SUMMARY
oll-like-receptors (TLRs) play a significant role in the generation of a specific innate immune response against invading pathogens. TLR2 and TLR4 signaling contributes to infection-induced inflammation in periodontal disease (PD) and atherosclerosis. Increased observational studies points towards a relationship between PD and atherosclerosis, but the role of TLR2 and TLR4 in recognition of multiple oral pathogens and their modulation of host response leading to atherosclerosis are not clear. We evaluated the role of TLR2 and TLR4 signaling on induction of both PD and atherosclerosis in TLR2−/− and TLR4−/− mice to polymicrobial infection with periodontal pathogens Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, and Fusobacterium nucleatum. Polybacterial infections have established gingival colonization in TLR2−/− and TLR4−/− mice and induction of pathogen-specific IgG immune response. But TLRs deficiency dampened accelerated alveolar bone resorption (ABR) and intrabony defects, indicating a central role in infection-induced PD. Periodontal bacteria disseminated from gingival tissue to the heart and aorta through intravascular dissemination, however, there was no increase in atherosclerosis progression in aortic arch. Polybacterial infection does not alter levels of serum risk factors oxidized LDL, nitric oxide, and lipid fractions in both mice. Polymicrobial-infected TLR2−/− mice demonstrated significant levels (p < 0.05- p < 0.01) of Th2 (TGFβ1, MIP-3α, IL-13) and Th17 (IL-17, IL-21, IL-22, IL-23) splenic T cell cytokine responses. Increased heat shock protein expression, hspa1a Hsp70 was observed for both TLR2−/− and TLR4−/− mice. This study supports a role for TLR2 and TLR4 in PD and atherosclerosis, corroborating an intricate association between two inflammatory diseases.
Keywords: Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, Fusobacterium nucleatum, polymicrobial infection, TLR2−/− mice, TLR4−/− mice, periodontal disease, atherosclerosis
INTRODUCTION
Periodontal disease represents a multifactorial microbial dysbiotic disease with a complex interplay between the host immune response, subgingival microbes and environmental factors (Cullinan et al., 2003). Both the initiating infectious agents and the host immunological responses in periodontal disease modulate the progression of periodontal disease. Similarly, there is an increasing body of evidence showing this intricate interaction between infection and innate immunity on initiation and acceleration of chronic inflammatory atherosclerotic vascular disease (ASVD) (Libby, 2012) which is the leading cause of death globally. Innate immunity constitutes the first line of defense against invading pathogens, and it is programmed to detect highly conserved molecular motifs called pathogen-associated molecular patterns (PAMPs) via specialized receptors. The innate immune system has a broad range of specificity allowing detection of foreign pathogens, and detection of infectious agents relies to a great extent on a family of evolutionarily conserved pattern recognition receptors (PRRs), known as the Toll-like receptors (TLRs), which have a crucial role in early host defense against invading pathogens(Takeda & Akira, 2005). Amongst several families of PPRs, TLRs are the best-characterized. TLR2 and TLR4 are located on the cell surface and are recruited to phagosomes after activation (Underhill et al., 1999).
Over the past decade, a major change has occurred in understanding of the mechanisms responsible for the development and progression of atherosclerosis, leading to an increasing recognition of atherosclerosis as an inflammatory disease (Ross, 1999). Numerous groups have tried to investigate the mechanisms for inflammation in driving early plaque growth and later progression of atherosclerotic lesions to unstable or ruptured plaque, the cause for sudden heart attack and thrombotic occlusions. Mullick et al., (Mullick et al., 2008) revealed that TLR2 expression is increased on the surface of endothelial cells at sites prone to development of atherosclerosis, such as the inner curvature of the aortic arch in LDLR−/− mice. Work by Higashimori et al., (Higashimori et al., 2011) found that TLR2 deficiency diminishes foam cell accumulation in lesion-prone areas of the aorta of ApoE−/− mice yielding further support for a pathogenic role for TLR2 signaling in murine models of atherosclerosis.
An increase in TLR4 gene expression was observed human atherosclerotic plaques and stimulation of human monocyte-derived macrophages with oxLDL in culture increased gene expression of TLR4 (Xu et al., 2001). Genetic deletion of TLR4 greatly reduces atherosclerotic lesion development and macrophage infiltration, which is accompanied by decreased levels of inflammation-promoting IL-12 and MCP-1 (Michelsen et al., 2004). A related study by Choi et al., (Choi et al., 2009) found that TLR4 detects minimally oxidized low-density lipoprotein, acts as a mediator of macropinocytosis and, eventually plays a role in formation of pathognomonic “foam cells” the lipid-filled and highly inflammatory macrophages that accumulate in the aortic intimal layer. Interestingly, TLR4 was found to contribute to foam cell formation to a greater extent than TLR2 (Higashimori et al., 2011).
The mechanisms undertaken by various mucosal pathogens like Porphyromonas gingivalis to evade regulatory barriers to initiate inflammatory bone resorption are not yet understood. This organism induces an immunological tolerance designed to suppress the host inflammatory cytokine production after bacterial infection. This is one of the principle events behind the development of tolerance is the down-regulation of TLR signaling components. These events are vital especially at non-sterile mucosal surfaces such as oral cavity (Dubois et al., 2005). A down-regulated host response increases potential risk of enhanced bacterial survival and replication.
In their initial examination of the effects of oral P. gingivalis infection on atherosclerosis development, Gibson, et al., (Gibson et al., 2004) detected elevated expression of TLRs 2 and 4 in aortic arch tissues of mice infected with invasive P. gingivalis. In contrast to previous studies on TLR2 (Mullick et al., 2005), when ApoE−/− mice deficient in TLR4 were infected with P. gingivalis they were paradoxically more susceptible to developing atherosclerosis, presenting with increased levels of inflammatory Th17 cells (Hayashi et al., 2012). Madan and Amar (Madan & Amar, 2008) demonstrated that ApoE+/− TLR2+/+ mice on a high fat diet and/or infected with P. gingivalis exhibited an unstable atherosclerotic plaque phenotype and cellular composition, which was abolished in mice heterozygous for, or completely lacking TLR2. However, periodontal disease results due to polymicrobial dysbiosis and subgingival plaque consists of a complex multi-species consortium of bacteria including P. gingivalis, T. denticola, T. forsythia, F. nucleatum and numerous bacterial species have been detected in human atherosclerotic plaques (Haraszthy et al., 2000, Fiehn et al., 2005). Whether TLR2 and TLR4 deficiencies play a major role in induction of both periodontal disease and atherosclerosis to polymicrobial infection is unknown.
With this study we have evaluated the role of TLR2 and TLR4 deficiencies in polymicrobial infection-induced periodontal disease and atherosclerosis. We have used our polymicrobial model of periodontal disease (Kesavalu et al., 2007, Rivera et al., 2013, Velsko et al., 2015) to assess the TLR2 and 4 signaling in induction of periodontal disease and atherosclerosis in TLR2−/− mice and TLR4−/− mice. This chronic infection model of periodontal disease mimics human infection which is characterized by multiple concurrent oral infections. Further, our study serves as a unique platform to better understand the nuances of two chronic inflammatory diseases, periodontal disease and atherosclerosis. The aim of this study was to demonstrate the role of TLR2 and TLR4 signaling deficiencies critical in periodontal inflammation, bone resorption, systemic inflammation, and progression of atherosclerosis.
