Abstract
Pb exposure is associated with cognitive deficits including Attention Deficit Hyperactivity Disorder (ADHD) in children and alters auditory temporal processing in humans and animals. Serotonin has been implicated in auditory temporal processing and previous studies from our laboratory have demonstrated that developmental Pb decreases expression of serotonin (5-HT) in the adult murine lateral superior olive (LSO). During development, certain non-serotonergic sensory neurons, including auditory LSO neurons, transiently take up 5-HT through the serotonin reuptake transporter (SERT). The uptake of 5-HT is important for development of sensory systems. This study examines the effect of Pb on the serotonergic system in the LSO of the early postnatal mouse. Mice were exposed to moderate Pb (0.01mM) or high Pb (0.1mM) throughout gestation and postnatal day 4 (P4) and P8. We found that Pb exposure prolongs the normal developmental expression of 5-HT by LSO neurons and this is correlated with expression of SERT on LSO cell bodies. The prolonged expression of 5-HT by postnatal LSO neurons is correlated with decreased synaptic immunolabeling within the LSO. This Pb-associated decrease in synaptic density within the LSO could contribute to the auditory temporal processing deficits and cognitive deficits associated with developmental Pb exposure.
Keywords: Lead acetate, serotonin reuptake transporter (SERT), serotonin, superior olivary nuclei, auditory system, development
1.1 Introduction
Lead (Pb) is a widespread environmental pollutant with neurotoxic effects causing neurobehavioral and cognitive deficits in humans (Finkelstein et al., 1998; Lanphear et al., 2000; Canfield et al., 2003). The developing central nervous system (CNS) is particularly susceptible to environmental Pb exposure (Landrigan and Todd, 1994; Moreira et al., 2001), suggesting that Pb may alter critical stages of development. Currently, the Centers for Disease Control (CDC) states that there is no safe blood lead level in children and that action be initiated for children with blood Pb levels above 5µg/dL (http://www.cdc.gov/nceh/lead/; 2/2/16). Blood Pb levels lower than 10 µg/dL have been shown to produce cognitive and neurobehavioral disorders such as attention deficit hyperactivity disorder (ADHD), and dyslexia (Glotzer et al., 1995; Lanphear et al., 2000; Bellinger and Bellinger, 2006; Braun et al., 2006; Bellinger, 2008). Even very low blood Pb levels (below 2 µg/dL) have been recently shown to be a risk factor for ADHD (Ha et al., 2009; Kim et al., 2013). Pb exposure is also associated with deficits in central auditory temporal processing (Finkelstein et al., 1998; Lurie et al., 2006; Jones et al., 2008). Of significance to the current study, children with either dyslexia or ADHD have also been shown to have deficits in auditory temporal processing (Breier et al., 2003; Facoetti et al., 2003; Putter-Katz et al., 2005; Wright and Conlon, 2009; Jafari et al., 2015).
The cellular mechanism that underlies these Pb-induced cognitive and neurobehavioral dysfunctions are largely unknown, however serotonin (5-HT) may play a role. 5-HT has been implicated in the modulation of auditory temporal processing (Hurley et al., 2002; Hurley and Pollak, 2005; Papesh and Hurley, 2015) although the role of 5-HT during central auditory development has not been fully elucidated. Studies in our laboratory have found that low-level Pb exposure during development decreases 5-HT and vesicular monoamine transporter 2 (VMAT2) immunostaining in brainstem auditory nuclei, particularly in the lateral superior olive (LSO) (Fortune and Lurie, 2009). Thus the effect of Pb on cognitive function could be mediated through the serotonergic system.
It is well established that during brain development, certain non-serotonergic neurons transiently express 5-HT. These neurons include the principal projection neurons of sensory systems such as the auditory, visual, and somatosensory systems (Gaspar et al., 2003). These neurons cannot synthesize 5-HT, instead, they accumulate 5-HT through uptake by SERT, a high affinity 5-HT transporter, which is also transiently expressed in these same neurons (Gaspar et al., 2003; Narboux-Neme et al., 2008; Thompson, 2008). In the auditory system, a subset of LSO neurons transiently express 5-HT from postnatal day 1 (P1) to P8 in wild-type mice, and from embryonic day 18 to P10 in Monoamine Oxidase A (MAOA) knockout mice (Cases et al., 1998; Thompson, 2006). Because Pb exposure decreases 5-HT within the developing LSO, we hypothesized that Pb disrupts the normal transient uptake of 5-HT by LSO neurons during early postnatal development.
The current study was undertaken to determine whether developmental Pb exposure alters the normal transient uptake of 5-HT by developing LSO neurons. Brainstem sections from postnatal control and Pb-exposed mice were quantified for 5-HT and SERT and total brainstem levels of 5-HT were measured by HPLC analysis. Because our previous studies had found that Pb exposure reduced synaptic development in the adult LSO (Fortune and Lurie, 2009), we also quantified synaptophysin immunlabeling (SYP) in Pb exposed postnatal mice to determine if Pb-induced changes in the 5-HT system resulted in decreased numbers of synapses in the developing LSO neurons.
We found that Pb prolongs the length of time that developing LSO neurons express 5-HT until at least P8. The expression of 5-HT in LSO neurons is correlated with the expression of SERT on LSO cell bodies, and total brainstem levels of 5-HT increased with the moderate dose of developmental Pb exposure. Pb also decreased immunoreactivity for SYP, demonstrating that the Pb-induced disruption of 5-HT accumulation in LSO neurons is correlated with impaired synaptic maturation within the LSO.
Materials and Methods
2.1 Chronic lead exposure to CBA/CaJ mice
Breeding pairs of CBA/CaJ mice were obtained from the Jackson Laboratory (Bar Harbor, MA). Mice were maintained in microisolator units and kept in the University of Montana specific pathogen free animal facility. Cages, bedding, and food were sterilized by autoclaving and mice were handled with aseptic gloves. Mice were allowed food and water ad libitum. All animal use was in accordance with NIH and University of Montana IACUC guidelines. Thirteen breeding pairs of CBA mice were randomly assigned to three groups having unlimited access to water (pH 3.0) containing 0 mM (control), 0.01 mM (moderate) or 0.1 mM (high) Pb acetate. Offspring were exposed to Pb throughout gestation and through the dam’s milk until sacrifice. The concentrations of Pb yielded blood Pb levels of 8.0 ± 0.4 µg/dL in moderate Pb, and 42.3 ± 1.97 µg/dL in high Pb P21 mice.
Blood was collected from deeply anesthetized mice by retro-orbital puncture. Blood Pb levels were measured by the Montana Health Department in Helena, MT. The means for the No Pb group include values of <1.0 which were included in the data set as equal to 1.0 (data not shown) (Prins et al., 2010).
2.1.2 Tissue preparation for Immunohistochemistry
Mice were deeply anesthetized using 2’,2’,2’-tribromoethanol (TBE) and perfused transcardially with 4% Na-periodate-lysine-paraformaldehyde fixative (PLP, final concentrations 0.01M sodium periodate, 0.075M lysine-HCl, 2.1% paraformaldehyde, 0.037M phosphate). Brains were removed and post-fixed in PLP overnight at 4°C, rinsed 3 times for 10 minutes each in phosphate buffered saline (PBS) and transferred to a 20% sucrose solution in PBS for 2 days at 4°C. Finally, brains were transferred to a 1:1 mixture of a 20 % sucrose solution in PBS and Optimal Cutting Temperature (O.C.T.) compound (Sakura Finetek, Torrance, CA) for 1–2 days at 4°C. Brains were embedded into 1.5 cm square embedding cups filled with O.C.T. compound, and then frozen in dry ice and 100% ethanol and stored at −20°C. Ten-micron tissue sections were cut on a Thermo Shandon Cryotome Cryostat (Thermo Shandon, Pittsburg, PA) and a one in three series was collected for each brain. Alternate sections were labeled with thionin and compared to the immunolabeled sections to confirm the identity and location of LSO neurons.
2.1.3 Immunohistochemistry
Alternate sections from brains from no Pb, moderate Pb, and high Pb (n=5–8 per treatment group) were thawed to room temperature and rinsed in PBS three times for 10 minutes each. Sections were then permeabilized for 30 minutes in 0.5% Triton X-100 in PBS and blocked for 30 minutes with 4% of the appropriate normal serum (Vector Laboratories, Burlingame, CA) in PAB (1% sodium azide, 0.5% bovine serum albumin in PBS) and incubated with primary antibody for 1–4 days in a humid chamber at 4°C. The sections were rinsed in PBS three times for 10 minutes each and incubated with the appropriate secondary antibody, Alexa Fluor-488, 568, 594, 633; 1:400, or 488 Avidin-Biotin complex; 1:500 (Invitrogen, Grand Island, NY) in PAB for 1 hour at room temperature in the dark. Sections were then rinsed in PBS followed by distilled water and coverslipped with FluorSaverTM (Calbiochem®, San Diego, CA) and stored at 4°C. The primary antibodies used for immunohistochemitry were as follows: rabbit polyclonal anti-5-HT (1:10,000); goat polyclonal anti-5-HT (1:500), rabbit polyclonal anti-SERT (1:250), mouse monoclonal anti-Synaptophysin (1:12,000).
2.1.4 Antibodies
The rabbit polyclonal against serotonin (5-HT) was raised in rabbit against serotonin coupled to bovine serum albumin with paraformaldehyde (Cat. No. 20080, ImmunoStar Inc., Hudson, WI). No cross-reactivity of serotonin antisera was seen with 5-hydroxytrytophan, 5-hydroxyindole-3-acetic acid, and dopamine (manufacturer’s specifications). The immunostar 5-HT antibody labels our mouse brainstem tissue in a staining pattern that is virtually identical to other studies that use the same antiserum to label the mouse superior olive and inferior colliculus (Hurley et al., 2002; Thompson, 2006) and has been previously well characterized in our system (Fortune and Lurie, 2009). In addition, preadsorption with the 5-HT/bovine serum albumin (BSA) conjugate protein (20 µg/ml, Cat. No. 20081, ImmunoStar Inc., Hudson, WI) abolishes all immunoreactivity in contrast to preadsorption with BSA, which does not affect immunostaining.
