Abstract
Attention is fundamentally important for sensory systems to focus on behaviourally relevant stimuli. It has therefore been an important field of study in human psychology and neuroscience. Primates, however, are not the only animals that might benefit from attention-like processes. Other animals, including insects, also have to use their senses and select one among many stimuli to forage, avoid predators and find mates. They have evolved different mechanisms to reduce the information processed by their brains to focus on only relevant stimuli. What are the mechanisms used by insects to selectively attend to visual and auditory stimuli? Do these attention-like mechanisms achieve the same functions as they do in primates? To investigate these questions, I use an established framework for investigating attention in non-human animals that proposes four fundamental components of attention: salience filters, competitive selection, top-down sensitivity control and working memory. I discuss evidence for each of these component processes in insects and compare the characteristics of these processes in insects to what we know from primates. Finally, I highlight important outstanding questions about insect attention that need to be addressed for us to understand the differences and similarities between vertebrate and insect attention.
Keywords: selective attention, visual search, cocktail party, bee, dragonfly, cricket
1. Introduction
In a world abundant with information, sensory faculties are undoubtedly a boon to any organism. Yet one only needs to recall a crowded restaurant or a busy highway to appreciate that our sensory capacities could be overloaded with a flood of information if we lacked the capacity to discriminate between stimuli and attend to the ones of interest. We are not alone in having to face this problem. Other animals also use a variety of sensory modalities and are often faced with a multitude of different stimuli. Insects are no exception (figure 1). A female cricket searching for a potential mate must recognize and locate one of several calling males, a situation not dissimilar to a human cocktail party problem [1–3]. A bee searching for a rewarding flower among non-rewarding flowers is dealing with a visual search task akin to those well studied in human attention studies [4–6]. Given these common problems, insects should surely also be well served by processes that reduce the stimulus set available to them to a subset of salient and relevant stimuli. Unsurprisingly, a growing body of literature has been providing us with behavioural and, increasingly, neurophysiological evidence of these processes in insects.
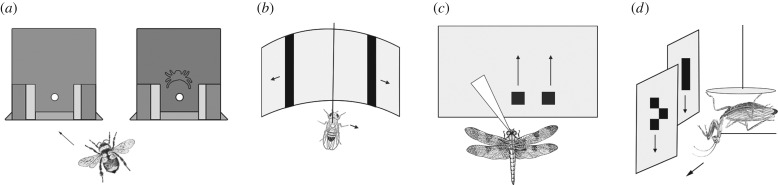
Figure 1.
Examples of selective attention in insects. (a) Bumblebees perform visual search tasks to choose between flowers (public domain image by Bernie Kohl). (b) Bushcrickets choose between multiple signallers in ‘cocktail party’-like auditory scenes (image © Natasha Mhatre). (c) Dragonflies selectively choose between different prey (image © Natasha Mhatre).
Do these processes in insects serve similar functions as those well studied in primates? Do they, for example, show both top-down influences and bottom-up effects? Could we see inattention blindness [7] and change blindness [8] in insects? While we do not yet have definite answers to all these questions, there is a case to be made for the utility of studying these processes in insects and primates in a common framework. Firstly, any common mechanisms give us information about fundamental neural solutions that evolve in response to similar problems. Secondly, the points of difference are equally interesting, revealing alternative means of filtering out distractors and choosing between stimuli, thus providing us with processes that might potentially be easier to implement in computer vision or robotics. Differences should also highlight which mechanisms are unique to humans and other primates—what makes primate attention unique? Finally, given the relatively simpler nervous systems of insects and the considerably simpler ethical issues involved, these processes might be more tractable to direct neurophysiological and genetic investigation in insects, thus opening up several new avenues for the study of attention-like processes.
Comparing these processes in humans and animals naturally entails a problem of definition. Attention has been variously defined in human neuroscience and psychology—as a possession of the mind by one of several stimuli [9], as a spotlight that is focused on one region compared with others [10] or as a competition between multiple inputs for recognition [11] to cite a few examples. It has also been used to describe several phenomena including centrally controlled voluntary direction of focus and stimulus-driven shifts of focus. However, one common theme that all these definitions share is the ability to select one input stimulus from several. For this review, I define attention-like processes as those that in any way limit the perception of stimuli to a subset. While some of these processes might serve a simpler ‘filtering’ function [12], they still could be called attention-like in that they reduce the information that brains need to process. I further look for selective attentional processes—defined as those that specifically lead to certain stimuli being preferred over others that are nonetheless perceived equally well when presented alone. It is important to highlight that this definition does not make any assumptions about the underlying mechanisms and these could involve either central or peripheral processes.
I review attention-like processes in two sensory modalities: vision and hearing. Throughout the review, I also explicitly look for processes that functionally resemble known processes in primates as well as evidence for processes that might be analogous to top-down (endogenous) control of or bottom-up (exogenous) capture of attention. To do this requires a comparative framework and I make use of Knudsen's [13] comparative framework for investigating the mechanistic basis for attention. The relevance to insects of some of the component process in the framework could be debated and future studies would no doubt be needed to evaluate the relevance of some of these components (e.g. the distinction between bottom-up and top-down attention) in insects. The framework is, however, based on well-established studies and approaches to studying attention. It thus forms an important starting point for the understanding of attention-like processes in insects. This framework proposes four fundamental component processes to attention: salience filters, competitive selection, top-down sensitivity control and working memory. All these components work to achieve attention by selecting specific signals over others in the environment. Salience filters are bottom-up filters that enhance signals of adaptive importance. Competitive selection is the process by which the filtered signals subsequently compete for access to be stored in the memory. Top-down control regulates the strengths of these signals during competitive selection. Working memory is a temporary, dynamic form of memory, which signals need to access and be stored for further analysis. These are thus the components that determine which signals are finally accessed by the central neural processes and which signals an organism attends to. I focus on each of these component processes and discuss evidence for them in insects.
