Abstract
Chronic kidney disease has multiple etiologies, but its single, hallmark lesion is renal fibrosis. CD39 is a key purinergic enzyme in the hydrolysis of ATP and increased CD39 activity on regulatory T cells (Treg) is protective in adriamycin-induced renal fibrosis. We examined the effect of overexpression of human CD39 on the development of renal fibrosis in the unilateral ureteric obstructive (UUO) model, a model widely used to study the molecular and cellular factors involved in renal fibrosis. Mice overexpressing human CD39 (CD39Tg) and their wild-type (WT) littermates were subjected to UUO; renal histology and messenger RNA (mRNA) levels of adenosine receptors and markers of renal fibrosis were examined up to 14 days after UUO. There were no differences between CD39Tg mice and WT mice in the development of renal fibrosis at days 3, 7, and 14 of UUO. Relative mRNA expression of the adenosine A2A receptor and endothelin-1 were higher in CD39Tg than WT mice at day 7 post UUO, but there were no differences in markers of fibrosis. We conclude that human CD39 overexpression does not attenuate the development of renal fibrosis in the UUO model. The lack of protection by CD39 overexpression in the UUO model is multifactorial due to the different effects of adenosinergic receptors on the development of renal fibrosis.
Keywords: Renal fibrosis, Adenosine receptor, CD39, Transforming growth factor-ß, Unilateral ureteric obstruction
Introduction
The incidence and prevalence of chronic kidney disease (CKD) is increasing worldwide. The morbidity and mortality associated with CKD is devastating: individuals with CKD are at high risk for premature death and progression to end stage kidney disease [1, 2]. Renal fibrosis is the histological hallmark of CKD and is the final common pathway regardless of the inciting etiology [3]. Renal fibrosis is typified by fibroblast stimulation and collagen deposition. The current lack of direct anti-fibrotic therapies drives the search for therapeutic targets to ameliorate renal fibrosis.
The purinergic enzyme, CD39 (ectonucleoside triphosphate diphosphohydrolase-1), is expressed predominantly on vascular endothelium. Following injury, the nucleotides ATP and ADP are extruded from injured cells into the extracellular space where they are hydrolyzed by the ectoenzymes CD39 and CD73 to generate adenosine. Mice overexpressing human CD39 (CD39Tg) have increased ability to hydrolyze ATP as evidenced by increased generation of AMP and adenosine levels following administration of intravenous collagen compared to wild-type (WT) (C57BL/6) littermates [4]. BALB/c CD39Tg mice are protected from adriamycin-induced renal injury and fibrosis; an effect mediated by increased CD39 levels on Tregs [5]. Whether increased CD39 overexpression impacts in other models of renal fibrosis is not known.
The unilateral ureteric obstructive (UUO) model is a high-throughput, reproducible model of renal fibrosis [3]. It is an attractive experimental model because it is species- and strain-independent and demonstrates changes that mimic the pathology of human progressive renal disease. The mechanism of injury in this model is predominantly due to increased tubular hydrostatic pressure from urinary obstruction [6]. The UUO model is commonly used as a benchmark to study the numerous molecular and cellular factors involved in renal fibrosis. However, there have been no studies of the effects of increased CD39 activity on the development of renal fibrosis in the UUO model.
There is conflicting evidence for a role for purinergic receptors in the pathogenesis of the UUO model. ATP is the ligand for both the P2X7 and P2X4 receptors and in the UUO model, these interactions have opposing effects: P2X7KO mice develop less fibrosis whereas P2X4KO mice have an exaggerated fibrotic response compared to WT mice [7–10]. Similarly, there is also conflicting evidence for a role for adenosine receptors in the pathogenesis of the UUO model. A2AR KO mice develop increased renal fibrosis and increased inflammatory cell infiltrate compared to WT mice on days 3 and 7 of UUO; however, by day 14, the extent of fibrosis was comparable in both groups [9]. Dai et al. demonstrated that A2BR KO mice developed less renal fibrosis after 14 days of UUO compared to WT mice, indicating a pro-fibrotic role of the A2BR in the UUO model [11]. These data suggest that the A2AR may modulate the early stages of UUO, whereas the A2BR plays a more significant role in the latter stages of fibrosis.
