Abstract
Guanosine, a guanine-based purine, has been shown to exert beneficial roles in in vitro and in vivo injury models of neural cells. Guanosine is released from astrocytes and modulates important astroglial functions, including glutamatergic metabolism, antioxidant, and anti-inflammatory activities. Astrocytes are crucial for regulating the neurotransmitter system and synaptic information processes, ionic homeostasis, energy metabolism, antioxidant defenses, and the inflammatory response. Aging is a natural process that induces numerous changes in the astrocyte functionality. Thus, the search for molecules able to reduce the glial dysfunction associated with aging may represent an approach for avoiding the onset of age-related neurological diseases. Hence, the aim of this study was to evaluate the anti-aging effects of guanosine, using primary astrocyte cultures from newborn, adult, and aged Wistar rats. Concomitantly, we evaluated the role of heme oxygenase 1 (HO-1) in guanosine-mediated glioprotection. We observed age-dependent changes in glutamate uptake, glutamine synthetase (GS) activity, the glutathione (GSH) system, pro-inflammatory cytokine (tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β)) release, and the transcriptional activity of nuclear factor kB (NFkB), which were prevented by guanosine in an HO-1-dependent manner. Our findings suggest guanosine to be a promising therapeutic agent able to provide glioprotection during the aging process. Thus, this study contributes to the understanding of the cellular and molecular mechanisms of guanosine in the aging process.
Keywords: Aging, Adult/aged astrocytes, Guanosine, Heme oxygenase 1
Introduction
Guanine-based purines are known to act as extracellular signaling molecules, exerting trophic and neuroprotective roles in in vitro and in vivo experimental models [1–5]. Guanosine, more specifically, has been shown to induce numerous beneficial cellular responses in several brain injuries, such as seizures, hypoxia, anxiety-like behavior, ischemia, and glucose deprivation [1, 6–9]. In addition to demonstrating the ability to modulate glutamatergic metabolism, avoiding the overactivation of glutamate receptors, and exerting antioxidant and anti-inflammatory activities [10–12], guanosine can also modulate several signaling pathways to provide neuroprotection [1, 13, 14]. However, despite the increasing evidence of the protective effects of guanosine in neural cells, its mechanism of action is not fully understood.
Our group has previously demonstrated the interplay between guanosine and the enzyme heme oxygenase 1 (HO-1) [12, 14], which is the major enzyme responsible for the conversion of heme into CO and the antioxidant products biliverdin and bilirubin [15, 16]. It has been reported that HO-1 may be a therapeutic target in the aging process and/or neurodegenerative diseases. Increased HO-1 activity correlates with protection against stressful conditions, such as hypoxia/ischemia, oxidative stress, and neuroinflammation [12, 15, 16]. Furthermore, HO-1 counteracts the transcriptional activity of nuclear factor kappa B (NFkB), which is the master regulator of oxidative stress and the inflammatory response [17–19].
Aging is a natural process that induces numerous changes in the brain functionality, including alterations in synaptic efficacy, changes in neuron-glia communication with consequent impairment in cerebral activities, and increases in reactive oxygen species (ROS) and inflammatory mediators [20–22]. Understanding and managing these alterations may be an important strategy to extend a healthy life span. As such, the association among oxidative stress, inflammation, and aging is based on complex molecular and cellular changes that have only just begun to be understood. As the HO-1 signaling pathway appears to play a role in some of these changes, this enzyme emerges an important therapeutic target to protect against the aging process [15].
Astrocytes, the main class of glial cells, participate in a diverse range of central nervous system (CNS) functions, including the regulation of neurotransmitter systems, synaptic information processing (as part of the tripartite synapse), ionic homeostasis, energy metabolism, antioxidant defenses, and the inflammatory response [23–27]. Thus, to study features of adult and aged brains, our group has established a routine technique for the primary culture of astrocytes obtained from non-neonatal rats, because the metabolic, oxidative, and inflammatory properties of cells derived from newborn and adult/aged animals are distinctive [21, 22, 28, 29]. We have previously shown that this tool can be employed to study different astrocytic roles in the CNS, as the cells present classical astroglial markers, such as glial fibrillary acidic protein (GFAP), S100B, and glutamine synthetase (GS) expression and activity. Moreover, they express glutamate transporters, such as the glutamate-aspartate transporter (GLAST) and glutamate transporter 1 (GLT-1), being able to respond to neuroprotective and neurotoxic stimuli [27, 30, 31].
Considering the essential role of astrocytes in brain functionality and the differences between immature and aged brain as well as the protective effects of guanosine on glial cells, the aim of this study was to evaluate the anti-aging effects of guanosine in primary astrocyte cultures. We evaluated glutamate uptake, GS activity, glutathione (GSH), and glutathione disulfide (GSSG) levels, the activities of glutathione peroxidase (GPx), reductase (GR), and glutamate cysteine ligase (GCL), levels of tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β), and the transcriptional activity of NFkB p65 in cortical primary astrocyte cultures from newborn, adult, and aged Wistar rats (1, 90, and 180 days old, respectively). Additionally, we investigated whether HO-1 is involved in the glioprotective effects of guanosine.
Materials and methods
Chemicals
Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12) and other materials for cell culture were purchased from Gibco/Invitrogen (Carlsbad, CA, USA). Papain was acquired from Merck (Darmstadt, Germany). L-[3H]-glutamate was from Amersham/GE Healthcare (Little Chalfont, UK). DNase, cysteine, albumin, γ-glutamylhydroxamate, reduced glutathione, zinc protoporphyrin IX (ZnPP IX), and guanosine were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were from common commercial suppliers.
Animals
Male Wistar rats (1, 90, and 180 days old) were obtained from our breeding colony (Department of Biochemistry, UFRGS, Brazil), maintained under a controlled environment (12-h light/12-h dark cycle; 22 ± 1 °C; ad libitum access to food and water). All animal experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Federal University of Rio Grande do Sul Animal Care and Use Committee (process number 24419).
Cell culture preparation and maintenance
Male Wistar rats (1, 90, and 180 days old) were sacrificed by decapitation and had their cerebral cortices aseptically dissected and meninges removed. The tissue was digested using trypsin only—for newborn tissue—or trypsin and papain—for adult tissue—at 37 °C as previously described [21]. After mechanical dissociation and centrifugation, the cells were resuspended in DMEM/F12 [10 % fetal bovine serum (FBS), 15 mM HEPES, 14.3 mM NaHCO3, 1 % Fungizone®, and 0.04 % gentamicin], plated on 6- or 24-well plates precoated with poly-L-lysine and cultured at 37 °C in a 5 % CO2 incubator. The cells were seeded at a density of 3–5 × 105 cells/cm2. Twenty-four hours later, the culture medium was exchanged; during the first week, the medium was replaced once every 2 days, and from the second week on, once every 4 days. From the third week on, the astrocytes received medium supplemented with 20 % FBS until they reached confluence (at approximately the fourth week). No dibutyryl cAMP was added to the culture medium in order to observe the naive response of the cells. Specific proteins of neurons and microglia were examined in order to determine the purity of the astrocyte culture, which was around 95 % (data not shown).
