Abstract
The cerebellum has been shown to be important for skill learning, including the learning of motor sequences. We investigated whether cerebellar transcranial direct current stimulation (tDCS) would enhance learning of fine motor sequences. Because the ability to generalize or transfer to novel task variations or circumstances is a crucial goal of real world training, we also examined the effect of tDCS on performance of novel sequences after training. In Study 1, participants received either anodal, cathodal or sham stimulation while simultaneously practising three eight-element key press sequences in a non-repeating, interleaved order. Immediately after sequence practice with concurrent tDCS, a transfer session was given in which participants practised three interleaved novel sequences. No stimulation was given during transfer. An inhibitory effect of cathodal tDCS was found during practice, such that the rate of learning was slowed in comparison to the anodal and sham groups. In Study 2, participants received anodal or sham stimulation and a 24 h delay was added between the practice and transfer sessions to reduce mental fatigue. Although this consolidation period benefitted subsequent transfer for both tDCS groups, anodal tDCS enhanced transfer performance. Together, these studies demonstrate polarity-specific effects on fine motor sequence learning and generalization.
This article is part of the themed issue ‘New frontiers for statistical learning in the cognitive sciences’.
Keywords: transcranial direct current stimulation, cerebellum, motor skill learning, sequence learning
1. Introduction
Individuals can implicitly acquire the structure of visuomotor response sequences through practice. An extensive body of research using the serial reaction time (SRT) task has shown that reaction time for a repeating sequence of keystrokes in response to locations is reduced compared with that of novel sequences, even when subjects are not aware of the sequence (e.g. [1–3]). Additionally, memory-impaired patients show normal sequence learning in this task, indicating that learning can occur independently of brain structures that support declarative memory including the hippocampus [1,4–6]. Learning structure in the form of motor sequences appears to depend on plasticity in brain regions involved in motor control, including the basal ganglia, motor cortical areas and the cerebellum [7–9]. While these areas certainly interact to produce effective motor behaviour, it is possible that they contribute to different aspects of learning. For example, practising visuomotor sequences leads to improvement that is sequence-specific, but it can also result in transfer to a new sequence. This transfer can be negative, in that learning the original sequence could interfere with performance of new sequences, or it could be positive, with better performance on new sequences than would be seen in a naive subject. Practice could lead to formation of a ‘learning set’ whereby benefits of practice on sequences would facilitate learning of new sequential structures. Here, we are particularly interested in those components of the visuomotor sequence-learning system that can support transfer to new sequences. Using transcranial direct current stimulation (tDCS), we can manipulate brain activity to examine the causal role of distinct brain areas in different processes supporting statistical learning, including those related to the generalization of sequence learning.
In a typical sequence-learning study, participants practise a repeating sequence during a training session. Response times will usually decrease over time; this speed-up reflects non-specific and sequence-specific learning. The presentation of a novel sequence or randomized elements after training is used as a way to determine the amount of sequence-specific learning. Participants' response times should increase when given a new order of elements if a repeating sequence that was learned has disappeared. However, we were interested in maximizing transfer performance on novel sequences and therefore have used training procedures that facilitate transfer. Following work showing that interleaved practice of tasks is beneficial to learning, we have found in previous work using the SRT task that presenting sequences in an interleaved order (e.g. A-B-C, B-C-A, C-B-A, where each letter represents a different sequence) is beneficial for transfer performance on both repetitive and interleaved novel sequences, compared with a repetitive order (e.g. A-A-A, B-B-B, C-C-C) [10]. The non-repetitive order may lead to greater benefits for learning owing to more opportunities for comparison and contrasting among the sequences [11,12]. This could facilitate encoding of the patterns by allowing the learner to more easily detect the differences and similarities among the different sequences. Interleaved training may therefore lead to fundamentally different representations of the individual sequences [12], which would also enable improved generalization to novel patterns. We next conducted a neuroimaging study to investigate the neural bases of transfer to interleaved novel sequences after interleaved training. Blood oxygen level-dependent (BOLD) activity in the cerebellum during practice and transfer were positively associated with transfer performance. Based on another study that suggests internal models representing specific input–output mappings might be blended together when faced with a novel tool [13], representations of specific learned sequences might be formed through error-based learning and then the relevant features from those representations could be applied when novel sequences are encountered. Those participants who were better at transfer may be more sensitive to error signals and therefore have more refined internal models that allow for improved transfer performance on novel sequences. Interleaved training could improve the development of internal models and therefore ultimately lead to an improved foundation for transfer performance.
Although our research highlights the role of the cerebellum in transfer learning, other previous work has shown that the cerebellum is crucial for motor sequence learning, which is supported by the fact that sequence learning is abolished or impaired in patients with cerebellar damage [14–16]. Healthy participants also exhibit cerebellar activity during motor sequence learning (see meta-analysis in [17]) and cerebellar grey matter volume has been associated with the rate of improvement in sequence learning [18]. The cerebellum is hypothesized to be crucial to the instantiation of internal models of different actions [19]. In particular, feed-forward control requiring anticipation of upcoming events in a sequence may be a role for the cerebellum [16,20,21]. Using a visuomotor tracking task, Imamizu and co-workers [22] have shown that the cerebellum is activated in response to errors, which could act to refine the internal model. Similar to some other sequence-learning studies [23–25], BOLD activity in some regions of the cerebellum was highest during the beginning of learning and decreased over time, suggesting less reliance on error feedback as learning continued. Although Imamizu et al.'s study [22] did not examine sequence learning, their results might also imply that errors can shape internal models and can allow for anticipatory responding as different sequences are learned. Furthermore, the cerebellum's role in prediction is not limited to motor tasks, but may also form internal models of mental representations [26]. This hypothesis is based on many studies that have found cerebellar involvement in more cognitive tasks [27] and the uniform microanatomy throughout the cerebellum that suggest similar computations in both motor and cognitive domains [28]. Probably more relevant to sequence learning and generalization, the cerebellum is involved in cognitive functions such as applying rules that constrain responses [29] and language [30].
We hypothesize that deeper encoding (promoted by interleaved training) of the abstract rules that can shape expectancies of forthcoming elements in the task would benefit transfer, as they are common to the trained and novel sequences and thus might be more beneficial to encode [31]. Because the cerebellum appears to be involved in feed-forward control and sequence detection, it could be a promising target for tDCS in facilitating transfer learning to novel motor sequences. tDCS is a non-invasive brain stimulation technique that induces small membrane potential changes that can act to facilitate or inhibit learning, depending on the polarity of stimulation [32]. In particular for cerebellar tDCS, polarity-specific effects have been demonstrated, with anodal stimulation facilitating excitability and cathodal stimulation resulting in diminished excitability [33]. Other studies indicate that the effects of cerebellar tDCS are specific to the cerebellum, without significant spread to the occipital cortex [34–36] and do not significantly affect autonomic function [37,38]. It has been hypothesized that tDCS may affect Purkinje cells [33,39], which receive error signals from climbing fibres and in turn modulate deep cerebellar nuclei output to the cortex.
Only one study thus far has examined the effect of cerebellar tDCS on sequence learning [39]. As a pre-stimulation measure, participants were given a repeating sequence to practice and then a retention test. They then received 20 min of anodal or sham stimulation, and then performed the same task as before as a post-stimulation test 35 min after the end of stimulation. Total learning during the course of practice and retention of the practised sequence were examined. After sham stimulation, participants did not show significant learning over practice or sequence-specific learning. By contrast, participants demonstrated greater total learning and sequence-specific knowledge during practice after anodal tDCS, suggesting that the cerebellar stimulation enhanced subsequent acquisition of the same task. Cerebellar tDCS therefore may have improved error detection during subsequent acquisition [33]. However, the lack of a cathodal stimulation group leaves it unclear as to whether this effect is polarity-specific or a general enhancement regardless of the polarity of stimulation.
