Abstract
Background
Plant WRKY transcription factors play pivotal roles in diverse biological processes but most notably in plant defense response to pathogens. Sheath blight represents one of the predominant diseases in rice. However, our knowledge about the functions of WRKY proteins in rice defense against sheath blight is rather limited.
Results
Here we demonstrate that the expression of Oryza sativa WRKY80 gene (OsWRKY80) is rapidly and strongly induced upon infection of Rhizoctonia solani, the causal agent of rice sheath blight disease. OsWRKY80 expression is also induced by exogenous jasmonic acid (JA) and ethylene (ET), but not by salicylic acid (SA). OsWRKY80-GFP is localized in the nuclei of onion epidermal cells in a transient expression assay. Consistently, OsWRKY80 exhibits transcriptional activation activity in a GAL4 assay in yeast cells. Overexpression of OsWRKY80 in rice plants significantly enhanced disease resistance to R. solani, concomitant with elevated expression of OsWRKY4, another positive regulator in rice defense against R. solani. Suppression of OsWRKY80 by RNA interference (RNAi), on the other hand, compromised disease resistance to R. solani. Results of yeast one-hybrid assay and transient expression assay in tobacco cells have revealed that OsWRKY80 specifically binds to the promoter regions of OsWRKY4, which contain W-box (TTGAC[C/T]) or W-box like (TGAC[C/T]) cis-elements.
Conclusions
We propose that OsWRKY80 functions upstream of OsWRKY4 as an important positive regulatory circuit that is implicated in rice defense response to sheath blight pathogen R. solani.
Electronic supplementary material
The online version of this article (doi:10.1186/s12284-016-0137-y) contains supplementary material, which is available to authorized users.
Keywords: Disease resistance, Rhizoctonia solani, Transcription factor, WRKY protein, Oryza sativa
Background
In the natural environment, plants are frequently confronted with diverse biotic and abiotic stresses that detrimentally affect their growth and development. Among them, pathogen attack is one of the most limiting factors of crop productivity and quality, and consequently poses a serious threat to agricultural industry worldwide. To ensure survival, plants have evolved intricate and robust mechanisms to respond to pathogen invasion through their innate immune system. Plant innate immune system is comprised of two interconnected branches. The first branch is pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI), initiated by the recognition of molecular signatures of certain pathogens (e.g., bacterial flagellin and fungal chitin oligosaccharide). PTI often activates downstream mitogen-activated protein kinase (MPK) cascades and defense response genes. The second branch is effector-triggered immunity (ETI), which is a more accelerated defense response than PTI and is triggered by host-resistance (R) protein-mediated recognition of pathogen effectors (Jones and Dangl 2006). PTI- and ETI-mediated defense responses in plants are modulated mainly by three signaling hormone molecules, salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) (Tsuda et al. 2009). There are both synergistic and antagonistic interactions between SA and JA/ET signaling pathways during plant immune progression (Kunkel and Brooks 2002; Mur et al. 2006). This apparent discrepancy reflects the complexity of plant defense mechanisms (Kim et al. 2006). Moreover, the expression of downstream defense-related genes is crucial for the establishment of plant immune responses. The interaction between plants and pathogens eventually leads to extensive transcriptional reprogramming of plant defense-responsive genes (Eulgem 2005), indicating that transcription factors play a pivotal role in plant disease resistance.
The transcription factor families involved in plant defense responses include TGA family of basic domain-leucine zipper (bZIP), ethylene response factor (ERF), MYB, WRKY and Whirly family proteins (Eulgem 2005). WRKY proteins are one of the largest families of transcription factors in plants with 72-74 members in Arabidopsis (Ülker and Somssich 2004; Dong et al. 2003) and over 100 members in rice (Wu et al. 2005; Ross et al. 2007). The WRKY factors are characterized by their conserved DNA-binding WRKY domains consisting of a highly conserved WRKYGQK stretch in N-termini, and a zinc-finger motif (C-X4-5-C-X22-23-H-X1-H or C-X7-C-X23-H-X1-C) in C-termini (Eulgem et al. 2000). The WRKY domain generally binds to the W-box (C/T)TGAC(C/T) or W-box like cis-elements in the promoters of target genes (Eulgem et al. 2000; Maleck et al. 2000). According to the number of WRKY domains and the features of zinc-finger motifs, the WRKY protein family is categorized into three distinct groups (I, II and III). Group II is further divided into five subgroups (IIa to IIe) based on the presence of additional short conserved structural motifs outside of the WRKY domain (Eulgem et al. 2000).
Loss- and gain-of-function studies have revealed that WRKYs act in a complex signaling network as both positive and negative regulators of various biological processes, but most notably in biotic stress responses (Pandey and Somssich 2009). To date, at least 13 Oryza sativa WRKY (OsWRKY) genes are known to positively regulate rice resistance against pathogens, such as Magnaporthe oryzae, Rhizoctonia solani and Xanthomonas oryzae pv oryzae (Xoo) (Cheng et al. 2015; Wang et al. 2015; Choi et al. 2015; Hwang et al. 2016). For instance, OsWRKY13 activates SA-dependent defense response whereas suppresses JA-dependent response, in mediating rice defense response to bacterial blight and fungal blast pathogens (Qiu et al. 2007). OsWRKY45 positively regulates systemic acquired resistance (SAR) in an SA-dependent manner (Shimono et al. 2007; Shimono et al. 2012). More recently, it has been reported that OsWRKY51 functions as a positive transcriptional regulator in defense signaling against Xoo by direct binding to the promoter of defense related gene, OsPR10a (Hwang et al. 2016). By contrast, although the transcription of OsWRKY28, 62, and 76 is upregulated upon pathogen infection, their protein products act to repress plant defense response against rice fungal blast or bacterial blight pathogens (Peng et al. 2008; Delteil et al. 2012; Chujo et al. 2013; Yokotani et al. 2013). More intriguingly, OsWRKY45-1 derived from subspecies japonica acts as a negative regulator, whereas its allele OsWRKY45-2 derived from subspecies indica as a positive regulator in the interactions between rice and bacterial pathogens such as Xoo and X. oryzae pv oryzicola. Nevertheless, both OsWRKY45 alleles function as positive regulators in the defense against fungal pathogen M. oryzae (Tao et al. 2009). WRKYs often work in concert in plant defense response to pathogens. OsWRKY42 has been characterized as a negative regulator functioning downstream of OsWRKY13. OsWRKY42-OsWRKY13 together with OsWRKY45-2 form a WRKY transcriptional regulatory cascade in the rice- M. oryzae interaction (Cheng et al. 2015). The multiple roles of WRKYs suggest that the complex signaling and transcriptional networks of biotic stress responses require concerted regulation. Coordinated modulation of WRKY proteins as positive and negative regulators could also enable the proper amplitude and duration of plant response to minimize detrimental effects on plant growth and development during pathogen attack (Pandey and Somssich 2009).
Blast, caused by M. oryzae, bacterial blight, caused by Xoo, and sheath blight, caused by R. solani are considered to be three major diseases in rice. Among those characterized OsWRKY genes, at least 12 and 10 genes have been shown to function as either positive or negative regulators in rice resistance against M. oryzae and Xoo, respectively. However, only 2 OsWRKY genes (OsWRKY30 and OsWRKY4) have been shown to mediate the defense responses against R. solani (Peng et al. 2012; Wang et al. 2015). Additionally, rice sheath blight is a necrotrophic disease (Zhao et al. 2008). The strategies of resistance to necrotrophs are distinct from those against biotrophs, and likely involved in defense mechanisms mediated by JA/ET-dependent signaling routes (Bari and Jones 2009). To elucidate the regulatory roles of rice WRKY factors in defense response to the sheath blight fungus, we have analyzed the expression profiles of rice WRKY family under R. solani infection and methyl jasmonate (MeJA) treatment. We have identified several pathogen- and JA-inducible WRKY genes, including OsWRKY30 and OsWRKY4, which are positive regulators in rice resistance to R. solani (Peng et al. 2012; Wang et al. 2015). In this report, we investigate the expression pattern of OsWRKY80 gene in response to exogenous defense-related phytohormones JA, ET and SA and R. solani challenge. We have found that OsWRKY80 is a nuclear-localized transcriptional activator. Compared to wild-type plants, the OsWRKY80 overexpression rice plants are more resistant whereas knockdown (RNAi) lines are more susceptible to R. solani attack. In addition, we have found opposing expression pattern of OsWRKY4 in gain- and loss-of function OsWRKY80 plants, respectively. We have further demonstrated that OsWRKY80 specifically binds to the W-box, or W-box like cis-elements in the promoter of the OsWRKY4 gene. Our findings suggest that OsWRKY80 functions upstream of OsWRKY4 and together this module acts as a positive regulatory circuit in the rice defense response against sheath blight disease.
