Abstract
In neutrophils, adenosine triphosphate (ATP) release and autocrine purinergic signaling regulate coordinated cell motility during chemotaxis. Here, we studied whether similar mechanisms regulate the motility of breast cancer cells. While neutrophils and benign human mammary epithelial cells (HMEC) form a single leading edge, MDA-MB-231 breast cancer cells possess multiple leading edges enriched with A3 adenosine receptors. Compared to HMEC, MDA-MB-231 cells overexpress the ectonucleotidases ENPP1 and CD73, which convert extracellular ATP released by the cells to adenosine that stimulates A3 receptors and promotes cell migration with frequent directional changes. However, exogenous adenosine added to breast cancer cells or the A3 receptor agonist IB-MECA dose-dependently arrested cell motility by simultaneous stimulation of multiple leading edges, doubling cell surface areas and significantly reducing migration velocity by up to 75 %. We conclude that MDA-MB-231 cells, HMEC, and neutrophils differ in the purinergic signaling mechanisms that regulate their motility patterns and that the subcellular distribution of A3 adenosine receptors in MDA-MB-231 breast cancer cells contributes to dysfunctional cell motility. These findings imply that purinergic signaling mechanisms may be potential therapeutic targets to interfere with the motility of breast cancer cells in order to reduce the spread of cancer cells and the risk of metastasis.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-016-9531-6) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Purinergic signaling, ATP, Adenosine, Adenosine receptor, Cell motility
Introduction
Despite the widespread use of tumor screening and aggressive multimodality treatment that have reduced mortality, breast cancer remains the most common form of cancer in women and a major source of morbidity and mortality [1]. Metastasis plays a key role in breast cancer morbidity. Because of this, much interest has focused on the mechanisms underlying the motility of breast cancer cells.
Since its original description as an extracellular signaling molecule in the central nervous system, adenosine triphosphate (ATP) has been shown to regulate the functional responses of a wide variety of cell systems [2]. ATP itself can bind to two families of purinergic receptors (P2X and P2Y). Membrane-bound ectonucleotidases hydrolyze ATP to adenosine, which in turn activates P1 adenosine receptors. Adenosine receptors comprise four different members: A1, A2a, A2b, and A3 receptors [2]. We have previously demonstrated a key role for A3 receptors in the regulation of neutrophil chemotaxis [3–7]. Specifically, we found that stimulation of neutrophils causes the release of cellular ATP. Localized release of ATP at the leading edge of neutrophils and the conversion of ATP to adenosine and autocrine stimulation of A3 adenosine receptors are required for cell polarization and the coordinated migration of neutrophils in a chemotactic gradient field (Supplemental Fig. 1) [3–8]. Mounting evidence suggests that similar purinergic signaling mechanisms regulate the migration of other cell types [9–12]. Therefore, we wondered whether purinergic signaling mechanisms could also regulate the motility of breast cancer cells.
In support of this notion, extracellular ATP and purinergic receptors have been shown to cause calcium influx and the activation of multiple MAP kinases involved in signaling pathways associated with cell proliferation and migration of MCF-7 breast cancer cells [13, 14]. Similarly, the ATP breakdown product adenosine can activate calcium signaling in MDA-MB-231 breast cancer cells, and the ectonucleotidase CD73, which converts extracellular adenosine monophosphate (AMP) to adenosine, was shown to promote breast cancer growth and metastasis [15–17]. In light of our previous work, these reports support the concept that purinergic signaling can indeed contribute to the motility and metastasis of breast cancer cells.
In the current study, we investigated whether a purinergic signaling system similar to the one that regulates neutrophil chemotaxis can also be found in breast cancer cells. Our results indicate that both cell types can release cellular ATP, which promotes autocrine feedback mechanisms that involve A3 adenosine receptors and regulate the motility patterns of these cells. Our work also revealed that interfering with the endogenous purinergic signaling mechanisms of breast cancer cells blocks cell motility, which suggests that purinergic signaling mechanisms are potential drug targets to arrest breast cancer cells and reduce the risk of metastasis.
Materials and methods
Materials
DPCPX, CSC, MRS 1754, CPA, CGS 21680, IB-MECA, and pentostatin were obtained from Tocris Bioscience (Ellisville, MI). All other reagents were from Sigma-Aldrich, unless otherwise stated.
Cell culture
MDA-MB-231 and MCF-7 cells were from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10 % fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco, Thermo Fisher Scientific, Waltham, MA) at 37 °C and 5 % CO2. Cells were cultured for a maximum of 15 passages to minimize the risk of experimental variance due to mutations. This is particularly important as there are contradictory reports about the expression levels of adenosine receptor subtypes in breast cancer cell lines [18–20]. Prior to experiments, the cells were trypsinized and washed once with serum-containing medium and three times with serum-free medium containing 20 mM HEPES and 0.1 % bovine serum albumin (BSA). Cells were resuspended in serum-free medium and incubated overnight. Human mammary epithelial cells (HMEC; Lonza, Rockland, ME) were cultured in complete medium provided by the manufacturer. Because these cells require multiple growth factors in order to survive, they were not serum starved prior to experiments. Neutrophils were isolated from the peripheral blood of healthy human subjects as previously described [3]. The use of human subjects was approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center.
Gene silencing
MDA-MB-231 cells were treated with 50 nM siRNA targeting the A3 adenosine receptor or negative control siRNA (Silencer® Select Pre-Designed siRNA, Ambion, Thermo Fisher Scientific) using Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific) according to the manufacturer’s instructions and incubated for 48 h.
Microscopy
Unless otherwise specified, all experiments were performed with an inverted Leica DMIRB microscope (Leica, Wetzlar, Germany) equipped with a PSMI-2 stage incubator controlled by a TC-202A temperature controller (Harvard Apparatus, Holliston, MA). Acquisition was done with a Hamamatsu Orca II camera (Hamamatsu, Hamamatsu City, Japan) and OpenLab software (Improvision, Coventry, UK). P1 receptor immunofluorescence images were captured using a Zeiss LSM 510 Meta confocal microscope made available by the Microscopy Core Facility at BIDMC funded through the Harvard Digestive Diseases Center. Image analysis was done using ImageJ software (National Institutes of Health).
Quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) and treated with 1 U/μg RNase-free DNAse (Invitrogen). First-strand cDNA was synthesized using Superscript III First-strand Synthesis SuperMix (Invitrogen) according to the manufacturer’s instructions. Real-time polymerase chain reaction (PCR) was performed on a Mastercycler ep realplex (Eppendorf, Hauppauge, NY) using a QuantiTect SYBR Green PCR kit and predesigned and validated QuantiTect primer sets (Qiagen, Valencia, CA). Gene expression was normalized against β-actin mRNA levels, and the comparative C t method was used for relative quantification of gene expression.
Immunocytochemistry
MDA-MB-231 cells were cultured overnight on polylysine-coated Lab-Tek eight-well chamber glass slides (Nunc Inc., Naperville, IL). The cells were permeabilized with Triton X-100, washed, and fixed in ice-cold 3.7 % formaldehyde for 15 min. This was followed by blocking with 1 % BSA in Hanks’ balanced salt solution and staining with fluorescence-labeled antibodies and with phalloidin to identify F-actin [21]. The antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA; A1 receptor), Abcam (Cambridge, MA; A2a receptor), EMD Millipore (Billerica, MA; A2b receptor), and Alpha Diagnostic International (San Antonio, TX; A3 receptor) and were used according to the manufacturers’ directions. These antibodies have been extensively characterized and previously used by our group in several studies [3, 22–25].
