Abstract
Polycystic kidney diseases are characterized by numerous renal cysts that continuously enlarge resulting in compression of intact nephrons and tissue hypoxia. Recently, we have shown that hypoxia-inducible factor (HIF)-1α promotes secretion-dependent cyst expansion, presumably by transcriptional regulation of proteins that are involved in calcium-activated chloride secretion. Here, we report that HIF-1α directly activates expression of the purinergic receptor P2Y2R in human primary renal tubular cells. In addition, we found that P2Y2R is highly expressed in cyst-lining cells of human ADPKD kidneys as well as PKD1 orthologous mouse kidneys. Knockdown of P2Y2R in renal collecting duct cells inhibited calcium-dependent chloride secretion in Ussing chamber analyses. In line with these findings, knockdown of P2Y2R retarded cyst expansion in vitro and prevented ATP- and HIF-1α-dependent cyst growth. In conclusion, P2Y2R mediates ATP-dependent cyst growth and is transcriptionally regulated by HIF-1α. These findings provide further mechanistic evidence on how hypoxia promotes cyst growth.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-016-9532-5) contains supplementary material, which is available to authorized users.
Keywords: Polycystic kidney disease, Cyst growth, Chloride secretion, ATP, P2Y2R, Hypoxia, HIF-1α
Introduction
Polycystic kidney diseases comprise a number of genetic disorders that are characterized by the development of bilateral renal cysts [1]. The most common form, autosomal dominant polycystic kidney disease (ADPKD), affects 1 in 400–1000 [1]. In ADPKD, cysts enlarge continuously over time which results in compression of adjacent normal parenchyma and often causes the need for renal replacement therapy [2]. Cyst growth is driven by proliferation of the cyst-lining cells and fluid secretion across the epithelium into the cyst lumen. [3] Fluid secretion is largely mediated by transepithelial transport of chloride which, in part, depends on activation of calcium-activated chloride channels [3]. We have shown that hypoxia is a typical feature of polycystic kidneys especially in later stages of disease and have recently demonstrated that it promotes calcium-activated chloride secretion through stabilization of the hypoxia-inducible factor-1α (HIF-1α) [4, 5]. Since HIF-1α mainly acts as a transcription factor, we postulated that it may transcriptionally regulate genes that mediate calcium-activated chloride conductance. Calcium-dependent chloride secretion is typically induced by extracellular ATP which stimulates purinergic Gq-coupled P2Y receptors or ATP-gated P2X receptors, thereby resulting in an increase of intracellular calcium which then activates calcium-activated chloride channels [6–8]. ATP has been detected in cyst fluid in concentrations sufficient to activate various P2 receptors, and it has been postulated to be involved in cyst enlargement [9]. In line with this assumption, we and others have shown that in vitro cyst growth depends on extracellular ATP and can be inhibited by non-selective inhibitors of G-protein-coupled P2Y receptors [10, 11]. However, the molecular identity of the P2 receptor mediating ATP-dependent cyst growth has remained elusive so far.
In view of these findings, we hypothesized that HIF-1α-dependent cyst expansion may in part be mediated through an enhanced expression of purinergic receptors and therefore aimed at identifying a P2 receptor that is (i) regulated in a HIF-1α-dependent manner and (ii) mediates renal cyst expansion.
Results
The purinergic receptor P2Y2R is a target gene of HIF-1α in renal tubular cells
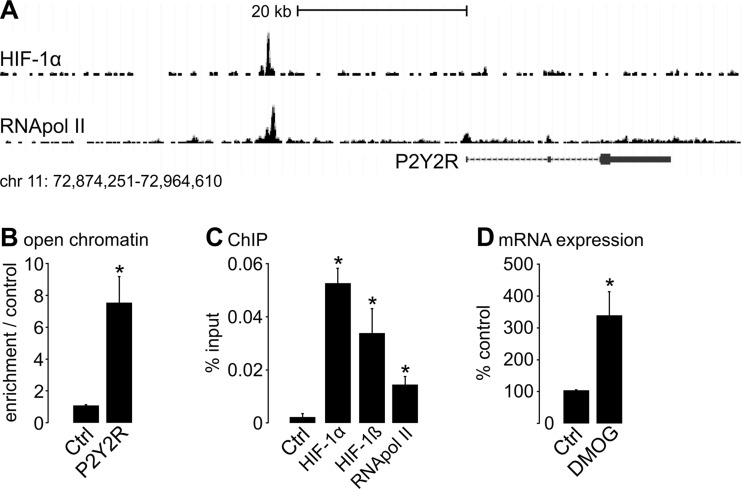
In order to identify purinergic receptors that are regulated in a HIF-dependent manner, we screened data obtained by chromatin immunoprecipitation of HIF subunits coupled to next-generation high-throughput sequencing (ChIP-seq) performed in human MCF-7 (Michigan Cancer Foundation-7) breast adenocarcinoma cells [12]. Inspecting the gene loci of P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14), we discovered significant HIF-1α binding events in an intergenic region approximately 23 kb upstream of the P2Y2R gene (Fig. 1a). HIF-binding at this locus was associated with increased binding of RNA polymerase II suggesting interaction of this regulatory element with a nearby promoter. In addition to P2Y receptors, ATP-gated P2X receptors have been proposed to affect renal cyst growth [13] and P2X7R has been shown to affect cystogenesis in PKD2-deficient zebrafish [14]. Therefore, we also inspected the genes coding for P2X receptors for HIF-binding events. However, none of the seven known P2X receptors (P2X1-7R) qualified as a potential HIF-target gene in MCF-7 cells (data not shown) [12]. Next, we aimed to evaluate HIF-1α-dependent regulation of P2Y2R in renal tubular cells. To this end, we used human primary tubular cells (hPTECs) isolated from healthy parts of the kidneys of patients that were nephrectomized because of kidney cancer [15]. We first performed formaldehyde-assisted isolation of regulatory elements (FAIRE) assays in hPTECs. We observed high levels of open chromatin at the HIF-binding region indicating gene regulatory potential (Fig. 1b). In line with the data obtained from MCF-7 cells, stabilization of HIF-1α by pharmacological inhibition of HIF-prolyl-hydroxylases (PHD) with dimethyloxalylglycine (DMOG) [12] resulted in binding of HIF-1α and its dimerization partner HIF-1ß to the intergenic genomic region upstream of P2RY2 in hPTECs (Fig. 1c). In addition, binding of RNA polymerase II was also enhanced confirming interaction with the transcriptional machinery (Fig. 1c). Furthermore, HIF stabilization in hPTECs resulted in increased P2Y2R messenger RNA (mRNA) expression (Fig. 1d). In summary, these data indicate that P2Y2R gene expression is directly regulated by HIF-1α in renal tubular cells.