Results
Cloning and Sequence Analysis of OsWRKY80 cDNA
Several nomenclature systems of rice WRKY genes were proposed in the past by independent research groups (Zhang et al. 2004; Wu et al. 2005; Zhang and Wang 2005). To avoid the conflicts and confusion, the rice WRKY-working group has redefined the WRKY gene nomenclature based on the CGSNL (Committee on Gene Symbolization, Nomenclature and Linkage, Rice Genetics Cooperative) rules (Rice WRKY Working Group 2012). In the present study, we isolated a full-length cDNA of OsWRKY80 gene. The OsWRKY80 gene is located on chromosome 3 and designated with the locus number Loc_Os03g63810. This OsWRKY80 is not to be confused with a previously reported OsWRKY80 gene by Li et al. (2009) and Ricachenevsky et al. (2010), which is now designated as OsWRKY90 (LOC _Os09g30400) according to the new CGSNL nomenclature. The obtained cDNA sequence of OsWRKY80 was 1392 bp in length, containing an ORF of 1164 bp, encoding a polypeptide of 387 amino acid residues. Structure analysis revealed that the deduced OsWRKY80 consisted of one classic conserved WRKY domain with a zinc finger motif of C-X5-C-X23-H-X1-H, indicating that it belongs to the WRKY group II-e family (Eulgem et al. 2000). BLAST analysis revealed that OsWRKY80 shared the highest homology with previously uncharacterized OsWRKY37 (54.8%, LOC_Os04g50920) and AtWRKY14 (50.7%, At1g30650), respectively.
Expression of OsWRKY80 is Induced by JA, ET and R. Solani
WRKY transcription factors are frequently implicated in the regulation of plant immune responses (Pandey and Somssich 2009). To reveal if OsWRKY80 might be involved in the plant responses to biotic stresses, the expression of OsWRKY80 was examined after the inoculation of R. solani. The OsWRKY80 transcription was induced by R. solani at 1 h and peaked at 24 h (Fig. 1).
Fig. 1.
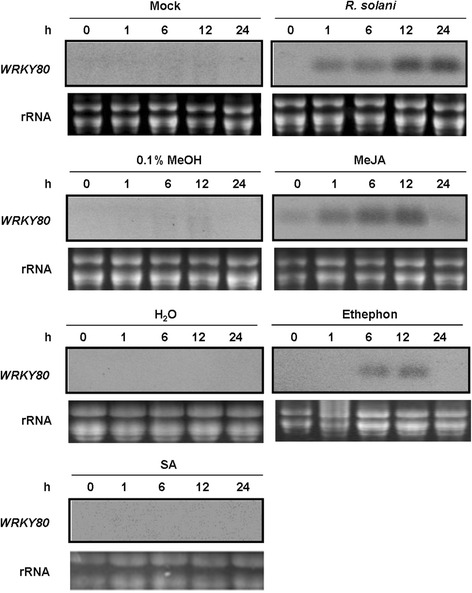
RNA gel blot analysis for expression of OsWRKY80 in response to pathogen infection and chemicals. Total RNA was extracted from leaves of 3-week-old rice seedlings at the indicated time intervals after treatments. A 10 μg aliquot of total RNA was loaded per lane. The ethidium bromide stain of rRNA is shown for assessment of equal loading
To determine the possible involvement of OsWRKY80 in hormone-mediated defense signaling pathways, we also monitored the expression of OsWRKY80 following treatment with exogenously applied SA, JA and ET. As shown in Fig. 1, the expression of OsWRKY80 was rapidly induced by JA within 1 h, peaked at 12 h, and sharply declined to basal levels at 24 h. The OsWRKY80 transcripts were also noticeably induced by an ethylene precursor ethephon during 6–12 h before returning to basal levels at 24 h. However, exogenous SA application exerted no effects on the expression of OsWRKY80.
The strong induction of OsWRKY80 expression by JA, ET and pathogen suggests that this gene may be involved in JA/ET-dependent defense signaling pathways.
OsWRKY80 is Localized in the Nucleus
To determine the subcellular localization of the OsWRKY80 protein, we generated an OsWRKY80-GFP fusion gene under the control of the constitutive CaMV 35S promoter, and transiently expressed in onion epidermal cells via particle bombardment. As shown in Fig. 2, OsWRKY80-GFP was exclusively localized in the nucleus. By contrast, the GFP protein alone as a control was found throughout in the cytoplasm. The observation indicates that WRKY80 is a nuclear protein.
Fig. 2.
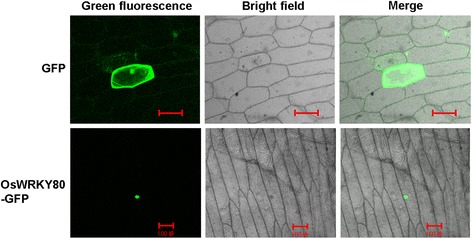
Nuclear localization of OsWRKY80. Onion epidermial cells were transformed with plasmids expressing GFP (top), or WRKY80-GFP fusion protein (bottom) and observed after 2 d under UV light (left panel) and white light (middle panel); Right panel is the merge of fluorescence and light. Bar = 100 μm
OsWRKY80 Acts as a Transcriptional Activator in Yeast Cells
Based on the prediction using DNAStar software package, OsWRKY80 contains a C-terminal acidic region (pI = 4.0) with 6 consecutive glutamines (Q6) and 8 consecutive threonines (S8) that may function as a transcriptional activation domain (Triezenberg 1995; Schwechheimer and Bevan 1998). To determine if OsWRKY80 has transcriptional activation activity, we fused the full-length OsWRKY80 in frame to the GAL4 DNA binding domain in the pGBKT7 vector and transformed into yeast strain AH109. Empty vector pBD and pBD-WRKY4 (Wang et al. 2015) were used as negative and positive control, respectively. The results showed that cells transformed with pBD-WRKY80 grew well on synthetic SD-Trp-Ade-His selection media (Fig. 3a), indicating that OsWRKY80 is a transcriptional activator in yeast cells. Next, to define the transcriptional activation domain of OsWRKY80, we generated a series of deletion constructs of OsWRKY80 (pBD-dN1, -dN2, -dC1, -dC2 and -dN1C1), and conducted the same analysis. The results indicated that pBD-dN1 (deletion of 60 amino acid residues from the N-terminus), pBD-dC2 (deletion of 102 amino acid residues from the C-terminus) and pBD-dN1C1 (deletion of both N1 and C1) significantly reduced the transcription activity by 27.8% (p < 0.05), 72.1% and 92.9% (p < 0.01), respectively, as indicated by α-galactosidase activity assay. Interestingly, the α-galactosidase activity of pBD-dC1, which contains Q6 and S8 and is 25 aa longer than pBD-dC2, was significantly higher (p < 0.05) than that of pBD-dC2 (Fig. 3b), indicating that Q6 and S8 are important for the transcriptional activation activity. Together these findings suggest that both the N- and the C-terminal region are required for the full transcriptional activation activity of OsWRKY80.
Fig. 3.
