Abstract
In jawed vertebrates, genes of the major histocompatibility complex (MHC) play a key role in immunity by encoding cell-surface proteins that recognize and bind non-self antigens. High variability at MHC suggests that these loci may also function in social signalling such as mate choice and kin recognition. This requires that MHC genotype covaries with some perceptible phenotypic trait. In mammals and fish, MHC is signalled chemically through volatile and non-volatile peptide odour cues, facilitating MHC-dependent mate choice and other behaviours. In birds, despite evidence for MHC-dependent mating, candidate mechanisms for MHC signalling remain largely unexplored. However, feather preen wax has recently been implicated as a potential source of odour cues. We examined whether the chemical composition of preen wax correlates with MHC class IIβ genotypes of wild song sparrows (Melospiza melodia). Pairwise chemical distance reflected amino acid distance at MHC for male–female dyads, although not for same-sex dyads. Chemical diversity did not reflect MHC diversity. We used gas chromatography–mass spectrometry (GC-MS) to characterize preen wax compounds, and identified four wax esters that best reflect MHC similarity. Provided songbirds can detect variation in preen wax composition, this cue may allow individuals to assess MHC compatibility of potential mates.
Keywords: chemical communication, gas chromatography–mass spectrometry, major histocompatibility complex, preen oil, uropygial gland, genetic similarity
1. Introduction
In jawed vertebrate animals, the major histocompatibility complex (MHC) is a key component of immune defence. MHC genes encode cell-surface proteins that recognize and bind foreign peptides (antigens), and present them to T cells to initiate an adaptive immune response [1]. MHC genotype determines the range of antigens to which individuals can respond. Thus, pathogen-mediated selection at MHC often favours locally adapted alleles [2], rare alleles [3] or certain combinations of alleles (e.g. heterozygote advantage [4]).
In the light of the adaptive importance of MHC, sexual selection should favour mechanisms by which receivers can assess MHC profiles—and thus the quality or compatibility—of potential mates. Indeed, MHC influences mating behaviour or preferences in all major vertebrate groups, including fish [5], reptiles [6], amphibians [7], mammals [8] and birds [9]. Although not universal [10], these taxonomically widespread patterns imply that MHC profile often varies with some detectable aspect of phenotype.
MHC signalling has been most extensively studied in mammals and fish, taxa in which olfaction is well developed. In mice, for example, MHC peptide ligands [11] and volatile non-peptides [12] occur in urine, and can be smelled by conspecific receivers. Similarly, female three-spined sticklebacks (Gasteroseus aculeatus) assess MHC profiles of potential mates based on their MHC peptide ligands [5]. Chemical communication in birds has received comparatively little study, because birds are generally considered microsmatic relative to other vertebrates [13]. However, recent evidence suggests that chemical signalling may be more important in avian communication than previously thought [14,15]. Preen wax is secreted from the avian uropygial gland and functions in plumage maintenance [16], but may also generate semiochemical odour cues. In black-legged kittiwakes (Rissa tridactyla), seabirds with well-developed olfaction, pairwise similarity of preen wax chemical profiles correlates with genetic similarity at neutral loci and at MHC [17,18]. Whether preen wax reflects MHC profiles in other birds—in particular, songbirds (Passeri), in which chemical communication is much less well documented—remains an open question. However, recent work has established that preen wax chemical profiles vary within and between songbird species [19,20]. Further, songbirds can perceive species and sex differences in preen wax composition [19,21], making this substance a strong candidate mechanism for chemosignalling.
We examined whether dissimilarity in chemical profiles of preen wax correlates with dissimilarity in MHC genotype in song sparrows (Melospiza melodia). Females in the study population adjust levels of parental care based on neutral-locus (microsatellite) similarity to their mates [22], suggesting the presence of some mechanism to assess relatedness. We quantified pairwise chemical distances between individuals' preen wax secretions, measured via gas chromatography, and genetic distances at MHC, measured via next-generation sequencing of the hypervariable peptide-binding region (PBR) of MHC class II, exon 2. We also examined whether chemical diversity of preen wax reflects diversity at MHC. Finally, to further explore the chemical basis by which MHC profiles might be communicated, we identified specific subsets of compounds that best signalled MHC dissimilarity.