METHODS
Bacterial Strains and Inocula
P. gingivalis ATCC 53977, T. denticola ATCC 35404, T. forsythia ATCC 43037, and F. nucleatum ATCC 49256 were grown at 37°C for 2 days, as previously described (Rivera et al., 2013, Velsko et al., 2015). Bacteria were suspended in equal proportions in reduced transport fluid (RTF) – 4% carboxymethylcellulose (CMC), and this mixture was used for oral infection of TLR2−/− as well as TLR4−/− mice, as described previously (Rivera et al., 2013, Velsko et al., 2015).
TLR2−/− and TLR4−/− Mouse Infection and Oral Plaque Sampling
The Institutional Animal Care and Use Committee at the University of Florida approved all mice-related procedures and experiments (IACUC, protocol #201304539). Eight-week old male B6.129-Tlr2tm1Kir/J and C.C3-Tlr4Lps-d/J mice were obtained from the Jackson Laboratories (Bar Harbor, ME). Upon arrival, TLR2 and 4 mice were allowed to adapt to the new environment for at least 1 week prior to initiation of any experiment (Rivera et al., 2013). Mice were housed under specific-pathogen-free facility in University of Florida vivarium. Twenty-four mice were randomly assigned to the infection group and twenty-four to the control group for both TLR2−/− and TLR4−/− groups. The infected group orally inoculated by lavage with 109 total cells per mL in RTF-4% CMC as previously described (Rivera et al., 2013, Velsko et al., 2015), while sham-infected mice were mock-infected with RTF-4% CMC only. At twenty-four weeks of infection, mice were euthanized and sera, jaws and internal organs were collected.
Detection of Bacterial Genomic DNA
Oral microbial samples were taken one week after each infection with a sterile cotton swab, stored in 150μl TE (Tris-EDTA) buffer, and processed as previously described (Rivera et al., 2013, Chukkapalli et al., 2014, Velsko et al., 2014, Velsko et al., 2015). Tissues for DNA analysis were collected at sacrifice and stored in RNAlater at −80°C, and processed as previously described (Chukkapalli et al., 2014, Chukkapalli et al., 2015a, Velsko et al., 2014, Velsko et al., 2015). All PCR was performed as previously described (Rivera et al., 2013) using the following primers, which detect bacterial species-specific 16S rDNA: P. gingivalis forward 5’-GGT AAG TCA GCG GTG AAA CC-3’, reverse 5’-ACG TCA TCC ACA CCT TCC TC-3’ , T. denticola forward 5’-TAATACCGAATGTGCTCATTTACAT-3’, reverse 5’-CTGCCATATCTCTATGTCATTGCTCTT-3’, T forsythia forward 5′-AAAACAGGGGTTCCGCATGG-3′, reverse 5′-TTCACCGCGGACTTAACAGC-3′ , F. nucleatum forward 5’-TAAAGCGCGTCTAGGTGGTT-3’, reverse 5’-ACAGCTTTGCGACTCTCTGT-3’ (Rivera et al., 2013, Chukkapalli et al., 2014, Velsko et al., 2014, Velsko et al., 2015). Bold letters were altered from the reference for a 100% match with strain ATCC 49256.
Serum Antibody Analysis
Serum was collected on sacrifice and stored at −20°C. P. gingivalis-specific, T. denticola-specific, T. forsythia-specific, and F. nucleatum-specific IgM and IgG antibody titers were determined by ELISA (Rivera et al., 2013, Velsko et al., 2015) using whole cell P. gingivalis, T. denticola, T. forsythia or F. nucelatum as antigen. Serum was diluted 1:100 while secondary goat anti-mouse conjugated to alkaline phosphatase (Bethyl Laboratories, Inc. Montgomery, TX) was used at a 1:5000 dilution. Absorbance of each well was read at OD405 using a Bio-Rad Microplate Reader, and a standard curve was used to determine the titer.
Morphometric Analysis of Alveolar Bone Resorption
Histomorphometric analysis of ABR and interproximal intrabony defects on tooth surface was performed as previously described (Chukkapalli et al., 2014, Velsko et al., 2015) with the following modifications. Cementum was not stained with methylene blue prior to measuring bone resorption. Digital images of both buccal and lingual root surfaces of all molar teeth were captured under a 10×stereo dissecting microscope (SteReo Discovery V8; Carl Zeiss Microimaging, Inc, Thornwood, NY), after superimposition of buccal and lingual cusps to ensure reproducibility and consistency. The surface perimeters of cementoenamel junction (CEJ) i.e., the location where the enamel that covers the anatomical crown of teeth and the cementum that covers anatomical root of teeth meet and alveolar bone crest (ABC) were traced using the calibrated line tool (AxioVision LE 29A software version 4.6.3.). Two examiners blinded to the study performed all measurements twice at separate times. The means of the measurements were obtained for each of the two quadrants. Total ABR was calculated by adding bone resorption calculated on maxilla palatal, maxilla buccal, and mandible lingual alveolar bone. The percentages of interproximal intrabony defects were calculated for all molar tooth surfaces, where percent values indicate the number of sites found to contain intrabony defects per total number of sites analyzed per group. Mice broken jaws or jaws missing molar teeth were excluded from the total number of sites.
Morphometric Analysis of Aortic Atherosclerosis
Hearts (n = 6) and aortas (n = 6) were fixed in 10% neutral buffered formalin and embedded in paraffin. Specimens were then sectioned and assessed for atherosclerotic plaque as previously described (Chukkapalli et al., 2014, Chukkapalli et al., 2015a, Velsko et al., 2014, Velsko et al., 2015). Plaque area, intimal thickness, medial thickness and calculated intimal/medial thickness ratios were measured by a reviewer blinded to the infection and mouse strain using an Olympus DP71 microscope and ImagePro MC 6.0 software standardized to the microscopic objective.
Fluorescence in situ Hybridization (FISH)
FISH was used to detect bacteria that were metabolically active within aortic tissues using bacterial species-specific probes to ribosomal 16S RNA (Moter & Gobel, 2000). FISH was performed on aorta tissue sections as described previously (Chukkapalli et al., 2014, Velsko et al., 2014), using the following specific probes labeled with Alexafluor-568 (Invitrogen, Carlsbad, CA): POGI 5’-CAATACTCGTATCGCCCGTTATTC-3’, TDEN 5’-CATGACTACCGTCATCAAAGAAGC -3’ , B(T)AFO 5’-CGTATCTCATTTTATTCCCCTGTA-3’ , and FUSO 5’-CTAATGGGACGCAAAGCTCTC-3’ , which detect P. gingivalis, T. denticola, T. forsythia, and F. nucleatum, respectively. Tissues were counter-stained with DAPI (ThermoScientific, Asheville, NC) and mounted in Mowiol® 4–88 (Sigma-Aldrich Corp, St. Louis, MO). Data were acquired at 63X on a Leica microscope. Images were processed using Image J (NCBI).
Flow Cytometry
On sacrifice, whole spleens of infected and sham-infected mice (n = 6) were collected in RPMI-1640 supplemented with 10% FBS, 1% sodium pyruvate, 1% non-essential amino acids and 1% L-glutamine (RPMI-complete). Spleens were homogenized and cells frozen in RPMI-10% DMSO at −80°C for analysis. For flow cytometric analysis of T cell subtypes, cell staining and analysis were performed as previously described (Velsko et al., 2015).
