Abstract
Glenoid osteochondral defects can be a significant source of pain and disability in an active population. Many treatments are available, but most joint-preserving procedures are limited to debridement, abrasion chondroplasty, or marrow-stimulation techniques, all of which depend on healthy underlying bone and none of which address underlying bony pathology. Osteochondral autograft transfer has been a successful form of treatment for lesions in the knee, elbow, and ankle, especially when subchondral bone is involved. We describe an arthroscopic method of treating glenoid osteochondral lesions with an osteochondral autograft transfer using a graft from the patient's ipsilateral knee. This technique addresses both cartilage and osseous pathology with minimal morbidity and provides a good biological restorative option for patients with isolated glenoid osteochondral defects.
A juxta-articular bone cyst1 located within the glenoid can be a source of pain and disability especially in an active population. In patients who have been treated for glenohumeral instability, cystic and avascular changes may develop around the glenoid, usually in areas corresponding to previous suture anchor placement or trauma. The mechanism of cyst formation is unknown, but it may develop as a local reaction to suture anchor material within bone2 or from a previous glenoid fracture.3 Cyst formation around a suture anchor is commonly asymptomatic and does not interfere with activity. However, juxta-articular cysts can alter the integrity of the articular cartilage and may affect the vascularity of the subchondral bone, resulting in avascular necrosis and an osteochondral defect on the glenoid. This defect may contribute to pain and disability with activity and continued instability because of loss of anatomic concavity of the glenoid with a potential for loss of chondrolabral containment.4 Indications for this procedure are evolving but include continued pain and disability caused by juxta-articular cysts after failed nonoperative treatment.
We describe treatment of posterior glenoid osteochondral defects with avascular subchondral cystic bone using an osteochondral autograft transfer (OATS) (Video 1). Conventionally, OATS is used in the lower extremity to repair osteochondral defects in the knee and ankle, as well as in the upper extremity to restore defects in the elbow. Although there are many different cartilage restoration procedures available, OATS is unique in that it replaces osteochondral defects with autogenous hyaline cartilage atop healthy, live bone, which can result in increased longevity and maintenance of structural integrity.5 This report describes transfer of intact osteochondral plugs from a non–weight-bearing portion of the knee to the damaged osteochondral area of the posterior glenoid in addition to revision capsulolabral repair.
Technique
Step 1: Preoperative Workup
Many patients with glenoid subchondral and juxta-articular cysts are asymptomatic. Therefore care and discernment must be taken to confirm the source of pain in a patient with these findings. Nonoperative treatment should be used with an initial focus on periscapular stabilization and strengthening, as well as cuff strengthening. The decision to pursue operative management should be made after all nonoperative options have failed. Once other sources of pain have been ruled out, the patient can then be counseled on the proposed plan to restore the osseous and cartilage defect within the glenoid using the OATS system. We prefer to take donor plugs from the sulcus terminalis along the distal lateral trochlea because this area provides a flat chondral surface and is involved minimally in knee kinematics. If the patient's daily activity or occupation obligates deep knee flexion (baseball catcher, wrestler, and so on), then one can consider other areas of the knee that are less involved in everyday activity. We have found that, for most patients, using grafts from the sulcus terminalis on the lateral trochlea provides a flat cartilaginous surface with minimal morbidity. When the procedure is performed correctly, most patients have minimal long-term morbidity from donor harvest at the sulcus terminalis of the knee.6, 7
Step 2: Operative Setup
After the induction of general anesthesia, the patient is examined, which is standard for all arthroscopic shoulder procedures. The patient is positioned in the lateral decubitus position using a beanbag (Olympic Vac-Pac; Natus Medical, Planegg, Germany) and an arm positioner (Spider2 Limb Positioner; Smith & Nephew, Andover, MA). In addition, the patient's ipsilateral lower extremity is prepared and draped in preparation for autograft harvest. A usual posterior portal is established approximately 1 cm medial and 2 cm distal to the posterolateral acromial border. A 4-mm arthroscope (Synergy; Arthrex, Naples, FL) is introduced, and additional anterior portals are established using an outside-in technique under direct visualization with the use of a switching stick (Arthrex). The anterosuperior portal through the rotator interval is established first, approximately 1 cm inferior to the anterior acromion. A standard diagnostic arthroscopy is performed within the glenohumeral joint, and any concurrent pathology is treated appropriately. If there is labral pathology near the osteochondral lesion, it is wise to delay its fixation until after the OATS procedure to help with visualization and access to the glenoid. We also use a second slightly lateralized midglenoid anterior portal through the rotator interval as a working portal along with the established posterior portal, whereas the anterior-superior portal is used for viewing.