2. Material and methods
(a). Field methods and sample collection
We used seed-baited Potter traps to capture 60 adult song sparrows (19 females, 41 males) at their breeding grounds near Newboro, Ontario, Canada (44.633° N, 76.330° W) between 14 April 2014 and 6 May 2014. This period corresponds to pair formation and early nesting (first return from wintering grounds 3 April; first egg 8 May). Upon capturing each bird, we applied gentle pressure to the uropygial gland at the base of the tail to express approximately 5–10 µl of preen wax. We collected preen wax in an unheparinized glass capillary tube, snapped the capillary tube to fit inside a sterile 1.5 ml microcentrifuge tube, and stored at −20°C. We used brachial venipuncture to collect approximately 25 µl of blood, which we blotted onto high-wet-strength filter paper saturated with 0.5 M EDTA. We determined sex based on the presence (male) or absence (female) of a cloacal protuberance, supplemented by wing chord measurements. We outfitted birds with unique combinations of coloured leg bands, then released them. In most cases, sex was further confirmed by field observations of sex-specific behaviours including singing, nest building or copulation solicitation.
(b). Genetic analysis of major histocompatibility complex
We used polymerase chain reaction (PCR) to amplify the second exon of MHC class II, using a degenerate forward primer (SospMHCint1f; 5′-AGY GGG GAY CCG GGG TGG-3′) and the reverse primer Int2r.1 [23] to bind within introns 1 and 2, respectively. In addition to the priming sequences, each primer included an adaptor sequence for the Illumina MiSeq platform, four wobble bases, and a unique ‘barcode’ of eight bases to assign recovered sequences to individuals. PCR conditions are detailed in the electronic supplementary material.
We confirmed amplification by agarose gel electrophoresis, then pooled products into a library, which we sent for next-generation sequencing using 300 bp paired-end reads on an Illumina MiSeq (London Regional Genomics Centre). We used a pipeline [24] to assign sequences to individuals and collapse into clusters of identical reads, and filtered out chimeric sequences (generally fewer than 0.1% of reads) through de novo checking in UCHIME [25]. We established an error rate of 1% and discarded sequences occurring below this threshold (electronic supplementary material). To confirm that at least some alleles are transcribed, we used primers SongEX1F.2 and SongEX3R.1 [26] to amplify cDNA from two individuals, then compared their genomic DNA- versus cDNA-derived sequences (electronic supplementary material).
We aligned nucleotide sequences in MEGA v. 7.0 [27], and trimmed out intron sequence based on comparison to other songbird sequences in GenBank. Trimming resulted in alleles of 70–74 amino acids (median = 73), corresponding to the entire putative second exon. We generated a maximum-likelihood phylogeny of alleles within each grouping (males only, females only, both sexes) in MEGA v. 7.0 [27], using a WAG model [28] with five discrete gamma categories. We calculated amino acid distances between all pairwise combinations of individuals using the phylogenetic comparison tool UniFrac [29]. Because genetic data were binary (allele presence versus absence) rather than continuous, we calculated unweighted UniFrac distances using the R package GUNIFRAC [30].