Serum Atherosclerosis Risk-factor Measurements
Assessment of the serum lipid profile, oxidized LDL (oxLDL), amyloid A (SAA) , and nitric oxide (NO) was performed on sera collected on sacrifice from infected (n = 6) and sham-infected (n = 6) mice. Procedures were performed as previously described (Chukkapalli et al., 2014, Chukkapalli et al., 2015a, Velsko et al., 2015).
RT2 Profiler PCR Array
Expression levels of 84 genes known to be involved in TLR signaling pathways were examined in aortas of infected (n = 3) and control (n = 3) mice in both TLR2−/− and TLR4−/− groups by qRT-PCR with the RT2 Profiler Mouse TLR Signaling Pathway PCR Array (SABiosciences, Valencia, CA). Tissues were processed and the array was performed as previously described (Chukkapalli et al., 2014, Chukkapalli et al., 2015a, Velsko et al., 2015). Data were analyzed using the PCR Array Data Analysis V4 excel worksheet, downloaded from the SABiosciences website.
Mouse TH1/Th2/Th17 Cytokine Array
Serum was collected from infected and control TLR2−/− and TLR4−/− mice on sacrifice. Sera from each group (n = 5) used to detect 18 cytokines on the Ray Biotech Mouse Th1/Th2/Th17 Cytokine Array (RayBiotech, Inc, Norcross, GA), according to the manufacturer’s protocol. Array slides were read with a GenePix 4400 scanner, using GenePix Pro 7.2.29.002 software. Results were analyzed using the RayBio Analysis Tool excel sheet.
Statistical Analysis
Unpaired, two-tailed Student’s T test was used to assess for statistical significance of serum antibody levels, serum lipid profile, SAA, NO, flow cytometry and horizontal ABR data, with GraphPad Prism 5.0 software. ELISA, flow cytometry, and horizontal ABR graphs show mean with standard deviation. ANOVA was used to determine significance for histology measurements and immunohistochemistry cell counts using the Statview program and post hoc PLSD analysis; graphs are presented as mean ± standard error. P < 0.05 was considered statistically significant.
RESULTS
Polymicrobial infection and periodontal disease progression
TLR2−/−, TLR4−/− mice were infected with polybacterial inoculum, the gingival surface was swabbed 4 days after each infection cycle and bacterial species-specific PCR was performed on plaque samples to test for the presence or absence of bacterial genomic DNA to monitor bacterial colonization and infection. Bacterial colonization of a majority of TLR2−/− mice gingival surfaces with all four bacterial species was confirmed by the seventh infection period, while half of TLR4−/− mice gingival surfaces demonstrated colonization with P. gingivalis, T. denticola, F. nucleatum, and T. forsythia (Table 1). Sterile cotton swabbing of the infected mice gingival surface may not be reflective of the subgingival microflora, the presence of 4 anaerobic species 4 days after oral infection suggests that the organisms were viable and adherent, as dead or non-adherent bacteria would be removed by feeding, drinking and saliva.
Table 1.
Distribution of gingival plaque samples positive for periodontal pathogens gDNA by PCR.
| Positive gingival plaque Samples | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Poly Infection | 1a | 2 | 3a | 4 | 5a | 6a | 7a |
| TLR2−/− (n = 12) | Pg/Td/Tf/Fn Control | 0/7/0/2 | NC | 0/2/0/11 | NC | 0/0/0/0* | 9/0/6/11* | 8/9/8/9* |
| 0/0/0/0 | NC | 0/0/0/0 | NC | 0/0/0/0 | NC | NC* | ||
|
| ||||||||
| TLR4−/− (n = 10) | Pg/Td/Tf/Fn Control | 0/9/0/0 | NC | 0/0/0/9 | NC | 0/0/0/0† | 4/0/4/5† | NC† |
| 0/0/0/0 | NC | 0/0/0/0 | NC | 0/0/0/0‡ | NC‡ | NC‡ | ||
n = 11,
n = 8,
n = 9.
NC – not collected gingival plaque samples to allow the bacterial biofilm to develop without disruption, attach gingival surface, invade epithelial cells, and multiply. Pg - P. gingivalis, Td -T. denticola, Tf - T. forsythia and Fn - F. nucleatum. The first value corresponds to the number of mice that tested positive for P. gingivalis genomic DNA, the second value to the number of mice that tested positive for T. denticola genomic DNA, the third value to the number of mice that tested positive for T. forsythia genomic DNA and the fourth value to the number of mice that tested positive for F. nucleatum genomic DNA at each time point.
Indicate time points at which oral microbial samples were collected (1, 3, 5, 6 and 7 infection cycles) following polymicrobial infection for determination of microbial colonization by species specific PCR analysis.
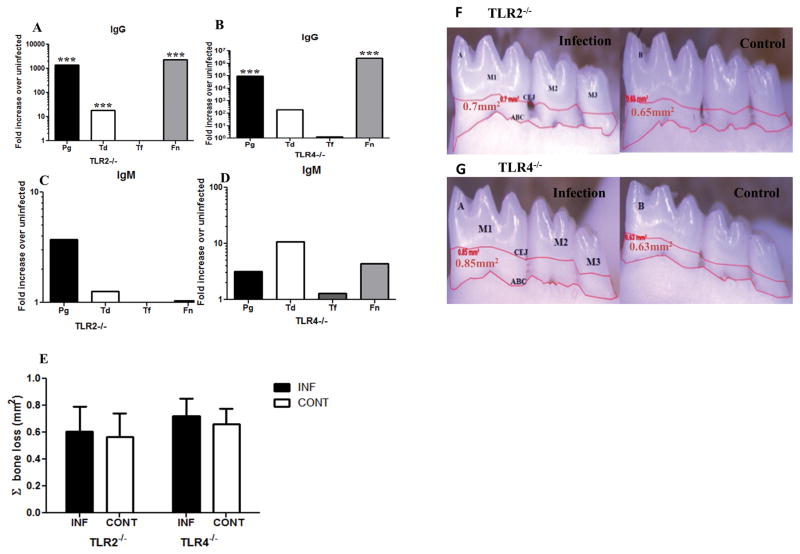
A strong serum IgG response to P. gingivalis, T. denticola and F. nucleatum was detected in TLR2−/− mice (p < 0.001, Fig. 1A), while TLR4−/− mice demonstrated significant IgG titers only to P. gingivalis and F. nucleatum (p < 0.001, Fig. 1B). The serum IgM response of both mouse groups to all four bacterial species was not significant (Fig. 1C & D). These data suggest that T. forsythia poorly colonizes mice in the absence of either TLR2 or TLR4, and subsequently no humoral immune response is mounted against it. Alveolar bone resorption is the result of gingival inflammatory processes, and mice lacking TLR2 or TLR4 receptors have dampened inflammatory responses. As we expected, 24-week-polymicrobial infection did not result in significantly enhanced ABR in either TLR2−/− or TLR4−/− mice, relative to their uninfected control mice in the maxilla palatal, maxilla buccal, and mandible lingual sides of the oral cavity (Fig. 1E – G). Interproximal intrabony defects in humans which suggest more localized patterns of bone resorption on the tooth surface were not significantly different in infected TLR2−/− mice than uninfected control mice while in TLR4−/− mice intrabony defects were almost similar to uninfected control mice (Table 2).