The goat polyclonal against serotonin (5-HT) was used for the double-label experiments and was raised in goat against serotonin coupled to bovine serum albumin with paraformaldehyde (Cat. No. 20079, ImmunoStar Inc., Hudson, WI). No cross-reactivity of serotonin antisera was seen with 5-hydroxytrytophan, 5-hydroxyindole-3-acetic acid, and dopamine (manufacturer’s specifications). This goat polyclonal 5-HT antibody labels our mouse brainstem sections in a staining pattern similar to that of the rabbit polyclonal 5-HT antibody. In addition, preadsorption with the 5-HT/bovine serum albumin (BSA) conjugate protein (20 µg/ml, Cat. No. 20081, ImmunoStar Inc., Hudson, WI) eliminates all immunoreactivity, whereas preadsorption with BSA does not affect immunostaining (data not shown).
The rabbit polyclonal antibody against the serotonin transporter (SERT) was raised in rabbit against synthetic peptide sequence corresponding to amino acids (602–622) of rat 5-HT transporter coupled to keyhole limper hemocyanin (Cat. No. 24330, ImmunoStar Inc., Hudson, WI). The SERT antibody labels our mouse brainstem sections in a staining pattern virtually identical to that observed in the mouse superior olive using the same antibody (Thompson and Thompson, 2009).
The rabbit polyclonal against Tryptophan Hydroxylase (TPH) was raised in sheep against recombinant rabbit tryptophan hydroxylase, isolated as inclusion bodies from E. coli and purified by preparative SDS PAGE (Cat. No. AB1556, Chemicon, USA). The TPH antibody labels our mouse brainstem sections in a staining pattern that is virtually identical to that observed in the dorsal raphe using the same antibody (Maguire et al., 2014).
The goat polyclonal against Monoamine Oxidase A (MAOA) was raised in goat against a peptide mapping near the C-terminus of MAO-A of human origin (Cat. No. SC18396; Santa Cruz Biotechnology, USA). The MAOA antibody co-localizes with the TPH antibody in dorsal raphe neurons in our mouse brain sections in a similar pattern to locus coerulius neurons that are double labeled with TPH antibodies and the same MAO-A antibody as used in the present study (Sader-Mazbar et al., 2013).
The mouse monoclonal antibody against synaptophysin was raised against in the vesicular fraction of bovine brain against the SY38 epitope; a pentapeptide repeat structure in the C-terminal cytoplasmic tail of synaptophysin (Cat. No. MAB5258, Millipore, Temecula, CA). This synaptophysin antibody labels our mouse brainstem sections in a staining pattern that is virtually identical to the pattern seen in the ferret superior olive (Alvarado et al., 2004), and the rat superior olive (immunolabeled with a different mouse monoclonal antibody against synaptophysin (Sigma; (Caminos et al., 2007)). The Millipore synpatophysin antibody has also been characterized in our mouse system (Fortune and Lurie, 2009).
2.1.5 Tissue Imaging and analysis
All fluorescent slides were viewed at either a 40x or a 60x objective using either a Bio-Rad Radiance 2000 Confocal microscope or an Olympus FV 1000 Fluoview Confocal microscope. Quantitative analysis for immunostaining was performed as previously described (Fortune and Lurie, 2009). Briefly, two to five sections per animal were analyzed from the middle of the nucleus of interest by an observer blinded as to treatment group. Images were collected and then converted from color tiff files to black and white 12-bit tiff files, and the integrated optical density (IOD) of the immunostaining was measured using MediaCybernetics Image-Pro software (Bethesda, MD). IOD measurements were used for quantification of immunostaining because it analyzes both the area of immunostained tissue that met threshold as well as the intensity of the immunostaining. A threshold of immunostaining in control (No Pb) animals was set for each antibody such that all immunoreactivity met threshold, and was used as a comparison with the Pb treatment groups. Immunostaining within the LSO in control and Pb-exposed mice was then quantified and averaged. To separately measure the IOD of 5-HT immunostaining in the LSO neuronal cell bodies and processes, a threshold for object size was also set such that either only neuronal cell bodies or processes were selected for IOD measurement. The immunostaining within three to five random areas (430 µm2) within the LSO in control and Pb-exposed mice was then quantified and averaged. Statistical differences in immunostaining between control and Pb-exposed mice were analyzed GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA).
2.1.6 Sample preparation for HPLC analysis
At each time point of assessment, brains from mice (five per treatment group) were quickly dissected and the forebrain was removed with a coronal cut separating dorsally the superior and inferior colliculus. After dissection, the remaining basal cerebral cortex and cerebellum were gently removed from the separated brainstem under the dissecting microscope. The remaining brainstem includes the inferior colliculus, and the ventral and dorsal brainstem areas. The dissected tissue was immediately weighed, and quickly frozen in the liquid nitrogen. Tissue was collected from controls (n=5), moderate Pb (n=5) and high Pb (n=5) mice. The brainstems from each treatment group was combined and homogenized by sonication in 500 µl of perchloric acid (0.5M) containing 3,4-dihydroxybenzylamine (DBA, 31 ng/mL) (Branson Sonifier 150, Branson, Danbury, CT), and stored at −20 °C until ready to use.
2.1.7 RP-HPLC analysis of 5HT
5-HT levels were measured using reverse phase high performance liquid chromatography (RPHPLC) with electrochemical detection. The homogenates were centrifuged at 14,000 × g for 20 min at 4°C and the supernatants filtered through a Millex® hydrophilic LCR (PTFE) 0.45 um filter (Millipore, Bedford, MA). Sample filtrates were loaded into autosampler vials and were placed into an ESA Model 5 autosampler (ESA, Chelmsford, MA). An OmniSpher 5 C18 chromatographic column (Varian Inc., Lake forest, CA) with a length of 25 cm and an internal diameter of 4.6 mm was used to separate analytes. The mobile phase consisted of water:acetonitrile (9:1, vol/vol) containing 0.15 M monochloroacetic acid, 0.12 M sodium hydroxide, 0.6 mM EDTA and 1.30 mM sodium octyl sulfate, and the pH was adjusted to 3.2 with glacial acetic acid. A constant flow rate of 1ml/min was maintained and the column effluent was analyzed with a Model 5600A ESA CoulArray® electrochemical detector (ESA, Chelmsford, MA). Potentials of the three ESA Model 6210 four channel electrochemical cells, placed in series, were as follows: (channels 1 through 5)-50, 0, 25, 100, 200 mV, (6 through 12) 300 mV. 5-HIAA and 5-HT were monitored at 200 mV. The ratio of the peak heights produced by 5-HT were compared to the peak height produced by DBA (internal standard) in the samples and used to obtain the analyte levels from a calibration curve. Data was expressed as micrograms of analyte per gram of wet tissue weight (µg/g wt).
2.1.8 Statistical Analysis
Data are expressed as mean ± SEM and were analyzed using a one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test; P < 0.05 was considered significant.
Results
3.1 Mouse blood Pb levels
The present study used three different doses of Pb in drinking water; the no Pb control, moderate Pb (0.01 mM), and high Pb (0.1 mM). The blood lead levels (mean ± SEM) of the mice were measured as: No Pb controls (≤1.38 ± 0.14 µg/dL), moderate Pb (8.0 ± 0.4 µg/dL), and high Pb (42.3 ± 1.97 µg/dL). Neither the high nor the moderate dose of Pb resulted in any lost litters or changes in postnatal body size and weight, and were considered sub-toxic doses. In addition, the blood lead levels of the high Pb animals have been used to demonstrate Pb’s neurotoxic effects in rodents (Gilbert et al., 1996; Lasley and Gilbert, 1996, 2000). Blood Pb levels in our moderate exposure are slightly higher than the newly established guidelines for blood lead levels in humans (5 µg/dL).
3.1.2 Pb exposure prolongs the time that developing LSO neurons transiently express 5-HT
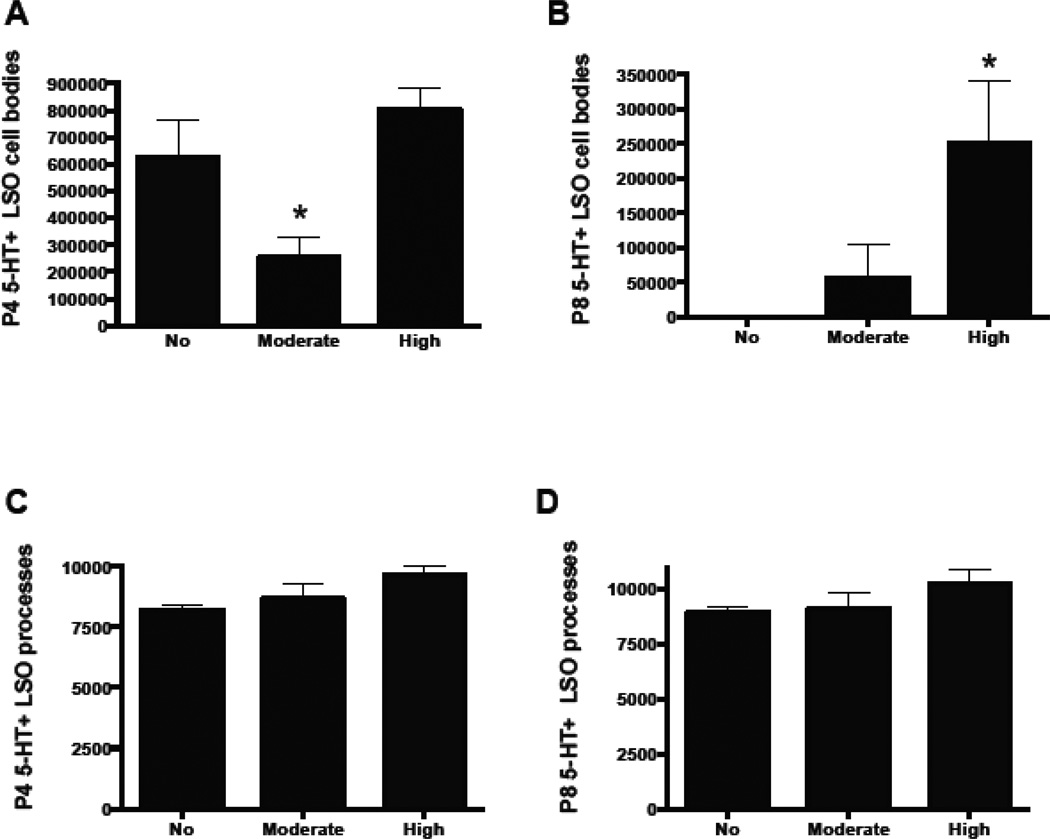
In order to determine whether developmental Pb exposure affects the normal transient expression of 5-HT by LSO neurons in early postnatal ages, control and Pb-exposed brainstem sections from postnatal day (P) 4 and P8 were immunostained for 5-HT and both LSO cell bodies and processes were quantified. Many 5-HT immunopositive cell bodies were observed in the LSO of control and high Pb mice at P4 (Figure 1A, C; 2A). Moderate Pb exposure resulted in LSO somata with significantly less 5-HT immunoreactivity (Figure 1B, 2A) compared with controls and the high Pb animals, suggesting that the moderate dose of Pb delayed the uptake of 5-HT by LSO neurons. In contrast to the 5-HT staining in LSO neuronal cell bodies, Pb exposure does not significantly change the 5-HT staining in LSO processes at P4 (Figure 2C).