Although we are especially interested in enhancing transfer performance, which would suggest the use of anodal stimulation, we have included a cathodal stimulation condition in order to be able to better interpret the results. As anodal and cathodal stimulation appear to have differential effects on cerebellar excitability [33], we hypothesize that there will be polarity-specific effects on learning, such that anodal cerebellar tDCS will facilitate learning and cathodal tDCS will have an inhibitory effect on learning. More specifically, we anticipate that anodal tDCS will lead to improvements in transfer performance, whereas cathodal tDCS will have a detrimental effect on transfer. Furthermore, as in [39], we expect to see a facilitatory effect of anodal tDCS on overall learning and sequence-specific learning, whereas cathodal tDCS would have the opposite effect. In this way, tDCS may have a facilitatory or inhibitory effect on error detection by affecting Purkinje cells [33], leading ultimately to stronger or weaker representations (internal models) in the cerebellum.
2. Study 1
In this study, we administered cerebellar tDCS during interleaved practice of three motor sequences. Immediately after practice and concurrent stimulation were finished, participants were given three novel sequences in an interleaved order during the same visit.
3. Material and methods
(a). Participants
Eighty-two young adults with a mean age of 21.28 years (s.d.age = 4.52 years; 45 women, 36 men, 1 declined to state; 69 right-handed, 10 left-handed, 3 ambidextrous) were recruited and underwent an informed consent process as approved by the Institutional Review Board at UCLA. Inclusion requirements were that participants were between the ages of 18 and 35 years, had normal or corrected-to-normal vision, and were able to make quick movements with fingers, hands or arms. Exclusion criteria were a current medical, neurological or psychiatric diagnosis; chronic medication (excluding contraceptive pills) that could affect sensory processing, movement or cognition; or metals located in the head. Participants rated handedness on a modified questionnaire based on the Edinburgh Handedness Inventory [40]. Participants who did not perform the task with 90% accuracy or greater during practice, or 75% accuracy or greater during transfer were excluded from the analysis. Accuracy was determined by taking the average number of correct key presses over the six blocks of practice or transfer. These criteria were determined a priori and were meant to rule out participants exhibiting large speed–accuracy trade-offs or those who did not follow instructions concerning accuracy. However, we set a lower threshold for the transfer session in order to be able to capture accuracy changes owing to stimulation, if any. One participant was excluded owing to technical difficulties with the stimulation device; four participants were excluded owing to use of an incorrect montage; and 12 more were excluded owing to low accuracy during the task. This yielded a final count of 65 participants (Mage = 21.11 years, s.d.age = 3.91 years; 33 women, 31 men, 1 not stated; 58 right-handed, 5 left-handed and 2 ambidextrous). Participants were compensated with course credit or with cash at a rate of $15 per hour.
(b). Behavioural task and procedure
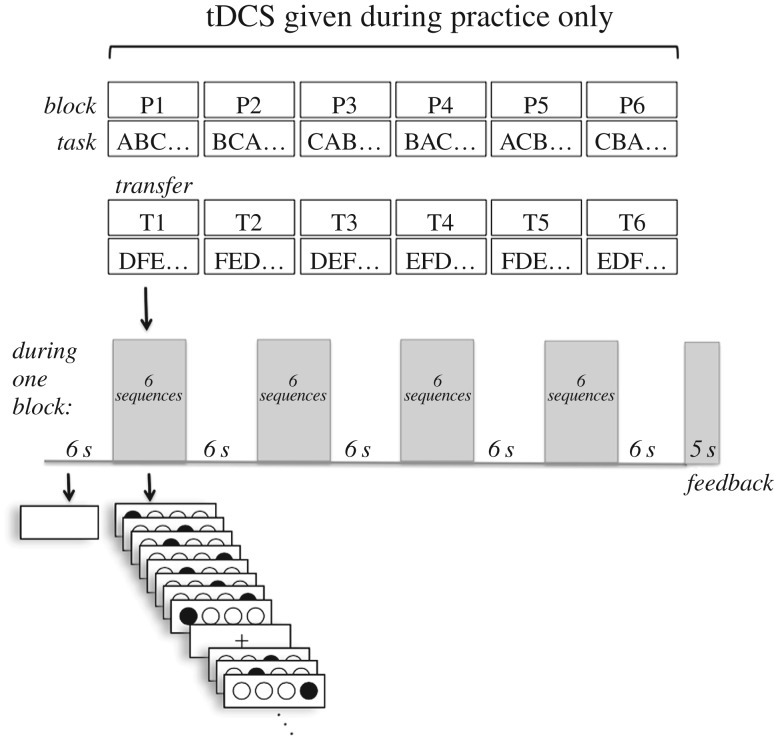
Stimulus presentation and data collection were performed on a 2.6 GHz Macintosh computer using Matlab release 2012 with the Psychophysics Toolbox extensions [41–43]. Four white circles outlined in black were presented on a white background. A target circle was filled with the colour black as a cue for the participant to respond by pressing the spatially corresponding key. The other three circles remained white while the target circle was filled. The participant had 800 ms to respond by pressing a key. An error was recorded if the key press was incorrect or if no key was pressed within the 800 ms response interval. Once a response was made, the target circle turned white for the remainder of the 800 ms. At the end of the response interval, the next target circle turned black. For each sequence, each of the four possible stimulus cues appeared twice for a total of eight elements. Once all eight elements of a sequence were presented, a fixation cross lasting 600 ms appeared before the onset of the next sequence. Between every six sequences, a fixation cross lasting 6 s appeared, and turned red for the final 2 s to alert participants to upcoming sequences.
Two sets of three eight-item sequences were devised so that for each participant, one set was presented during the practice session and the other set during the transfer session. The order of sets was counterbalanced across participants. Sequences could not contain trills (e.g. 1-2-1-2), consecutive runs (e.g. 1-2-3-4) or immediate repetitions (e.g. 2-2). Each element appeared twice within each sequence. The practice session was divided into six blocks of 24 sequences each; thus, each of the three sequences was presented 48 times for a total of 144 sequence presentations in each session. At the end of each block, feedback appeared on the screen for 5 s that showed the average key press reaction time (RT) in milliseconds and the percentage of correct key presses for that block. If the percentage correct was equal to or greater than 90%, a message appeared indicating that performance was satisfactory. However, if the percentage correct was below 90%, a message appeared encouraging the participant to aim for greater accuracy in the following blocks. The format of the transfer session was the same as the practice session except that three novel sequences from the opposite sequence set were presented.
All participants received a non-repeating, intermixed order of sequences during the practice and transfer sessions. The order of the sequences within every group of six sequences that occurred between the 6 s fixation crosses was determined pseudorandomly with the constraints that a sequence could not repeat and that each sequence must appear twice.
At the beginning of the experiment, participants were seated in front of the computer at a comfortable distance of their choosing in a private testing room and were instructed to place the four fingers of the dominant hand on the four consecutive keys C, V, B and N of a keyboard (for a right-handed person, the index finger would be on C, whereas for a left-handed person, the index finger would be on N). If the participant was ambidextrous, the right hand was used. On the screen, instructions told the participants to respond as quickly as possible but also to aim for an accuracy rate of 90% or better. They were informed that they would receive intermittent feedback, and should use it to improve performance. Participants were not aware of practice or transfer schedules they were to receive, nor that novel sequences (the transfer session) would be presented later. After the instructions were read, the participant went through a short practice session. Sequences presented for the practice session were consecutive runs (e.g. 1-2-3-4-3-2-1-2). Once the instructions and the practice session were complete and participants confirmed that they understood the task, participants began the actual experiment. Figure 1 illustrates the experimental procedure and stimuli.
Figure 1.
Participants practised three sequences in an interleaved order (blocks P1–P6) and then received three novel sequences in an interleaved order (blocks T1–T6). Transcranial direct current stimulation (tDCS) was delivered to the cerebellum for the duration of the practice session. In Study 1, transfer occurred immediately after practice and concurrent stimulation. In Study 2, transfer occurred after a 24 h delay. Each letter A–F represents a different eight-element sequence.