In mice deficient of the enzyme adenosine deaminase (ADA), which converts adenosine to inosine, high levels of renal adenosine content lead to increased renal fibrosis via the A2BR [11]. In a model of angiotensin II-induced hypertension, renal fibrosis develops through increased activity of CD73 and adenosine generation. The A2BR is upregulated and downstream signalling markers such as hypoxia-inducible factor 1α (HIF-1α) and endothelin-1 (ET-1) promote fibrosis [12]. Together these data demonstrate the complex and inter-related roles of the purinergic and adenosinergic pathways in the UUO model. We have previously reviewed the evolving role of adenosine signaling in the development of renal fibrosis [13]. In this study, we expand on this existing literature and define the role of CD39 on the development of renal fibrosis in the model of UUO. The aim of this study was to determine whether CD39Tg mice [4] are protected in the UUO model. We examined the development of renal fibrosis in CD39Tg and WT mice together with a range of fibrotic biomarkers. Expression of HIF-1α and ET-1 at baseline, days 3 and 7 post UUO were assessed in order to explore the potential mechanisms of renal fibrosis in this model.
Methods
Animals
CD39Tg mice and C57BL/6 WT littermates were bred and housed at Bioresources Centre, St Vincent’s Hospital Melbourne. The human CD39 transgene (hCD39) is under the control of the mouse H-2Kb promoter, which promotes expression on all nucleated cells. The level of CD39 overexpression is increased on circulating cells (as demonstrated by flow cytometry) [4, 14] and in solid organs (as demonstrated by immunohistochemistry) such as the heart [4], lung [4], liver [15], pancreatic islets [16], and kidneys, especially on the vasculature [17]. CD39Tg mice have the same phenotype as WT littermates and have similar body weights and breeding patterns [4]. The St. Vincent’s Hospital Melbourne Animal Ethics Committee approved all procedures.
UUO model
Male mice aged 10–14 weeks were anesthetized using ketamine and xylazine (16 and 8 mg/kg, respectively). A midline laparotomy was performed and the left ureter was ligated and sectioned between two Silk 4.0 ligatures. The abdomen was closed in two layers with silk-2.0 sutures, and mice were allowed to recover over a heat pad overnight with full access to food and water. Cohorts of mice were euthanized at baseline (no surgery), days 3, 7, and 14 of UUO for tissue analysis. Kidneys were harvested and cut in half lengthways; one half was fixed in 10 % formalin and embedded in paraffin and the other half was stored in RNAlater® solution.
Histology
Morphological assessment on hematoxylin and eosin (H&E)-stained paraffin sections (3 μm) and scoring of fibrosis on Masson’s trichrome-stained paraffin sections (3 μm) were performed at baseline and on days 3, 7, and 14 of UUO. For scoring of renal fibrosis, each Masson’s trichrome-stained section was scanned using an Aperio ScanScope (Leica Biosystems, North Ryde, New South Wales, Australia) to generate a digitized image of the whole section. Fibrosis was quantified in a blinded fashion using the positive pixel count algorithm and expressed as a positivity score, which is the ratio of the area of positive staining compared to the total area of the section.
Quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR)
qRT-PCR was performed on RNA extracted from kidneys collected at baseline and on days 3 and 7 of UUO. qRT-PCR was not performed on RNA extracted from kidneys collected at day 14 of UUO because of the low yield and poor quality of RNA extracted at this time point. RNA was isolated with TRIzol® according to the manufacturer’s instructions. RNA concentration and quality were measured using a NanoDrop ND-1000 spectrophotometer. Genomic DNA contamination was reduced by DNase treatment using a Turbo-DNA free kit® (Life Technologies, Carlsbad, CA, USA).
qRT-PCR was a two-step process. The first-strand complementary DNA (cDNA) was generated in a reaction volume of 22 μL of 1 μg oligo (dT), 1 μg random hexamers, 1 μg of RNA, and sterile water. After incubation for 10 min at 70 °C, a 28 μL reaction mix comprising of 0.5 mM dNTPs, 4 U/μL SuperScript III recombinant reverse transcriptase, 0.8 U/μL RNaseOUT recombinant ribonuclease inhibitor, 5 mM DTT and 1× first-strand buffer was added. Reverse transcription was then performed at 42 °C for 60 min and at 70 °C for 10 min to generate cDNA.
cDNA was diluted 10-fold to a working concentration in water, then analyzed using TaqMan® primer-probe sets (Table 1) in the LightCycler® 480 RT-PCR System. The PCR conditions were 10 min of pre-incubation at 95 °C, followed by 45 cycles of amplification at 95 °C for 15 s and 60 °C for 60 s. Cycle threshold (Ct) was corrected against the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The relative expression of each gene was calculated as follows: relative expression = 2 −Δ Ct, from which −Δ Ct is calculated as:
Table 1.