2. Salience filters
A quick and easy way to reduce the perception of distractors and noise in the surroundings is to implement neural filters that preferentially select for signals of interest. These would effectively enable stimulus-driven access to further neural processing for stimuli of special relevance. In humans, such filters perform low-level extraction of scene features such as colour [14]. In most insect systems, the wavelength sensitivity of photoreceptors or frequency tuning of auditory afferent neurons automatically serves as a filter of this kind. The resultant behaviour can be compared to exogenous orientation where certain stimuli access attention through bottom-up, sensory processes [15].
Neural afferent tuning curves bias nervous systems towards certain types of signals over others and thus could be seen to serve as an attention-like filtering process [16] albeit not one that achieves selective attention. In the cricket Teleogryllus commodus, for example, the ascending auditory interneurons (AN1) are not always perfectly tuned to the average frequency of male mating calls. However, they are still preferentially tuned to signals within 1.2 kHz of this frequency [17] enhancing the saliency of these signals relative to environmental noise. The sharpness of this tuning also varies between cricket species; species from species-rich rainforest communities (e.g. Paroecanthus podagrosus) have sharper frequency tuning than temperate species (e.g. Gryllus campestris and Gryllus bimaculatus) [18]. In the former, the frequency tuning of the same auditory interneuron (AN1) can preferentially increase signal-to-noise ratios by as much as 26 dB, but even in the latter, tuning achieves ratios of 10–16 dB. The frequency tuning of auditory afferent neurons has thus evolved to be a more effective saliency filter in environments where perceiving relevant signals is more difficult. In addition to this preferential tuning, stimulus-specific adaptations to noisy backgrounds can enable AN1 in at least one bushcricket (Mecopoda elongata) to detect relevant ‘novel’ signals at frequencies that differ from the noise [19]. The sensitivity of photoreceptors also biases insect visual behaviour towards particular colours. Bees preferentially choose colours in the blue range of their visible spectrum even if trained on other colours [20]. Colours in this region of the spectrum also appear to dominate behavioural responses and interfere with other learning tasks [21]. Fruit flies (Drosophila) also have a preference for UV light, which is governed by the peripheral nervous system [22]. Thus, even the tuning of peripheral sensory systems biases organisms' responses between still perceptible stimuli, even before any choice is made centrally.
3. Competitive selection
While filters certainly increase the signal-to-noise ratio of important signals, they do not achieve selective attention. The latter involves suppressing or ‘outcompeting’ the response to irrelevant stimuli that are still clearly perceivable independently [23]. This would be the case where an individual is faced not just with the signal and noise but with two or more signals or targets of interest and must respond to one or the other. A cricket female that hears the mating calls of two different conspecifics [1,2,24,25] or a bee selecting between two different flowers [5,26] both face a similar problem. Similarly, mantises or dragonflies faced with multiple individual prey must be able to track one while ignoring others [27,28]. We should therefore expect to find selective attentional mechanisms operating in all of these situations.
In humans, visual spatial attention has been investigated as a limited resource, which organisms confine to a particular visual region [10]. Spatial cues lead to humans confining attention to specific regions of the visual field. In insects, the spatial location of targets can indeed serve to focus positioning behaviour in fruit flies, bees, dragonflies and hoverflies [29–32]. Experiments investigating this typically present the insect with multiple visual targets like stripes on different sides of its visual field and measure the orienting behaviour of the insect. Flies (Drosophila melanogaster) in these experiments can control their behaviour to orient towards stimuli in particular parts of their visual field while not showing responses to other visual stimuli [29]. In more recent experiments [33], test stimuli are preceded by a briefly presented visual cue. Subsequently, two vertical stripes moving in opposite directions are presented as test stimuli (figure 2b). One of them is presented at the cued position while the other is presented at an uncued position. Flies in these experiments are more likely to follow the motion of the test stimuli in the cued position [33]. These experiments show that flies restrict their responses to visual regions that have previously been cued and fit a definition of visual spatial attention [10].
Figure 2.
Experimental tests of visual competitive selective attention in insects. (a) Competing artificial flowers with the possibility of predation by ‘robotic spiders’ test selective attention of free-flying bumblebees to colour and shape during simultaneous tasks (after [34]). (b) Moving stripes in a cylindrical flight arena are used to test spatial attention and response to prior cuing in flies during tethered flight (after [33]). (c) Competing targets in different areas of the visual field are used during electrophysiological investigations into selective attention in dragonflies (after [28]). (d) Targets that differ in depth and shape compete for selective attention as measured by the saccades of tethered praying mantises (after [27]). All insect images are public domain images from Wikimedia Commons where details of authors and usage are available [35–38].
External cues and spatial location can thus bias insect visual behaviour. Visual attention in humans also exploits cues inherent in the stimuli [6]. Human visual search uses several cues like colour, orientation, size and motion to more efficiently find targets [6]. Insects use a variety of visual cues including depth, shape and colour to select between targets. Mantises have been shown to be capable of preferentially orienting to targets based on their depth and shape (figure 2d) [27]. In these experiments, mantises are presented with two targets (vertical rectangles) moving downward and their response is measured by which target they saccade towards (figure 2d). When one target is presented on a screen that is closer to the mantis and an identical target is presented on one further away, mantises make more saccades to the closer target. If two targets are presented at the same depth but one has a ‘worm-like’ shape while the other is a rectangle, then the mantises preferentially saccade to the former. In control experiments with two rectangular targets at the same depth, however, they perceive and saccade to both the rectangular targets [27].