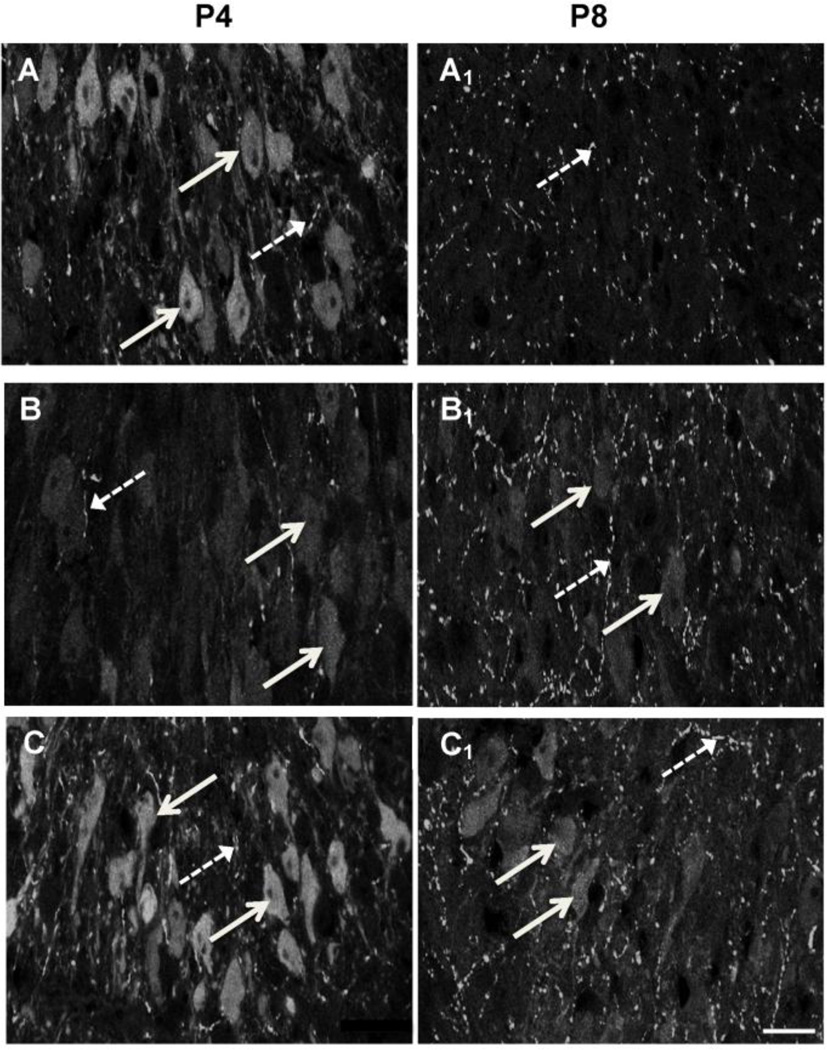
Figure 1.
5-HT immunofluorescence labeling in somata and processes within the LSO of postnatal day (P) 4 and P8 mice. A–C (P4): A) P4 LSO neurons are immunopositive for 5-HT (solid arrows) and some LSO processes are also immunopositive (broken arrows). B) P4 LSO neurons are immunopositive for 5-HT following moderate Pb treatment (arrows), but the immunoreactivity is decreased compared to the no Pb controls. 5-HT+ LSO processes are similar to the no Pb control (broken arrows). C) P4 high Pb LSO neurons have similar 5-HT immunoreactivity compared to the no Pb controls (arrows). Broken arrows=5-HT+ LSO processes. A1–C1 (P8): A1) By P8, control LSO neurons are no longer immunopositive for 5-HT (arrows) although there are many immunopositive LSO processes (broken arrows). Both the moderate (B1) and high (C1) Pb exposure results in 5-HT+ LSO neurons (arrows) and processes (broken arrows). Thus Pb extends the developmental window in which LSO neurons are immunopositive for 5-HT through P8. Bar=15 µ.
Figure 2.
Quantification of 5-HT immunostaining in LSO cell bodies and processes. At P4, moderate Pb exposure significantly decreases 5-HT expression in LSO neurons compared to controls while high Pb results in 5-HT expression that is similar to controls (A). At P8, the high dose of Pb results in significantly more 5-HT immunoreactivity in LSO cell bodies compared to controls, while the moderate dose of Pb shows a trend towards increased immunostaining (B). Neither the moderate nor the high dose of Pb affected the 5-HT immunostaining of LSO processes at either P4 (C) or P8 (D). Graphs represent mean IOD ± SEM. *p<0.05; ANOVA with Dunnett’s post hoc test.
By P8, non-Pb exposed LSO neuronal cell bodies have lost their immunoreactivity for 5-HT, indicating that the transient expression of 5-HT by normal developing LSO neurons is over by P8 (Figure 1A1). However, both the moderate and the high dose of Pb result in continued expression of 5-HT in LSO neurons (Figure 1B1, C1, 2B). Similar to P4, 5-HT in LSO processes remains unaffected by Pb exposure at P8 (Figure 2D). Thus, Pb exposure prolongs the period of time that LSO neurons transiently express 5-HT through P8. However, LSO neurons do not continue to be immunopositive for 5-HT indefinitely. By P21, both control and Pb exposed LSO neurons show no expression of 5-HT (Fortune and Lurie, 2009).
It is important to note that LSO neurons appear to be a specific target of Pb, because 5-HT immunostaining in processes within LSO remains unchanged with Pb exposure. In addition, LSO neurons were the only auditory neurons within the brainstem that immunolabeled for 5-HT during the early postnatal period. Neurons in the cochlear nucleus and the Medial Nucleus of the Trapezoid Body (MNTB) did not express 5-HT at any time during postnatal development (data not shown).
3.1.3 LSO neurons at P4 do not synthesize or degrade 5-HT
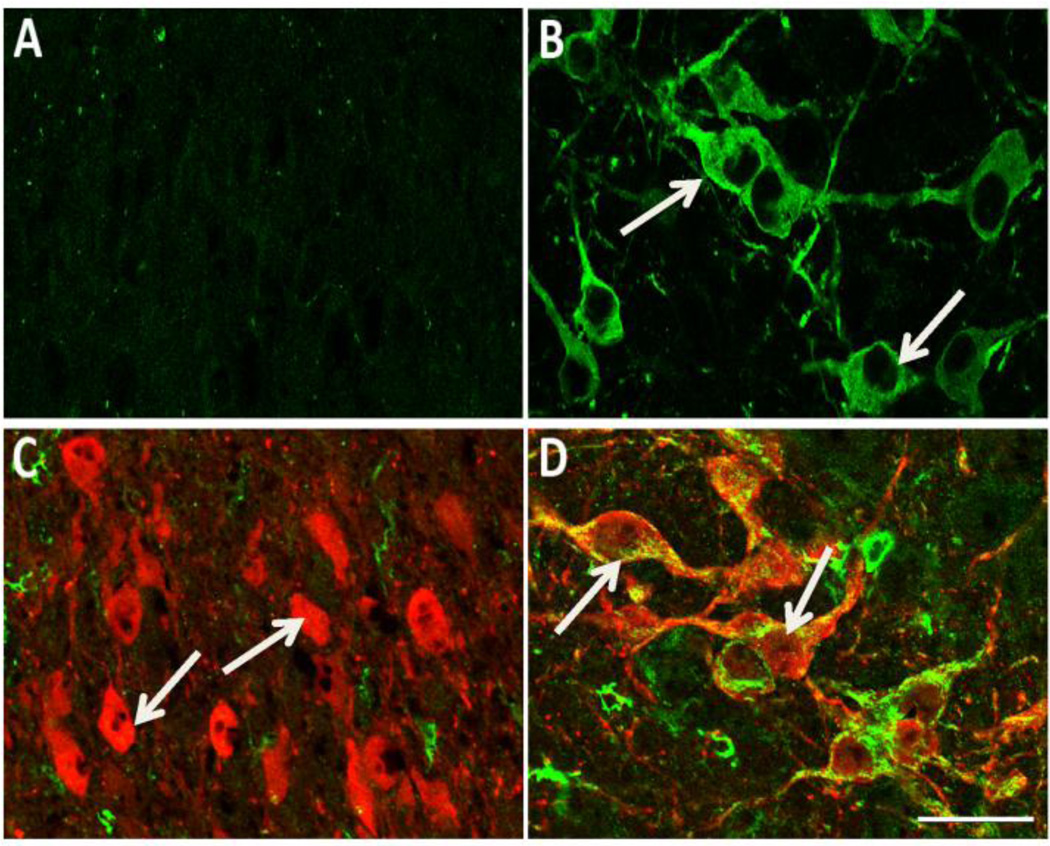
To rule out any possibility that the Pb-induced alterations in 5-HT immunostaining in LSO neurons during early postnatal development is the result of changes in 5-HT synthesis and/or degradation, brainstem sections from control and Pb exposed mice were immunostained for the 5-HT synthesizing enzyme, tryptophan hydroxylase (TPH). LSO neurons have been found to be immunonegative for TPH in P6 MAOA k/o mice (Thompson and Thompson, 2009). In agreement with these studies, LSO somata were immunonegative for TPH in our P4 control mice (Figure 3A) as well as in Pb exposed mice (data not shown). As expected, neurons in the raphe nuclei located in the same brainstem section were immunopositive for TPH (Figure 3B). Thus, LSO neurons at P4 do not appear to be able to synthesize 5-HT. That being the case, then extracellular 5-HT must be taken up by transport in developing LSO neurons. In serotononergic neurons that synthesize 5-HT, the 5-HT that is taken up from the extracellular space by SERT is rapidly degraded by monoamine oxidase (MAOA) (Vitalis et al., 2002). It is possible that Pb is affecting MAOA in LSO neuronal cell bodies, resulting in less degradation of the 5-HT that is taken up by the cell bodies. MAO activity can be modulated by Pb (Devi et al., 2005) and therefore could be a potential mechanism by which Pb changes 5-HT levels in LSO neurons during postnatal development. We therefore examined whether the 5-HT-positive LSO neurons observed at P4 also contain MAOA. Double-labeling for 5-HT and MAOA in the LSO of P4 control mice demonstrated that 5-HT-positive LSO somata were immunonegative for MAOA (Figure 3C). LSO somata were immunonegative in Pb exposed mice as well (data not shown). In contrast, adjacent raphe neurons were immunopositive for both 5-HT and MAOA (Figure 3D).
