Abstract
The physiological performance of a reef-building coral is a combined outcome of both the coral host and its algal endosymbionts, Symbiodinium. While Orbicella annularis—a dominant reef-building coral in the Wider Caribbean—is known to be a flexible host in terms of the diversity of Symbiodinium types it can associate with, it is uncertain how this diversity varies across the Caribbean, and whether spatial variability in the symbiont community is related to either O. annularis genotype or environment. Here, we target the Symbiodinium-ITS2 gene to characterize and map dominant Symbiodinium hosted by O. annularis at an unprecedented spatial scale. We reveal northwest–southeast partitioning across the Caribbean, both in terms of the dominant symbiont taxa hosted and in assemblage diversity. Multivariate regression analyses incorporating a suite of environmental and genetic factors reveal that observed spatial patterns are predominantly explained by chronic thermal stress (summer temperatures) and are unrelated to host genotype. Furthermore, we were able to associate the presence of specific Symbiodinium types with local environmental drivers (for example, Symbiodinium C7 with areas experiencing cooler summers, B1j with nutrient loading and B17 with turbidity), associations that have not previously been described.
Keywords: symbiont diversity, Zooxanthellae, environmental drivers, coral bleaching, denaturing gel gradient electrophoresis, Internal Transcribed Spacer 2
1. Background
Symbiodinium, dinoflagellate endosymbionts present in many marine invertebrates, play key roles on tropical coral reefs. Their symbioses with reef-building corals underpin a diverse and productive ecosystem, enabling the establishment of reefs in oligotrophic tropical waters. Symbiodinium translocate organic compounds, generated by photosynthesis, to their coral hosts and can account for 50–70% of reef primary production [1]. Importantly, they also promote calcium carbonate skeletal deposition in their coral hosts, helping to build three-dimensional structure, a process vital to maintaining coral reef ecosystem function [2].
Despite their important role, the diversity and distribution of Symbiodinium taxa are still in the process of being described for many host organisms. Molecular techniques reveal diversity in excess of 400 taxa, nested within nine genetically divergent groups (clades A–I) in what was once thought to be a single pandemic species. The ecological consequences of this diversity are not fully understood [3], but evidence suggests that association with certain Symbiodinium species may affect aspects of coral colony physiology, principally calcification rate and bleaching thresholds [4]. With rising sea temperatures threatening the future of reefs globally [5], recognition of the variation in the coral–endosymbiont association is vital for understanding the capacity of corals to survive and maintain healthy reef growth in a rapidly changing climate [6].
Scleractinian corals predominantly host Symbiodinium clade C, and also associate with clades A, D and (particularly in the Caribbean) B [7]. Although the molecular systematics are complex and ecologically relevant units of diversity are continually being refined [8,9], the genetic markers used for identification are deemed capable of resolving reproductively isolated lineages, conventionally referred to as ‘sub-clades’ or ‘types’. Mapping the distribution of these lineages in and among corals has revealed major partitions based on geography [10,11], bathymetry [12], habitat [13–15] and, importantly, host species [16], with further divisions driven by environmental factors, such as irradiance [17], turbidity [14] and temperature [11,15]. Observed spatial partitioning and experimental manipulations have aided the formation of hypotheses regarding various physiological traits conveyed by different Symbiodinium types.
Here, we focus on characterizing and mapping the distribution of Symbiodinium assemblages hosted by the Caribbean coral Orbicella annularis. Orbicella is the dominant framework-building coral in the tropical Western Atlantic, and its growth supports diverse communities and creates important reef structure [18]. All Orbicella species acquire their Symbiodinium through open modes—from the environment—rather than inheriting them from parents. This allows the coral to be a flexible host, unlike many other Caribbean corals that show fidelity to a single symbiont taxon. Orbicella annularis readily associates with types from at least four genetically distinct taxa (A, B, C and D [17,19,20]), and single colonies frequently host mixed assemblages, with A/C and B/C mixes most common [16,21]. Generally, associations appear stable from year to year [22], although longer-term (decadal) shifts in community composition have been observed [23]. Most studies of O. annularis sampled from either one or two restricted geographical locations [14,16] and/or have only classified Symbiodinium to a limited genetic level [19]. More detailed information is needed to assess the relative importance of host relatedness and environmental drivers in determining Symbiodinium type(s) hosted by this key coral species.
2. Hypotheses
This study explores the hypothesis that (i) Symbiodinium assemblages exhibit spatial structuring in O. annularis across the Wider Caribbean, and (ii) structuring is driven by (H1) environmental heterogeneity, (H2) diversity within the coral host or (H3) geographical isolation.
(a). Null hypothesis (H0): symbiont communities exhibit spatial homogeneity across the Caribbean region within Orbicella annularis
The current known geographical spread of symbiont types in shallow water hosts does not provide conclusive evidence for spatial patterning within O. annularis. Symbiodinium B1 is frequently the dominant symbiont in O. annularis colonies [7,14,17,24] and, along with less commonly occurring A-types [23], tend to be hosted by O. annularis in high-light environments (e.g. unshaded colony tops). Types belonging to clade C are also prevalent in this species, many of which are associated with lower-irradiance habitats, such as deeper reef areas (i.e. >10–15 m) [16,25] or the shaded sides of colonies [17,19]. As this study is concerned with the geographical rather than bathymetric distribution of Symbiodinium, symbiont communities may fail to exhibit any form of spatial structuring at the selected taxonomic resolution and sampling depth (approx. 6 m). Subsequently, we might expect to observe an almost uniform distribution of B1-dominated communities across the coral's range.
(b). Alternate hypotheses (HX): symbiont communities exhibit spatial structuring across the Caribbean within Orbicella annularis
Previous studies have shown O. annularis hosting different symbiont communities across the Caribbean basin (e.g. between Panama and Belize [14], Barbados and the Mesoamerican Barrier Reef [16], and Florida and the Bahamas [24]), yet the details of how this pattern is extrapolated across the region is not known. If spatial partitioning is detected by this study, we hypothesize that observed patterns might be explained by the following.
H1 = environmental heterogeneity. Light and temperature are recognized as environmental controls on Symbiodinium distribution in some hosts, yet few studies examine other environmental drivers of symbiont partitioning, such as water quality, which was shown to play a role in determining symbiont assemblages hosted by a Great Barrier Reef (GBR) coral [26]. Here, we tested a suite of environmental drivers to identify those that could explain spatial partitioning.