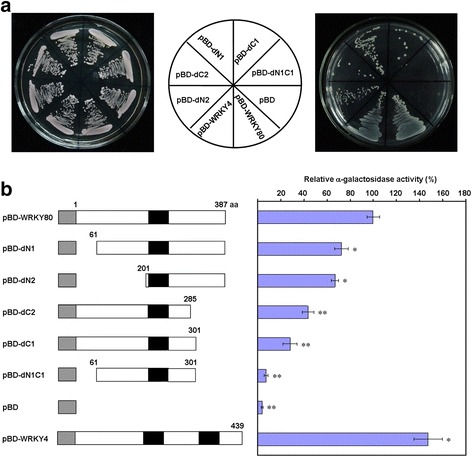
Transcriptional activation activity of OsWRKY80 in yeast cells. The full encoding sequence and deletion derivatives of OsWRKY80 were fused in frame to the GAL4 binding domain (BD) in pBDKT7 (pBD) to generate various vectors for yeast transformation. a The constructed vectors were transformed into yeast AH109 strain, and grew on the selective medium at 30°C for 3 d. Yeast cells carrying different constructs grew on SD-Trp medium (left panel), or SD-Trp-Ade-His (right panel). Middle panel, schematic distribution of yeast cells carrying different vectors. b Assay for a-galactosidase activity. Empty vector pBD and pBD-WRKY4 (Wang et al, 2015) were used as negative and positive control, respectively. The enzymatic activity of cells carrying pBD-WRKY80 was set as 100%. Data are represented as mean values ± SE for three replicates. *, ** indicate a significant difference at P < 0.05 and 0.01, respectively, between the transformant for pBD-WRKY80 and other vectors according to Duncan’s multiple range test. Grey, black rectangles represent BD in pGBKT7 and WRKY domains, respectively. The numbers in each construct are the start and end positions of translation product of OsWRKY80
Modulation of Rice Resistance to Sheath Blight Fungus by OsWRKY80
The induction of OsWRKY80 expression in response to R. solani and exogenous JA and ET suggests its role for plant innate immunity. To test this possibility, we generated transgenic rice T2 lines ectopically expressing OsWRKY80 driven by maize (Zea mays) ubiquitin promoter. RNA interference (RNAi) lines that express a 302-bp inverted-repeat sequence of OsWRKY80 coding region were also generated. The construct for OsWRKY80-overexpression (OX) or RNAi was introduced into the rice cultivar Xiushui 11 by Agrobacterium-mediated transformation, and the expression of OsWRKY80 in transgenic rice plants was determined by Northern blot and qRT-PCR analysis, respectively. Together, 10 and 12 independent transgenic lines of OX and RNAi, respectively, were obtained. Compared to those in the WT plants, OsWRKY80 transcripts were evidently increased in different OsWRKY80 OX lines (Fig. 4a). qRT-PCR revealed that the transcripts of OsWRKY80 were significantly reduced in the RNAi lines (Fig. 4b). All OsWRKY80 OX plants exhibited dwarfism and less crown roots compared with the WT plants (Fig. 5). However, no significant differences in the growth and morphology were observed between RNAi lines and the WT plants (data not shown).
Fig. 4.
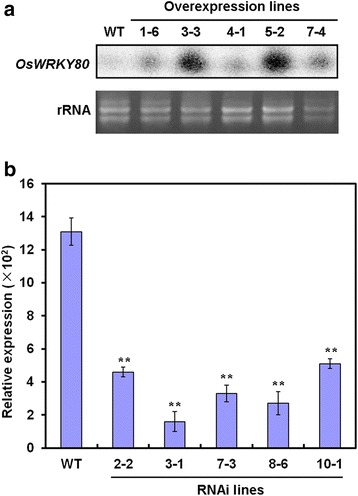
Analysis of the expression levels of OsWRKY80 in transgenic rice lines under normal conditions. Total RNA was prepared from leaves of 4-week-old wild-type (WT) and T2 homozygous progeny of OsWRKY80-overexpressing and RNAi transgenic rice seedlings. a The expression of OsWRKY80 in different overexpression lines was analyzed by RNA gel blot. A 10 μg aliquot of total RNA was loaded per lane. The ethidium bromide stain of rRNA is shown for assessment of equal loading. b The expression of OsWRKY80 in different RNAi lines was determined by qRT-PCR analysis. The mRNA levels of OsWRKY80 were calculated relative to those of rice Actin1 (AK071586). Data are represented as mean values ± standard error (SE) for three replicates. ** indicates a significant difference at P < 0.01 between the transgenic and wild-type plants according to Duncan’s multiple range test
Fig. 5.
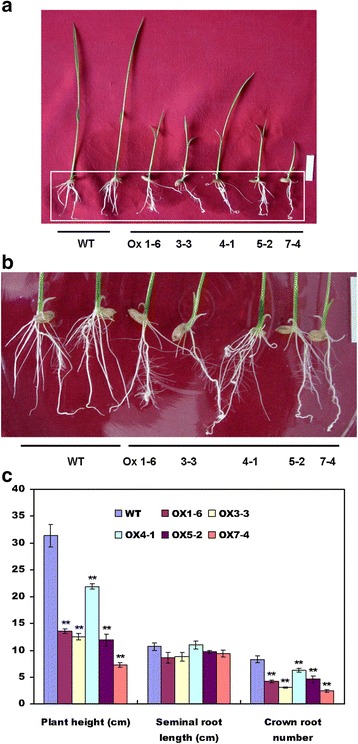
The phenotype of transgenic rice plants overexpressing OsWRKY80. a Plants (T2 generation) grew on ½ MS (containing 0.5% agar) for 14 d. Root architechure of each line in a box with white line is shown in (b). c Histogram showing plant height, seminal root length, crown root number of plants. At least 20 plants of each line were analyzed. Data are represented as mean values ± SE for three replicates. *, ** indicate a significant difference at P < 0.05, 0.01, respectively, between the transgenic and wild-type plants according to Duncan’s multiple range test. OX, overexpression lines; WT, wild-type plants
Next, we selected representative OsWRKY80 OX (OX3-3 and 5-2) and RNAi lines (3-1 and 8-6) for further disease resistance tests. Four-week-old rice plants grown in greenhouse were inoculated with R. solani GD118 strain, and disease symptoms were evaluated 14 days post-inoculation (DPI). The results showed that OsWRKY80 OX plants exhibited reduced susceptible lesions and disease severity compared with the WT plants (Fig. 6a, b). Consistently, the fungal growth in the OX lines was 55.2% (OX3-3) to 65.5% (OX5-2) lower than that in WT plants at 7 DPI (Fig. 6c). By contrast, the RNAi lines exhibited increased susceptibility to the fungal pathogen compared with the WT plants. These findings suggest that OsWRKY80 is a positive modulator of plant defense against the sheath blight fungus.
Fig. 6.
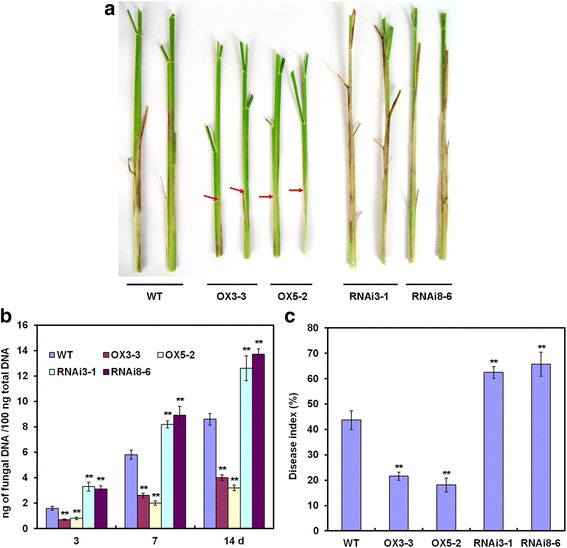
Resistance phenotypes of OsWRKY80-overexpressing and RNAi transgenic rice plants to Rhizoctonia solani. a Disease symptoms in wild-type (WT), OsWRKY80-overexpressing (lines OX3-3 and 5-2), and RNAi (lines 3-1 and 8-6) plants at 14 days after inoculation (DPI) with Rhizoctonia solani GD118. b Progression of sheath blight disease evaluated by quantitating R. solani genomic DNA using qPCR analysis. The amount of R. solani 28S rDNA was calculated relative to rice RUBQ1 (AF184279) DNA. c Disease severity was evaluated as disease index at 14 DPI. Data are represented as mean values ± standard error (SE) for three replicates according to Duncan’s multiple range test (** P < 0.01). 20 plants for each genotype were used for each repetition
OsWRKY80 Positively Regulates OsWRKY4 Expression
Altered resistance phenotypes of OX and RNAi plants (Fig. 6) suggest that OsWRKY80 might control a subset of defense-related genes. To identify potential OsWRKY80 target genes, we evaluated the expression of several well-characterized defense-related genes in WT, OsWRKY80-OX and -RNAi plants. Interestingly, the results were highly similar to those obtained from the study of OsWRKY4 (Wang et al. 2015). The expression of PR1a, PR1b, PR5 and PR10/PBZ1 was elevated in the OsWRKY80-OX lines compared with the WT plants. In contrast, the expression of these marker genes was decreased in the OsWRKY80 RNAi lines (Fig. 7). However, the transcript levels of PR3, LOX, AOS2, PAL/ZB8 and CHS were not significantly different in the transgenic lines compared with the WT plants. These observations suggest that a subset of defense responsive genes is under the control of OsWRKY80 either directly or indirectly. It is noteworthy that these genes are also JA, or ET-responsive (Agrawal et al. 2001; Mei et al. 2006; Wang et al. 2015).