High-performance liquid chromatography
HMEC or MDA-MB-231 cells (5 × 105 cells/well) were cultured overnight in 24-well polylysine-coated tissue culture plates. The kinetics of ATP hydrolysis were characterized by adding ATP (5 μM) and measuring ATP, adenosine diphosphate (ADP), AMP, and adenosine after different time intervals with high-performance liquid chromatography (HPLC) as described elsewhere [26]. Briefly, 150-μl aliquots of sample or of a nucleotide standard solution was incubated with 1 M chloroacetaldehyde and 25 mM Na2HPO4 in a final reaction volume of 200 μl at 72 °C for 30 min. Samples were placed on ice, alkalinized with 50 μl of 0.5 M NH4HCO3, and analyzed as previously described using a Waters HPLC system (Milford, MA) equipped with a fluorescence detector [3, 26]. Inosine concentrations in cell culture supernatants were detected with a UV absorbance detector at 254 nm.
Cell motility assay
Cells (2.5 × 104 cells/well) suspended in serum-free culture media containing 0.1 % BSA were placed in Lab-Tek II eight-well chamber glass slides pre-coated with 20 μg/ml bovine fibronectin and incubated overnight at 37 °C and 5 % CO2. In some experiments, poly-d-lysine was used to coat the chamber slides. The next day, cells were washed once with serum-free culture medium and reagents were added at the indicated concentrations. Cell migration was tracked by time-lapse video microscopy. Sequential images were acquired at 12-min intervals for up to 24 h and migration paths were analyzed using ImageJ software. Migration velocity was calculated as the total distance traveled by a cell divided by time. Velocities of at least 30 randomly selected cells per well were averaged to produce a mean velocity for each well. Each well was treated as a data point and experiments were repeated with at least three wells per assay condition, unless otherwise stated. Staining with propidium iodide (10 μM) was done to assess cell viability of control cells and of adenosine-treated cells. Viability was consistently >97 %.
Statistical analyses
Data are shown as the mean ± standard deviation (SD) of at least three independent experiments, if not stated otherwise. Statistical comparisons were done using Student’s t test when comparing two groups or one-way analysis of variance (ANOVA) followed by Holm–Sidak’s test for multiple comparisons. Differences between groups were considered statistically significant at p < 0.05.
Results
MDA-MB-231 cells display uncoordinated motility patterns
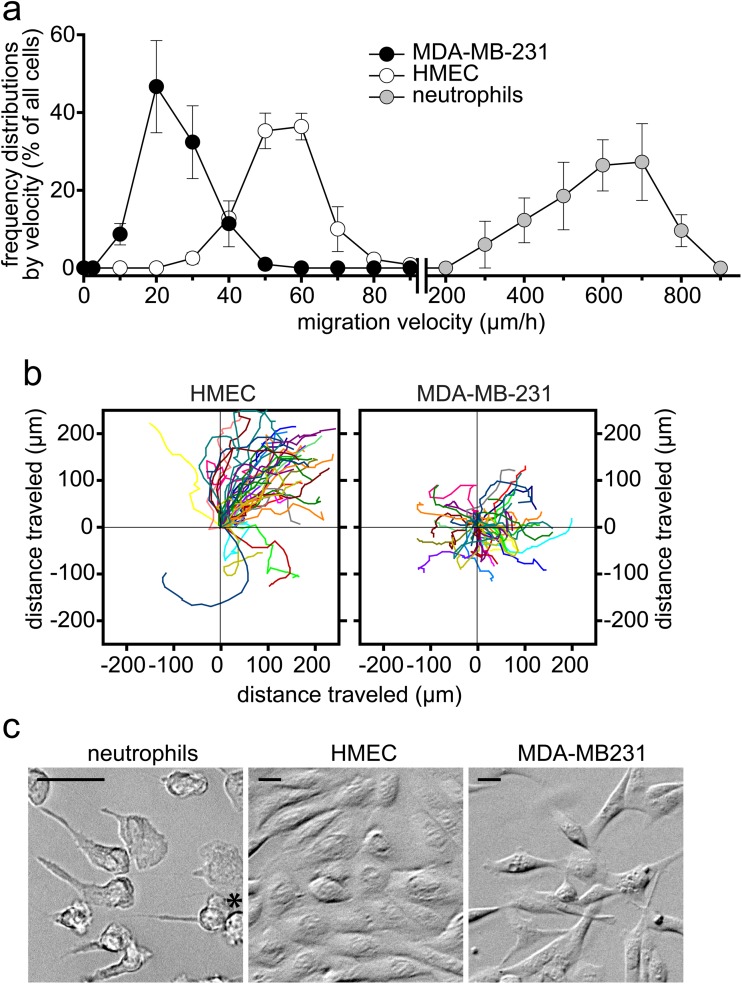
We have previously demonstrated that neutrophil chemotaxis in response to chemokine receptor stimulation is regulated by cellular ATP release and autocrine purinergic signaling [3, 8]. Breast cancer cells express the chemokine receptor CXCR4, and the CXCR4 receptor ligand SDF-1α has been reported to contribute to metastasis of breast cancer by recruiting cancer cells to secondary organs such as the lungs [27–30]. However, in our hands, SDF-1α failed to induce directed migration or chemotaxis of MDA-MB-231 cells (data not shown). Instead, we observed a considerable degree of random baseline motility of unstimulated MDA-MB-231 cells, suggesting that random migration and not chemotaxis toward SDF-1α is the primary mode of motility of MDA-MB-231 cells and that increased random motility could be involved in the spread of breast cancer cells. In order to gain a better understanding of the motility patterns of breast cancer cells, we compared the movements of MDA-MB-231 cells, healthy HMEC, and human neutrophils. HMEC and MDA-MB-231 cells showed clear differences in their migration velocities, cell shapes, and their modes of motility (Fig. 1 and Video 1). The average migration velocity of HMEC was between 50 and 80 μm/h, while the velocity of MDA-MB-231 cells was considerably slower, with an average of ∼20 μm/h (Fig. 1a). In contrast, the velocity of healthy neutrophils in a chemotactic gradient field was about ten times faster than the speed of HMEC and MDA-MB-231 cells (Fig. 1a). While HMEC clustered and migrated as large coherent sheets of cells in a single direction to generate a collective layer of confluent epithelial cells, MDA-MB-231 cells lacked collective behavior, migrated as individual cells, and often moved across each other (Fig. 1b and Video 1). While the shape of HMEC resembled that of neutrophils with a single leading edge and a trailing section, MDA-MB-231 cells assumed spike-like or triangular cell shapes with multiple leading edges that seemed to prompt frequent changes in the direction of cell movement, contributing to an overall disorganized motility pattern which differed considerably from that of healthy cells (Fig. 1c and Video 1).
Fig. 1.
