on inflammation-related proteins in blood samples collected during their first postnatal month) and 889 (92%) agreed to participate. Procedures for this study were approved by the institutional review boards of participating institutions.
Maternal and newborn characteristics
Mothers reported their own characteristics. We have previously described the standard procedures used for defining gestational age, birth weight, and growth restriction (birth weight Z-score) (O'Shea et al., 2009).
ASD assessment
Diagnostic assessment of ASD was conducted with three well-validated measures, administered sequentially. Children meeting standardized criteria on a given measure were further assessed with the next measure.
First, study participants were screened for ASD symptoms with the Social Communication Questionnaire (SCQ) (Rutter, Bailey, & Lord, 2003), a parent-report screener that assesses ASD symptoms observed at any time in the child’s life. The SCQ includes 39 ratings for children with simple sentence speech, and 33 ratings for those without simple sentence speech. To increase screener sensitivity, a score ≥ 11, recommended by the authors for individuals at higher-than-normal risk for ASD was used instead of the standard criterion of ≥ 15.
Second, children who met SCQ screening criteria were evaluated with the Autism Diagnostic Interview–Revised (ADI-R) (Le Couteur, Lord, & Rutter, 2003), an in-depth parent interview that assesses symptoms in the core domains of communication, social, and repetitive behavior, and classifies autism based on 30 to 36 ratings, depending on the child’s language level. Because the ADI-R diagnostic algorithm classifies autism only, and not the less severe diagnosis of PDD-NOS or ASD, slightly relaxed cutoffs were used to ensure identification of less severely affected children. These modified criteria (Risi et al., 2006) are described in Table 2. The ADI-R is not recommended for the diagnosis of ASD in children with a developmental age less than 2 years because of poor specificity in discriminating between children with ASD and children with global neurodevelopmental disability.
Table 2.
Sample characteristics by ASD classification. These are column percents.
| ASD+ (n=61) |
ASD- (n=796) |
||
|---|---|---|---|
| Maternal characteristics at birth | |||
| Age, years | < 21 | 8 | 13 |
| 21–35 | 70 | 67 | |
| > 35 | 21 | 20 | |
| Education, years | ≤ 12 (high school) | 47 | 40 |
| > 12, < 16 | 14 | 24 | |
| ≥ 16 (≥ college) | 39 | 36 | |
| Maternal IQ Z-score | ≤ −2 | 3 | 4 |
| > −2, ≤ −1 | 5 | 7 | |
| > −1, ≤ 1 | 61 | 69 | |
| > 1 | 23 | 15 | |
| missing | 8 | 5 | |
| Single marital status | Yes | 36 | 40 |
| Public insurance | Yes | 33 | 34 |
| Racial identity | White | 62 | 64 |
| Black | 30 | 25 | |
| Other | 8 | 12 | |
| Hispanic | Yes | 7 | 10 |
| Newborn characteristics | |||
| Sex | Male | 67 | 50 |
| Gestational age, weeks | 23–24 | 43 | 18 |
| 25–26 | 41 | 45 | |
| 27 | 16 | 36 | |
| Birth weight, grams | ≤ 750 | 59 | 35 |
| 751–1000 | 28 | 45 | |
| > 1000 | 13 | 20 | |
| Birth weight Z-score | < −2 | 13 | 5 |
| ≥ −2, < −1 | 10 | 13 | |
| ≥ −1 | 77 | 81 | |
Finally, children who met criteria for autism or ASD on the ADI-R were assessed with the Autism Diagnostic Observation Schedule, Second Version (ADOS-2) (Lord et al., 2012), which served as the criterion measure of ASD in this study. The ADOS-2 is a semi-structured, observation protocol in which the examiner interacts with the child to assess social-communicative and repetitive behavior symptoms. One of three modules of the ADOS-2 is administered based on the child’s language level. Each ADOS-2 module is scored with a diagnostic algorithm comprised of 10 social affective and 4 repetitive behavior items that best discriminate children with ASD from typically developing children and non-ASD children with intellectual disability. Each algorithm provides threshold criteria for autism as well as the less severe classification of ASD based on the combined scores for social affect and repetitive behavior. Because diagnostic thresholds vary across the ADOS-2 modules, each algorithm also generates calibrated severity scores, ranging from 1 to 10, which are comparable across modules (Gotham, Pickles, & Lord, 2009). The ADOS-2 is not a valid measure of ASD in children with severe uncorrected visual or hearing impairment or severe motor impairment, as such deficits would significantly limit the child’s ability, for example, to make eye contact, to orient in the direction of another person’s voice or shift of gaze, or to use communicative gestures, and to engage in several other behaviors that are evaluated in an ADOS-2 assessment of ASD.