(c). Chemical analysis of preen wax
We used gas chromatography with flame ionization detection (GC-FID) to separate and quantify chemical compounds in preen wax. Capillary tubes containing preen wax samples were transferred to glass vials and the waxes dissolved in 3 ml of chloroform. A mixture of alkane retention time standards (C19, C30 and C36, 25 ng each in 5 µl) was added to a 100 µl aliquot of each sample. For GC-FID analysis, 1 µl samples were injected into a 5% phenyl methyl siloxane column (DB-5, Agilent Technologies; 30 m × 0.32 µm ID × 0.25 µm film thickness) on an Agilent 6890N instrument. Samples were injected at 70°C (held for 1 min), ramped to 130°C at 20°C per minute, ramped to 320°C at 4°C per minute, then held at 320°C for 10 min. Hydrogen was used as a carrier gas at 2.5 ml min−1. For preen wax compound identification, a representative sample was analysed by gas chromatography–mass spectrometry (GC-MS), on a Varian 3800 GC coupled with a Varian MS220 ion trap mass spectrometer. We used the same GC parameters as for the GC-FID analysis, except that He was used as a carrier gas at 1 ml min−1. Monoesters were identified on the basis of their [M]+ ion and the fatty acid–alcohol composition determined by the presence of a protonated fatty acid fragment [31]. A mock extraction prepared from an empty glass capillary tube stored inside a microcentrifuge tube, and subsequently extracted as above, yielded no signal in the GC-MS (data not shown).
Because samples varied in the volume of preen wax collected, we quantified relative peak sizes based on peak area relative to that of the full chromatogram. We retained data from peaks comprising at least 0.1% of total chromatogram area [17]. To prevent large peaks from disproportionately influencing distance measures [18], we normalized data using the deconstand function in the R package VEGAN [32]. We generated matrices of standardized Bray–Curtis dissimilarity based on chemical distances between all same-sex (male–male, 41 × 41; female–female, 19 × 19) and cross-sex dyads (male–female, 41 × 19) using bcdist in the R package ECODIST [33].
(d). Data analysis
We assessed correlations between Bray–Curtis dissimilarity of preen wax and amino acid distance at MHC separately for male–male and female–female dyads, using Mantel tests (mantel in VEGAN [32]) with 9999 permutations. Using partial Mantel tests to control for capture date did not alter the statistical significance of results, so below we present results derived from simple Mantel tests. Because the pairwise matrix for male–female dyads was not square, a Mantel test was not possible, so we used Spearman's correlation permutation test (perm.cor.test in the R package JMUOUTLIER [34]), run with 10 000 permutations.
To determine whether individual diversity at MHC (number of alleles) influences chemical diversity of preen wax, we used two measures of chemical diversity for each individual: number of chromatogram peaks and Shannon's diversity index (calculated using diversity in VEGAN [32]). We used simple linear regression to model each measure as a function of MHC diversity.
We used BIOENV [35] implemented in PRIMER 7 [36] to identify the subset of preen wax components that best reflect MHC distance [37]. This approach considers all possible combinations of variables (peaks) at increasing levels of complexity up to a user-specified maximum (here, eight peaks), and identifies those that maximize the rank correlation between two distance matrices (here, chemical and genetic distance).
Analyses were run in R v. 3.3.1 [38]. Values are reported as means ± s.e.m., and all statistical tests were two-tailed.
3. Results
Across 60 birds, we detected 250 unique DNA sequences (18.47 ± 0.41 alleles per individual) and 69 preen wax peaks (30.05 ± 0.50 peaks per individual). Figure 1 shows a sample chromatogram. Males and females did not differ in chemical richness (no. peaks: t58 = 1.64, p = 0.11), but pairwise chemical distances were lower for male–male dyads (0.329 ± 0.003) than for female–female (0.384 ± 0.008) or male–female dyads (0.370 ± 0.005; Kruskal–Wallis test, H2,2717 = 63.82, p < 0.0001). Consistent with findings from a closely related species [31], GC-MS indicated that all peaks corresponded to monoesters of varying chain lengths, with total carbon numbers ranging from 23 to 39 (electronic supplementary material, table S1).
Figure 1.
Annotated total ion chromatogram from GC-MS analysis of song sparrow preen wax. Peak numbers correspond to the total number of carbons in the compound(s) contributing to the peak; peak letters indicate a subset of monoesters within a given total carbon number category. Asterisks denote peaks for which chemical distance best reflects genetic distance (table 1). IS: internal standard. Chemical compositions of peaks are detailed in electronic supplementary material, table S1.