H2 = host genetics. Spatial structuring of symbiont communities has been correlated with genetic structuring within their coral hosts across several sites along the GBR [12]. However, studies that have compared the population structures of Caribbean cnidarian hosts and their endosymbiotic partners have not found clear links between host and symbiont distributions, with Symbiodinium populations connected across geographical regions that divide the host [27].
H3 = geographical distance. Environmental and genetic factors may not adequately explain patterns in the distribution of symbionts hosted by O. annularis. Partitioning of clade C across the Caribbean has been shown to be a product of ecological radiations from different areas [8]. Symbiodinium community partitioning (if detected) may best be explained by a combination of host genetics, environmental and geographical factors, perhaps indicating reproductive isolation.
In addressing these hypotheses, we provide the most comprehensive dataset that exists for this species: samples originate from 33 shallow-water sites, stretching approximately 3000 km east–west from Barbados to Belize, and approximately 1800 km north–south from the Bahamas to Colombia (figure 1). By elucidating contemporary biogeographic patterns, our data contribute insights into Symbiodinium genetic diversity and distribution in a key Caribbean reef-building coral, while improving our understanding of the patterns of endosymbiont diversity underpinning coral reef resilience.
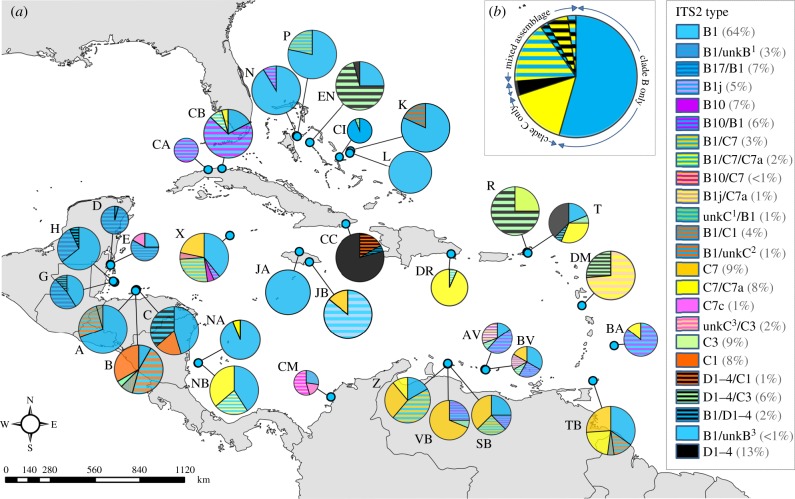
Figure 1.
Map depicting proportions of Symbiodinium B (blue colours), C (yellow) and D (black) types, and combinations (stripes) hosted by Orbicella annularis populations at 33 sites (identified with letters; see table 1 for site information) across the Caribbean and the Bahamas. Only dominant types (or combinations of types) are represented; unk = unknown type. Pie chart size reflects colony sample size (minimum 11, maximum 24, total n = 632), numbers in parentheses indicate proportion of total colonies that each type was dominant in. More blue (clade B) is apparent in the northwest, with more mixed assemblages dominated by clade C (yellow) in the southeast. Inset: pie chart representing clade types found hosted by O. annularis for the entire Wider Caribbean area.
3. Methodology
Orbicella annularis was sampled from 33 sites across the Caribbean and the Bahamas between 2004 and 2007 (figure 1 and table 1). Single 1 cm2 fragments were chiselled from each of the 30 independent O. annularis colonies [28]. Colony tops were targeted to avoid sampling intra-colony Symbiodinium zonation, and collections were limited to shallow depths [17,19]. To explore symbiont diversity, a nuclear ribosomal gene, Internal Transcribed Spacer 2 (ITS2), commonly used to resolve Symbiodinium types [29,30], was targeted. ITS2 has some limitations in its ability to resolve (e.g. B1 [31]) and detect (e.g. D1–4 [32]) certain types, but is a widely used and well-regarded marker, appropriate for exploring broad patterns in symbiont diversity to a taxonomic level relevant to this study. Coral and symbiont DNA were extracted together using DNeasy tissue kits (Qiagen), and prevailing Symbiodinium-ITS2 types within each O. annularis individual were identified using denaturing gel gradient electrophoresis (DGGE) and direct sequencing (following [7]; see electronic supplementary material). Prominently stained gel bands (or pairs of bands) were scored as the dominant symbiont [33] against an ITS2 standard run on each gel. Representatives of every discrete, prominent band were excised under UV-transillumination, cleaned and sequenced (Macrogen) to resolve ITS2 type. Host population genetics were explored using microsatellite markers for six loci [28].
Table 1.
Sampling locations, site names, sampling dates and depths, along with percentage colonies at each site that hosts either exclusively clade B, C or D types, or a mix of types, and species richness and diversity (Simpson's D index) for each site. ‘n.a.’ denotes data not available.