Key press RT was measured as the time from cue onset to key press. The eight key press RTs for each sequence were summed to obtain the total RT to complete the sequence to be used in data analysis. The number of errors in each block was also recorded.
(c). Transcranial direct current stimulation procedure
tDCS was delivered by a 9 V battery-driven ActivaDose Iontophoresis Delivery Unit produced by ActivaTek, Inc. Two carbon electrodes were inserted into 5 × 7 cm2 sponges soaked in saline solution and held in place on the scalp with plastic and rubber straps. The active electrode was centred 2 cm below the inion in order to target the cerebellum [6,12]. The reference electrode was placed on the cheek ipsilateral to the dominant hand in order to keep the path of current flow similar relative to the hand being used. In both real and sham stimulation conditions, stimulation was automatically ramped up from 0 milliamps (mA) to 2.0 mA over 20 s and the behavioural task began once stimulation reached 2.0 mA. In real stimulation conditions, 2.0 mA current was delivered for 20 min and then automatically ramped down to 0 mA. In sham stimulation conditions, 2.0 mA current was delivered for only 30 s and then switched to 0.1 mA for the remainder of the 20 min. Each participant experienced only one tDCS condition (anode, cathode or sham stimulation). Stimulation was only applied during the practice session of the behavioural task. At the end of the practice session and the concurrent tDCS, the stimulation device was turned off but the electrodes remained in place while the participants completed the transfer session. The researcher remained in the testing room with the participant during the entire experiment to monitor stimulation.
For each participant, the median RT of each block to complete each sequence during practice and transfer was calculated. As a significant difference in RTs at the beginning of the task was found between the anodal and sham groups (p = 0.044), the mean of the median RTs to complete each sequence for each block during the practice and the transfer sessions was normalized to the first practice block's average median RT so that any initial differences in RT between stimulation groups were controlled for. Normalization was performed by dividing each block's average median RT by the first practice block's average median RT. Figure 2 shows the normalized RTs and the average key press error rates for each stimulation group throughout the practice and transfer sessions. Analyses performed on normalized RT data excluding the first block to which the RT scores were normalized, and analyses on the raw RT data are presented in electronic supplementary material, S1. The results are similar regardless of the way in which the RT data are analysed.
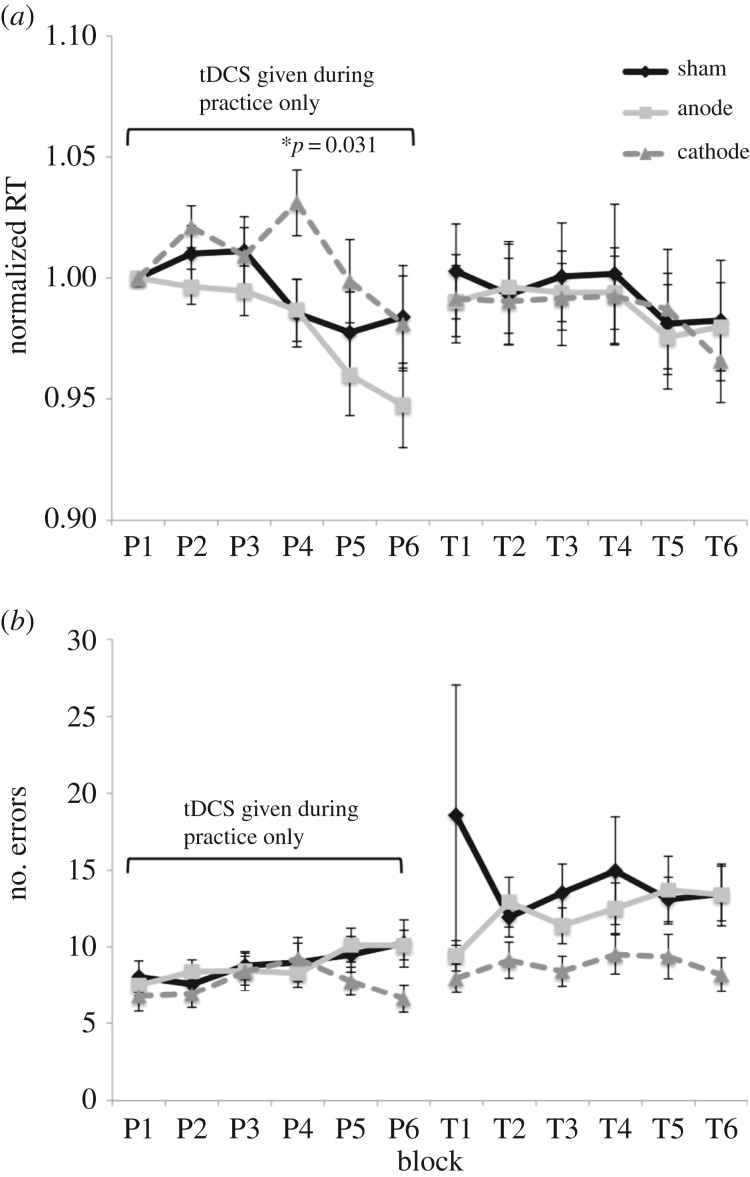
Figure 2.
Study 1 results. (a) RTs normalized to the first practice block RT (P1) to control for any pre-existing differences in performance. There was an interaction between tDCS group and block, such that the cathodal group was significantly slower at P4 than the other two groups. (b) The average number of key press errors during practice and transfer sessions. There was a main effect of tDCS during transfer (p = 0.045) such that the cathodal group made fewer errors than the other two groups after receiving stimulation. (The large variance for the sham group at T1 is owing to one participant who approached 0% accuracy during this block, suggesting fingers were shifted over the wrong keys. The participant's accuracy subsequently improved enough to meet the criteria for inclusion in data analysis.) The practice session consists of P1 through P6, where the number indicates the block. The transfer session consists of T1 through T6.
4. Results
Using normalized RT as the dependent variable, total learning during practice and transfer were examined for each stimulation group by testing for a main effect of block. Sequence-specific learning was defined as the difference between the normalized RTs of the first transfer block and the final practice block. Finally, transfer learning was defined as the difference between the normalized RTs of the first practice block and the first transfer block. The same learning scores were calculated using the average number of key press errors for the relevant blocks.
The normalized RTs from practice and transfer sessions were analysed with MANOVAs, with tDCS condition as a between-subjects factor and practice block as a within-subjects factor. MANOVAs were preferred as the assumption of sphericity was not met, and sphericity indices were less than 0.85, suggesting that MANOVAs would be more powerful than univariate corrections [44]. A significant interaction between tDCS condition and practice block was found, F10,116 = 2.34, p = 0.015. Post hoc univariate ANOVAs and t-tests were then conducted to look for significant differences in RT among the tDCS groups at each practice block. A significant main effect of tDCS condition was found for the fourth practice block, F2,62 = 3.67, p = 0.031. This was because the cathodal group was significantly slower than the sham group at this point, t40 = 2.35, p = 0.024, and significantly slower than the anodal group as well, t42 = 2.37, p = 0.023. However, the anodal group was not significantly different from the sham group, t42 = 0.071, p = 0.944. No other significant differences between RTs among tDCS conditions during practice were found, all p's ≥ 0.170. This pattern of results indicates that the cathodal group slowed during practice in comparison to the first practice block, but this effect was eliminated later in practice. A main effect of block was found during practice, F5,58 = 5.139, p = 0.001, indicating that participants generally were faster over time. No main effect of tDCS condition was found during practice, p = 0.201. For subsequent stimulation-free performance during transfer, no interaction was found between tDCS condition and block, p = 0.944. Furthermore, no main effects of tDCS condition or block were found on normalized RTs during the transfer session, p = 0.959 and p = 0.202, respectively. Finally, there were no differences among tDCS groups in sequence-specific learning, p = 0.432, or transfer learning scores, p = 0.861.