Figure 3.
LSO neurons of P4 mice do not express tryptophan hydroxylase (TPH) or monoamine oxidase-A (MAOA). A, B: The LSO (A) does not contain any TPH (green) immunopositive somata unlike neurons in the raphe pallidus (B) where there are many immunopositive neurons (arrows). C, D: Double immunolabeling of 5-HT (red) and MAOA (green) shows that the 5-HT positive neurons in the LSO (C) do not contain any MAOA positive somata (arrows), unlike neurons in the raphe pallidus where there is obvious double label (yellow) within many neurons (D; arrows). Scale bar = 30 µ.
These results confirm that LSO neurons do not have the synthetic machinery necessary for either the synthesis or the degradation of 5-HT and suggest that the Pb-induced changes in 5-HT immunostaining within LSO neurons is due to the modulation of SERT on LSO neuronal cell bodies.
3.1.4 SERT expression in the developing LSO
One possible explanation for the Pb-induced prolongation in 5-HT expression by LSO neurons may be that Pb affects the expression of SERT. It has been previously shown that LSO neurons take up 5-HT transiently during development through the transient expression of SERT on LSO neuronal cell bodies (Cases et al., 1998; Thompson, 2006). If Pb extends the period of time that LSO neurons express SERT, then we would expect to see an prolonged uptake of 5-HT into LSO neurons.
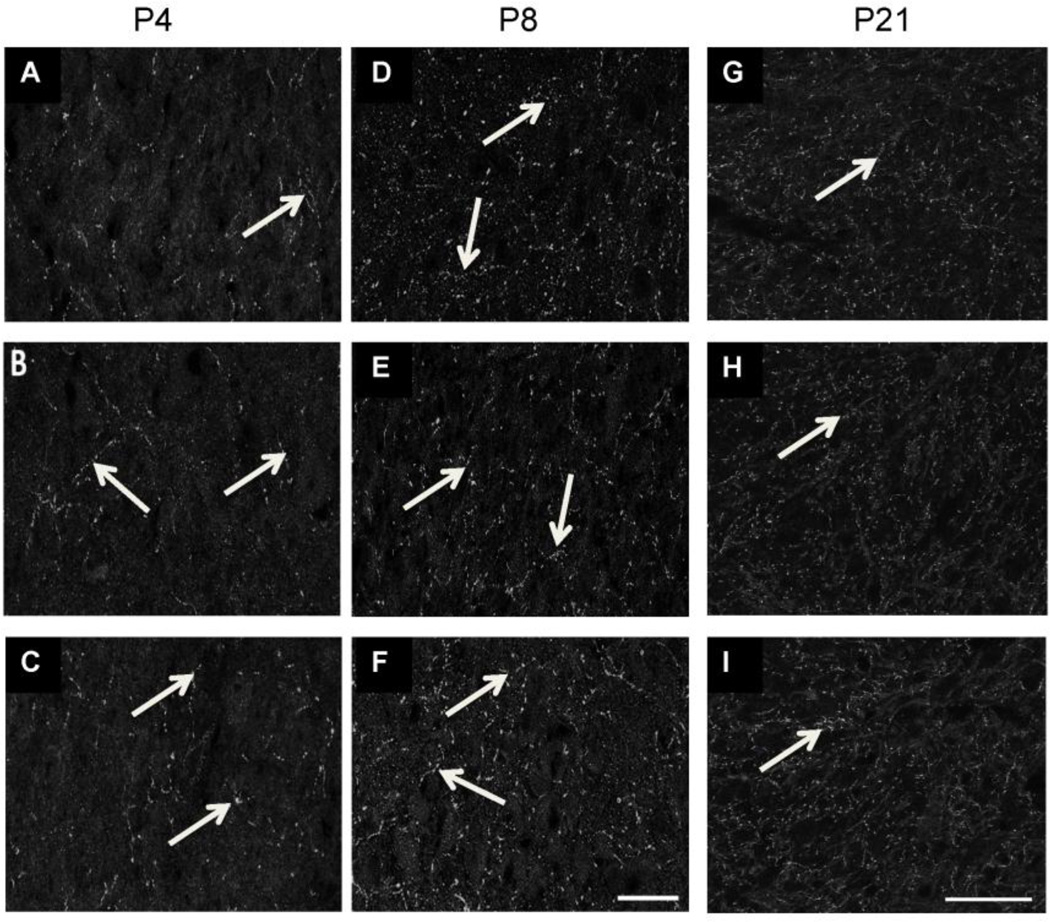
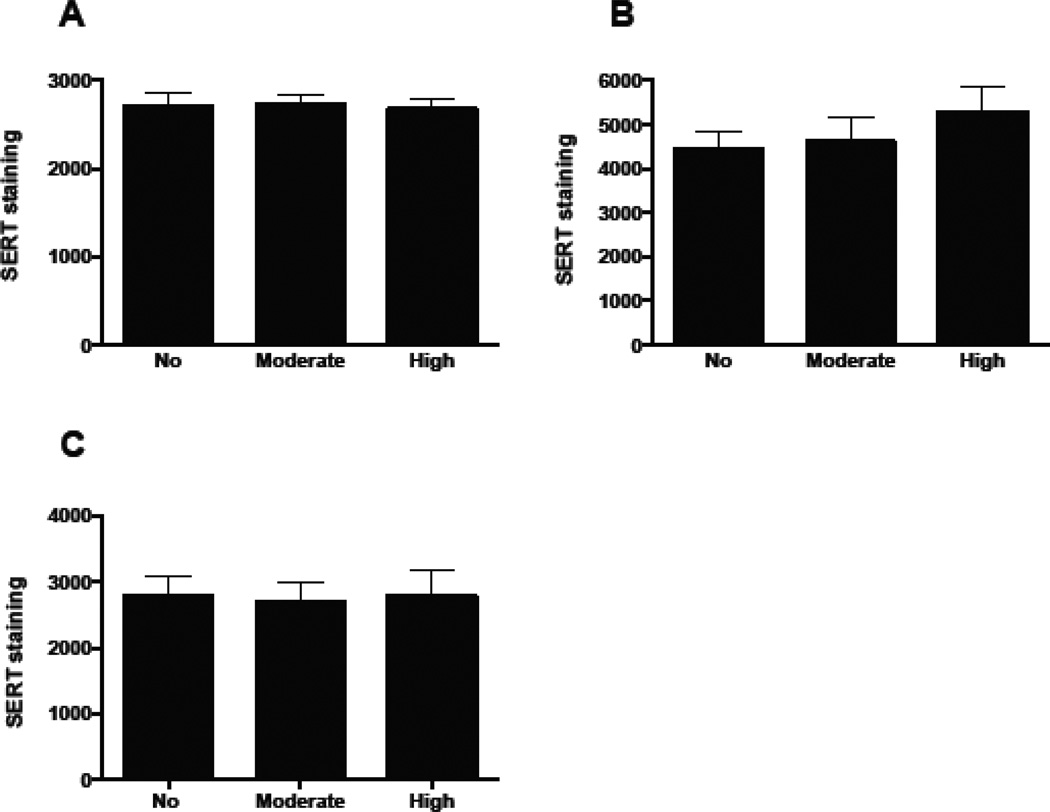
We first immunostained brainstem sections from control and Pb exposed P4, P8, and P21 mice to determine if Pb affected the overall expression of SERT in LSO. Total levels of SERT immunoreactivity within the LSO (which can include SERT immunoreactivity within axons of passage from the Raphe and astrocytes) did not change with Pb exposure (Figures 4 & 5), although the amount of SERT immunostaining increases slightly from P4-P8 (Figure 5).
Figure 4.
SERT immunolabeling in LSO. Pb does not result in significant changes in total SERT expression within the LSO at either P4 (A–C) or P8 (D–F) or P21 (G–I) (arrows). Controls (A, D, G), Moderate Pb (B, E, H), High Pb (C, F, I). Scale bar = 30µ (A–F), Scale bar = 50µ (GI).
Figure 5.
Quantification of SERT immunostaining confirms that Pb does not significantly affect total SERT immunoreactivity in the LSO. However, the amount of SERT immunoreactivity is higher at P8 (B) compared to P4 (A) and P21 (C). Graphs represent mean IOD ± SEM. *p<0.05; ANOVA with Dunnett’s post hoc test.