MDA-MB-231 cells display a disorganized motility pattern. a The velocities of individual HMEC, MDA-MB-231 cells, and neutrophils were assessed by live cell microscopy and the results plotted to show the frequency distribution of cell speeds. Neutrophil chemotaxis was induced by generating a chemotactic gradient field with a micropipette loaded with 100 nM N-formyl-methionyl-leucyl-phenylalanine (fMLP). Data shown are means ± SD of n = 3 (HMEC) or n = 4 (neutrophils and MDA-MC-231 cells) separate experiments, each comprising tracking data from at least 40 individual cells. b Cell motility patterns of HMEC and MDA-MB-231 cells were analyzed from tracking data acquired over 5 h of time-lapse video microscopy (see also related Video 1). Migration paths of n = 40 cells aligned with their origins at x = y = 0 μm are shown. c Cell shapes of migrating neutrophils, HMEC, and MDA-MB-231 cells. Left panel: The asterisk indicates the position of the micropipette tip loaded with 100 nM fMLP to generate a chemotactic gradient field. Scale, 20 μm
Breast cancer cells express ectonucleotidases that promote adenosine formation
Neutrophils in a chemotactic gradient field release ATP through pannexin-1 (panx1) channels. The released ATP stimulates P2Y2 receptors that contribute to gradient sensing and delineate the direction of cell migration [3, 7]. Ectonucleotidases such as alkaline phosphatase, CD39, and CD73 convert the released ATP to adenosine, which promotes autocrine stimulation of A3 receptors at the front of cells and A2a receptors at the back [3, 4, 7, 8]. Both receptors elicit excitatory and inhibitory feedback mechanisms, respectively, that maintain cell polarity and increase the migration speed of neutrophils in a chemotactic gradient field (Supplemental Fig. 1) [3, 4, 8]. We wondered whether similar purinergic signaling mechanisms define the motility patterns of breast cancer cells. Therefore, we studied the expression of purinergic signaling molecules in MDA-MB-231 cells, HMEC, and MCF-7 cells, which is a breast cancer cell line with less metastatic potential than MDA-MB-231 cells. Our quantitative PCR (qPCR) results showed that all three cell types express mRNA of most of the known purinergic receptor subtypes (Supplemental Fig. 2a, b). However, the expression patterns of these receptors differ among the three cell lines, suggesting that the peculiar migration patterns of breast cancer cells might be linked to the particular purinergic signaling components expressed in these cells. Notably, the mRNA levels of the adenosine receptors were higher in MDA-MB-231 and MCF-7 cells when compared to HMEC (Supplemental Fig. 2b). This finding is in agreement with previous reports that breast tumors overexpress A3 receptors [31]. We found that HMEC and MDA-MB-231 cells possess transcripts for panx1 and panx2, but not panx3 (Supplemental Fig. 2c), which suggests that panx1 may be a primary ATP release channel in these breast cancer cells.
Multiple families of membrane-bound ectonucleotidases can degrade the released ATP in the extracellular space. These ectonucleotidases are found on the cell surfaces of virtually all mammalian cell types, including neutrophils and cancer cells [15–17, 32, 33]. The largest families of ectonucleotidases are the ectonucleoside triphosphate diphosphohydrolase (ENTPD) and ectonucleotide pyrophosphatase/phosphodiesterase (ENPP) families that hydrolyze nucleotide di- and triphosphates. The number of phosphate residues removed and the kinetic properties and substrate preferences vary from enzyme to enzyme [34, 35]. Alkaline phosphatase, which is highly expressed in neutrophils, can convert ATP directly to adenosine, while CD73 converts AMP to adenosine [33]. In order to determine whether the expression of ectonucleotidases in breast cancer cells could be involved in their unique motility patterns, we used qPCR analysis to compare ectonucleotidase transcripts in HMEC and MDA-MB-231 cells. Both cell types contained mRNA species encoding numerous members of the ectonucleotidase families (Fig. 2a). However, transcripts of ENPP1 and CD73, which convert ATP to AMP and AMP to adenosine, respectively, were more abundant in MDA-MB-231 cells than in HMEC. On the other hand, mRNA levels for ENTPD2, an enzyme that converts ATP to ADP, were higher in HMEC than in MDA-MB-231 cells. Neither cell type expressed mRNA encoding ENTPD1 (also referred to as CD39), which is the major enzyme that facilitates adenosine formation in neutrophils [4]. These findings suggest that MDA-MB-231 cells are better equipped than HMEC to generate adenosine from ATP released into the extracellular space.
Fig. 2.
MDA-MB-231 cells possess ectonucleotidases that facilitate adenosine formation. a Ectonucleotidase expression profiles of HMEC and MDA-MB-231 cells were characterized by qPCR (n = 2). The preferred ATP hydrolysis products of key enzymes are indicated by arrows. b–e The ability of HMEC and MDA-MB-231 cells to hydrolyze ATP was assessed by incubating equal numbers of HMEC and MDA-MB-231 cells with exogenous ATP (5 μM) and measuring the concentrations of the remaining ATP (b) and its breakdown products ADP (c), AMP (d), and adenosine (ADO) (e) with HPLC after the indicated incubation times. ATP was added immediately (∼3 s) before the first sample was taken for HPLC analysis (t = 0 min). Values are expressed as the mean ± SD of n = 3 experiments; *p < 0.05, HMEC vs. MDA-MB-231 (t test)
To test this possibility, we compared the kinetics of ATP breakdown by HMEC and MDA-MB-231 cells. HMEC and MDA-MB-231 cells were incubated with equal concentrations (5 μM) of exogenously added ATP and the concentrations of the remaining ATP and its hydrolytic breakdown products in cell supernatants were determined after different times with HPLC (Fig. 2b–e). Both cell types were equally efficient in converting ATP (Fig. 2b), but the two cell types differed in the primary breakdown products they generated. HMEC converted ATP mainly to ADP (Fig. 2c). MDA-MB-231 cells generated comparatively little ADP but about twice the amount of adenosine that HMEC produced (Fig. 2e). These findings support the concept that MDA-MB-231 cells generate an adenosine-rich microenvironment that preferentially promotes autocrine P1 receptor stimulation.
Endogenous adenosine formation and autocrine A3 receptor stimulation contribute to MDA-MB-231 cell motility
Neutrophils generate local ATP and adenosine microenvironments at the front and back of cells to facilitate coordinated cell migration [3, 8]. In analogy, we hypothesized that endogenous adenosine formation could regulate the motility of breast cancer cells. In support of this idea, we found that the migration velocity of MDA-MB-231 cells significantly decreased upon inhibition of ATP release with the panx1 inhibitor carbenoxolone (CBX) or upon removal of extracellular adenosine with adenosine deaminase (ADA). ADA converts adenosine to inosine, which has been reported to act as another agonist of A3 receptors [36]. However, we found that the inosine concentrations in our cell culture supernatants were below the detection limit (∼100 nM) even when cells were treated with ADA (Supplemental Fig. 3a). Moreover, inosine addition did not alter the motility of MDA-MB-231 cells even at concentrations as high as 50 μM (Supplemental Fig. 3b). Taken together with previous reports of the low efficacy of inosine at A3 receptors [37], our results suggest that removal of adenosine and not production of inosine is responsible for the inhibitory effect of ADA on cell motility. This is also supported by the finding that the ADA inhibitor pentostatin increased MDA-MB-231 migration velocity by about 40 % (Fig. 3a). Taken together, these results suggest that the migration of MDA-MB-231 cells involves autocrine stimulation of adenosine receptors. In order to test which of the four known adenosine receptor subtypes is involved, we assessed the migration velocity of MDA-MB-231 cells in the presence of specific P1 receptor antagonists. The antagonists of A1 (DPCPX), A2a (CSC), and A2b receptors (MRS 1754) had no significant effect on MDA-MB-231 cell motility (Fig. 3a). However, the A3 receptor antagonist MRS 1191 significantly reduced cell migration velocity by 40–50 % compared to untreated MDA-MB-231 cells (Fig. 3a). These results indicate that endogenous stimulation of A3 receptors is involved in the regulation of MDA-MB-231 cell motility.
Fig. 3.