Next, accuracy rates were analysed with MANOVAs, using the number of key press errors as the dependent variable. During practice, there was no interaction between tDCS condition and block for practice, p = 0.223, nor any main effects of tDCS condition or block on the number of errors, p = 0.463 and p = 0.108, respectively. This indicates that despite the increase in RT during practice for the cathodal group, it was not accompanied by a significant change in accuracy. During the stimulation-free transfer session, there was no interaction between tDCS condition and block for practice on accuracy rate, p = 0.736, nor a main effect of block, p = 0.738. There was a main effect of tDCS condition during transfer, F2,62 = 3.27, p = 0.045, such that the cathodal group had significantly fewer errors. No significant differences were found among tDCS groups in sequence-specific learning, p = 0.394, or transfer learning scores, p = 0.328.
5. Study 2
Participants in the first tDCS study performed practice and transfer sequences in one visit. Although the majority of participants were able to perform with high accuracy in both sessions, participants from all tDCS conditions (sham and real) often commented on being tired or bored. It is possible that mental fatigue overcame any effects of stimulation, so for the following study, we added a period of 24 h in between practice and transfer. We also asked participants to rate their level of attention and level of mental fatigue before and after tDCS using visual analogue scales (VASs) to ensure no differences occurred between sham and real stimulation groups.
By testing for transfer a full day after stimulation, we were able to examine effects on learning separate from the online effects or immediate aftereffects of stimulation on performance. Given our hypothesis that increased cerebellar excitability during practice leads to better transfer, Study 2 compared only sham and anodal tDCS conditions.
6. Material and methods
(a). Participants
A total of 38 participants with a mean age of 20.55 years (s.d.age = 2.13 years; 18 women; 35 right-handed) were recruited from the undergraduate student population at UCLA. The same inclusion and exclusion criteria were applied as in Study 1, and all participants underwent an informed consent process as approved by the Institutional Review Board at UCLA. Two participants were excluded owing to low accuracy during practice, one participant was excluded owing to incomplete data and one participant felt dizzy during the initial ramp-up of stimulation and the study was stopped. Thus, our final sample consisted of 34 participants (Mage = 20.32 years, s.d.age = 1.82 years, 17 women, 31 right-handed), with 20 participants in the anodal stimulation group and 14 participants in the sham group.
(b). Behavioural task and transcranial direct current stimulation procedure
The behavioural task was the same as described previously with some exceptions. First, the experiment took place over two visits. Once the practice session was finished, participants were asked to return 24 h later to complete the experiment. The next day during the transfer session, participants performed the three novel sequences. On VASs consisting of 100 mm horizontal lines, participants were asked to draw one vertical line on each to indicate levels of mental fatigue and attention. The number of millimetres out of 100 mm indicated by the participants' vertical line was recorded. Participants were asked to complete the two VASs three times: once during the first visit before practice and concurrent tDCS, again during the first visit immediately after practice and tDCS, and finally during the second visit before performing novel sequences.
7. Results
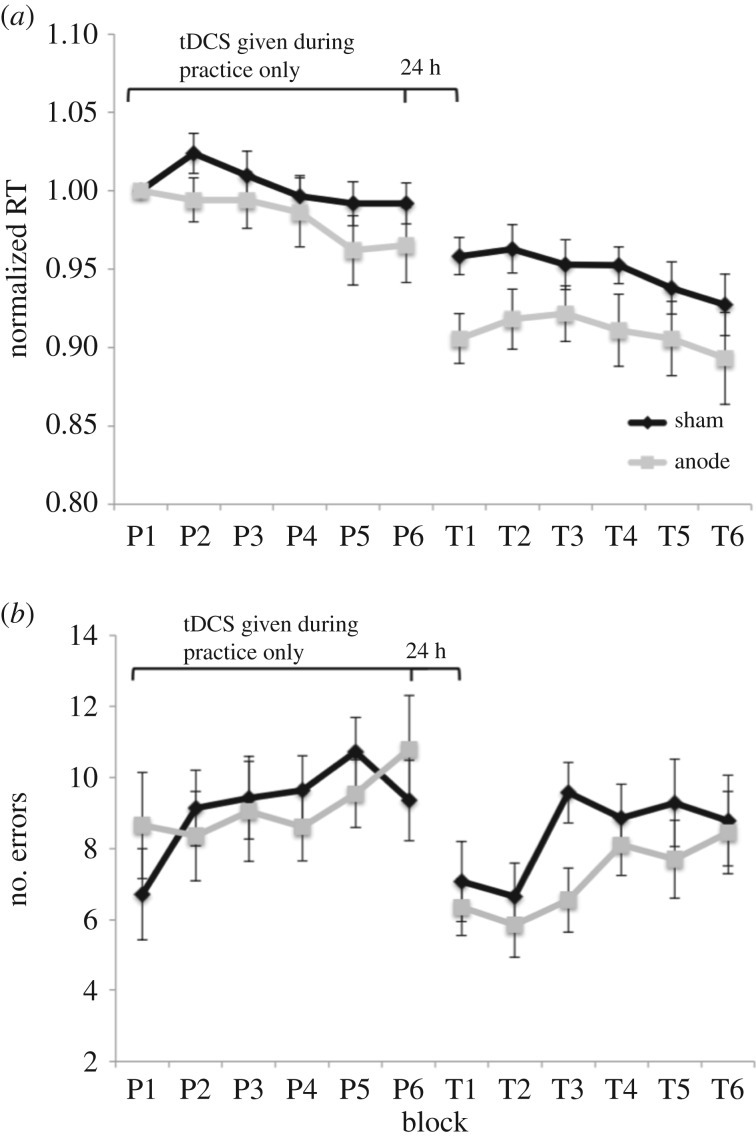
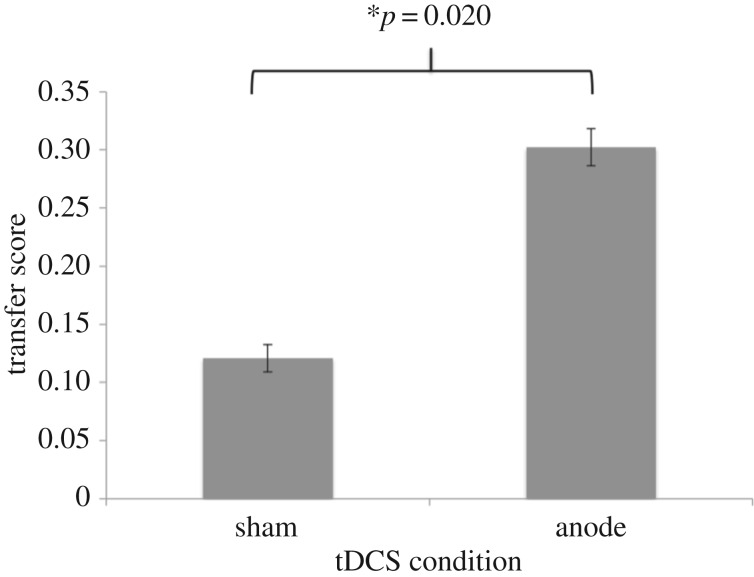
Figure 3 shows the normalized RTs and the error rates throughout the practice session and the transfer session on the following day for the anodal and sham groups. Transfer learning and sequence-specific learning were evaluated as described for Study 1. MANOVAs did not reveal a significant interaction between tDCS condition and practice block, p = 0.699, nor a significant interaction between tDCS condition and transfer block, p = 0.900, meaning that both groups were similar in their rates of learning during practice and during transfer. There were small trends toward main effects of block during practice and transfer, suggesting learning by both tDCS groups over time, p = 0.089 and p = 0.096, respectively. We also did not observe an effect of tDCS condition during practice, p = 0.358, or transfer, p = 0.138. We next examined transfer learning by comparing the RTs of the first practice and first transfer blocks for each of the stimulation groups. As shown in figure 4, both the sham and anodal groups showed significant positive transfer, t13 = 3.49, p = 0.004, and t19 = 5.95, p < 0.001, respectively. Furthermore, there was a significant difference between transfer performance of the sham versus anodal groups, t32 = 2.45, p = 0.020. Finally, no significant differences were found between stimulation groups for sequence-specific learning, p = 0.281. Both the sham and anodal groups performed better at the beginning of the transfer session than at the end of the practice session, t13 = 2.17, p = 0.049 and t19 = 3.58, p = 0.002, respectively. This finding suggests that knowledge expressed in the second session was not specific to sequences in the practice session. Electronic supplementary material, S1 contains additional analyses on normalized and raw RT data. As for Study 1, the results are similar regardless of the way the data are analysed.