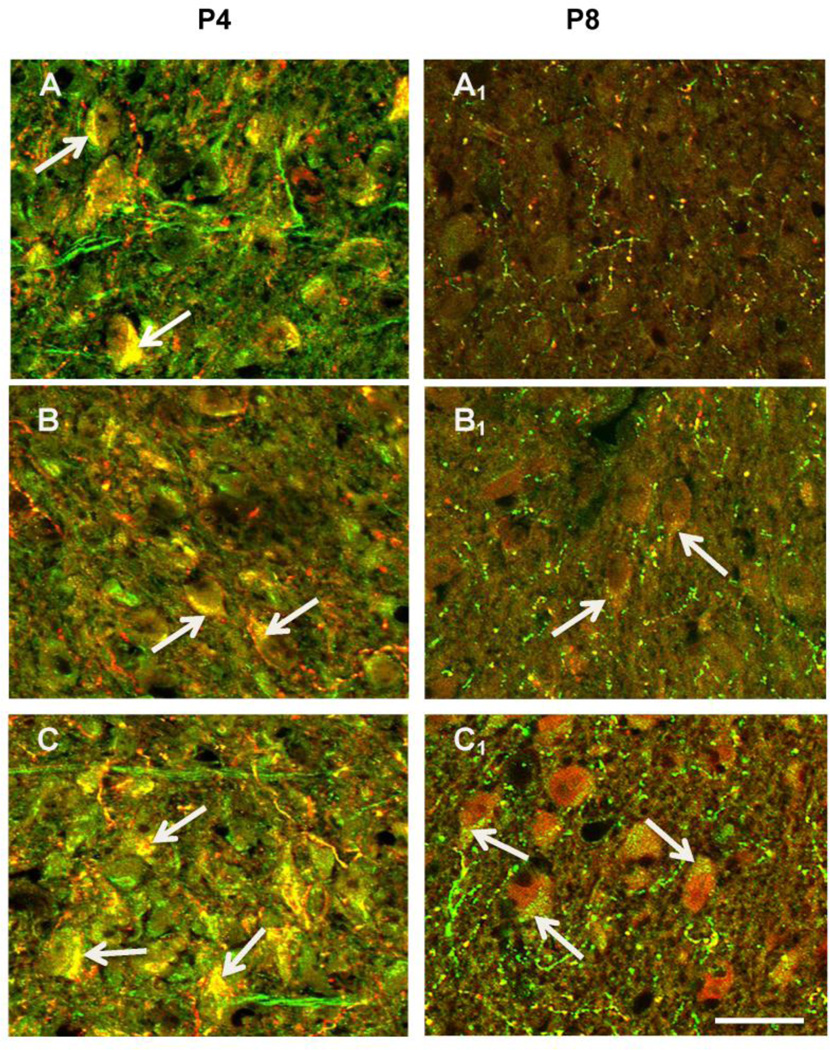
However, because neuronal processes and astrocytes also express SERT (Hirst et al., 1998; Malynn et al., 2013) staining the entire LSO for SERT might not pick up subtle changes in SERT distribution that could occur on LSO neuronal cell bodies following Pb exposure. Therefore, brainstem sections from control and Pb exposed P4 and P8 mice were double immunostained for 5-HT and SERT. We found that LSO neuronal cell bodies and processes were double-labeled for both 5-HT and SERT in control P4 and P8 mice. Figure 6 illustrates that at P4, LSO neuronal cell bodies in control, moderate, and high Pb all double label for 5-HT and SERT, suggesting that 5-HT is taken up by LSO neurons by uptake through the SERT transporter. It is worth noting that SERT expression appears to be lower in the moderate dose of Pb at P4, which may explain the lower levels of 5-HT in LSO neurons compared to the controls and high dose of Pb. By P8, control LSO neuronal cell bodies are no longer double labeled for 5-HT and SERT, and these cell bodies no longer express 5-HT (Figure 6A1). Both the moderate and the high dose LSO neuronal cell bodies continue to be immunopositive both 5-HT and SERT, again suggesting that the prolonged expression of 5-HT in LSO neuronal cell bodies in Pb exposed mice is due to the prolonged expression of SERT (Figure 6B1 &B2).
Figure 6.
Micrographs of LSO double-labeled for 5-HT (red) and SERT (green) in the LSO in P4 and P8 mice. Neurons in LSO at P4 are double labeled for both 5-HT and SERT (arrows) in control, moderate Pb, and high Pb animals. By P8, control neurons are no longer double-labeled (A1), but LSO neuron in both the moderate (B1) and high Pb (C1) treatment groups continue to be double-labeled for both 5-HT and SERT. Scale bar = 15µ.
Taken together, these results indicate that Pb prolongs the period of normal developmental uptake of 5-HT through continued expression of SERT in LSO neurons. It is important to note that the effect of Pb on SERT appears to be specific for SERT expression on LSO somata. Total levels of SERT immunoreactivity within the LSO (which includes cell bodies and processes) did not change with Pb exposure (Figures 4 & 5). This suggests that Pb specifically targets SERT expression on LSO neuronal cell bodies.
3.1.5 Pb exposure decreases synaptophsyin (SYP) staining in LSO
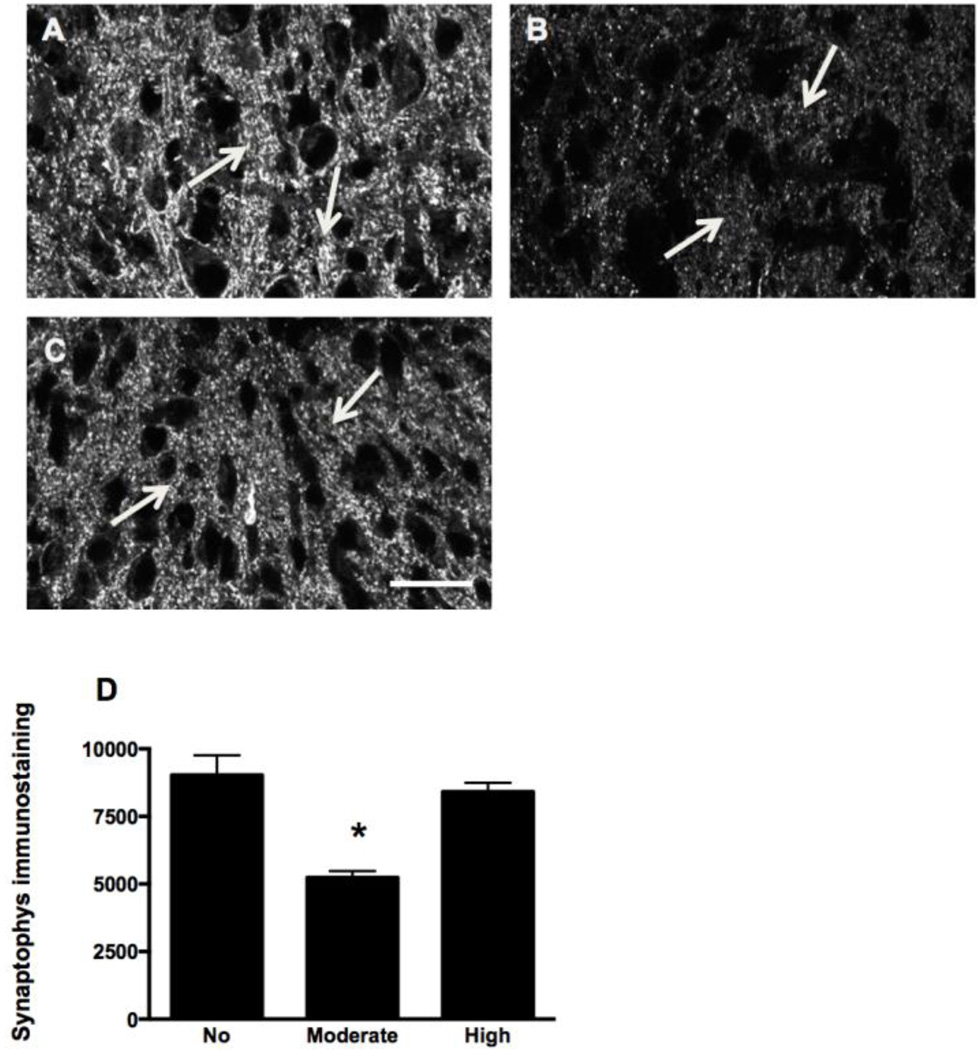
An important question is whether the Pb-induced extension of SERT expression on LSO neurons alters the structure and/or function of LSO neurons in the adult animal. Our previous studies found that both the moderate and high dose of Pb reduces SYP staining in LSO in P21 mice (Fortune and Lurie 2009) indicating that developmental Pb exposure reduces synaptic density in the adult LSO. However, it was not known when during development the decrease of SYP occurred. Therefore, brainstem sections from control and Pb treated mice were immunlabeled with SYP at P4 and P8 and synaptophysin staining quantified in LSO. At P4, no sSYP staining is observed in LSO (data not shown). By P8, the moderate dose of Pb induced a significant decrease in SYP labeling compared to controls at this time point (Figure 7). Interestingly, SYP labeling in the high Pb group was similar to controls at P8, but all Pb groups show decreased staining by P21. Thus, the moderate dose of Pb appears to induce an early decrease of SYP labeling LSO.
Figure 7.
Pb exposure decreases synaptophysin (SYP) immunolabeling in the LSO of P8 mice. A–C: Immunoreactivity for SYP (arrows) decreases with moderate Pb (B) compared to control (A). High Pb (C) does not result in changes in the SYP expression in the P8 LSO. D: Quantification of SYP immunostaining in the LSO confirms there is a statistically significant decrease in the moderate Pb treatment group. Graphs represent mean IOD ± SEM. *p<0.05; ANOVA with Dunnett’s post hoc test. Scale bar = 30 µ.
