Abstract
Morphine creates a neuroinflammatory response and enhances release of the proinflammatory cytokines like interleukin-1β (IL-1β), which compromises morphine analgesia as well as induces morphine tolerance. In this study, we attempted to investigate the mechanisms of morphine induced IL-1β synthesis and release. Microglial cells were treated with morphine (100 μM) once daily for 3 days. Control groups underwent the same procedure but received sterile saline injection instead of morphine. Toll-like receptor 4 (TLR4) and P2X4 receptor (P2X4R) signaling were analyzed using Western blot; immunofluorescence was used to detect the signaling of CD68; real-time RT-PCR and ELISA kit was used to measure the messenger RNA and protein synthesis and release level of IL-1β. Morphine enhanced IL-1β synthesis and P2X4R protein expression. TLR4 were responsible for morphine-induced IL-1β synthesis, while morphine-induced IL-1β release was via P2X4R. Morphine-induced IL-1β release is mediated by endocytosis of TLR4. These results indicated that TLR4 and P2X4R pathways mediated IL-1β synthesis and release in microglia followed chronic morphine. TLR4 internalization is the main mechanism of morphine-induced microglia activation and IL-1β release.
Keywords: Morphine, Microglia, Toll-like receptor 4, P2X4 receptors, Interleukin-1β
Introduction
Morphine induces potent analgesia and is the gold standard for the treatment of moderate-to-severe pain. However, the chronic use of morphine leads to tolerance and hyperalgesia which is a clinical challenge for managing chronic pain. Microglia, resident macrophages within the central nervous system (CNS), are ubiquitously distributed throughout the brain and spinal cord. Microglial activation is one of the most important mechanisms for morphine tolerance and hyperalgesia [1]. Activated microglia are a source of proinflammatory cytokines such as interleukin-1β (IL-1β) which may weaken morphine analgesia and contributes to the development of morphine tolerance [2].
IL-1β precursors do not have a clear signal peptide for synthesis and secretion and none of them is found in the Golgi [3]. So IL-1β belongs to a so-called leaderless secretory protein group lacking a secretory signal sequence. Previous studies have demonstrated that the secretion of IL-1β is as an active, mature cytokine through activation of different receptors [4]. The synthesis of IL-1β precursor (pro-IL-1β) is induced by stimulation of TLR4, and the release of mature IL-1β cleaved by a cysteine protease called caspase-1 is induced by stimulation of P2X purinoreceptors by ATP [5, 6]. However, the mechanisms of morphine-induced IL-1β release in microglia are still unclear.
Microglia express μ-opioid receptors, as well as TLR4 and P2X receptors [6, 7]. TLR4 are key initiators of innate and adaptive immune responses through production of proinflammatory cytokines and up-regulation of costimulatory molecules [7]. Though some studies indicate that TLR4 is not involved in morphine tolerance and microglial activation [8, 9], many evidences have proved that morphine creates neuroinflammation not via μ-opioid receptors, but through activation of TLR4 signaling [10–12]. Thus, morphine-induced proinflammatory glial activation via TLR4 could potentially provide an explanation for tolerance to the analgesic effects of morphine. The TLR4 agonist, LPS, can induce endosomal trafficking of TLR4 leading to lysosomal degradation and signal termination [13]. However, it is unknown how morphine induces proinflammatory microglial activation and IL-1β release via TLR4.
P2X receptors are a distinct family of ATP-gated ion channels which appear to be key players in microglia activation and IL-1ß release [14]. P2X7-dependent IL-1b release plays a critical role in LPS-activated microglia, but in inactivated microglial cells, P2X7 receptor shows little functional activity [15, 16]. Microglia are co-expression of P2X4R with P2X7 receptor, and recent evidence has indicated a structural interaction between P2X4 and P2X7 receptors, and the expression of P2X4R leads to facilitation of P2X7-dependent release of IL-1β [17, 18]. Chronic morphine enhances microglial P2X4R signaling which makes a crucial contribution to pathologically enhanced pain processing [19, 20].
The present study was designed to determine the modulatory effect of morphine on IL-1β production and possible mechanisms in microglia. We hypothesize that chronic morphine increases IL-1β release via chronic increases in P2X4R signaling which induced by TLR4 endocytosis. To this end, we assessed the effect of P2X4 antagonism and endocytosis inhibitor on morphine-induced microglia activation and IL-1β release.
Materials and methods
Microglia primary culture
Primary culture was prepared as described [21]. Briefly, mixed glial culture was isolated using P1–3 rat cortex and maintained for 10–14 days in DMEM medium (ATCC, USA) containing 10 % fetal bovine serum (Invitrogen, USA) plus 50 U/mL penicillin G and 50 μg/mL streptomycin sulfate (GIBCO-BRL, Paisley, UK). Microglia separated by gentle shaking were plated in T-75-cm2 culture flasks (Corning, USA) and maintained at 37 °C in a humidified incubator under 5 % CO2 atmosphere.