Cellular treatments
In order to investigate how astrocytes react to guanosine stimulus, we treated astrocyte cultures with 100 μM of guanosine for 24 h in DMEM/F12 with 1 % FBS at 37 °C in a 5 % CO2 incubator. This concentration and time are in accordance with previous studies [14]. To explore the involvement of HO-1 signaling pathway in the glioprotective effects of guanosine, we previously incubated astrocytes with 10 μM of ZnPP IX (a HO-1 inhibitor) for 1 h, in the presence or absence of guanosine. After cellular treatments, the evaluations described below were performed. To measure membrane integrity, cells were incubated with propidium iodide. To evaluate glutamate uptake after L-[2,3-3H] glutamate incorporation, cells were lysed with NaOH. For GS activity and GSH levels, cells were lysed in a sodium phosphate buffer with KCl (140 mM). The extracellular medium was used to measure cytokines’ release. The nuclear fraction from cell cultures was isolated and used to measure NFkB p65 levels.
Immunofluorescence analysis
Immunofluorescence was performed as described previously by our group [27]. Briefly, cell cultures were fixed with 4 % paraformaldehyde for 20 min and permeabilized with 0.1 % Triton X-100 in PBS for 5 min at room temperature. After blocking overnight with 4 % albumin, the cells were incubated overnight with anti-GFAP (1:400) and anti-GLT-1 (1:400) at 4 °C; this was followed by PBS washes and incubation with a specific secondary antibody conjugated with Alexa Fluor® 488 (green staining) for 1 h at room temperature. For all immunostaining-negative controls, the reactions were performed by omitting the primary antibody. No reactivity was observed when the primary antibody was excluded. Cell nuclei were stained with 0.2 μg/ml of 4′,6′-diamidino-2-phenylindole (DAPI). The cells were visualized with a Nikon inverted microscope, and the images were transferred to a computer with a digital camera (Sound Vision Inc.).
Membrane integrity
Membrane integrity was assessed by fluorescent image analysis (Nikon inverted microscope using a TE-FM Epi-Fluorescence accessory) of propidium iodide (PI) uptake (at 7.5 μM) at 37 °C in an atmosphere of 5 % CO2 in DMEM/F12 supplemented with 1 % FBS.
Glutamate uptake
The glutamate uptake was performed as previously described with some modifications [27]. Briefly, the cells were rinsed once with PBS and were incubated at 37 °C in Hank’s balanced salt solution (HBSS) containing the following components (in mM): 137 NaCl, 5.36 KCl, 1.26 CaCl2, 0.41 MgSO4, 0.49 MgCl2, 0.63 Na2HPO4, 0.44 KH2PO4, 4.17 NaHCO3, and 5.6 glucose, adjusted to pH 7.4. The assay was started by the addition of 0.1 mM L-glutamate and 0.33 μCi/ml L-[2,3-3H] glutamate. The incubation was stopped after 7 min by removal of the medium and rinsing twice the cells with ice-cold HBSS. The cells were then lysed in a solution containing 0.5 M NaOH. Incorporated radioactivity was measured in a scintillation counter. Sodium-independent uptake was determined using ice-cold N-methyl-D-glucamine instead of sodium chloride. Sodium-dependent glutamate uptake was obtained by subtracting the sodium-independent uptake from the total uptake.
Glutamine synthetase activity
The enzymatic assay was performed as previously described [27]. Briefly, cell homogenate (0.1 ml) was added to 0.1 ml of the reaction mixture containing (in mM) 10 MgCl2, 50 L-glutamate, 100 imidazole-HCl buffer (pH 7.4), 10 2-mercaptoethanol, 50 hydroxylamine-HCl, and 10 ATP and incubated for 15 min (37 °C). The reaction was stopped by the addition of 0.4 ml of a solution containing (in mM) 370 ferric chloride, 670 HCl, and 200 trichloroacetic acid. After centrifugation, the absorbance of the supernatant was measured at 530 nm and compared to the absorbance generated using standard quantities of γ-glutamylhydroxamate treated with a ferric chloride reagent. The activity was expressed as μmol/mg protein/h.
Glutathione content
GSH and GSSG levels were assessed as previously described [27]. Astrocyte lysate suspended in a sodium phosphate buffer with 140 mM KCl was diluted containing a 100 mM sodium phosphate buffer (pH 8.0) containing 5 mM EDTA. To measure GSH, the protein was precipitated with 1.7 % meta-phosphoric acid. The supernatant was assayed with o-phthaldialdehyde (at a concentration of 1 mg/ml methanol) at 22 °C for 15 min. Fluorescence was measured using excitation and emission wavelengths of 350 and 420 nm, respectively. A calibration curve was performed with standard GSH solutions at concentrations ranging from 0 to 500 μM. The results are expressed in nmol/mg protein.
To measure GSSG levels, cell lysate was incubated during 20 min with 0.05 M of N-ethylmaleimide. After, 1 M NaOH was added as well as o-phthaldialdehyde (at a concentration of 1 mg/ml methanol) at 22 °C for 15 min. Fluorescence was measured using excitation and emission wavelengths of 350 and 420 nm, respectively. A calibration curve was performed with standard GSSG solutions at concentrations ranging from 0 to 50 μM. The results are expressed in nmol/mg protein.
Glutathione peroxidase activity
GPx (EC 1.11.1.9) activity was measured using the RANSEL kit from Randox (Autrim, UK). The concentration of GPx in the lysed cells was assessed as the decrease in absorption at 340 nm, reflecting the oxidation of NADPH to NADP+, which occurs during the conversion of GSH to GSSG. The results are expressed as U/mg protein (U = international unit, in accordance with the kit).
Glutathione reductase activity
GR (EC 1.8.1.7) activity was measured using the Randox commercial kit (Autrim, UK). The enzyme activity was determined after monitoring the NADPH disappearance at 340 nm in medium containing 200 mM sodium phosphate buffer, pH 7.5, 6.3 mM EDTA, 1 mM GSSG, 0.1 mM NADPH, and cell lysate. The results are expressed as U/mg protein (U = international unit, in accordance with the kit).
