Abstract
It is widely accepted that the c-Fos gene has a role in proliferation and differentiation of bone cells. ATP-induced c-Fos activation is relevant to bone homeostasis, because nucleotides that are present in the environment of bone cells can contribute to autocrine/paracrine signalling. Gut hormones have previously been shown to have an effect on bone metabolism. In this study, we used the osteoblastic Saos-2 cell line transfected with a c-Fos-driven reporter stimulated with five gut hormones: glucose inhibitory peptide (GIP), glucagon-like peptide-1 (GLP-1), glucagon-like peptide-2 (GLP-2), ghrelin and obestatin, in the presence or absence of ATP. In addition, TE-85 cells were used to determine the time course of c-Fos transcript induction following stimulation with GLP-1, and GLP-2 with or without ATP, using reverse transcription qPCR. The significant results from the experiments are as follows: higher level of c-Fos induction in presence of GIP, obestatin (p = 0.019 and p = 0.011 respectively), and GIP combined with ATP (p < 0.001) using the luciferase assay; GLP-1 and GLP-2 combined with ATP (p = 0.034 and p = 0.002, respectively) and GLP-2 alone (p < 0.001) using qPCR. In conclusion, three of the gut peptides induced c-Fos, providing a potential mechanism underlying the actions of these hormones in bone which can be directed or enhanced by the presence of ATP.
Keywords: ATP, GIP, GLP-1, GLP-2, Osteoblasts, c-Fos
Introduction
Several studies have reported that c-Fos has a role in proliferation and differentiation of bone cells [1, 2]. ATP released from osteoblasts may activate P2 receptors on osteoclasts and/or other lining osteoblasts and could be a mechanism by which these cells establish cross talk for the control of bone remodelling, in addition to other paracrine factors as well as a direct cell-to-cell contact [3].
The rationale for the experiments described in this paper is based on the actions of gut hormones as systemic regulators and their reported direct effects on bone cells [4, 5]. Animal models [6] and clinical trials [7–11] have mainly shown an increase in the markers for bone formation. On the other hand, to our knowledge, there are no reports focused on the measurement of c-Fos induction elicited by gut hormones and ATP in osteoblastic cells; however, there is evidence that these regulators are able to activate the transcription of c-Fos. In this regard, glucose inhibitory peptide (GIP) and glucagon-like peptide-1 (GLP-1) have been shown to induce c-Fos transcriptional activation in the presence of glucose, showing a synergistic pattern, when they were added to pancreatic beta-cell cultures [12]. Also, GIP combined with Xenin-25 caused a significant increase in the number of c-Fos-positive cells in the arcuate nucleus of a knockout mice model (lacking GIP-producing K cells) [13]. Another report showed that administration of low-dose GLP-1 combined with glucagon inhibited food intake-induced c-Fos expression in the area postrema and amygdala [14]. Moreover, the effect of glucagon-like peptide-2 (GLP-2) on c-Fos has been studied in primary rat astroglial cell cultures [15], and it has been demonstrated that GLP-2 has a role in hypertension regulation evaluated by c-Fos induction [16, 17]. Ghrelin (GHR) and obestatin (OB) can also modulate c-Fos expression, GHR at neuronal level and so alter feeding patterns in rats and OB) in mouse embryonic fibroblast and adipose-like cells. [18, 19].