Fig. 7.
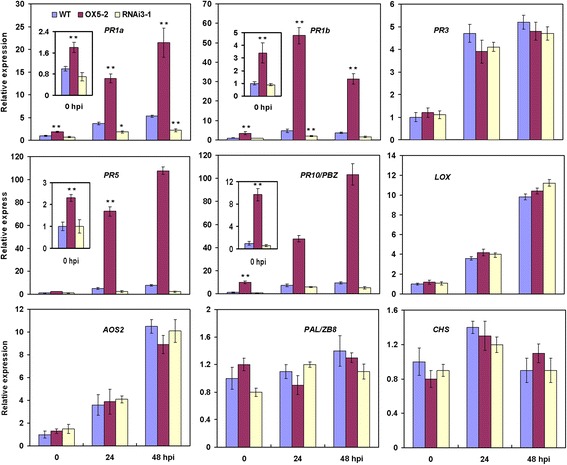
Changes in the expression of defense-related genes in OsWRKY80-overexpressing and RNAi transgenic rice plants. Four-week-old wild-type (WT), OsWRKY80-overexpression (line 5-2) and RNAi (line 3-1) plants were inoculated with R. solani race GD118, and RNA samples were generated from the fourth leaves of each genotype. The time course of gene expression was determined by qRT-PCR analysis. Transcription levels are expressed as the ratio to the level of transcript at 0 h in WT. Data are represented as mean values ± SE for three replicates. Asterisks indicate a significant difference between the transgenic plant and corresponding wild type within the same treatment (* P < 0.05, ** P < 0.01 according to Duncan’s multiple range test). hpi, hours postinoculation
OsWRKY80 and OsWRKY4 display similar expression patterns in response to JA, ET and R. solani (Fig. 1; Wang et al. 2015). Moreover, they regulate the expression of the same subsets of JA-, or ET-responsive defense-associated genes (Fig. 7). These findings raise a possibility that OsWRKY4 and OsWRKY80 coordinately modulate rice defense response to R. solani possibly via a JA/ET signaling pathway. To test this hypothesis, we evaluated in OsWRKY80-OX and -RNAi lines the expression of OsWRKY4 and OsWRKY30 genes, which have been previously characterized as postive modulators in rice defense response to R. solani (Peng et al. 2012; Wang et al. 2015). Only OsWRKY4 was upregulated in the OsWRKY80-OX lines and downregulated in the OsWRKY80-RNAi lines (Fig. 8). However, no changes in OsWRKY80 expression were observed in OsWRKY4 transgenic plants. Likewise, the expression levels of OsWRKY80 and OsWRKY30 in the OsWRKY4 transgenic lines, or those of OsWRKY80 and OsWRKY4 in the OsWRKY30 transgenic lines were not significantly changed (data not shown). These findings suggest that OsWRKY4 acts downstream of OsWRKY80, and OsWRKY30 may act independently of OsWRKY80 and OsWRKY4 in the defense response pathway against rice sheath blight.
Fig. 8.
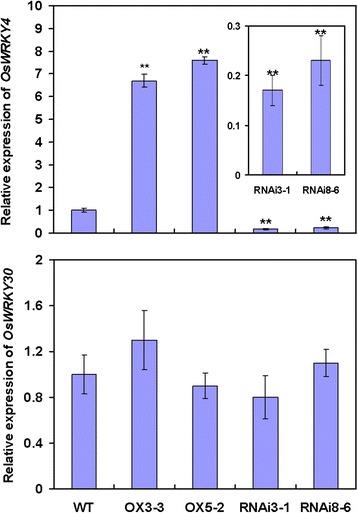
Expression of OsWRKY4 and 30 in OsWRKY80 transgenic rice lines by qRT-PCR analysis. RNA samples were prepared from leaves of 4-week-old wild-type (WT) and T2 homozygous progeny of OsWRKY80-overexpressing and RNAi transgenic rice seedlings. Expression was normalized to that of rice Actin1(AK071586). The transcript level from the wild-type plants was set to 1. Data are represented as mean values ± SE for three replicates. ** indicates a significant difference at P < 0.01 between the transgenic and wild-type plants according to Duncan’s multiple range test
WRKY proteins specifically bind the W-boxes or W-box like elements containing the TGAC core sequence which often exist in the promoters of many defense-related genes, including WRKY genes themselves (Eulgem et al. 2000; Maleck et al. 2000; Turck et al. 2004). Promoter analysis using the PLACE database (http://tenor.dna.affrc.go.jp/) revealed that 2 W-box and 7 W-box like sequences were distributed in the 1.5 kb-promoter of OsWRKY4 (Wang et al. 2015; Fig. 10a). To test whether OsWRKY80 specifically binds to W-box cis-elements in the promoter of OsWRKY4 in planta, we conducted a transient expression assay by agro-infiltration of Nicotiana benthamiana leaves (Yang et al. 2000). The full (P1) and 5’-trucated (P2, P3 and P4) promoters of the OsWRKY4 gene were cloned into a reporter vector with dual luciferases, and infiltrated alone or together with a modified pCambia1301 in which OsWRKY80 is expressed under the control of a maize ubiquitin promoter (Fig. 9a). Results showed that OsWRKY80 significantly enhanced reporter activity driven by the intact promoter (P1) of OsWRKY4, but not by the 5’-deleted P2, P3 and P4 fragments (Fig. 9b). These findings indicate that OsWRKY80 can bind to the promoter of OsWRKY4 in plant cells, and the deleted sequence between P1 and P2 fragments plays a key role in OsWRKY80-mediated OsWRKY4 expression.
Fig. 10.
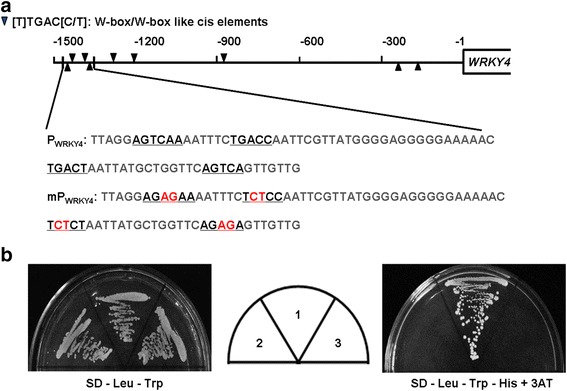
OsWRKY80 binds to OsWRKY4 promoter in yeast. a Distribution of W-box and W-box like elements in the OsWRKY4 promoter, 1.5 Kb upstream from the translational start site. Black triangles indicate W-box or W-box like elements. A 79-nucleotide-long promoter fragment, which contains four W-box or W-box like elements, was indicated, and used for DNA-binding test in yeast. The W-box or W-box like elements are underlined and in black. Bases in red were indicated as the mutant sites. b Yeast cells were co-transformed with a bait vector, containing a promoter fragment in (a) fused to a HIS2 reporter gene, and a prey vector, containing OsWRKY80 fused to a GAL4 activation domain. Left, yeast cells carrying different constructs were grown for 2 d at 30°C in SD-Leu-Trp agar medium. Middle, schematic distribution of yeast cells carrying different vectors; 1, OsWRKY80 + PWRKY4; 2, OsWRKY80 + mPWRKY4; 3, PWRKY4 only. Right panel, positive interactions between OsWRKY80 and target DNA fragments were verified in SD-Leu-Trp-His agar medium with 30 mM 3-amino-1, 2, 4-triazole (3-AT) for suppression of background growth
Fig. 9.