| country | identifier | reef name | depth | collection date | no. samples | relative abundance of clades (%) |
dominant symbiont at each site | richness | diversity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | C | D | B + C | B + D | C + D | B + C + D | |||||||||
| Honduras | A | Seaquest | 4.0 | Oct 2004 | 23 | 70 | 0 | 0 | 30 | 0 | 0 | 0 | B1 | 3 | 0.42 |
| B | Sandy Bay | 6.0 | Oct 2004 | 24 | 17 | 33 | 0 | 50 | 0 | 0 | 0 | B1/C1 | 6 | 0.20 | |
| C | Western Wall | 4.5 | Oct 2004 | 22 | 82 | 18 | 0 | 0 | 0 | 0 | 0 | B1 | 3 | 0.42 | |
| Belize | D | Coral Gardens | 4.5 | Jan 2006 | 24 | 79 | 0 | 0 | 0 | 21 | 0 | 0 | B17 | 4 | 0.31 |
| E | Eagle Ray | 2.0 | Jan 2006 | 16 | 69 | 0 | 6 | 0 | 25 | 0 | 0 | B1/B17 | 5 | 0.23 | |
| G | Long Cay | 6.0 | Jan 2006 | 17 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | B1/B17 | 4 | 0.30 | |
| H | West Reef | 3.5 | Jan 2006 | 14 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | B1/B17 | 4 | 0.29 | |
| Bahamas | CI | Conception Island | 18.6 | May 2007 | 16 | 94 | 0 | 0 | 6 | 0 | 0 | 0 | B1 | 4 | 0.55 |
| EN | Exumas North | 7.9 | Apr 2007 | 24 | 25 | 0 | 0 | 0 | 4 | 50 | 21 | D1–4 | 5 | 0.20 | |
| K | Seahorse Reef | 3.4 | June 2006 | 22 | 82 | 0 | 0 | 18 | 0 | 0 | 0 | B1 | 3 | 0.49 | |
| L | Snapshot Reef | 2.7 | June 2006 | 16 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | B1 | 2 | 0.79 | |
| N | School House Reef | 3.5 | June 2006 | 23 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | B1 | 3 | 0.50 | |
| P | Propeller Reef | 3.0 | June 2006 | 23 | 78 | 0 | 0 | 22 | 0 | 0 | 0 | B1 | 3 | 0.57 | |
| Nicaragua | NA | White Hole | 9.0 | Sep 2007 | 16 | 44 | 0 | 0 | 56 | 0 | 0 | 0 | B1 | 5 | 0.22 |
| NB | Chavo | 10.0 | Sep 2007 | 22 | 18 | 36 | 0 | 45 | 0 | 0 | 0 | B1/C7a | 5 | 0.22 | |
| Colombia | CM | Palo 1 | 8.0 | Oct 2005 | 11 | 9 | 73 | 0 | 18 | 0 | 0 | 0 | C7c | 4 | 0.27 |
| Cuba | CA | Baracoa | 4.0 | Sep 2007 | 24 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | B10 | 3 | 0.47 |
| CB | Bacunayagua | 4.0 | Sep 2007 | 23 | 87 | 0 | 0 | 13 | 0 | 0 | 0 | B10 | 5 | 0.33 | |
| CC | Siboney | 4.0 | Sep 2007 | 24 | 0 | 0 | 71 | 0 | 13 | 17 | 0 | D1–4 | 7 | 0.34 | |
| Cayman | X | Rum Point | 5.0 | Jul 2007 | 23 | 48 | 9 | 0 | 43 | 0 | 0 | 0 | B1/C7 | 4 | 0.34 |
| Dominican Republic | DR | Bayahibe | 6.0 | Oct 2007 | 15 | 0 | 93 | 0 | 7 | 0 | 0 | 0 | C7a | 5 | 0.29 |
| Jamaica | JA | Drunkenmans Cay | 8.0 | Sep 2007 | 18 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | B1 | 3 | 0.68 |
| JB | Dairy Bull | 8.0 | Sep 2007 | 21 | 86 | 14 | 0 | 0 | 0 | 0 | 0 | B1/B8 | 4 | 0.36 | |
| Barbados | BA | Victor's Reef | 11.8 | July 2007 | 14 | 86 | 14 | 0 | 0 | 0 | 0 | 0 | B1j/B1 | 4 | 0.38 |
| BVI | R | Ginger Island | n.a. | Nov 2006 | 24 | 0 | 4 | 0 | 0 | 0 | 79 | 17 | D1–4 | 6 | 0.22 |
| T | Beef Island | n.a. | Nov 2006 | 16 | 25 | 31 | 38 | 6 | 0 | 0 | 0 | B1/D1–4 | 6 | 0.14 | |
| Curaçao | SB | Snakebay | 6.7 | Oct 2005 | 16 | 31 | 38 | 0 | 31 | 0 | 0 | 0 | C7 | 5 | 0.31 |
| VB | Vaersenbay | 6.5 | Oct 2005 | 16 | 6 | 69 | 0 | 25 | 0 | 0 | 0 | C7 | 7 | 0.20 | |
| Z | Buoy 1 | 4.7 | Oct 2005 | 18 | 11 | 39 | 0 | 50 | 0 | 0 | 0 | B1/C7 | 4 | 0.31 | |
| Dominica | DM | Grande Savane | 12.0 | Aug 2007 | 19 | 0 | 74 | 0 | 0 | 0 | 0 | 26 | C7a | 8 | 0.16 |
| Tobago | TB | Buccoo Reef | 3.0 | Sep 2007 | 23 | 52 | 13 | 0 | 35 | 0 | 0 | 0 | C1 | 7 | 0.21 |
| Venezuela | AV | Cayo de Agua | n.a. | Aug 2007 | 13 | 38 | 0 | 0 | 62 | 0 | 0 | 0 | B1j/C7a | 6 | 0.20 |
| BV | Dos Mosquises | n.a. | Aug 2007 | 12 | 42 | 17 | 0 | 42 | 0 | 0 | 0 | B1j/C7a | 7 | 0.18 | |
Maps (figure 1), ordination plots (figure 2) and cluster analyses (figure 3) were used to visualize patterns in the dominant Symbiodinium assemblage composition across the Caribbean. To add statistical support to emergent spatial patterns, SADIE (Spatial Analysis by Distance IndicEs; a statistical approach designed for assessing the patterning of count data from spatially referenced locations) was performed on the count data for symbiont types identified [34]. SADIE generated maps showing the spatial distribution of each type (figure 3; see electronic supplementary material for details).
Figure 2.
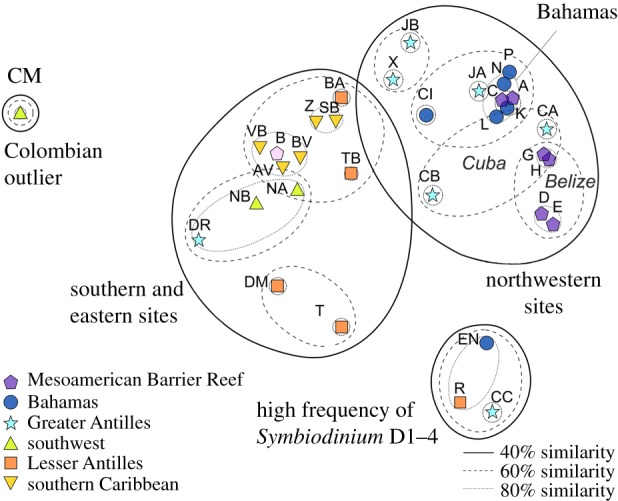
A two-dimensional MDS of square root transformed Symbiodinium type abundance data, based on Bray–Curtis similarities (stress = 0.12). Letters indicate site identifier code; site symbols denote ecoregion. Superimposed clusters were based on a dendrogram (not shown) of site similarities.