Glutamate cysteine ligase activity
GCL (EC 6.3.2.2) was assayed according to Seelig et al., with slight modifications [32]. Cell lysate, suspended in a sodium phosphate buffer containing 140 mM KCl, was diluted with 100 mM sodium phosphate buffer (pH 8.0) containing 5 mM EDTA. The enzyme activity was determined after monitoring the NADH oxidation at 340 nm in sodium phosphate/KCl (pH 8.0) containing 5 mM Na2-ATP, 2 mM phosphoenolpyruvate, 10 mM L-glutamate, 10 mM L-α-aminobutyrate, 20 mM MgCl2, 2 mM Na2-EDTA, 0.2 mM NADH, and 17 μg of pyruvate kinase/lactate dehydrogenase. The results are expressed in nmol/mg protein/min.
Inflammatory response
The TNF-α levels were measured in extracellular medium using rat TNF-α ELISA kit from PeproTech. The levels of IL-1β were measured in extracellular medium using rat ELISA kit from eBioscience. The results are expressed in ng/ml.
NFkB transcriptional activity
The levels of NFkB p65 in the nuclear fraction determine the activity of NFkB. Astrocyte nuclear fraction was isolated from lysed cells with Igepal CA-630 and centrifugation (following the manufacturer’s instructions) and assayed using an ELISA commercial kit from Invitrogen (USA). The results were expressed as percentages relative to the basal conditions.
Protein assay
Protein content was measured using bicinchoninic acid method with bovine serum albumin as a standard [33].
Statistical analyses
Data were statistically analyzed using two-way analysis of variance (ANOVA), followed by the Tukey’s test. P values <0.05 were considered significant. All analyses were performed using the Statistical Package for Social Sciences (SPSS) software version 17.0.
Results
Adult astrocytes present typical glial markers
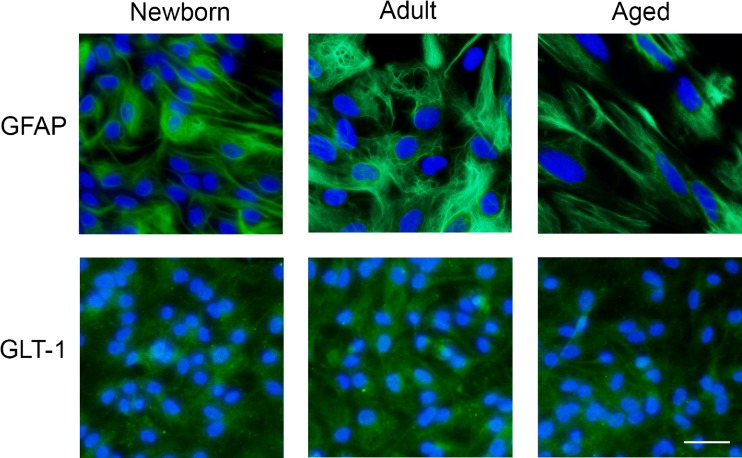
In order to confirm the purity of the cultures, we performed immunofluorescence for GFAP and GLT-1 (Fig. 1), two classical markers of cytoskeleton and glutamate transport, respectively, attesting the astrocytic phenotype of our cultures.
Fig. 1.
Astrocytic characterization. Cortical astrocytes present GFAP and GLT-1. Representative images of astrocytes from newborn, adult, and aged Wistar rats show intense cytoplasmic immunolabeling for GFAP (95 % GFAP-positive cells) and GLT-1. Immunofluorescence was performed as described in the “Materials and methods” section. All images are representative fields from three independent experiments. Scale bar = 50 μm
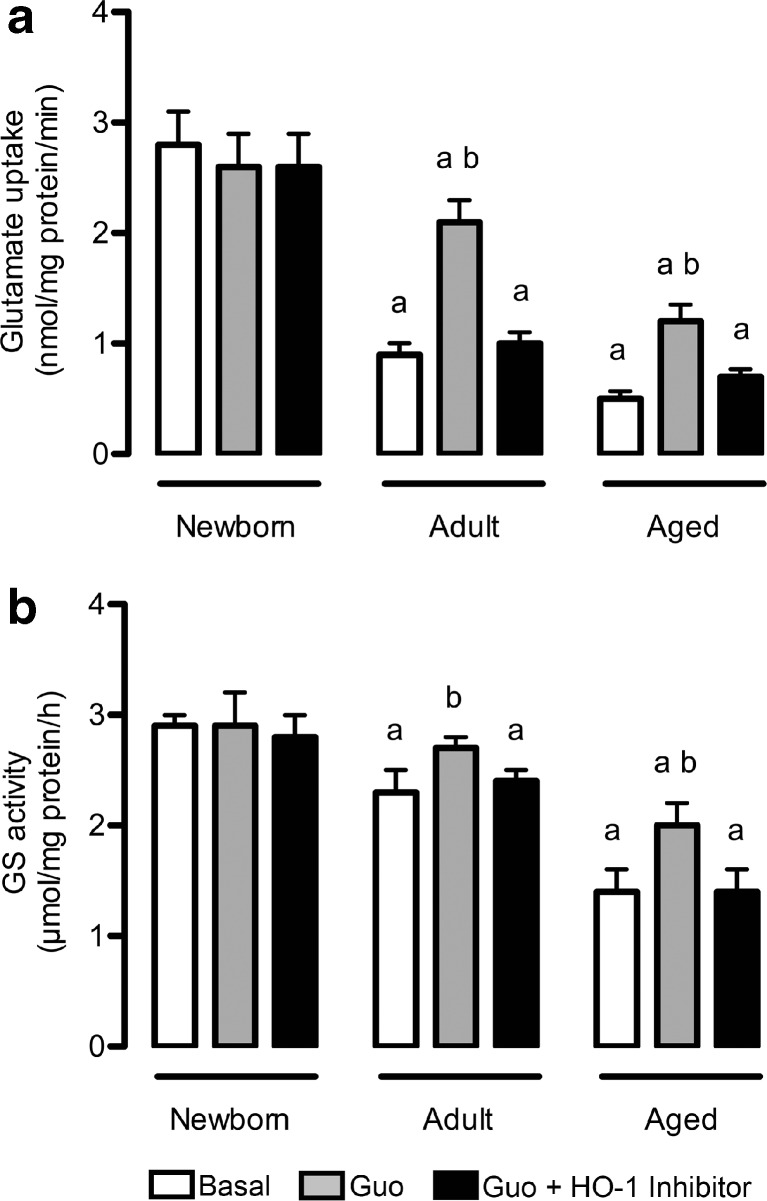
Effects of guanosine on glutamate uptake and GS activity
The regulation of glutamate in astrocyte cultures was assessed through glutamate uptake (Fig. 2a) and GS activity (Fig. 2b). Firstly, we demonstrated an age-dependent decrease in glutamate uptake in cortical astrocytes (Fig. 2a). In line with this, guanosine treatment partially prevented this effect, inducing an increase (around 130 %, P < 0.01) in glutamate uptake in adult and aged cultures, compared to newborn cultures. Because guanosine modulates HO-1 [12, 14], we investigated whether its effect on glutamate uptake was dependent on HO-1 activity using an HO-1 inhibitor (ZnPP IX), which totally abolished the effect of guanosine. Interestingly, newborn astrocytes did not present significant changes after guanosine exposure, indicating that guanosine might act as a protective signaling molecule in disturbances of cellular homeostasis, such as those occurring during pathological aging processes.