TaqMan primer-probe sets used in qRT-PCR assays
| Gene Assay Number |
|---|
| A1R Mm01308023_m1 |
| A2AR Mm00802075_m1 |
| A2BR Mm00839292_m1 |
| A3R Mm01296602_m1 |
| CD39 Mm00515447_m1 |
| CD73 Mm00501910_m1 |
| Collagen I Mm00483888_m1 |
| Endothelin-1 Mm00438656_m1 |
| GAPDH Mm99999915_g1 |
| hCD39 Hs00969559_m1 |
| HIF-1α Mm00468869_m1 |
| KIM-1 Mm00506686_m1 |
| TGFβ Mm01178820_m1 |
Statistical analysis
Data are expressed as means ± SEM. Comparison of two or more groups with baseline was performed using ANOVA of log transformed data, with Dunnett’s test for multiple comparisons with control. Comparisons between WT and CD39Tg mice were performed using the Student’s t test. All statistical analyses were performed using GraphPad Prism software and a two-tailed P < 0.05 was considered significant.
Results
Progressive renal injury and fibrosis develop following UUO
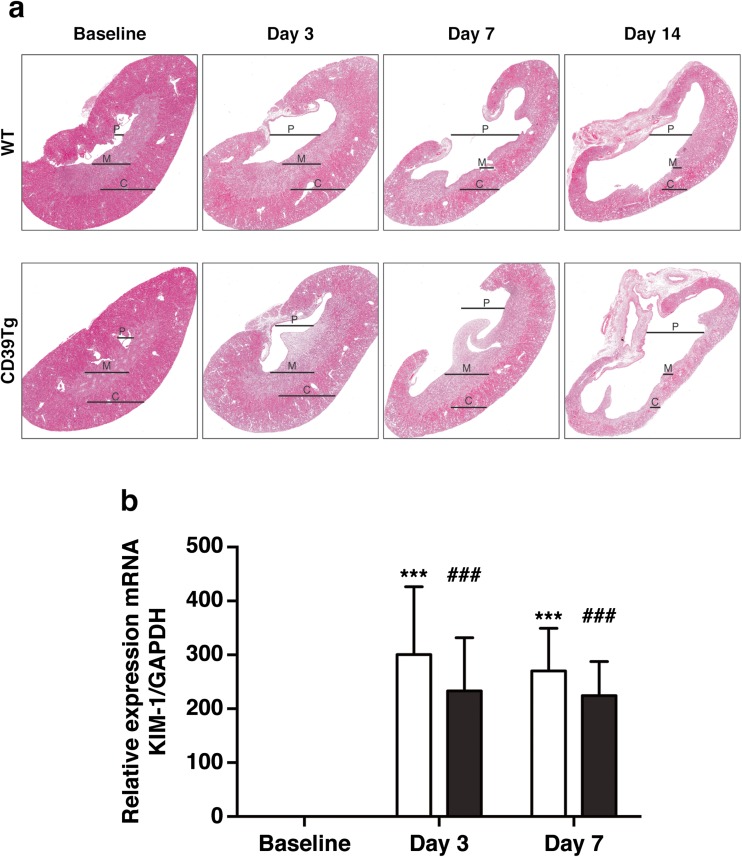
Tubular structure following UUO was examined in H&E sections (Fig. 1a). The most striking abnormality was progressive dilation of the renal pelvis and reduced renal medullary and cortical volume from days 3 to 14 following UUO, which was similar in both WT and CD39Tg mice. The degree of tubular injury was confirmed by KIM-1 expression, a marker of tubular injury. Both WT and CD39Tg mice showed increased renal expression of KIM-1 mRNA at days 3 and 7 after UUO in comparison with baseline; there were no differences between WT and CD39Tg mice at any time point (Fig. 1b).
Fig. 1.