The only exceptions to the ASD assessment procedure described above were made for 9 children who did not meet ADI-R criteria for ASD, but who were evaluated with the ADOS-2 because the child had a prior clinical diagnosis of ASD or the child was thought likely to meet ASD criteria based on the site psychologist’s clinical observation during cognitive testing of the child. For two additional children who met ADOS-2 diagnostic criteria for ASD, the parent did not complete the ADI-R interview.
All personnel who conducted the ADI-R and ADOS-2 received research-level training in their use and established high inter-rater reliability with an experienced trainer. All ADOS-2 administrations were video recorded and independently scored by a second rater with autism diagnostic and ADOS-2 expertise (R.M.J.), who did not have knowledge of the child’s SCQ and ADI-R results and prior clinical history. In cases of ADOS-2 scoring disagreements, a consensus score was reached after review and discussion of the videoed data. Item-by-item inter-rater agreement on the 14 scores comprising each of the ADOS-2 diagnostic algorithms was on average .93 (SD = .12). Of 90 ADOS-2 assessments, inter-rater disagreement and consensus scoring resulted in 4 changes of classification, 3 from non-ASD to ASD and 1 from ASD to non-ASD, Cohen’s K = .90.
Intellectual ability and sensorimotor status
Intellectual ability (IQ) was assessed with the School-Age Differential Ability Scales – II (DAS-II) (Elliott, 2007) Verbal (VIQ) and Nonverbal Reasoning (NVIQ) scales. Intellectual disability (ID) was defined as having both VIQ and NVIQ below a standard score of 70. Severe gross motor dysfunction was defined as Level 5 (i.e., no self-mobility) on the Gross Motor Function Classification System (GMFCS) (Palisano et al., 1997). A child was considered to have severe visual impairment if the parent reported uncorrectable functional blindness in both eyes. No participant had a significant, uncorrected hearing impairment.
Data analyses
The prevalence of ASD in the ELGAN cohort was calculated with 95% confidence intervals and compared to the prevalence of ASD in the general population estimated by the CDCP as 1.5% with a chi-square goodness of fit test. Differences in the prevalence of ASD among ELGANs by gestational age were evaluated with a chi-square test of independence, and presented with 95% confidence intervals. Differences in the proportion of ELGANs with ASD compared to those without ASD who had verbal IQ and nonverbal IQ below 70 were also evaluated with chi-square tests of independence. Chi-square goodness of fit tests were used to compare the prevalence of ID among ELGANs with ASD to the prevalence of ID among children with ASD in the general population (30%), and to compare the sex ratio of ELGANs with ASD to the sex ratio in the general ASD population (4:1) (CDC, 2014).
RESULTS
Sample description
Table 1 compares the maternal and child characteristics of children who did and did not participate in the 10-year follow-up evaluation. From among the 1198 children who survived to 10 years of age, those who did not participate (n = 309) were more likely to have indicators of social advantage, such as lower maternal education and receipt of public health insurance. Social disadvantage has been identified as a risk factor for both ASD (DiGuiseppi et al., 2016; Rai et al., 2012) and preterm birth (Glinianaia et al., 2013). In contrast, these same children did not differ from those who participated in newborn characteristics, including sex, gestational age, and birth-weight Z-score, a measure of fetal growth restriction.
Table 1.
Characteristics of the 1198 children from the ELGAN birth cohort who survived to age 10 years who were not recruited, who were recruited but did not participate, and who were recruited and did participate in the 10-year follow-up evaluation. These are column percents.