Fig. 2.
Guanosine alters glutamatergic metabolism in mature astrocytes. Cells were incubated in DMEM/F12 1 % FBS in the presence or absence of 100 μM guanosine (Guo) for 24 h. Alternatively, cells were co-incubated with an HO-1 inhibitor. Glutamate uptake (a) and GS activity (b) were measured as described in the Materials and methods section. Data represent the mean + SEM of four independent experiments performed in triplicate. Differences between groups were analyzed statistically using two-way ANOVA, followed by Tukey’s test. Values of P < 0.05 were considered significant. a indicates differences from Newborn control; b indicates difference within the same group
An age-dependent decrease in GS activity, compared to newborn cultured astrocytes, was also observed (Fig. 2b), while guanosine induced an age-related increase in GS activity in adult and aged astrocytes (17 and 42 %, respectively), compared to that seen in newborn astrocytes. The presence of the HO-1 inhibitor was also able to block this effect, restoring the activity to control values. The HO-1 inhibitor had no effect per se on glutamate uptake and GS activity (data not shown).
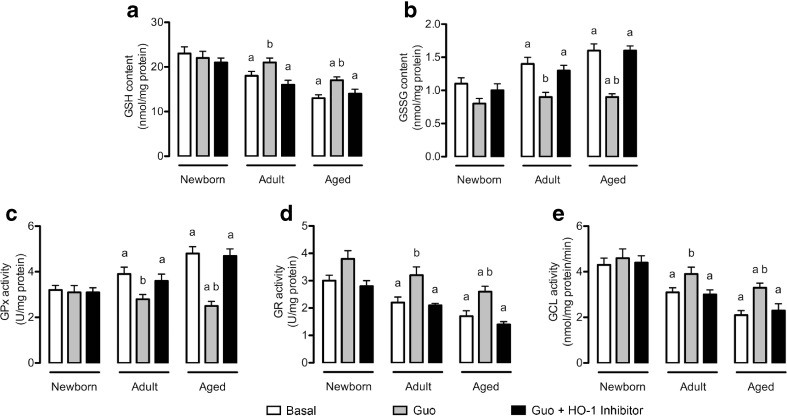
Guanosine modulated GSH system
GSH is the main non-enzymatic antioxidant defense molecule in the CNS and closely associated with glutamate metabolism; the levels of this antioxidant decreased significantly with age (Fig. 3a). Guanosine prevented this effect, increasing the levels of GSH in adult and aged cultured astrocytes. Once again, the HO-1 inhibitor abolished the effect of guanosine.
Fig. 3.
Guanosine alters the GSH system in mature astrocytes. Cells were incubated in DMEM/F12 1 % FBS in the presence or absence of 100 μM guanosine (Guo) for 24 h. Alternatively, cells were co-incubated with an HO-1 inhibitor. Content of GSH (a) and GSSG (b) as well as activities of GPx (c), GR (d), and GCL (e) were measured as described in the Materials and methods section. Data represent the mean + SEM of four independent experiments performed in triplicate. Differences between groups were analyzed statistically using two-way ANOVA, followed by Tukey’s test. Values of P < 0.05 were considered significant. a indicates differences from newborn control; b indicates difference within the same group
Additionally, we measured the levels of GSSG, which increased in an age-dependent manner (Fig. 3b). Guanosine restored the GSSG levels via the HO-1 pathway, indicating that guanosine contributes to maintain the GSH system levels. Furthermore, we evaluated the activities of the main enzymatic antioxidant defenses associated with GSH homeostasis, GPx, and GR. GPx activity increased with aging, and guanosine prevented this effect in an HO-1-dependent manner (Fig. 3c). In contrast, GR activity decreased with aging, and guanosine was able to prevent this decrease via the HO-1 pathway (Fig. 3d).
Subsequently, we found that the activity of GCL, the first enzyme required for GSH synthesis, decreased with aging (Fig. 3e). Guanosine also prevented this effect, which was abolished by the HO-1 inhibitor. The HO-1 inhibitor per se had no effect on assays related to the GSH system.
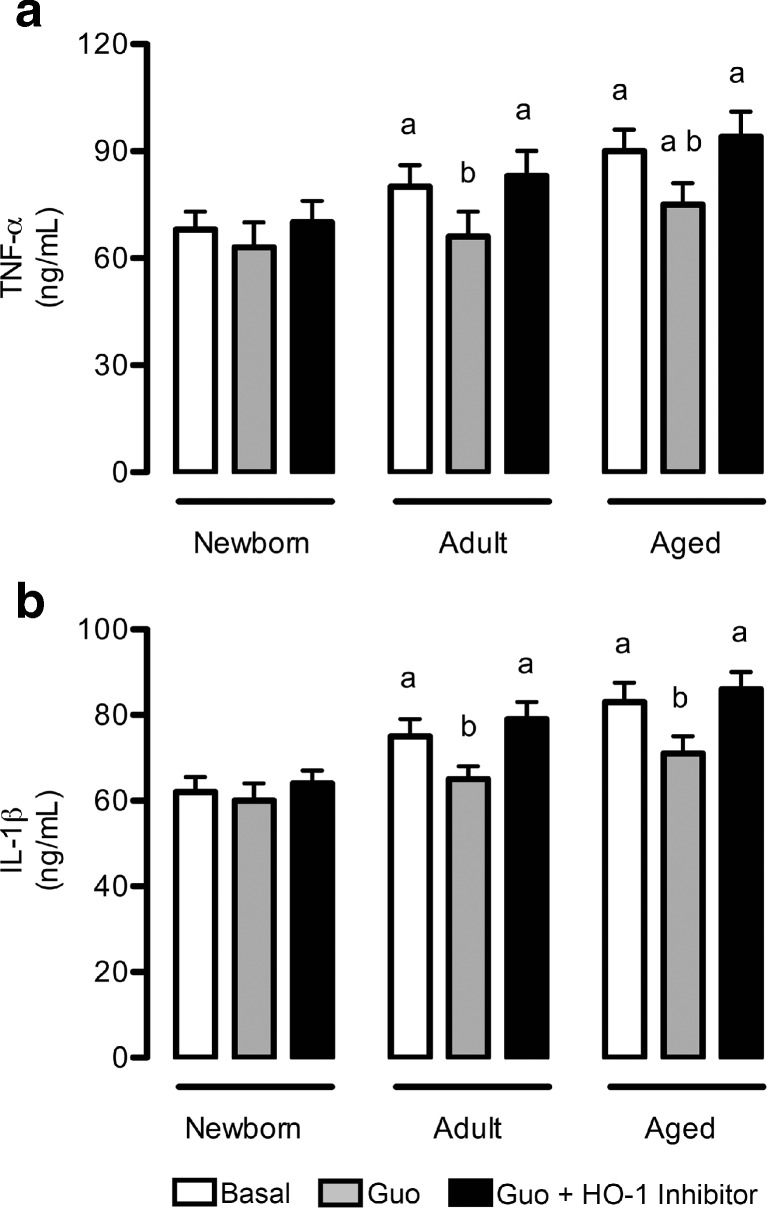
Effects of guanosine on the inflammatory response
As shown in Fig. 4, there was an age-dependent increase in pro-inflammatory cytokines’ release, namely of TNF-α (Fig. 4a) and IL-1β (Fig. 4b). Guanosine was able to induce a decrease in both TNF-α and IL-1β levels in adult and aged cultured astrocytes. This anti-inflammatory effect of guanosine was mediated by HO-1. Because changes in cell integrity were not observed through PI incorporation (data not shown), the increased levels of cytokines most likely resulted from secretion.