Colour can also be an important cue, which some insects use to discriminate targets associated with learnt reward from unrewarding distractors. For example, bumblebees (Bombus terrestris) that have learnt to associate targets of particular colours with a sucrose reward, ignore differently coloured distractors in visual search paradigms [5]. They manage to choose only the rewarding colours even when there are multiple rewarding colours and distractor colours presented. Here too, the distractor colours were colours the bees could perceive and respond to if they were rewarding, indicating that they were selecting between still perceivable colours. Interestingly, honeybees (Apis mellifera) trained on one colour take longer to choose the target colour as the number of distractors increases [4]. They thus seem to be searching for targets serially. This is a marked difference from the parallel visual search mechanisms we see in humans and other vertebrates where target detection is independent of the number of distractors [39–41]. Bumblebees in similar experiments, however, appear to be capable of parallel visual search. This has led to the suggestion that there might be different mechanisms of selective attention at play in bumblebees and honeybees [42].
The neural basis for some of these discriminations is becoming clearer and there has been considerable progress in this respect in recent years. In the dragonfly (Hemicordulia tau), neuronal mechanisms have been identified that are good candidates for selective attentional processing [28]. Small targets presented individually with vertical motion in the central or peripheral regions of the visual field (figure 2c) are represented independently in an identified binocular neuron in the midbrain. When presented together, the neuron tracks either one target or the other and shows the same signature representation for this target as when it was presented alone—rather than a sum or an average of the two representations. When this neuron is presented with targets of differing saliency (in terms of size or contrast), targets that are more salient suppress responses to other targets in a competitive manner thus enabling visual selective attention for salient targets [43]. This clearly shows a suppression of one perceivable target in favour of another, bringing to mind analogous responses in primates [44].
We find a similar exploitation of auditory cues to selectively attend to signals of interest in cocktail-party-like situations such as dense orthopteran choruses where auditory attention becomes important. In these choruses, multiple males simultaneously call to attract females [45]. Females need to be able to process the input from all perceivable signals to recognize signals and localize individual males. Males often interact with each other and adjust their calls relative to each other in order to either overlap or alternate with the calls of other males [45–47]. Responding to all males in a chorus could lead to long delays without calling and thus reduced mating opportunities. They would therefore benefit if they selectively attended to a subset of neighbours. Thus, both males and females would benefit from selective attention to a restricted number of signallers. Experiments that investigate this record neural and behavioural responses to simultaneous playback of calling song (figure 3). Typically, two or more speakers play out calling songs that differ in specific characteristics. The differential responses to the different calls are then observed to investigate whether the responses are selective to one or the other call. Behaviourally, these responses would be call timing adjustment by the males or phonotaxis towards the call by the females.
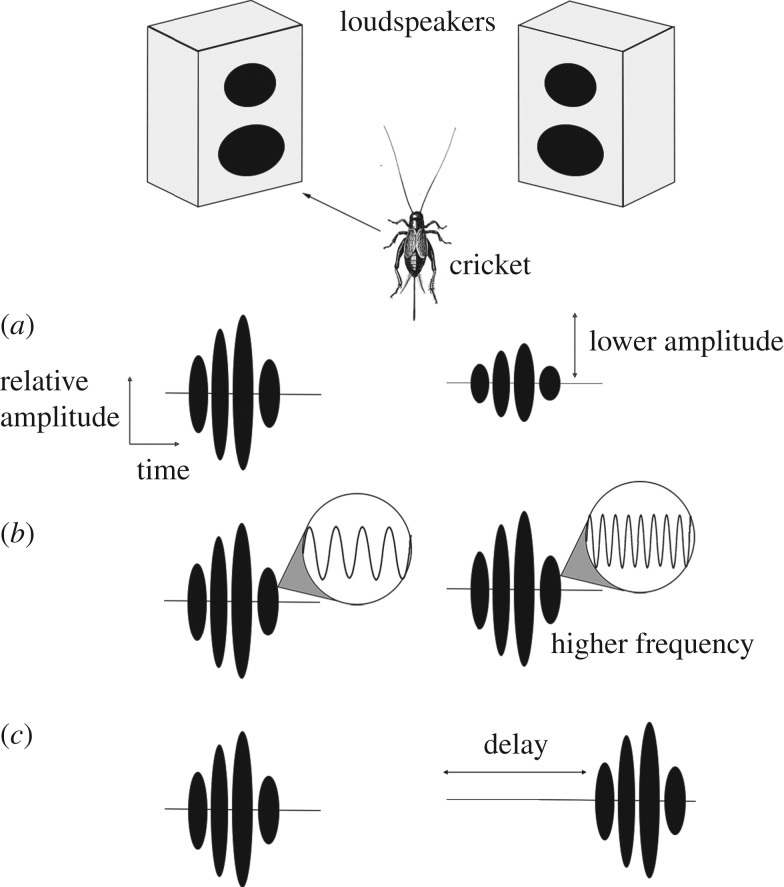
Figure 3.
Experimental tests of auditory competitive selection in insects. Speakers present crickets or bushcrickets choices between calls. The measured outputs are either neural representation of the signals or behavioural responses. Behavioural responses include phonotaxis for females (depicted here) or calls by the males. The calls are here depicted as oscillograms of chirps that differ in (a) relative amplitude (see [1,24]), (b) frequency (see [48]) or (c) timing (see [2,49,50]), all of which are cues used by crickets or bushcrickets to selectively attend to calls in their environment.