Chemical distance of preen wax was positively related to MHC amino acid distance for male–female dyads (correlation permutation test; Spearman's r = 0.111, p = 0.002; figure 2). This relationship was not significant, however, for same-sex dyads (Mantel test; male–male: r = −0.058, p = 0.80; female–female: r = 0.145, p = 0.10). Genetic diversity at MHC (number of alleles) did not predict chemical richness (no. peaks:  , p = 0.58) or diversity (Shannon index:
, p = 0.58) or diversity (Shannon index: 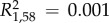 , p = 0.78).
, p = 0.78).
Figure 2.
Pairwise chemical distances (Bray–Curtis dissimilarity) in preen wax composition of cross-sex song sparrow dyads reflect genetic distances at MHC class II. Solid line shows least-squares regression.
BIOENV identified a best combination of four peaks at which chemical and genetic dissimilarity matrices were maximally correlated (r = 0.222; table 1). Each of these peaks also occurred repeatedly in the top combinations for other subset sizes (k = 1–8), further suggesting that they are strong candidates for chemosignalling MHC genotype. All four peaks occurred in the sample used for GC-MS, and could thus be identified (electronic supplementary material, table S1). Two were pure substances (peak 32c was a C15 acid esterified to a C17 alcohol, hereafter C15 : C17, i.e. heptadecanyl pentadecanoate; peak 33c was C19 : C14, i.e. tetradecanyl nonadecanoate). The other two peaks (28b, 34d) were monoester mixtures with the same total carbon number but varying lengths of acid versus alcohol components.
Table 1.
BIOENV analysis identified subsets of peaks that maximize the rank correlation between chemical (preen wax) and genetic (MHC) distance matrices. The top-ranked subset comprised four peaks (28b, 32c, 33c and 34d), each of which occurred frequently in other subset sizes (denoted in italics). Chemical composition of peaks is described in electronic supplementary material, table S1.
| subset size | Mantel's r | peaks |
|---|---|---|
| 1 | 0.125 | 35c |
| 2 | 0.190 | 28b, 34d |
| 3 | 0.212 | 28b, 32c, 34d |
| 4 | 0.222 | 28b, 32c, 33c, 34d |
| 5 | 0.221 | 28b, 32c, 33c, 34d, 35b |
| 6 | 0.219 | 28b, 32c, o, 33c, 34d, 36c |
| 7 | 0.219 | 28b, 32c, o, 33c, 34d, 35b, 36c |
| 8 | 0.215 | 28b, 32c, o, 33c, 34d, 35b, 36c, 37b |
4. Discussion
Two conditions are needed for animals to use chemical cues in assessing MHC profiles. First, these cues must covary with MHC; second, animals must be able to perceive such cues. Whereas both conditions are met in fish [5] and mammals [11], evidence for chemosignalling in birds has until recently been lacking. Similarity in preen wax composition has recently been shown to reflect MHC similarity in seabirds [18], and our findings show for the first time that the preen wax of songbirds conveys comparable information. Determining whether song sparrows perceive this variation in chemical signatures, much less use it in mating or other contexts, will require behavioural testing. Still, findings that other songbirds mate non-randomly at MHC [9] and that birds in our study population adjust parental effort based on overall genetic similarity to their mates [22] suggest that some cue exists.
The mechanism by which chemical similarity of preen wax reflects pairwise similarity at MHC remains uncertain [18]. MHC genotype may influence microbial community composition within the uropygial gland, which might generate individual variation in odour profiles either directly or via differences in metabolites [18,39]. Uropygial glands of a closely related species (dark-eyed junco) harbour bacteria capable of synthesizing wax esters (Acinetobacter spp.) and others (Burkholderia spp., Pseudomonas spp.) that may metabolize preen wax into breakdown products of fatty acids and alcohols [40,41].