Fig. 4.
Guanosine decreases pro-inflammatory cytokine levels in mature astrocytes. Cells were incubated in DMEM/F12 1 % FBS in the presence or absence of 100 μM guanosine (Guo) for 24 h. Alternatively, cells were co-incubated with an HO-1 inhibitor. Levels of TNF-α (a) and IL-1β (b) were measured as described in the “Materials and methods” section. Data represent the mean + SEM of four independent experiments performed in triplicate. Differences between groups were analyzed statistically using two-way ANOVA, followed by Tukey’s test. Values of P < 0.05 were considered significant. a indicates differences from newborn control; b indicates difference within the same group
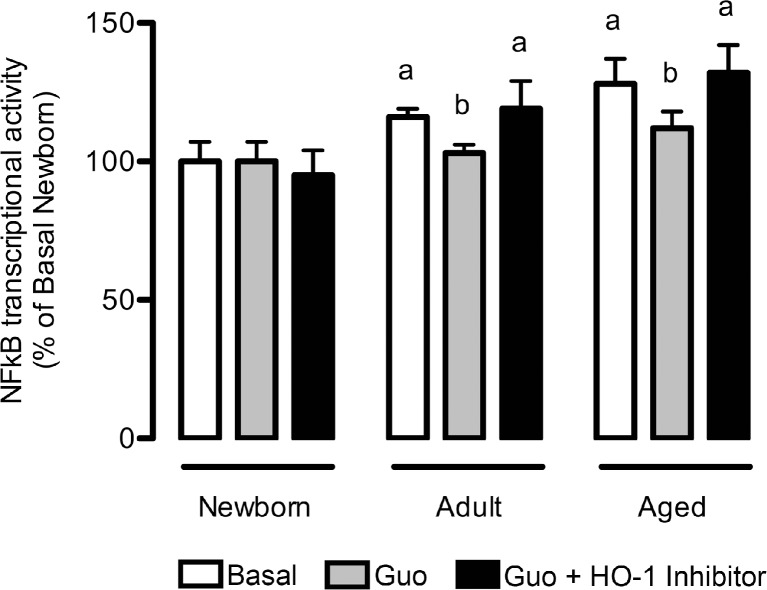
The NFkB signaling pathway is involved in the anti-aging effect of guanosine
The levels of NFkB, a transcription factor involved in numerous biological activities, including the inflammatory response, demonstrated a significant increase with aging (Fig. 5). However, guanosine decreased the transcriptional activity of NFkB p65 in adult and aging astrocytes. In the presence of the HO-1 inhibitor, guanosine did not reduce NFkB activation. Although the HO-1 pathway is upstream of NFkB signaling, the HO-1 inhibitor per se did not present any effect on NFkB levels (data not shown).
Fig. 5.
Guanosine decreases NFkB transcriptional activity in mature astrocytes. Cells were incubated in DMEM/F12 1 % FBS in the presence or absence of 100 μM guanosine (Guo) for 24 h. Alternatively, cells were co-incubated with an HO-1 inhibitor. The NFkB transcriptional activity was measured as described in the “Materials and methods” section. Data represent the mean + SEM of four independent experiments performed in triplicate. Differences between groups were analyzed statistically using two-way ANOVA, followed by Tukey’s test. Values of P < 0.05 were considered significant. a indicates differences from newborn control; b indicates difference within the same group
Discussion
The aging process correlates with brain biochemical, cellular, and molecular changes, including alterations in glutamate metabolism, oxidative stress, and the inflammatory response, and these events involve the modulation of several signaling pathways [34–36]. The development of preventive treatments that can protect the brain from pathological aging is of importance, since these cells may become dysfunctional and lead to the onset of neurodegenerative diseases.
Guanosine has been studied in a variety of experimental models, including seizures, hypoxia, glucose deprivation, oxidative injury, and inflammatory conditions [4, 5, 37–40]. Because guanosine may be released from glial cells, modulating important glial functions, astrocytes emerge as central players in the protective actions of guanosine [12, 14]. Recently, we reported that guanosine has glioprotective effects, and here, we show, for the first time, the anti-aging effect of guanosine on classical astroglial parameters. Moreover, the precise mechanism of guanosine neural/glioprotection is not completely understood, and elucidation of its cellular and molecular targets is needed. In line with this, some data indicate that the extracellular effects of guanosine might involve the activation of intracellular signaling pathways, such as G proteins, MAPK, and HO-1 [12–14, 41, 42].
The HO-1 pathway has been reported to be active and to operate as a fundamental defensive mechanism for cells exposed to stressful conditions [15, 42, 43]. Accordingly, HO-1 is closely associated with nuclear factor erythroid-derived 2-like 2 (Nrf-2), which controls the master regulator of the redox state and inflammatory response, the transcription factor NFkB [17]. In our previous reports, we demonstrated that HO-1 expression was increased by guanosine, mediating their putative glutamatergic, antioxidant, and anti-inflammatory activities [12, 14]. Additionally, HO-1 may induce GSH biosynthesis, which protects glutamate transporters from oxidative damage. Moreover, the beneficial effects of HO-1 are predominantly expressed in the mature brain; therefore, our cultured astrocytes from adult/aged rats might provide answers about the role of guanosine in aging and neurodegenerative diseases [44, 45].