Two important characteristics that could enable such selective responses are the intensity and the frequency of the signals (figure 3a,b). Males of many species, for example, respond only to the loudest neighbours [2,51]. This appears to involve a sliding threshold of selective attention, sensitive to the relative intensities of neighbouring males [2,51]. Thus, selective attention here appears to depend on a comparison of stimuli rather than a simple thresholding operation. In at least one bushcricket species (Mecopoda ‘Chirper’), however, signallers might respond to all neighbours that call above a given intensity, thus using a fixed intensity threshold [25]. Evidence from neurophysiology also shows that in both crickets (Teleogryllus oceanicus) and bushcrickets (Tettigonia viridissima), an auditory interneuron—the omega neuron—represents only the loudest signal on the ipsilateral side [1,24]. Stimuli that are perfectly well represented when played alone are no longer represented when played simultaneously with a louder signal. This process is driven by an intensity-dependent hyperpolarization of the AN1 in response to signals. Louder signals cause a larger and longer-lasting hyperpolarization that is not overcome by the response to softer signals [1,24]. Crickets and bushcrickets thus make use of a low-level gain-dependent mechanism that enables selective attention to specific signals in the presence of several calling males. This mechanism appears to be sensitive not only to the intensity but also to the duration of the signal, filtering out signals with lower duration in favour of higher duration signals [24]. Such a hyperpolarization-driven process bears intriguing similarities to the competitive selection for visual targets recorded from the dragonfly neuron [43]. This suggests that this might be a common and perhaps simple solution that insect nervous systems evolve in response to tasks and environments that demand selective attention. It is also interesting to observe that the filtering out of signals occurs in peripheral neurons, so that no higher level processing actually chooses between two signals. This might be a strategy that insect nervous systems have evolved to enable functional selective attention despite having limited neural processing power. We thus have filtering by the salience filters, followed by selectivity implemented in peripheral sensory systems.
Another auditory cue that insects could attend to in principle is frequency. Humans and other vertebrates separate different streams of auditory input based on their frequency differences in a process called auditory stream segregation [52–55]. Attention has been implicated in this process although its importance is still debated [56]. Crickets and bushcrickets typically can hear sounds in two frequency ranges—a low frequency range for intraspecific communication and a high frequency range to hear predators [57]. Within the low frequency range, female crickets and bushcrickets can clearly respond differentially between auditory signals with differing frequencies [58]. This could, however, be achieved using a saliency filter: the frequency tuning of the auditory neurons causes signals that are not at the preferred frequency to be poorly represented [59]. Signals like bat calls and mating song that differ in temporal pattern are, however, both represented well in an auditory interneuron—TN1 or the T-neuron—in at least one bushcricket (Neoconocephalus retusus) when presented independently. When both signals are presented together, the same interneuron selectively represents bat calls but only if present in a different frequency range to mating song. Thus, bushcrickets use frequency cues to selectively encode calls of bats even in the presence of calls of conspecific males that they can perceive [48]. In this, insects show a primitive form of auditory stream segregation not dissimilar to that seen in vertebrates [53–55]. In bushcrickets, a primitive version of this process thus achieves functional selective attention to predator calls over mating song.
Finally, one cue that seems to influence both visual and auditory selective attention is the timing of the signal (figure 3c). Timing has been shown to be important also in human attention [60]. In these experiments, observers are presented with a series of characters and instructed to attend to one of them. While attending to this character, they find it difficult to report or process any characters that follow it within a short duration of time, which is called an ‘attentional blink’ [60,61]. What evidence is there for such an attentional blink in insects? Females of several species of crickets and bushcrickets show a clear preference for leading signals compared with following signals arriving a few milliseconds after [49,62,63]. Leading signals are preferentially represented in bushcricket AN1 and following signals of the same intensity are suppressed until after the leading signal has stopped [50]. This time-sensitivity is achieved by AN1 competitively inhibiting the response of contralateral auditory neurons with a hyperpolarization. This hyperpolarization is then not overcome by the neural response to a following signal of equal intensity [50]. The inability of crickets and bushcrickets to process chirps immediately after any given chirp thus resembles an attentional blink [60]. Similar behavioural responses are seen in firefly flashing in response to visual flashes [64], but the neural mechanisms underlying this response are still unclear.
4. Top-down sensitivity control
While there is ample evidence to show that insects can and do respond selectively to a variety of cues, a key question is whether these cues elicit a response purely exogenously (using bottom-up mechanisms) or whether insects orient to these cues in a top-down, endogenous fashion. In humans, for example, higher-order processes such as learning and memory affect attention [65,66]. Neural response in the cortex also correlates with attentional sensitivity [11,67]. Is there evidence for higher-order processes influencing selective attention in insects?
Studies of bee visual search show that they modify foraging behaviour in response to experience [68,69], indicating that learning and memory processes do influence orienting and selective discriminations in bees. For example, in response to colours associated with aversive quinine solution, bumblebees become more accurate at avoiding these colours and take longer to make their choices [68]. The change in reaction time in particular is important as this is typically used to measure attentional differences in visual search experiments [70]. Similarly, when faced with dual tasks of avoiding predatory attacks from ‘robotic spiders’ [34] and discriminating between lower and higher rewarding flowers (figure 2a), bees perform poorly on the latter task if the spiders in the former task are cryptic [26]. However, if the second task involves discriminating rewarding flowers and flowers associated with quinine, they change their behaviour. In these experiments, they do learn to make the selective discriminations required from both tasks [26]. Prior learning experience can also modify bees' natural preference for global information and lead to them selectively attending to local information over global features in a stimulus [71]. These examples seem to make a clear case for learning and memory influencing a bee's visual search behaviour during foraging tasks.