Chemical composition of preen wax is influenced by multiple factors beyond MHC genotype, including diet [31] and seasonal variation in endocrine profiles [42]. For male–male dyads, the effects of seasonal variation in androgens on chemical profiles may have outweighed and obscured effects of MHC. For cross-sex and female–female dyads, we found correlations between MHC and chemical distances (r = 0.11 and 0.15, respectively) comparable to those of free-living seabirds (r = 0.12 and 0.08, respectively [18]). Captive studies, standardizing diet and photoperiod, should generate stronger correlations between MHC and preen wax composition. However, studies on free-living animals (this study, [18]) are useful because they permit estimating the degree to which chemosignals reflect MHC under natural conditions. Finally, whereas our study focused on the hypervariable PBR of class II MHC, other genetic factors such as MHC class I and non-MHC loci may also influence chemical signatures.
Of the preen wax compounds detected in this study, probably only a subset can be detected through olfaction. Behavioural experiments represent a key next step in identifying which, if any, compounds might function in chemosignalling. Of particular interest are the four wax esters that best predict MHC genotype (heptadecanyl pentadecanoate, tetradecanyl nonadecanoate and the mixtures of 28-carbon and 34-carbon waxes), and their fatty acid and alcohol metabolites.
We found a maximum of 26 MHC class II alleles in a single individual, implying at least 13 loci. This is within the range of diversity reported for other New World nine-primary oscines (e.g. 39 alleles in a single individual, implying at least 20 loci, in common yellowthroats Geothlypis trichas [43]). Extensive duplication at the avian MHC has generated multiple expressed loci, but also several non-transcribed pseudogenes [23]. Indeed, our comparison of gDNA to cDNA profiles suggests that not all gDNA alleles are transcribed: this almost certainly introduces variation into our analyses. However, MHC expression varies with tissue type and infection status [44]; thus our estimate of 16–37% of gDNA alleles being transcribed probably underestimates the proportion of loci that are functional.
The salience of MHC genotype to fitness in songbirds [45] suggests that selection should favour the ability to signal and assess MHC profiles. The relationship between MHC and chemical distances for mixed-sex dyads suggests that provided song sparrows can detect chemical cues, this information should be useful in the context of mate choice, regardless of whether a self-referent or a known-kin criterion is used. By contrast, chemical cues do not appear to reflect MHC diversity. Our findings implicate preen secretions as potential semiochemicals in songbirds, a group in which chemical communication has only recently been explored. Further testing is warranted to determine if songbirds can perceive MHC-related variation in chemical profiles. Still, our findings suggest that chemosignalling may be more taxonomically widespread than previously thought, and could help to maintain adaptive genetic diversity in natural populations.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Queen's University Biological Station for logistic support; David Carter, André Lachance, John Loggie and Trish Tully for advice; and Bita Azad, Alannah Lymburner, Heather MacGillivray and Dominique Potvin for assistance.
Ethics
Animal work was approved by the University of Western Ontario Animal Use Subcommittee (protocols 2008-054 and 2015-047 to EAM-S) and conducted under the required federal permits.
Data accessibility
MHC allele sequences have been submitted to GenBank (accession numbers KX263957–KX264148 for 138 previously described sequences; KX375230–KX375341 for 112 newly described sequences). Other supporting data are on the Dryad Digital Repository [46].
Authors' contributions
J.W.G.S. and E.A.M.-S. designed the study. T.R.K. and E.A.M.-S. conducted field sampling. J.W.G.S. confirmed the sex of individuals in the field. J.W.G.S. and M.J.W. conducted genetic analysis. G.B.G. oversaw bioinformatic analyses. M.A.B. performed GC-FID and GC-MS. J.W.G.S. performed statistical analyses, in consultation with E.A.M.-S. J.W.G.S. and E.A.M.-S. drafted the manuscript and all authors gave approval for submission.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by Discovery Grants to E.A.M.-S. (award 293123-2012RGPIN) and M.A.B. (award 157930-2012RGPIN) from the Natural Sciences and Engineering Research Council of Canada (NSERC); and by a Hesse Award from the American Ornithologists' Union and a Taverner Award from the Society of Canadian Ornithologists to J.W.G.S.