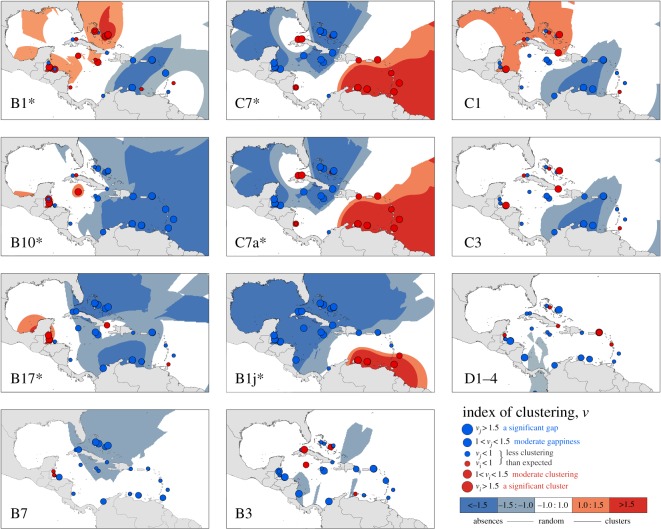
Figure 3.
SADIE index of aggregation plots highlighting areas and sites that showed significant positive (large red circles) and negative (large blue circles) clustering in terms of the presence/absence of Symbiodinium-ITS2 variants. Coloured areas highlight neighbouring groups of sites that share high (or low) degrees of clustering. Six ITS2 variants (B1, B17, B10, B1j, C7 and C7a) showed significant spatial patterns; *p < 0.05.
A distance-based linear regression analysis (DISTLM in PRIMER) was used to model the relationship between multivariate response variables (i.e. derived from representation of each Symbiodinium type among colonies at each site) and a suite of 15 predictors (electronic supplementary material, table S1), including environmental, geographical, temporal and host genetic variables, at the level of reef site. Marginal tests explored the amount of variability explained by each parameter considered independently. A ‘BEST’ model selection procedure was used to identify the combination of available predictor variables that best explained symbiont assemblage partitioning.
A RELATE test was used to further explore the relationship between coral host diversity and symbiont diversity at the multivariate and colony level (DISTLM targeted population level only and assessed univariate descriptors of the host). Resemblance matrices were generated for Symbiodinium count data and for host microsatellite allele score data for six loci (based on pairwise individual genetic distance estimated in GENALEX, 12 allele scores), for 567 individual O. annularis colonies [35]. The RELATE test compared the two matrices to assess the relationship between these two multivariate datasets.
4. Results
(a). Symbiodinium assemblage diversity
The prevailing Symbiodinium assemblages of 632 O. annularis colonies were characterized for 33 sites, with an average of 18 holobiont assemblages resolved per site (figure 1). Fifteen dominant Symbiodinium types were identified (see electronic supplementary material, table S2 for GenBank accession numbers), nested within clades A–D. These were found in different combinations in each colony, with distinct DGGE banding profiles representing different symbiont assemblages.
Symbiont assemblages were dominated by Symbiodinium B1; 64% of corals sampled (405 colonies) hosted B1, 35% (221 colonies) exclusively (figure 1). B17 was the second most commonly occurring B-type, followed by B10, B1j, B3 and three unidentified types (unkB); in total, clade B was found in 70% of the corals sampled (442 colonies). Of the 70%, 80% (355 colonies) hosted Symbiodinium B-types exclusively, with the remainder hosting a B/C mix (72 colonies) or combinations of B/D or B/C/D (15 colonies). Almost 40% of colonies (236 colonies) hosted clade C (predominantly Symbiodinium C7a, C7 and C7c, and also C3 and C1, plus four–unkC–that could not be reliably identified), and 13% (83 colonies) hosted Symbiodinium trenchii, aka D1–4, the only known Caribbean D-type. One sample harboured A13, the only clade A type found.
(b). Symbiodinium distribution patterns
Mapping the dominant symbiont type(s) revealed an emergent pattern in terms of the distribution (figure 1). Assemblages dominated by the most commonly occurring clade B-types (blue), B1, B17 and B10, showed a northwesterly distribution, while C types (yellow), particularly C7 and C7a, appeared more frequently in the eastern and southern Caribbean. D1–4-dominated assemblages were spread across six geographically dispersed sites.
Statistical analyses provided robust support to the northwest/southeast divide in the symbiont distribution identified in the mapping exercise. First, patterns of a symbiont assemblage across sites were explored using ordination plots and a cluster analysis (figure 2). Sites clustered at the 40% similarity level: southeastern populations (more diverse assemblages more often dominated by mixes of C7, C7a, B1 and B1j); central and northwestern sites (including all Cayman, Belize and Jamaican sites, all but one Bahamian and all but one Cuban site—dominated by B1); and a final group comprising three sites heavily (more than 75% of colonies) dominated by D1–4.
Geographical distance became important at the 80% level, with sites less than 100 km apart grouping, such as Belizean sites D and E (located on Caye Caulker), G and H (from Glovers Atoll), and Z and SB (neighbouring sites in Curaçao). More isolated sites (more than 100 km from any other site) hosted less similar assemblages.
SADIE analyses added further statistical support to a northwest/southeast break (figure 3; electronic supplementary material, tables S3 and S4) and were important in identifying the symbiont taxa responsible for driving this biogeographic pattern. Significant spatial patterning was identified in six symbiont taxa (electronic supplementary material, table S3): B1, B17 and B10 were more prevalent in the northwest than the southeast, while C7, C7a and B1j demonstrated the strongest partitioning (electronic supplementary material, table S3), with notable absences from most northern sites (figure 3; electronic supplementary material, table S4). No evidence of any Caribbean-wide spatial patterning was shown for D1–4 symbionts, or any other dominant type (e.g. B3, C3, C1; electronic supplementary material, table S3, figure 3). Site-specific clustering values generated by a separate cluster analysis (electronic supplementary material, table S4) confirmed that B1, B10 and B17 occurred more frequently in Belize (B17, B10) and some Honduran sites (B1), as well as most Bahamas sites (B1), but occurred less frequently than expected in easterly BVI and Curaçao sites. Meanwhile, C7, C7a and B1j were all more commonly hosted in the Lesser Antilles (southeast) and were lacking from corals across the Greater Antilles, the Bahamas and Mesoamerican Barrier Reef (figure 3; electronic supplementary material, table S4). Only one site, R, Ginger Island (BVI), reported a high cluster value for D1–4 (electronic supplementary material, table S4).
(c). Environmental, geographical and genetic drivers of spatial patterning
Chronic thermal stress (maximum monthly mean sea temperature, °C, 1981–2010; electronic supplementary material, figure S1) was identified as the best single predictor of symbiont assemblage in marginal tests, explaining 19% of the observed variability (pseudo-F = 7.25, p < 0.001; electronic supplementary material, table S5). Both geographical distance (pseudo-F = 5.89, p < 0.001) and phosphate concentration (pseudo-F = 3.54, p = 0.011) were also identified as predictors, explaining 16% and 10% of the assemblage variation, respectively. Combining variables improved their explanatory power, with the BEST algorithm identifying a model containing seven variables (distance, acute thermal stress, chronic thermal stress, turbidity, nitrate and phosphate concentration, and sampling year) capable of explaining 54% of the variation (electronic supplementary material, table S5).