What evidence is there then for the involvement of brain structures in selective attention? The bulk of evidence addressing this comes from fruit flies. The mushroom bodies, one of the central brain structures involved in learning and memory in insects [72–74] appear to be important for fruit flies to selectively fixate on visual targets at lower contrasts or in the presence of visual noise [75]. Mutant flies, which are mushroom-body-deficient, are also poor at selectively fixating visual targets in the presence of olfactory distractors compared with wild-type flies [75]. Flies with specific genetic defects in their mushroom bodies also show a change in how they orient between stimuli: they show a more linear, graded response to changing parameters of the stimuli as opposed to the more abrupt changes seen in wild-type individuals [76]. The latter is the response one would expect if neural representations of stimuli were competing for working memory. There would typically be a temporal attentional window before each new stimulus could access the working memory. Selective orienting would therefore be expected to shift after discrete time intervals corresponding to this temporal window and not gradually as seen in the mutant flies. Active switching between competing stimuli with a temporal window has been argued to indicate attentional switching based on an endogenous drive with top-down control [77]. Such a temporal attentional window has also been associated with an ‘attention span’ and has been studied as such in fruit flies [78]. In these experiments, flies that had turned towards stimuli on one side retained a bias for turning towards this side. They lost this bias and endogenously switched their orienting to stimuli in another spatial location only after a period of time. This time period has been termed their ‘attention span’ and was typically about 4 s in wild-type flies. In flies that have mutations previously associated with defects in selective attention, this reduced to about 1 s. Thus, these experiments hold promise for the genetic investigation of endogenous control over orienting in insects during selective attention.
Recordings of local field potentials from fly brains also indicate attentional modulation. These field potentials are modulated by learnt salience (due to heat or odour) of a target with an increase in power in response to salience compared with baseline stimuli [79]. The shape of the modulation is similar to modulated neural responses to preferred stimulus features in primates [67]. In both cases, neural response curves have a fixed width in response to change in position or orientation of the stimulus. With attentional modulation, this width remains the same. The height of the curve, however, is greater at the salient position or orientation, indicating increased response for the salient position or orientation of the stimulus. Studies of fly brain local field potentials have also shown that they respond even in the absence of a behavioural orienting response [80]. These experiments made use of stimuli that flickered at specific frequencies (i.e. frequency-tagged stimuli) and found modulations of the local field potentials at these frequencies in the fly brain even when the fly itself was not responding behaviourally. This suggests that, as with humans [81], behaviour is not a prerequisite for these selective attentional processes in flies. Frequency-tagged stimuli have also been used in one study on honeybees [82]. In this experiment, recordings of visually evoked potentials were made from different regions of the bee brain while bees fixated on one or the other frequency-tagged bar. The recordings showed selective neural responses in the optic lobe, but not the central brain, when the bees endogenously shifted fixation between the bars. Selective attention in bee brain thus preceded behavioural choices and seems to occur at an early stage in bee visual processing.
Local field potentials, which provide population level outputs, and single neuron recordings such as those in the dragonfly are of course not mutually exclusive approaches. Studying attention-like processes at these different levels help provide a clearer picture of how they might operate in the brain. It is also important to study the different stages of visual processing from the optic lobes to the central brain for a complete picture of selective attention. A recent study [83] that looked at target tracking in fruit flies recorded local field potentials from multiple brain regions during two different conditions. In the first ‘closed loop’ condition, the fly could control the position of a frequency-tagged stimulus it saw on a screen with its own tracking movements. In the second, ‘open-loop’ condition, the same movement pattern of the stimulus was replayed back to the fly but it could not control how the pattern changed. Comparing these two conditions tells us how neural processing implements endogenous volitional control of the external world view separated out from the neural response to the view itself. This comparison showed that during closed loop presentations, the optic lobes and central brain had similar responses. In open loop replay, however, these brain areas had different responses when the fly was turning; coherence across brain areas appears to be important when flies endogenously respond to external stimuli but not when viewing replay. While this study does not explicitly address selective attention, it shows how both peripheral processes and top-down sensitivity could be implicated in endogenous control over orienting behaviour. Evidence from some recent studies not explicitly investigating selective attention also suggests that multiple brain structures might influence visual selective attention in insects [84]. These include the fan shaped body and the ellipsoid body in the central complex of the insect brain. Neural correlates of visual processing resembling selective attention have been seen in all these structures. They have therefore been suggested to represent stages that lead to selective visual attention or alternatively a brain-wide network of neurons governing selective attention [84].
In summary, therefore, there appears to be a fair amount of evidence for the involvement of different brain structures in selective visual attention in insects. We have less evidence for top-down control of selective auditory attention, which appears to be achieved more peripherally. How the selectivity of visual stimuli is achieved through various stages in the neural processing and effected in behaviour is still an important open question. It would also be important to investigate how brain structures influence sensitivity to stimuli. Addressing these questions with the cutting-edge tools currently available to investigate fly neural processing could be an exciting step forward in the study of insect attention.
5. Working memory
Working memory has been suggested to contribute to attention by itself comprising competitive processes as well as by the fact that processes could compete for control over or access to working memory [13,66]. It is therefore important to consider whether insects could also have a similar capacity. There is some evidence that crickets remember prior calls while choosing between multiple males [85]. The time course of this memory is however, unknown and it is as yet unclear whether this would qualify as auditory working memory. Visual working memory in insects, has however, been demonstrated in bees. Studies in the honeybee have used delayed matching to sample [86,87] or reversal learning [88] paradigms to probe short-term memory capabilities of bees. In both paradigms, bees were required to remember a target for varying intervals of time and were tested to see at what interval of time this short-term memory of the target would be abolished. These studies have shown that bees indeed do have short-term memory capabilities including a visual working memory interval that lasts around 6–9 s [86]. Field studies of flower choice by bumblebees also seem to indicate similar memory dynamics [89]. Another study in bumblebees has also shown that they can switch between multiple learnt targets with latencies shorter than this duration of working memory [5], suggesting that they can perhaps simultaneously have more than one visual search image in their working memory. There thus appears to be good evidence for visual working memory in at least two insect species.