Figure 1. Periodontal disease establishment in TLR2−/− and TLR4−/− mice after 24 weeks of chronic gingival infection with periodontal pathogens.
(A & B) Serum IgG levels in TLR2−/− and TLR4−/− mice. (C & D) Serum IgM levels in TLR2−/− and TLR4−/− mice. Each bar indicates fold increase over uninfected mice. (E) Total alveolar bone resorption in TLR2−/− and TLR4−/− mice after 24 weeks of chronic periodontal infection. Each bar indicates the mean horizontal ABR. (F). Representative mandible lingual view of 24 week infected TLR2−/− and uninfected control mice. (G) Representative mandible lingual view of 24 week infected TLR4−/− and uninfected control mice. Measurements were made between the cementoenamel junction (CEJ), and alveolar bone crest (ABC) of three molar teeth by three independent individuals blinded to the treatment group. Pg–P. gingivalis, Td–T. denticola, Tf–T. forsythia, Fn–F. nucleatum. *** p < 0.001. M1, M2, M3 indicates molar tooth. INF indicates infected and CONT indicates uninfected control mice. Data points and error bars represent means ± SD for infected (n = 6) and uninfected control (n = 6) mice.
Table 2.
Intrabony defect in infected and sham-infected TLR2−/− and TLR4−/− mice.
| Mouse/infection | TLR2−/− | TLR4−/− |
|---|---|---|
| Pg/Td/Tf/Fn | (4/198)* 2%** | (16/198) 8% |
| Control | (4/216) 1.8% | (12/150) 8% |
Number of positive sites/total number of sites examined.
Percentage of positive sites.
Dissemination of Periodontal Pathogens
To determine if the bacteria disseminated intravascular to systemic organs from gingival tissue, we isolated genomic DNA from heart, aorta, liver, spleen, kidney, lung, and brain, and ran PCR to detect the presence of bacterial genomic DNA. Bacteria disseminated similarly between the two mouse strains, with highest bacterial presence in the hearts and aortas of both TLR 2 and 4 deficient mice (Table 3). There was a significant bacterial presence in the kidney of TLR2−/− mice and this may reflect removal of the bacteria from the blood stream, while the bacteria detected in the lungs may be the result of inhalation in addition to intravascular dissemination. Similar to the gingival bacterial colonization/infection, T. forsythia was detected in fewer organs than the other three species, which may be a result of fewer adherences to gingival tissue and invasion of gingival epithelium. Fluorescence in situ hybridization was performed on TLR2−/− and TLR4−/− infected mice aortas to detect live bacterial invasion of the aorta, but no bacterial morphology were observed in the aortic sections examined (n = 6) (data not included).
Table 3.
Distribution of tissue samples positive for P. gingivalis, T. denticola, T. forsythia and F. nucleatum gDNA by PCR.
| Group | Infection | Positive systemic tissue samples | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Heart | Aorta | Liver | Spleen | Kidney | Lung | Brain | ||
| n = 11 | n = 6 | n = 11 | n = 5 | n = 11 | n = 11 | n = 11 | ||
| TLR2−/− | Pg/Td/Tf/Fn | 8/4/5/1 | 5/1/3/0 | 1/0/0/0 | 0/0/0/0 | 9/2/0/11 | 4/3/0/4 | 0/0/0/0 |
| Control | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | |
|
| ||||||||
| n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | n = 7 | ||
| TLR4−/− | Pg/Td/Tf/Fn | 7/2/0/2 | 6/3/0/5 | 0/2/1/1 | 0/0/0/0 | 1/1/0/0 | 3/0/1/1 | 0/0/0/0 |
| Control | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | |
Pg – P. gingivalis, Td – T. denticola, Tf – T. forsythia, Fn – F. nucleatum. The first value corresponds to the number of mice that tested positive for P. gingivalis genomic DNA, the second value to the number of mice that tested positive for T. denticola genomic DNA, the third value to the number of mice that tested positive for T. forsythia genomic DNA and the fourth value to the number of mice that tested positive for F. nucleatum genomic DNA. None of the sham-infected (control) mice were tested positive for any of the bacterial genomic DNA.
Polymicrobial infection and progression of atherosclerotic plaque
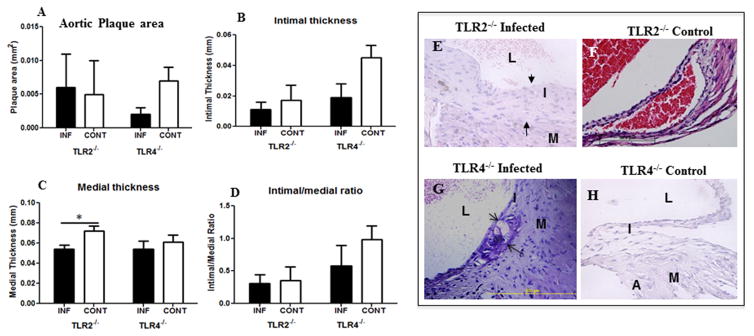
To determine how polymicrobial infection induces atherosclerosis progression in TLR2 and TLR4 deficient mice, we assessed the atherosclerotic plaque accumulation in the aorta at the level of the aortic valve. As expected, the TLR2−/− mice exhibited no significant difference in plaque area (Fig. 2A), intimal thickness (Fig. 2B), or intimal-medial thickness ratio between infected and uninfected control mice (Fig. 2D) except significant medial thickness in TLR2−/− mice (Fig. 2C). The infected TLR4−/− mice, however, exhibited smaller plaque area (Fig. 2A), intimal thickness (Fig. 2B), medial thickness (Fig. 2C), and intimal-medial thickness ratios than uninfected control mice aorta (Fig. 2D), although these values did not reach statistical significance. These studies demonstrate that in the absence of TLR2 and TLR4 signaling there is no increase in atherosclerotic plaque accumulation between infected and uninfected control mice (Fig. 2E–H).
Figure 2. Polybacterial infection-induced changes in the aortic tissue after 24 weeks of chronic gingival infection.
(A) Bar graph of total aortic plaque area (mm2) in infected TLR2−/−, TLR4−/−, and uninfected TLR2−/−, TLR4−/−mice. (B) Bar graph showing intimal layer thickness area (mm) in infected TLR2−/− and TLR4−/− mice compared to uninfected control mice. (C) Bar graph showing medial layer thickness area (mm) in infected TLR2−/− and TLR4−/− mice compared to uninfected control mice. (D) Bar graph showing intimal/medial thickness ratio in infected TLR2−/− and TLR4−/− mice compared to uninfected control mice. (E) Representative cross section of 24 weeks polybacterial-infected TLR2−/− mouse aorta. (F) Representative cross section of 24 weeks uninfected control TLR2−/− mouse aorta. (G) Representative cross section of 24 weeks polybacterial-infected TLR4−/− mouse aorta. (H). Representative cross section of 24 weeks uninfected control TLR4−/− mouse aorta. L-lumen; I- intimal layer; M-medial layer; A- adventitial layer; and arrow indicate plaque. INF indicates infected (n = 6) and CONT indicates uninfected mice (n = 6).