Real-time RT-PCR
Total RNA was extracted by an RNeasy Mini Kit (Qiagen) according to the manufacturer’s instruction. cDNA was synthesized by RT2 Easy First Strand cDNA Synthesis Kit (Qiagen) according to the manufacturer’s instruction. The primers for IL-1β and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from SABioscience (Frederick). qPCR was performed on a CFX96™ Real-Time PCR detection system (Bio-Rad) using the SYBR Green method. The data were analyzed by the ΔΔCt method.
IL-1β ELISA
After drug treatment, cell lysates and medium were collected for measure IL-1β. Measurement of microglial IL-1β was performed using mouse IL-1β commercial kits (R&D Systems). The kit has a detection range of 2–500 pg/ml. Assays were performed following the manufacturer’s instructions and read by a spectrophotometer plate reader (Molecular Devices) at 450 nm. Data were normalized to the control.
Western blot analysis
Cultured microglia were lysed in 100 μl of cold lysis buffer (50 mM Tris-HCl pH 7.4, 1 mM EDTA, 150 mM NaCl, 1 % NP- 40, 1 mM PMSF, 1 mg/mL Aprotinin, 1 mg/mL Leupeptin, 1 mg/mL Pepstatin A, 10 mM NaF, 1 mM Na3VO4). Protein samples was subjected to 4 %–12 % SDS-polyacrylamide gradient gel (Bio-Rad) and transferred onto a polyvinylidene difluoride membrane followed by immunoblotting. The blots were blocked for 1 h at room temperature in 5 % non-fat dry milk-Phosphate Buffered Saline (PBS)-0.1 % Tween-20 and incubated with rabbit antibody to TLR4 (1:500, Abcam), P2X4R (1:500, Alomone) overnight in 5 % non-fat dry milk-TBST at 4 °C, followed by incubation with Donkey Anti-Goat IgG peroxidase (HRP)-conjugated secondary antibody (1:10,000, Alomone). Data were normalized to the internal control GAPDH. All treatments were completed at least three times.
Cell surface biotinylation
Prior to surface protein biotinylation, all reagents were cooled to 4 °C. The cells were washed four times with ice-cold phosphate buffered saline (PBS) followed by incubation with 0.5 mg/mL EZ-link Sulfo-NHS-LC-biotin (Pierce, PerBio) in 4 mL ice-cold PBS per flask on a rotating wheel for 30 min at 4 °C. Glycine was added to quench the reaction and cells were centrifuged and then lysed in 2 % Triton X-100-containing lysis buffer containing complete protease inhibitors (Roche). Protein was measured using Bio-Rad Protein Assay and equal amounts of protein were incubated with streptavidin beads (Pierce, PerBio) overnight at 4 ° to pull down all biotinylated proteins. Beads were washed four times and boiled in gel sample buffer to release biotinylated proteins. Equal amounts of biotinylated protein was loaded per lane and electrophoresed on 4–12 % SDS-PAGE gels (Bio-Rad).
Immunofluorescence for microglia
Microglia cells were fixed for 15 min at room temperature in PBS containing 3 % paraformaldehyde before being permeabilized for 5 min at 4 °C with 0.2 % Triton X-100. Before antibody labeling, the cells were incubated for 1 h at room temperature in a blocking solution containing 3 % fetal bovine serum (FBS) and 0.5 % Triton-20. Cells were then incubated over 48 h at 4 °C with rat anti-CD68 (1:100, AbD Serotec). Subsequently, the cells were rinsed three times in PBS before being incubated for 1 h with FITC-conjugated goat anti-mouse IgG FITC (1:200, Sigma). The anti-fade DAPI (1:1000, Sigma) solution should be added and observed with a fluorescence microscope (Carl Zeiss).
Statistical analysis
Analysis of variance was carried out using one-way ANOVA with Dunnett’s post hoc test. All experiments were completed at least three times and are presented as mean ± standard deviation of the mean. Significance was determined at a level of p < 0.05.
Results
Morphine enhances IL-1β synthesis via TLR4
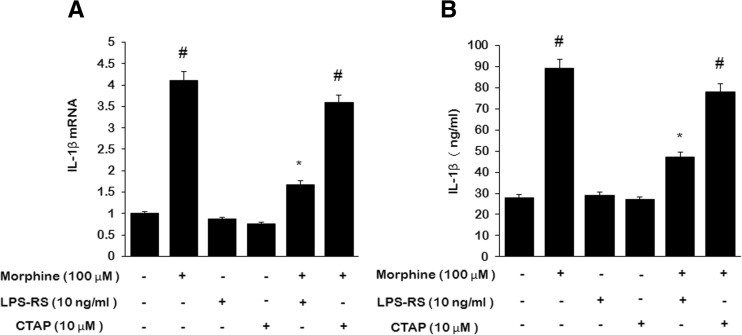
We first examined the effect of morphine on IL-1β synthesis in microglia. Quantitative RT-PCR (qRT-PCR) and ELISA were performed respectively to investigate the effect of morphine on IL-1β mRNA and protein level. One-hundred-micromole morphine potently increased IL-1β mRNA expression (Fig. 1a) and protein (Fig. 1b) in microglial cells at 24 h. Pretreatment of microglia with 100 μM LPS-RS, a selective TLR4 antagonist, before treatment with 100 μM morphine inhibited morphine-induced IL-1β mRNA expression and IL-1β protein synthesis. While 10 μM CTAP, a μ-opioid receptor selective antagonist, had no effect on morphine-induced IL-1β mRNA and protein elevations. This suggests that TLR4, while not μ-opioid receptors, are involved in morphine-induced IL-1β synthesis.