We aimed to investigate whether or not the aforementioned five gut hormones were able to induce c-Fos in the presence or absence of ATP in two osteoblastic cell lines: in Saos-2 using a luciferase assay and in TE-85 using a real-time PCR assay,
Methods
Luciferase assay
This assay was used to test the effects of GIP, GHR and OB on Saos-2 cells since these have been demonstrated to express reliable levels of the receptors for those gut peptides [5]. Briefly, the Saos-2 cells had been previously transfected with the c-Fos-luciferase reporter gene, in which the full c-Fos promoter spanning positions −711 to −1 was linked to the firefly luciferase as the reporter gene, [20]. Transfected Saos-2 cells were stored in liquid nitrogen until needed. They were defrosted and expanded in culture medium ((DMEM), Invitrogen, Paisley, UK) containing a selective agent (Geneticin®, Sigma, Gillingham, UK) to favour the growth of transfected cells over the wild type. Then, cells were passaged to 96-well plates (white walls) and settled for 24 h, serum deprived for 24 h and induced for 4 h with gut peptides in the presence or absence of ATP, diluted in DMEM serum free. Then, attached cells were washed in PBS, lysed and kept stored in −80 °C. After 24 h, cells were defrosted at room temperature, and the assay was performed as follows: 100 μL of luciferase reagent (Promega, Southampton, UK) was added to each well, and the chemiluminescence was measured every 10 ms, for 10 s in an Anthos luminometer microplate reader (Anthos Labtech Instruments, Salzburg, Austria). Two working concentrations of gut hormones were used: 10−9 and 10−8 M; ATP was used at 10−5 M; PTH 10−9 M was used as a positive control. Comparisons were performed per treatment against its paired control (i.e. DMEM with and without ATP, no added gut peptides). Experiments were repeated twice using ten replicates each time. The cell line Saos-2 was part of the cell bank in the Human Anatomy and Cell Biology Department, University of Liverpool, and its osteoblastic lineage has been tested previously using mRNA expression of bone markers [5].
RT-qPCR
This procedure was performed on TE-85 cells, and the peptides under scrutiny were GLP-1 and GLP-2, since TE 85 cells have been shown to respond to these hormones. [5]. Briefly, cells were passaged into 6-well plates, seeded and grown until confluence and serum deprived for 24 h. After that time, cells were washed in PBS and treated with the gut peptides with or without ATP 10−5 M for 15, 30, 60 and 120 min. Then, cells were lysed with Tri Reagent®-chloroform (Invitrogen, UK); RNA was extracted and reverse transcribed. cDNA was analysed for c-Fos level of expression, using beta-actin as reference gene.
The gut peptides were used at a concentration of 10−8 M and ATP was added at a final concentration of 10−5 M. DMEM plus ATP (D + A), 10 % foetal calf serum DMEM (D + S) and PTH 10−9 M were used as positive controls. The low induction control (LIC) was DMEM without peptides or ATP. The results were normalised to fold change, using DMEM without ATP and are expressed as the average of fold change of 5–6 replicates in the iCycler iQ instrument (Bio-Rad, Hertfordshire, UK). The cell line TE-85 was part of the cell bank in the Human Anatomy and Cell Biology Department, University of Liverpool; its osteoblastic features have been tested previously using mRNA expression of bone markers [5].
Statistical analysis
The comparisons were analysed with SPSS software, using ANOVA with Bonferroni post hoc testing or unpaired t tests to compare the means of the tests obtained between groups. The equality of variances in different samples was assessed using the test of Levene. The significance was set at p values less than 0.05. Results are presented as fold change ± standard error of the mean (SEM).
Results
Luciferase assay: induction of c-Fos in Saos-2 osteoblastic cell line
c-Fos expression was higher with GIP 10−8 M treatment either in the presence or absence of ATP (p = 0.019 and p < 0.001, respectively) when compared to respective controls (Fig. 1a). In the cases of GLP-1, GLP-2 and GHR treatments, no differences were observed in any experiment. OB only displayed a significant increase when a 10−8 M treatment was present in the culture medium (p = 0.011), but the addition of ATP did not cause any significant change in the activation of c-Fos (Fig. 1b).
Fig. 1.