| Survived to 10 years | Row N |
||||
|---|---|---|---|---|---|
| Not recruited |
Recruited | ||||
| Did not participate |
Participated | ||||
| Maternal characteristics at birth | |||||
| Age, years | < 21 | 19 | 13 | 13 | 170 |
| 21–35 | 64 | 78 | 67 | 802 | |
| > 35 | 17 | 9 | 20 | 226 | |
| Education, years | ≤ 12 (high school) | 51 | 55 | 41 | 506 |
| > 12, < 16 | 23 | 26 | 23 | 270 | |
| ≥ 16 (≥ college) | 26 | 19 | 35 | 376 | |
| Single marital status | Yes | 50 | 58 | 41 | 513 |
| Public insurance | Yes | 50 | 60 | 35 | 464 |
| Racial identity | White | 53 | 45 | 63 | 706 |
| Black | 29 | 38 | 26 | 322 | |
| Other | 18 | 17 | 11 | 151 | |
| Hispanic | Yes | 22 | 14 | 13 | 147 |
| Newborn characteristics | |||||
| Sex | Male | 53 | 56 | 51 | 621 |
| Gestational age, weeks | 23–24 | 20 | 14 | 21 | 245 |
| 25–26 | 46 | 60 | 45 | 553 | |
| 27 | 34 | 26 | 34 | 400 | |
| Birth weight, grams | ≤ 750 | 36 | 27 | 37 | 436 |
| 751–1000 | 38 | 64 | 43 | 520 | |
| > 1000 | 26 | 9 | 20 | 242 | |
| Birth weight Z-score | < −2 | 3 | 1 | 6 | 62 |
| ≥ −2, < −1 | 9 | 14 | 14 | 153 | |
| ≥ −1 | 87 | 84 | 81 | 938 | |
| Maximum column N | 232 | 77 | 889 | 1198 | |
Of 889 children in the sample, 26 children were excluded from an assessment of ASD, 17 because of severe motor impairment and severe ID, 7 for functional blindness, and 2 for severe motor impairment, blindness, and ID combined. Of these 26 children, 19 did not achieve basal IQ scores on the DAS-II, indicative of a nonverbal mental age of approximately 3.5 years or lower.
One child who met SCQ criteria and 5 children who met both SCQ and ADI-R criteria for ASD did not return to complete the ASD assessment, and were not included in the final sample. Of these 6 children, 4 did not achieve basal IQ scores, 1 scored in the mild ID range, and 1 family refused IQ testing.
Of the 857 children assessed for ASD, 13 (1 ASD positive, 12 ASD negative) did not have an IQ assessment. As shown in Table 2, maternal characteristics did not differ between children with and without ASD, but children with ASD tended to have lower gestational age, birth weight, and birth weight Z-scores.
Prevalence of ASD by SCQ, ADI-R, and ADOS-2
Of 857 ELGANs who were assessed for ASD, 106 (12.4%) screened positive on the SCQ, and 79 (9.2%) met ADI-R criteria for ASD. An additional 2 children for whom ADI-R data were not collected, and 9 ADI-R-negative children were further evaluated because of prior clinical diagnosis or current clinical suspicion. Of these 90 children, 61 children met ADOS-2 criteria for ASD, resulting in a sample prevalence of 7.1% (95% CI=5.5–9.0), significantly higher than the 1.5% prevalence in the general population (χ2GOF(1) = 183.1, P < .001). (Of the 9 ADI-R-negative children who were assessed with the ADOS-2, 4 met ADOS-2 criteria for ASD. Of the 2 children who were not assessed with the ADI-R, both met ADOS-2 criteria for ASD.)
ASD occurred more frequently with lower gestational age (χ2IND(2) = 23.0, P < .001). The frequency of ASD was 15.0% (95% CI=10.0–21.2) among children born during the 23rd and 24th weeks of gestation, 6.5% (95% CI=4.2–9.4) among those born during the 25th and 26th weeks of gestation, and 3.4% (95% CI=1.6–6.1) among children born during the 27th week of gestation (see Figure 1). When the frequency of ASD by gestational age was limited to children in the sample without ID, a similar pattern was observed: 10.1% (95% CI=5.7–16.1), 4.8% (95% CI=2.8–7.5), and 1.0% (95% CI=0.4–3.6) for the gestational age categories of 23 – 24, 25 – 26, and 27 weeks, respectively.
Figure 1.