Fig. 1.
P2Y2R is a target gene of HIF-1α in human primary renal tubular cells. a HIF-1α and RNA polymerase II (RNApol II) ChIP-seq signals at the P2Y2R gene locus on chromosome 11 in MCF-7 cells indicate binding of both factors to a region approximately 23 kb upstream of the transcriptional start site of P2Y2R. HIF-1α was stabilized by 1 mM DMOG. b FAIRE assays indicate the presence of open chromatin at this site compared to a putative non-regulatory site in hPTECs. n = 3 experiments from independent hPTEC cultures. c ChIP experiments using antibodies targeting HIF-1α, HIF-1ß, and RNApol II in hPTECs reveal binding of HIF-1 to the regulatory region and interaction with the transcriptional machinery. HIF-1α was stabilized by 1 mM DMOG. n = 3 experiments from independent hPTEC cultures. d Expression qPCR for P2Y2R in lysates from hPTECs exposed to vehicle or 1 mM DMOG for 16 h. n = 13 experiments from independent hPTEC cultures. Asterisk indicates comparison to control (Ctrl)
P2Y2R is expressed in cyst-lining epithelial cells
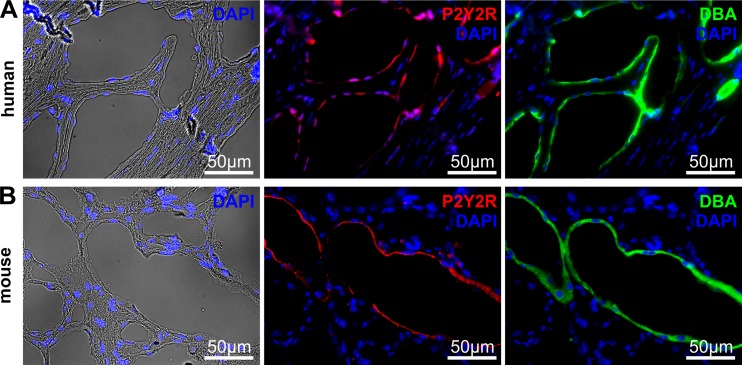
In the kidney, P2Y2R has been shown to be expressed in the apical membrane of principal cells of the collecting duct [16, 17], where most of the cysts originate in ADPKD [10, 18, 19]. In addition, P2Y2R mRNA has been detected in primary ADPKD cells from humans [20]. We analyzed human ADPKD kidney tissue and found a positive staining for P2Y2R in the apical membrane of cysts that also stained positive for Dolichos biflorus agglutinin (DBA), a marker for collecting duct cells [21] (Fig. 2a). A similar expression pattern was found in a PKD1 orthologous mouse model (Fig. 2b). In order to validate antibody specificity, we analyzed P2Y2R staining in P2Y2R knockout and wildtype mice. While DBA-positive tubules in wildtype mice stained positive, staining was absent in P2Y2R knockout kidney sections (Supplemental Fig. S1). These data suggest that P2Y2R is expressed in cyst-lining cells originating from the collecting duct.
Fig. 2.
P2Y2R is expressed in cyst-lining cells in vivo. Representative photos of a human ADPKD kidney and b PKD1 orthologous mouse knockout kidney stained for P2Y2R (red), nuclei (DAPI; blue) and Dolichos biflorus agglutinin (DBA; green) showing expression of P2Y2R in the apical membrane of DBA-positive cysts
P2Y2R is localized in the apical membrane of principal-like MDCK cells and its expression level depends on HIF
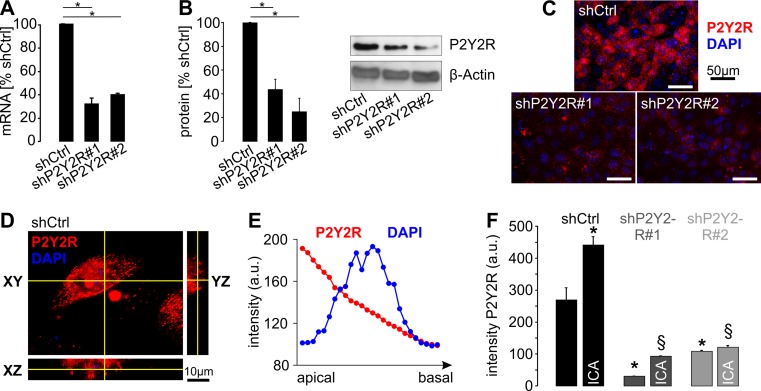
Next, we used a subclone of Madin Darby Canine Kidney cells resembling principal cells of the collecting duct (plMDCK cells) which are capable of forming cysts within a collagen matrix that grow in a secretion-dependent manner [10]. P2Y2R mRNA and protein could be detected in these cells, and expression levels were significantly reduced by stable transfection with two distinct shRNAs directed against canine P2Y2R (Fig. 3a–c). Next, we analyzed the subcellular localization of P2Y2R in polarized plMDCK cells and found a predominant, but not exclusive localization in the apical membrane (Fig. 3d–f). We then tested if HIF stabilization affects P2Y2R expression in plMDCK cells. Cells were incubated with the PHD inhibitor 2-(1-chloro-4-hydroxyisoquinoline-3-carboxamido) acetate (ICA) which is more feasible for long-term experiments than DMOG due to less toxicity [5, 22]. ICA led to an increase of P2Y2R signal in the apical membrane of plMDCK cells in control-transfected cells whereas ICA had no or only little effect in P2Y2R-deficient cells (Fig. 3f). These data show that P2Y2R is preferentially localized in the apical membrane of plMDCK cells and that its expression is enhanced by HIF stabilization.