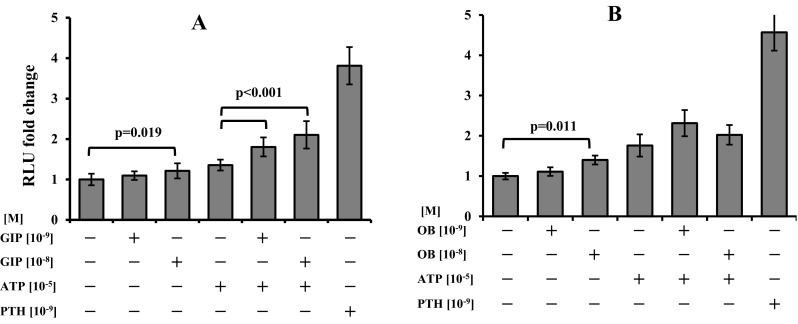
c-Fos induction in transfected Saos-2 cells. a GIP alone (p = 0.019) and combined with ATP (p < 0.001) induced significant higher levels of c-Fos. b OB treatment increased c-Fos, when 10−8 M was present (p = 0.011), but no significant changes were observed in the presence of ATP. PTH was used as a positive control of induction. Data were normalised to control and results are expressed as the average of fold change, n = 10, ±SEM
RT-qPCR: time course of c-Fos expression in TE-85 osteoblastic cell line
The effect of GLP-1 and GLP-2 was tested in TE-85, a cell line with a consistent expression pattern for those receptors. The results for all the positive controls (D + A, D + S and PTH) were consistently higher than the LIC at different point times (Fig. 2a), and each of them showed different patterns of expression.
Fig. 2.
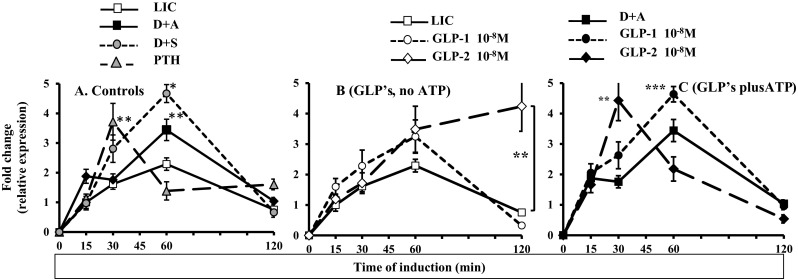
Time course patterns exhibited by c-Fos induction. a Time course of c-Fos induction after LIC, D + A, D + S, PTH: a maximum peak at 60 min and depletion at 120 min were observed in LIC, D + A and D + S. PTH induced a maximum induction at 30 min and depletion at 60 min. b A maximum peak at 60 min and depletion at 120 min were observed after GLP-1, with no significant differences compared to LIC; GLP-2 had similar increase as GLP-1 at 60 min, but no depletion was observed at 120 min, with higher levels that GLP-1 and LIC. c GLP1 combined with ATP 10−5 M induced a maximum peak at 60 min and depletion at 120 min; GLP-2 had an increase at 30 min, and depletion was observed at 60 min. Results are expressed as the average of fold change, n = 6, ±SEM. Comparisons were performed against the paired time points (*p < 0.001, **p < 0.01, ***p < 0.05 compared with their respective control, either LIC or D + A at their respective point times). LIC low induction control (DMEM alone), D + A DMEM plus ATP, D + S DMEM plus 10 % FCS (positive control), PTH PTH 10−9 M (positive control)
Next, the analysis of the patterns after the induction with gut peptides (no ATP added) showed that treatment with GLP-1 prompted the highest expression of c-Fos at 60 min of exposure, with depletion at 120 min; this was similar to LIC. GLP-2 treatment also increased the induction of c-Fos at 60 min and was sustained up to 120 min, with a significant difference in relation to LIC (p < 0.001) (Fig. 2b). The observations of peptide treatments combined with ATP showed that the combination of GLP-1 plus ATP increased c-Fos induction from 30 min, but a significant difference was observed at its highest peak at 60 min (p = 0.021), with depletion at 120 min. In the case of GLP-2 combined with ATP, the pattern of expression showed a significant increase at 30 min when compared against the control (D + A) (p = 0.002), and also a significant difference was observed when compared to GLP-1 combined with ATP at 30 min (p = 0.034) (Fig. 2c). To sum up, the induction of c-Fos caused by GLPs with added ATP followed similar patterns to those for the well-known inductors of c-Fos (D + A, D + S, PTH).