Figure 3.
Study 2 results. (a) RTs normalized to the first practice block RT (P1) to control for any pre-existing differences in performance. (b) The average number of key press errors during practice and transfer sessions. The practice session consists of P1 through P6, where the number indicates the block. The transfer session consists of T1 through T6.
Figure 4.
In Study 2, there was a significant difference between transfer scores of the sham and anodal tDCS groups, such that the anodal tDCS group showed greater positive transfer than the sham group.
MANOVAs were also carried out to examine accuracy. There was a trend during practice towards a main effect of block on the number of key press errors, F5,28 = 2.16, p = 0.087, suggesting that participants tended to make more errors over time. There was no main effect of tDCS condition, p = 1.00, nor an interaction between tDCS condition and block during practice, p = 0.678. During transfer, there was a significant main effect of block, F5,28 = 2.79, p = 0.037, indicating that both groups made significantly more errors as the transfer session progressed. However, there was not a main effect of tDCS condition, p = 0.303, nor an interaction between tDCS condition and block, p = 0.512. There were no significant differences between the tDCS conditions for sequence-specific or transfer scores, p = 0.319 and p = 0.246, respectively.
The ratings of mental fatigue and attention levels were analysed next. There was a main effect of the time of the VAS for mental fatigue, F2,31 = 5.19, p = 0.011, meaning that participants in both the sham and anodal groups gave ratings of higher mental fatigue immediately after practice compared to immediately before transfer, p = 008. There was also a trend towards a significant main effect of test time for attention, F2,31 = 2.93, p = 0.068, such that participants tended to rate themselves as more attentive before transfer in comparison to before practice, p = 0.024. However, there were no main effects of tDCS on ratings of mental fatigue and attention, p = 0.547 and p = 0.130, respectively, and no interactions with tDCS and VAS rating time for mental fatigue and attention, p = 0.654 and p = 0.489, respectively.
8. Discussion
In the experiments reported here, tDCS applied to the cerebellum was shown to affect performance and learning of motor sequences. In Study 1, the finding that cathodal stimulation impaired performance of the sequences during practice but improved accuracy during transfer is a novel finding concerning tDCS and sequence learning. In Study 2, anodal tDCS improved transfer learning in comparison to sham.
The participants who received cathodal stimulation initially exhibited longer RTs, but they performed as quickly as the sham and anodal groups during the transfer session. At transfer, the sham and anodal groups exhibit increased error rates but the cathodal group appears to maintain a similar level of accuracy achieved in practice. This pattern may suggest that cathodal tDCS induces a more conservative criterion for movement execution. It could have led to initially slower RTs owing to processes such as a longer internal simulation time. However, while anodal and sham group participants appear to eventually sacrifice some accuracy to move quickly during the transfer session, the cathodal group is able to maintain a relatively low level of errors. One imaging study examining the neural bases of voluntary focus on speed or accuracy while performing a task found that the cerebellum was part of a network of regions involved in response preparation and decision making that was more activated when the participants focused on speed [45]. It is possible that cathodal tDCS acted to increase the threshold for motor execution, which initially led to slower RTs but better accuracy in relation to the sham and anodal tDCS groups after the consolidation period.
The online effect of cathodal stimulation on RTs does not appear to be inhibitory for the entire length of practice, as the initial increase in practice RTs was eliminated during the final two practice blocks. An improvement in accuracy is also seen later during the transfer session. It is possible that homeostatic plasticity mechanisms are responsible for these changes owing to neurons engaging in compensatory regulation of their excitability. One study examining anodal stimulation of 1.0 mA delivered to the primary motor cortex (M1) for either 13 or 26 min found evidence of homeostatic plasticity [46]. M1 excitability increased after 13 min of tDCS, which was the expected excitatory effect of anodal tDCS. By contrast, M1 excitability was reduced after 26 min of stimulation. Thus, there appears to be a reversal in the expected effect of tDCS between 13 and 26 min. In the current experiment, stimulation only lasted for 20 min, and the fourth practice block (where the slowest average median RT occurs) took place between approximately the 10th and 14th minute of stimulation. The decrease in RTs in the fifth and sixth practice blocks is consistent with a reversal in the direction of excitability change between 13 and 26 min. However, homeostatic plasticity mechanisms could have been engaged after a certain amount of time to counteract the excitability shift owing to cathodal stimulation. Synaptic scaling, a type of homeostatic plasticity, can be caused by prolonged changes in neuronal excitation and serves to reduce or increase the strength of all synapses accordingly [47]. This could possibly explain why increasingly faster RTs at the end of practice and improved accuracy during the transfer session become apparent. It is important to note that the comparison of the findings in [46] to those of this study should be interpreted cautiously. Although they measured changes in cortical excitability, we have inferred changes in excitability owing to changes in performance. Additionally, the sites and intensities of stimulation were different, although it is hypothesized that Purkinje cells are polarized in a similar fashion as motor cortical neurons by an applied electric field (for a brief overview, see [48]).
Another recent study examined the effects of cerebellar tDCS on a sequential pinch task [49]. Over three consecutive days, anodal, cathodal, or sham tDCS was given during practice of the task. Anodal stimulation to the cerebellum was found to improve acquisition starting on the first day, mainly by reducing error rates. Our finding of an online effect of stimulation is consistent with theirs. However, it is unclear why we observed an online effect of cathodal stimulation on RT and an offline effect on accuracy whereas they observed an online effect of anodal stimulation. These results together might suggest that cathodal stimulation does not lead to the exact behavioural inverse of anodal tDCS [49], and might depend on characteristics of the task. Differences in task or stimulation protocol may also explain the lack of effects of anodal tDCS on total learning during practice or sequence-specific learning, which was not consistent with the enhanced learning that Ferrucci et al. [39] observed after anodal tDCS was applied after the baseline SRT task. We stimulated during practice of the sequences whereas they stimulated between two sessions of SRT task performance. The effect of stimulation might also be modulated by the relative difficulty of the task, as we presented three interleaved sequences that could have led to greater interference whereas only one repeating sequence was presented by Ferrucci and co-workers.
Our results concerning transfer performance are consistent with the idea that the cerebellum is important for sequence detection and anticipatory responses. This would also predict improvement for sequence-specific learning, but we did not observe sequence-specific learning nor effects of stimulation on sequence-specific learning. It is typical for participants to show learning throughout practice and sequence-specific learning in the SRT task, and we have seen these occur in addition to transfer learning in our own prior work with multiple interleaved sequences [10]. Despite that, the lack of sequence-specific learning was not completely surprising. Training with interleaved sequences is relatively difficult and additionally the tDCS set-up may have been generally distracting for all groups. We expect that with longer training times, decreases in RT during practice and sequence-specific learning would have been observed, and perhaps effects of tDCS also on sequence-specific learning. The results of Study 2 highlight the importance of distinguishing between learning and performance in motor learning studies. While the conditions of practice revealed no measureable improvement, learning necessarily occurred during the practice session given the improvement measured the following day over initial performance.