Step 3: Debridement of Cysts and Determination of Access Angle (α)
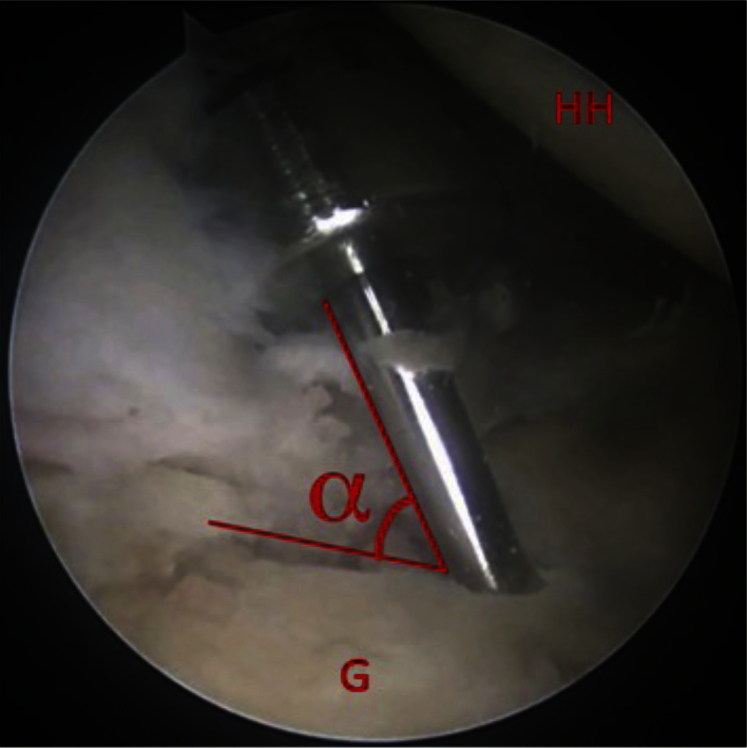
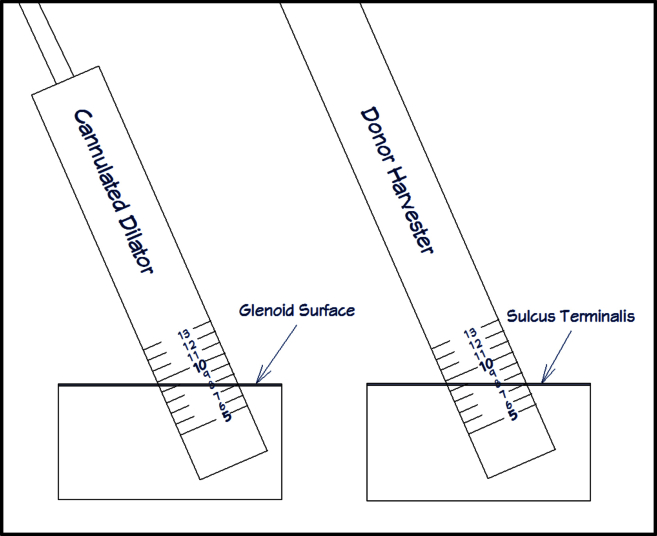
Once the cartilaginous defect is located, a probe is used to assess the underlying bone structure and locate the border of the bony defect. A calibrated probe (3.4-mm hook probe with markings; Arthrex) is useful for measuring the defect size. After the lesion is assessed, the decision is made to continue with an OATS procedure based on the size of the lesion and integrity of the underlying bone. An 18-gauge 4-inch spinal needle (BD, Franklin Lakes, NJ) is then used to establish an accessory percutaneous portal placed lateral and inferior to the posterior corner of the acromion that will provide the most perpendicular angle to the posterior-inferior portion of the glenoid defect. The humeral head can be maneuvered either anteriorly or posteriorly depending on the location of the lesion, thus establishing a reproducible angle of access to the glenoid. The portal is established with a longitudinal split through the infraspinatus, which is repaired side to side arthroscopically at the end of the procedure. The guide pin for the OATS system (Arthrex) is inserted into the defect using a pin driver through this accessory portal. The angle created between the glenoid surface and the guide pin is then determined and will be used later during autograft harvest. Because the arthroscopic image is 2-dimensional, the arthroscope can be moved around the guide pin until the most acute angle (referred to as α) is observed. Because the humeral head impedes direct perpendicular access to the glenoid, this angle (α) is usually less than 90° (Fig 1). The cannulated headed low-profile reamer (Arthrex) is placed over the guide pin by hand gently through the infraspinatus. The smooth shaft of this cannulated reamer helps maximize perpendicularity while protecting the chondral surface of the humeral head. Glenoid reaming is then initiated and taken to a depth of 8 to 10 mm. A cannulated dilator from the OATS kit (Arthrex) is inserted into the newly created recipient site to confirm depth and angle. The α angle is then assessed more specifically by measuring the depth of the dilator at the front and back (e.g., 7 mm and 10 mm, respectfully, as shown in Fig 2). We are currently in the process of developing an arthroscopic goniometer that can identify this angle with accuracy