6. Conclusion
Evidence from diverse fields makes a case arguing that insects have several mechanisms in place to enable attention-like and selective attentional capabilities—from sensory filters to higher-order brain processing. Knudsen's [13] framework for a mechanistic basis for attention argues for four fundamental component processes to attention: salience filters, competitive selection, top-down sensitivity control and working memory. It seems clear that at least some insects could fulfil all these criteria required to qualify as having attentional mechanisms. Behavioural evidence unequivocally shows that insects can and do restrict behavioural responses to specific stimuli of interest while simultaneously ignoring other perceivable stimuli. Substantial progress has already been made in identifying neuronal processes that underlie the deployment and modulation of both auditory and, more recently, visual selective attention in insects with evidence for both salience filters and competitive selection between neural representations. Recent studies have also revealed genetic changes that affect selective attention and top-down control on attention-like processes. Importantly, we find functional analogues of spatial attention, competitive selection, attentional blinks, auditory stream segregation and serial and parallel visual search in insects. Thus, several behaviours and neural processes have formal parallels with primate correlates of attention and we are at a stage where some of the fundamental questions of selective attention processing can be investigated in insects with both direct neurophysiology and genetic manipulation. In addition, the development of cutting-edge technological tools has allowed studies to begin making brain recordings from multiple regions in the brain while tracking freely moving insects as they respond to stimuli on virtual reality screens [32,83,90,91]. These technologies hold the promise for major advances in understanding the neural processing of selective attention in freely behaving insects.
Yet while much progress has been made in recent years, much still remains to be addressed in the study of attention-like and selective attentional processes in insects. Several important outstanding questions need to be investigated to establish the nature and function of selective attention in insects. One of the primary questions is whether insects have top-down (endogenous) control of selective attention, especially in the auditory domain. Both behavioural and neural evidence seems to suggest that insects do have some endogenous control of selective attention but further investigation is required into the underlying mechanisms. Another important question is the locus of attention—whether the influence of attention-like processes is seen only in peripheral sensory processes or whether central processes choose between two perceived stimuli. A related question is how and where decisions or choices are made between two stimuli and how such choices are integrated with selective attention at different levels from the periphery to the central brain. Some of the neurophysiological studies indicate that selective attention in insects is implemented in the periphery [1,82], while others demonstrate the involvement of higher brain structures [79,84]. It would therefore be important to test for the possibility for central choice between different representations of stimuli in experiments. One way of testing this behaviourally could be to use successive tests where success in the second test relies on the insect perceiving a stimulus it did not selectively attend to in the first. Success on the second task would show that representations of unattended stimuli are preserved and can be used for other tasks. In addition, we know, from attentional studies in humans that the locus of attention can vary depending on the difficulty of the tasks [92,93]. It remains to be seen if this is true for insects. Experiments investigating this would need to test insects with easier (e.g. fewer distractors) and more difficult (e.g. more distractors) tasks. Selective attention would be predicted to be achieved by more central processes in the former and by more peripheral processes in the latter. We also need more detailed studies establishing whether and how selective attention in insects modifies their detection and discrimination thresholds for stimuli. Finally, an important area that requires more research is how selective attention in insects operates across different modalities. Recent evidence indicates that, at least for some tasks, selective attention in foraging bees is allocated separately for different modalities such as vision and olfaction [94]. The details of the interactions between selective attention allocation for different tasks in different modalities are, however, still unknown. The study of insect attention thus holds tremendous potential for future research with scope for further fascinating discoveries about the fundamental processes governing attention in diverse systems.
Acknowledgements
I am grateful to Dr Michael Proulx, Prof. Heiner Römer and Prof. Jenny Read for helpful comments on earlier drafts of the manuscript. I also thank Dr Natasha Mhatre for use of two photographs in figure 1.
Competing interests
I declare I have no competing interests.
Funding
I am supported by a College for Life Sciences Fellowship at the Wissenschaftskolleg zu Berlin, Institute for Advanced Study.