Fig. 3.
P2Y2R is localized in the apical membrane of plMDCK cells, and its expression is regulated by HIF-1α. a Quantification of P2Y2R mRNA in plMDCK cell clones stably transfected with two distinct shRNAs directed against P2Y2R (shP2Y2R#1 and shP2Y2R#2) compared with MDCK cells stably transfected with scrambled shRNA (shCtrl) serving as control (n = 4). b Quantification of P2Y2R protein expression in P2Y2R knockdown and control cells described in a (n = 5). Right panel shows representative Western blot. c Representative photos of plMDCK cells competent (shCtrl) or deficient for P2Y2R (shP2Y2R#1 and #2) that were grown on permeable supports and stained for P2Y2R (red) and nuclei (DAPI; blue). d Representative z-stack analysis of plMDCK cells stained for P2Y2R (red) and DAPI (blue) in control-transfected, polarized plMDCK cells showing P2Y2R signals predominantly at the top of the cells and much less pronounced at the bottom. e Representative quantification of P2Y2R signal (red) and DAPI (blue) along the z-axis from the apical to the basal side of the cell. f Quantification of the apical P2Y2R signal intensity in P2Y2R competent (shCtrl) and deficient (shP2Y2R#1 and #2) plMDCK cells in the absence and presence of 10 μM ICA. Asterisk indicates comparison to non-treated control-transfected cells. Section sign indicates comparison to ICA-treated control-transfected cells
P2Y2R mediates chloride secretion of plMDCK cells
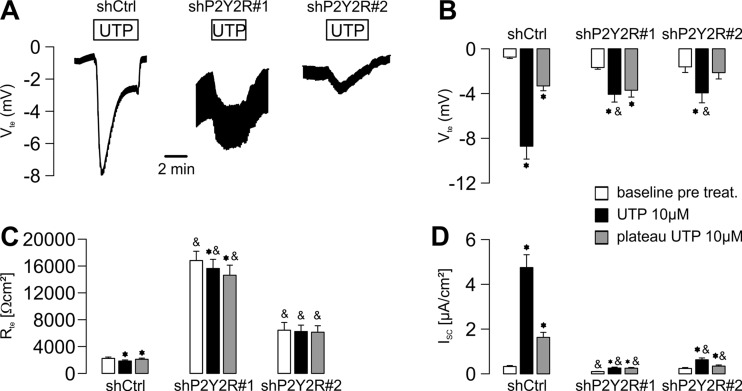
Since cyst growth is largely driven by apical chloride secretion of cyst epithelial cells [3], we next tested if P2Y2R expression affects chloride conductance of plMDCK cells. Recently, we have shown that application of either UTP or ATP at both, the apical and the basolateral side of plMDCK cells, results in transepithelial chloride secretion [23]. In line with these data, control-transfected plMDCK cells showed a strong negative deflection of the transepithelial potential upon addition of apical UTP (Fig. 4a, b) resulting in an increase of the equivalent short-circuit current (Fig. 4d) reflecting apical chloride secretion [23]. Knockdown of P2Y2R mRNA resulted in markedly reduced chloride secretion (Fig. 4a–d). These data indicate that P2Y2R mediates chloride secretion of cyst-forming plMDCK cells.
Fig. 4.
P2Y2R mediates calcium-dependent chloride secretion of plMDCK cells. plMDCK cells were grown as polarized monolayers on permeable supports for 9–15 days. Then, cells were mounted into a perfused micro Ussing chamber. a Representative original recordings of open-circuit Ussing chamber experiments using control-transfected plMDCK cells (shCtrl) and P2Y2R-deficient cells (shP2Y2R#1 and shP2Y2R#2). Application of 10 μM UTP at the luminal side resulted in a strong negative deflection of the transepithelial potential (V te) in control-transfected cells and much less pronounced in P2Y2R-deficient cells. b Quantification of baseline V te prior to application of UTP (white bars), maximum deflection of V te initially after application of 10 μM UTP (black bars), and V te plateau in the presence of UTP (gray bars). c Summary of the corresponding changes in transepithelial resistance (R te). d Summary of the calculated equivalent short-circuit currents (I sc). N = 6. Asterisk indicates comparison to baseline values prior to incubation with UTP. Ampersand indicates comparison to control-transfected cells
P2Y2R is involved in plMDCK cyst growth and mediates ATP- and HIF-dependent cyst expansion
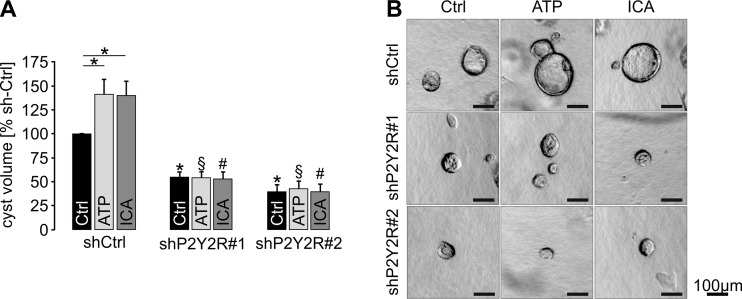
We next tested the impact of P2Y2R on plMDCK cyst growth within a collagen matrix. P2Y2R-deficient cells showed significantly reduced cyst growth under control conditions (Fig. 5). Administration of extracellular ATP resulted in increased cyst sizes of control-transfected cells whereas cyst growth of P2Y2R-deficient cells remained unaffected (Fig. 5). Likewise, ICA augmented enlargement of P2Y2R-competent cysts whereas it had no impact on cyst growth of P2Y2R-deficient cells (Fig. 5). These data reveal a role of P2Y2R in in vitro cyst growth and indicate that P2Y2R mediates ATP- and HIF-dependent cyst expansion.