Fig. 1.
Morphine enhanced IL-1β synthesis which is inhibited by LPS-RS. Microglia were treated for 24 h with 0 or 10 μM CTAP or 10 ng/ml LPS-RS, then 0 or 100 μM morphine was added for 24 h. a IL-1β mRNA relative to GAPDH. b IL-1β protein in microglial cells lysates. (#p < 0.05, compared with saline; *p < 0.05, compared with morphine; n = 6)
Morphine increases IL-1β release via P2X4R
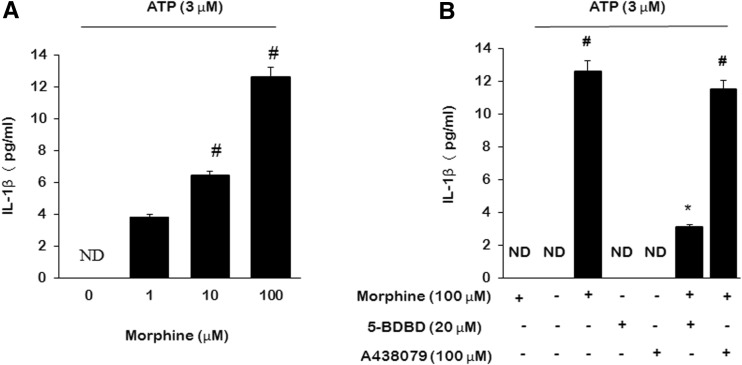
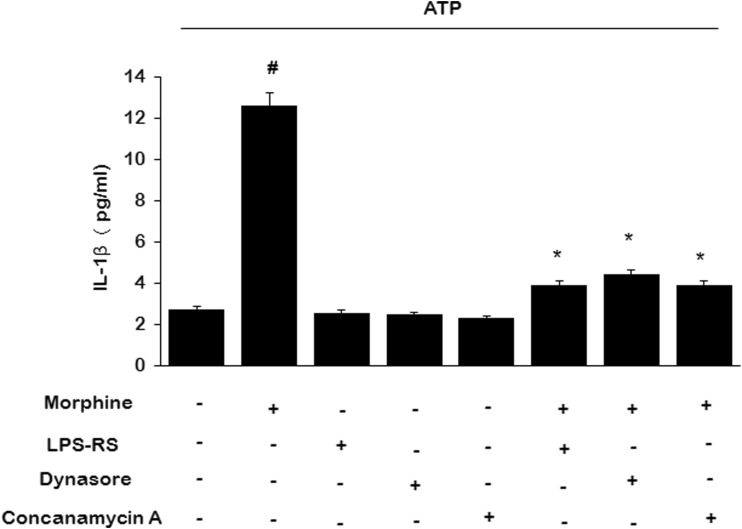
Following 3 days of morphine treatment (100 μM, once daily), no IL-1β was detected in the culture media. However, IL-1β release was dramatically induced by 3 μM ATP stimulation for 4 h dose-dependently (Fig. 2a). ATP stimulation did not further influence the IL-1β mRNA expression (data not shown). To assess the role of ionotropic purinergic receptors on IL-1β release in the presence of morphine stimulation, we pretreated microglial cells with 20 μM 5-BDBD (P2X4R antagonist) or 100 μM A438079 (P2X7 receptor antagonist) for 30 min before ATP stimulation, we found that it was 5-BDBD, while not A438079, reduced morphine-induced IL-1β release (Fig. 2b).
Fig. 2.