It has been found that interleaved practice is more beneficial for retention and transfer for a variety of motor skills in comparison to repetitive practice [50,51]. In Study 2, it appears that anodal tDCS to the cerebellum was able to increase the benefit of interleaved training on transfer performance. Both the sham and anodal tDCS groups appeared to benefit from a 24 h period of consolidation as demonstrated by significant positive transfer achieved by both groups. Consolidation would better support transfer performance than the more labile memory traces that were probably present in Study 1 owing to a lack of a prolonged consolidation period. However, the anodal tDCS group demonstrated greater transfer than the sham group. This difference does not appear to be owing to effects of tDCS on levels of attention or mental fatigue. There was no difference in subjective ratings between the two stimulation groups, and the effects of stimulation were apparent the next day, which is unlikely to be a non-specific enhancement of attention or arousal. This result is similar to other research indicating that the effects of tDCS are better observed at a delay [35,39,52] and to Study 1 in that no differences between the sham and anodal group were observed on the same day. It is possible that the aftereffect of cerebellar tDCS, which can last for up to 30 min after stimulation is over [33], could be directly enhancing consolidation processes. However, although the cerebellum may be involved in the consolidation of adaptation skills [53,54], consolidation of motor sequence tasks seem to be more dependent on the striatum [55] and/or M1 [56] while the cerebellum may be more crucial for early learning [17].
Thus, we instead hypothesize that anodal tDCS in combination with interleaved learning may facilitate the instantiation of more generalized internal models during acquisition by increasing the prediction error sensitivity of Purkinje cells [33]. It has been hypothesized that interleaved learning allows for more opportunities to compare and contrast different tasks, leading to better encoding of abstract features that would be useful to retrieve during transfer [11,12]. Both sham and anodal groups practised sequences in an interleaved fashion, but perhaps anodal stimulation led to improved detection and encoding of general rules and features that were relevant to the practised and novel sequences in the task. The encoding of more abstract rules during practice is not inconsistent with the idea that learning-specific representations are instantiated in the cerebellum [13], and it could be that interleaving facilitates the integration of these abstract features into the model for more generalized predictive ability. Consolidation also took place for both stimulation groups, which appeared to be beneficial even without stimulation because both the sham and anodal groups showed positive transfer, but consolidation of a more developed internal model would better support transfer performance. It is also possible that the cerebellum may encode rules for mapping spatial locations onto motor responses [57] and it may also be important for shifting attention to different locations [58] and rapid stimulus-response remapping [59]. Our work is consistent with the hypothesis that the cerebellum encodes internal models that ultimately allow for improved cognitive control [26]. In our task, there were general rules that govern practice and transfer sequence structure and presentation (e.g. no trills within a sequence, one sequence cannot consecutively repeat) and these higher-order aspects may have been encoded in the cerebellum. For example, in our task the learner might generate a prediction of the upcoming sequential element based on the mental model of task structure that had developed through training. This could aid retention performance on trained sequences, but we believe that interleaved training would also lead to better encoding of more abstract features. Once the learner realizes there are multiple sequences, the rules that an element cannot consecutively repeat or that a sequence cannot consecutively repeat could lead to better predictions of the upcoming response to make.
9. Conclusion
Manipulating cerebellar excitability during practice of visuomotor sequences in the SRT task affected both learning and performance. Our results suggest that enhancing plasticity in the cerebellum during practice leads to the formation of a learning set for visuomotor sequences as measured by this task. This may be a specific role of the cerebellum in motor sequence learning, with other structures supporting sequence-specific representations. Further research with more extensive training and more complex motor skills is needed to test this idea, along with comparison of the effects of cerebellar and motor cortical tDCS.
The SRT task has typically been used as a way to examine implicit learning (e.g. [1]), but a few studies have already taken advantage of the SRT task to examine learners' ability to anticipate upcoming sequential elements while considering statistical structure [5,60,61]. Implicit and statistical learning studies share a common goal in understanding the extraction of regularities in the environment during learning [62]. The cerebellum may play a role in optimizing performance in a learned context that requires feed-forward control and prediction, in both motor and cognitive domains. Statistical learning may in part be supported by chunking (e.g. [63,64]), which is typically thought to be a function of the basal ganglia [65–67]. Computations of transitional probabilities that could enable chunk formation might be formed in cortical areas and an efference copy would be sent to the cerebellum [26]. The mental model of probabilities formed in the cortex could be used to anticipate upcoming events. This hypothesis is consistent with work suggesting that computations of transitional probabilities are carried out by temporal and parietal regions [68–70]. Research showing that the cerebellum supports prediction in language [30,71] and the application of higher-order rules [29] might suggest a role for the cerebellum in representing abstract rules, such as transitional probabilities that could guide responses, for more efficient performance in a learned context. Techniques such as tDCS that can influence behaviour through modulation of brain activity could be used to test specific hypotheses concerning the particular causal role of the cerebellum and other cortical areas in statistical learning.
Supplementary Material
Acknowledgements
The authors thank Walter Dunn for insightful discussion.
Ethics
All participants underwent an informed consent process as approved by the UCLA Institutional Review Board.
Data accessibility
The datasets supporting this article have been uploaded to the Figshare repository, https://dx.doi.org/10.6084/m9.figshare.c.3517458.
Authors' contributions
R.E.S. designed the studies, contributed to data collection, analysed and interpreted data, and drafted the manuscript. J.K.S. collected data and contributed to data analysis. A.D.W. and B.J.K. contributed to the design of the studies, interpretation of the data and revision of the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
Support was provided in part by a grant from the National Science Foundation (BCS-1634157) to B.J.K.