References
- 1.Trowsdale J. 1995. Both man & bird & beast: comparative organization of MHC genes. Immunogenetics 41, 1–17. ( 10.1007/BF00188427) [DOI] [PubMed] [Google Scholar]
- 2.Evans ML, Neff BD, Heath DD. 2010. MHC-mediated local adaptation in reciprocally translocated Chinook salmon. Conserv. Genet. 11, 2333–2342. ( 10.1007/s10592-010-0119-3) [DOI] [Google Scholar]
- 3.Takahata N, Nei M. 1990. Allelic genealogy under overdominant and frequency-dependent selection and polymorphism of major histocompatibility complex loci. Genetics 124, 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penn DJ, Damjanovich K, Potts WK. 2002. MHC heterozygosity confers a selective advantage against multiple-strain infections. Proc. Natl Acad. Sci. USA 99,11 260–11 264. ( 10.1073/pnas.162006499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milinski M, Griffiths S, Wegner KM, Reusch TBH, Haas-Assenbaum A, Boehm T. 2005. Mate choice decisions of stickleback females predictably modified by MHC peptide ligands. Proc. Natl Acad. Sci. USA 102, 4414–4418. ( 10.1073/pnas.0408264102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller HC, Moore JA, Nelson NJ, Daugherty CH. 2009. Influence of major histocompatibility complex genotype on mating success in a free-ranging reptile population. Proc. R. Soc. B 276, 1695–1704. ( 10.1098/rspb.2008.1840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos DH, Williams RN, Gopurenko D, Bulut Z, Dewoody JA. 2009. Condition-dependent mate choice and a reproductive disadvantage for MHC-divergent male tiger salamanders. Mol. Ecol. 18, 3307–3315. ( 10.1111/j.1365-294X.2009.04242.x) [DOI] [PubMed] [Google Scholar]
- 8.Wedekind C, Seebeck T, Bettens F, Paepke AJ. 1995. MHC-dependent mate preferences in humans. Proc. R. Soc. Lond. B 260, 245–249. ( 10.1098/rspb.1995.0087) [DOI] [PubMed] [Google Scholar]
- 9.Freeman-Gallant CR, Meguerdichian M, Wheelwright NT, Sollecito SV. 2003. Social pairing and female mating fidelity predicted by restriction fragment length polymorphism similarity at the major histocompatibility complex in a songbird. Mol. Ecol. 12, 3077–3083. ( 10.1046/j.1365-294X.2003.01968.x) [DOI] [PubMed] [Google Scholar]
- 10.Westerdahl H. 2004. No evidence of an MHC-based female mating preference in great reed warblers. Mol. Ecol. 13, 2465–2470. ( 10.1111/j.1365-294X.2004.02238.x) [DOI] [PubMed] [Google Scholar]
- 11.Brennan PA, Zufall F. 2006. Pheromonal communication in vertebrates. Nature 444, 308–315. ( 10.1038/nature05404) [DOI] [PubMed] [Google Scholar]
- 12.Kwak J, Opiekun MC, Matsumura K, Preti G, Yamakazi K, Beauchamp GK. 2009. Major histocompatibility complex-regulated odortypes: peptide-free urinary volatile signals. Physiol. Behav. 96, 184–188. ( 10.1016/j.physbeh.2008.10.003) [DOI] [PubMed] [Google Scholar]
- 13.Jones RB, Roper TJ. 1997. Olfaction in the domestic fowl: a critical review. Physiol. Behav. 62, 1009–1018. ( 10.1016/S0031-9384(97)00207-2) [DOI] [PubMed] [Google Scholar]
- 14.Balthazart J, Taziaux M. 2009. The underestimated role of olfaction in avian reproduction? Behav. Brain Res. 200, 248–259. ( 10.1016/j.bbr.2008.08.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caro SP, Balthazart J, Bonadonna F. 2015. The perfume of reproduction in birds: chemosignaling in avian social life. Horm. Behav. 68, 25–42. ( 10.1016/j.yhbeh.2014.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob J, Ziswiler V. 1982. The uropygial gland. In Avian biology (eds Farner DS, King JR, Parkes KC), pp. 199–314. New York, NY: Elsevier. [Google Scholar]