Discussion
Bone turnover has been described as a process in which localised parts of the skeleton are remodelled through the process of activation-resorption-formation. However, this may pose a question on the mechanisms that localised signals use to stimulate the bone remodelling foci. One of these signals may be the presence of extracellular nucleotides resulting in c-Fos signalling. Also, it has been shown that along with the local stimuli, some hormones act to increase the rate of bone turnover activation, triggering the transcription of c-Fos, which plays an important part in bone turnover [21].
From the experiments described here, it was observed that GIP alone was a stimulus for c-Fos expression at its highest concentration. However, when ATP was present, the induction of c-Fos was much higher reaching up to 70 % over the control. This finding is relevant since GIP has been reported to exert effects on bone tissue, and this could be done through the increase of cAMP response element binding (CREB) phosphorylation [22]. An enhancement and/or synergy of this cascade can be predicted in the presence of extracellular nucleotides, suggesting that GIP systemic effects can be directed by the release of nucleotides to the bone surroundings. Therefore, multiple pathways involving P2 and GIP receptors can coordinate a synergistic induction of gene expression in osteoblasts triggering direct responses from osteoblasts to modulate bone formation, involving c-Fos and ATP, to exert a more efficient signalling for bone remodelling.
Also, it was shown that the combination of GLP-1 and ATP was capable of provoking an increased transcription of c-Fos in osteoblastic cells, suggesting that in states of high energy like those exhibited after feeding (GLP-1 is released after a meal and the circulating levels increase rapidly [23]), this peptide could be involved in bone turnover helped by the presence of ATP and by triggering mechanisms that generate the transcription of c-Fos and subsequent activation of bone remodelling in specific places. However, the lack of response at bone metabolism levels observed in some clinical trials [8] and the link of GLP-1 to bone turnover may be explained by a factor that acts in a more localised manner, like ATP.
In addition, GLP-2 induced a response in the presence of ATP, prompting a c-Fos peak at 30 min. Unlike GLP-1, GLP-2 has shown more responses in bone turnover in terms of decreasing bone resorption markers [9–11] and its involvement in bone turnover may be explained in terms of intracellular cascades triggered by GLP-2 itself, being modulated by ATP.
This enhancement of the induction in the presence of ATP (i.e. GIP, GLP-1 and GLP-2) suggests a synergistic mechanism between nucleotides and these systemic regulators to “switch on” localised cell signals, which in turn can modulate bone remodelling processes [24], in certain feeding/fasting states. In this regard, in terms of energy homeostasis, after a meal, the levels of these three hormones are increased, and trigger a number of systemic responses (e.g. insulin release) and intracellular cascades [22, 25]. Also, levels of ATP are related to nutrient ingestion and play a role in the appetite behaviour [26, 27]. Once ATP is present in the extracellular milieu, it functions as an autocrine/paracrine signal and binding to P2Y receptors leads to further c-Fos activation which in turn activates transcriptional and regulation factors related to cell differentiation [20]. Together, these factors are likely to be combined to prompt responses from bone cells, as there is a body of evidence of the effects of the gut hormones on bone metabolism modulation [4–11].
Although a set of five hormones was evaluated, a feature of the current study is that the evaluation was carried out using two different methodologies. These data come from cell lines, and we acknowledge that subsequent studies on primary cell culture could provide stronger data on the role of c-Fos when bone cells are grown in presence of gut hormones and ATP, since the osteoblastic differentiation stage may modulate P2 receptor expression and hence the observed responses [28]. There is a well-established heterogeneity in expression of P2 receptors in primary cell cultures [29] and thus studies on established well-characterised cell lines are important in elucidating complex signalling pathways. Our results underscore the ability of the gut hormones to generate systemic signals in the digestive tract that are translated as localised signals to act on bone remodelling foci.