Fig. 5.
P2Y2R is involved in ATP- and HIF-dependent plMDCK cyst growth. Stably control-transfected plMDCK cells (shCtrl) and plMDCK cells stably deficient for P2Y2R (shP2Y2R#1 and shP2Y2R#2) were grown within a collagen I matrix in the presence of 10 μM forskolin to form cysts for 5 days. Additionally, medium was supplemented with either 10 μM ATP or 10 μM ICA. a Cyst volumes relative to control-tranfected cells incubated with control medium (Ctrl; set 100 %) were determined from seven individual experiments comprising the analysis of approximately 230–400 cysts per condition. b Representative cysts within the collagen matrix at day 5. Asterisk indicates comparison to non-treated control-transfected cysts. Section sign indicates comparison to ATP-treated control-transfected cysts. Number sign indicates comparison to ICA-treated control-transfected cysts
Discussion
Cyst expansion and subsequent compression of adjacent nephrons has been suggested as the main driving force for the decline of renal function in PKD [2]. There is significant interest therefore in identifying mechanisms that retard cyst growth in order to prevent patients from developing renal failure. Strategies that aimed at inhibition of cyst cell proliferation by targeting mTOR have not been successful so far [24, 25]. In contrast, pharmacological inhibition of the vasopressin 2 receptor (V2R) to reduce transepithelial cyst secretion attenuated cyst expansion and improved renal function in ADPKD patients [26]. However, the effects were quantitatively modest and the treatment is associated with relevant side effects. Thus, there remains a need to further unravel molecular mechanisms of cyst expansion that are potentially targetable.
Stimulation of V2R leads to an increase of intracellular cAMP which then results in activation of apical CFTR chloride channels [27]. Besides cAMP-dependent secretion, calcium-activated chloride secretion is also involved in cyst expansion and both act synergistically [3, 10]. Stimulation of calcium-dependent chloride secretion is typically induced by extracellular ATP which has been shown to accumulate in cyst fluids up to concentrations of 10 μM [9]. Ten micromolars ATP is sufficient to stimulate Gq-coupled P2Y-receptors. This leads to an increase of intracellular calcium which then activates calcium-activated chloride channels, including Anoctamin 1 (ANO1) [7, 9, 23, 28]. In addition, we have shown that stimulation of the Gq-coupled P2Y2R also activates CFTR and that ANO1 and CFTR interact functionally [29, 30]. Here, we show that the purinergic receptor P2Y2R mediates ATP-dependent chloride secretion of cyst-forming plMDCK cells. Since P2Y2R is mainly localized in the apical membrane of plMDCK cells and mouse and human cyst epithelium in vivo, it is tempting to speculate that luminal ATP may stimulate apical P2Y2R and thereby activate chloride secretion. However, since P2Y2R is also expressed in the basolateral membrane of plMDCK cells and chloride secretion could be induced by application of ATP at both, the luminal or basolateral membrane of plMDCK cells [23], we cannot rule out that cyst expansion is also promoted by basolateral ATP. Cyst growth may also be affected by the cysts’ micro-environment. Cyst epithelial cells can become severely hypoxic, presumably due to compression of the interstitial vasculature [4, 31, 32]. We have previously shown that hypoxia leads to stabilization of HIF-1α in cyst-lining cells which then promotes calcium-dependent chloride secretion [4, 5]. Since HIF-1α is a transcriptional activator, we postulated that HIF-1α may induce the expression of molecules that are involved in chloride secretion. In line with this assumption, P2Y2R provides the first direct link between hypoxia, HIF-1α, and cyst expansion. Previously, we found ANO1 to be upregulated in vivo upon pharmacological stabilization of HIF-1α [5]. In addition, we could show that ANO1 is involved in cyst growth in vitro [23]. Furthermore, expression of KCa3.1, a basolateral calcium-activated potassium channel which essentially provides the electrochemical driving force for secretion-dependent MDCK cyst expansion [33], was also increased upon HIF stabilization in MDCK cells [5]. However, unlike P2Y2R, both proteins seem to be upregulated in an indirect manner since they are no direct HIF-1α target genes [12]. CFTR expression was neither directly nor indirectly upregulated by HIF-1α [5, 12]. Apart from the impact of oxygen supply, we have also shown recently that glucose concentration affects in vitro cyst growth and transcriptional regulation of ANO1 and P2Y2R, thereby promoting calcium-dependent chloride secretion [34]. Whether glucose-dependent upregulation of P2Y2R is mediated in a HIF-dependent or -independent way deserves further investigation. An ATP-dependent increase of intracellular calcium may not only be mediated by G-protein-coupled P2Y receptors but also by ATP-gated P2X receptors [35]. P2X7R has been shown to be involved in cystogenesis of PKD2-deficient zebrafish [14]. We did not detect HIF-binding signals at the P2X7R gene and other P2X receptor genes in MCF-7 cells suggesting no direct effect of HIF on the expression of this gene. However, this does not exclude an indirect influence of HIF and hypoxia on the function of P2X7R in cyst expansion.
The plMDCK cyst model used in this study has limitations. Since plMDCK cells lack mutations in PKD1 and PKD2, a potential impact of mutations in both genes on P2Y2R remains elusive. Specific pharmacological inhibitors of P2Y2R are not available so far, which precludes an investigation of its potential role in vivo in animal models for PKD. However, there is significant interest and success in developing compounds that specifically target P2 receptor subtypes [36]. Therefore, there is a good chance that selective P2Y2R inhibitors will be available in the future, and we propose that these should then be tested for their effect on cyst growth in vivo. Also, specific HIF inhibitors are not available so far, but our data add to the accumulating evidence that such drugs could be useful for retarding the progression of cystic kidney disease. Since expression of HIF-1α significantly correlates with the progression of the cystic disease [4, 5], inhibition of HIF-dependent pathways could be a therapeutic option in particular for advanced stages of PKD.