and reproducibility. Until this is made available, the angle can be assessed most accurately by using a cannulated dilator placed into the reamed defect. Figure 3 shows the cannulated dilator within a simulated defect in a cadaveric specimen. The dilator is used to obtain specific measurements that can be reproduced at the time of graft harvest. This example shows the depth of the reamed defect to be 13 mm and 17 mm on either side of the dilator. Although this cadaveric example shows the reaming to be deeper than desired, it is the 4-mm differential that is important to remember and will be used during harvest to match the reamed angle with the harvest angle. Figure 3 is a schema of the reamed defect with the dilator in place. As stated previously, the differential between measurements on either side of the dilator is translated to the harvester at the time of donor harvest. In the example shown in Figure 3, one would use the measurements of 7 mm and 10 mm obtained from either side of the dilator and replicate them at the time of donor harvest. It is important to note that it may be necessary to purposefully ream the defect at an angle, especially if the glenoid defect is located on the perimeter. Reaming at a direct perpendicular angle to a lesion on the perimeter can result in the reamer exiting the glenoid neck, which is undesirable. The arthroscope is maintained in the joint with fluid running and portal setup intact to ensure visualization is not disturbed by bleeding from a depressurized joint.
Fig 1.
Guide pin insertion into glenoid defect to determine angle of access (α) for autograft harvest. (G, glenoid; HH, humeral head.)
Fig 2.
Cannulated dilator placed into reamed glenoid defect. The measurement differential of 3 mm obtained from the dilator is translated to the harvester at the time of donor harvest. Because the dilator shows a 3-mm differential (7 mm and 10 mm), the harvester should have the same differential at the time of harvest to match the angle of the reamed glenoid osteochondral defect.
Fig 3.
An alignment rod is used to verify the angle of insertion (α). A calibrated alignment rod can assist in determining the angle by comparing maximum and minimum depth measurements on opposite sides of the rod. (For example, in this figure, the measurements are 13 mm and 17.5 mm.) Depth measurements can be used to verify both the depth and the angle of autograft before insertion.