Endogenous adenosine formation and autocrine stimulation of A3 receptors contribute to MDA-MB-231 cell motility. a MDA-MB-231 cells were treated with CBX (10 μM), adenosine deaminase (ADA; 10 U/ml), pentostatin (100 nM), DPCPX (A1 antagonist, 20 nM), CSC (A2a antagonist, 200 nM), MRS 1754 (A2b antagonist, 100 nM), or MRS 1191 (A3 antagonist, 20 nM) and the velocity of random cell motility was assessed by time-lapse microscopy. At least 30 randomly selected cells were tracked over 16 h to calculate the mean velocity for each well. Results represent the mean values ± SD of n = 4–10 separate experiments. Statistical comparisons were done with one-way ANOVA; *p < 0.05. b MDA-MB-231 cells were stained with antibodies for the different P1 receptor subtypes (green) and with phalloidin (red) to evaluate F-actin accumulation at the leading edge. Note arrows indicating A3 receptor accumulation at multiple leading edges. The histograms depict the fluorescence intensity distributions of adenosine receptors (green) and F-actin (red) along the region of interest marked in the merged image. Images were taken with a Zeiss LSM 510 confocal microscope using a ×63 objective. Scale bar, 20 μm
In neutrophils, we found that A3 adenosine receptors translocate to the front of polarized cells, where their autocrine stimulation promotes cell migration speed [3]. Disruption of A3 receptor translocation to the leading edge or disruption of the spatiotemporally coordinated stimulation of adenosine receptors by endogenously generated adenosine impairs neutrophil migration [3, 8]. Based on these findings, we decided to study the distribution of adenosine receptors across the cell surface of MDA-MB-231 cells. Leading edges were identified by phalloidin staining of F-actin. We detected all four adenosine receptor subtypes on the cell surface of MDA-MB-231 cells (Fig. 3b). A1, A2a, and A2b receptors were uniformly distributed across the cell surface. However, A3 receptors accumulated at membrane regions enriched with F-actin, suggesting preferential accumulation of A3 receptors at the leading edges of MDA-MB-231 cells. In contrast to HMEC and neutrophils, which typically possess only a single leading edge, we found that MDA-MB-231 cells often possess multiple leading edges that correspond to the corners of angular cells (Fig. 3b; see arrows).
These observations suggest that, like in neutrophils, A3 receptors associate with leading edges of MDA-MB-231 cells but that they contribute to the peculiar migration pattern of the breast cancer cells. Alternating stimulation of A3 receptors at different leading edges could cause the frequent directional changes and overall dysfunctional motility patterns of MDA-MB-231 cells (Video 1).
Exogenous adenosine elicits shape changes and spreading of MDA-MB-231 cells
We previously reported that the addition of adenosine to neutrophils inhibits chemotaxis by obscuring the endogenous purinergic feedback mechanisms that regulate chemotaxis [3]. The findings described above suggest that similar endogenous mechanisms involving A3 adenosine receptors regulate the motility of MDA-MB-231 cells. We therefore hypothesized that addition of exogenous adenosine could interfere with the migration of these breast cancer cells. If so, treatment with adenosine would be expected to stretch cancer cells in different directions by simultaneously stimulating A3 receptors on their multiple leading edges. Analysis of the cell shapes of MDA-MB-231 cells in the presence or absence of adenosine revealed four main cell groups: spherical, flat rounded, spindle-shaped, and polygonal. The distribution of these groups changed upon addition of adenosine (Fig. 4a). Exogenous adenosine shifted the proportion from rounded and spindle-shaped cells to >40 % of cells with polygonal shapes (Fig. 4b). This supports the concept that simultaneous stimulation of multiple A3 receptor-enriched leading edges does indeed promote cell stretch and the flattening of cancer cells. This conclusion was further supported by a significant increase in the cell surface area of MDA-MB-231 cells in response to adenosine treatment (Fig. 4c).
Fig. 4.
Exogenous adenosine elicits shape changes and spreading of MDA-MB-231 cells. a Cell shapes of MDA-MB-231 cells treated or not (contol) with 10 μM adenosine (ADO) for 6 h (×10 objective). Scale bar, 50 μm. b Four different groups were identified and their frequency distributions were assessed in the presence or absence of 10 μM adenosine. All cells (150–200) in a randomly selected microscopic field were analyzed. Data show the mean values ± SD of n = 4 separate experiments; *p < 0.05 (t test). c Surface area of cells treated or not with adenosine. Data show the mean values ± SD of n = 4 separate experiments, each comprising 150–200 cells; *p < 0.05 (t test)
Addition of adenosine blocks the motility of breast cancer cells
To further test our hypothesis that addition of adenosine interferes with breast cancer cell motility, we treated cells with adenosine and assessed cell motility by time-lapse video microscopy. Addition of adenosine (>1 μM) suppressed migration velocity by >50 % (Fig. 5a). Similarly, addition of exogenous ATP dose-dependently reduced random motility of MDA-MB-231 cells (Fig. 5b). ATP at a concentration of 100 μM diminished the baseline velocity of MDA-MB-231 cells from 16 ± 2 to about 5 μm/h. In contrast to ATP, the non-hydrolyzable ATP analog ATPγS slightly increased cell motility at low concentrations (1 nM) and had a slighly suppressive effect at higher concentrations (>10 μM). Taken together, these results suggest that much of the suppressive effect of ATP results from its breakdown to adenosine. At concentrations of 10 μM and higher, adenosine blocked MDA-MB-231 cell motility, diminishing average velocity from 20 to ∼5 μm/h (Fig. 5c and Video 2). Based on previous reports, adenosine increases cell adhesion to extracellular matrix proteins such as fibronectin [15, 38]. To test whether such effects accounted for reduced motility in our experiments, we also used poly-d-lysine-coated slides that bind cells in a nonspecific manner [39]. Exogenous adenosine had similar effects on cell shape (Supplemental Fig. 4a, b) and motility (Supplemental Fig. 4c) regardless of whether cells were attached to poly-d-lysine or fibronectin. While cells on poly-d-lysine-coated slides moved significantly more slowly than cells attached to fibronectin, the inhibitory effect of adenosine was almost identical under both conditions (50–60 %; Supplemental Fig. 4c). This suggests that adenosine inhibits cell motility not by increasing cell adhesion but by inducing cell spreading in opposing directions.
Fig. 5.
Addition of adenosine arrests breast cancer cell motility. a, b The motility of MDA-MB-231 cells was assessed by time-lapse microscopy over a period of 3 h after the addition of adenosine (a), ATP, or the non-hydrolyzable ATP analog ATPγS (b). The velocity of random migration was calculated from the tracks of individual cells (n = 24–30 cells). Values are expressed as the mean ± SD of n = 3 experiments and statistical comparisons were done with one-way ANOVA; *p < 0.05 vs. untreated control. c, d Motility of MDA-MB-231 cells (c) or MCF-7 cells (d) in the absence (control) or presence of 10 μM adenosine was assessed by time-lapse video microscopy over a period of 3 h and individual cell tracks (n = 30) were aligned with their origins at coordinate x = y = 0 μm (see related Video 2)
We wondered whether this pronounced inhibitory effect was restricted to MDA-MB-231 cells or whether other breast cancer cells are also sensitive to adenosine. Therefore, we studied how adenosine affects the motility of MCF-7 cells, which are a non-triple negative breast cancer cell line that expresses estrogen receptors. With an average migration speed of <8 μm/h, MCF-7 cells were about two times slower than MDA-MB-231 cells (Fig. 5d and Supplemental Fig. 4d). Adenosine at concentrations ranging from 1 to 100 μM significantly reduced the migration velocity of MCF-7 cells (Supplemental Fig. 4d). However, migration velocity dropped by a maximum of ∼30 %, which was considerably less when compared to MDA-MB-231 cells.
Adenosine reversibly arrests MDA-MB-231 cells through A3 receptor stimulation
As described above, we found that autocrine stimulation of A3 adenosine receptors by endogenously produced adenosine promotes MDA-MB-231 cell motility. Next, we tested whether the suppressive effect of exogenous adenosine on MDA-MB-231 cell motility is mediated by these A3 receptors. MDA-MB-231 cells were treated with agonists of A3 receptors or of other adenosine receptor subtypes and cell motility was assessed. The A3 receptor agonist IB-MECA blocked cell motility, recapitulating the effect of exogenous adenosine on breast cancer cells (Fig. 6a). In contrast, agonists of A1 (CPA) and A2a (CGS 21680) receptors had no significant effects on cell motility. Silencing of A3 receptors with siRNA reduced the inhibitory effect of adenosine on cell migration velocity (Fig. 6b). This supports the notion that exogenous adenosine arrests MDA-MB-231 cell motility by simultaneous stimulation of A3 receptors on multiple leading edges.