a Representative H&E-stained sections of kidneys from WT and CD39Tg mice at baseline and at days 3, 7, and 14 following UUO. Progressive UUO injury was associated with increased dilation of the renal pelvis (P) and significant loss of medullary (M) and cortical (C) volume in both WT and CD39Tg mice. Image magnification is ×20 original image. b Relative expression of KIM-1 mRNA in kidney at baseline and at days 3 and 7 following UUO in WT and CD39Tg mice. KIM-1 mRNA levels were increased above baseline at days 3 and 7 following UUO in both WT (open bar) and CD39Tg (black bar) mice, and there were no differences between WT and CD39Tg mice at any time point. (***P < 0.001 vs. WT baseline; ###P < 0.001 vs. CD39Tg baseline)
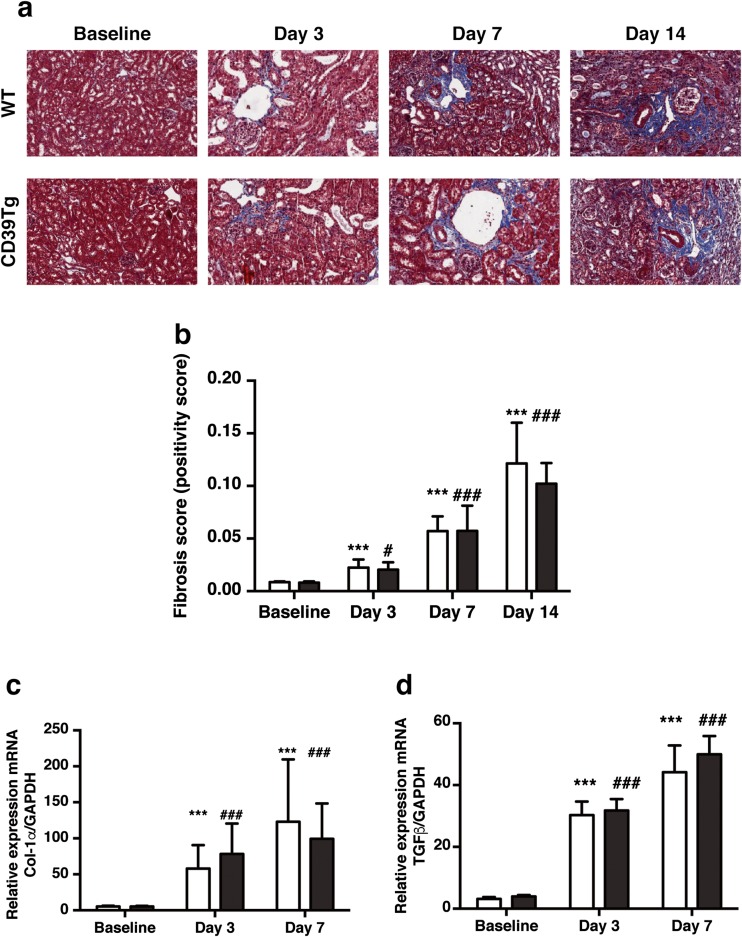
Renal fibrosis was examined by Masson’s trichrome staining of paraffin sections at baseline and at days 3, 7, and 14 following UUO (Fig. 2a). Both WT and CD39Tg mice developed renal fibrosis from day 3, but there were no differences in renal fibrosis scores between WT mice and CD39Tg mice at any time point (Fig. 2b).
Fig. 2.
a Representative Masson’s trichrome-stained sections of kidneys from WT and CD39Tg mice at baseline and at days 3, 7, and 14 following UUO. Areas of renal fibrosis (blue staining) increased following UUO in both WT and CD39Tg mice. Image magnification is ×40 original image. b Renal fibrosis score at baseline and at days 3, 7, and 14 following UUO in WT and CD39Tg mice. Fibrosis scores were increased above baseline at days 3, 7, and 14 following UUO in both WT (open bar) and CD39Tg (black bar) mice, and there were no differences between WT and CD39Tg mice at any time point. Data are described as means ± SEM, n = 4–7. (***P < 0.001 vs. WT baseline; #P < 0.05, ###P < 0.001 vs. CD39Tg baseline). c, d Relative expression of Col-1α and TGFβ mRNA in kidney at baseline and at days 3 and 7 following UUO in WT and CD39Tg mice. Col-1α (2c) and TGFβ (2d) mRNA levels were increased above baseline at days 3 and 7 following UUO in both WT (open bar) and CD39Tg (black bar) mice, and there were no differences between WT and CD39Tg mice at any time point in either Col-1α or TGFβ mRNA levels. Data are described as means ± SEM, n = 4 (***P < 0.001 vs. WT baseline; ###P < 0.001 vs. CD39Tg baseline)
In parallel, the relative renal expression of the fibrotic marker Col-1α and the pro-fibrotic factor TGFβ mRNA were elevated in both WT and CD39Tg mice at days 3 and 7 following UUO compared to baseline. There were no differences in Col-1α and TGFβ mRNA levels between WT and CD39Tg mice at any time point following UUO (Fig. 2c, d).