References
- 1.Pollack GS. 1988. Selective attention in an insect auditory neuron. J. Neurosci. 8, 2635–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenfield MD, Snedden WA. 2003. Selective attention and the spatio-temporal structure of orthopteran choruses. Behaviour 140, 1–26. ( 10.1163/156853903763999863) [DOI] [Google Scholar]
- 3.Cherry E. 1953. Some experiments on the recognition of speech, with one and with two ears. J. Acoust. Soc. Am. 25, 975–979. ( 10.1080/00335558008248231) [DOI] [Google Scholar]
- 4.Spaethe J, Tautz J, Chittka L. 2006. Do honeybees detect colour targets using serial or parallel visual search? J. Exp. Biol. 209, 987–993. ( 10.1242/jeb.02124) [DOI] [PubMed] [Google Scholar]
- 5.Nityananda V, Pattrick JG. 2013. Bumblebee visual search for multiple learned target types. J. Exp. Biol. 216, 4154–4160. ( 10.1242/jeb.085456) [DOI] [PubMed] [Google Scholar]
- 6.Wolfe JM, Horowitz TS. 2004. What attributes guide the deployment of visual attention and how do they do it? Nat. Rev. Neurosci. 5, 495–501. ( 10.1038/nrn1411) [DOI] [PubMed] [Google Scholar]
- 7.Simons DJ, Chabris CF. 1999. Gorillas in our midst: sustained inattentional blindness for dynamic events. Perception 28, 1059–1074. ( 10.1068/p281059) [DOI] [PubMed] [Google Scholar]
- 8.Levin T, Simons DJ. 1997. Change blindness. Trends Cogn. Sci. 1, 261–267. ( 10.1016/S1364-6613(97)01080-2) [DOI] [PubMed] [Google Scholar]
- 9.James W. 1890. The principles of psychology. New York, NY: Holt. [Google Scholar]
- 10.Posner MI. 1980. Orienting of attention. Q. J. Exp. Psychol. 32, 3–25. ( 10.1080/00335558008248231) [DOI] [PubMed] [Google Scholar]
- 11.Desimone R, Duncan J. 1995. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222. ( 10.1146/annurev.ne.18.030195.001205) [DOI] [PubMed] [Google Scholar]
- 12.Wehner R. 1987. ‘Matched filters’—neural models of the external world. J. Comp. Physiol. A 161, 511–531. ( 10.1007/BF00603659)3316619 [DOI] [Google Scholar]
- 13.Knudsen EI. 2007. Fundamental components of attention. Annu. Rev. Neurosci. 30, 57–78. ( 10.1146/annurev.neuro.30.051606.094256) [DOI] [PubMed] [Google Scholar]
- 14.Itti L, Koch C. 2001. Computational modelling of visual attention. Nat. Rev. Neurosci. 2, 1–11. ( 10.1038/35058500) [DOI] [PubMed] [Google Scholar]
- 15.Proulx MJ. 2007. Bottom-up guidance in visual search for conjunctions. J. Exp. Psychol. Hum. Percept. Perform. 33, 48–56. ( 10.1037/0096-1523.33.1.48) [DOI] [PubMed] [Google Scholar]
- 16.Proulx MJ. 2007. Turning on the spotlight: do attention and luminance contrast affect neuronal responses in the same way? J. Neurosci. 27, 13 043–13 044. ( 10.1523/JNEUROSCI.4378-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kostarakos K, Hennig MR, Römer H. 2009. Two matched filters and the evolution of mating signals in four species of cricket. Front. Zool. 6, 22 ( 10.1186/1742-9994-6-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt AKD, Riede K, Römer H. 2011. High background noise shapes selective auditory filters in a tropical cricket. J. Exp. Biol. 214, 1754–1762. ( 10.1242/jeb.053819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kostarakos K, Roemer H. 2015. Neural mechanisms for acoustic signal detection under strong masking in an insect. J. Neurosci. 35, 10 562–10 571. ( 10.1523/JNEUROSCI.0913-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gumbert A. 2000. Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behav. Ecol. Sociobiol. 48, 36–43. ( 10.1007/s002650000213) [DOI] [Google Scholar]
- 21.Morawetz L, Svoboda A, Spaethe J, Dyer AG. 2013. Blue colour preference in honeybees distracts visual attention for learning closed shapes. J. Comp. Physiol. A 199, 817–827. ( 10.1007/s00359-013-0843-5) [DOI] [PubMed] [Google Scholar]
- 22.Karuppudurai T, et al. 2014. A hard-wired glutamatergic circuit pools and relays UV signals to mediate spectral preference in Drosophila. Neuron 81, 603–615. ( 10.1016/j.neuron.2013.12.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Swinderen B. 2011. Attention in Drosophila. Int. Rev. Neurobiol. 99, 51–85. ( 10.1016/B978-0-12-387003-2.00003-3) [DOI] [PubMed] [Google Scholar]
- 24.Römer H, Krusch M. 2000. A gain-control mechanism for processing of chorus sounds in the afferent auditory pathway of the bushcricket Tettigonia viridissima (Orthoptera; Tettigoniidae). J. Comp. Physiol. A 186, 181–191. ( 10.1007/s003590050018) [DOI] [PubMed] [Google Scholar]
- 25.Nityananda V, Stradner J, Balakrishnan R, Römer H. 2007. Selective attention in a synchronising bushcricket: physiology, behaviour and ecology. J. Comp. Physiol. A 193, 983–991. ( 10.1007/s00359-007-0251-9) [DOI] [PubMed] [Google Scholar]
- 26.Wang M-Y, Ings TC, Proulx MJ, Chittka L. 2013. Can bees simultaneously engage in adaptive foraging behaviour and attend to cryptic predators? Anim. Behav. 86, 859–866. ( 10.1016/j.anbehav.2013.07.029) [DOI] [Google Scholar]
- 27.Rossel S. 1996. Binocular vision in insects: how mantids solve the correspondence problem. Proc. Natl Acad. Sci. USA 93, 13 229–13 232. ( 10.1073/pnas.93.23.13229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiederman SD, O'Carroll DC. 2012. Selective attention in an insect visual neuron. Curr. Biol. 23, 1–6. ( 10.1016/j.cub.2012.11.048) [DOI] [PubMed] [Google Scholar]
- 29.Wolf R, Heisenberg M. 1980. On the fine structure of yaw torque in visual flight orientation of Drosophila melanogaster II. A temporally and spatially variable weighting function for the visual field (‘visual attention’). J. Comp. Physiol. A 80, 69–80. ( 10.1007/BF00613749) [DOI] [Google Scholar]
- 30.Collett TS, Land MF. 1975. Visual control of flight behaviour in the hoverfly, Syritta pipiens L. J. Comp. Physiol. A 6, 1–66. ( 10.1007/BF01464710) [DOI] [Google Scholar]
- 31.Olberg RM, Seaman RC, Coats MI, Henry AF. 2007. Eye movements and target fixation during dragonfly prey-interception flights. J. Comp. Physiol. A 193, 685–693. ( 10.1007/s00359-007-0223-0) [DOI] [PubMed] [Google Scholar]
- 32.Taylor GJ, Paulk AC, Pearson TWJ, Moore RJD, Stacey JA, Ball D, van Swinderen B, Srinivasan MV. 2015. Insects modify their behaviour depending on the feedback sensor used when walking on a trackball in virtual reality. J. Exp. Biol. 218, 3118–3127. ( 10.1242/jeb.125617) [DOI] [PubMed] [Google Scholar]
- 33.Sareen P, Wolf R, Heisenberg M. 2011. Attracting the attention of a fly. Proc. Natl Acad. Sci. USA 108, 7230–7235. ( 10.1073/pnas.1102522108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ings TC, Chittka L. 2008. Speed–accuracy tradeoffs and false alarms in bee responses to cryptic predators. Curr. Biol. 18, 1520–1524. ( 10.1016/j.cub.2008.07.074) [DOI] [PubMed] [Google Scholar]
- 35.Bumblebee. Wikimedia Commons. See https://commons.wikimedia.org/wiki/File:PSM_V45_D033_Orange_banded_bumblebee.jpg.