Effect of gingival infection on inflammatory mediators
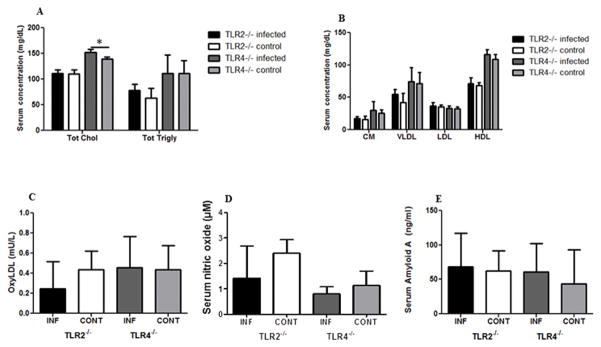
We have previously demonstrated that gingival polymicrobial infection alters the serum lipid profile to a more proatherogenic state (Rivera et al., 2013, Chukkapalli et al., 2015b). The total serum cholesterol and total serum triglycerides were not altered by infection in TLR2−/− mice, while infected TLR4−/− mice had significantly higher total serum cholesterol than uninfected TLR4−/− mice (Fig. 3A). Both total cholesterol and total triglycerides were higher in TLR4−/− mice than in TLR2−/− mice, suggesting that TLR4-mediated inflammatory signaling maintain lipid levels. Infection in TLR2−/− and TLR4−/− mice had no effect on the serum lipid fractions chylomicrons (CM), very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), or high-density lipoprotein (HDL) (Fig. 3B), although TLR2−/− mice had lower chylomicrons, VLDL and HDL concentrations than TLR4−/− mice.
Figure 3. Polybacterial infection-induced changes in serum inflammatory mediators after 24 weeks of chronic oral infection.
(A) Effects of gingival infection with periodontal pathogens on serum levels of total cholesterol and total triglycerides in TLR2−/− and TLR4−/− mice. (B) Effects of gingival infection with periodontal pathogens on serum levels of lipid fractions (CM Chylomicrons, VLDL, LDL, and HDL). (C) Changes in serum levels of oxidized LDL levels in infected TLR2−/− and TLR4−/− mice compared to uninfected control mice. (D) Changes in serum levels of nitric oxide levels in infected TLR2−/− and TLR4−/− mice compared to uninfected control mice. (E) Changes in serum levels of serum amyloid A (SAA) levels in infected TLR2−/− and TLR4−/− mice compared to uninfected control mice. The data are shown as the mean + SD (n = 6 in each group), * p < 0.05. INF indicates infected and CONT indicates uninfected mice.
We have similarly shown that polymicrobial infection can significantly alter serum inflammatory mediators oxidized LDL (oxyLDL), serum nitric oxide (NO) (Chukkapalli et al., 2015b), and serum amyloid A (Rivera et al., 2013) in ApoE−/− mice, which may create an atherosclerosis-prone environment. We assessed the influence of TLR2 and 4 deficiencies on these markers to determine if inflammatory signaling through either TLR enhances these markers. We found that infection did not modulate significant alteration of oxyLDL, NO or SAA levels in either TLR2−/− or TLR4−/− mice (Fig. 3C–E). OxyLDL was slightly lower in infected TLR2−/− mice than uninfected controls, although the levels in infected and control TLR4−/− mice were comparable. Serum NO was slightly less in infected TLR2−/− and TLR4−/− mice than uninfected control mice, while SAA was nearly identical levels across all four mouse groups.
We additionally assessed the levels of cytokines present in the sera of infected and uninfected control TLR2−/− and TLR4−/− mice to determine if oral polymicrobial infection elevated serum inflammatory cytokine profile. We found no significant difference in the levels of all Th1 (IFNγ, IL-1β, IL-6, IL-12p70, IL-28, TNFγ), Th2 (IL-2, IL-4, IL-5, IL-10, IL-28, TGFβ, MIP-3α, IL-13), and Th17 (IL-17, IL-21, IL-22, IL-23) cytokines measured between infected and uninfected control mice for either TLR2 or TLR4 knockouts, indicating both TLR receptors are critical for induction of inflammation following bacterial infection. These results further substantiate the critical role of TLR signaling during polymicrobial infection (Table 4).
Table 4.
Serum cytokines altered during gingival infection with four periodontal bacteria.
| TLR2−/− | TLR4−/− | ||||
|---|---|---|---|---|---|
| Type | Cytokine | Infected | Uninfected | Infected | Uninfected |
| Th1 | IFNγ | 2.05±0.30 | 2.68±1.3 | 2.06±0.92 | 1.96±0.26 |
| IL-1β | 25.9±5.9 | 29.9±16 | 27.6±4.2 | 22.1±4.2 | |
| IL-6 | 23.8±1.5 | 22.6±0.80 | 24.3±2.4 | 23.1±1.2 | |
| IL-12p70 | 24.5±1.7 | 26.9±3.08 | 25.6±4.1 | 30.0±1.5 | |
| IL-28 | 29.6±1.5 | 31.5±3.3 | 32.5±5.06 | 35.4±2.42 | |
| TNFα | 62.4±8.5 | 45.6±20.79 | 44.2±4.5 | 69.6±19 | |
| Th2 | IL-2 | 16.0±1.1 | 15.8±0.53 | 16.6±0.58 | 16.1±1.7 |
| IL-4 | 100.1±7.4 | 99.4±13 | 105.2±29 | 108.6±8.9 | |
| IL-5 | 85.7±0.14 | 85.3±1.0 | 87.1±2.9 | 86.7±0.81 | |
| IL-10 | 24.6±0.71 | 24.8±1.7 | 26.1±1.5 | 27.6±1.2 | |
| IL-28 | 29.6±1.5 | 31.5±3.3 | 32.5±5.06 | 35.4±2.42 | |
| TGFβ1 | 7.59±0.32 | 7.18±0.74 | 7.56±0.32 | 7.94±0.37 | |
| MIP-3α | 38.9±3.28 | 41.10±5.2 | 33.9±1.8 | 31.8±1.3 | |
| IL-13 | 4.8±0.099 | 4.82±0.09 | 5.00±0.26 | 4.84±0.03 | |
| Th17 | IL-17 | 34.9±0.40 | 32.7±0.94 | 31.7±3.9 | 33.6±0.58 |
| IL-21 | 5.25±0.04 | 5.21±0.12 | 5.31±0.21 | 7.19±2.6 | |
| IL-22 | 4.58±0.01 | 4.56±0.02 | 4.59±0.00 | 4.99±0.59 | |
| IL-23 | 1.82±0.38 | 1.69±0.21 | 1.50±0.47 | 2.19±0.42 | |
Values are mean x10 (pg/ml) ± SD x10 , n = 4 infected, n = 3 control.
Additionally, we assessed cytokine production by cultured, unstimulated splenocytes to determine if infection induced a peripheral T cell response, and if specific TLR deficiency altered this response. We found that infected TLR2−/− mice splenocytes express significantly elevated levels of Th-17-type cytokines IL-17, IL-21, IL-22 (p < 0.05 - p < 0.01) and IL-23 (p < 0.05) as well as IL-13, IFNγ, MIP-3α and TGFβ1 (p < 0.05 - p < 0.01) compared to uninfected control mice (Table 4). However, infected TLR4−/− mice did not express significantly different levels of any of the Th1, Th2, and Th17 cytokines examined (Table 5). The significant increased expression of Th17 cytokines suggest that intact TLR4 signaling in TLR2 deficient mice in response to a polymicrobial infection may promote a pro-inflammatory Th17 response.
Table 5.