3.1.6 Moderate Pb exposure increases total 5-HT levels in the brainstem in adult animals
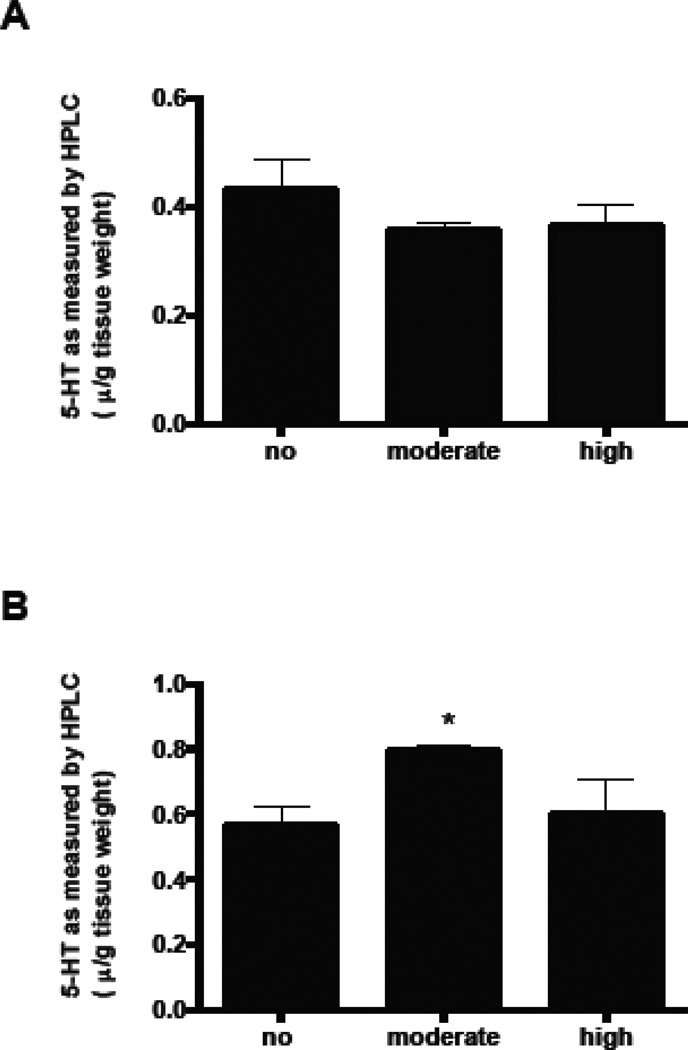
LSO neurons appear to be a specific target for Pb. They express 5-HT through P8 presumably due to uptake through SERT, and show decreased SYP staining through adulthood. However, it is important to determine whether total brainstem levels of 5-HT are changed with Pb exposure, or whether Pb is only altering the expression of SERT. Therefore, 5-HT was measured in crude brainstem fractions (which contains all of the serotontergic raphe nuclei) of P 4 and P21 mice by HPLC analysis. Pb exposure did not result in significant changes in brainstem levels of 5-HT compared to controls at P4 (Figure 8). This suggests that the Pb-induced changes in the transient accumulation of 5-HT by LSO neurons at P4 are not the result of changes in total brainstem 5- HT levels. In contrast, at P21, the moderate, but not the high dose of Pb resulted in a significant increase in 5-HT (Figure 8).
Figure 8.
5-HT levels in brainstem, expressed as µg/g wet tissue. A) 5-HT levels in P4 mice. B) 5-HT levels in P21 mice. Graphs represent mean ± SEM. * denotes significant change from the no Pb group at p<0.05; ANOVA with Dunnett’s post hoc test.
Discussion
4.1 Pb exposure prolongss the transient expression of 5-HT by LSO neurons
The current study demonstrates that Pb exposure prolongs the transient expression of 5-HT by LSO neuronal cell bodies through postnatal day 8. In control mice, 5-HT expression in LSO cell bodies disappears by P8, however, the Pb-exposed mice continue to express 5-HT through P8. The expression of 5-HT by Pb-exposed LSO cell bodies is not permanent, by P21 both control and Pb-exposed LSO neurons are immune-negative for 5-HT. Thus Pb prolongs the normal developmental period during which LSO neuronal cell bodies are immunopositive for 5-HT.
LSO neurons are not considered to be intrinsic serotonergic neurons. They project to the ipsilateral and contralateral inferior colliculus (IC) and use glycine and glutamate as a neurotransmitter (Thompson and Schofield, 2000). When brain levels of 5-HT are increased 6–9 fold compared to wild type (MAOA k/o mice), a subset of LSO neuronal cell bodies do transiently express 5-HT immunostaining starting from embryonic day 18 (E18). This immunoreactivity reaches maximal levels from birth (P0) to postnatal day 7 (P7) and then disappears by P10 (Cases et al., 1998). More recently, 5-HT immunoreactive LSO somata have been found in wild type mice at P1 which then disappears by P8 (Thompson, 2006).
The 5-HT containing LSO neurons do not have the capacity for synthesizing 5-HT, as evidenced by the lack of tryptophan hydroxylase (TPH), a rate-limiting enzyme in 5-HT synthesis (Thompson and Thompson, 2009, and the current study). Instead, it is thought that LSO neurons take up extracellular 5-HT through SERT. In MAOA k/o mice, 5-HT and SERT staining is colocalized in LSO cell bodies at P0 and P5-6, and then all staining in the LSO somata for both proteins disappears by P15 (Thompson and Thompson, 2009). We see similar co-localization of 5-HT and SERT immunoreactivity postnatally, that then disappears.
4.1.1 Pb induced changes in SERT expression in LSO somata
The current study found that the changes in 5-HT expression that were induced by Pb in LSO neurons were associated with similar changes in SERT expression. For example, at P4, control and Pb-exposed LSO neuronal somata are double-labeled with both 5-HT and the SERT. The moderate dose of Pb appears to result in a lower level of SERT expression on LSO neurons compared to the control and high dose of Pb, which could explain why LSO neurons exposed to the moderate dose of Pb have a delayed uptake of serotonin. Why the moderate dose of Pb differs from the high dose has yet to be determined, but previous studies in the developing hippocampus have documented that Pb has a dose-dependent bimodal effect on the developing hippocampus (Slomianka et al., 1989). Our results also show a dose-dependent bimodal effect in the developing LSO.
By P8, control LSO cell bodies have lost their SERT immunostaining and are also immunenegative for 5-HT. A recent study of gene expression profiles in the developing Superior Olivary Complex (SOC) in the rat found that SERT was upregulated in the P4 SOC compared to the P25 SOC (Ehmann et al., 2013), lending additional support to the hypothesis that SERT expression is responsible for the transient uptake of 5-HT by developing LSO neurons.
The current study found that developmental Pb exposure prolongs the expression of SERT on LSO cell bodies through P8, and these LSO neurons remain immunopositive for 5-HT at P8. In addition, Pb appears to specifically target LSO neurons. The serotonergic processes in LSO that are thought to originate from the raphe nuclei remain unaffected by Pb exposure. It will be very interesting to determine what happens in LSO if this transient uptake of 5-HT is eliminated using SSRI inhibitors. One might expect to see major alterations in synaptic structure in the LSO.
4.1.2 Pb and SERT expression
The mechanism by which LSO somata transiently express SERT is not clear. The active (functional) SERT is located mainly in axon terminals and along the axons of 5-HT neurons (Zhou et al., 1998). In 5-HT neurons, the transcription factor Pet1 directly activates the transcription of genes that encode TPH, ADAC (L-amino acid decarboxylase), and SERT (Hendricks et al., 1999). However, this gene is not found in neurons transiently expressing SERT (Pfaar et al., 2002). In non-serotonergic thalamocortical neurons that transiently accumulate 5-HT through SERT, SERT proteins are transiently expressed in the axons and axon terminals, and are responsible for taking up 5-HT from the extracellular space and the 5-HT is then transported back to the cell bodies (Lebrand et al., 1996; Lebrand et al., 1998). However, the factors regulating SERT expression in non-serotonergic neurons are poorly understood. Nonetheless, there are several ways in which Pb might be affecting SERT expression.
One mechanism by which Pb could be affecting SERT expression might be that Pb targets p38 MAP kinase (MAPK). Studies have found that inhibition of p38 MAP kinase decreases SERT proteins in the plasma membrane (Samuvel et al., 2005). In addition, when p38 MAPK expression is decreased by siRNAs, there is a concomitant decrease in cell surface expression of SERT. Taken together, these studies suggest that a decrease in p38 MAPK leads to a downregulation of SERT in the plasma membrane (Samuvel et al., 2005). Pb has been shown to activate the p38 MAPK pathway through phosphorylation of both ERK 1/2 and p38 MAPK (Cordova et al., 2004). Thus, it is possible that Pb activates p38 MAPK, leading to increased expression of SERT in LSO neuronal cell membranes. Although why developing LSO neuronal cell bodies appear to be a specific target for Pb remains to be determined.
A second potential mechanism for how Pb might be affecting SERT expression involves thyroid hormones. Thyroid hormones have been shown to regulate the transient expression of SERT in thalamocortical neurons (Auso et al., 2001). In normal mice, the transient expression of the SERT gene disappears by P11, whereas its expression persists until P15 in hypothyroid rats (Auso et al., 2001). This effect of hypothyroidism is specific for the transient expression SERT, because there is no general delay in brain maturation and SERT is not changed in serotonergic raphe neurons. Prolonged expression of SERT in hypothyroid rats also leads to reduced axon terminal arborization and synaptogenesis within the cortical barrel fields, resulting in a smaller barrel area. Interestingly, Pb has been shown to reduce free thyroxine (FT4) levels in Pb exposed adolescents whose average blood Pb level is 7.3 ± 2.92 µg/dL (Dundar et al., 2006). In addition, Pb exposure reduces 131I uptake in animals and impairs the release of thyroidstimulating hormone (TSH) in children (Slingerland, 1955; Huseman et al., 1987). Therefore, a Pb-induced impairment in thyroid function is one potential mechanism by which Pb might prolong the transient expression of 5-HT and SERT in non-serotonergic LSO neurons. Future studies are needed to elucidate the precise mechanism by which Pb modulates the transient expression of SERT on LSO neuronal cell bodies.
4.1.3 Pb exposure decreases synaptophysin labeling in LSO
The extension of the developmental window whereby Pb-exposed LSO neurons continue to express both SERT and 5-HT is correlated with decreased SYP labeling in both the P8 and P21 LSO. Thus Pb exposure results in decreased synaptic density within the adult LSO that can first be observed at P8. Recently, maternal Pb exposure in mice has been shown to decrease SYP expression in the hippocampus the Pb-exposed offspring at P21, indicating that Pb also affects synaptogenesis in non-sensory areas (Li et al., 2015).
The transient expression of 5-HT by non-serotonergic neurons in sensory systems has been well studied. In other sensory systems such as rodent cortical barrel fields, precise regulation of the transient uptake of 5-HT by non-serotonergic sensory neurons has been shown to be critical for the proper formation of highly topographically organized sensory maps (Gaspar et al., 2003). Studies suggest that this transient uptake of 5-HT by non-serotonergic sensory neurons is necessary to remove 5-HT from the extracellular space, thereby maintaining proper extracellular concentrations of 5-HT during a critical period of development. It is well known that 5-HT functions as a neurotrophic factor during brain development prior to the time when it plays a role as a neurotransmitter (Luo et al., 2003). Depletion of 5-HT levels during development has a long lasting effect on synaptogenesis and brain maturation and therefore maintaining proper 5-HT levels during the critical period of brain development is crucial (Alvarez et al., 2002; Luo et al., 2003).