The six symbiont types (B1, B17, B1j, B10, C7a and C7) that demonstrated significant spatial patterning across the region (electronic supplementary material, table S3) were investigated independently. Negative linear relationships were identified between chronic thermal stress and the proportion of O. annularis colonies hosting clade C7 (F = 13.84, R2 = 0.41, p = 0.0001) and, to a lesser extent, C7a and B1j (electronic supplementary material, table S6); these three symbionts showed the strongest degree of spatial partitioning. A positive relationship between the occurrence of type B1j (the symbiont with the most strongly structured distribution, electronic supplementary material, table S3) and phosphate concentration was also identified (electronic supplementary material, table S6). B1 and B10 abundances were positively related to wave exposure, while B17 was more prevalent in turbid areas (electronic supplementary material, table S6).
Univariate metrics describing host genetics were not important in explaining symbiont assemblages in the regression analyses. Moreover, the greatest host genotypic richness was in the Bahamas—the poorest area in terms of symbiont diversity—and subsequently no association between symbiont richness and host genotypic richness was found (F = 0.65, R2 = 0.023, p = 0.322). Clonemates—O. annularis colonies with identical genotypes—were found only within sites and were dominated by the same symbiont(s) in 58% of cases (electronic supplementary material, table S7).
The RELATE analysis explored the relationship between symbiont assemblage similarity and host genetic distance matrices, and generated a Spearman's ρ of −0.12. This implied a weak positive relationship (p < 0.001) between host genetic relatedness and symbiont assemblage similarity. Repeating this analysis using different measures of symbiont assemblage (e.g. dominant symbiont types, clade level) produced similar results. When the same analysis was used to test symbiont community composition resemblance matrices against geographical distance matrices, a stronger relationship emerged (ρ = 0.244, p < 0.001).
5. Discussion
Mapping of symbiont distributions (supported by spatial analyses) confirmed the alternate hypotheses (HX) of spatial partitioning in Symbiodinium hosted by shallow water O. annularis across the Wider Caribbean. This was manifest as a northwest–southeast biogeographic divide, with commonly occurring types B1, B17 and B10 more prevalent in the northwest and C7, C7a and B1j in the southeast. This partitioning was best explained by chronic thermal stress (H1), further supported by evidence that C7, C7a and B1j tended to be absent from the sites experiencing warmer summers. Distance (H3) became important at smaller (less than 100 km) spatial scales, and in more fine-scale analyses. Both local environment and distance were more important than host genotype (H2) in explaining the symbiont assemblage, suggesting different mechanisms driving the distributions of the symbiont and its host.
(a). Patterns of Symbiodinium diversity in Orbicella annularis
Reported Caribbean-wide distributions of endosymbiont types corroborated well with more localized previous studies on O. annularis. B1 has been previously reported at sites across this host's entire latitudinal range [7,14,17,33] and was the dominant Symbiodinium type in this study.
The distributions of other Symbiodinium B types (e.g. B10 in Cuba [22], B17 in Belize [16]) also supported previous studies [23] (see electronic supplementary material for details). Despite being associated with deeper/shaded colonies, types C7c, C7 and C7a occurred in reasonable abundance in our samples (agreeing with [8]), with C7 and C7a showing southeasterly distributions (figure 3). C7 has previously been reported in the western Caribbean, whereas C7a has a more easterly distribution [8,16], with overlap around Curaçao [8]. Data from the current study largely supported this (electronic supplementary material).
Not all taxa exhibited spatial partitioning: the distribution of S. trenchii (D1–4) was homogeneous across the sampling region with no evidence of clustering (figure 3). Symbiodinium trenchii made up a minor component (13%) of the total Caribbean-wide assemblages surveyed (but see [32]), and was detected only in abundance (more than 55% of colonies) at 6 of the 33 sites (dominating only three). Other studies report comparable abundances in the Caribbean, with D1–4 typically representing approximately 10% of the Symbiodinium community [32]. Associated with thermal stress events, the ‘patchy’ distribution of S. trenchii may be explained by temporary dominance at the time of sampling [25]. This is probably due to its role as an invasive opportunist [36]. Repeated sampling from these sites to test the temporal stability of the occurrence of this species would be required to explore this hypothesis.
(b). H1: environmental drivers of host/symbiont biogeography
Chronic thermal stress (an indicator of routine ambient summer temperature; electronic supplementary material, figure S1) was the single most informative environmental covariate identified (electronic supplementary material, table S4), agreeing with other studies that identify temperature as a key determinant of distribution [11]. For example, where multiple environmental drivers of symbiosis were tested in Acropora millepora at comparable regional scales (1400 km) along the GBR [26], ‘long term’ (9-year average) SST was similarly identified as explaining the most variation in symbiont assemblage (10.8%), with summer SST and SST anomalies explaining a further 6.9% and 5.4%, respectively. Likewise, other temperature metrics remained important for explaining symbiont assemblage in this study; for example, acute thermal stress added explanatory power and was retained in most models.
An association between C7 and, to a lesser extent, C7a and B1j with chronic thermal stress was also found, suggesting that these endosymbionts may be more restricted in their distribution by thermal sensitivity than Symbiodinium B1 (which demonstrated a broader geographical spread), at least in O. annularis. An alternative explanation is that C7/C7a—usually associated with deeper O. annularis [16,25]—are able to tolerate living in shallower colonies only in areas where summer maxima are less extreme. Partitioning of symbiont assemblages between areas with cooler or warmer summers might influence regional bleaching performance, perhaps explaining why O. annularis found in cooler conditions fare worse under stress [37]. The increase in temperatures across the Caribbean due to climate change may also explain the reported replacement of C7/C7a endosymbionts by B1/B101 variants in O. annularis in the US Virgin Islands [23].
Phosphate concentration was also identified as a predictor, supporting Caribbean and GBR studies that identified water quality as a driver of symbiont community partitioning [14,26]. While turbidity was not an explanatory factor on its own, it was included in the best model and was found to be related to the distribution of B17 in Belize (electronic supplementary material, table S6). Meanwhile, B1j was found in greater abundance in southerly sites where phosphate levels were higher (perhaps linked to river run-off and upwelling). Phosphates are known to inhibit calcification in corals [38,39], and Symbiodinium are thought to remove phosphate with the addition of phosphate and nitrate boosting symbiont numbers [1]. However, as nitrate concentration was not found to be informative, we suggest that this pattern is more likely to be related to latitudinal gradients and river flow in the southeastern Caribbean.