Splenocyte cytokine concentration in TLR2−/− and TLR4−/− infected and sham-infected mice.
| TLR2−/− | TLR4−/− | ||||
|---|---|---|---|---|---|
| Type | Cytokine | Infected | Uninfected | Infected | Uninfected |
| Th1 | IFNγ | 1.8±0.42* | 1.1±0.13 | 1.54±0.12 | 1.74±0.25 |
| IL-1β | 22.9±1.3 | 18.9±1.9 | 23.42±1.8 | 21.5±4.7 | |
| IL-6 | 22.3±1.6 | 22.0±0.13 | 21.54±0.41 | 22.5±0.21 | |
| IL-12p70 | 24.5±4.8 | 24.1±3.1 | 32.07±1.2 | 32.4±0.66 | |
| IL-28 | 36.1±1.1 | 31.6±0.56 | 38.60±1.2 | 37.7±1.9 | |
| TNFα | 49.4±6.6 | 34.8±8.5 | 43.70±.34 | 62.9±1.2 | |
| Th2 | IL-2 | 17.8±1.5 | 14.6±1.9 | 15.47±0.69 | 15.3±0.68 |
| IL-4 | 10.9±20 | 87.1±8.3 | 12.48±7.3 | 12.1±7.8 | |
| IL-5 | 85.3±0.72 | 83.5±0.22 | 8.50±0.64 | 8.49±0.64 | |
| IL-10 | 29.1±2.6 | 22.6±2.0 | 28.59±0.47 | 28.3±1.2 | |
| IL-28 | 36.1±1.1 | 31.6±0.56 | 38.60±1.2 | 37.7±1.9 | |
| TGFβ1 | 7.90±0.097* | 7.0±0.39 | 7.577±0.12 | 7.75±0.21 | |
| MIP-3α | 30.7±0.28** | 28.7±0.25 | 30.57±0.074 | 29.7±0.42 | |
| IL-13 | 4.99±0.057** | 4.75±0.0002 | 4.90±0.05 | 4.95±0.03 | |
| Th17 | IL-17 | 35.7±1.07** | 31.4±2.1 | 31.78±0.82 | 32.9±0.51 |
| IL-21 | 5.59±0.079** | 4.98±0.14 | 5.40±0.21 | 5.49±0.032 | |
| IL-22 | 4.60±0.026* | 0.46±0.003 | 4.56±0.011 | 4.55±0.001 | |
| IL-23 | 1.94±0.52* | 1.19±0.12 | 1.31±0.13 | 1.79±0.15 | |
Values are mean x103(pg/ml) ± SD x103, n = 4 infected, n = 3 control.
p < 0.05 infected vs. control,
p < 0.01 infected vs. control.
Infection-induced changes in aortic TLR signaling gene expression
We have previously reported that monoinfection with P. gingivalis and T. denticola induces gene expression changes in aortic tissues in genes known to contribute to atherosclerotic plaque development (Chukkapalli et al., 2014, Chukkapalli et al., 2015b, Velsko et al., 2014, Velsko et al., 2015). Here we investigated the gene expression changes in TLR signaling pathways in aortic tissues, to determine how signaling changes when the major periodontal pathogen-sensing TLRs are not expressed. We found that in TLR2−/− mice, which are able to signal through TLR4, seventeen of eighty-four genes examined by array had a fold change of > 2.5, while one gene, Lta had a fold change of < −2.5 (Table 6). On the other hand, in TLR4−/− mice, which have intact TLR2 signaling, one gene had a fold change of > 2.5, while five genes had a fold change of < −2.5, one of which, Il-6, had a p-value < 0.0001 (Table 6). Three genes, Hspd1, Il2, Jun, have statistically significant p-values, but fold changes were below the cut-off levels (Table 6).
Table 6.
Polymicrobial infection-induced aortic TLR signaling gene expression changes.
| TLR2−/− mice | TLR4−/− mice | ||||
|---|---|---|---|---|---|
| Genes | p-value | Fold change | Genes | p-value | Fold change |
|
|
|
||||
| Ccl2 | 0.3054 | 2.53 | Fos | 0.1158 | −3.28 |
| Chuk | 0.3037 | 3.65 | Hspa1a | 0.3813 | 2.51 |
| Clec4e | 0.3732 | 4.37 | Ifng | 0.1007 | −2.56 |
| Csf2 | 0.2618 | 3.51 | Il-1α | 0.3490 | −5.36 |
| Fos | 0.1637 | 3.61 | Il-6 | 0.0001 | −2.78 |
| Hmgb1 | 0.3351 | 3.12 | Il-12α | 0.0583 | −4.79 |
| Hspa1a | 0.3604 | 6.72 | Hspd1 | 0.0390 | 1.46 |
| Ifnb1 | 0.3725 | 4.32 | Il2 | 0.0253 | −1.76 |
| Il-1β | 0.3698 | 3.04 | Jun | 0.0023 | −2.22 |
| Il-2 | 0.3762 | 2.59 | |||
| Il-6 | 0.3710 | 4.51 | |||
| Il-10 | 0.3802 | 3.04 | |||
| Lta | 0.5684 | −3.00 | |||
| Mapk8 | 0.3011 | 3.41 | |||
| Muc13 | 0.3784 | 3.98 | |||
| Myd88 | 0.3801 | 2.60 | |||
| Ptgs2 | 0.3748 | 3.69 | |||
| Ube2v1 | 0.3785 | 3.28 | |||
Polymicrobial-infected and sham-infected control TLR2−/− and TLR4−/− mouse aortic tissue samples were processed and analyzed as described in methods. Bold letters indicate genes significantly altered in TLR4−/− mice.
The difference in the number of genes affected by infection in TLR2−/− and TLR4−/− mice indicates that signaling through TLR4 is more pronounced in infection. As this array included TLR4, we know that in the absence of TLR2, TLR4 expression was not greatly elevated, demonstrating only a 1.21-fold increase in expression, suggesting a minimal change in surface expression of the protein. Interestingly, of the 6 genes that were altered in TLR4−/− mice, 5 were down-regulated, as was TLR2, -1.45-fold, while the majority of genes altered in TLR2−/− mice were up-regulated.
DISCUSSION
Atherosclerosis is a chronic inflammatory disease characterized by inflammation in arterial intima and lipid retention (Libby, 2002, Libby, 2012). Innate immune signaling mechanisms are thought to play a central role in the pathogenesis of atherosclerosis as they involve the activation of pattern-recognition receptors (PRR) and induction of inflammatory responses (Hansson et al., 2002). Periodontal disease is a significant source of chronic inflammatory mediators and TLRs play an instructive role in the development of innate and T cell adaptive responses to periodontal pathogens. The cells of the periodontal tissues (gingival epithelium) also express TLRs, and TLR sensing and signaling plays a pivotal role in maintaining the periodontal health. Cells of the periodontium express different TLRs and actively participate in the immune response against dental plaque bacteria. These shared inflammatory processes may partly explain the association between atherosclerosis and periodontal disease.
Although there is clear evidence for a role of TLR2- and TLR4-induced inflammation in development of both periodontal disease and atherosclerosis, the precise role of either in promoting inflammatory responses to polymicrobial infections is not clear. Surface components of all four of the bacterial species we used are known to stimulate either TLR2, P. gingivalis fimbriae (Hajishengallis et al., 2008), T. denticola major sheath protein (Nussbaum et al., 2009), T. forsythia BspA (Inagaki et al., 2006), F. nucleatum FomA (Toussi et al., 2012) or TLR4, P. gingivalis LPS (Darveau et al., 2004), F. nucleatum LPS (Grenier & Grignon, 2006), indicating the potential of a polymicrobial infection to strongly stimulate TLR signaling.