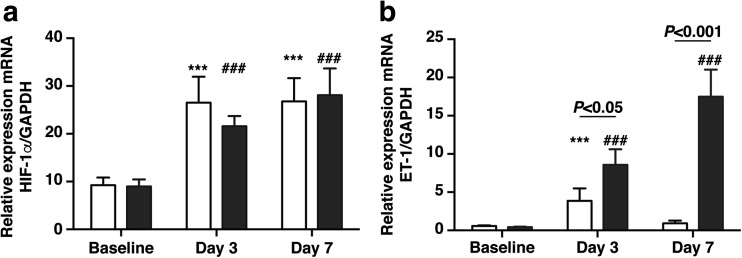
At the transcriptional mRNA level, the relative expression of HIF-1α mRNA was significantly higher at days 3 and 7 post UUO in WT and CD39Tg mice compared to baseline (Fig. 3a). There were no differences in renal HIF-1α mRNA levels between WT and CD39Tg mice at any time point. Renal ET-1 mRNA levels were significantly higher at day 3 following UUO in WT mice compared to baseline, and at days 3 and 7 in CD39Tg mice, compared to baseline (Fig. 3b). CD39Tg mice had higher renal ET-1 mRNA expression than WT mice at both days 3 and 7 following UUO.
Fig. 3.
a, b Relative expression of HIF-1α and ET-1 mRNA in kidney at baseline and at days 3 and 7 following UUO in WT and CD39Tg mice. HIF-1α (3a) mRNA levels were increased above baseline at days 3 and 7 following UUO in both WT (open bar) and CD39Tg (black bar) mice, and there were no differences between WT and CD39Tg mice at any time point. ET-1 (3b) mRNA levels were increased above baseline at days 3 and 7 following UUO in CD39Tg mice, but only at day 3 in WT mice, and ET-1 mRNA levels were higher in CD39Tg than WT mice at both days 3 and 7. Data are described as mean ± SEM, n = 4 (***P < 0.001 vs. WT baseline; ###P < 0.001 vs. CD39Tg baseline)
Relative mRNA expression of CD39, CD73, and adenosine receptors following UUO
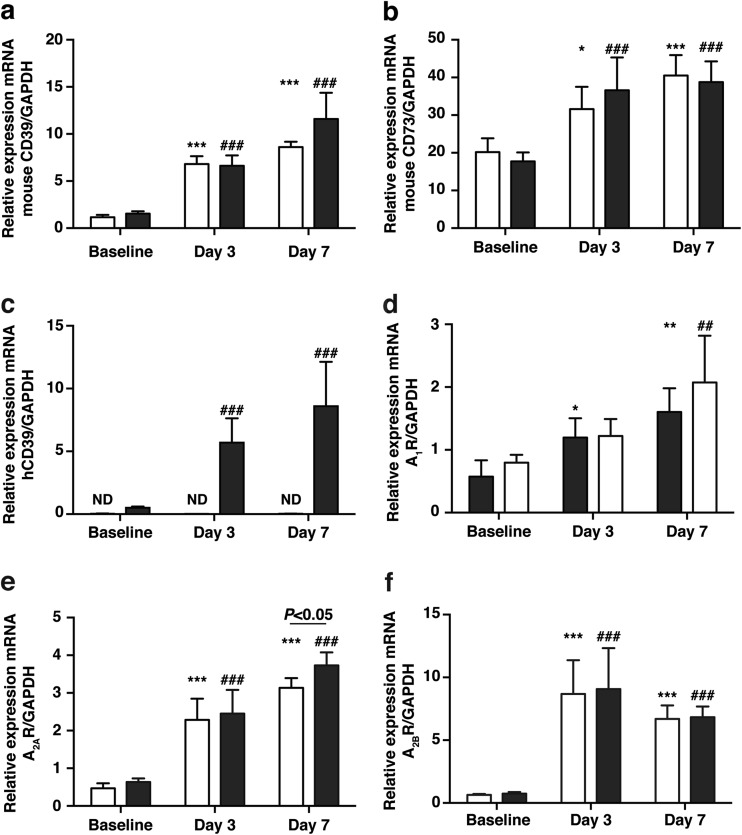
Given the dynamic nature of CD39 and CD73 expression in response to various insults, the relative renal expression of endogenous CD39 and CD73 mRNA was determined. Both were elevated in the kidneys of WT and CD39Tg mice at days 3 and 7 following UUO, compared to baseline. There were no differences in endogenous CD39 and CD73 mRNA levels between WT and CD39Tg mice at any time point (Fig. 4a, b). However, as expected, human CD39 expression was not detectable in WT kidney and renal expression of human CD39 mRNA was significantly higher at days 3 and 7 following UUO, compared to baseline (Fig. 4c).