Step 4: Osteochondral Harvest From Ipsilateral Knee
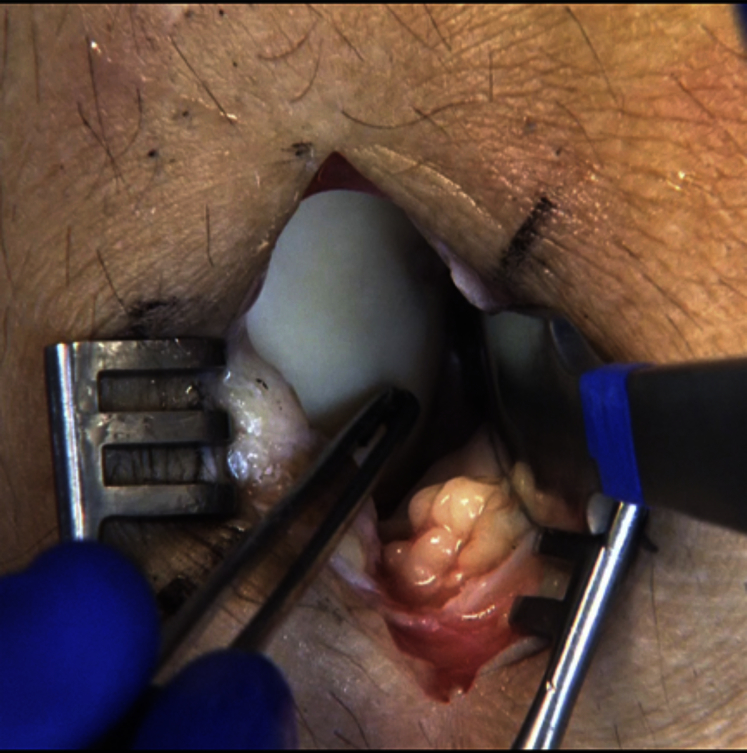
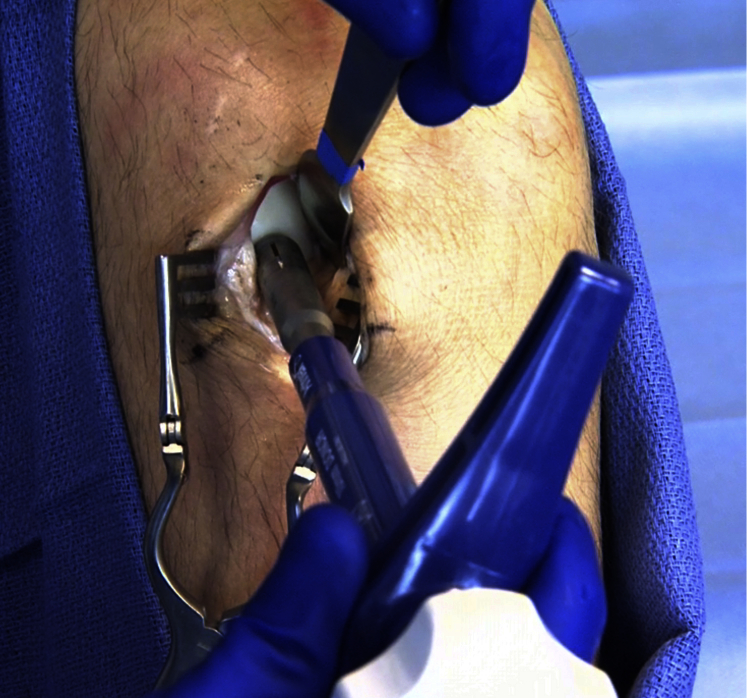
The previously sterilized ipsilateral lower extremity is then addressed, and a small 2- to 3-cm longitudinal incision is made over the distal lateral trochlea of the knee. A small longitudinal arthrotomy is performed exposing the sulcus terminalis at the junction of the lateral trochlea and lateral femoral condyle (Fig 4). By use of the appropriately sized harvester (Donor Harvester; Arthrex), a donor plug is harvested in close approximation to the same α angle corresponding to the previously reamed recipient plug angle (Fig 5). The measurement differential obtained from the dilator in the recipient site is now used to match the angle of harvest to the reamed recipient site angle (Fig 3). It is helpful at this point to use a dry arthroscope to visualize the harvester as it penetrates into the femoral condyle. Using the arthroscope in this manner helps to accurately identify the angle of harvest by closely assessing the depth differential as the cartilage is penetrated by the harvester. With the arthroscope closely visualizing the harvester, minor adjustments can be made as harvesting is initiated. This procedure can be repeated along the sulcus terminalis to harvest multiple donor plugs if the size of the glenoid defect necessitates. Depending on the size of each plug, one can usually harvest up to 3 grafts from the sulcus terminalis if needed. The donor site defects are then filled with pre-sized allograft plugs (Alloplug Frozen Backfill Plug; Arthrex) measuring 1 mm larger than the harvested size to ensure a press fit or, alternatively, can be left unfilled. A layered closure is then performed on the knee incision to complete the harvest procedure. Local anesthetic is injected around the incision, the area is covered with a soft dressing, and ice is applied to the knee postoperatively as is our standard regimen. Physical therapy is initiated postoperatively focusing on range of motion and quadriceps strengthening.