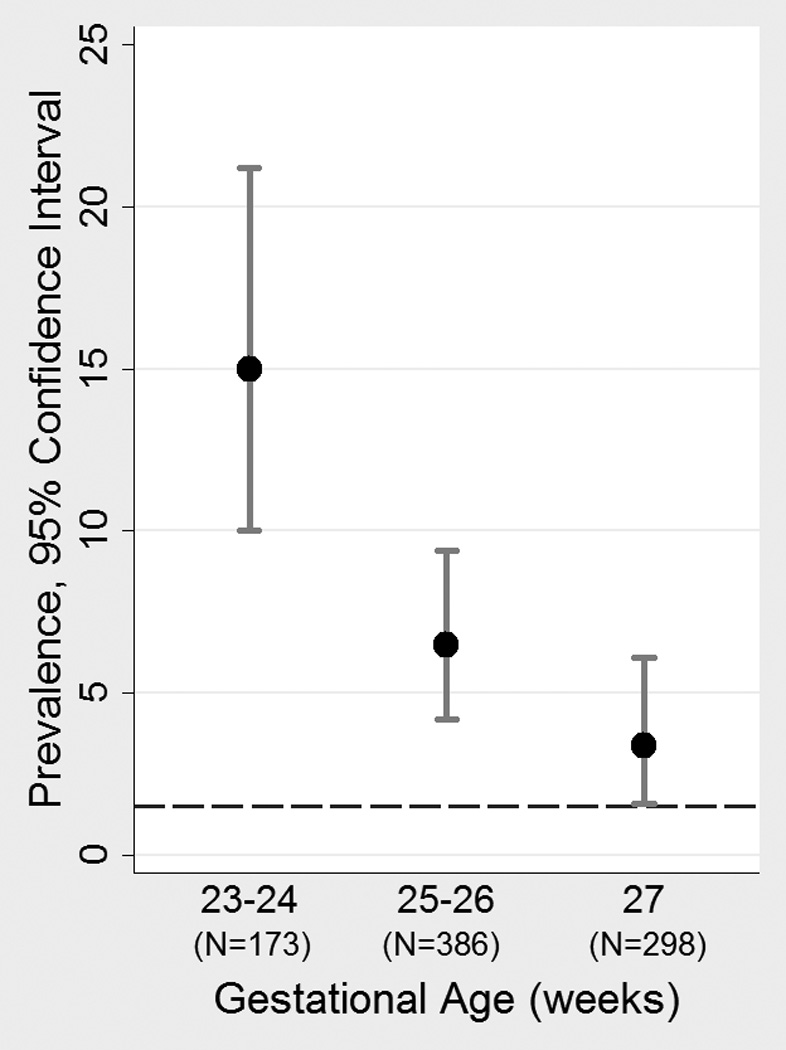
Prevalence (with 95% confidence interval) of ASD by gestational age category. Dashed horizontal line represents ASD prevalence in the U.S. population.
Among children meeting criteria for ASD, all median ADI-R and ADOS-2 scores exceeded cutoffs for the more severe classification of autism, and were similar across IQ groups (Table 3). In addition, both lower and higher IQ groups had median ADOS-2 calibrated symptom scores of 8, in the “high” range of symptom severity (Gotham et al., 2009). (In contrast, the median calibrated symptom score for the 31 children who did not meet ADOS-2 ASD criteria was 1, in the “minimal-to-no-evidence” range of severity.) All but 2 of the 61 children who met criteria for ASD had repetitive behaviors on the ADOS-2. One of these two children had repetitive behaviors reported on the ADI-R.
Table 3.
SCQ, ADI-Ra and ADOS-2b median (and 25th and 75th percentile) scores for lower and higher IQ children meeting criteria for ASD (n = 60)c.
| IQ < 70e (n=24) |
IQ ≥ 70f (n=36) |
Row n | |
|---|---|---|---|
| SCQ total score | 23 (17, 27) | 18 (13, 24) | 58 |
| ADI-R social total | 26 (18, 28) | 20 (13, 25) | 58 |
| ADI-R communication totald | |||
| ADI-R verbal communication total | 18.5 (15, 22) | 13 (9, 18) | 47 |
| ADI-R nonverbal communication total | 14 (11, 14) | --- | 11 |
| ADI-R repetitive behaviors total | 4 (3, 8) | 5 (2,7) | 58 |
| ADOS-2 Module 1 | |||
| Social affect | 15 (12, 17) | --- | 14 |
| Restricted and repetitive behaviors | 6 (3, 7) | --- | 14 |
| Total | 21 (18, 22) | --- | 14 |
| ADOS-2 Module 2 | |||
| Social affect | 13 (11.5, 16) | 10g | 5 |
| Restricted and repetitive behaviors | 4.5 (3,6) | 7g | 5 |
| Total | 17 (16, 20.5) | 17g | 5 |
| ADOS-2 Module 3 | |||
| Social affect | 11 (10,16) | 10 (7, 15) | 41 |
| Restricted and repetitive behaviors | 3.5 (0, 4) | 3 (2, 5) | 41 |
| Total | 15.5 (13, 17) | 14 (9, 19) | 41h |
| ADOS-2 symptom severity score | 8 (6.5, 9) | 8 (4, 9.5) | 60 |
An ADI-R classification of autism requires meeting or exceeding standardized cutoffs for social, communication, and repetitive behaviors of 10, 8, and 3, respectively. ADI-R classifications of ASD (Risi et al., 2006) were calculated as follows: meets autism cutoff for social and meets cutoff on either communication or repetitive behavior; meets cutoffs for social and communication or meets cutoff for social and within 2 points of communication cutoff; or meets cutoff for communication and within 2 points of social cutoff or within 1 point of both social and communication cutoffs.