Morphine enhanced IL-1β release which is inhibited by 5-BDBD. Microglia were treated for 3 days with 100 μM morphine; then were treated with 20 μM 5-BDBD or 100 μM A438079 for 30 min; then were coincubated with 3 μM ATP for 4 h. a IL-1β release following different doses of morphine treatment. b IL-1β release inhibited by 5-BDBD. (#p < 0.05, compared with saline; *p < 0.05, compared with morphine; n = 6). ND no detect
Morphine enhances surface P2X4 expression via TLR4
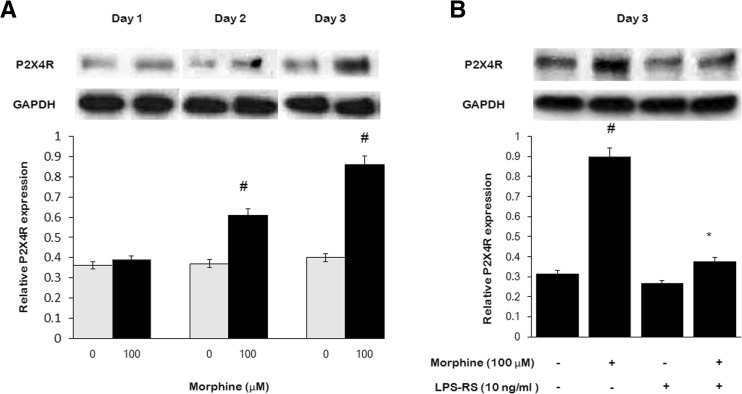
Chronic morphine treatment (100 μM, once daily) resulted in a time-dependent increasing of surface P2X4R expression in microglia (Fig. 3a). We went on to investigate whether the activation of TLR4 interferes with surface P2X4R expression followed by chronic morphine. Microglia were incubated in morphine-supplemented (100 μM, once daily) DMEM in combination with the TLR4 selective antagonist LPS-RS (100 nM, once daily) for 3 days. Morphine-induced increasing of surface P2X4R expression was significantly inhibited in the presence of LPS-RS in microglia, indicating that the effect of morphine on surface P2X4R expression was mediated via the TLR4 (Fig. 3b).
Fig. 3.
Morphine enhances P2X4R expression in microglia. Microglia were treated with morphine (100 μM, once daily) or together with LPS-RS (10 ng/ml, once daily) for 1, 2, or 3 days, lysed, and subjected to Western blot analysis. a Morphine induced time-dependent increasing of P2X4R expression. b P2X4R expression was inhibited with LPS-RS treatment for 3 days. (#p < 0.05, compared with saline; *p < 0.05, compared with morphine; n = 6)
Morphine decreases surface TLR4 expression through endocytic pathway
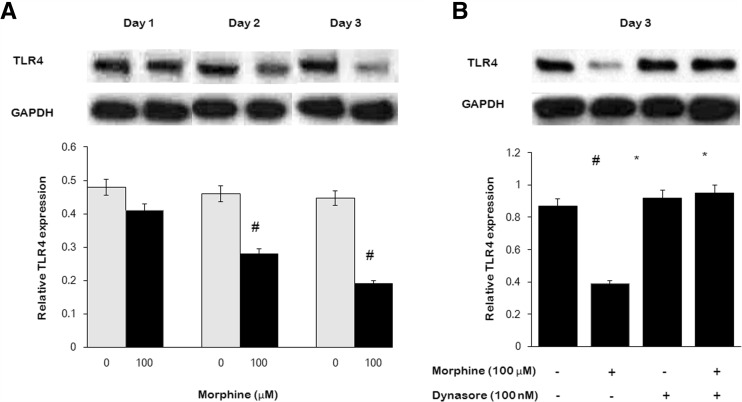
Morphine at 100 μM once daily × 3 days decreased surface TLR4 expression in microglia time-dependently (Fig. 4a). To investigate whether the endocytosis of TLR4 involved in decreasing of TLR4 modulated by morphine, microglia were incubated in morphine-supplemented (100 μM, once daily) DMEM in combination with the endocytic inhibitor dynasore (100 nM, once daily) for 3 days. Morphine-induced decreasing of TLR4 was significantly inhibited in the presence of dynasore, indicating that the effect of morphine on decreasing of surface TLR4 signaling was mediated via the endocytic pathway (Fig. 4b).
Fig. 4.
Morphine decreased TLR4 expression in microglia. Microglia were treated with morphine (100 μM, once daily) or together with endocytic inhibitor dynasore (100 nM, once daily) for 1, 2, or 3 days, lysed, and subjected to Western blot analysis. a Morphine-induced time-dependent decreasing of TLR4 expression. b Morphine-induced TLR4 decreasing was inhibited by dynasore treatment. (#p < 0.05, compared with saline; *p < 0.05, compared with morphine; n = 6)
Inhibition of the endocytic pathway decreases surface P2X4 expression
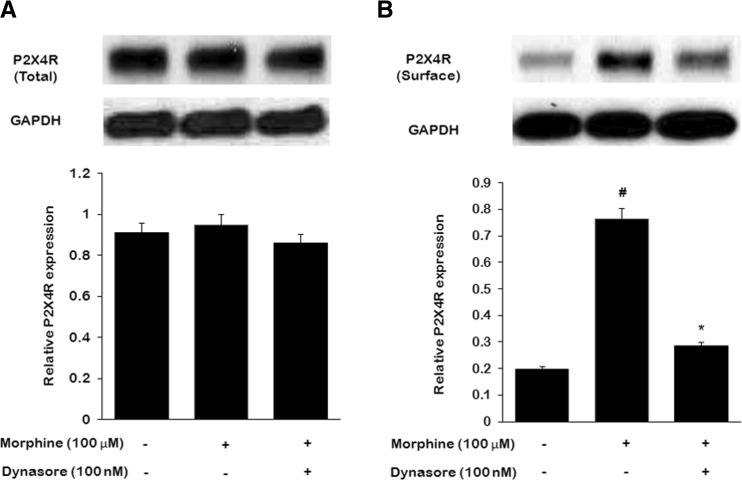
To elucidate whether the endocytic pathway was involved in the effects of morphine on P2X4R expression by microglia, we utilized dynasore to incubate microglia with morphine and then examined total and surface P2X4 expression. We found that microglia activation with morphine did not lead to an increase in total P2X4 protein levels (Fig. 5a), while lead to an increase in surface P2X4 expression (Fig. 5b). Dynasore effectively blocked the increase of microglial surface P2X4R expression (Fig. 5b). These results suggest that endocytic pathway could mediate the enhancing effects of morphine on surface P2X4R signaling.