Fig. 6.
Adenosine reversibly inhibits breast cancer cell motility through A3 receptors. a MDA-MB-231 cells were treated with adenosine (10 μM), CPA (selective A1 agonist, 50 nM), CGS 21680 (selective A2a agonist, 50 nM), or IB-MECA (selective A3 agonist, 20 nM) and the velocity of random cell motility was assessed by time-lapse video microscopy. At least 30 randomly selected cells were tracked to calculate the mean velocity for each well. Results represent the mean values ± SD of n = 4–10 separate experiments. Statistical comparisons were done with one-way ANOVA; *p < 0.05. b MDA-MB-231 cells were treated with siRNA targeting the A3 receptor or with nonsense control siRNA and the velocity of random cell motility in the presence or absence of adenosine (10 μM) was assessed. Results are expressed as percentage of the random cell motility of cells treated with the respective siRNA but not with adenosine. Mean values ± SD of n = 4 separate experiments are shown; *p < 0.05 (t test). c MDA-MB-231 cells were treated with adenosine (10 μM) at the indicated time point (t = 4 h). At t = 8 h, adenosine was washed out and motility was observed for another 16 h (see also related Video 3). Data represent the mean values ± SEM of n = 60 cells of two independent experiments
To test whether the effect of adenosine is reversible, we studied the motility of MDA-MB-231 cells under the microscope before and after addition of 10 μM adenosine. After 4 h, we removed adenosine by washing cells with fresh media and continued to record cell motility for another 18 h (Fig. 6c). Adenosine rapidly blocked cell motility to ∼4 μm/h, and removal of adenosine allowed cell motility to gradually recover to ∼70 % of the baseline velocity of untreated MDA-MB-231 cells (Fig. 6c and Video 3). However, adenosine did not alter the viability of cells that were treated with adenosine for up to 16 h. Viability remained >97 % in both adenosine-treated and untreated controls, indicating that the reduction in cell migration speed is not due to cytotoxic effects of A3 receptor stimulation [20]. These findings demonstrate that adenosine has a powerful yet reversible inhibitory effect on MDA-MB-231 cell migration, which is consistent with the concept that A3 receptors define the motility of MDA-MB-231 cells and that exogenous stimulation of A3 receptors arrests cell motility.
Discussion
Our previous work has shown that neutrophils possess a highly structured and tightly regulated purinergic signaling system that defines various aspects of chemotaxis [3, 7, 8, 40]. Our present study revealed that MDA-MB-231 breast cancer cells possess a variant of this purinergic signaling system. Breast cancer cells share with neutrophils the ability to release ATP into the extracellular space and to convert that ATP to adenosine, which influences their motility patterns by feedback through A3 adenosine receptors. However, unlike neutrophils, which release ATP from a single leading edge [3, 41], we found that MDA-MB-231 cells form multiple leading edges, suggesting that they release ATP in a disorganized fashion, resulting in alternating stimulation of A3 receptors at these different leading edges and the peculiar motility pattern of these cells.
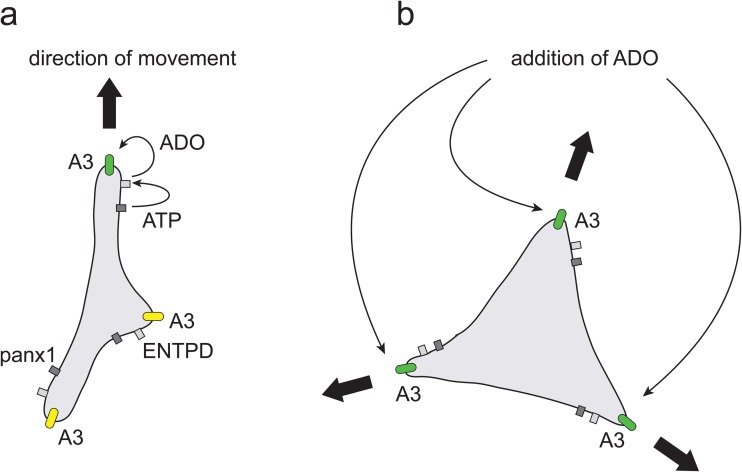
Based on our findings, we conclude that endogenous stimulation of A3 receptors is involved in the apparently random cell movement of MDA-MB-231 cells. Depending on the sites at which ATP is released and adenosine is formed, adjacent A3 receptors promote cell migration in that particular direction (Fig. 7a). Based on our model, we propose that changes in the sites of ATP release and A3 receptor stimulation contribute to the frequent directional changes that are characteristic of the random motility pattern of MDA-MB-231 cells. This model is also consistent with the inhibitory effect of exogenous adenosine on MDA-MB-231 cell motility. Simultaneous stimulation of A3 receptors at all leading edges of a cell prompts its spread in opposing directions and a net inhibition of MDA-MB-231 cell motility (Fig. 7b). Our model therefore offers an explanation for the seemingly contradictory finding that both A3 receptor agonists and antagonists can inhibit MDA-MB-231 cell migration. Overexpression of CD73 and increased adenosine formation in human breast cancer cells have been shown to cause cell adhesion to extracellular matrix proteins [15, 38]. The effect of adenosine on cell migration did not differ when cells were attached to fibronectin or poly-d-lysine, and the inhibitory effect of adenosine on cell motility was reversible upon removal of adenosine. These findings suggest that the effect of adenosine on cell motility is not merely a result of increased adherence to fibronectin.
Fig. 7.
Proposed model of the regulation of MDA-MB-231 cell migration by endogenous and exogenous stimulation of A3 receptors. a ATP release through pannexin1 (panx1) channels and adenosine formation by ectonucleotidases (ENTPD) result in endogenous A3 receptor stimulation at the nearest leading edge and random motility of MDA-MB-231 cells in the corresponding direction. b Exogenous adenosine simultaneously stimulates A3 receptors on all leading edges of a cell, causing the cell to spread out in opposing directions and thus arresting MDA-MB-231 cell motility
However, several previous studies have shown that extracellular adenosine can also impair T cell-mediated immune responses in cancer [15–17, 42, 43]. Stagg et al. and Zhou et al. found that CD73, the ectoenzyme responsible for endogenous adenosine formation, contributes to breast cancer metastasis [15–17]. In agreement with these reports, we found that MDA-MB-231 cells overexpress CD73 and differ from HMEC in that they possess more efficient means than HMEC to hydrolyze extracellular ATP to adenosine (Fig. 2). The tumor microenvironment is rich in extracellular ATP [44, 45]. Depending on the ectonucleotidases present, high levels of extracellular ATP and its breakdown proct adenosine could entrap cancer cells within the tumor environment or promote random motility and the spread of cancer cells outside of tumors. For example, cancer cells overexpressing CD73 may have a greater chance to escape the tumor environment and promote metastasis than cells that express lower levels of CD73. Thus, altered expression of CD73 and other molecules that regulate the nucleotide environment of cancer cells may contribute to metastasis and an overall insidious cell phenotype that allows invasion of the extracellular matrix and the spread of tumors to other organs. This may explain the findings by Stagg et al. who reported that CD73 expression contributes to in vitro cancer cell migration in a transwell chamber model and to tumor growth and metastasis in vivo [44, 45]. However, the tumor-enhancing pro-migratory and pro-metastatic properties of CD73 are controversial. Recent studies have shown that CD73 overexpression is associated with increased resistance to treatment and adhesive and proliferative responses of head and neck squamous cell carcinoma, but that CD73 overexpression is not associated with metastasis [46]. On the other hand, loss of CD73 seems to favor endometrial tumor progression [47]. These conflicting results point to differences in the enzymatic activity of CD73 or the expression profiles of adenosine receptors in these different tumors.