Fig 4.
Identification of sulcus terminalis on lateral femoral condyle of a right knee. Identification is performed by finding the sulcus terminalis ridge at the most distal aspect of the lateral trochlea. This ridge marks the most distal aspect of graft harvest.
Fig 5.
Graft harvest from ipsilateral knee at border of sulcus terminalis. Harvest is performed at an angle (α) with the cartilage to match the previously established access angle at the glenoid.
Step 5: Insertion of Donor Plug Into Recipient Site
The graft is removed from the harvest site, inspected, and then carefully inserted into the clear delivery tube from the disposable kit (Arthrex). Before placement into the clear delivery tube, the length of the graft is made to be 1 mm shorter than the recipient depth to allow for slight recession of the graft. The bony edges of the graft are chamfered with scissors to allow for easier placement. In addition, care is taken to note the rotation of the plug within the delivery tube so that the graft will be oriented to match the recipient hole, thereby ensuring the chondral surface will be as flush as possible with the surrounding intact glenoid cartilage. After this step, the delivery tube with the graft loaded is inserted into the joint through the posterolateral accessory portal. Once the correct orientation of the graft is established arthroscopically, the graft is then gently pushed out of the delivery tube and into its recipient site. It is important to note that once the graft has entered the recipient site beyond halfway, it is difficult to adjust rotation; therefore, precise rotation and orientation of the graft should be established early during insertion. Once 50% of the graft has been inserted, the rotation is established and cannot be changed without risk of damage to the graft (Fig 6). If the graft were to be inserted mal-oriented, a removal device could be used from the same kit (disposable OATS kit; Arthrex) and the process repeated.
Fig 6.
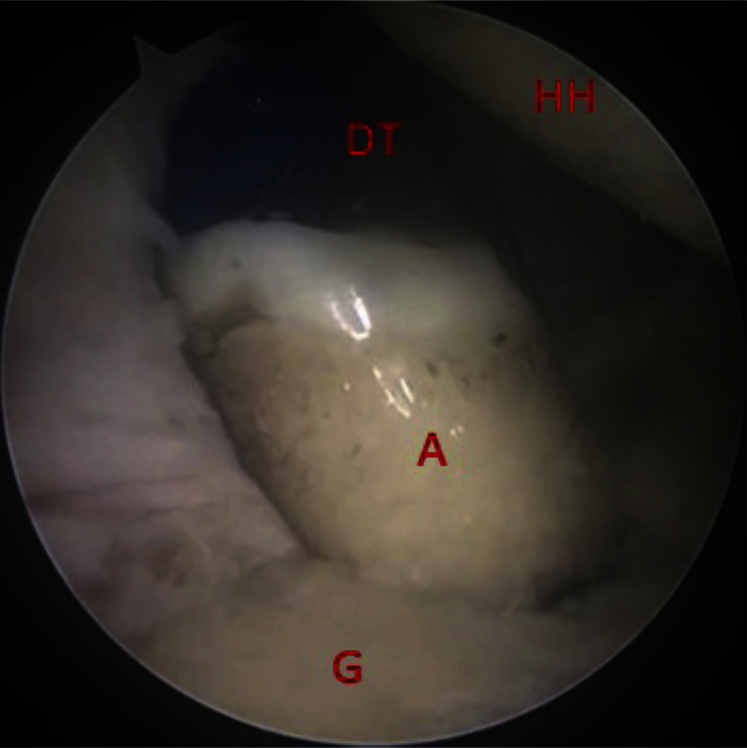
Osteochondral autograft insertion into glenoid defect using clear delivery tube to ensure correct orientation. (A, osteochondral autograft plug; DT, graft delivery tube; G, glenoid; HH, humeral head.)