Fig. 5.
The effect of endocytosis on P2X4R expression in primary microglia treated with morphine. Microglia were treated with morphine (100 nM, once daily) or together with endocytic inhibitor dynasore (100 nM, once daily) for 3 days. a Western blot analysis of total P2X4R signaling in microglial cells. b Western blot analysis of surface P2X4R signaling in microglial cells. (#p < 0.05, compared with saline; *p < 0.05, compared with morphine; n = 6)
Morphine enhances microglial CD68 expression through endocytic pathway
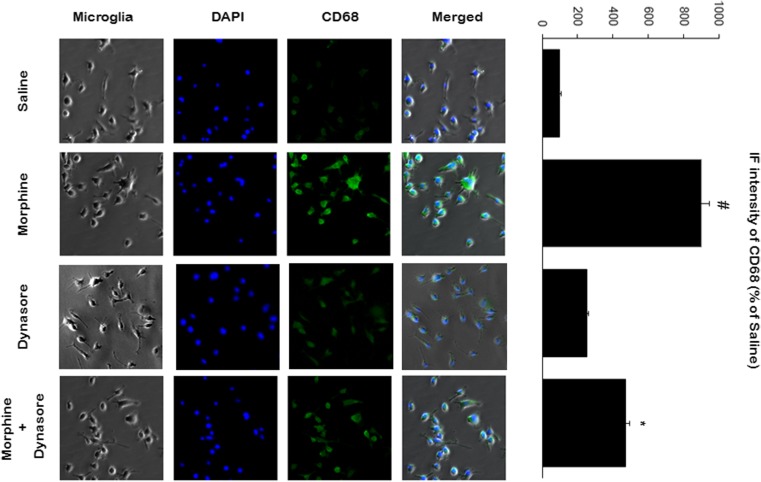
We went on to investigate the stimulatory effect of morphine on microglial activation. As shown in Fig. 6, morphine (100 μM, once daily × 3 days ) stimulation of microglial cells resulted in an increase in CD68 expression, which is a microglia activation marker and also one of the lysosomal membrane markers. To evaluate whether the morphine-induced changes in CD68 signaling were endocytic pathway-dependent, we used endocytic inhibitor, dynasore (100 nM, once daily × 3 days). Morphine-induced CD68 increased signaling was reduced with dynasore treatment together with morphine (Fig. 6), indicating that the effect of morphine on microglial activation was mediated via endocytic inhibitor dynasore.
Fig. 6.
Morphine increased CD68 expression in microglia. Microglia were treated with morphine (100 μM, once daily × 3) or together with endocytic inhibitor dynasore (100 nM, once daily × 3) and subjected to immunofluorescence. Morphine induced the increase of CD68 signaling, while this effect induced of by morphine was inhibited by dynasore. The quantification was performed by using immunofluorescence (IF) intensity values of CD68 protein in individual microglia (n = 3)
Discussion
Our studies presented here are the first to assess the signaling mechanisms through which morphine modulates microglial IL-1β synthesis and release. In those studies, we demonstrated that (1) morphine enhances IL-1β synthesis via TLR4, while enhances IL-1β release via P2X4R; and (2) morphine enhances P2X4R protein expression through TLR4 endocytosis. Together, these results suggest that TLR4 and P2X4R are all critical to the activity of microglia, enhancing the morphine-induced IL-1β synthesis and release in microglial cells.
Morphine leads to microglial activation and neuroinflammation through receptors signaling cascade [20]. Although it was long assumed that morphine-induced microglial activation must be mediated via activation of classic opioid receptors, recent data from Hutchinson et al. [10, 22] contested this assumption. Their studies have suggested that such proinflammatory effects of morphine are not via classic opioid receptors, while via TLR4 signaling. So the mechanism of morphine-induced neuroinflammation which leads to inflammatory cytokine release has remained a mystery. We demonstrated that morphine enhances IL-1β synthesis via TLR4, while stimulating IL-1β release via P2X4R. Furthermore, we suggest a novel interaction between P2X4R, IL-1β release, and TLR4 in microglia, which is likely to be mediated via the endocytosis pathways of TLR4.