Both P. gingivalis and T. denticola are known to subvert TLR signaling, which is thought to contribute to periodontal disease pathogenesis. In human monocytes, P. gingivalis binding to CXCR4 inhibited TLR2-dependent MyD88 signaling (Hajishengallis et al., 2013), while in neutrophils P. gingivalis actively induced proteasomal degradation of MyD88 (Maekawa et al., 2014), which inhibited neutrophil phagocytosis and may promote bacterial community dysbiosis by preventing immune-mediated clearance (Maekawa et al., 2014). T. denticola was likewise shown to interfere with TLR2 initiated signaling, albeit in gingival epithelial cells, that resulted in decreased expression of human β-defensins (Shin et al., 2010), which could reduce pathogen clearance and promote gingival tissue degradation.
However, in our infection model, TLR2 deficiency did not enhance either local or systemic inflammation in polymicrobial-infected mice. In prior work we have demonstrated a marked increase in atherosclerosis and alveolar bone resorption in hyperlipidemic mice. We observed no difference in ABR, atherosclerotic plaque area, or any of the serum atherosclerosis risk factors, which suggests that loss of TLR2-initiated signaling can protect against inflammation and subsequent disease development in infections with multiple bacterial species.
Our observation of increased expression of heat shock proteins, especially Hsp70 (hspa1a) common among both TLR2−/− and TLR4−/− mice points towards the role these stress response molecules play in atherosclerosis. Hsp70 has been previously shown to have the potential to foster an anti-inflammatory environment which might attenuate atherosclerosis (George et al., 1998, Zhou et al., 2001, Binder et al., 2002). The mechanisms by which HSP induce these atheroprotective effects include the inherent anti-inflammatory properties of Hsp70 and/or through direct effects on the biology and functional status of endothelial cells (Pockley et al., 2009). However, additional studies will be necessary to determine whether altered HSP levels can be directly correlated with greater stress resistance and matrix proteolysis.
We recently published a novel integrin β6−/− mouse model of gingival infection-induced periodontal disease in which we investigated infection-induced gene expression changes in the aorta (Velsko et al., 2015), and found that expression of Tlr1 and Tlr9 were altered in aortic tissues in the presence of periodontal disease, while Tlr2 and Tlr4 were not. Although the mouse model was a different genetic background from those used here, our findings suggest that Tlr1 and Tlr9 signaling may be more important in periodontal disease-influenced atherosclerosis than either Tlr2 or Tlr4, and future studies should investigate mice deficient in these TLRs. Involvement of TLR9 is intriguing as this receptor is found intracellularly in endosomes and senses microbial DNA, as opposed to cell surface markers TLR2 and TLR4 that sense bacterial surface components. Involvement of TLR9 suggests that intracellular bacterial invasion is a more potent driver of inflammation than bacterial-host cell surface interactions. It is also important to note the differences in these TLR knockout examining polymicrobial infection studies and those using P. gingivalis alone (Hayashi et al., 2012, Hayashi et al., 2010). Bacterial-bacterial interactions may change gene expression of the interacting organisms (Tan et al., 2014, Aruni et al., 2011, Sarkar et al., 2014) and therefore alter protein expression and behavior of the bacteria, such that bacterial-host cell interactions in polymicrobial infections may vary significantly from those observed in single-species infections (Shin et al., 2013).
Further it is of significance to note that periodontal tissues are constantly exposed to microbes, and tissue homeostasis is mediated by a balance between continual turnovers of microbial and host cells. This constant activity provides a platform for the continuous presence of nucleic acids in the periodontium. It is of significance to understand how innate immune cells integrate signals especially in a dysbiotic ecosystem like that of polymicrobial-induced periodontal disease. Recent reports have revealed that microbial nucleic acid sensing, specifically TLR9, plays a novel role in periodontal disease pathogenesis (Hajishengallis & Sahingur, 2014). It was also revealed that TLR9 deficiency can affect the extent of inflammatory responses despite similar TLR2 and TLR4 expression through crosstalk via downstream signaling pathways (Kim et al., 2015). Endothelial cells also express TLR9 and bacterial DNA activation of endothelial cells via TLR9 has been reported to promote neutrophil chemotaxis (El Kebir et al., 2009). Furthermore, heart failure due to polymicrobial sepsis was ameliorated in TLR9−/− mice compared to their WT counterparts (Lohner et al., 2013). It has been reported that TLR3 and TLR7 play protective roles in atherosclerosis (Cole et al., 2011, Salagianni et al., 2012). These observations highlight the significance to look beyond extracellular TLRs and potentiate the initiation of future studies to understand the intricate role endosomal TLRs plays in initiation of atherosclerosis.
CONCLUSIONS
In conclusion, our study highlights the involvement of multiple innate immune mediators in promoting the inflammatory response against chronic periodontal bacterial infection. Future studies should be initiated to expand our understanding of the intimate interlacing of various convergent innate immune pathways to better define new therapeutic strategies.
Acknowledgments
This work was supported by NIH National Institute for Dental and Craniofacial Research NIDCR R01DE020820. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We acknowledge Dr. Donghang for his assistance in histology tissue processing.
Footnotes
Conflict of interest statement
We have no financial conflicts of interest.