Astrocytes play a central role in neuronal transmission, providing the removal of glutamate from the synaptic cleft by high-affinity transporters [46, 47]. This function may avoid excitotoxicity, whose incidence increases in age-related neurodegenerative diseases [22, 34, 48]. Thus, we showed that glutamate uptake activity decreased with aging and that guanosine was able to restore glutamate uptake in an age-dependent manner, emerging as a promising pharmacological tool for the prevention of the progression of neurodegeneration associated with aging. Reinforcing our data, the effects of guanosine originated exclusively from mature astrocyte cultures, which presented decreased GSH levels, in turn, possibly impairing the glutamate transporters [45]. Interestingly, as previously demonstrated under oxidative and inflammatory conditions, the anti-aging effect of guanosine in glial cells was dependent on the HO-1 pathway.
Glutamate may be a precursor of GSH as well as of glutamine via the GS enzyme, thus entering the glutamate-glutamine cycle [49, 50]. GS activity presented an age-dependent decrease in astrocyte cultures, which may impair neuronal activity. In addition, the aging process is associated with increased oxidative/nitrosative stress, and GS activity is very sensitive to this condition [51, 52]. Our data reveal that guanosine prevented the decrease in GS activity, reinforcing its antioxidant activity as well as its role in glutamate metabolism. Corroborating our previous findings, the effect of guanosine on GS was mediated by HO-1.
There is ample evidence to connect disturbances in GSH metabolism with aging, as reduced GSH content in the brain is associated with altered cognitive functions [53]. Accordingly, guanosine prevented the age-related decrease in GSH content via the HO-1 pathway. Moreover, the increased GSH in glial cells confers protection against age-related neurological diseases, such as Alzheimer’s and Parkinson’s diseases [54]. Additionally, disturbances in GSH homeostasis are associated with GSH-GSSG redox cycling in astrocytes. Thus, during pathological conditions, GR can become rate limiting for GSH-GSSG redox cycling, which causes a transient increase in cellular GSSG levels, as observed in adult and aged cultured astrocytes.
Guanosine effectively contributes to maintain the biosynthesis of GSH and also modulates the GPx and GR activities. With regard to GSH-GSSG redox cycling, GPx catalyzes the reduction in peroxides, generating GSSG, which is subsequently reduced to GSH by GR [53, 54]. Finally, GCL was found to decrease with aging; GCL catalyzes the formation of the dipeptide γ-glutamylcysteine and is the rate-limiting step in cellular GSH synthesis. Guanosine also modulated GCL, supporting the de novo synthesis of GSH, maintaining high cellular GSH levels, and acting as a glioprotective molecule. In addition, the transcription of GCL is regulated by the Nrf2/HO-1 system, the signaling pathway through which guanosine might exert its glioprotective role.
The aging process is commonly associated with an increased inflammatory response in the CNS. Accordingly, astrocytes are key players in brain immunity, because they sense and amplify inflammatory signals [26, 55]. Moreover, the depletion of GSH in glial cells also induces neuroinflammation [56]. Consistent with the anti-inflammatory role of GSH, we showed that classical pro-inflammatory cytokines, such as TNF-α and IL-1β, increased with aging in cortical astrocytes. These cytokines act in the acute inflammatory response and have a critical role in chronic inflammation, commonly associated with age-related neurological diseases. Guanosine was able to prevent the increase in TNF-α and IL-1β levels through the HO-1 pathway, and, for the first time, we demonstrated the anti-inflammatory effect of guanosine in the aging process. In addition, our findings are in accordance with recent publications that suggest an anti-epileptic activity of guanosine partly via astrocyte modulation in a cross talk between inflammation and glutamatergic system [57–59].
TNF-α and IL-1β are known activators of the NFkB signaling pathway, which is intricately involved in inflammation [18, 60]. Thus, in accordance with the increased inflammatory response in aging, we also showed increased transcriptional activity of NFkB p65. Guanosine controlled NFkB activation in mature astrocytes in an HO-1-dependent mechanism. Additionally, numerous studies have shown that suppressing pro-inflammatory astroglial NFkB signaling could improve clinical outcomes in cases of neuroinflammatory-related diseases [61, 62]; thus, the inhibitory effect of guanosine on NFkB is consistent with its glioprotective role.
In summary, we herein demonstrate that the astrocyte culture model developed by our group is a powerful tool for investigating age-related changes in astrocytic functionality, such as glutamate metabolism, oxidative stress, and the inflammatory response. Additionally, we showed, for the first time, that guanosine has anti-aging effects, reinforcing its potential as a glioprotective molecule in the aging process. Thus, this study contributes to the understanding of the cellular and molecular mechanisms of guanosine in brain aging.