In contrast to the motility enhancing effect of endogenous adenosine, we found that exogenous adenosine at comparatively high concentrations can inhibit the migration of the breast cancer cell lines we tested. Importantly, these effects of adenosine were well within the adenosine concentration range that has been measured in the tumor microenvironment [48]. In line with these findings, Virtanen et al. recently described an inhibitory effect of exogenous adenosine on cancer cell invasion and migration [18]. They suggested that receptor-independent mechanisms were responsible for this effect. Interestingly, A2b receptors were the only adenosine receptor subtype they found to be expressed in the MDA-MB-231 cell line they studied. Others have shown that A2a receptors mediate the growth and migration of MDA-MB-231 cells in response to adenosine [49]. While there are controversial reports about the adenosine receptor subtypes expressed in MDA-MB-231 cells, we found all four subtypes, including A3 receptors. This is consistent with findings reported by others [20]. The discrepancy between all these studies suggests that MDA-MB-231 cells, and probably other cancer cell lines, can mutate considerably and alter the profiles of purinergic signaling components they express.
The A3 receptor agonist IB-MECA has been shown to inhibit the metastasis of prostate cancer [50]. Our current study demonstrates that IB-MECA can significantly impair the motility of MDA-MB-231 breast cancer cells, which suggests that A3 receptor agonists may be able to reduce breast cancer metastasis. This is supported by recent in vitro studies with an elegant microfluidic 3D model of extravasation of breast cancer cells in organotypic microenvironments. Blocking of A3 receptors increased the extravasation of breast cancer cells in this model [51]. IB-MECA and other A3 receptor agonists also inhibit proliferation in a number of different cancer models, including bone-residing breast cancer in an animal model of surgery-induced metastasis [52–55]. Moreover, A3 receptor agonists have been in clinical trials for metastatic hepatocellular carcinoma [56, 57]. Based on our current findings, the effect of A3 agonists may not only be restricted to cell proliferation but could also involve cell motility.
In summary, we conclude that purinergic signaling mechanisms contribute to the peculiar motility patterns of MDA-MB-231 breast cancer cells and possibly other cell lines. The purinergic signaling mechanisms of these cells are potential therapeutic targets to reduce the metastatic potential of breast cancer cells. However, further work and extensive preclinical studies will be necessary to test whether these concepts are translatable into clinically relevant treatment strategies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Regulation of neutrophil chemotaxis by autocrine purinergic signaling. Stimulation of chemoattractant receptors, such as formyl peptide receptors (FPR), through bacterial peptides (e.g., N-formyl-methionyl-leucyl-phenylalanine, fMLP) induces the release of ATP from neutrophils through pannexin-1 (panx1) channels and autocrine stimulation of P2Y2 receptors at the site that first encounters the chemoattractant. This autocrine feedback amplifies the chemotactic signal and promotes cell polarization within the chemotactic gradient field. Panx1, CD39 and A3 adenosine receptors accumulate at the leading edge while A2a receptors translocate toward the back of the cell. Hydrolysis of ATP by the ectonucleotidases CD39 and CD73 leads to the formation of adenosine. At the leading edge, adenosine promotes forward migration through stimulation of A3 receptors, whereas stimulation of A2a receptors at the back of neutrophils provides an inhibitory signal that promotes the retraction of the trailing edge. Adenosine is removed by intracellular uptake (not shown) or breakdown to inosine by adenosine deaminase (ADA) [3, 8, 40] (GIF 121 kb)
Expression profiles of components of the purinergic signaling complex in HMEC, MDA-MB-231, and MCF-7 cells. a–c qPCR was performed to compare mRNA levels of P2 (a) and P1 (b) receptors in HMEC, MDA-MB-231, and MCF-7 cells and the expression of the three pannexin (panx) channel isoforms in HMEC and MDA-MB-231 cells (c). Values are expressed as means ± SD of triplicates; n.d., not detected (GIF 78 kb)
Inosine does not affect random migration velocity of breast cancer cells. a Inosine levels in the cell culture supernatant of MDA-MB-231 cells treated for 30 min with ADA (10 U/ml), adenosine (ADO; 10 μM), or vehicle control were determined by HPLC (n = 3); n.d., not detectable. b The velocity of random migration of MDA-MB-231 cells treated with the indicated concentrations of inosine was calculated from the tracks of individual cells (n = 30) that were recorded for 16 h by time-lapse video microscopy. Results are expressed as means ± SD of n = 3 separate experiments; *p < 0.05, one-way ANOVA (GIF 23 kb)
Adenosine has similar effects on migration velocity of breast cancer cells that are attached to fibronectin or poly-D-lysine. a MDA-MB-231 cells attached to poly-d-lysine-coated glass chamber slides were treated or not with 10 μM adenosine (ADO) for 6 h (10× objective; scale bar, 50 μm). b Four different types of cell shapes of MDA-MB-231 cells attached to poly-d-lysine were defined as shown in Fig. 4 and the frequency distributions assessed in the presence or absence of 10 μM adenosine. All cells (150–200) in a randomly selected microscopic field were analyzed. Data show mean values ± SD of n = 3 separate experiments; *p < 0.05, t test. c The velocity of random migration of MDA-MB-231 cells attached to fibronectin or poly-d-lysine and treated with 10 μM adenosine or vehicle control was calculated from the tracks of individual cells (n = 30) recorded for 16 h by time-lapse video microscopy. d Random migration velocity of MCF-7 cells treated with the indicated concentrations of adenosine was determined as described for panel c. Results are expressed as mean ± SD of n = 3 separate experiments; *p < 0.05, one-way ANOVA (GIF 76 kb)
Motility patterns of HMEC and MDA-MB-231 cells. Random motility of MDA-MB-231 cells (left) and HMEC (right) was monitored for 15 h using an inverted microscope with a 10× objective. Frame rate: 5 frames/h; scale bar: 80 μm (AVI 3294 kb)
Adenosine blocks MDA-MB-231 cell motility. Random migration of MDA-MB-231 cells in the presence (right) and absence (left) of adenosine (10 μM) was recorded for 3 h using a Leica DMIRB microscope with a 10× objective (frame rate: 10 frames/h; scale bar: 50 μm) (AVI 780 kb)
Adenosine treatment causes cell spreading and the reversible arrest of the motility of MDA-MB-231 cells. Migrating MDA-MB-231 cells exposed to adenosine for 4 h recover their motility after adenosine washout with fresh medium. Cells were incubated for 4 h in serum-free growth medium, 10 μM adenosine was added at t = 4 h, and the medium was changed again to adenosine-free medium at t = 8 h. The period when adenosine was present is marked in yellow. Cell migration was monitored using a Leica DMIRB microscope with a 10× objective (frame rate: 10 frames/h; scale bar: 80 μm) (AVI 2929 kb)
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (GM-51477, GM-60475, AI-072287, AI-080582, and T32 GM-103702) to WGJ and a grant from the German Research Foundation (LE-3209/1-1) to CL. The authors wish to thank Dr. Jeffrey Segall for his advice and valuable comments.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Carola Ledderose, Marco M. Hefti and Yu Chen contributed equally to this work.