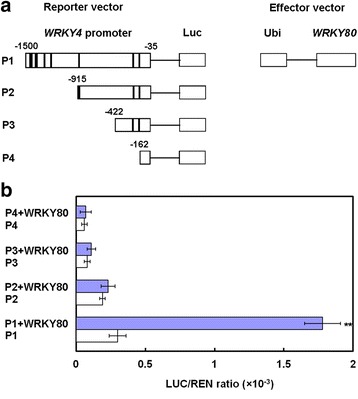
Transient assay for the interaction between OsWRKY80 and the promoter of OsWRKY4 in tobacco. a Diagram of the promoter of WRKY4 showing W-box or W-box like elements (bold vertical lines) in different regions. b Transient expression assay in tobacco. Full-length (P1) and 5’-deleted (P2, P3 and P4) promoters of OsWRKY4 were constructed into the report vector, and OsWRKY80 was cloned into the effect vector, respectively. LUC, Firefly luciferase activity; REN, Renilla luciferase activity (used as the control). Data are represented as mean values ± SE for three replicates. Asterisks indicate a significant difference (** P < 0.01) between the co-infiltration with reporter and effector vectors and the infiltration with reporter vectors alone according to Duncan’s multiple range test
Furthmore, we used the 5’ region (-1429-1356 bp) of OsWRKY4 promoter, which contains 4 tandem W-box or W-box like elements, and the same region with mutations as baits and performed yeast one-hybrid assays. The interactions between OsWRKY80 and these promoter fragments were determined by yeast growth on agar media (-Trp, -Leu, -His) supplemented with 30 mM 3-AT for suppression of leaky growth. As shown in Fig. 10b, cells cotransformed with OsWRKY80 and the native OsWRKY4 promoter fragments grew well on the selective media, whereas those with the corresponding mutant promoter fragments could not grow. These results again indicate that OsWRKY80 could specifically bind to the promoter of OsWRKY4. Interestingly, additional yeast one-hybrid analysis revealed that OsWRKY80 could not bind its own promoter in yeast cells (data not shown), suggesting that OsWRKY80 does not regulate its own expression.
Discussion
Emerging evidence has highlighted the importance of WRKY factors as positive or negative regulators in rice disease resistance networks (Pandey and Somssich 2009). Global gene expression profiling has revealed a large number of WRKY genes that are rapidly induced or repressed upon pathogen infection, suggesting that these WRKY genes may contribute to the regulation of rice response to pathogen infection (Ryu et al. 2006; Bagnaresi et al. 2012; Wei et al. 2013). Moreover, some rice WRKY factors have been characterized to be involved in plant defense responses (Pandey and Somssich 2009; Jimmy and Babu 2015; Phukan et al. 2016). However, the roles of WRKY transcription factors in rice denfense against sheath blight is quite limited. Overexpression of OsWRKY30 enhanced resistance to R. solani and M. oryzae possibly by activating several downstream genes, including JA biosynthesis-related genes (Peng et al. 2012). OsWRKY4 acts as a transcriptional activator in modulating defense response against R. solani (Wang et al. 2015). Both OsWRKY4 and OsWRKY30 belong to group I family. Here, we have added a novel OsWRKY80 gene to the list of WRKY defense regulators in rice- R. solani interaction. Our results demonstrate that overexpression of OsWRKY80 significantly enhanced, whereas suppression of OsWRKY80 by RNAi markedly compromised sheath blight resistance. Furthermore, we show that OsWRKY80 acts upstream of the previously characterized OsWRKY4 in defense signaling pathway against R. solani. On the basis of these results, we conclude that OsWRKY80-OsWRKY4 regulatory circuit plays a positive role in rice defense response to sheath blight infection.
Compared to other groups, a higher percentage of rice WRKY Group II proteins are involved in plant defense responses to pathogens. A systematic expression analysis of OsWRKY genes revealed that the expression of one-third of tested genes was upregulated in response to an incompatible interaction between rice and M. grisea. Among the inducible OsWRKY genes, 8 genes belong to the members of Group II subfamily (Ryu et al. 2006). Moreover, 10 out of 16 OsWRKY genes, which have been characterized to mediate plant innate or induced immunity in rice, belong to Group II. For instance, OsWRKY13 positively mediates rice defense responses against both Xoo and M. grisea (Qiu et al. 2007). OsWRKY6, 13/03, 51 and 71 also positively mediate rice resistance to Xoo (Choi et al. 2015; Liu et al. 2005; Hwang et al. 2016; Liu et al. 2007). On the other hand, some Group IIa proteins, such as OsWRKY28, 62 and 76 are transcriptional repressors, and they act as negative regulators in rice response to M. grisea or Xoo (Chujo et al. 2013; Peng et al. 2008; Yokotani et al. 2013). In the present study, OsWRKY80, a member of the IIe subgroup protein, has also been identified as a positive modulator of rice resistance to sheath blight. Together these results suggest that Group II WRKY proteins represent a major force in mediating plant defense responses against various pathogens. In support of this notion, Arabidopsis Group IIe AtWRKY22 and 29 have been shown as important downstream components of a MAPK pathway that confers resistance to both bacterial and fungal pathogens (Asai et al. 2002). Conversely, AtWRKY27 negatively modulates symptom development caused by Ralstonia solanacearum infection (Mukhtar et al. 2008).
To date, only a few investigations have revealed regulatory cascades amongst different WRKYs. OsWRKY45-2, functioning as a transcriptional activator, directly activates WRKY13. OsWRKY13 functioning as a transcriptional repressor, in turn suppresses OsWRKY42. The three WRKYs form a transcriptional regulatory cascade in the defense signaling pathway against M. oryzae. In the present study, we have identified a new OsWRKY80-OsWRKY4 regulatory circuit in the rice defense signaling pathway to R. solani. First, OsWRKY80 and OsWRKY4 control the same subset of defense-related genes such as PR1a, PR1b, PR5 and PR10/PBZ1, suggesting that the two regulators may be located in the same pathway. Second, the transcript levels of OsWRKY4 were activated by OsWRKY80-overexpression, whereas suppressed by OsWRKY80 RNAi, suggesting that OsWRKY4 may be a target gene of OsWRKY80. By contrast, no changes in the expression of OsWRKY80 were observed in OsWRKY4-overexpressing or -RNAi transgenic rice plants. Finally, the promoter region of the OsWRKY4 gene contains 9 W-box or W-box like elements. Yeast one-hybrid assay and transient expression analysis in tobacco cells results showed that OsWRKY80 could specifically bind to the OsWRKY4 promoter, and the 5’ promoter region containing W-box, or W-box like elements is responsible for the binding activity. Together these lines of evidence clearly suggest that OsWRKY80 acts upstream of OsWRKY4 by regulating its expression.
Functional studies have suggested an intricate regulatory network involved in both WRKY transcription factors and phytohormone signaling pathways. WRKY proteins frequently act as key components and interact with diverse partners related to hormone signaling pathways (Jiang and Yu 2015). In plants, pathogen attack often triggers multiple defense-response signaling pathways mediated by SA, JA and ET. Multiple lines of evidence have also revealed the roles of WRKY factors in modulating the balance between SA- and JA/ET-mediated signaling pathways. For instance, AtWRKY33, a positive regulator of JA/ET-mediated defense response signaling and a negative regulator of SA-mediated defense response signaling, plays an important role in plant defense against necrotrophic pathogens (Zheng et al. 2006). By contrast, OsWRKY13 appears to promote SA-dependent and suppress JA-dependent defense responses, acting in a convergent point of the two defense signal pathways (Qiu et al. 2007). Recently, we have identified OsWRKY4 as a crucial positive regulator in JA/ET-mediated defense signaling pathway (Wang et al. 2015). In this study, OsWRKY80 and WRKY4 gene exhibited similar expression pattern induced by JA, ET and R. solani, bu not by SA (Fig. 1; Wang et al. 2015). More importantly, OsWRKY80 directly binds the promoter of the OsWRKY4 gene, suggesting that OsWRKY4 acts downstream of OsWRKY80 in the defense signaling pathway. These findings strongly suggest that OsWRKY80 may affect defense responses through JA/ET-mediated signaling pathway.