Interleukin-1β (IL-1β) is a potent pro-inflammatory cytokine, central to the pathogenesis of acute and chronic morphine-induced inflammatory reaction of the central nervous system (CNS) which plays a major role in the induction and development of morphine tolerance [23, 24]. Using IL-1β receptor antagonist in the spinal cord reduced neuropathic pain-like behavior in animal models of peripheral nerve injury and increased the intensity and duration of morphine-induced analgesia [24]. Morphine releases interleukin-1, which heightens pain sensitivity and requires more morphine to treat the pain, thereby beginning a cycle of increased morphine dosages and increased pain. Combining morphine with a drug that blocks the release of interleukin-1 can provide long-term pain relief without increased morphine dosages [25]. Understanding the mechanisms of morphine-induced IL-1β generation may therefore identify new targets for anti-IL-1β therapies and contribute to the understanding of morphine-induced tolerance.
The synthesis and release of IL-1β may be mediated by some underlying cellular and molecular mechanisms [26], but the mechanisms are poorly understood. A two-signal model has been proposed to explain IL-1β synthesis and release which is a tightly controlled process. The first signal triggers synthesis of pro-IL-1β, a precursor form protein, by transcriptional induction; the second stimulus leads to caspase-1-dependent cleavage of pro-IL-1β and then release of mature IL-1β [27]. So despite pro-IL-1 mRNA transcripts and proteins were strongly induced already after 24 h of morphine priming in microglia, no mature IL-1β were detected in the culture medium which is consistent with previous findings [28]. In murine microglia, LPS does not promote the release of mature IL-1β, and the cells require a secondary stimulus such as ATP [29–31]. Here, we show that extracellular ATP regulates IL-1β: a dramatic release of IL-1β occurs soon after the exposure to ATP and IL-1β level was significantly higher in the morphine group. It means that morphine increases the synthesis and release of IL-1β, but morphine is not sufficient to promote the maturation and externalization of cellular IL-1β (Fig. 7).
Fig. 7.
A dramatic release of IL-1β occurring soon after the exposure to ATP and IL-1β level was significantly higher in the morphine group
Microglial cells express several purinoceptors, such as P2X4R, P2X7R, P2Y2R, P2Y6R, and P2Y12R [32]. Purinergic signaling is involved in microglial activation both as initiators and modulators. Activated microglia adopt a phenotype characterized by increased expression of P2X4R and the P2X4R+ state are a central player in mechanisms for neuropathic pain which shares the same mechanisms as morphine-induced hyperalgesia [33]. Hyperalgesia induced by peripheral nerve injury does not occur in P2X4R-deficient mice [34]. We found that P2X4R signaling was getting stronger with the extension of morphine treatment in microglia, and the expression of P2X4R signaling was inhibited by LPS-RS, a small-molecule inhibitor of TLR4. Together, these results suggest that there were some associations between TLR4 internalization and surface exposure of P2X4 receptors.
P2X4 signaling may play a critical role in the inflammatory responses in the neuropathic pain [2]. P2X4R activation has been suggested to mediate IL-1β released in microglia [35]. P2X4 knock-out (KO) mice showed impaired inflammasome signaling in the cord, resulting in decreased levels of IL-1β [36]. The mechanism may be that P2X4R activation induces increased intracellular calcium concentrations, calcium as a second messenger activation of mitogen-activated protein kinases, and release of proinflammatory cytokines [37, 38]. Our results provide evidence that P2X4R, while not P2X7R, expressed by microglia after chronic morphine treatment promote IL-1β release. But previous studies revealed that P2X7 receptor activation leads to the release of IL-1ß from microglia [33, 39]. We think the main reason is due to a different microglia state. In previous studies, it is the acute activation of microglial TLR4 by LPS. Activated microglia up-regulate a myriad of cell surface activation antigens and produce innate cytokines and chemokines. In this state, microglia rapidly switch to a ramified appearance, characterized by transcriptional and functional remodeling where P2X7 plays a critical role in IL-1ß release from LPS-primed microglia [40]. While in the healthy CNS, microglia normally exist in a quiescent state, characterized by a small soma and ramified processes where P2X7 receptor shows little or no functional activity [16, 41]. In our study, it is a chronic and long-term active effect of morphine on microglia. Indeed, similarly, P2X4R expressed by morphine-activated resting microglia promote IL-1ß release. In short, P2X receptors appear to be key players in microglia activation and IL-1ß release, both in their resting and activated states.
The signaling receptor for LPS is TLR4/MD-2 receiving LPS from CD14. TLR4 are key initiators of innate and adaptive immune responses through production of proinflammatory cytokines and chemokines. Excessive responses toward LPS may lead to life-threatening complications such as septic shock [7, 42]. Endocysis and endosomal degradation of the LPS receptor complex is indispensable for signal termination and LPS-associated antigen presentation, thus controlling both innate and adaptive immunity through TLR4 [43, 44]. Little is known about whether the mechanisms serve important functions for morphine-induced proinflammatory response. In our study, we found that chronic morphine treatment decreased TLR4 expression time-dependently. To further investigate whether endocytosis was involved in this process, we added dynasore, a specific endocytic pathway inhibitor, to the incubation medium with chronic morphine. We found that dynasore could inhibit the declination of TLR4 expression induced by morphine. At the same time, chronic opioid stimulation was found to up-regulate the expression of CD68, a microglia activation marker, and the up-regulation was also suppressed by dynasore. These results indicate that TLR4 is the target receptor of morphine-induced proinflammatory actions which modulates wide-ranging aspects of morphine pharmacology including morphine analgesic tolerance, morphine-induced hyperalgesia, and opioid respiratory depression.