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Financiadora de Estudos e Projetos (FINEP)-Instituto Brasileiro de Neurociências (IBN Net) 01.06.0842-00, Universidade Federal do Rio Grande do Sul (UFRGS), and Instituto Nacional de Ciência e Tecnologia para Excitotoxicidade e Neuroproteção (INCTEN/CNPq).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- 1.Schmidt AP, Lara DR, Souza DO. Proposal of a guanine-based purinergic system in the mammalian central nervous system. Pharmacol Ther. 2007;116(3):401–416. doi: 10.1016/j.pharmthera.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Ciccarelli R, Di Iorio P, Giuliani P, D’Alimonte I, Ballerini P, Caciagli F, Rathbone MP. Rat cultured astrocytes release guanine-based purines in basal conditions and after hypoxia/hypoglycemia. Glia. 1999;25(1):93–98. doi: 10.1002/(SICI)1098-1136(19990101)25:1<93::AID-GLIA9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 3.Chang R, Algird A, Bau C, Rathbone MP, Jiang S. Neuroprotective effects of guanosine on stroke models in vitro and in vivo. Neurosci Lett. 2008;431(2):101–105. doi: 10.1016/j.neulet.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 4.Dal-Cim T, Ludka FK, Martins WC, Reginato C, Parada E, Egea J, Lopez MG, Tasca CI. Guanosine controls inflammatory pathways to afford neuroprotection of hippocampal slices under oxygen and glucose deprivation conditions. J Neurochem. 2013;126(4):437–450. doi: 10.1111/jnc.12324. [DOI] [PubMed] [Google Scholar]
- 5.Bettio LE, Gil-Mohapel J, Rodrigues AL. Guanosine and its role in neuropathologies. Purinergic Signal. 2016 doi: 10.1007/s11302-016-9509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frizzo ME, Lara DR, Dahm KC, Prokopiuk AS, Swanson RA, Souza DO. Activation of glutamate uptake by guanosine in primary astrocyte cultures. Neuroreport. 2001;12(4):879–881. doi: 10.1097/00001756-200103260-00051. [DOI] [PubMed] [Google Scholar]
- 7.Ganzella M, de Oliveira ED, Comassetto DD, Cechetti F, Cereser VH, Jr, Moreira JD, Hansel G, Almeida RF, Ramos DB, Figueredo YN, Souza DG, Oses JP, Worm PV, Achaval M, Netto CA, Souza DO. Effects of chronic guanosine treatment on hippocampal damage and cognitive impairment of rats submitted to chronic cerebral hypoperfusion. Neurol Sci. 2012 doi: 10.1007/s10072-011-0872-1. [DOI] [PubMed] [Google Scholar]
- 8.Hansel G, Ramos DB, Delgado CA, Souza DG, Almeida RF, Portela LV, Quincozes-Santos A, Souza DO. The potential therapeutic effect of guanosine after cortical focal ischemia in rats. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0090693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quincozes-Santos A, Bobermin LD, de Souza DG, Bellaver B, Goncalves CA, Souza DO. Gliopreventive effects of guanosine against glucose deprivation in vitro. Purinergic Signal. 2013;9(4):643–654. doi: 10.1007/s11302-013-9377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lara DR, Schmidt AP, Frizzo ME, Burgos JS, Ramirez G, Souza DO. Effect of orally administered guanosine on seizures and death induced by glutamatergic agents. Brain Res. 2001;912(2):176–180. doi: 10.1016/S0006-8993(01)02734-2. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt AP, Lara DR, de Faria MJ, da Silveira PA, Onofre Souza D. Guanosine and GMP prevent seizures induced by quinolinic acid in mice. Brain Res. 2000;864(1):40–43. doi: 10.1016/S0006-8993(00)02106-5. [DOI] [PubMed] [Google Scholar]
- 12.Bellaver B, Souza DG, Bobermin LD, Goncalves CA, Souza DO, Quincozes-Santos A. Guanosine inhibits LPS-induced pro-inflammatory response and oxidative stress in hippocampal astrocytes through the heme oxygenase-1 pathway. Purinergic Signal. 2015;11(4):571–580. doi: 10.1007/s11302-015-9475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Iorio P, Ballerini P, Traversa U, Nicoletti F, D’Alimonte I, Kleywegt S, Werstiuk ES, Rathbone MP, Caciagli F, Ciccarelli R. The antiapoptotic effect of guanosine is mediated by the activation of the PI 3-kinase/AKT/PKB pathway in cultured rat astrocytes. Glia. 2004;46(4):356–368. doi: 10.1002/glia.20002. [DOI] [PubMed] [Google Scholar]
- 14.Quincozes-Santos A, Bobermin LD, Souza DG, Bellaver B, Goncalves CA, Souza DO. Guanosine protects C6 astroglial cells against azide-induced oxidative damage: a putative role of heme oxygenase 1. J Neurochem. 2014;130(1):61–74. doi: 10.1111/jnc.12694. [DOI] [PubMed] [Google Scholar]
- 15.Cuadrado A, Rojo AI. Heme oxygenase-1 as a therapeutic target in neurodegenerative diseases and brain infections. Curr Pharm Des. 2008;14(5):429–442. doi: 10.2174/138161208783597407. [DOI] [PubMed] [Google Scholar]
- 16.Quincozes-Santos A, Bobermin LD, Latini A, Wajner M, Souza DO, Goncalves CA, Gottfried C. Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0064372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who’s listening? Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shih RH, Wang CY, Yang CM. NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci. 2015;8:77. doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107(3):247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitteldorf J. Is programmed aging a cause for optimism? Curr Aging Sci. 2015;8(1):69–75. doi: 10.2174/1874609808666150422112826. [DOI] [PubMed] [Google Scholar]
- 21.Souza DG, Bellaver B, Raupp GS, Souza DO, Quincozes-Santos A. Astrocytes from adult Wistar rats aged in vitro show changes in glial functions. Neurochem Int. 2015 doi: 10.1016/j.neuint.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Jiang T, Cadenas E. Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell. 2014;13(6):1059–1067. doi: 10.1111/acel.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maragakis NJ, Rothstein JD. Mechanisms of disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2(12):679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- 24.Ransom BR, Ransom CB. Astrocytes: multitalented stars of the central nervous system. Methods Mol Biol. 2012;814:3–7. doi: 10.1007/978-1-61779-452-0_1. [DOI] [PubMed] [Google Scholar]
- 25.Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27(12):735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28(3):138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Souza DG, Bellaver B, Souza DO, Quincozes-Santos A. Characterization of adult rat astrocyte cultures. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0060282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellaver B, Souza DG, Souza DO, Quincozes-Santos A. Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9880-8. [DOI] [PubMed] [Google Scholar]
- 29.Stanimirovic DB, Ball R, Small DL, Muruganandam A. Developmental regulation of glutamate transporters and glutamine synthetase activity in astrocyte cultures differentiated in vitro. Int J Dev Neurosci. 1999;17(3):173–184. doi: 10.1016/S0736-5748(99)00028-3. [DOI] [PubMed] [Google Scholar]
- 30.Souza DG, Bellaver B, Hansel G, Arus BA, Bellaver G, Longoni A, Kolling J, Wyse AT, Souza DO, Quincozes-Santos A. Characterization of amino acid profile and enzymatic activity in adult rat astrocyte cultures. Neurochem Res. 2016 doi: 10.1007/s11064-016-1871-7. [DOI] [PubMed] [Google Scholar]