These findings could be relevant from a point of view of clinical management of bone loss secondary to inadequate nutrient intake or changes in the feeding patterns (e.g. gastric bypass) [30]. Currently, P2 receptor agonists are in development, which could provide new therapeutic agents for bone disease [31]. Furthermore, there are other potential strategies for increasing ATP locally in bone, including ultrasound [32], and possibly vibration and exercise, which administered postprandially could be used to exploit the positive interaction with gut hormones to promote bone growth.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was partly supported by a grant from the National Council of Science and Technology (CONACyT, scholarship/grant 206349/148591), Mexico.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Grigoriadis AE, Wang ZQ, Cecchini MG, et al. C-fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266(80-):443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 2.Liedert A, Kaspar D, Blakytny R, et al. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun. 2006;349:1–5. doi: 10.1016/j.bbrc.2006.07.214. [DOI] [PubMed] [Google Scholar]
- 3.Gartland A, Orriss IR, Rumney RMH, et al. Purinergic signalling in osteoblasts. Front Biosci. 2012;17:16–29. doi: 10.2741/3912. [DOI] [PubMed] [Google Scholar]
- 4.Bollag RJ, Zhong Q, Ding KH, et al. Glucose-dependent insulinotropic peptide is an integrative hormone with osteotropic effects. Mol Cell Endocrinol. 2001;177:35–41. doi: 10.1016/S0303-7207(01)00405-1. [DOI] [PubMed] [Google Scholar]
- 5.Pacheco-Pantoja EL, Ranganath LR, Gallagher J, et al. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011;11:12. doi: 10.1186/1472-6793-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie D, Cheng H, Hamrick M, et al. Glucose-dependent insulinotropic polypeptide receptor knockout mice have altered bone turnover. Bone. 2005;37:759–769. doi: 10.1016/j.bone.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Meier C, Schwartz AV, Egger A, Lecka-Czernik B. Effects of diabetes drugs on the skeleton. Bone. 2015 doi: 10.1016/j.bone.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Bunck MC, Poelma M, Eekhoff EM, et al. Effects of vildagliptin on postprandial markers of bone resorption and calcium homeostasis in recently diagnosed, well-controlled type 2 diabetes patients. J Diabetes. 2012;4:181–185. doi: 10.1111/j.1753-0407.2011.00168.x. [DOI] [PubMed] [Google Scholar]
- 9.Henriksen DB, Alexandersen P, Bjarnason NH, et al. Role of gastrointestinal hormones in postprandial reduction of bone resorption. J Bone Miner Res. 2003;18:2180–2189. doi: 10.1359/jbmr.2003.18.12.2180. [DOI] [PubMed] [Google Scholar]
- 10.Henriksen DB, Alexandersen P, Byrjalsen I, et al. Reduction of nocturnal rise in bone resorption by subcutaneous GLP-2. Bone. 2004;34:140–147. doi: 10.1016/j.bone.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Henriksen DB, Alexandersen P, Hartmann B, et al. Four-month treatment with GLP-2 significantly increases hip BMD: a randomized, placebo-controlled, dose-ranging study in postmenopausal women with low BMD. Bone. 2009;45:833–842. doi: 10.1016/j.bone.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Susini S, Van Haasteren G, Li S, et al. Essentiality of intron control in the induction of c-fos by glucose and glucoincretin peptides in INS-1 beta-cells. FASEB J. 2000;14:128–136. [PubMed] [Google Scholar]
- 13.Wice BM, Wang S, Crimmins DL, et al. Xenin-25 potentiates glucose-dependent insulinotropic polypeptide action via a novel cholinergic relay mechanism. J Biol Chem. 2010;285:19842–19853. doi: 10.1074/jbc.M110.129304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker J, McCullough K, Field BCT, et al. Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala. Int J Obes. 2013;37:1391–1398. doi: 10.1038/ijo.2012.227. [DOI] [PubMed] [Google Scholar]