References
- 1.Nissen MJ, Bullemer P. 1987. Attentional requirements of learning: evidence from performance measures. Cogn. Psych. 19, 1–32. ( 10.1016/0010-0285(87)90002-8) [DOI] [Google Scholar]
- 2.Grafton ST, Hazeltine E, Ivry R. 1995. Functional mapping of sequence learning in normal humans. J. Cogn. Neurosci. 7, 497–510. ( 10.1162/jocn.1995.7.4.497) [DOI] [PubMed] [Google Scholar]
- 3.Reber PJ, Squire LR. 1994. Parallel brain systems for learning with and without awareness. Learn. Mem. 1, 217–229. ( 10.1101/lm.1.4.217) [DOI] [PubMed] [Google Scholar]
- 4.Reber PJ, Squire LR. 1998. Encapsulation of implicit and explicit memory in sequence learning. J. Cogn. Neurosci. 10, 248–263. ( 10.1162/089892998562681) [DOI] [PubMed] [Google Scholar]
- 5.Curran T. 1997. Higher-order associative learning in amnesia: evidence from the serial reaction time task. J. Cogn. Neurosci. 9, 522–533. ( 10.1162/jocn.1997.9.4.522) [DOI] [PubMed] [Google Scholar]
- 6.Vandenberghe M, Schmidt N, Fery P, Cleeremans A. 2006. Can amnesic patients learn without awareness? New evidence comparing deterministic and probabilistic sequence learning. Neuropsychologia 44, 1629–1641. ( 10.1016/j.neuropsychologia.2006.03.022) [DOI] [PubMed] [Google Scholar]
- 7.Hikosaka O, Miyashita K, Miyachi S, Sakai K, Lu X. 1998. Differential roles of the frontal cortex, basal ganglia, and cerebellum in visuomotor sequence learning. Neurobiol. Learn. Mem. 70, 137–149. ( 10.1006/nlme.1998.3844) [DOI] [PubMed] [Google Scholar]
- 8.Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehéricy S, Benali H. 2009. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 199, 61–75. ( 10.1016/j.bbr.2008.11.012) [DOI] [PubMed] [Google Scholar]
- 9.Penhune VB, Steele CJ. 2012. Parallel contributions of cerebellar, striatal, and M1 mechanisms to motor sequence learning. Behav. Brain Res. 226, 579–591. ( 10.1016/j.bbr.2011.09.044) [DOI] [PubMed] [Google Scholar]
- 10.Shimizu RE, Wu AD, Knowlton BJ. 2016. Cerebellar activation during motor sequence learning is associated with subsequent transfer to new sequences. Behav. Neurosci. ( 10.1037/bne0000164) [DOI] [PubMed] [Google Scholar]
- 11.Shea JB, Morgan RL. 1979. Contextual interference effects on the acquisition, retention, and transfer of a motor skill. J. Exp. Psychol. Hum. Learn. 5, 179–187. ( 10.1037/0278-7393.5.2.179) [DOI] [Google Scholar]
- 12.Shea JB, Zimny ST. 1983. Context effects in memory and learning movement information. Adv. Psychol. Res. 12, 345–366. ( 10.1016/S0166-4115(08)61998-6) [DOI] [Google Scholar]
- 13.Imamizu H, Higuchi S, Toda A, Kawato M. 2007. Reorganization of brain activity for multiple internal models after short but intensive training. Cortex 43, 338–349. ( 10.1016/S0010-9452(08)70459-3) [DOI] [PubMed] [Google Scholar]
- 14.Pascual-Leone A, Grafman J, Clark K, Stewart M, Massaquoi S, Lou JS, Hallett M. 1993. Procedural learning in Parkinson's disease and cerebellar degeneration. Ann. Neurol. 34, 594–602. ( 10.1002/ana.410340414) [DOI] [PubMed] [Google Scholar]
- 15.Shin JC, Ivry RB. 2003. Spatial and temporal sequence learning in patients with Parkinson's disease or cerebellar lesions. J. Cogn. Neurosci. 15, 1232–1243. ( 10.1162/089892903322598175) [DOI] [PubMed] [Google Scholar]
- 16.Molinari M, Leggio MG, Solida A, Ciorra R, Misciagna S, Silveri MC, Petrosini L. 1997. Cerebellum and procedural learning: evidence from focal cerebellar lesions. Brain 120, 1753–1762. ( 10.1093/brain/120.10.1753) [DOI] [PubMed] [Google Scholar]
- 17.Bernard JA, Seidler RD. 2013. Cerebellar contributions to visuomotor adaptation and motor sequence learning: an ALE meta-analysis. Front. Hum. Neurosci. 7, 140–153. ( 10.3389/fnhum.2013.00027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steele CJ, Scholz J, Douaud G, Johansen-Berg H, Penhune VB. 2012. Structural correlates of skilled performance on a motor sequence task. Front. Hum. Neurosci. 6, 289 ( 10.3389/fnhum.2012.00289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolpert DM, Miall RC, Kawato M. 1998. Internal models in the cerebellum. Trends Cogn. Sci. 2, 338–347. ( 10.1016/S1364-6613(98)01221-2) [DOI] [PubMed] [Google Scholar]
- 20.Molinari M, Chiricozzi FR, Clausi S, Tedesco AM, De Lisa M, Leggio MG. 2008. Cerebellum and detection of sequences, from perception to cognition. Cerebellum 7, 611–615. ( 10.1007/s12311-008-0060-x) [DOI] [PubMed] [Google Scholar]
- 21.Ohyama T, Nores WL, Murphy M, Mauk MD. 2003. What the cerebellum computes. Trends Neurosci. 4, 222–227. ( 10.1016/S0166-2236(03)00054-7) [DOI] [PubMed] [Google Scholar]
- 22.Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Ryousuke T, Pütz B, Yoshioka T, Kawato M. 2000. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403, 192–195. ( 10.1038/35003194) [DOI] [PubMed] [Google Scholar]
- 23.Friston KJ, Frith CD, Passingham RE, Liddle PF, Frackowiak RSJ. 1992. Motor practice and neurophysiological adaptation in the cerebellum: a positron tomography study. Proc. R. Soc. Lond. B 248, 223–228. ( 10.1098/rspb.1992.0065) [DOI] [PubMed] [Google Scholar]
- 24.Hazeltine E, Grafton ST, Ivry R. 1997. Attention and stimulus characteristics determine the locus of motor-sequence encoding. A PET study. Brain 120, 123–140. ( 10.1093/brain/120.1.123) [DOI] [PubMed] [Google Scholar]
- 25.Doyon J, Song AW, Karni A, Lalonde F, Adams MW, Ungerleider LG. 2001. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc. Natl Acad. Sci. USA 99, 1017–1022. ( 10.1073/pnas.022615199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito M. 2006. Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci. 9, 304–313. ( 10.1038/nrn2332) [DOI] [PubMed] [Google Scholar]
- 27.Stoodley CJ. 2012. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum 11, 352–365. ( 10.1007/s12311-011-0260-7) [DOI] [PubMed] [Google Scholar]
- 28.Ramnani N. 2006. The primate cortico-cerebellar system: anatomy and function. Nat. Rev. Neurosci. 7, 511–522. ( 10.1038/nrn1953) [DOI] [PubMed] [Google Scholar]
- 29.Balsters JH, Laird AR, Fox PT, Eickhoff SB. 2013. Bridging the gap between functional and anatomical features of cortico-cerebellar circuits using meta-analytic connectivity modeling. Hum. Brain Mapp. 35, 3152–3169. ( 10.1002/hbm.22392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moberget T, Gullesen EH, Andersson S, Ivry RB, Endestad T. 2014. Generalized role for the cerebellum in encoding internal models: evidence from semantic processing. J. Neurosci. 34, 2871–2878. ( 10.1523/JNEUROSCI.2264-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obayashi S. 2004. Possible mechanism for transfer of motor skill learning: implication of the cerebellum. Cerebellum 3, 204–211. ( 10.1080/14734220410018977) [DOI] [PubMed] [Google Scholar]
- 32.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. 2003. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiol. 533, 293–301. ( 10.1113/jphysiol.2003.049916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galea JM, Jayaram G, Ajagbe L, Celnik P. 2009. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J. Neurosci. 29, 9115–9122. ( 10.1523/JNEUROSCI.2184-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parazzini M, Rossi E, Ferrucci R, Liorni I, Priori A, Ravazzani P. 2014. Modelling the electric field and the current density generated by cerebellar transcranial DC stimulation in humans. Clin. Neurophysiol. 125, 577–584. ( 10.1016/j.clinph.2013.09.039) [DOI] [PubMed] [Google Scholar]
- 35.Ferrucci R, Marceglia S, Vergari M, Cogiamanian F, Mrakic-Sposta S, Mameli F, Zago S, Barbieri S, Priori A. 2008. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. J. Cogn. Neurosci. 20, 1687–1697. ( 10.1162/jocn.2008.20112) [DOI] [PubMed] [Google Scholar]
- 36.Rampersad SM, Janssen AM, Lucka F, Aydin U, Lanfer B, Lew S, Wolters CH, Stegeman DF, Oostendorp TF. 2014. Simulating transcranial direct current stimulation with a detailed anisotropic human head model. IEEE Trans. Neural Syst. Rehabil. Eng. 22, 441–452. ( 10.1109/TNSRE.2014.2308997) [DOI] [PubMed] [Google Scholar]
- 37.Parazzini M, Rossi E, Rossi L, Priori A, Ravazzani P. 2013. Numerical estimation of the current density in the heart during transcranial direct current stimulation. Brain Stimul. 6, 457–459. ( 10.1016/j.brs.2012.05.007) [DOI] [PubMed] [Google Scholar]
- 38.Parazzini M, Rossi E, Rossi L, Priori A, Ravazzani P. 2013. Evaluation of the current density in the brainstem during transcranial direct current stimulation with extra-cephalic reference electrode. Clin. Neurophysiol. 124, 1039–1040. ( 10.1016/j.clinph.2012.09.021) [DOI] [PubMed] [Google Scholar]
- 39.Ferrucci R, et al. 2013. Modulating human procedural learning by cerebellar transcranial direct current stimulation. Cerebellum 12, 485–492. ( 10.1007/s12311-012-0436-9) [DOI] [PubMed] [Google Scholar]
- 40.Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. ( 10.1016/0028-3932(71)90067-4) [DOI] [PubMed] [Google Scholar]
- 41.Brainard DH. 1997. The psychophysics toolbox. Spat. Vis. 10, 433–436. ( 10.1163/156856897X00357) [DOI] [PubMed] [Google Scholar]
- 42.Pelli DG. 1997. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat. Vis. 10, 437–442. ( 10.1163/156856897X00366) [DOI] [PubMed] [Google Scholar]
- 43.Kleiner M, Brainard D, Pelli D.2007. What’s new in PsychToolbox-3? [PDF document]. Retrieved from See http://www.kyb.mpg.de/fileadmin/user_upload/files/publications/attachments/ECVP2007-Kleiner-slides_5490%5b0%5d.pdf .