References
- Aruni AW, Roy F, Fletcher HM. Filifactor alocis has virulence attributes that can enhance its persistence under oxidative stress conditions and mediate invasion of epithelial cells by porphyromonas gingivalis. Infect Immun. 2011;79:3872–3886. doi: 10.1128/IAI.05631-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder CJ, Chang MK, Shaw PX, et al. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- Choi SH, Harkewicz R, Lee JH, et al. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukkapalli SS, Rivera MF, Velsko IM, et al. Chronic oral infection with major periodontal bacteria Tannerella forsythia modulates systemic atherosclerosis risk factors and inflammatory markers. Pathog Dis. 2015a;73 doi: 10.1093/femspd/ftv009. pii: ftv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukkapalli SS, Rivera MF, Velsko IM, et al. Invasion of oral and aortic tissues by oral spirochete Treponema denticola in ApoE(−/−) mice causally links periodontal disease and atherosclerosis. Infect Immun. 2014;82:1959–1967. doi: 10.1128/IAI.01511-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukkapalli SS, Velsko IM, Rivera-Kweh MF, Zheng D, Lucas AR, Kesavalu L. Polymicrobial Oral Infection with Four Periodontal Bacteria Orchestrates a Distinct Inflammatory Response and Atherosclerosis in ApoEnull Mice. PLoS One. 2015b;10:e0143291. doi: 10.1371/journal.pone.0143291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JE, Navin TJ, Cross AJ, et al. Unexpected protective role for Toll-like receptor 3 in the arterial wall. Proc Natl Acad Sci U S A. 2011;108:2372–2377. doi: 10.1073/pnas.1018515108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan MP, Hamlet SM, Westerman B, Palmer JE, Faddy MJ, Seymour GJ. Acquisition and loss of Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans and Prevotella intermedia over a 5-year period: effect of a triclosan/copolymer dentifrice. J Clin Periodontol. 2003;30:532–541. doi: 10.1034/j.1600-051x.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- Darveau RP, Pham TT, Lemley K, et al. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect Immun. 2004;72:5041–5051. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Goubier A, Joubert G, Kaiserlian D. Oral tolerance and regulation of mucosal immunity. Cell Mol Life Sci. 2005;62:1322–1332. doi: 10.1007/s00018-005-5036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kebir D, Jozsef L, Pan W, Wang L, Filep JG. Bacterial DNA activates endothelial cells and promotes neutrophil adherence through TLR9 signaling. J Immunol. 2009;182:4386–4394. doi: 10.4049/jimmunol.0803044. [DOI] [PubMed] [Google Scholar]
- Fiehn NE, Larsen T, Christiansen N, Holmstrup P, Schroeder TV. Identification of periodontal pathogens in atherosclerotic vessels. J Periodontol. 2005;76:731–736. doi: 10.1902/jop.2005.76.5.731. [DOI] [PubMed] [Google Scholar]
- George J, Afek A, Gilburd B, et al. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis. 1998;138:147–152. doi: 10.1016/s0021-9150(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Gibson FC, 3rd, Hong C, Chou HH, et al. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:2801–2806. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]
- Grenier D, Grignon L. Response of human macrophage-like cells to stimulation by Fusobacterium nucleatum ssp. nucleatum lipopolysaccharide. Oral Microbiol Immunol. 2006;21:190–196. doi: 10.1111/j.1399-302X.2006.00278.x. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, McIntosh ML, Nishiyama SI, Yoshimura F. Mechanism and implications of CXCR4-mediated integrin activation by Porphyromonas gingivalis. Mol Oral Microbiol. 2013;28:239–249. doi: 10.1111/omi.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Sahingur SE. Novel inflammatory pathways in periodontitis. Adv Dent Res. 2014;26:23–29. doi: 10.1177/0022034514526240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Wang M, Liang S, Triantafilou M, Triantafilou K. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc Natl Acad Sci U S A. 2008;105:13532–13537. doi: 10.1073/pnas.0803852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- Hayashi C, Madrigal AG, Liu X, et al. Pathogen-mediated inflammatory atherosclerosis is mediated in part via Toll-like receptor 2-induced inflammatory responses. J Innate Immun. 2010;2:334–343. doi: 10.1159/000314686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi C, Papadopoulos G, Gudino CV, et al. Protective role for TLR4 signaling in atherosclerosis progression as revealed by infection with a common oral pathogen. J Immunol. 2012;189:3681–3688. doi: 10.4049/jimmunol.1201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashimori M, Tatro JB, Moore KJ, Mendelsohn ME, Galper JB, Beasley D. Role of toll-like receptor 4 in intimal foam cell accumulation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:50–57. doi: 10.1161/ATVBAHA.110.210971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki S, Onishi S, Kuramitsu HK, Sharma A. Porphyromonas gingivalis vesicles enhance attachment, and the leucine-rich repeat BspA protein is required for invasion of epithelial cells by "Tannerella forsythia". Infect Immun. 2006;74:5023–5028. doi: 10.1128/IAI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavalu L, Sathishkumar S, Bakthavatchalu V, et al. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect Immun. 2007;75:1704–1712. doi: 10.1128/IAI.00733-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PD, Xia-Juan X, Crump KE, Abe T, Hajishengallis G, Sahingur SE. Toll-Like Receptor 9-Mediated Inflammation Triggers Alveolar Bone Loss in Experimental Murine Periodontitis. Infect Immun. 2015;83:2992–3002. doi: 10.1128/IAI.00424-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohner R, Schwederski M, Narath A, et al. Toll-like receptor 9 promotes cardiac inflammation and heart failure during polymicrobial sepsis. Mediators Inflamm. 2013;2013:261049. doi: 10.1155/2013/261049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan M, Amar S. Toll-like receptor-2 mediates diet and/or pathogen associated atherosclerosis: proteomic findings. PLoS One. 2008;3:e3204. doi: 10.1371/journal.pone.0003204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Krauss JL, Abe T, et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 2014;15:768–778. doi: 10.1016/j.chom.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KS, Wong MH, Shah PK, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moter A, Gobel UB. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J Microbiol Methods. 2000;41:85–112. doi: 10.1016/s0167-7012(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Mullick AE, Soldau K, Kiosses WB, Bell TA, 3rd, Tobias PS, Curtiss LK. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum G, Ben-Adi S, Genzler T, Sela M, Rosen G. Involvement of Toll-like receptors 2 and 4 in the innate immune response to Treponema denticola and its outer sheath components. Infect Immun. 2009;77:3939–3947. doi: 10.1128/IAI.00488-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Calderwood SK, Multhoff G. The atheroprotective properties of Hsp70: a role for Hsp70-endothelial interactions? Cell Stress Chaperones. 2009;14:545–553. doi: 10.1007/s12192-009-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera MF, Lee JY, Aneja M, et al. Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoE(null) mice. PLoS One. 2013;8:e57178. doi: 10.1371/journal.pone.0057178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Salagianni M, Galani IE, Lundberg AM, et al. Toll-like receptor 7 protects from atherosclerosis by constraining "inflammatory" macrophage activation. Circulation. 2012;126:952–962. doi: 10.1161/CIRCULATIONAHA.111.067678. [DOI] [PubMed] [Google Scholar]
- Sarkar J, McHardy IH, Simanian EJ, Shi W, Lux R. Transcriptional responses of Treponema denticola to other oral bacterial species. PLoS One. 2014;9:e88361. doi: 10.1371/journal.pone.0088361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, Baek KJ, Choi YS, Choi Y. A periodontal pathogen Treponema denticola hijacks the Fusobacterium nucleatum-driven host response. Immunol Cell Biol. 2013;91:503–510. doi: 10.1038/icb.2013.35. [DOI] [PubMed] [Google Scholar]
- Shin JE, Kim YS, Oh JE, Min BM, Choi Y. Treponema denticola suppresses expression of human {beta}-defensin-3 in gingival epithelial cells through inhibition of the toll-like receptor 2 axis. Infect Immun. 2010;78:672–679. doi: 10.1128/IAI.00808-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Tan KH, Seers CA, Dashper SG, et al. Porphyromonas gingivalis and Treponema denticola exhibit metabolic symbioses. PLoS Pathog. 2014;10:e1003955. doi: 10.1371/journal.ppat.1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussi DN, Liu X, Massari P. The FomA porin from Fusobacterium nucleatum is a Toll-like receptor 2 agonist with immune adjuvant activity. Clin Vaccine Immunol. 2012;19:1093–1101. doi: 10.1128/CVI.00236-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Hajjar AM, et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- Velsko IM, Chukkapalli SS, Rivera MF, et al. Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PLoS One. 2014;9:e97811. doi: 10.1371/journal.pone.0097811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velsko IM, Chukkapalli SS, Rivera-Kweh MF, et al. Periodontal pathogens invade gingiva and aortic adventitia and elicit inflammasome activation in alphavbeta6 integrin-deficient mice. Infect Immun. 2015;83:4582–4593. doi: 10.1128/IAI.01077-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XH, Shah PK, Faure E, et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- Zhou X, Caligiuri G, Hamsten A, Lefvert AK, Hansson GK. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:108–114. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]