For example, embryonic or neonatal depletion of 5-HT has been shown to decrease synaptic density, reduce spine density and complexity of cortical pyramidal neurons and hippocampal dentate granule cells (Mazer et al., 1997; Vitalis et al., 2007), and alter the neural morphology of somatosensory cortical barrel fields (Bennett-Clarke et al., 1994). In addition, knockout of SERT in thalamacortical neurons in mice results in long-term changes in spatial organization of cortical neurons, alterations in the pattern of thalamacortical neurons, and changes in the dendritic organization in sensory cortex (Chen et al., 2015). Thus disruption of SERT expression leads to excessive 5-HT and results in permanent impairments in the developing sensory cortex (Chen et al., 2015).
The present study demonstrates that Pb exposure prolongs the normal uptake of 5-HT by LSO neurons. Thus, an intriguing hypothesis is that Pb shifts the extracellular concentrations of 5-HT during a critical window of neuronal development, thereby resulting in disrupted axonal arborization and synaptogenesis in LSO. In support of this, we found that Pb decreases synaptophysin immunostaining within the LSO of P8 and P21 mice. In addition, our moderate dose of Pb in the current study results in an increase in total 5-HT within the P21 brainstem, which might also contribute to the decreased SYP immunoreactivity observed in the P21 LSO (Fortune and Lurie, 2009). A limitation of the current study is that Pb was present in the drinking water throughout gestation and through P21. Thus we do not know the critical time during development when Pb has its effect on the LSO. Future studies will address this by exposing the developing mice to defined windows of Pb exposure.
4.1.4 5-HT and synaptogenesis
Finally, in addition to its action as a trophic factor, 5-HT could modulate spontaneous activity in the LSO, thereby affecting LSO development. The application of 5-HT results in prolonged bursts of spontaneous inhibitory postsynaptic currents (IPSCs) in the developing gerbil LSO and intriguingly, this effect in not seen after P8 (Fitzgerald and Sanes, 1999). Thus, Pb could reduce the normal extracellular concentrations of 5-HT during the early postnatal period due to increased uptake into LSO neurons. This might lead to a decrease in IPSCs in the developing gerbil LSO, which could then alter proper axonal arborization and synapse formation. It is also worth noting that in the adult mouse brainstem, 5-HT does modulate the auditory brainstem response (ABR). Systemic serotonin depletion results in reduced ABR latencies, particularly for lower and mid-range frequencies (Papesh and Hurley, 2015).
4.1.5 Summary and Conclusion
The current study demonstrates that Pb prolongs the normal developmental uptake of 5-HT by LSO neurons during a critical period of development, through the prolonged expression of SERT in LSO. SERT appears to be a target for Pb, and the extended uptake of 5-HT by LSO neurons is also correlated with the decreased expression of SYP and an increase in total 5-HT in the adult LSO. So how could the modulation of the serotonergic system by Pb be relevant to auditory processing in Pb-exposed children? Pb exposure in children has been linked to attention deficit disorder (Ha et al., 2009; Kim et al., 2013), and children with attention deficit disorder have deficits in the temporal processing of both speech and non-speech stimuli at the level of the brainstem (Jafari et al., 2015). Thus, developmental Pb exposure in humans could modulate 5- HT in the developing auditory system, leading to auditory temporal processing deficits. The effect of manipulating 5-HT levels on the developing human auditory system has not been examined, however, infants exposed to selective serotonin reuptake inhibitors (SSRIs) in utero show lower motor scores, more CNS stress signs, and higher arousal than unexposed infants (Salisbury et al., 2016). Of depressed pregnant women who seek treatment, one-third of them will take SSRIs. Increasing evidence suggests that exposure to SSRIs early in development alters the function of the serotonergic system and affects multiple systems including emotional, motor, and circadian (Maciag et al., 2006; McAllister et al., 2012). Thus, prenatal SSRI exposure has profound effects on infants, suggesting that prenatal regulation of 5-HT and SERT is essential for the normal development of the brain.
Our study demonstrates that Pb exposure modulates the serotonergic system in the developing LSO. Additional studies are needed to fully elucidate the cellular and molecular mechanisms of the effect of Pb on the developing LSO, and the long-term consequences to the adult auditory system.
Highlights.
Developmental Pb exposure prolongs transient expression of 5-HT in postnatal murine LSO neurons.
Pb-induced 5-HT expression correlates with expression of SERT on LSO cell bodies.
Prolonged expression of 5-HT after Pb exposure leads to decreased synapses in LSO.
Decreased LSO synapses might contribute to cognitive deficits linked to Pb exposure.
Acknowledgments
We would like to thank Diane Brooks for her expert technical assistance. Supported by NIH NCRR P20 RR17670, NIH P20 RR015583 (D.I.L.; F.C.P.). NIA grant RO1AG031184-01 to F.C.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarado JC, Fuentes-Santamaria V, Henkel CK, Brunso-Bechtold JK. Alterations in calretinin immunostaining in the ferret superior olivary complex after cochlear ablation. The Journal of comparative neurology. 2004;470:63–79. doi: 10.1002/cne.11038. [DOI] [PubMed] [Google Scholar]
- Alvarez C, Vitalis T, Fon EA, Hanoun N, Hamon M, Seif I, Edwards R, Gaspar P, Cases O. Effects of genetic depletion of monoamines on somatosensory cortical development. Neuroscience. 2002;115:753–764. doi: 10.1016/s0306-4522(02)00484-0. [DOI] [PubMed] [Google Scholar]
- Auso E, Cases O, Fouquet C, Camacho M, Garcia-Velasco JV, Gaspar P, Berbel P. Protracted expression of serotonin transporter and altered thalamocortical projections in the barrelfield of hypothyroid rats. Eur J Neurosci. 2001;14:1968–1980. doi: 10.1046/j.0953-816x.2001.01815.x. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Very low lead exposures and children's neurodevelopment. Curr Opin Pediatr. 2008;20:172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Bellinger AM. Childhood lead poisoning: the torturous path from science to policy. J Clin Invest. 2006;116:853–857. doi: 10.1172/JCI28232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Leslie MJ, Lane RD, Rhoades RW. Effect of serotonin depletion on vibrissa-related patterns of thalamic afferents in the rat's somatosensory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:7594–7607. doi: 10.1523/JNEUROSCI.14-12-07594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ Health Perspect. 2006;114:1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier JI, Fletcher JM, Foorman BR, Klaas P, Gray LC. Auditory temporal processing in children with specific reading disability with and without attention deficit/hyperactivity disorder. J Speech Lang Hear Res. 2003;46:31–42. doi: 10.1044/1092-4388(2003/003). [DOI] [PubMed] [Google Scholar]
- Caminos E, Garcia-Pino E, Martinez-Galan JR, Juiz JM. The potassium channel KCNQ5/Kv7.5 is localized in synaptic endings of auditory brainstem nuclei of the rat. The Journal of comparative neurology. 2007;505:363–378. doi: 10.1002/cne.21497. [DOI] [PubMed] [Google Scholar]
- Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Lebrand C, Giros B, Vitalis T, De Maeyer E, Caron MG, Price DJ, Gaspar P, Seif I. Plasma membrane transporters of serotonin, dopamine, and norepinephrine mediate serotonin accumulation in atypical locations in the developing brain of monoamine oxidase A knock-outs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:6914–6927. doi: 10.1523/JNEUROSCI.18-17-06914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ye R, Gargus JJ, Blakely RD, Dobrenis K, Sze JY. Disruption of Transient Serotonin Accumulation by Non-Serotonin-Producing Neurons Impairs Cortical Map Development. Cell reports. 2015 doi: 10.1016/j.celrep.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova FM, Rodrigues AL, Giacomelli MB, Oliveira CS, Posser T, Dunkley PR, Leal RB. Lead stimulates ERK1/2 and p38MAPK phosphorylation in the hippocampus of immature rats. Brain research. 2004;998:65–72. doi: 10.1016/j.brainres.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Devi CB, Reddy GH, Prasanthi RP, Chetty CS, Reddy GR. Developmental lead exposure alters mitochondrial monoamine oxidase and synaptosomal catecholamine levels in rat brain. Int J Dev Neurosci. 2005;23:375–381. doi: 10.1016/j.ijdevneu.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Dundar B, Oktem F, Arslan MK, Delibas N, Baykal B, Arslan C, Gultepe M, Ilhan IE. The effect of long-term low-dose lead exposure on thyroid function in adolescents. Environmental research. 2006;101:140–145. doi: 10.1016/j.envres.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Ehmann H, Hartwich H, Salzig C, Hartmann N, Clement-Ziza M, Ushakov K, Avraham KB, Bininda-Emonds OR, Hartmann AK, Lang P, Friauf E, Nothwang HG. Time-dependent gene expression analysis of the developing superior olivary complex. The Journal of biological chemistry. 2013;288:25865–25879. doi: 10.1074/jbc.M113.490508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facoetti A, Lorusso ML, Paganoni P, Cattaneo C, Galli R, Umilta C, Mascetti GG. Auditory and visual automatic attention deficits in developmental dyslexia. Brain Res Cogn Brain Res. 2003;16:185–191. doi: 10.1016/s0926-6410(02)00270-7. [DOI] [PubMed] [Google Scholar]
- Finkelstein Y, Markowitz ME, Rosen JF. Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain research Brain research reviews. 1998;27:168–176. doi: 10.1016/s0165-0173(98)00011-3. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KK, Sanes DH. Serotonergic modulation of synapses in the developing gerbil lateral superior olive. J Neurophysiol. 1999;81:2743–2752. doi: 10.1152/jn.1999.81.6.2743. [DOI] [PubMed] [Google Scholar]