- 17.Leclaire S, Merkling T, Raynaud C, Mulard H, Bessiére JM, Lhuillier E, Hatch SA, Danchin E. 2012. Semiochemical compounds of preen secretion reflect genetic make-up in a seabird species. Proc. R. Soc. B 279, 1185–1193. ( 10.1098/rspb.2011.1611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leclaire S, van Dongen WFD, Voccia S, Merkling T, Ducamp C, Hatch SA, Blanchard P, Danchin E, Wagner RH. 2014. Preen secretions encode information on MHC similarity in certain sex-dyads in a monogamous seabird. Sci. Rep. 4, 6920 ( 10.1038/srep06920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whittaker DJ, Soini HA, Atwell JW, Hollars C, Novotny MV, Ketterson ED. 2010. Songbird chemosignals: volatile compounds in preen gland secretions vary among individuals, sexes, and populations. Behav. Ecol. 21, 608–614. ( 10.1093/beheco/arq033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y-H, Du Y-F, Zhang J-X. 2013. Uropygial gland volatiles facilitate species recognition between two sympatric sibling bird species. Behav. Ecol. 24, 1271–1278. ( 10.1093/beheco/art068) [DOI] [Google Scholar]
- 21.Whittaker DJ, Richmond KM, Miller AK, Kiley R, Burns CB, Atwell JA, Ketterson ED. 2011. Intraspecific preen oil odor preferences in dark-eyed juncos (Junco hyemalis). Behav. Ecol. 22, 1256–1263. ( 10.1093/beheco/arr122) [DOI] [Google Scholar]
- 22.Potvin DA, MacDougall-Shackleton EA. 2009. Parental investment amplifies effects of genetic complementarity on growth rates in song sparrows, Melospiza melodia. Anim. Behav. 78, 943–948. ( 10.1016/j.anbehav.2009.07.023) [DOI] [Google Scholar]
- 23.Edwards SV, Gasper J, March M. 1998. Genomics and polymorphism of Agph-DAB1, an Mhc class II B gene in red-winged blackbirds (Agelaius phoeniceus). Mol. Biol. Evol. 15, 236–250. [DOI] [PubMed] [Google Scholar]
- 24.Gloor GB, Hummelen R, Macklaim JM, Dickson RJ, Fernandes AD, MacPhee R, Reid G. 2010. Microbiome profiling by Illumina sequencing of combinatorial sequence-tagged PCR products. PLoS ONE 5, e15406 ( 10.1371/journal.pone.0015406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. 2011. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200. ( 10.1093/bioinformatics/btr381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguilar A, Edwards SV, Smith TB, Wayne RK. 2006. Patterns of variation in MHC class II β loci of the little greenbul (Andropadus virens) with comments on MHC evolution in birds. J. Hered. 97, 133–142. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. ( 10.1093/molbev/msw054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whelan S, Goldman N. 2001. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 18, 691–699. ( 10.1093/oxfordjournals.molbev.a003851) [DOI] [PubMed] [Google Scholar]
- 29.Lozupone C, Knight R. 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71, 8228–8235. ( 10.1128/AEM.71.12.8228-8235.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Bittinger K, Charlson ES, Hoffmann C, Lewis J, Wu GD, Collman RG, Bushman FD, Li H. 2012. Associating microbiome composition with environmental covariates using generalized UniFrac distances. Bioinformatics 28, 2106–2113. ( 10.1093/bioinformatics/bts342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas RH, Price ER, Seewagen CL, Mackenzie SL, Bernards MA, Guglielmo CG. 2010. Use of TLC-FID and GC-MS/FID to examine the effects of migratory state, diet and captivity on preen wax composition in White-throated Sparrows Zonotrichia albicollis. Ibis 152, 782–792. ( 10.1111/j.1474-919X.2010.01050.x) [DOI] [Google Scholar]