Step 6: Graft Assessment and Labral Repair
Once the graft is seated into its recipient site, the arthroscope is used to verify that there is no prominence of the graft (Fig 7). This technique can then be repeated as needed to address any further defects. We prefer to slightly overlap our grafts to increase graft stability and optimize lesion coverage. On completion of the autograft transfer, labral injuries are addressed. Care is taken not to insert any suture anchors into the graft because this may compromise the graft integrity. In addition, large portals through the rotator cuff are closed arthroscopically in a side-to-side manner with a suture-shuttling device (SutureLasso; Arthrex).
Fig 7.
Final inspection of graft after insertion into glenoid defect. (A, autograft; G, glenoid; HH, humeral head.)
Step 7: Postoperative Regimen
On completion of the procedure, a simple sling (Shoulder Immobilizing Sling; Hely-Weber, Irving, TX) is placed, and we initiate elbow, wrist, and hand active range of motion on postoperative day 1. We do not feel the need to protect the osteochondral grafts any differently than our usual capsulolabral repairs. Physical therapy is initiated following our usual protocols, in which gentle passive and active-assisted range-of-motion exercises are begun along with simple scapular stabilizing exercises such as shoulder shrugs and scapular retractions. The knee is also addressed with formal therapy including attention to early patellar mobilization, glides, and the regaining of quadriceps control as soon as possible. A brace is not necessary for the knee, and weight bearing is allowed as tolerated on postoperative day 1.
Discussion
Treatment of cartilage lesions within the glenoid remains a difficult task for shoulder surgeons. Unlike the knee, options for cartilage restoration in the glenoid are limited and without long-term follow-up. Microfracture has shown positive results at an average of 28 months' follow-up in patients with isolated defects8; however, this requires healthy subchondral bone and, at best, results in filling of the lesion with suboptimal fibrocartilage. Another option is autologous chondrocyte implantation (ACI). Romeo et al.9 described the first case report of a 16-year-old patient with a unipolar humeral cartilaginous defect that they treated with ACI using the intercondylar notch in the ipsilateral knee for chondrocyte harvest. Although favorable short-term results were reported, this procedure is costly and subjects the patient to 2 separate procedures with the implantation requiring an open approach. Moreover, ACI requires healthy subchondral bone and is contraindicated in areas of avascular necrosis or juxta-articular cysts. Buchmann et al.10 subsequently reported on 4 patients who underwent glenohumeral ACI. Of these patients, 2 had isolated humeral defects, one had an isolated glenoid defect, and one had bipolar defects. Although preoperative outcome scores were not reported, the average postoperative Constant score and American Shoulder and Elbow Surgeons score were 83.25 and 95.33, respectively.
Another option that has recently been described is micronized allogeneic cartilage matrix implantation.11 This technique involves securing the allogeneic graft over a microfractured glenoid surface with fibrin glue. Advantages of this technique include an all-arthroscopic approach and the ability to accomplish the technique in a single-stage procedure. Disadvantages include cost of allograft preparation, requirement of healthy subchondral bone, and lack of long-term results.
Cartilage restoration options for glenoid defects are limited and usually require healthy subchondral bone. Few options remain for defects that contain compromised subchondral bone. Using our technique, we perform OATS into glenoid defects that contain damaged subchondral bone. This technique results in hyaline cartilage and simultaneously treats the diseased underlying bone. This is a relatively simple, minimally invasive and reproducible technique that can be applied to symptomatic glenoid chondral defects in which the subchondral bone is damaged. There are, however, risks that are unique to this technique. Reaming the glenoid defect can result in glenoid vault perforation, as well as glenoid fracture. Both of these can have devastating results, and if fracture occurs, conversion to an open approach may be required for fixation. Glenoid vault perforation, if relatively small, may not necessarily require any further treatment if the graft and glenoid rim remain stable. An advantage, however, unique to this technique is that it does not require healthy subchondral bone for incorporation, and once incorporation occurs, the defect is filled with type 2 hyaline cartilage. Table 1 lists surgical pearls and pitfalls for quick reference.