References
- 1.Merrill RM, Sloan A. Risk-adjusted female breast cancer incidence rates in the United States. Cancer Epidemiol. 2012;36:137–140. doi: 10.1016/j.canep.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 4.Corriden R, Chen Y, Inoue Y, Beldi G, Robson SC, Insel PA, Junger WG. Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J Biol Chem. 2008;283:28480–28486. doi: 10.1074/jbc.M800039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue Y, Chen Y, Hirsh MI, Yip L, Junger WG. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock. 2008;30:173–177. doi: 10.1097/shk.0b013e318160dad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Junger WG. Purinergic regulation of neutrophil chemotaxis. Cell Mol Life Sci. 2008;65:2528–2540. doi: 10.1007/s00018-008-8095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3:ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao Y, Chen Y, Ledderose C, Li L, Junger WG. Pannexin 1 channels link chemoattractant receptor signaling to local excitation and global inhibition responses at the front and back of polarized neutrophils. J Biol Chem. 2013;288:22650–22657. doi: 10.1074/jbc.M113.476283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 10.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, Robaye B, Conley PB, Kim HC, Sargin S, Schön P, Schwab A, Hanley PJ. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal. 2010;3:ra55. doi: 10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- 12.Müller T, Robaye B, Vieira RP, Ferrari D, Grimm M, Jakob T, Martin SF, Di Virgilio F, Boeynaems JM, Virchow JC, Idzko M. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65:1545–1553. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 13.Wagstaff SC, Bowler WB, Gallagher JA, Hipskind RA. Extracellular ATP activates multiple signalling pathways and potentiates growth factor-induced c-fos gene expression in MCF-7 breast cancer cells. Carcinogenesis. 2000;21:2175–2181. doi: 10.1093/carcin/21.12.2175. [DOI] [PubMed] [Google Scholar]
- 14.Stefano L, Rossler OG, Griesemer D, Hoth M, Thiel G. P2X(7) receptor stimulation upregulates Egr-1 biosynthesis involving a cytosolic Ca(2+) rise, transactivation of the EGF receptor and phosphorylation of ERK and Elk-1. J Cell Physiol. 2007;213:36–44. doi: 10.1002/jcp.21085. [DOI] [PubMed] [Google Scholar]
- 15.Zhou P, Zhi X, Zhou T, Chen S, Li X, Wang L, Yin L, Shao Z, Ou Z. Overexpression of Ecto-5′-nucleotidase (CD73) promotes T-47D human breast cancer cells invasion and adhesion to extracellular matrix. Cancer Biol Ther. 2007;6:426–431. doi: 10.4161/cbt.6.3.3762. [DOI] [PubMed] [Google Scholar]
- 16.Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, Smyth MJ. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011;71:2892–2900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 17.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virtanen SS, Kukkonen-Macchi A, Vainio M, Elima K, Härkönen PL, Jalkanen S, Yegutkin GG. Adenosine inhibits tumor cell invasion via receptor-independent mechanisms. Mol Cancer Res. 2014;12:1863–1874. doi: 10.1158/1541-7786.MCR-14-0302-T. [DOI] [PubMed] [Google Scholar]
- 19.Chung H, Jung JY, Cho SD, Hong KA, Kim HJ, Shin DH, Kim H, Kim HO, Shin DH, Lee HW, Jeong LS, Kong G. The antitumor effect of LJ-529, a novel agonist to A3 adenosine receptor, in both estrogen receptor-positive and estrogen receptor-negative human breast cancers. Mol Cancer Ther. 2006;5:685–692. doi: 10.1158/1535-7163.MCT-05-0245. [DOI] [PubMed] [Google Scholar]
- 20.Panjehpour M, Karami-Tehrani F. Adenosine modulates cell growth in the human breast cancer cells via adenosine receptors. Oncol Res. 2007;16:575–585. doi: 10.3727/000000007783629981. [DOI] [PubMed] [Google Scholar]
- 21.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 22.Inoue Y, Chen Y, Pauzenberger R, Hirsh MI, Junger WG. Hypertonic saline up-regulates A3 adenosine receptor expression of activated neutrophils and increases acute lung injury after sepsis. Crit Care Med. 2008;36:2569–2575. doi: 10.1097/CCM.0b013e3181841a91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bulger EM, Tower CM, Warner KJ, Garland T, Cuschieri J, Rizoli S, Rhind S, Junger WG. Increased neutrophil adenosine a3 receptor expression is associated with hemorrhagic shock and injury severity in trauma patients. Shock. 2011;36:435–439. doi: 10.1097/SHK.0b013e318231ee2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buira SP, Albasanz JL, Dentesano G, Moreno J, Martin M, Ferrer I, Barrachina M. DNA methylation regulates adenosine A(2A) receptor cell surface expression levels. J Neurochem. 2010;112:1273–1285. doi: 10.1111/j.1471-4159.2009.06538.x. [DOI] [PubMed] [Google Scholar]
- 25.Datino T, Macle L, Qi XY, Maguy A, Comtois P, Chartier D, Guerra PG, Arenal A, Fernandez-Aviles F, Nattel S. Mechanisms by which adenosine restores conduction in dormant canine pulmonary veins. Circulation. 2010;121:963–972. doi: 10.1161/CIRCULATIONAHA.109.893107. [DOI] [PubMed] [Google Scholar]
- 26.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 28.Rhodes LV, Short SP, Neel NF, Salvo VA, Zhu Y, Elliott S, Wei Y, Yu D, Sun M, Muir SE, Fonseca JP, Bratton MR, Segar C, Tilghman SL, Sobolik-Delmaire T, Horton LW, Zaja-Milatovic S, Collins-Burow BM, Wadsworth S, Beckman BS, Wood CE, Fuqua SA, Nephew KP, Dent P, Worthylake RA, Curiel TJ, Hung MC, Richmond A, Burow ME. Cytokine receptor CXCR4 mediates estrogen-independent tumorigenesis, metastasis, and resistance to endocrine therapy in human breast cancer. Cancer Res. 2011;71:603–613. doi: 10.1158/0008-5472.CAN-10-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueda Y, Neel NF, Schutyser E, Raman D, Richmond A. Deletion of the COOH-terminal domain of CXC chemokine receptor 4 leads to the down-regulation of cell-to-cell contact, enhanced motility and proliferation in breast carcinoma cells. Cancer Res. 2006;66:5665–5675. doi: 10.1158/0008-5472.CAN-05-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zlotnik A. New insights on the role of CXCR4 in cancer metastasis. J Pathol. 2008;215:211–213. doi: 10.1002/path.2350. [DOI] [PubMed] [Google Scholar]
- 31.Panjehpour M, Hemati S, Forghani MA. Expression of A1 and A3 adenosine receptors in human breast tumors. Tumori. 2012;98:137–141. doi: 10.1177/030089161209800119. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Shukla A, Namiki S, Insel PA, Junger WG. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J Leukoc Biol. 2004;76:245–253. doi: 10.1189/jlb.0204066. [DOI] [PubMed] [Google Scholar]
- 33.Kukulski F, Levesque SA, Sevigny J. Impact of ectoenzymes on p2 and p1 receptor signaling. Adv Pharmacol. 2011;61:263–299. doi: 10.1016/B978-0-12-385526-8.00009-6. [DOI] [PubMed] [Google Scholar]
- 34.Robson SC, Sevigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin X, Shepherd RK, Duling BR, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest. 1997;100:2849–2857. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/S0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Zhou X, Zhou T, Ma D, Chen S, Zhi X, Yin L, Shao Z, Ou Z, Zhou P. Ecto-5′-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J Cancer Res Clin Oncol. 2008;134:365–372. doi: 10.1007/s00432-007-0292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazia D, Schatten G, Sale W. Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J Cell Biol. 1975;66:198–200. doi: 10.1083/jcb.66.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ledderose C, Bao Y, Zhang J, Junger WG. Novel method for real-time monitoring of ATP release reveals multiple phases of autocrine purinergic signaling during immune cell activation. Acta Physiol (Oxford) 2015;213:334–345. doi: 10.1111/apha.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antonioli L, Blandizzi C, Pacher P, Haskó G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer. 2013;13:842–857. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 43.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 45.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res. 2008;14:5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 46.Bonnin N, Armandy E, Carras J, Ferrandon S, Battiston-Montagne P, Aubry M, Guihard S, Meyronet D, Foy JP, Saintigny P, Ledrappier S, Jung A, Rimokh R, Rodriguez-Lafrasse C, Poncet D (2016) MiR-422a promotes loco-regional recurrence by targeting NT5E/CD73 in head and neck squamous cell carcinoma. Oncotarget. doi:10.18632/oncotarget.9829 [DOI] [PMC free article] [PubMed]