Fig. 4.
a, b Relative expression of endogenous CD39 and CD73 mRNA in kidney at baseline and at days 3 and 7 following UUO in WT and CD39Tg mice. Endogenous CD39 (4a) and endogenous CD73 (4b) mRNA levels were increased above baseline at days 3 and 7 following UUO in both WT (open bar) and CD39Tg (black bar) mice, and there were no differences between WT and CD39Tg mice at any time point in either CD39 or CD73 mRNA levels. Data are described as means ± SEM, n = 4 (*P < 0.05, ***P < 0.001 vs. WT baseline; ##P < 0.01, ###P < 0.001, vs. CD39Tg baseline). c Relative expression of human CD39 (hCD39) mRNA in kidney at baseline and at days 3 and 7 following UUO in WT and CD39Tg mice. hCD39 transgene mRNA levels were increased above baseline at days 3 and 7 following UUO in CD39Tg (black bar) mice, whereas the transgene was not detectable (ND) in WT (open bar) mice. Data are described as means ± SEM, n = 4 (###P < 0.001 vs. CD39Tg baseline). d, e, f Relative expression of A1R, A2AR, and A2BR mRNA in kidney at baseline and at days 3 and 7 following UUO in WT and CD39Tg mice. A1R, A2AR,, and A2BR mRNA levels were increased above baseline at days 3 and 7 following UUO in both WT (open bar) and CD39Tg (black bar) mice, although the increase in A1R mRNA levels in CD39Tg mice did not reach statistical significance at day 3. There were no differences in A1R, A2AR, and A2BR mRNA levels between WT and CD39Tg mice at any time point except for higher A2AR mRNA levels in CD39Tg mice at day 7. Data are described as means ± SEM, n = 4 (*P < 0.05, **P < 0.01, ***P < 0.001 vs. WT baseline; ##P < 0.01, ###P < 0.001 vs. CD39Tg baseline, ns = non-significant)
The relative expression of the adenosine receptors in the kidney was determined during the course of UUO. The A1R, A2AR, and A2BR mRNA were increased above the baseline in both CD39Tg and WT mice at days 3 and 7 following UUO, although the increase in A1R mRNA expression in CD39Tg mice at day 3 failed to achieve statistical significance (Fig. 4d). There were no differences between WT and CD39Tg mice in renal A1R, A2AR, and A2BR mRNA expression at any time point except for the A2AR level at day 7, which was higher in CD39Tg than WT mice. A3R mRNA was undetectable at all time points in the kidneys of WT and CD39Tg mice.
Discussion
In contrast to the protection from adriamycin-induced nephropathy provided by increased CD39 expression [5], human CD39 transgene expression did not influence either tubular injury (as evidenced by KIM-1 expression) or the development of renal fibrosis in the UUO model. Our finding of increased renal A1R, A2AR, and A2BR mRNA levels after UUO was in agreement with a previous report using a rat model [18], although A3R mRNA was undetectable, likely due to the very low level of expression of this receptor in mouse kidney [19]. Renal HIF-1α and ET-1 mRNA levels were also increased following UUO, as previously described [20, 21]. Despite the similar renal injury and fibrosis of WT and CD39Tg mice, we found higher renal A2AR mRNA levels at day 7 post UUO and higher ET-1 mRNA levels at days 3 and 7 in CD39Tg mice than WT mice.
In adriamycin-induced renal injury and fibrosis, Treg cells are protective and depletion of these cells promotes renal injury [22]. Specifically, human CD39 transgene expression by Treg conferred increased protection from adriamycin-induced injury compared to WT Tregs [5]. On the contrary, in the UUO model, total depletion of CD4+ T cells with an anti-CD4 monoclonal antibody protects WT mice from UUO induced renal fibrosis [23]. This demonstrates the opposing role of T cells in the two models of renal fibrosis. CD39Tg mice on the C56BL/6 background have a relative CD4+ lymphopenia due to defective T-cell maturation in the thymus [15]. However, our demonstration of similar renal fibrosis in WT and CD39Tg mice with UUO indicates that relative CD4+ lymphopenia does not affect renal fibrosis in the same way as total depletion of CD4+ T cells.