In resting C8-B4 microglia, P2X4R are predominantly present within intracellular vesicular compartments, including lysosomes [45]. From a preexisting lysosomal pool, P2X4R can be trafficked to the surface membrane by procedures that induce endolysosomal secretion [46]. Similar to previous reports, we found that morphine increased surface and functional P2X4Rs expression without altering total P2X4R protein levels, while inhibiting endocytosis could eliminate the up-regulation of P2X4Rs in morphine-activated microglia. In addition, chronic morphine induces the up-regulation of CD68 and it was also suppressed by dynasore. CD68 is also a member of the lysosome associated membrane protein (LAMP) family, where CD68 predominantly localizes to lysosomes and endosomes, which reveals lysosomal activity, both in intracellular lysosomal metabolism and extracellular cell-pathogen interactions [47]. This indicated that morphine-induced TLR4 activation-dependent internalization increases lysosomal activity in freshly isolated microglia which may stimulate P2X4R from lysosomes to the microglial surface membrane.
Conclusion
The data presented here identify a novel interaction between TLR4 and P2X4R pathways mediating microglial activation and IL-1β release followed chronic morphine. This is the first study of the intracellular trafficking of TLR4 in microglia by chronic morphine stimuli and presents a picture whereby microglia is activated and P2X4R is up-regulated which is responsible for the production of IL-1β. This study is an integral first step toward assessing the role of TLR4 internalization in morphine tolerance and hyperalgesia which may prove to be valuable targets for the development of therapeutics aimed at attenuating morphine-induced microglia activation and IL-1β release or targets for novel analgesics compared with current therapies.
Acknowledgments
The authors’ work is supported by grants from the Affiliated Hospital of Qingdao University. We are grateful to Zejun Niu and Zhiqiang Qu for their technical assistance.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests. The authors alone are responsible for the content and writing of this paper. This study was partially supported by the Qingdao University.
Ethical approval
The experimental protocols were approved by the Animal Care and Protection Committee of Qingdao University. Our use of animals conformed to our Institution’s and Country’s animal welfare laws and our studies were approved.
References
- 1.Bekhit MH. Opioid-induced hyperalgesia and tolerance. Am J Ther. 2010;17(5):498–510. doi: 10.1097/MJT.0b013e3181ed83a0. [DOI] [PubMed] [Google Scholar]
- 2.Hutchinson MR, Coats BD, Lewis SS, et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun. 2008;22(8):1178–1189. doi: 10.1016/j.bbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubartelli A, Cozzolino F, Talio M, et al. A novel secretory pathway for interleukin 1b, a protein lacking a signal sequence. EMBO J. 1990;9(5):1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubartelli A, Bajetto A, Allavena G, et al. Posttranslational regulation of interleukin 1b secretion. Cytokine. 1993;5(3):117–124. doi: 10.1016/1043-4666(93)90050-F. [DOI] [PubMed] [Google Scholar]
- 6.Hickman SE, Khouri JE, Greenberg S, et al. P2Z adenosine triphosphate receptor activity in cultured human monocyte-derived macrophages. Blood. 1994;84(8):2452–2456. [PubMed] [Google Scholar]
- 7.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 8.Mattioli TA, Leducpessah H, Skelhornegross G, et al. Toll-like receptor 4 mutant and null mice retain morphine-induced tolerance, hyperalgesia, and physical dependence. PLoS One. 2014;9(9):e97361. doi: 10.1371/journal.pone.0097361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukagawa H, Koyama T, Kakuyama M, et al. Microglial activation involved in morphine tolerance is not mediated by toll-like receptor 4. J Anesth. 2013;27(1):93–97. doi: 10.1007/s00540-012-1469-4. [DOI] [PubMed] [Google Scholar]
- 10.Hutchinson MR, Zhang Y, Shridhar M, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24(1):83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens CW, Aravind S, Das S, et al. Pharmacological characterization of LPS and opioid interactions at the toll-like receptor 4. Br J Pharmacol. 2013;168(6):1421–1429. doi: 10.1111/bph.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eidson LN, Murphy AZ. Blockade of toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J Neurosci. 2013;33(40):15952–15963. doi: 10.1523/JNEUROSCI.1609-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husebye H, Halaas Ø, Stenmark H, et al. Endocytic pathways regulate toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25(4):683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue K. Purinergic systems in microglia. Cell Mol Life Sci. 2008;65(19):3074–3080. doi: 10.1007/s00018-008-8210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber FC, Esser PR, Müller T, et al. Lack of the purinergic receptor P2X7 results in resistance to contact hypersensitivity. J Exp Med. 2010;207(12):2609–2619. doi: 10.1084/jem.20092489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphreys BD, Dubyak GR. Modulation of P2X7 nucleotide receptor expression by pro-and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol. 1998;64(2):265–273. doi: 10.1002/jlb.64.2.265. [DOI] [PubMed] [Google Scholar]