SA and JA/ET signaling pathways mediate resistance against different types of microbial pathogens. SA is usually involved in resistance against biotrophic pathogens (Hammond-Kosack and Parker 2003). On the other hand, the synergistic action of JA and ET is usually induced by necrotrophic pathogens and insects (Bari and Jones 2009). AtWRKY33 is a positive regulator of JA responses. Ectopic overexpression of AtWRKY33 increases resistance to necrotrophic fungal pathogens Botrytis cinerea and Alternaria brassicicola, concomitant with reduced expression of JA-regulated gene PDF1.2 (Zheng et al. 2006). Shimono et al. (2007) reported that overexpression of OsWRKY45, a positive regulator in BTH-induced disease resistance by mediating SA signaling, enhanced resistance against hemibiotrophic M. oryzae and biotrophic Xoo, but not against necrotic R. solani (Shimono et al. 2012). The results presented here showed that OsWRKY80 plays a positive role in the resistance to a necrotic fungal pathogen (Fig. 5). These findings are consistent with the facts that OsWRKY80 activates OsWRKY4 expression, and subsequently activates JA/ET-dependent defense responses, thus protecting rice plants from the necrotic fungus. Thus, both dicots and monocots may share similar defense mechanisms to diverse pathogens through discrete phytohormone-mediated signal pathways.
Conclusions
This study provides data on the role of OsWRKY80 in defense responses. Our results clearly demonstrated that OsWRKY80 contributes to activating defense responses to the rice sheath blight fungus by directly controlling OsWRKY4 via JA/ET-mediated signal pathway. To gain more insights into the integral regulatory network mediated by OsWRKY80 and OsWRKY4, further investigations are needed to explore additional functional linkages with MPK cascades and other novel components.
Methods
Plant Material, Growth Conditions and Chemical Treatments
Rice plants (Oryza sativa L. japonica cv. Xiushui 11) were grown in a greenhouse with day/night cycle of 14/10 h, 28°C/25°C, photosynthetically active radiation (PAR) of 100 μmol m-2 s-1, and relative humidity around 85% in hydroponic culture, as previously described by Peng et al. (2012).
Three-week-old rice seedlings were sprayed with 1 mM SA, 100 μM MeJA and 1 mM ethephon (an ethylene generator) until liquid dripped off the leaves. Control plants were treated in the same way with diluted water or 0.1% methanol (for MeJA only). Leaf samples were harvested at given time points after treatments for total RNA extraction.
Pathogen Inoculation and Disease Investigation
Inoculation with R. solani (race GD118) was carried out according to Wang et al. (2009). Sheath blight disease progression was quantified by measurement of fungal genomic 28S rDNA relative to a rice RUBQ1 gene (AF184279) (Wang et al. 2000) using quantitative real-time PCR (qRT-PCR) analysis (Sayler and Yang 2007). The primer sequences are listed in Additional file 1: Table S1. Symptom development was observed after 14 d and rated on a 0–9 scale based on leaf area affected (Rush et al. 1976). The disease index was calculated by using the formula:
Isolation of OsWRKY80 cDNA
The full-length cDNA of OsWRKY80 was amplified from the total RNA extracted from MeJA-infected rice seedlings by RT-PCR using specific primer pairs: forward primer, 5’- AATACTGAATAGGCAGCAGCAACA -3’ and reverse primer, 5’- GCACAGGCGACCATCATATCATAT -3’. The primers were designed according to the annotated OsWRKY80 gene (Loc_Os03g63810) including its longest open reading frame (ORF). The PCR products were cloned to a pUCmT vector and sequenced for verification.
Gene Expression Analysis
Total RNA was extracted using PureYield™ RNA Midiprep System (Promega). Reverse transcription was performed using 2 μg of total RNA treated with DNase I (Invitrogen) and SuperScript reverse transcriptase II (Invitrogen) according to the manual. qRT-PCR was performed as previously described (Peng et al. 2011). Each experiment was repeated independently three times. Rice Actin1 (AK071586) was used as internal reference (Qiu et al. 2008). Northern blot analysis was carried out as described by Sambrook et al. (1989). The GenBank accession numbers of the defense-related genes examined in the qRT-PCR analysis are as follows: PR1a (AJ278436), PR1b (AK107926), PR3 (D16221), PR5 (OSU77657), PR10/PBZ1 (D38170), LOX (D14000), AOS2 (AY062258), CHS (NM_001058538) and PAL/ZB8 (KF556681). The gene-specific primers for gene expression analysis are listed in Additional file 1: Table S1.
Subcellular Localization
The coding sequence of OsWRKY80 was fused in frame to the N-terminus of an enhanced green fluorescent protein gene (eGFP) in p35S: eGFP (Wang et al. 2007) to generate p35S: WRKY80-eGFP construct. The primers are as follows: 5’-TTGGATCC ATGGATATGATGGAGGAGGA-3’ and 5’-TCACTCGAGGAACTTGTGCCACTGATGATCA-3’ with an underlined BamH I site and Sal I site, respectively. The empty p35S: eGFP vector was used as control. The fusion and the control constructs were transformed into onion (Allium cepa) epidermis cells by particle bombardment using PDS-1000/He (BIO-RAD) (Xie et al. 2003). The transformed cells were incubated on 1/2 MS medium at 28°C for 2 d, and GFP signals were detected by a confocal fluorescence microscope (Bio-Rad MRC 1024).
Transactivation Activity Assay in Yeast
The coding region of OsWRKY80 and its truncated fragments were amplified by PCR and fused to the GAL4 DNA binding-domain (BD) vector pGBKT7 (Clontech) to generate pBD-WRKY80, -dN1 (61-387), -dN2 (201-387), -dC1 (1-301), -dC2 (1-285) and -dN1C1 (61-301) constructs. The PCR primers are listed in Additional file 2: Table S2. The empty vector pGBKT7 was used as negative control. The yeast strain AH109 was transformed with different constructs and grown on SD-Trp-Ade-His selective medium at 30°C for 3 d. The α-galactosidase activity assay was performed with the transformed cell lines grown in liquid SD-Trp medium using p-nitrophenyl α-D-galactopyranoside as a substrate according to the manual.
Generation of Transgenic Plants
The full-length coding sequence of OsWRKY80 was digested with BamH I and Sac I and inserted into a modified pCambia1301 vector under the control of the constitutive maize (Zea mays) ubiquitin promoter (Wang et al. 2007). To construct a plasmid for OsWRKY80 RNAi, part of the WRKY80 cDNA (302 bp, nucleotides 775 to 1076) was amplified by PCR and used to construct self-complementary hairpin vector pCo-Ubi: dsWRKY80 after several steps of enzyme digestions and ligations. The hairpin structure, which is composed of the sense and antisense of OsWRKY80 cDNA fragments seperated by a catalase intron, was put under the control of constitutive maize ubiquitin promoter. The resulting constructs were introduced into rice calli of cultivar Xiushui 11 by the Agrobacterium-mediated transformation method (Hiei et al. 1994).
Promoter Fragments-Binding in Yeast one-Hybrid System
For analysis of the putative W-box (TTGAC[C/T]) or W-box like (TGAC[C/T]) cis-elements in promoters, the 1500 nucleotide sequences upstream of the transcription initiation sites of genes were used to search the PLACE (Plant Cis-acting Regulatory DNA Elements) database available online (http://tenor.dna.affrc.go.jp/).
OsWRKY80 coding region was amplified and in frame fused with the GAL4 activation domain of pGADT7-rec2 prey vector (Clontech), forming pGAD-WRKY80. Construction of pHIS2 vector, yeast cotransformation and growth were performed as previously described (Wang et al. 2015).
Transient Expression Assay in Tobacco (Nicotiana Benthamiana)
The construct for generation of transgenic plants described above was used as the effector vector for transient expression assay. The intact (about 1500 bp) and 5′-deleted promoters of OsWRKY4 were amplified and constructed into the reporter vector pGreenII0800-LUC (Hellens et al. 2005). The recombinant reporter and effector plasmids, or reporter plasmids alone, were transferred into the Agrobacterium GV3101 lines, and infiltrated into the N. benthamiana leaves as described previously by Yang et al. (2000). The Firefly and Renilla luciferase activities were measured using a Dual Luciferase assay kit (Promega) according to the manufacturer’s instructions. The primers used are listed in Additional file 3: Table S3.
Statistical Analysis
Data are represented as mean values ± standard error (SE) for three replicates. Analysis of variance (one-way ANOVA) and multiple comparisons of differences between treatments (Duncan’s multiple range test, p < 0.05, 0.01) were performed using SPSS for Windows version 11.5 (SPSS Inc.).