We observed an increase in mRNA expression of endogenous CD39 and CD73 in the WT and CD39Tg mice within 3 days of UUO, along with an increase in CD39 transgene expression in CD39Tg mice. We hypothesize, although are unable to confirm, that these increases in CD39 and CD73 expression contributed to increased adenosine generation. Furthermore, tissue non-specific alkaline phosphatase (TNAP), an ecto-nucleotidase present in the kidney [24], can generate adenosine from AMP in addition to CD73 [25]. It is difficult to fully elucidate that the contribution of the CD39 transgene expression to adenosine generation as the overexpression of CD39 is predominantly on the renal vasculature [17]. Whole kidney measurements of nucleotide and adenosine content in the kidneys following UUO will not be able to accurately quantify the isolated increase in adenosine level in the vascular compartment compared to the whole kidney. Although the intravascular nucleotide and adenosine level could not be measured, the absence of this data does not change the current understanding, which is that CD39 transgene expression does not influence the development of renal fibrosis in the UUO model.
The higher renal ET-1 mRNA expression in CD39Tg than WT mice at days 3 and 7 following UUO may have been a consequence of the increased adenosine generation in the intravascular space by CD39 overexpression leading to enhanced A2BR signalling. A2BR activation promotes upregulation of ET-1 mRNA expression, similar to the A2BR-mediated stimulation of ET-1 production described by Zhang et al. in mice with angiotensin II-induced hypertension [12]. At day 7 post UUO, the expression of A2AR was elevated in CD39Tg mice compared to WT mice. Increased A2AR activity reduces the development of renal fibrosis in the early periods of UUO injury [9]. It could be that the pro-fibrotic actions of ET-1 were counterbalanced by the anti-fibrotic effects of increased renal A2AR expression in the CD39Tg mice with UUO. Therefore, additional studies with specific adenosine receptor inhibitors are required to establish the role of adenosine signaling in the development of renal fibrosis in this model.
In conclusion, in contrast to the protective effects of overexpression of CD39 in adriamycin-induced nephropathy, we showed that CD39 transgene expression did not influence either tubular injury or renal fibrosis in the UUO model. This observation is reconciled by the disparate pathophysiology involved in these two models of renal fibrosis. The lack of protection by CD39 overexpression in the UUO model is multifactorial due to the differential and opposing effects of the adenosinergic receptors and T-cell immunity on the development of renal fibrosis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Alan C, Steven C, Martin G, Kirsten H, Alexandra J, Stephen, Mc, Paul S, White S (2010) The economic impact of end-stage renal disease in Australia. projections to 2020.
- 2.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 3.Eddy A, López-Guisa J, Okamura D, Yamaguchi I. Investigating mechanisms of chronic kidney disease in mouse models. Pediatr Nephrol. 2012;27(8):1233–1247. doi: 10.1007/s00467-011-1938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dwyer KM, Robson SC, Nandurkar HH, Campbell DJ, Gock H, Murray-Segal LJ, Fisicaro N, Mysore TB, Kaczmarek E, Cowan PJ, d’Apice AJ. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest. 2004;113(10):1440–1446. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang YM, McRae JL, Robson SC, Cowan PJ, Zhang GY, Hu M, Polhill T, Wang Y, Zheng G, Wang Y, Lee VWS, Unwin RJ, Harris DCH, Dwyer KM, Alexander SI. Regulatory T cells participate in CD39-mediated protection from renal injury. Eur J Immunol. 2012;42(9):2441–2451. doi: 10.1002/eji.201242434. [DOI] [PubMed] [Google Scholar]
- 6.Forbes MS, Thornhill BA, Minor JJ, Gordon KA, Galarreta CI, Chevalier RL. Fight-or-flight: murine unilateral ureteral obstruction causes extensive proximal tubular degeneration, collecting duct dilatation, and minimal fibrosis. Am J Physiol Renal Physiol. 2012;303(1):F120–F129. doi: 10.1152/ajprenal.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goncalves RG, Gabrich L, Rosario A Jr, Takiya CM, Ferreira MLL, Chiarini LB, Persechini PM, Coutinho-Silva R, Leite M Jr (2006) The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int 70(9):1599–1606 [DOI] [PubMed]