Table 1.
Surgical Pearls and Pitfalls
| Pearls |
| The surgeon should use a guide pin into the glenoid defect to establish the access angle (α). In addition, he or she should use a cannulated acorn reamer over the guide pin to maintain consistency of the angle. |
| After harvest, Alloplug backfill plugs (Arthrex) should be used to fill the defect created from harvesting the autograft. The plugs should be trimmed with a rongeur or with Mayo scissors before insertion to match the cartilage angle. The backfill plug should be recessed approximately 1 mm to safely avoid any prominences. |
| The portals should be made large enough to insert the graft easily with minimal resistance. Large portals through the rotator cuff should be made longitudinally along the fibers and closed in a side-to-side manner at the conclusion of the procedure. |
| The surgeon should establish and verify the position and orientation of the graft arthroscopically before insertion into the recipient site. If a graft plug is malpositioned or inserted in an undesirable location, the plug can be removed with a removal device (Arthrex) from the disposable osteochondral autograft transfer kit and the procedure repeated with another graft. |
| Repair of any labral pathology should be postponed until after the insertion of the graft (or grafts) because maximum humeral mobility is desired for site preparation and graft insertion. |
| Pitfalls |
| If the osteochondral lesion is situated on the rim of the glenoid, reaming perpendicular to the glenoid face may seem desirable; however, this may result in the reamer perforating the glenoid neck. Therefore, intentionally angling the reamer may be necessary to avoid this pitfall. |
| When positioning the patient, one should be sure to leave the ipsilateral knee well exposed so that it can be prepared and draped in preparation for the graft harvest. |
| The surgeon must ensure that the orientation of the graft is correct before insertion into the recipient site. Once the graft has been inserted, it is difficult to change the orientation of the graft. Use of a clear graft delivery tube (Arthrex) is critical to determine graft orientation before insertion. If the graft is inserted incorrectly, it must be removed and another graft placed. |
Lee and Harryman3 treated a patient with a juxta-articular glenoid cyst after a fracture with an osteochondral autograft plug harvested from the ipsilateral humeral head. In their case report, the patient was able to return to painless normal function, lacking only 20° of internal rotation at both 0° and 90° of abduction as compared with the opposite extremity. As stated previously, our preference is to harvest from the knee at the distal lateral trochlea just proximal to the sulcus terminalis using a mini-open approach, which has been shown to cause minimal morbidity6 and is easily reproducible. Garretson et al.7 showed that the distal lateral trochlea has the lowest contact pressure among the lateral knee sites and is a desired location of autograft harvest. In a study by Nishimura et al.,6 12 patients underwent osteochondral autograft transport for severe osteochondritis dissecans of the humeral capitellum. Each patient received donor autograft plugs from the ipsilateral knee at the sulcus terminalis. Ten patients reported a pain score of zero on a visual analog scale at 3 months postoperatively. At 6 months postoperatively, all patients had re-established at least 80% of their preoperative extensor strength, and at 24 months, there were no radiographic signs of knee arthritis.6 In addition, this mini-open harvest technique allows direct visualization of the harvest and offers fine-tuning of the approach angle to match the previously established α angle at the glenoid. The clinical outcomes at our institution are relatively short-term, but favorable results have been shown. As with other glenoid cartilage restoration or preservation techniques, larger studies with longer-term follow-up are required to evaluate this technique fully.
Footnotes
The authors report the following potential conflict of interest or source of funding: D.J.W. receives research support from Ceterix and Zimmer.
Supplementary Data
Surgical technique for arthroscopic osteochondral autograft transfer into glenoid osteochondral defect using graft from ipsilateral knee. The patient is positioned in the lateral decubitus position. A left shoulder is shown, with an anterior viewing portal through the rotator interval. After a standard diagnostic evaluation of the shoulder is performed, the decision is made to perform an osteochondral autograft transfer. A guide pin is used to establish the angle of approach for the transfer. Once established, an osteochondral autograft is harvested from the patient's ipsilateral knee at the sulcus terminalis. Care is taken to ensure that the angle of graft harvest is the same as the angle determined by the guide pin. The harvest site is filled with allograft, and the glenoid is then reamed with a cannulated reamer over the guide pin. The pin is removed, and the autograft is inserted arthroscopically. Portals are then closed arthroscopically and instruments removed.