(c). H2: genetic drivers of host/symbiont biogeography
Host genotype could not explain symbiont community variability, leading us to reject hypothesis H2. No clear relationship between coral host genetic diversity and the richness of its symbiont community was apparent at the site level (DISTLM analysis), although a weak but significant association identified at the colony level (RELATE analyses) suggests an underlying environmental variable indirectly driving both factors. While breaks in the genetic structuring of O. annularis host populations [28] (also evident in Acropora sp. [40] and soft corals [27]) suggest a common biogeographic divide for many Caribbean organisms around the Mona Passage (an area of strong current flow between Puerto Rico and Hispaniola), this did not align well with symbiont distributions, with many Symbiodinium types present on both sides of the divide. Studies of other Caribbean cnidarian hosts also fail to identify a clear link between host and symbiont population structures, with Symbiodinium populations often connected across geographical features—like the Mona Passage—that divide the host [27]. For example, no association was found between the population genetics of Symbiodinium type C and host (Sinularia flexibilis) genotype on the GBR [41], or symbiont assemblages and Acropora spp. hosts across Micronesia [42], suggesting that different factors affect the recruitment of host and symbiont. In this study, it seems likely that the spawning nature of O. annularis means host and symbiont genetic diversity are not closely coupled.
(d). H3: geographical distance drivers of host/symbiont biogeography
Geographical distance played a significant role in the partitioning of Symbiodinium community variation in this study. At the population level, the distance remained important in explaining symbiont variation in a single regression (explaining 16% of community variability). Distance was also selected as a variable in the best-fit regression model (alongside environmental parameters explaining 53% of variability). This provides support for a theory of patterns of population structure reflecting hydrodynamic circulation, as observed in clade C on the GBR [41]. While not explaining as much diversity as environmental drivers, when combined, distance parameters accounted for 20% of the symbiont assemblage variability, and were more important than the host genotype or temporal variability. Furthermore, the distance measures corresponded better with the higher-resolution symbiont community measures in the RELATE analyses. Presumably, this finding reflects the natural variation in the reservoir of free-living Symbiodinium available in the local environment, which in turn may reflect evolutionary radiations [8] or local environmental drivers [42].
Another explanation for the abundance of B1 in the northwest might be responses to past environmental change [43], such as marine extinctions in the western tropical Atlantic at the Pliocene/Pleistocene boundary, a hypothesis supported by the low level of ITS2 sequence divergence recorded in clade B [16,31]. Future warming of the Caribbean may even facilitate the expansion of the current range of B1 into the southern and eastern Caribbean [23]. With regions in the east (including the Dominican Republic and Curaçao) harbouring demonstrably higher-than-average community richness and diversity compared with the Bahamas, the Mesoamerican Barrier Reef and Jamaica, a gradual shift towards more B1-dominated assemblages could lessen the arsenal of available symbionts hosted within southeastern O. annularis populations, perhaps compromising their ability to respond to environmental disturbances.
Comparable studies that focus on symbiont biogeography have generally identified host specificity and depth zonation as key determinants of Symbiodinium variability [33]. The distributions described by this study are unlikely to be mirrored by other Caribbean corals, as most species demonstrate higher specificity than O. annularis in the formation of symbioses. Spatial diversity of symbionts in O. annularis is known to be greater at shallower depths (less than 8 m), and so patterns described here are unlikely to be maintained in colonies residing at deeper bathymetries, where C7 and C7a types tend to be exclusively harboured [33].
6. Conclusion
Identifying and mapping symbiont partitioning within a cnidarian host at this unprecedented scale is a key step in advancing our understanding of Symbiodinium diversity, distribution and evolution, and the potential responses of corals to future environmental change. Additionally, our results have identified a correlation between occurrences of Symbiodinium C7 and C7a types and milder summer temperatures, and B1j and B17 with water quality factors. Differences in O. annularis bleaching incidences on the Mesoamerican Barrier Reef in 1995 have been shown to be driven by the variation in the observed distribution of Symbiodinium communities, with fore-reef O. annularis undergoing a higher percentage of bleaching compared with back-reef habitats, as a greater proportion of corals hosted Symbiodinium C-types, which are less tolerant to stress [44]. Knowing that bleaching can vary depending on the Symbiodinium type(s) predominating at a site and that tolerance to bleaching roughly follows D, A>B>>C [17,25], we can extrapolate the results of the current study to predict Caribbean-wide bleaching patterns. However, it must be recognized that ITS2 markers give only a broad overview and that deeper resolution of species [31] is needed to improve the accuracy of ecological/evolutionary conclusions drawn.
The northwest–southeast Caribbean divide identified is likely to be of major consequence as corals are forced to adapt to changing environmental conditions, and may create patchiness in the severity of bleaching across the Caribbean, particularly during global bleaching events (as under way at the time of writing). Our findings on a dominant Caribbean reef-building species will help in explaining and predicting future bleaching patterns. Finally, these data provide a novel baseline that will support valuable insights into evolution-in-action in a coral species that hosts multiple symbiont assemblages.
Supplementary Material
Acknowledgement
We thank our editors and reviewers for helpful comments on the manuscript.
Ethics
The project was assessed and approved by the Department of Biosciences Ethics Committee, University of Exeter, UK. The research was deemed compatible with UK Home Office regulations and a licence to undertake the research was not required. The necessary permits for collection and export of coral samples were provided by the Department of Fisheries, Nassau, the Bahamas; Fisheries Department, Belize City, Belize; Secretaria de Agricultura y Ganaderia Despacho Ministerial, Tegucigalpa, Honduras; Department of Public Health, Willemstad, Curaçao; Government of Colombia. CITES import permits and Animal Health Licences were provided by the Department for the Environment, Food and Rural Affairs, Bristol, UK.
Data accessibility
Additional methods and description of results and four tables are included as the electronic supplementary material.
Authors' contributions
N.L.F., P.J.M. and J.R.S. collected field data; E.V.K., N.L.F. and L.T. carried out the molecular laboratory work with support of S.D. and O.H.-G.; I.C. provided all environmental data; E.V.K. and L.T. participated in molecular analyses and sequence alignments; E.V.K. carried out the statistical analyses with guidance from J.-C.O. and I.C.; E.V.K. drafted the manuscript; J.R.S., P.J.M. and N.L.F. conceived the study; E.V.K., J.R.S. and L.T. designed the study; E.V.K. and J.R.S. coordinated the study; and all authors helped refine the manuscript. All authors gave final approval for publication.