An ADOS-2 Module 1 classification of autism requires a total score ≥ 16 for children with no-to-few words and ≥ 12 for children with some words. An ADOS-2 Module 1 classification of ASD requires a total score ≥ 11 for children with no-to-few words and ≥ 8 for children with some words. ADOS-2 Module 2 classifications of autism and ASD require total scores ≥ 9 and 8, respectively. ADOS-2 Module 3 classifications of autism and ASD require total scores ≥ 9 and 7, respectively.
IQ was not available for one Module 3 child.
Only children with simple sentence speech (n = 47) are evaluated for verbal communication on the ADI-R.
Both verbal and nonverbal IQ < 70.
Either or both verbal and nonverbal IQ ≥ 70.
Single observation.
Of 42 children administered ADOS-2 Module 3, 6 had IQ < 70. One child was missing both VIQ and NVIQ data, and was therefore not included in this table.
Associated characteristics of ASD
Of the 61 children who met ASD criteria on the ADOS-2, 14 were administered Module 1 (no words or single words), 5 Module 2 (phrase or simple sentence speech), and 42 Module 3 (complex sentence speech), indicating that the majority of the sample spoke fluently.
Children diagnosed with ASD were much more likely to have verbal (χ2IND(1) = 94.8, P < .001) and nonverbal IQ (χ2IND(1) = 79.8, P < .001) below 70, in the ID range, than children not diagnosed with ASD (Table 4). Among those with ASD, 40% (n = 24) had both verbal and nonverbal IQ < 70, and 60% (n = 36) had either (n = 13) or both (n = 23) verbal and nonverbal IQ ≥ 70. Of the 13 children who had either verbal or nonverbal IQ ≥ 70, 10 had nonverbal IQ ≥ 70 and 3 had verbal IQ ≥ 70.
Table 4.
Child verbal and nonverbal IQa by ASD classification. These are column percents.
| ASD+ (n=61)a |
ASD- (n=796)b |
||
|---|---|---|---|
| Verbal IQ | < 55 | 42 | 3 |
| 55–69 | 14 | 8 | |
| 70–84 | 19 | 19 | |
| ≥ 85 | 25 | 71 | |
| Nonverbal IQ | < 55 | 27 | 2 |
| 55–69 | 18 | 6 | |
| 70–84 | 27 | 23 | |
| ≥ 85 | 28 | 69 | |
One child missing both VIQ and NVIQ data, and one child missing VIQ data (NVIQ = 122) because of inattention/noncompliance.
11 children missing IQ data.
ASD co-occurred with ID in 24/844 or 2.8% (95% CI=1.8–4.2) of the entire sample, and with IQ in the borderline range or above in 36/844 or 4.3% (95% CI=3.0–5.9) of the sample. The 40% prevalence of ID among study participants with ASD was not significantly different from the 30% prevalence, χ2GOF(1) = 2.5, P = .11, among individuals with ASD reported by the CDCP (CDC, 2014).
Among ELGANs with ASD, the ratio of males (n = 41) to females (n = 20) was 2.1 to 1 (95% CI=1.2:1–3.5:1), significantly lower than the 4:1 ratio estimated by the CDCP (CDC, 2014) χ2GOF(1) = 6.6, P = .01. This sex ratio was similar among individuals with ID (2.4:1) and those without ID (2.0:1).
Of the 24 children with ASD whose verbal and nonverbal IQ scores were below 70, 46% (n = 11) failed to achieve a basal IQ score, indicative of a nonverbal mental age of 3.5 years or lower. In the clinical impression of both ADOS-2 reviewers, all 11 of these children met DSM-5 criteria for ASD because their ASD-related behaviors were not better accounted for by ID than by ASD. However, because the specificity of ASD diagnoses declines with decreasing mental age (Risi et al., 2006), we also estimated the prevalence of ASD in the ELGAN sample after excluding children (n = 13; 11 ASD positive, 2 ASD negative) for whom we could not confirm a nonverbal mental age of at least 2 years. This yielded a frequency of 50/844 or 5.9% (95% CI=4.4–7.7), still more than 3 times higher than the U.S. population estimate.