- 15.Velázquez E, Blázquez E, Ruiz-Albusac JM. Synergistic effect of glucagon-like peptide 2 (GLP-2) and of key growth factors on the proliferation of cultured rat astrocytes. Evidence for reciprocal upregulation of the mRNAs for GLP-2 and IGF-I receptors. Mol Neurobiol. 2009;40:183–193. doi: 10.1007/s12035-009-8080-1. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki-Hamada S, Yuri Y, Hoshi M, Oka J-I. Immunohistochemical determination of the site of antidepressant-like effects of glucagon-like peptide-2 in ACTH-treated mice. Neuroscience. 2015;294:156–165. doi: 10.1016/j.neuroscience.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki-Hamada S, Ito K, Oka JI. Neuronal Fos-like immunoreactivity associated with dexamethasone-induced hypertension in rats and effects of glucagon-like peptide-2. Life Sci. 2013;93:889–896. doi: 10.1016/j.lfs.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Inhoff T, Mönnikes H, Noetzel S, et al. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides. 2008;29:2159–2168. doi: 10.1016/j.peptides.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JV, Jahr H, Luo C-W, et al. Obestatin induction of early-response gene expression in gastrointestinal and adipose tissues and the mediatory role of G protein-coupled receptor, GPR39. Mol Endocrinol. 2008;22:1464–1475. doi: 10.1210/me.2007-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowler WB, Dixon CJ, Halleux C, et al. Signaling in human osteoblasts by extracellular nucleotides. Their weak induction of the c-fos proto-oncogene via Ca2+ mobilization is strongly potentiated by a parathyroid hormone/cAMP-dependent protein kinase pathway independently of mitogen-activated p. J Biol Chem. 1999;274:14315–14324. doi: 10.1074/jbc.274.20.14315. [DOI] [PubMed] [Google Scholar]
- 21.Orriss IR, Burnstock G, Arnett TR. Purinergic signalling and bone remodelling. Curr Opin Pharmacol. 2010;10:322–330. doi: 10.1016/j.coph.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Trümper A, Trümper K, Trusheim H, et al. Glucose-dependent insulinotropic polypeptide is a growth factor for beta (INS-1) cells by pleiotropic signaling. Mol Endocrinol. 2001;15:1559–1570. doi: 10.1210/mend.15.9.0688. [DOI] [PubMed] [Google Scholar]
- 23.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Buckley KA, Wagstaff SC, McKay G, et al. Parathyroid hormone potentiates nucleotide-induced [Ca2+]i release in rat osteoblasts independently of Gq activation or cyclic monophosphate accumulation. A mechanism for localizing systemic responses in bone. J Biol Chem. 2001;276:9565–9571. doi: 10.1074/jbc.M005672200. [DOI] [PubMed] [Google Scholar]
- 25.Tsuboi T, da Silva Xavier G, Holz GG, et al. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells. Biochem J. 2003;369:287–299. doi: 10.1042/bj20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch JE, Ji H, Osbakken MD, Friedman MI. Temporal relationships between eating behavior and liver adenine nucleotides in rats treated with 2,5-AM. Am J Phys. 1998;274:R610–R617. doi: 10.1152/ajpregu.1998.274.3.R610. [DOI] [PubMed] [Google Scholar]
- 27.Beauvieux M-C, Roumes H, Robert N, et al. Butyrate ingestion improves hepatic glycogen storage in the re-fed rat. BMC Physiol. 2008;8:19. doi: 10.1186/1472-6793-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orriss IR, Knight GE, Ranasinghe S, et al. Osteoblast responses to nucleotides increase during differentiation. Bone. 2006;39:300–309. doi: 10.1016/j.bone.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 29.Dixon CJ, Bowler WB, Walsh CA, Gallagher JA. Effects of extracellular nucleotides on single cells and populations of human osteoblasts: contribution of cell heterogeneity to relative potencies. Br J Pharmacol. 1997;120:777–780. doi: 10.1038/sj.bjp.0700961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Brethauer S. Metabolic bone changes after bariatric surgery. Surg Obes Relat Dis. 2015;11:406–411. doi: 10.1016/j.soard.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Kaebisch C, Schipper D, Babczyk P, Tobiasch E. The role of purinergic receptors in stem cell differentiation. Comput Struct Biotechnol J. 2015;13:75–84. doi: 10.1016/j.csbj.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayton MJ, Dillon JP, Glynn D, et al. Involvement of adenosine 5’-triphosphate in ultrasound-induced fracture repair. Ultrasound Med Biol. 2005;31:1131–1138. doi: 10.1016/j.ultrasmedbio.2005.04.017. [DOI] [PubMed] [Google Scholar]