In summary, we propose HIF-dependent regulation of P2Y2R as a novel mechanism of cyst expansion. Targeting this pathway may be of interest beyond PKD. For instance, P2Y2R has been reported to play a role in acute ischemic heart injury [37] and hypoxic liver injury [38].
Methods
Cell culture
Human primary tubular epithelial cells (hPTECs) were isolated from renal cortical tissues collected from healthy parts of tumor nephrectomies. Isolated cells represent a mixture of proximal and distal tubular cells with a ratio of approximately 50:50 %, as described previously [15]. Isolation of human cells from healthy parts of tumor nephrectomies was approved by the local ethics committee (Reference number 3755, Ethik-Kommission der Medizinischen Fakultät der Friedrich-Alexander Universität Erlangen-Nürnberg). Cortex tissue was cut into 1 mm3 pieces and digested with collagenase type II (Gibco, Karlsruhe, Germany) and DNase I grade II (Roche Diagnostics, Mannheim, Germany) for 60 min. Next, cell suspension was sieved through 100 and 70 μm meshes. Cells were seeded in epithelial cell selective medium (DMEM/Ham’s F12 medium containing 2 mM L-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, insulin-transferrin-selenium supplement, 10 ng/ml epidermal growth factor, 36 ng/ml hydrocortisone, and 4 pg/ml triiodothyronine) in the presence of 0.5 % FCS. After 1–2 days, medium was replaced by FCS-free medium. plMDCK cells were maintained in modified MEM containing Earl’s balanced salt solution supplemented with 2 mmol/l L-glutamine, 10 % heat-inactivated FCS, 50 IU/ml penicillin, and 50 mg/ml streptomycin.
Chromatin immunoprecipitations and FAIRE assay
ChIP-seq data from MCF-7 breast adenocarcinoma cells were obtained from publicly available data sets (accession numbers: GSE28352 and E-MTAB-1995) as described previously [12, 39]. Chromatin immunoprecipitations (ChIP) and FAIRE assays were performed as described previously [40] using antibodies against HIF-1α (Cayman Chemicals, Cay10006421), HIF-1β (Novus Biologicals, NB100-110) and RNA polymerase II (Santa Cruz, SC-899). Control serum was used as negative control.
Generation of P2Y2R-deficient plMDCK cells
Primers complementary to two distinct regions of Canis familiaris P2Y2R (accession number XP_542321.2) were cloned BglII and XhoI into pSUPERIOR vector (Oligoengine, Seattle, WA). Correct cloning was verified by sequencing. As a negative control, pSUPERIOR containing a scrambled sequence was purchased from Oligoengine. plMDCK cells were transfected with Fugene (Roche, Mannheim, Germany) according to the manufacturer’s instructions. Colonies were picked after 2 weeks of treatment with G-418 (500 μg/ml; PAA Laboratories, Coelbe, Germany).
Primer sequences used for shRNA directed against P2Y2R
The following primers were used for shP2Y2R: 5′-GGATCCCCGGGATGAGCTAGGCTACAA GTTTCAAGAGAACTTGTAGCCTAGCTCATCCCTTTTTCTCGAG-3′ and 3′-CCTAGGGGCC CTACTCGATCCGATGTTCAAAGTTCTCTTGAACATCGGATCGAGTAGGGAAAAAGAGCTC-5′ (sequence 1); and 5′-GGATCCCCGCCGTTTCAATGAGGACTTCATTCAAGAGATGAA GTCCTCATTGAAACGGCTTTTTCTCGAG-3′ and 3′-CCTAGGGGCGGCAAAGTTACTCCT GAAGTAAGTTCTCTACTTCAGGAGTAACTTTGCCGAAAAAGAGCTC-5′ (sequence 2).
Analysis of knockdown efficiency
RNA preparation from P2Y2R deficient plMDCK cells was performed by the use of the peqGOLD TriFast (peqlab/VWR International GmbH, Erlangen, Germany) according to the manufacturer’s instructions. SYBR-Green-based real-time PCR was performed using StepOnePlus (Applied Biosystems, Foster City, CA, USA). mRNA expression levels were normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) using the ΔΔCt method. All primer sequences are listed in Supplemental Table S1.
Protein was extracted from plMDCK cells using RIPA buffer (1 % Trition X-100, 1 % sodium deoxycholate, 0.1 % SDS, 150 mM NaCl, 10 mM EDTA, 50 mM Tris/HCl pH 7.2). Thirty micrograms of proteins were used for immunodetection of P2Y2R. Proteins were probed with primary P2Y2R goat-polyclonal antibody (1:1000; Santa Cruz Biotechnology Inc., Dallas, TX, USA). Binding of the primary antibody was visualized by incubation with horseradish peroxidase-conjugated secondary polyclonal rabbit anti-goat antibody (1:1000, Dako, Hamburg, Germany). P2Y2R signals were normalized to ß-actin stain (Sigma-Aldrich).
Collection of human renal ADPKD tissue and patient characteristics
Kidney specimens of seven patients (six men, one woman; age, 55.6 ± 9.3 years (mean ± s.d.)) were obtained as described previously [23]. Briefly, tissue was fixed immediately after nephrectomy in 3 % paraformaldehyde (pH 7.4). Six patients were on hemodialysis at the time of nephrectomy, thus representing rather late stages of ADPKD. Collection and analysis of tissue samples were approved by the local ethics committee.