DISCUSSION
We used research-validated diagnostic instruments to assess the prevalence of ASD in a prospectively-followed cohort of 889 10-year-old children born extremely preterm. Excluding children with severe sensory and motor impairment, we confirmed prior reports (Hack et al., 2009; Johnson et al., 2010; Pinto-Martin et al., 2011; Treyvaud et al., 2013) that children born significantly preterm are at substantially increased risk of ASD. We found a 7.1 prevalence of ASD in our total cohort of children born at 23 to 27 weeks of gestational age, a more than fourfold increase over the 1.5% prevalence reported by the CDCP for 8-year-olds in the general U.S. population in 2010 (CDC, 2014).
Our findings of a significantly increased risk of ASD in the ELGAN cohort confirm those from prior studies (Johnson et al., 2010; Pinto-Martin et al., 2011), but also advance them in two critical ways. First, we excluded from our target sample children with significant sensory, motor, and intellectual disability, enhancing the specificity of our estimates by ensuring that ASD classifications could not be better accounted for by global neurodevelopmental disability than by ASD. Second, in addition to in-depth parent interviewing with the ADI-R, we required that all our cases be confirmed by direct behavioral observation with the ADOS-2, consensus-scored by two expert autism clinicians. Johnson et al. (2010) used the Differential and Well Being Assessment (Goodman, Ford, Richards, Gatward, & Meltzer, 2000), a parent interview administered by telephone or online as the criterion measure of ASD in an extremely low gestational age sample, and estimated an ASD prevalence of 8%. Using the same assessments as used in the current study, Pinto-Martin et al. (2011) obtained an ASD prevalence estimate of 5% in a low birth weight (< 2000 grams) cohort, which had a mean gestational age of 31 weeks, but did not confirm all identified cases with standardized behavioral observational testing.
In the ELGAN cohort, the IQ distribution among children with ASD was not markedly different from that of the general population of children with ASD in the U.S. (CDC, 2014). Although the overall prevalence of IQ < 70 in the ELGAN cohort, as in other extremely preterm cohorts, was much higher than in unselected samples, the prevalence of ASD among children with an IQ < 70 was consistent with that previously reported (Bryson, Bradley, Thompson, & Wainwright, 2008). Thus, the increased ASD prevalence in this sample might be associated, in part, with neurodevelopmental processes related to intellectual disability. However, 60% of our sample who met rigorous diagnostic criteria for ASD did not have severe cognitive or sensorimotor impairment.
The increased risk of ASD for boys relative to girls among ELGANs was lower than CDCP U.S. population estimates and ratios reported in some (Glasson et al., 2004; Maimburg & Vaeth, 2006) but not all (Sandin et al., 2013) retrospective studies of children with ASD born preterm. Contrary to prior findings (Schendel & Bhasin, 2008), the relatively low male-to-female risk ratio among ELGANs was not specifically associated with ID. We offer two explanations for a lower male-to-female risk ratio among ELGANs. First, community diagnostic practice is now recognized to be less sensitive to ASD in girls, resulting in their under-representation in autism research samples (Frazier, Georgiades, Bishop, & Hardan, 2014). Second, the decreased sex ratio could reflect the role of environmental factors in the pathology of ASD among ELGANs. For example, preterm birth and its antecedents might disrupt a prenatal hormonal milieu that otherwise serves as a protective factor against ASD-promoting exposures for girls born at term (Gore, Martien, Gagnidze, & Pfaff, 2014; Werling & Geschwind, 2013).
ASD risk has previously been found to increase with each week of decreasing gestational age among children born from 24 to 34 weeks gestation (Kuzniewicz et al., 2014). In the ELGAN cohort, we found a similar gestational age gradient of ASD risk within the relatively narrower gestational age range of 23 to 27 weeks, even among children who did not have intellectual disability. Accordingly, low gestational age can be seen as a marker of the immaturity and vulnerability of the central nervous system as well as other physiological systems that function to protect the developing brain and, when perturbed, put the developing fetus at risk (Dammann & Leviton, 1999; Leviton, Blair, Dammann, & Allred, 2005; Sanders & Harvey, 2008). Thus, the exposures that promote preterm birth might also promote ASD. For example, maternal and fetal inflammation associated with processes leading to extremely preterm birth (Bastek et al., 2011; Leviton, Allred, Yamamoto, Fichorova, & Investigators, 2012; Wei, Fraser, & Luo, 2010) also appear to be antecedents of ASD (Lee et al., 2015; Onore, Careaga, & Ashwood, 2012). In addition, neonatal systemic inflammation, which occurs much more commonly among very preterm newborns than in children born at term, has also been associated with ASD (Krakowiak et al.; Masi et al., 2015). Beyond inflammation, preterm birth and the development of ASD may be associated through other unrecognized processes, such as epigenetic activation in response to environmental exposures (Tordjman et al., 2014).