Competing interests
All authors declare that there are no conflicting or competing interests relating to this article.
Funding
This project was funded primarily by an NERC grant, no. NE/E010393/1 (J.R.S. and P.J.M.), European Union FP7 project Future of Reefs in a Changing Environment (FORCE) under grant agreement no. 244161 (P.J.M. and J.R.S.) and a University of Exeter studentship (E.V.K.).
References
- 1.Muscatine L, Porter JW. 1977. Reef corals: mutualistic symbioses adapted to nutrient-poor environments. BioScience 27, 454–460. ( 10.2307/1297526) [DOI] [Google Scholar]
- 2.Kennedy EV, et al. 2013. Avoiding coral reef functional collapse requires local and global action. Curr. Biol. 23, 912–918. ( 10.1016/j.cub.2013.04.020) [DOI] [PubMed] [Google Scholar]
- 3.Heath KD, Stinchcombe JR. 2014. Explaining mutualism variation: a new evolutionary paradox? Evolution 68, 309–317. ( 10.1111/evo.12292) [DOI] [PubMed] [Google Scholar]
- 4.Ortiz JC, González-Rivero M, Mumby PJ. 2013. Can a thermally tolerant symbiont improve the future of Caribbean coral reefs? Glob. Change Biol. 19, 273–281. ( 10.1111/gcb.12027) [DOI] [PubMed] [Google Scholar]
- 5.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. ( 10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 6.Ortiz JC. 2008. Coral holobiont community structure: how much have we missed by focusing only in the coral host? In Proc. of the Int. Soc. for Coral Reef Studies, 2008, Miami, FL, USA.
- 7.LaJeunesse TC. 2002. Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar. Biol. 141, 387–400. ( 10.1007/s00227-002-0829-2) [DOI] [Google Scholar]
- 8.Thornhill DJ, Lewis AM, Wham DC, LaJeunesse TC. 2014. Host-specialist lineages dominate the adaptive radiation of reef coral endosymbionts. Evolution 68, 352–367. ( 10.1111/evo.12270) [DOI] [PubMed] [Google Scholar]
- 9.Arif C, Daniels C, Bayer T, Banguera-Hinestroza E, Barbrook A, Howe CJ, LaJeunesse TC, Voolstra CR. 2014. Assessing Symbiodinium diversity in scleractinian corals via next-generation sequencing-based genotyping of the ITS2 rDNA region. Mol. Ecol. 23, 4418–4433. ( 10.1111/mec.12869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaJeunesse TC, Bhagooli R, Hidaka M, DeVantier L, Done T, Schmidt GW, Fitt WK, Hoegh-Guldberg O. 2004. Closely related Symbiodinium spp. differ in relative dominance in coral reef host communities across environmental, latitudinal and biogeographic gradients. Mar. Ecol. Prog. Ser. 284, 147–161. ( 10.3354/meps284147) [DOI] [Google Scholar]
- 11.Tonk L, Sampayo EM, Weeks S, Magno-Canto M, Hoegh-Guldberg O. 2013. Host-specific interactions with environmental factors shape the distribution of Symbiodinium across the Great Barrier Reef. PLoS ONE 8, e68533 ( 10.1371/journal.pone.0068533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bongaerts P, Riginos C, Ridgway T, Sampayo EM, van Oppen MJH, Englebert N, Vermeulen F, Hoegh-Guldberg O. 2010. Genetic divergence across habitats in the widespread coral Seriatopora hystrix and its associated Symbiodinium. PLoS ONE 5, e10871 ( 10.1371/journal.pone.0010871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green EA, Davies SW, Matz MV, Medina M. 2014. Quantifying cryptic Symbiodinium diversity within Orbicella faveolata and Orbicella franksi at the Flower Garden Banks, Gulf of Mexico. PeerJ 2, e386 ( 10.7717/peerj.386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garren M, Walsh S, Caccone A, Knowlton N. 2006. Patterns of association between Symbiodinium and members of the Montastraea annularis species complex on spatial scales ranging from within colonies to between geographic regions. Coral Reefs 25, 503–512. ( 10.1007/s00338-006-0146-1) [DOI] [Google Scholar]
- 15.LaJeunesse TC, Pettay DT, Sampayo EM, Phongsuwan N, Brown B, Obura DO, Hoegh-Guldberg O, Fitt WK. 2010. Long-standing environmental conditions, geographic isolation and host–symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium. J. Biogeogr. 37, 785–800. ( 10.1111/j.1365-2699.2010.02273.x) [DOI] [Google Scholar]
- 16.Finney J, Pettay D, Sampayo E, Warner M, Oxenford H, LaJeunesse T. 2010. The relative significance of host–habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodinium. Microb. Ecol. 60, 250–263. ( 10.1007/s00248-010-9681-y) [DOI] [PubMed] [Google Scholar]
- 17.Rowan R, Knowlton N, Baker A, Jara J. 1997. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388, 265–269. ( 10.1038/40843) [DOI] [PubMed] [Google Scholar]
- 18.Knowlton N, Weil E, Weigt LA, Guzman HM. 1992. Sibling species in Montastraea annularis, coral bleaching, and the coral climate record. Science 255, 330–333. ( 10.1126/science.255.5042.330) [DOI] [PubMed] [Google Scholar]
- 19.Toller WW, Rowan R, Knowlton N. 2001a. Zooxanthellae of the Montastraea annularis species complex: patterns of distribution of four taxa of Symbiodinium on different reefs and across depths. Biol. Bull. 201, 348–359. ( 10.2307/1543613) [DOI] [PubMed] [Google Scholar]
- 20.Rowan R, Knowlton N. 1995. Intraspecific diversity and ecological zonation in coral–algal symbiosis. Proc. Natl Acad. Sci. USA 92, 2850–2853. ( 10.1073/pnas.92.7.2850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemp D, Fitt W, Schmidt G. 2008. A microsampling method for genotyping coral symbionts. Coral Reefs 27, 289–293. ( 10.1007/s00338-007-0333-8) [DOI] [Google Scholar]
- 22.Thornhill D, Fitt W, Schmidt G. 2006. Highly stable symbioses among western Atlantic brooding corals. Coral Reefs 25, 515–519. ( 10.1007/s00338-006-0157-y) [DOI] [Google Scholar]