Experimental animals
All animal experiments were approved by local government authorities and conformed to the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. In this study, kidney sections of a tamoxifen-inducible kidney epithelium-specific Pkd1 deletion mouse model were used. Upon administration of tamoxifen to these mice (tam-KspCad-Cre;Pkd1lox;lox), a genomic fragment containing exons 2–11 of the Pkd1 gene is specifically deleted in renal epithelial cells and cysts are formed as described previously [41]. Tamoxifen (2 mg dissolved in 10 μl ethanol and 190 μl sunflower oil; Sigma-Aldrich) was administered for three consecutive days to mice at postnatal day 35 via intraperitoneal injection. Kidneys were extracted 90 days after induction with tamoxifen. Generation of P2Y2R knockout mice has been described previously [42].
plMDCK cyst model
In vitro cyst assays were performed as described previously [43]. In brief, plMDCK cells were resuspended as a single-cell suspension in type I collagen and filled into 24-well plates (3–6 wells per condition). Forskolin (Sigma-Aldrich) was added to the medium at day 0 and medium was changed every 2 days. After 5 days, two random visual fields per well were photographed with a Zeiss Primo Vert microscope and a Zeiss Axiocam 105 color camera (both Zeiss Microscopy GmbH, Jena, Germany). Cyst diameters of all captured cysts that were nearly spherical were measured with ImageJ (V. 1.48) and the use of a Wacom Tablet device. Cyst volume was then estimated using the formula for the volume of a sphere, 4/3πr 3.
Immunofluorescence staining
Confluent and polarized plMDCK cells kept on permeable inserts (Millicell, Millipore, Schwalbach, Germany) were rinsed in phosphate-buffered saline (PBS) supplemented with 0.9 mmol/l CaCl2 and 0.49 mmol/l MgCl2 (PBS+) and then fixed with paraformaldehyde (4 %). Glycine (200 mmol/l) in PBS+ was added to quench the excess aldehyde. Cells as well as human and mouse kidney sections were stained with P2Y2R goat-polyclonal antibody (1:500, Santa Cruz Biotechnology Inc., Dallas, TX, USA). Binding of the primary antibody was visualized by incubation with secondary polyclonal rabbit anti-goat antibody (1:1000, Dako, Hamburg, Germany) conjugated with AlexaFluor 488 (1:1000, Molecular Probes, Invitrogen, Darmstadt, Germany). Signals were captured with a BZ-9000 microscope (Keyence, Osaka, Japan), and the background correction algorithm in ImageJ (V.1.48) was applied.
Quantification of P2Y2R intensities in plMDCK cells
To quantify the fluorescence intensities, polarized plMDCK cells were stained for P2Y2R and photos were taken along the z-axis ranging from the apical to the basal membrane and vice versa to exclude bleaching artefacts by the use of a BZ-9000 microscope (Keyence, Osaka, Japan) in combination with a deconvolution algorithm of the BZ-9000 analyzer software (V.2.1., Keyence, Japan). Next, regions of interest (ROIs) were set for each cell (n = 5–7 per condition and single experimental procedure with a total of three individual experiments), and signal intensities of each ROI were acquired by blotting the histogram profile along the z-axis (ImageJ; V.1.48).
Ussing chamber experiments
plMDCK cells were grown as polarized monolayers on permeable supports (Millipore). Cells then were mounted into a perfused micro Ussing chamber, and the luminal and basolateral surfaces of the epithelium were perfused continuously with ringer solution [in mM: NaCl (145), KH2PO4 (0.4), K2HPO4 (1.6), glucose (5.6), MgCl2 (1), Ca-gluconate (1.3)] at a rate of 6 ml/min (chamber volume 2 ml). All experiments were carried out at 37 °C under open-circuit conditions. Transepithelial resistance (R te) was determined by applying short (1 s) current pulses (ΔI = 0.5 μA), and the corresponding changes in transepithelial voltage (V te) were recorded continuously. Values for V te were referred to the serosal side of the epithelium. R te was calculated according to Ohm’s law (R te = ΔV te/ΔI). The equivalent short-circuit current (I′sc) was calculated according to Ohm’s law from V te and R te (I′sc = V te/R te).
Statistical analysis
Data are expressed as mean ± SEM. The differences among groups were analyzed using one-way ANOVA, followed by a Bonferroni test for multiple comparisons. An unpaired t test was applied to compare the differences between two groups; a paired t test was used for matched observations. Wilcoxon signed-rank test for column statistics was used for relative values. P < 0.05 was considered statistically significant.
Electronic supplementary material
(DOCX 11 kb)
P2Y2R is expressed in collecting ducts of wildtype mouse kidneys. A Wildtype mouse kidney sections were stained for P2Y2R (red), nuclei (DAPI; blue) and DBA (green) showing apical staining of P2Y2R in axial (upper row) and cross sections (lower row) of DBA-positive tubules. B P2Y2R knockout kidneys were stained for P2Y2R (red), nuclei (DAPI; blue) and DBA (green). P2Y2R signal is absent in axial (upper row) and cross sections (lower row) of DBA-positive tubules. (JPEG 1942 kb)
Acknowledgments
We thank Barbara Teschemacher for excellent technical support. BB was supported by the Deutsche Forschungsgemeinschaft (DFG BU2918/2-1), the Else Kroener-Fresenius-Stiftung (2013_A299), the Center for Kidney and Blood Pressure Research Regensburg-Erlangen-Nuremberg (REN), and the Interdisciplinary Center for Clinical Research Erlangen (project F5). RS and KK were supported by the DFG (SFB699 A7/A12 and DFG KU756/12-1). JS received funding by the DFG (SCHO 1598/1) and the Else Kroener-Fresenius Stiftung (2014_EKES.11). The present work was performed by AK in fulfillment of the requirements for obtaining the degree “Dr. rer. nat.”