- 17.Ma W, Korngreen A, Weil S, et al. Pore properties and pharmacological features of the P2X receptor channel in airway ciliated cells. J Physiol. 2006;571(Pt 3):503–517. doi: 10.1113/jphysiol.2005.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qureshi OS, Paramasivam A, Jowie CH, et al. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J Cell Sci. 2007;120(Pt 21):3838–3849. doi: 10.1242/jcs.010348. [DOI] [PubMed] [Google Scholar]
- 19.Horvath RJ, DeLeo JA. Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J Neurosci. 2009;29(4):998–1005. doi: 10.1523/JNEUROSCI.4595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrini F, Trang T, Mattioli T, et al. Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl-homeostasis. Nat Neurosci. 2013;16(2):183–192. doi: 10.1038/nn.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuda M, Shigemoto-Mogami Y, Koizumi S, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 22.Hutchinson MR, Shavit Y, Grace PM, et al. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63(3):772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- 24.Wolf G, Gabay E, Tal M, et al. Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain. 2006;120(3):315–324. doi: 10.1016/j.pain.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Hebrew University of Jerusalem. How morphine can be given more effectively without having to increase dosages. Science Daily 2008; April 28.
- 26.Shavit Y, Wolf G, Goshen I, et al. Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain. 2005;115(1–2):50–59. doi: 10.1016/j.pain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Bauernfeind F, Ablasser A, Bartok E, et al. Inflammasomes: current understanding and open questions. Cell Mol Life Sci. 2011;68(5):765–783. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schäfers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-a induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23(7):2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269(21):15195–15203. [PubMed] [Google Scholar]
- 30.Ferrari D, Chiozzi P, Falzoni S, et al. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185(3):579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brough D, Le Feuvre RA, Iwakura Y, et al. Purinergic (P2X7) receptor activation of microglia induces cell death via an interleukin-1-independent mechanism. Mol Cell Neurosci. 2002;19(2):272–280. doi: 10.1006/mcne.2001.1054. [DOI] [PubMed] [Google Scholar]
- 32.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 33.Inoue K, Tsuda M. Microglia and neuropathic pain. Glia. 2009;57(14):1469–1479. doi: 10.1002/glia.20871. [DOI] [PubMed] [Google Scholar]
- 34.Burnstock G. Purinergic receptors and pain. Curr Pharmaceut Design. 2009;15:1717–1735. doi: 10.2174/138161209788186335. [DOI] [PubMed] [Google Scholar]
- 35.Cunha TM, Verri WA, Jr, Silva JS, et al. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 2005;102(5):1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Rivero Vaccari JP, Bastien D, Yurcisin G, et al. P2X4 receptors influence inflammasome activation after spinal cord injury. J Neurosci. 2012;32(9):3058–3066. doi: 10.1523/JNEUROSCI.4930-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwiebert LM, Rice WC, Kudlow BA, et al. Extracellular ATP signaling and P2X nucleotide receptors in monolayers of primary human vascular endothelial cells. Am J Physiol Cell Physiol. 2002;282(2):C289–C301. doi: 10.1152/ajpcell.01387.2000. [DOI] [PubMed] [Google Scholar]
- 38.Ferrari D, Chiozzi P, Falzoni S, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159(3):1451–1458. [PubMed] [Google Scholar]
- 39.Chessell IP, Hatcher JP, Bountra C, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114(3):386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 41.Biber K, Neumann H, Inoue K, et al. Neuronal ‘on’ and ‘off’ signals control microglia. Trends Neurosci. 2007;30(11):596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Waage A, Brandtzaeg P, Halstensen A, et al. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thieblemont N, Wright SD. Transport of bacterial lipopolysaccharide to the Golgi apparatus. J Exp Med. 1999;190(4):523–534. doi: 10.1084/jem.190.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Latz E, Visintin A, Lien E, et al. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem. 2002;277(49):47834–47843. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]
- 45.Toulme E, Garcia A, Samways D, et al. P2X4 receptors in activated C8-B4 cells of cerebellar microglial origin. J Gen Physiol. 2010;135(4):333–353. doi: 10.1085/jgp.200910336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toulme E, Soto F, Garret M, et al. Functional properties of internalization-deficient P2X4 receptors reveal a novel mechanism of ligand-gated channel facilitation by ivermectin. Mol Pharmacol. 2006;69(2):576–587. doi: 10.1124/mol.105.018812. [DOI] [PubMed] [Google Scholar]
- 47.Li S, Carolyn L, Christian S. Deletion of the murine scavenger receptor CD68. J Lipid Res. 2011;52(8):1542–1550. doi: 10.1194/jlr.M015412. [DOI] [PMC free article] [PubMed] [Google Scholar]