- 47.Bowser JL, Blackburn MR, Shipley GL, Molina JG, Dunner K, Jr, Broaddus RR. Loss of CD73-mediated actin polymerization promotes endometrial tumor progression. J Clin Invest. 2016;126:220–238. doi: 10.1172/JCI79380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57:2602–2605. [PubMed] [Google Scholar]
- 49.Zhou JZ, Riquelme MA, Gao X, Ellies LG, Sun LZ, Jiang JX. Differential impact of adenosine nucleotides released by osteocytes on breast cancer growth and bone metastasis. Oncogene. 2015;34:1831–1842. doi: 10.1038/onc.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jajoo S, Mukherjea D, Watabe K, Ramkumar V. Adenosine A(3) receptor suppresses prostate cancer metastasis by inhibiting NADPH oxidase activity. Neoplasia. 2009;11:1132–1145. doi: 10.1593/neo.09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci U S A. 2015;112:214–219. doi: 10.1073/pnas.1417115112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bar-Yehuda S, Stemmer SM, Madi L, Castel D, Ochaion A, Cohen S, Barer F, Zabutti A, Perez-Liz G, Del Valle L, Fishman P. The A3 adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-kappaB signal transduction pathways. Int J Oncol. 2008;33:287–295. [PubMed] [Google Scholar]
- 53.Fishman P, Bar-Yehuda S, Ardon E, Rath-Wolfson L, Barrer F, Ochaion A, Madi L. Targeting the A3 adenosine receptor for cancer therapy: inhibition of prostate carcinoma cell growth by A3AR agonist. Anticancer Res. 2003;23:2077–2083. [PubMed] [Google Scholar]
- 54.Fishman P, Bar-Yehuda S, Ohana G, Barer F, Ochaion A, Erlanger A, Madi L. An agonist to the A3 adenosine receptor inhibits colon carcinoma growth in mice via modulation of GSK-3 beta and NF-kappa B. Oncogene. 2004;23:2465–2471. doi: 10.1038/sj.onc.1207355. [DOI] [PubMed] [Google Scholar]
- 55.Varani K, Vincenzi F, Targa M, Paradiso B, Parrilli A, Fini M, Lanza G, Borea PA. The stimulation of A(3) adenosine receptors reduces bone-residing breast cancer in a rat preclinical model. Eur J Cancer. 2013;49:482–491. doi: 10.1016/j.ejca.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Stemmer SM, Benjaminov O, Medalia G, Ciuraru NB, Silverman MH, Bar-Yehuda S, Fishman S, Harpaz Z, Farbstein M, Cohen S, Patoka R, Singer B, Kerns WD, Fishman P. CF102 for the treatment of hepatocellular carcinoma: a phase I/II, open-label, dose-escalation study. Oncologist. 2013;18:25–26. doi: 10.1634/theoncologist.2012-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blad CC, von Frijtag Drabbe Kunzel JK, de Vries H, Mulder-Krieger T, Bar-Yehuda S, Fishman P, Ijzerman AP. Putative role of the adenosine A3 receptor in the antiproliferative action of N6-(2-isopentenyl)adenosine. Purinergic Signal. 2011;7:453–462. doi: 10.1007/s11302-011-9244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Regulation of neutrophil chemotaxis by autocrine purinergic signaling. Stimulation of chemoattractant receptors, such as formyl peptide receptors (FPR), through bacterial peptides (e.g., N-formyl-methionyl-leucyl-phenylalanine, fMLP) induces the release of ATP from neutrophils through pannexin-1 (panx1) channels and autocrine stimulation of P2Y2 receptors at the site that first encounters the chemoattractant. This autocrine feedback amplifies the chemotactic signal and promotes cell polarization within the chemotactic gradient field. Panx1, CD39 and A3 adenosine receptors accumulate at the leading edge while A2a receptors translocate toward the back of the cell. Hydrolysis of ATP by the ectonucleotidases CD39 and CD73 leads to the formation of adenosine. At the leading edge, adenosine promotes forward migration through stimulation of A3 receptors, whereas stimulation of A2a receptors at the back of neutrophils provides an inhibitory signal that promotes the retraction of the trailing edge. Adenosine is removed by intracellular uptake (not shown) or breakdown to inosine by adenosine deaminase (ADA) [3, 8, 40] (GIF 121 kb)
Expression profiles of components of the purinergic signaling complex in HMEC, MDA-MB-231, and MCF-7 cells. a–c qPCR was performed to compare mRNA levels of P2 (a) and P1 (b) receptors in HMEC, MDA-MB-231, and MCF-7 cells and the expression of the three pannexin (panx) channel isoforms in HMEC and MDA-MB-231 cells (c). Values are expressed as means ± SD of triplicates; n.d., not detected (GIF 78 kb)
Inosine does not affect random migration velocity of breast cancer cells. a Inosine levels in the cell culture supernatant of MDA-MB-231 cells treated for 30 min with ADA (10 U/ml), adenosine (ADO; 10 μM), or vehicle control were determined by HPLC (n = 3); n.d., not detectable. b The velocity of random migration of MDA-MB-231 cells treated with the indicated concentrations of inosine was calculated from the tracks of individual cells (n = 30) that were recorded for 16 h by time-lapse video microscopy. Results are expressed as means ± SD of n = 3 separate experiments; *p < 0.05, one-way ANOVA (GIF 23 kb)
Adenosine has similar effects on migration velocity of breast cancer cells that are attached to fibronectin or poly-D-lysine. a MDA-MB-231 cells attached to poly-d-lysine-coated glass chamber slides were treated or not with 10 μM adenosine (ADO) for 6 h (10× objective; scale bar, 50 μm). b Four different types of cell shapes of MDA-MB-231 cells attached to poly-d-lysine were defined as shown in Fig. 4 and the frequency distributions assessed in the presence or absence of 10 μM adenosine. All cells (150–200) in a randomly selected microscopic field were analyzed. Data show mean values ± SD of n = 3 separate experiments; *p < 0.05, t test. c The velocity of random migration of MDA-MB-231 cells attached to fibronectin or poly-d-lysine and treated with 10 μM adenosine or vehicle control was calculated from the tracks of individual cells (n = 30) recorded for 16 h by time-lapse video microscopy. d Random migration velocity of MCF-7 cells treated with the indicated concentrations of adenosine was determined as described for panel c. Results are expressed as mean ± SD of n = 3 separate experiments; *p < 0.05, one-way ANOVA (GIF 76 kb)
Motility patterns of HMEC and MDA-MB-231 cells. Random motility of MDA-MB-231 cells (left) and HMEC (right) was monitored for 15 h using an inverted microscope with a 10× objective. Frame rate: 5 frames/h; scale bar: 80 μm (AVI 3294 kb)
Adenosine blocks MDA-MB-231 cell motility. Random migration of MDA-MB-231 cells in the presence (right) and absence (left) of adenosine (10 μM) was recorded for 3 h using a Leica DMIRB microscope with a 10× objective (frame rate: 10 frames/h; scale bar: 50 μm) (AVI 780 kb)
Adenosine treatment causes cell spreading and the reversible arrest of the motility of MDA-MB-231 cells. Migrating MDA-MB-231 cells exposed to adenosine for 4 h recover their motility after adenosine washout with fresh medium. Cells were incubated for 4 h in serum-free growth medium, 10 μM adenosine was added at t = 4 h, and the medium was changed again to adenosine-free medium at t = 8 h. The period when adenosine was present is marked in yellow. Cell migration was monitored using a Leica DMIRB microscope with a 10× objective (frame rate: 10 frames/h; scale bar: 80 μm) (AVI 2929 kb)