- Fortune T, Lurie DI. Chronic low-level lead exposure affects the monoaminergic system in the mouse superior olivary complex. The Journal of comparative neurology. 2009;513:542–558. doi: 10.1002/cne.21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Mack CM, Lasley SM. Chronic developmental lead exposure increases the threshold for long-term potentiation in rat dentate gyrus in vivo. Brain research. 1996;736:118–124. doi: 10.1016/0006-8993(96)00665-8. [DOI] [PubMed] [Google Scholar]
- Glotzer DE, Freedberg KA, Bauchner H. Management of childhood lead poisoning: clinical impact and cost-effectiveness. Med Decis Making. 1995;15:13–24. doi: 10.1177/0272989X9501500104. guidelines C CDC blood lead guidelines. In. [DOI] [PubMed] [Google Scholar]
- Ha M, Kwon HJ, Lim MH, Jee YK, Hong YC, Leem JH, Sakong J, Bae JM, Hong SJ, Roh YM, Jo SJ. Low blood levels of lead and mercury and symptoms of attention deficit hyperactivity in children: a report of the children's health and environment research (CHEER) Neurotoxicology. 2009;30:31–36. doi: 10.1016/j.neuro.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst WD, Price GW, Rattray M, Wilkin GP. Serotonin transporters in adult rat brain astrocytes revealed by [3H]5-HT uptake into glial plasmalemmal vesicles. Neurochemistry international. 1998;33:11–22. doi: 10.1016/s0197-0186(05)80003-8. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin shifts first-spike latencies of inferior colliculus neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:7876–7886. doi: 10.1523/JNEUROSCI.1178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Thompson AM, Pollak GD. Serotonin in the inferior colliculus. Hearing research. 2002;168:1–11. doi: 10.1016/s0378-5955(02)00365-9. [DOI] [PubMed] [Google Scholar]
- Huseman CA, Moriarty CM, Angle CR. Childhood lead toxicity and impaired release of thyrotropin-stimulating hormone. Environmental research. 1987;42:524–533. doi: 10.1016/s0013-9351(87)80219-0. [DOI] [PubMed] [Google Scholar]
- Jafari Z, Malayeri S, Rostami R. Subcortical encoding of speech cues in children with attention deficit hyperactivity disorder. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2015;126:325–332. doi: 10.1016/j.clinph.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Jones LG, Prins J, Park S, Walton JP, Luebke AE, Lurie DI. Lead exposure during development results in increased neurofilament phosphorylation, neuritic beading, and temporal processing deficits within the murine auditory brainstem. The Journal of comparative neurology. 2008;506:1003–1017. doi: 10.1002/cne.21563. [DOI] [PubMed] [Google Scholar]
- Kim S, Arora M, Fernandez C, Landero J, Caruso J, Chen A. Lead, mercury, and cadmium exposure and attention deficit hyperactivity disorder in children. Environmental research. 2013;126:105–110. doi: 10.1016/j.envres.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Todd AC. Lead poisoning. West J Med. 1994;161:153–159. [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations < 10 microg/dL in US children and adolescents. Public health reports. 2000;115:521–529. doi: 10.1093/phr/115.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasley SM, Gilbert ME. Presynaptic glutamatergic function in dentate gyrus in vivo is diminished by chronic exposure to inorganic lead. Brain research. 1996;736:125–134. doi: 10.1016/0006-8993(96)00666-x. [DOI] [PubMed] [Google Scholar]
- Lasley SM, Gilbert ME. Glutamatergic components underlying lead-induced impairments in hippocampal synaptic plasticity. Neurotoxicology. 2000;21:1057–1068. [PubMed] [Google Scholar]
- Lebrand C, Cases O, Wehrle R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. The Journal of comparative neurology. 1998;401:506–524. [PubMed] [Google Scholar]
- Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S, Seif I, Gaspar P. Transient uptake and storage of serotonin in developing thalamic neurons. Neuron. 1996;17:823–835. doi: 10.1016/s0896-6273(00)80215-9. [DOI] [PubMed] [Google Scholar]
- Li N, Zhang P, Qiao M, Shao J, Li H, Xie W. The effects of early life lead exposure on the expression of P2X7 receptor and synaptophysin in the hippocampus of mouse pups. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements. 2015;30:124–128. doi: 10.1016/j.jtemb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Luo X, Persico AM, Lauder JM. Serotonergic regulation of somatosensory cortical development: lessons from genetic mouse models. Developmental neuroscience. 2003;25:173–183. doi: 10.1159/000072266. [DOI] [PubMed] [Google Scholar]
- Lurie DI, Brooks DM, Gray LC. The effect of lead on the avian auditory brainstem. Neurotoxicology. 2006;27:108–117. doi: 10.1016/j.neuro.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Maciag D, Simpson KL, Coppinger D, Lu Y, Wang Y, Lin RC, Paul IA. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EP, Mitchell EA, Greig SJ, Corteen N, Balfour DJ, Swinny JD, Lambert JJ, Belelli D. Extrasynaptic glycine receptors of rodent dorsal raphe serotonergic neurons: a sensitive target for ethanol. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:1232–1244. doi: 10.1038/npp.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malynn S, Campos-Torres A, Moynagh P, Haase J. The pro-inflammatory cytokine TNF-alpha regulates the activity and expression of the serotonin transporter (SERT) in astrocytes. Neurochemical research. 2013;38:694–704. doi: 10.1007/s11064-012-0967-y. [DOI] [PubMed] [Google Scholar]
- Mazer C, Muneyyirci J, Taheny K, Raio N, Borella A, Whitaker-Azmitia P. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain research. 1997;760:68–73. doi: 10.1016/s0006-8993(97)00297-7. [DOI] [PubMed] [Google Scholar]
- McAllister BB, Kiryanova V, Dyck RH. Behavioural outcomes of perinatal maternal fluoxetine treatment. Neuroscience. 2012;226:356–366. doi: 10.1016/j.neuroscience.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Moreira EG, Vassilieff I, Vassilieff VS. Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicol Teratol. 2001;23:489–495. doi: 10.1016/s0892-0362(01)00159-3. [DOI] [PubMed] [Google Scholar]
- Narboux-Neme N, Pavone LM, Avallone L, Zhuang X, Gaspar P. Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs) Neuropharmacology. 2008;55:994–1005. doi: 10.1016/j.neuropharm.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Papesh MA, Hurley LM. Modulation of auditory brainstem responses by serotonin and specific serotonin receptors. Hearing research. 2015;332:121–136. doi: 10.1016/j.heares.2015.11.014. [DOI] [PubMed] [Google Scholar]
- Pfaar H, von Holst A, Vogt Weisenhorn DM, Brodski C, Guimera J, Wurst W. mPet-1, a mouse ETS-domain transcription factor, is expressed in central serotonergic neurons. Development genes and evolution. 2002;212:43–46. doi: 10.1007/s00427-001-0208-x. [DOI] [PubMed] [Google Scholar]
- Prins JM, Brooks DM, Thompson CM, Lurie DI. Chronic low-level Pb exposure during development decreases the expression of the voltage-dependent anion channel in auditory neurons of the brainstem. Neurotoxicology. 2010;31:662–673. doi: 10.1016/j.neuro.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putter-Katz H, Kishon-Rabin L, Sachartov E, Shabtai EL, Sadeh M, Weiz R, Gadoth N, Pratt H. Cortical activity of children with dyslexia during natural speech processing: evidence of auditory processing deficiency. J Basic Clin Physiol Pharmacol. 2005;16:157–171. doi: 10.1515/jbcpp.2005.16.2-3.157. [DOI] [PubMed] [Google Scholar]
- Sader-Mazbar O, Loboda Y, Rabey MJ, Finberg JP. Increased L-DOPA-derived dopamine following selective MAO-A or -B inhibition in rat striatum depleted of dopaminergic and serotonergic innervation. British journal of pharmacology. 2013;170:999–1013. doi: 10.1111/bph.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury AL, O'Grady KE, Battle CL, Wisner KL, Anderson GM, Stroud LR, Miller-Loncar CL, Young ME, Lester BM. The Roles of Maternal Depression, Serotonin Reuptake Inhibitor Treatment, and Concomitant Benzodiazepine Use on Infant Neurobehavioral Functioning Over the First Postnatal Month. The American journal of psychiatry. 2016;173:147–157. doi: 10.1176/appi.ajp.2015.14080989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingerland DW. The influence of various factors on the uptake of iodine by the thyroid. J Clin Endocrinol Metab. 1955;15:131–141. doi: 10.1210/jcem-15-1-131. [DOI] [PubMed] [Google Scholar]
- Slomianka L, Rungby J, West MJ, Danscher G, Andersen AH. Dose-dependent bimodal effect of low-level lead exposure on the developing hippocampal region of the rat: a volumetric study. Neurotoxicology. 1989;10:177–190. [PubMed] [Google Scholar]
- Thompson AM. "Non-serotonergic" lateral superior olivary neurons of the neonatal mouse contain serotonin. Brain research. 2006;1122:122–125. doi: 10.1016/j.brainres.2006.08.126. [DOI] [PubMed] [Google Scholar]
- Thompson AM. Serotonin immunoreactivity in auditory brainstem neurons of the postnatal monoamine oxidase-A knockout mouse. Brain research. 2008;1228:58–67. doi: 10.1016/j.brainres.2008.06.091. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Schofield BR. Afferent projections of the superior olivary complex. Microsc Res Tech. 2000;51:330–354. doi: 10.1002/1097-0029(20001115)51:4<330::AID-JEMT4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Thompson GC. Experimental evidence that the serotonin transporter mediates serotonin accumulation in LSO neurons of the postnatal mouse. Brain research. 2009;1253:60–68. doi: 10.1016/j.brainres.2008.11.054. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Passemard S, Callebert J, Parnavelas JG. Embryonic depletion of serotonin affects cortical development. Eur J Neurosci. 2007;26:331–344. doi: 10.1111/j.1460-9568.2007.05661.x. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Fouquet C, Alvarez C, Seif I, Price D, Gaspar P, Cases O. Developmental expression of monoamine oxidases A and B in the central and peripheral nervous systems of the mouse. The Journal of comparative neurology. 2002;442:331–347. doi: 10.1002/cne.10093. [DOI] [PubMed] [Google Scholar]
- Wright CM, Conlon EG. Auditory and visual processing in children with dyslexia. Dev Neuropsychol. 2009;34:330–355. doi: 10.1080/87565640902801882. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Tao-Cheng JH, Segu L, Patel T, Wang Y. Serotonin transporters are located on the axons beyond the synaptic junctions: anatomical and functional evidence. Brain research. 1998;805:241–254. doi: 10.1016/s0006-8993(98)00691-x. [DOI] [PubMed] [Google Scholar]