- 31.Bellaver B, Souza DG, Souza DO, Quincozes-Santos A. Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol In Vitro. 2014;28(4):479–484. doi: 10.1016/j.tiv.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Seelig GF, Meister A. Glutathione biosynthesis; gamma-glutamylcysteine synthetase from rat kidney. Methods Enzymol. 1985;113:379–390. doi: 10.1016/S0076-6879(85)13050-8. [DOI] [PubMed] [Google Scholar]
- 33.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 34.Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev. 2001;122(1):1–29. doi: 10.1016/S0047-6374(00)00225-6. [DOI] [PubMed] [Google Scholar]
- 35.Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, Bates TE, Giuffrida Stella AM, Schapira T, Dinkova Kostova AT, Rizzarelli E. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33(12):2444–2471. doi: 10.1007/s11064-008-9775-9. [DOI] [PubMed] [Google Scholar]
- 36.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18(9):685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 37.Almeida RF, Comasseto DD, Ramos DB, Hansel G, Zimmer ER, Loureiro SO, Ganzella M, Souza DO. Guanosine anxiolytic-like effect involves adenosinergic and glutamatergic neurotransmitter systems. Mol Neurobiol. 2016 doi: 10.1007/s12035-015-9660-x. [DOI] [PubMed] [Google Scholar]
- 38.Vinade ER, Schmidt AP, Frizzo ME, Izquierdo I, Elisabetsky E, Souza DO. Chronically administered guanosine is anticonvulsant, amnesic and anxiolytic in mice. Brain Res. 2003;977(1):97–102. doi: 10.1016/S0006-8993(03)02769-0. [DOI] [PubMed] [Google Scholar]
- 39.Soares FA, Schmidt AP, Farina M, Frizzo ME, Tavares RG, Portela LV, Lara DR, Souza DO. Anticonvulsant effect of GMP depends on its conversion to guanosine. Brain Res. 2004;1005(1-2):182–186. doi: 10.1016/j.brainres.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 40.Ramos DB, Muller GC, Rocha GB, Dellavia GH, Almeida RF, Pettenuzzo LF, Loureiro SO, Hansel G, Horn AC, Souza DO, Ganzella M. Intranasal guanosine administration presents a wide therapeutic time window to reduce brain damage induced by permanent ischemia in rats. Purinergic Signal. 2016;12(1):149–159. doi: 10.1007/s11302-015-9489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dal-Cim T, Molz S, Egea J, Parada E, Romero A, Budni J, Martin de Saavedra MD, del Barrio L, Tasca CI, Lopez MG. Guanosine protects human neuroblastoma SH-SY5Y cells against mitochondrial oxidative stress by inducing heme oxigenase-1 via PI3K/Akt/GSK-3beta pathway. Neurochem Int. 2012;61(3):397–404. doi: 10.1016/j.neuint.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 42.Bau C, Middlemiss PJ, Hindley S, Jiang S, Ciccarelli R, Caciagli F, Diiorio P, Werstiuk ES, Rathbone MP. Guanosine stimulates neurite outgrowth in PC12 cells via activation of heme oxygenase and cyclic GMP. Purinergic Signal. 2005;1(2):161–172. doi: 10.1007/s11302-005-6214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8(3):240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 44.Butterfield DA, Barone E, Di Domenico F, Cenini G, Sultana R, Murphy MP, Mancuso C, Head E. Atorvastatin treatment in a dog preclinical model of Alzheimer’s disease leads to up-regulation of haem oxygenase-1 and is associated with reduced oxidative stress in brain. Int J Neuropsychopharmacol. 2012;15(7):981–987. doi: 10.1017/S1461145711001118. [DOI] [PubMed] [Google Scholar]
- 45.Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci. 1998;19(8):328–334. doi: 10.1016/S0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- 46.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/S0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 47.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32(1):1–14. doi: 10.1002/1098-1136(200010)32:1<1::AID-GLIA10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 48.Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27(5):717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Hertz L. Glutamate, a neurotransmitter—and so much more. A synopsis of Wierzba III. Neurochem Int. 2006;48(6-7):416–425. doi: 10.1016/j.neuint.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 50.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62(6):649–671. doi: 10.1016/S0301-0082(99)00060-X. [DOI] [PubMed] [Google Scholar]
- 51.Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98(3):641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- 52.Mates JM, Perez-Gomez C, Nunez de Castro I, Asenjo M, Marquez J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int J Biochem Cell Biol. 2002;34(5):439–458. doi: 10.1016/S1357-2725(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 53.Dringen R, Brandmann M, Hohnholt MC, Blumrich EM. Glutathione-dependent detoxification processes in astrocytes. Neurochem Res. 2014;40(12):2570–2582. doi: 10.1007/s11064-014-1481-1. [DOI] [PubMed] [Google Scholar]
- 54.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 55.Fernandes A, Falcao AS, Silva RF, Brito MA, Brites D. MAPKs are key players in mediating cytokine release and cell death induced by unconjugated bilirubin in cultured rat cortical astrocytes. Eur J Neurosci. 2007;25(4):1058–1068. doi: 10.1111/j.1460-9568.2007.05340.x. [DOI] [PubMed] [Google Scholar]
- 56.Lee M, Cho T, Jantaratnotai N, Wang YT, McGeer E, McGeer PL. Depletion of GSH in glial cells induces neurotoxicity: relevance to aging and degenerative neurological diseases. FASEB J. 2010;24(7):2533–2545. doi: 10.1096/fj.09-149997. [DOI] [PubMed] [Google Scholar]
- 57.Kovacs Z, Kekesi KA, Dobolyi A, Lakatos R, Juhasz G. Absence epileptic activity changing effects of non-adenosine nucleoside inosine, guanosine and uridine in Wistar Albino Glaxo Rijswijk rats. Neuroscience. 2015;300:593–608. doi: 10.1016/j.neuroscience.2015.05.054. [DOI] [PubMed] [Google Scholar]
- 58.Kovacs Z, Kekesi KA, Juhasz G, Dobolyi A. Modulatory effects of inosine, guanosine and uridine on lipopolysaccharide-evoked increase in spike-wave discharge activity in Wistar Albino Glaxo/Rijswijk rats. Brain Res Bull. 2015;118:46–57. doi: 10.1016/j.brainresbull.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Lakatos RK, Dobolyi A, Todorov MI, Kekesi KA, Juhasz G, Aleksza M, Kovacs Z. Guanosine may increase absence epileptic activity by means of A2A adenosine receptors in Wistar Albino Glaxo Rijswijk rats. Brain Res Bull. 2016;124:172–181. doi: 10.1016/j.brainresbull.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Kim YJ, Hwang SY, Oh ES, Oh S, Han IO. IL-1beta, an immediate early protein secreted by activated microglia, induces iNOS/NO in C6 astrocytoma cells through p38 MAPK and NF-kappaB pathways. J Neurosci Res. 2006;84(5):1037–1046. doi: 10.1002/jnr.21011. [DOI] [PubMed] [Google Scholar]
- 61.Verma IM. Nuclear factor (NF)-kappaB proteins: therapeutic targets. Ann Rheum Dis. 2004;63(Suppl 2):ii57–ii61. doi: 10.1136/ard.2004.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaltschmidt B, Kaltschmidt C. NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol. 2009;1(3):a001271. doi: 10.1101/cshperspect.a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]