- 8.Kim MJ, Turner CM, Hewitt R, Smith J, Bhangal G, Pusey CD, Unwin RJ, Tam FW. Exaggerated renal fibrosis in P2X4 receptor-deficient mice following unilateral ureteric obstruction. Nephrol Dial Transplant. 2014;29(7):1350–1361. doi: 10.1093/ndt/gfu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao H, Si L-Y, Liu W, Li N, Meng G, Yang N, Chen X, Zhou Y-G, Shen H-Y. The effects of adenosine A2A receptor knockout on renal interstitial fibrosis in a mouse model of unilateral ureteral obstruction. Acta Histochem. 2012 doi: 10.1016/j.acthis.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Garcia G, Truong L, Chen J, Johnson R, Feng L. Adenosine A2A receptor activation prevents progressive kidney fibrosis in a model of immune-associated chronic inflammation. Kidney Int. 2011;80:378–388. doi: 10.1038/ki.2011.101. [DOI] [PubMed] [Google Scholar]
- 11.Dai Y, Zhang W, Wen J, Zhang Y, Kellems RE, Xia Y. A2B adenosine receptor–mediated induction of IL-6 promotes CKD. J Am Soc Nephrol. 2011;22(5):890–901. doi: 10.1681/ASN.2010080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Zhang Y, Wang W, Dai Y, Ning C, Luo R, Sun K, Glover L, Grenz A, Sun H, Tao L, Zhang W, Colgan S, Blackburn M, Eltzschig HK, Kellems RE, Xia Y. Elevated CD73-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ Res. 2013;112:1466–1478. doi: 10.1161/CIRCRESAHA.111.300166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts VS, Cowan PJ, Alexander SI, Robson SC, Dwyer KM. The role of adenosine receptors A2A and A2B signaling in renal fibrosis. Kidney Int. 2014;86(4):685–692. doi: 10.1038/ki.2014.244. [DOI] [PubMed] [Google Scholar]
- 14.Dwyer KM, Mysore TB, Crikis S, Robson SC, Nandurkar H, Cowan PJ, D’Apice AJ. The transgenic expression of human CD39 on murine islets inhibits clotting of human blood. Transplantation. 2006;82(3):428–432. doi: 10.1097/01.tp.0000229023.38873.c0. [DOI] [PubMed] [Google Scholar]
- 15.Pommey S, Lu B, McRae J, Stagg J, Hill P, Salvaris E, Robson SC, d’Apice AJF, Cowan PJ, Dwyer KM. Liver grafts from CD39-overexpressing rodents are protected from ischemia reperfusion injury due to reduced numbers of resident CD4+ T cells. Hepatology. 2013;57(4):1597–1606. doi: 10.1002/hep.25985. [DOI] [PubMed] [Google Scholar]
- 16.Chia JS, McRae JL, Thomas HE, Fynch S, Elkerbout L, Hill P, Murray-Segal L, Robson SC, Chen JF, d’Apice AJ, Cowan PJ, Dwyer KM. The protective effects of CD39 overexpression in multiple low-dose streptozotocin-induced diabetes in mice. Diabetes. 2013;62(6):2026–2035. doi: 10.2337/db12-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crikis S, Lu B, Murray-Segal LM, Selan C, Robson SC, D’Apice AJ, Nandurkar HH, Cowan PJ, Dwyer KM. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant. 2010;10(12):2586–2595. doi: 10.1111/j.1600-6143.2010.03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Hwang L, Ha H. Adenosine receptors are up-regulated in unilateral ureteral obstructed rat kidneys. Transplant Proc. 2012;44:1166–1168. doi: 10.1016/j.transproceed.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 19.Roberts V, Lu B, Dwyer KM, Cowan PJ. Adenosine receptor expression in the development of renal fibrosis following ischemic injury. Transplant Proc. 2014;46(10):3257–3261. doi: 10.1016/j.transproceed.2014.09.151. [DOI] [PubMed] [Google Scholar]
- 20.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117(12):3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldman DL, Mogelesky TC, Chou M, Jeng AY. Enhanced expression of renal endothelin-converting enzyme-1 and endothelin-A-receptor mRNA in rats with interstitial fibrosis following ureter ligation. J Cardiovasc Pharmacol. 2000;36(5 Suppl 1):S255–S259. doi: 10.1097/00005344-200036051-00075. [DOI] [PubMed] [Google Scholar]
- 22.Wang YM, Zhang GY, Wang Y, Hu M, Wu H, Watson D, Hori S, Alexander IE, Harris DCH, Alexander SI. Foxp3-transduced polyclonal regulatory T cells protect against chronic renal injury from adriamycin. J Am Soc Nephrol. 2006;17(3):697–706. doi: 10.1681/ASN.2005090978. [DOI] [PubMed] [Google Scholar]
- 23.Tapmeier TT, Fearn A, Brown K, Chowdhury P, Sacks SH, Sheerin NS, Wong W. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 2010;78:351–362. doi: 10.1038/ki.2010.177. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8(3):437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson EK, Cheng D, Verrier JD, Janesko-Feldman K, Kochanek PM. Interactive roles of CD73 and tissue nonspecific alkaline phosphatase in the renal vascular metabolism of 5′-AMP. Am J Physiol Renal Physiol. 2014;307(6):F680–F685. doi: 10.1152/ajprenal.00312.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]