Acknowledgements
This research was funded by National Natural Science Foundation of China (No. 31301617), Project of Hunan Provincial Natural Science Foundation (No. 2016JJ3060), Project of Scientific Research Fund of Hunan Provincial Education Department (No. 15K045), and Project of China Scholarship Council (No. 201608430089).
Authors’ Contributions
XP performed the experiments of gene cloning and phenotype assays of transgenic plants. HW and XP designed the experiments. XT and HH generated T0, T1 and T2 generations of transgenic rice lines and performed the experiments of molecular analyses. DJ and XT performed analyzed the biochemical characteristics of OsWRKY80. HW and JCJ discussed the research and wrote the manuscript. All authors read and approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Additional files
Gene-specific primers for quantitative real-time PCR or Northern blot analysis. (DOC 48 kb)
Specific primers of transcriptional activity anlysis in yeast cells. (DOC 34 kb)
Specific primers for amplification of the full and 5’-deleted promoters of OsWRKY80. (DOC 29 kb)
References
- Agrawal GK, Rakwal R, Jwa NS. Differential induction of three pathogenesis-related genes, PR10, PR1b and PR5 by the ethylene generator ethephon under light and dark in rice (Oryza sativa L.) seedlings. J Plant Physiol. 2001;158:133–137. doi: 10.1078/0176-1617-00186. [DOI] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- Bagnaresi P, Biselli C, Orrù L, Urso S, Crispino L, Abbruscato P, Piffanelli P, Lupotto E, Cattivelli L, Valè G. Comparative transcriptome profiling of the early response to Magnaporthe oryzae in durable resistant vs susceptible rice (Oryza sativa L.) genotypes. PLoS One. 2012;7:e51609. doi: 10.1371/journal.pone.0051609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R, Jones JD. Role of plant hormones in plant defense responses. Plant Mol Biol. 2009;69:473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- Cheng H, Li H, Deng Y, Xiao J, Li X, Wang S. The WRKY45-2–WRKY13–WRKY42 transcriptional regulatory cascade is required for rice resistance to fungal pathogen. Plant Physiol. 2015;167:1087–1099. doi: 10.1104/pp.114.256016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Hwang SH, Fang IR, Kwon SI, Park SR, Ahn I, Kim JB, Hwang DJ. Molecular characterization of Oryza sativa WRKY6, which bindsto W-box-like element 1 of the Oryza sativa pathogenesis-related (PR) 10a promoter and confers reduced susceptibility to pathogens. New Phytol. 2015;208:846–859. doi: 10.1111/nph.13516. [DOI] [PubMed] [Google Scholar]
- Chujo T, Miyamoto K, Shimogawa T, Shimizu T, Otake Y, Yokotani N, Nishizawa Y, Shibuya N, Nojiri H, Yamane H, Minami E, Okada K. OsWRKY28, a PAMP-responsive transrepressor, negatively regulates innate immune responses in rice against rice blast fungus. Plant Mol Biol. 2013;82:23–37. doi: 10.1007/s11103-013-0032-5. [DOI] [PubMed] [Google Scholar]
- Delteil A, Blein M, Faivre-Rampant O, Guellim A, Estevan J, Hirsch J, Bevitori R, Michel C, Morel JB. Building a mutant resource for the study of disease resistance in rice reveals the pivotal role of several genes involved in defence. Mol Plant Pathol. 2012;13:72–82. doi: 10.1111/j.1364-3703.2011.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Chen C, Chen Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol. 2003;51:21–37. doi: 10.1023/A:1020780022549. [DOI] [PubMed] [Google Scholar]
- Eulgem T. Regulation of the Arabidopsis defense transcriptome. Trends Plant Sci. 2005;10(2):71–78. doi: 10.1016/j.tplants.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/S1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Parker JE. Deciphering plant pathogen communication: fresh perspectives for molecular resistance breeding. Curr Opin Biotechnol. 2003;14:177–193. doi: 10.1016/S0958-1669(03)00035-1. [DOI] [PubMed] [Google Scholar]
- Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Laing WA. Transient plant expression vectors for functional genomics, quantification of promoter activity and RNA silencing. Plant Methods. 2005;1:13. doi: 10.1186/1746-4811-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994;6:271–282. doi: 10.1046/j.1365-313X.1994.6020271.x. [DOI] [PubMed] [Google Scholar]
- Hwang SH, Kwon SI, Jang JY, Fang IL, Lee H, Choi C, Park S, Ahn I, Bae SC, Hwang DJ. OsWRKY51, a rice transcription factor, functions as a positive regulator in defense response against Xanthomonas oryzae pv. oryzae. Plant Cell Rep. 2016;35(9):1975–1985. doi: 10.1007/s00299-016-2012-0. [DOI] [PubMed] [Google Scholar]
- Jiang YJ, Yu DQ. WRKY transcription factors: links between phytohormones and plant processes. Sci Chin Life Sci. 2015;58(5):501–502. doi: 10.1007/s11427-015-4849-9. [DOI] [PubMed] [Google Scholar]
- Jimmy JL, Babu S. Role of OsWRKY transcription factors in rice disease resistance. Trop plant pathol. 2015;40:355–361. doi: 10.1007/s40858-015-0058-0. [DOI] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kim KC, Fan B, Chen Z. Pathogen-induced Arabidopsis WRKY7 is a transcriptional repressor and enhances plant susceptibility to Pseudomonas syringae. Plant Physiol. 2006;142:1180–1192. doi: 10.1104/pp.106.082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel BN, Brooks DM. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol. 2002;5:325–331. doi: 10.1016/S1369-5266(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Li NY, Chai RY, Guo ZJ. The disease resistance of rice regulated by OsWRKY80 gene. Acta Agric Shanghai. 2009;25(3):14–18. [Google Scholar]
- Liu XQ, Xian QB, Qian Q, Xiu JW, Ming SC, Cheng CC, Xiao QL, Xian QB, Qian Q, Xiu JW, Ming SC, Cheng CC (2005) OsWRKY03, a rice transcriptional activator that functions in defense signaling pathway upstream of OsNPR1. Cell Res 15(8):593-603 [DOI] [PubMed]
- Liu X, Xianquan B, Xiujie W, Chengcai C, Xiaoqiang L, Xianquan B, Xiujie W, Chengcai C (2007) OsWRKY71, a rice transcription factor, is involved in rice defense response. J Plant Physiol 164(8):969-979 [DOI] [PubMed]
- Maleck K, Levine A, Eulgem T, Morgan A, Schmid J, Lawton KA, Dangl JL, Dietrich RA. The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat Genet. 2000;26:403–410. doi: 10.1038/82521. [DOI] [PubMed] [Google Scholar]
- Mei C, Qi M, Sheng G, Yang Y. Inducible overexpression of a rice allene oxide synthase gene increases the endogenous jasmonic acid level, PR gene expression, and host resistance to fungal infection. Mol Plant Microbe Interact. 2006;19:1127–1137. doi: 10.1094/MPMI-19-1127. [DOI] [PubMed] [Google Scholar]
- Mukhtar MS, Deslandes L, Auriac MC, Marco Y, Somssich IE. The Arabidopsis transcription factor WRKY27 influences wilt disease symptom development caused by Ralstonia solanacearum. Plant J. 2008;56:935–947. doi: 10.1111/j.1365-313X.2008.03651.x. [DOI] [PubMed] [Google Scholar]
- Mur LA, Kenton P, Atzorn R, Miersch O, Wasternack C. The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 2006;140:249–262. doi: 10.1104/pp.105.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SP, Somssich IE. The role of WRKY transcription factors in plant immunity. Plant Physiol. 2009;150:1648–1655. doi: 10.1104/pp.109.138990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng XX, Hu YJ, Tang XK, Zhou PL, Deng XB, Wang HH, Guo ZJ. Constitutive expression of rice WRKY30 gene increases the endogenous jasmonic acid accumulation, PR gene expression and resistance to fungal pathogens in rice. Planta. 2012;236:1485–1498. doi: 10.1007/s00425-012-1698-7. [DOI] [PubMed] [Google Scholar]