- 23.Edmunds P, Pochon X, Levitan D, Yost D, Belcaid M, Putnam H, Gates R. 2014. Long-term changes in Symbiodinium communities in Orbicella annularis in St. John, US Virgin Islands . Mar. Ecol. Prog. Ser. 506, 129–144. ( 10.3354/meps10808) [DOI] [Google Scholar]
- 24.Thornhill DJ, Xiang Y, Fitt WK, Santos SR. 2009. Reef endemism, host specificity and temporal stability in populations of symbiotic dinoflagellates from two ecologically dominant Caribbean corals. PLoS ONE 4, e6262 ( 10.1371/journal.pone.0006262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaJeunesse TC, Smith RT, Finney J, Oxenford H. 2009. Outbreak and persistence of opportunistic symbiotic dinoflagellates during the 2005 Caribbean mass coral ‘bleaching’ event. Proc. R. Soc. B 276, 4139–4148. ( 10.1098/rspb.2009.1405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper TF, et al. 2011. Environmental factors controlling the distribution of Symbiodinium harboured by the coral Acropora millepora on the Great Barrier Reef. PLoS ONE 6, e25536 ( 10.1371/journal.pone.0025536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andras JP, Rypien KL, Harvell CD. 2013. Range-wide population genetic structure of the Caribbean sea fan coral, Gorgonia ventalina. Mol. Ecol. 22, 56–73. ( 10.1111/mec.12104) [DOI] [PubMed] [Google Scholar]
- 28.Foster NL, et al. 2012. Connectivity of Caribbean coral populations: complementary insights from empirical and modelled gene flow. Mol. Ecol. 21, 1143–1157. ( 10.1111/j.1365-294X.2012.05455.x) [DOI] [PubMed] [Google Scholar]
- 29.LaJeunesse TC. 2001. Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the its region: In search of a ‘species’ level marker. J. Phycol. 37, 866–880. ( 10.1046/j.1529-8817.2001.01031.x) [DOI] [Google Scholar]
- 30.Sampayo EM, Dove S, Lajeunesse TC. 2009. Cohesive molecular genetic data delineate species diversity in the dinoflagellate genus Symbiodinium. Mol. Ecol. 18, 500–519. ( 10.1111/j.1365-294X.2008.04037.x) [DOI] [PubMed] [Google Scholar]
- 31.Parkinson JE, Coffroth MA, LaJeunesse TC. 2015. New species of Clade B Symbiodinium (Dinophyceae) from the greater Caribbean belong to different functional guilds: S. aenigmaticum sp. nov. S. antillogorgium sp. nov. S. endomadracis sp. nov., and S. pseudominutum sp. nov. J. Phycol. 51, 850–858. ( 10.1111/jpy.12340) [DOI] [PubMed] [Google Scholar]
- 32.Kennedy EV, Foster NL, Mumby PJ, Stevens JR. 2015. Widespread prevalence of cryptic Symbiodinium D in the key Caribbean reef builder, Orbicella annularis. Coral Reefs 34, 519–531. ( 10.1007/s00338-015-1264-4) [DOI] [Google Scholar]
- 33.Warner ME, LaJeunesse TC, Robison JD, Thur RM. 2006. The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: potential implications for coral bleaching. Limnol. Oceanogr. 51, 1887–1897. ( 10.4319/lo.2006.51.4.1887) [DOI] [Google Scholar]
- 34.Perry JN. 1995. Spatial analysis by distance indices. J. Anim. Ecol. 64, 303–314. ( 10.2307/5892) [DOI] [Google Scholar]
- 35.Foster NL, Baums IB, Sanchez JA, Paris CB, Chollett I, Agudelo CL, Vermeij MJA, Mumby PJ. 2013. Hurricane-driven patterns of clonality in an ecosystem engineer: the Caribbean coral Montastraea annularis. PLoS ONE 8, e53283 ( 10.1371/journal.pone.0053283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pettay DT, Wham DC, Smith RT, Iglesias-Prieto R, LaJeunesse TC. 2015. Microbial invasion of the Caribbean by an Indo-Pacific coral zooxanthella. Proc. Natl Acad. Sci. USA 112, 7513–7518. ( 10.1073/pnas.1502283112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castillo KD, Helmuth BST. 2005. Influence of thermal history on the response of Montastraea annularis to short-term temperature exposure. Mar. Biol. 148, 261–270. ( 10.1007/s00227-005-0046-x) [DOI] [Google Scholar]
- 38.Kinsey DW, Davis PJ. 1979. Effect of elevated nitrogen and phosphorus on coral reef growth. Limnol. Oceanogr. 25, 935–940. ( 10.4319/lo.1979.24.5.0935) [DOI] [Google Scholar]
- 39.Yamashiro H. 1995. The effects of HEBP, an inhibitor of mineral deposition, upon photosynthesis and calcification in the scleractinian coral, Stylophora pistillata. J. Exp. Mar. Biol. Ecol. 191, 57–63. ( 10.1016/0022-0981(95)00045-S) [DOI] [Google Scholar]
- 40.Baums IB, Miller MW, Hellberg ME. 2005. Regionally isolated populations of an imperiled Caribbean coral, Acropora palmata. Mol. Ecol. 14, 1377–1390. ( 10.1111/j.1365-294X.2005.02489.x) [DOI] [PubMed] [Google Scholar]
- 41.Howells EJ, Oppen MJH, Willis BL. 2009. High genetic differentiation and cross-shelf patterns of genetic diversity among Great Barrier Reef populations of Symbiodinium. Coral Reefs 28, 215–225. ( 10.1007/s00338-008-0450-z) [DOI] [Google Scholar]
- 42.Davies SW, Wham D, Kanke MR, Matz MV. 2016. Ecological factors rather than physical barriers shape genetic structure of algal symbionts in Micronesian corals. bioRxiv. ( 10.1101/037994) [DOI] [Google Scholar]
- 43.LaJeunesse TC. 2005. ‘Species’ radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the miocene-pliocene transition. Mol. Biol. Evol. 22, 570–581. ( 10.1093/molbev/msi042) [DOI] [PubMed] [Google Scholar]
- 44.Walsh SM, McField M. 2005 Understanding patterns of bleaching in the Mesoamerican Reef: a collaborative effort to support resilience-based management. In Responding to global change: a reef manager's guide to coral bleaching (eds H Schuttenberg, P Marshall), pp. 83–84. Washington, DC: NOAA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional methods and description of results and four tables are included as the electronic supplementary material.