- 44.Algina J, Kesselman HJ. 1997. Detecting repeated measures effects with univariate and multivariate statistics. Psychol. Methods 2, 208–218 ( 10.1037/1082-989X.2.2.208) [DOI] [Google Scholar]
- 45.van Veen V, Krug MK, Carter CS. 2008. The neural and computational basis of controlled speed–accuracy tradeoff during task performance. J. Cogn. Neurosci. 20, 1952–1965. ( 10.1162/jocn.2008.2014) [DOI] [PubMed] [Google Scholar]
- 46.Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, Nitsche MA. 2013. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 6, 424–432. ( 10.1016/j.brs.2012.04.011) [DOI] [PubMed] [Google Scholar]
- 47.Turrigiano G. 2012. Homeostatic synaptic plasticity: Local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol. 4, 1–17. ( 10.1101/cshperspect.a005736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahman A, Toshev PK, Bikson M. 2014. Polarizing cerebellar neurons with transcranial direct current stimulation. Clin. Neurophysiol. 125, 435–438. ( 10.1016/j.clinph.2013.10.003) [DOI] [PubMed] [Google Scholar]
- 49.Cantarero G, Spampinato D, Reis J, Ajagbe L, Thompson T, Kulkarni K, Celnik P. 2015. Cerebellar direct current stimulation enhances on-line motor skill acquisition through an effect on accuracy. J. Neurosci. 35, 3285–3290. ( 10.1523/JNEUROSCI.2885-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magill RA, Hall KG. 1990. A review of the contextual interference effect in motor skill acquisition. Hum. Movement Sci. 9, 241–289. ( 10.1016/0167-9457(90)90005-X) [DOI] [Google Scholar]
- 51.Brady F. 2004. Contextual interference: a meta-analytic study. Percept. Mot. Skills 99, 116–126. [DOI] [PubMed] [Google Scholar]
- 52.Peters MAK, Thompson B, Merabet LB, Wu AD, Shams L. 2013. Anodal tDCS to V1 blocks visual perceptual learning consolidation. Neuropsychologia 51, 1234–1239. ( 10.1016/j.neuropsychologia.2013.03.013) [DOI] [PubMed] [Google Scholar]
- 53.Shadmehr R, Holcomb HH. 1997. Neural correlates of motor memory consolidation. Science 277, 821–825. ( 10.1126/science.277.5327.821) [DOI] [PubMed] [Google Scholar]
- 54.Debas K, et al. 2010. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc. Natl Acad. Sci. USA 107, 17 839–17 844. ( 10.1073/pnas.1013176107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Debas K, et al. 2014. Off-line consolidation of motor sequence learning results in greater integration within a cortico-striatal functional network. Neuroimage 99, 50–58. ( 10.1016/j.neuroimage.2014.05.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steele CJ, Penhune VB. 2010. Specific increases within global decreases: a functional magnetic resonance imaging investigation of five days of motor sequence learning. J. Neurosci. 30, 8332–8341. ( 10.1523/JNEUROSCI.5569-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Albert NB, Weigelt M, Hazeltine E, Ivry RB. 2007. Target selection during bimanual reaching to direct cues is unaffected by the perceptual similarity of the targets. J. Exp. Psychol. 33, 1107–1116. ( 10.1037/0096-1523.33.5.1107) [DOI] [PubMed] [Google Scholar]
- 58.Townsend J, Courchesne E, Covington J, Westerfield M, Harris NS, Lyden P, Lowry TP, Press GA. 1999. Spatial attention deficits in patients with acquired or developmental cerebellar abnormality. J. Neurosci. 19, 5632–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bischoff-Grethe AB, Ivry RB, Grafton ST. 2002. Cerebellar involvement in response reassignment rather than attention. J. Neurosci. 22, 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stadler MA. 1992. Statistical structure and implicit serial learning. J. Exp. Psychol. Learn. Mem. Cogn. 18, 318–327. ( 10.1037/0278-7393.18.2.318) [DOI] [Google Scholar]
- 61.Hunt RH, Aslin RN. 2001. Statistical learning in a serial reaction time task: access to separable statistical cues by individual learners. J. Exp. Psychol. 130, 658–680. ( 10.1037/0096-3445.130.4.658) [DOI] [PubMed] [Google Scholar]
- 62.Perruchet P, Pacton S. 2006. Implicit learning and statistical learning: one phenomenon, two approaches. Trends Cogn. Sci. 10, 233–238. ( 10.1016/j.tics.2006.03.006) [DOI] [PubMed] [Google Scholar]
- 63.Knowlton BJ, Squire LR. 1995. The information acquired during artificial grammar learning. J. Exp. Psychol. 20, 79–91. ( 10.1037/0278-7393.22.1.169) [DOI] [PubMed] [Google Scholar]
- 64.Knowlton BJ, Squire LR. 1996. Artificial grammar learning depends on implicit acquisition of both abstract and exemplar-specific information. J. Exp. Psychol. 22, 169–181. [DOI] [PubMed] [Google Scholar]
- 65.Graybiel AM. 1998. The basal ganglia and chunking of action repertoires. Neurobiol. Learn. Mem. 70, 119–136. ( 10.1006/nlme.1998.3843) [DOI] [PubMed] [Google Scholar]
- 66.Boyd LA, Edwards JD, Siengsukon CS, Vidoni ED, Wessel BD, Linsell MA. 2009. Motor sequence chunking is impaired by basal ganglia stroke. Neurobiol. Learn. Mem. 92, 35–44 ( 10.1016/j.nlm.2009.02.009) [DOI] [PubMed] [Google Scholar]
- 67.Sakai K, Kitaguchi K, Hikosaka O. 2003. Chunking during human visuomotor sequence learning. Exp. Brain Res. 152, 229–242. ( 10.1007/s00221-003-1548-8) [DOI] [PubMed] [Google Scholar]
- 68.McNealy K, Mazziotta JC, Dapretto M. 2006. Cracking the language code: neural mechanisms underlying speech parsing. J. Neurosci. 26, 7629–7639. ( 10.1523/JNEUROSCI.5501-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blakemore S, Rees G, Frith CD. 1998. How do we predict the consequences of our actions? A functional imaging study. Neuropsychologia 36, 521–529. ( 10.1016/S0028-3932(97)00145-0) [DOI] [PubMed] [Google Scholar]
- 70.Bischoff-Grethe A, Proper SM, Mao H, Daniels KA, Berns GS. 2000. Conscious and unconscious processing of nonverbal predictability in Wernicke's Area. J. Neurosci. 20, 1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lesage E, Morgan BE, Olson AC, Meyer AS, Miall RC. 2012. Cerebellar rTMS disrupts predictive language processing. Curr. Biol. 22, R794–R795. ( 10.1016/j.cub.2012.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded to the Figshare repository, https://dx.doi.org/10.6084/m9.figshare.c.3517458.