- Peng XX, Tang XK, Zhou PL, Hu YJ, Deng XB, He Y, Wang HH. Isolation and expression patterns of rice WRKY82 transcription factor gene responsive to both biotic and abiotic stresses. Agr Sci China. 2011;10:891–901. doi: 10.1016/S1671-2927(11)60074-6. [DOI] [Google Scholar]
- Peng Y, Bartley LE, Chen XW, Dardick C, Chern M, Ruan R, Canlas PE, Ronald PC. OsWRKY62 is a negative regulator of basal and Xa21-mediated defense against Xanthomonas oryzae pv. oryzae in rice. Mol Plant. 2008;1:446–458. doi: 10.1093/mp/ssn024. [DOI] [PubMed] [Google Scholar]
- Phukan UJ, Jeena GS, Shukla RK. WRKY transcription factors: molecular regulation and stress responses in plants. Front Plant Sci. 2016;7:760. doi: 10.3389/fpls.2016.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu D, Xiao J, Ding X, Xiong M, Cai M, Cao Y, Li X, Xu C, Wang S. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Interact. 2007;20:492–499. doi: 10.1094/MPMI-20-5-0492. [DOI] [PubMed] [Google Scholar]
- Qiu D, Xiao J, Xie W, Liu H, Li X, Xiong L, Wang S. Rice gene network inferred from expression profiling of plants overexpressing OsWRKY13, a positive regulator of disease resistance. Mol Plant. 2008;1:538–551. doi: 10.1093/mp/ssn012. [DOI] [PubMed] [Google Scholar]
- Ricachenevsky FK, Sperotto RA, Menguer PK, Fett JP. Identification of Fe-excess-induced genes in rice shoots reveals a WRKY transcription factor responsive to Fe, drought and senescence. Mol Biol Rep. 2010;37:3735–3745. doi: 10.1007/s11033-010-0027-0. [DOI] [PubMed] [Google Scholar]
- Rice WRKY Working Group Nomenclature report on rice WRKY’s. -Conflict regarding gene names and its solution. Rice. 2012;5:3. doi: 10.1186/1939-8433-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Liu Y, Shen QJ. The WRKY gene family in rice (Oryza sativa) J Integr Plant Biol. 2007;49:827–842. doi: 10.1111/j.1744-7909.2007.00504.x. [DOI] [Google Scholar]
- Rush MC, Hoff BJ, Mcllrath WO. Proceedings of the 16th Rice Technical Working Group, Lake Charles, Louisiana, USA. 1976. A uniform disease rating system for rice disease in the United States; p. 64. [Google Scholar]
- Ryu HS, Han M, Lee SK, Cho JI, Ryoo N, Heu S, Lee YH, Bhoo SH, Wang GL, Hahn TR, Jeon JS. A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Rep. 2006;25:836–847. doi: 10.1007/s00299-006-0138-1. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual. 2. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sayler RJ, Yang Y. Detection and quantification of Rhizoctonia solani AG-1 IA, the rice sheath blight pathogen, in rice using real-time PCR. Plant Dis. 2007;91:1663–1668. doi: 10.1094/PDIS-91-12-1663. [DOI] [PubMed] [Google Scholar]
- Schwechheimer C, Bevan M. The regulation of transcription factor activity in plants. Trends Plant Sci. 1998;3:378–383. doi: 10.1016/S1360-1385(98)01302-8. [DOI] [Google Scholar]
- Shimono M, Koga H, Akagi A, Hayashi N, Goto S, Sawada M, Kurihara T, Matsushita A, Sugano S, Jiang CJ, Kaku H, Inoue H, Takatsuji H. Rice WRKY45 plays important roles in fungal and bacterial disease resistance. Mol Plant Pathol. 2012;13:83–94. doi: 10.1111/j.1364-3703.2011.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono M, Sugano S, Nakayama A, Jiang CJ, Ono K, Toki S, Takatsuji H. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell. 2007;19:2064–2076. doi: 10.1105/tpc.106.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Liu H, Qiu D, Zhou Y, Li X, Xu C, Wang S. A pair of allelic WRKY genes play opposite roles in rice-bacteria interactions. Plant Physiol. 2009;151:936–948. doi: 10.1104/pp.109.145623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triezenberg SJ. Structure and function of transcriptional activation domains. Curr Opin Genet Dev. 1995;5:190–196. doi: 10.1016/0959-437X(95)80007-7. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Sato M, Stoddard T, Glazebrook J, Katagiri F. Network properties of robust immunity in plants. PLoS Genet. 2009;5:e1000772. doi: 10.1371/journal.pgen.1000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck F, Zhou A, Somssich IE. Stimulus-Dependent, promoter-specific binding of transcription factor WRKY1 to its native promoter and the defense-related gene PcPR1-1 in parsley. Plant Cell. 2004;16:2573–2585. doi: 10.1105/tpc.104.024810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ülker B, Somssich IE. WRKY transcription factors: From DNA binding towards biological function. Curr Opin Plant Biol. 2004;7:491–498. doi: 10.1016/j.pbi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Wang HH, Hao JJ, Chen XJ, Hao ZZ, Wang X, Lou YG, Peng YL, Guo ZJ. Overexpression of rice WRKY89 enhances ultraviolet B tolerance and disease resistance in rice plants. Plant Mol Biol. 2007;65:799–815. doi: 10.1007/s11103-007-9244-x. [DOI] [PubMed] [Google Scholar]
- Wang HH, Meng J, Peng XX, Tang XK, Zhou PL, Xiang JH, Deng XB. Rice WRKY4 acts as a transcriptional activator mediating defense responses toward Rhizoctonia solani, the causing agent of rice sheath blight. Plant Mol Biol. 2015;89:157–171. doi: 10.1007/s11103-015-0360-8. [DOI] [PubMed] [Google Scholar]
- Wang J, Jiang J, Oard JH. Structure, expression and promoter activity of two polyubiquitin genes from rice (Oryza sativa L.) Plant Sci. 2000;156:201–211. doi: 10.1016/S0168-9452(00)00255-7. [DOI] [PubMed] [Google Scholar]
- Wang ZB, Zuo SM, Li G, Chen XJ, Chen ZX, Zhang YF, Pan XB. Rapid identification technology of resistance to rice sheath blight in seedling stage. Acta Phytopathol Sin. 2009;39:174–182. [Google Scholar]
- Wei T, Ou B, Li J, Zhao Y, Guo D, Zhu Y, Chen Z, Gu H, Li C, Qin G, Qu LJ. Transcriptional profiling of rice early response to Magnaporthe oryzae identified OsWRKYs as important regulators in rice blast resistance. PLoS One. 2013;8:e59720. doi: 10.1371/journal.pone.0059720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KL, Guo ZJ, Wang HH, Li J. The WRKY Family of transcription factors in rice and Arabidopsis and their Origins. DNA Res. 2005;12:9–26. doi: 10.1093/dnares/12.1.9. [DOI] [PubMed] [Google Scholar]
- Xie C, Zhang JS, Zhou HL, Li J, Zhang ZG, Wang DW, Chen SY. Serine/threonine kinase activity in the putative histidine kinase-like ethylene receptor NTHK1 from tobacco. Plant J. 2003;33:385–393. doi: 10.1046/j.1365-313X.2003.01631.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li R, Qi M. In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J. 2000;22:543–551. doi: 10.1046/j.1365-313x.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- Yokotani N, Sato Y, Tanabe S, Chujo T, Shimizu T, Okada K, Yamane H, Shimono M, Sugano S, Takatsuji H, Kaku H, Minami E, Nishizawa Y. WRKY76 is a rice transcriptional repressor playing opposite roles in blast disease resistance and cold stress tolerance. J Exp Bot. 2013;64:5085–5097. doi: 10.1093/jxb/ert298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Wang LJ. The WRKY transcription factor superfamily : It s origin in eukaryotes and expansion in plants. BMC Evol Biol. 2005;5(1):1–12. doi: 10.1186/1471-2148-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZL, Xie Z, Zou X, Casaretto J, Ho TH, Shen QJ. A rice WRKY gene encodes a transcriptional repressor of the gibberellin signaling pathway in aleurone cells. Plant Physiol. 2004;134:1500–1513. doi: 10.1104/pp.103.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao CJ, Wang AR, Shi YJ, Wang LQ, Liu WD, Wang ZH, Lu GD (2008) Identification of defense-related genes in rice responding to challenge by Rhizoctonia solani. Theor Appl Genet 116:501–516 [DOI] [PubMed]
- Zheng Z, Qamar SA, Chen Z, Mengiste T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 2006;48:592–605. doi: 10.1111/j.1365-313X.2006.02901.x. [DOI] [PubMed] [Google Scholar]