References
- 1.Schajowicz F., Sainz M., Slullitel J. Juxta-articular bone cysts (intraosseous ganglia) J Bone Joint Surg Br. 1979;61:107–116. doi: 10.1302/0301-620X.61B1.422629. [DOI] [PubMed] [Google Scholar]
- 2.Spoliti M. Glenoid osteolysis after arthroscopic labrum repair with a bioabsorbable suture anchor. Acta Orthop Belg. 2007;73:107–110. [PubMed] [Google Scholar]
- 3.Lee S., Harryman D. Local arthroscopic bone grafting of a juxta-articular glenoid bone cyst. Arthroscopy. 1997;13:502–506. doi: 10.1016/s0749-8063(97)90131-6. [DOI] [PubMed] [Google Scholar]
- 4.Kim S.H., Noh K.C. Loss of chondrolabral containment of the glenohumeral joint in atraumatic posteroinferior multidirectional instability. J Bone Joint Surg Am. 2005;87:92–98. doi: 10.2106/JBJS.C.01448. [DOI] [PubMed] [Google Scholar]
- 5.Alford J., Cole B. Cartilage restoration, part 1. Am J Sports Med. 2005;33:295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura A., Morita A., Fukuda A., Kato K., Sudo A. Functional recovery of the donor knee after autologous osteochondral transplantation for capitellar osteochondritis dissecans. Am J Sports Med. 2011;39:838–842. doi: 10.1177/0363546510388386. [DOI] [PubMed] [Google Scholar]
- 7.Garretson R., Katolik L., Verma N., Beck P., Bach B., Cole B. Contact pressure at osteochondral donor sites in the patellofemoral joint. Am J Sports Med. 2004;32:967–974. doi: 10.1177/0363546503261706. [DOI] [PubMed] [Google Scholar]
- 8.Frank R., Van Thiel G., Slabaugh M., Romeo A., Cole B., Verma N. Clinical outcomes after microfracture of the glenohumeral joint. Am J Sports Med. 2010;38:772–781. doi: 10.1177/0363546509350304. [DOI] [PubMed] [Google Scholar]
- 9.Romeo A., Cole B., Mazzocca A., Fox J. Autologous chondrocyte repair of an articular defect in the humeral head. Arthroscopy. 2002;18:925–929. doi: 10.1053/jars.2002.36144. [DOI] [PubMed] [Google Scholar]
- 10.Buchmann S., Salzmann G., Glanzmann M. Early clinical and structural results after autologous chondrocyte transplantation at the glenohumeral joint. J Shoulder Elbow Surg. 2012;21:1213–1221. doi: 10.1016/j.jse.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 11.Shin J., Mellano C., Cvetanovich G., Frank R., Cole B. Treatment of glenoid chondral defect using micronized allogeneic cartilage matrix implantation. Arthrosc Tech. 2014;3:e519–e522. doi: 10.1016/j.eats.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Surgical technique for arthroscopic osteochondral autograft transfer into glenoid osteochondral defect using graft from ipsilateral knee. The patient is positioned in the lateral decubitus position. A left shoulder is shown, with an anterior viewing portal through the rotator interval. After a standard diagnostic evaluation of the shoulder is performed, the decision is made to perform an osteochondral autograft transfer. A guide pin is used to establish the angle of approach for the transfer. Once established, an osteochondral autograft is harvested from the patient's ipsilateral knee at the sulcus terminalis. Care is taken to ensure that the angle of graft harvest is the same as the angle determined by the guide pin. The harvest site is filled with allograft, and the glenoid is then reamed with a cannulated reamer over the guide pin. The pin is removed, and the autograft is inserted arthroscopically. Portals are then closed arthroscopically and instruments removed.