In conclusion, using the best available diagnostic instruments, and excluding children with severe sensorimotor and cognitive impairment from our sample, we found an increased prevalence of ASD among extremely preterm children at least 4 times higher than in the general population. In addition, ASD risk increased with lower gestational age. Whereas the distribution of IQ among ELGANs was not markedly different from term children with ASD, the male-to-female ratio was approximately half that found in most general population cohorts.
Acknowledgments
Financial Support: This study was supported by the National Institute of Neurological Disorders and Stroke (5U01NS040069-05; 2R01NS040069-06A2) and the National Institute of Child Health and Human Development (5P30HD018655-28).
REFERENCES
- Abel KM, Dalman C, Svensson AC, Susser E, Dal H, Idring S, Magnusson C. Deviance in fetal growth and risk of autism spectrum disorder. Am J Psychiatry. 2013;170(4):391–398. doi: 10.1176/appi.ajp.2012.12040543. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders : DSM-5. 5th. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- Bastek JA, Brown AG, Anton L, Srinivas SK, D'Addio A, Elovitz MA. Biomarkers of inflammation and placental dysfunction are associated with subsequent preterm birth. J Matern Fetal Neonatal Med. 2011;24(4):600–605. doi: 10.3109/14767058.2010.511340. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Bradley EA, Thompson A, Wainwright A. Prevalence of autism among adolescents with intellectual disabilities. Can J Psychiatry. 2008;53(7):449–459. doi: 10.1177/070674370805300710. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18674403. [DOI] [PubMed] [Google Scholar]
- CDC. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveillance Summaries. 2014;63(2):1–21. [PubMed] [Google Scholar]
- Dammann O, Leviton A. Brain damage in preterm newborns: might enhancement of developmentally regulated endogenous protection open a door for prevention? Pediatrics. 1999;104(3 Pt 1):541–550. doi: 10.1542/peds.104.3.541. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10469783. [DOI] [PubMed] [Google Scholar]
- DiGuiseppi CG, Daniels JL, Fallin DM, Rosenberg SA, Schieve LA, Thomas KC, Currie DW. Demographic profile of families and children in the Study to Explore Early Development (SEED): Case-control study of autism spectrum disorder. Disability and health journal. 2016 doi: 10.1016/j.dhjo.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD. Differential Ability Scales. 2nd. San Antonio, TX: Pearson Education; 2007. [Google Scholar]
- Frazier TW, Georgiades S, Bishop SL, Hardan AY. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J Am Acad Child Adolesc Psychiatry. 2014;53(3):329–340. e321–e323. doi: 10.1016/j.jaac.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasson EJ, Bower C, Petterson B, de Klerk N, Chaney G, Hallmayer JF. Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry. 2004;61(6):618–627. doi: 10.1001/archpsyc.61.6.618. [DOI] [PubMed] [Google Scholar]
- Glinianaia SV, Ghosh R, Rankin J, Pearce MS, Parker L, Pless-Mulloli T. No improvement in socioeconomic inequalities in birthweight and preterm birth over four decades: a population-based cohort study. BMC Public Health. 2013;13:345. doi: 10.1186/1471-2458-13-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41(5):645–655. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10946756. [PubMed] [Google Scholar]
- Gore AC, Martien KM, Gagnidze K, Pfaff D. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr Rev. 2014;35(6):961–991. doi: 10.1210/er.2013-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M, Taylor HG, Schluchter M, Andreias L, Drotar D, Klein N. Behavioral outcomes of extremely low birth weight children at age 8 years. J Dev Behav Pediatr. 2009;30(2):122–130. doi: 10.1097/DBP.0b013e31819e6a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Risch N. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Autism spectrum disorders in extremely preterm children. J Pediatr. 2010;156(4):525–531. e522. doi: 10.1016/j.jpeds.2009.10.041. [DOI] [PubMed] [Google Scholar]