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369(9569):1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 2.Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2011;7(10):556–566. doi: 10.1038/nrneph.2011.109. [DOI] [PubMed] [Google Scholar]
- 3.Terryn S, Ho A, Beauwens R, Devuyst O. Fluid transport and cystogenesis in autosomal dominant polycystic kidney disease. Biochim Biophys Acta. 2011;1812(10):1314–1321. doi: 10.1016/j.bbadis.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Bernhardt WM, Wiesener MS, Weidemann A, Schmitt R, Weichert W, Lechler P, Campean V, Ong AC, Willam C, Gretz N, Eckardt KU. Involvement of hypoxia-inducible transcription factors in polycystic kidney disease. Am J Pathol. 2007;170(3):830–842. doi: 10.2353/ajpath.2007.060455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchholz B, Schley G, Faria D, Kroening S, Willam C, Schreiber R, Klanke B, Burzlaff N, Jantsch J, Kunzelmann K, Eckardt KU. Hypoxia-inducible factor-1alpha causes renal cyst expansion through calcium-activated chloride secretion. J Am Soc Nephrol. 2014;25(3):465–474. doi: 10.1681/ASN.2013030209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kunzelmann K, Tian Y, Martins JR, Faria D, Kongsuphol P, Ousingsawat J, Thevenod F, Roussa E, Rock J, Schreiber R. Anoctamins. Pflugers Arch. 2011;462(2):195–208. doi: 10.1007/s00424-011-0975-9. [DOI] [PubMed] [Google Scholar]
- 7.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol. 2003;284(3):F419–F432. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- 8.Namkung W, Finkbeiner WE, Verkman AS. CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol Biol Cell. 2010;21(15):2639–2648. doi: 10.1091/mbc.E09-12-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson PD, Hovater JS, Casey CC, Fortenberry JA, Schwiebert EM. ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys. J Am Soc Nephrol. 1999;10(2):218–229. doi: 10.1681/ASN.V102218. [DOI] [PubMed] [Google Scholar]
- 10.Buchholz B, Teschemacher B, Schley G, Schillers H, Eckardt KU. Formation of cysts by principal-like MDCK cells depends on the synergy of cAMP- and ATP-mediated fluid secretion. J Mol Med (Berl) 2011;89(3):251–261. doi: 10.1007/s00109-010-0715-1. [DOI] [PubMed] [Google Scholar]
- 11.Turner CM, King BF, Srai KS, Unwin RJ. Antagonism of endogenous putative P2Y receptors reduces the growth of MDCK-derived cysts cultured in vitro. Am J Physiol Renal Physiol. 2007;292(1):F15–F25. doi: 10.1152/ajprenal.00103.2006. [DOI] [PubMed] [Google Scholar]
- 12.Schodel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011;117(23):e207–e217. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rangan G. Role of extracellular ATP and P2 receptor signaling in regulating renal cyst growth and interstitial inflammation in polycystic kidney disease. Front Physiol. 2013;4:218. doi: 10.3389/fphys.2013.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang MY, JK L, Tian YC, Chen YC, Hung CC, Huang YH, Chen YH, MS W, Yang CW, Cheng YC. Inhibition of the P2X7 receptor reduces cystogenesis in PKD. J Am Soc Nephrol. 2011;22(9):1696–1706. doi: 10.1681/ASN.2010070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keller C, Kroening S, Zuehlke J, Kunath F, Krueger B, Goppelt-Struebe M. Distinct mesenchymal alterations in N-cadherin and E-cadherin positive primary renal epithelial cells. PLoS One. 2012;7(8):e43584. doi: 10.1371/journal.pone.0043584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishore BK, Ginns SM, Krane CM, Nielsen S, Knepper MA. Cellular localization of P2Y (2) purinoceptor in rat renal inner medulla and lung. Am J Physiol Renal Physiol. 2000;278(1):F43–F51. doi: 10.1152/ajprenal.2000.278.1.F43. [DOI] [PubMed] [Google Scholar]
- 17.Lehrmann H, Thomas J, Kim SJ, Jacobi C, Leipziger J. Luminal P2Y2 receptor-mediated inhibition of Na+ absorption in isolated perfused mouse CCD. J Am Soc Nephrol. 2002;13(1):10–18. doi: 10.1681/ASN.V13110. [DOI] [PubMed] [Google Scholar]
- 18.Raphael KL, Strait KA, Stricklett PK, Miller RL, Nelson RD, Piontek KB, Germino GG, Kohan DE. Inactivation of Pkd1 in principal cells causes a more severe cystic kidney disease than in intercalated cells. Kidney Int. 2009;75(6):626–633. doi: 10.1038/ki.2008.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres VE. Cyclic AMP, at the hub of the cystic cycle. Kidney Int. 2004;66(3):1283–1285. doi: 10.1111/j.1523-1755.2004.00945.x. [DOI] [PubMed] [Google Scholar]
- 20.Xu C, Shmukler BE, Nishimura K, Kaczmarek E, Rossetti S, Harris PC, Wandinger-Ness A, Bacallao RL, Alper SL. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol. 2009;296(6):F1464–F1476. doi: 10.1152/ajprenal.90542.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Agati V, Trudel M. Lectin characterization of cystogenesis in the SBM transgenic model of polycystic kidney disease. J Am Soc Nephrol. 1992;3(4):975–983. doi: 10.1681/ASN.V34975. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Schley G, Turkoglu G, Burzlaff N, Amann KU, Willam C, Eckardt KU, Bernhardt WM. The protective effect of prolyl-hydroxylase inhibition against renal ischaemia requires application prior to ischaemia but is superior to EPO treatment. Nephrol Dial Transplant. 2012;27(3):929–936. doi: 10.1093/ndt/gfr379. [DOI] [PubMed] [Google Scholar]
- 23.Buchholz B, Faria D, Schley G, Schreiber R, Eckardt KU, Kunzelmann K. Anoctamin 1 induces calcium-activated chloride secretion and proliferation of renal cyst-forming epithelial cells. Kidney Int. 2014;85(5):1058–1067. doi: 10.1038/ki.2013.418. [DOI] [PubMed] [Google Scholar]