- 32.Dixon P. 2003. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930. ( 10.1111/j.1654-1103.2003.tb02228.x) [DOI] [Google Scholar]
- 33.Goslee SC, Urban DL. 2007. The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Softw. 22, p1–19.39. ( 10.18637/jss.v022.i07) [DOI] [Google Scholar]
- 34.Garren ST. 2016. jmuOutlier: Permutation tests for nonparametric statistics. R package version 1.1. See https://CRAN.R-project.org/package=jmuOutlier. [Google Scholar]
- 35.Clarke KR, Ainsworth M. 1993. A method of linking multivariate community structure to environmental variables. Mar. Ecol. Prog. Ser. 92, 205–219. ( 10.3354/meps092205) [DOI] [Google Scholar]
- 36.Clarke KR, Gorley RN. 2015. PRIMER v7: User manual/tutorial. Plymouth, UK: PRIMER-E. [Google Scholar]
- 37.Stoffel MA, Caspers BA, Forcada J, Giannakara A, Baier M, Eberhart-Phillips L, Müller C, Hoffmann JI. 2015. Chemical fingerprints encode mother-offspring similarity, colony membership, genetic quality, and relatedness in fur seals. Proc. Natl Acad. Sci. USA 112, E5005–E5012. ( 10.1073/pnas.1506076112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 39.Strandh M, Westerdahl H, Pontarp M, Canbäck B, Dubois MP, Miquel C, Taberlet P, Bonadonna F. 2012. Major histocompatibility complex class II compatibility, but not class I, predicts mate choice in a bird with highly developed olfaction. Proc. R. Soc. B 279, 4457–4463. ( 10.1098/rspb.2012.1562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishige T, Tani A, Takabe K, Sakai Y, Kato N. 2002. Wax ester production from Acinetobacter sp. strain M-1: ultrastructure of cellular inclusions and role of acyl coenzyme A reductase. Appl. Environ. Microbiol. 68, 1192–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whittaker DJ, Theis KR. 2016. Bacterial communities associated with junco preen glands: preliminary ramifications for chemical signaling. In Chemical signals in vertebrates 13 (eds Schulte BA, Goodwin TE, Ferkin MH), pp. 105–117. New York, NY: Springer. [Google Scholar]
- 42.Whittaker DJ, Soini HA, Gerlach NM, Posto AL, Novotny MV, Ketterson ED. 2011. Role of testosterone in stimulating seasonal changes in a potential avian chemosignal. J. Chem. Ecol. 37, 1349–1357. [DOI] [PubMed] [Google Scholar]
- 43.Bollmer JL, Dunn PO, Whittingham LA, Wimpee C. 2010. Extensive MHC class II B gene duplication in a passerine, the common yellowthroat Geothlypis trichas. J. Hered. 101, 448–460. ( 10.1093/jhered/esq018) [DOI] [PubMed] [Google Scholar]
- 44.Rohn WM, Lee YJ, Benveniste EN. 1996. Regulation of class II MHC expression. Crit. Rev. Immunol. 16, 311–330. ( 10.1615/CritRevImmunol.v16.i3.40) [DOI] [PubMed] [Google Scholar]
- 45.Dunn PO, Bollmer JL, Freeman-Gallant CR, Whittingham LA. 2013. MHC variation is related to a sexually selected ornament, survival, and parasite resistance in common yellowthroats. Evolution 67, 679–687. ( 10.1111/j.1558-5646.2012.01799.x) [DOI] [PubMed] [Google Scholar]
- 46.Slade JWG, Watson MJ, Kelly TR, Gloor GB, Bernards MA, MacDougall-Shackleton EA. 2016. Data from: Chemical composition of preen wax reflects major histocompatibility complex similarity in songbirds. Dryad Digital Respository. ( 10.5061/dryad.3574r) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
MHC allele sequences have been submitted to GenBank (accession numbers KX263957–KX264148 for 138 previously described sequences; KX375230–KX375341 for 112 newly described sequences). Other supporting data are on the Dryad Digital Repository [46].