- Krakowiak P, Goines PE, Tancredi DJ, Ashwood P, Hansen RL, Hertz-Picciotto I, Van de Water J. Neonatal Cytokine Profiles Associated with Autism Spectrum Disorder. Biological Psychiatry. doi: 10.1016/j.biopsych.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzniewicz MW, Wi S, Qian Y, Walsh EM, Armstrong MA, Croen LA. Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. J Pediatr. 2014;164(1):20–25. doi: 10.1016/j.jpeds.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Lampi KM, Lehtonen L, Tran PL, Suominen A, Lehti V, Banerjee PN, Sourander A. Risk of autism spectrum disorders in low birth weight and small for gestational age infants. J Pediatr. 2012;161(5):830–836. doi: 10.1016/j.jpeds.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, Mortensen PB. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol. 2005;161(10):916–925. doi: 10.1093/aje/kwi123. discussion 926–918. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Lord C, Rutter M. The autism diagnostic interview-revised (ADI-R) Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Lee BK, Magnusson C, Gardner RM, Blomstrom A, Newschaffer CJ, Burstyn I, Dalman C. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun. 2015;44:100–105. doi: 10.1016/j.bbi.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviton A, Allred EN, Yamamoto H, Fichorova RN, Investigators ES. Relationships among the concentrations of 25 inflammation-associated proteins during the first postnatal weeks in the blood of infants born before the 28th week of gestation. Cytokine. 2012;57(1):182–190. doi: 10.1016/j.cyto.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviton A, Blair E, Dammann O, Allred E. The wealth of information conveyed by gestational age. J Pediatr. 2005;146(1):123–127. doi: 10.1016/j.jpeds.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule–2 (ADOS-2) Los Angeles, CA: Western Psychological Corporation; 2012. [Google Scholar]
- Losh M, Esserman D, Anckarsater H, Sullivan PF, Lichtenstein P. Lower birth weight indicates higher risk of autistic traits in discordant twin pairs. Psychol Med. 2012;42(5):1091–1102. doi: 10.1017/S0033291711002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimburg RD, Vaeth M. Perinatal risk factors and infantile autism. Acta Psychiatr Scand. 2006;114(4):257–264. doi: 10.1111/j.1600-0447.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry. 2015;20(4):440–446. doi: 10.1038/mp.2014.59. [DOI] [PubMed] [Google Scholar]
- Moore GS, Kneitel AW, Walker CK, Gilbert WM, Xing G. Autism risk in small- and large-for-gestational-age infants. Am J Obstet Gynecol. 2012;206(4):314, e311–e319. doi: 10.1016/j.ajog.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N Investigators, E.s. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85(11):719–725. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26(3):383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9183258. [DOI] [PubMed] [Google Scholar]
- Pinto-Martin JA, Levy SE, Feldman JF, Lorenz JM, Paneth N, Whitaker AH. Prevalence of autism spectrum disorder in adolescents born weighing <2000 grams. Pediatrics. 2011;128(5):883–891. doi: 10.1542/peds.2010-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai D, Lewis G, Lundberg M, Araya R, Svensson A, Dalman C, Magnusson C. Parental socioeconomic status and risk of offspring autism spectrum disorders in a Swedish population-based study. J Am Acad Child Adolesc Psychiatry. 2012;51(5):467–476. e466. doi: 10.1016/j.jaac.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, Pickles A. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1094–1103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: a decade of new twin studies. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(3):255–274. doi: 10.1002/ajmg.b.31159. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Sanders EJ, Harvey S. Peptide hormones as developmental growth and differentiation factors. Dev Dyn. 2008;237(6):1537–1552. doi: 10.1002/dvdy.21573. [DOI] [PubMed] [Google Scholar]
- Sandin S, Kolevzon A, Levine SZ, Hultman CM, Reichenberg A. Parental and Perinatal Risk Factors for Autism. 2013:195–202. [Google Scholar]
- Schendel D, Bhasin TK. Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics. 2008;121(6):1155–1164. doi: 10.1542/peds.2007-1049. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Somogyi E, Coulon N, Kermarrec S, Cohen D, Bronsard G, Xavier J. Gene x Environment interactions in autism spectrum disorders: role of epigenetic mechanisms. Front Psychiatry. 2014;5:53. doi: 10.3389/fpsyt.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treyvaud K, Ure A, Doyle LW, Lee KJ, Rogers CE, Kidokoro H, Anderson PJ. Psychiatric outcomes at age seven for very preterm children: rates and predictors. Journal of Child Psychology and Psychiatry. 2013;54(7):772–779. doi: 10.1111/jcpp.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol. 2010;116(2 Pt 1):393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013;26(2):146–153. doi: 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]