- 24.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wuthrich RP. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363(9):820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 25.Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Horl WH, Obermuller N, Arns W, Pavenstadt H, Gaedeke J, Buchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363(9):830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 26.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS, Investigators TT. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reif GA, Yamaguchi T, Nivens E, Fujiki H, Pinto CS, Wallace DP. Tolvaptan inhibits ERK-dependent cell proliferation, Cl(−) secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am J Physiol Renal Physiol. 2011;301(5):F1005–F1013. doi: 10.1152/ajprenal.00243.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunzelmann K, Cabrita I, Wanitchakool P, Ousingsawat J, Sirianant L, Benedetto R, Schreiber R. Modulating Ca(2+) signals: a common theme for TMEM16, Ist2, and TMC. Pflugers Arch. 2016;468(3):475–490. doi: 10.1007/s00424-015-1767-4. [DOI] [PubMed] [Google Scholar]
- 29.Faria D, Schreiber R, Kunzelmann K. CFTR is activated through stimulation of purinergic P2Y2 receptors. Pflugers Arch. 2009;457(6):1373–1380. doi: 10.1007/s00424-008-0606-2. [DOI] [PubMed] [Google Scholar]
- 30.Ousingsawat J, Kongsuphol P, Schreiber R, Kunzelmann K. CFTR and TMEM16A are separate but functionally related Cl- channels. Cell Physiol Biochem. 2011;28(4):715–724. doi: 10.1159/000335765. [DOI] [PubMed] [Google Scholar]
- 31.Bello-Reuss E, Holubec K, Rajaraman S. Angiogenesis in autosomal-dominant polycystic kidney disease. Kidney Int. 2001;60(1):37–45. doi: 10.1046/j.1523-1755.2001.00768.x. [DOI] [PubMed] [Google Scholar]
- 32.Ow CP, Abdelkader A, Hilliard LM, Phillips JK, Evans RG. Determinants of renal tissue hypoxia in a rat model of polycystic kidney disease. Am J Physiol Regul Integr Comp Physiol. 2014;307(10):R1207–R1215. doi: 10.1152/ajpregu.00202.2014. [DOI] [PubMed] [Google Scholar]
- 33.Albaqumi M, Srivastava S, Li Z, Zhdnova O, Wulff H, Itani O, Wallace DP, Skolnik EY. KCa3.1 potassium channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;74(6):740–749. doi: 10.1038/ki.2008.246. [DOI] [PubMed] [Google Scholar]
- 34.Kraus A, Schley G, Kunzelmann K, Schreiber R, Peters DJ, Stadler R, Eckardt KU, Buchholz B. Glucose promotes secretion-dependent renal cyst growth. J Mol Med (Berl) 2016;94(1):107–117. doi: 10.1007/s00109-015-1337-4. [DOI] [PubMed] [Google Scholar]
- 35.Booth JW, Tam FW, Unwin RJ. P2 purinoceptors: renal pathophysiology and therapeutic potential. Clin Nephrol. 2012;78(2):154–163. doi: 10.5414/CN107325. [DOI] [PubMed] [Google Scholar]
- 36.Felix RA, Martin S, Pinion S, Crawford DJ. Development of a comprehensive set of P2 receptor pharmacological research compounds. Purinergic Signal. 2012;8(Suppl 1):101–112. doi: 10.1007/s11302-011-9270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hochhauser E, Cohen R, Waldman M, Maksin A, Isak A, Aravot D, Jayasekara PS, Muller CE, Jacobson KA, Shainberg A. P2Y2 receptor agonist with enhanced stability protects the heart from ischemic damage in vitro and in vivo. Purinergic Signal. 2013;9(4):633–642. doi: 10.1007/s11302-013-9374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carini R, Alchera E, De Cesaris MG, Splendore R, Piranda D, Baldanzi G, Albano E. Purinergic P2Y2 receptors promote hepatocyte resistance to hypoxia. J Hepatol. 2006;45(2):236–245. doi: 10.1016/j.jhep.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Choudhry H, Schodel J, Oikonomopoulos S, Camps C, Grampp S, Harris AL, Ratcliffe PJ, Ragoussis J, Mole DR. Extensive regulation of the non-coding transcriptome by hypoxia: role of HIF in releasing paused RNApol2. EMBO Rep. 2014;15(1):70–76. doi: 10.1002/embr.201337642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schodel J, Bardella C, Sciesielski LK, Brown JM, Pugh CW, Buckle V, Tomlinson IP, Ratcliffe PJ, Mole DR. Common genetic variants at the 11q13.3 renal cancer susceptibility locus influence binding of HIF to an enhancer of cyclin D1 expression. Nat Genet. 2012;44(4):420–425. doi: 10.1038/ng.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lantinga-van Leeuwen IS, Leonhard WN, van de Wal A, Breuning MH, Verbeek S, de Heer E, Peters DJ. Transgenic mice expressing tamoxifen-inducible Cre for somatic gene modification in renal epithelial cells. Genesis. 2006;44(5):225–232. doi: 10.1002/dvg.20207. [DOI] [PubMed] [Google Scholar]
- 42.Matos JE, Robaye B, Boeynaems JM, Beauwens R, Leipziger J. K+ secretion activated by luminal P2Y2 and P2Y4 receptors in mouse colon. J Physiol. 2005;564(Pt 1):269–279. doi: 10.1113/jphysiol.2004.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchholz B, Klanke B, Schley G, Bollag G, Tsai J, Kroening S, Yoshihara D, Wallace DP, Kraenzlin B, Gretz N, Hirth P, Eckardt KU, Bernhardt WM. The Raf kinase inhibitor PLX5568 slows cyst proliferation in rat polycystic kidney disease but promotes renal and hepatic fibrosis. Nephrol Dial Transplant. 2011;26(11):3458–3465. doi: 10.1093/ndt/gfr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 11 kb)
P2Y2R is expressed in collecting ducts of wildtype mouse kidneys. A Wildtype mouse kidney sections were stained for P2Y2R (red), nuclei (DAPI; blue) and DBA (green) showing apical staining of P2Y2R in axial (upper row) and cross sections (lower row) of DBA-positive tubules. B P2Y2R knockout kidneys were stained for P2Y2R (red), nuclei (DAPI; blue) and DBA (green). P2Y2R signal is absent in axial (upper row) and cross sections (lower row) of DBA-positive tubules. (JPEG 1942 kb)