- 36.Drosophila melanogaster. Wikimedia Commons. See https://commons.wikimedia.org/wiki/File:Drosm3.gif.
- 37.Dragonfly. Wikimedia Commons. See https://commons.wikimedia.org/wiki/File:Dragonfly_2_(PSF).svg#filelinks.
- 38.Praying Mantis. Wikimedia Commons. See https://commons.wikimedia.org/wiki/File%3APSM_V04_D731_Praying_mantis.jpg.
- 39.Treisman AM, Gelade G. 1980. A feature-integration theory of attention. Cogn. Psychol. 12, 97–136. ( 10.1016/0010-0285(80)90005-5) [DOI] [PubMed] [Google Scholar]
- 40.Orlowski J, Beissel C, Rohn F, Adato Y, Wagner H, Ben-shahar O. 2015. Visual pop-out in barn owls: human-like behavior in the avian brain. J. Vis. 15, 1–13. ( 10.1167/15.14.4.doi) [DOI] [PubMed] [Google Scholar]
- 41.Proulx MJ, Parker MO, Tahir Y, Brennan CH. 2014. Parallel mechanisms for visual search in zebrafish. PLoS ONE 9, e111540 ( 10.1371/journal.pone.0111540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morawetz L, Spaethe J. 2012. Visual attention in a complex search task differs between honeybees and bumblebees. J. Exp. Biol. 215, 2515–2523. ( 10.1242/jeb.066399) [DOI] [PubMed] [Google Scholar]
- 43.Wiederman SD, O'Carroll DC. 2012. Feature saliency in a dragonfly neuron. In Frontiers in Behavioural Neuroscience Conference Abstract: Tenth International Congress of Neuroethology, 5–10 August, University of Maryland, College Park, MD. See http://www.frontiersin.org/10.3389/conf.fnbeh.2012.27.00223/event_abstract?sname=Tenth_International_Congress_of_Neuroethology_1.
- 44.Reynolds JH, Chelazzi L, Desimone R. 1999. Competitive mechanisms subserve attention in macaque areas V2 and V4. J. Neurosci. 19, 1736–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenfield MD. 1994. Synchronous and alternating choruses in insects and anurans: common mechanisms and diverse functions. Am. Zool. 34, 605–615. ( 10.1093/icb/34.6.605) [DOI] [Google Scholar]
- 46.Nityananda V, Balakrishnan R. 2007. Synchrony during acoustic interactions in the bushcricket Mecopoda ‘Chirper’ (Tettigoniidae:Orthoptera) is generated by a combination of chirp-by-chirp resetting and change in intrinsic chirp rate. J. Comp. Physiol. A 193, 51–65. ( 10.1007/s00359-006-0170-1) [DOI] [PubMed] [Google Scholar]
- 47.Hartbauer M, Kratzer S, Steiner K, Römer H. 2005. Mechanisms for synchrony and alternation in song interactions of the bushcricket Mecopoda elongata (Tettigoniidae: Orthoptera). J. Comp. Physiol. A 191, 175–188. ( 10.1007/s00359-004-0586-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schul J, Sheridan RA. 2006. Auditory stream segregation in an insect. Neuroscience 138, 1–4. ( 10.1016/j.neuroscience.2005.11.023) [DOI] [PubMed] [Google Scholar]
- 49.Snedden WA, Greenfield MD. 1998. Females prefer leading males: relative call timing and sexual selection in katydid choruses. Anim. Behav. 56, 1091–1098. ( 10.1006/anbe.1998.0871) [DOI] [PubMed] [Google Scholar]
- 50.Romer H, Hedwig B, Ott SR. 2002. Contralateral inhibition as a sensory bias: the neural basis for a female preference in a synchronously calling bushcricket, Mecopoda elongata. Eur. J. Neurosci. 15, 1655–1662. ( 10.1046/j.1460-9568.2002.02003.x) [DOI] [PubMed] [Google Scholar]
- 51.Snedden WA, Greenfield MD, Jang Y. 1998. Mechanisms of selective attention in grasshopper choruses: who listens to whom? Behav. Ecol. Sociobiol. 43, 59–66. ( 10.1007/s002650050466) [DOI] [Google Scholar]
- 52.Bregman A. 1990. Auditory scene analysis: the perceptual organization of sound. Cambridge, MA: The MIT press. [Google Scholar]
- 53.Izumi A. 2002. Auditory stream segregation in Japanese monkeys. Cognition 82, B113–B122. ( 10.1016/S0010-0277(01)00161-5) [DOI] [PubMed] [Google Scholar]
- 54.Ma L, Micheyl C, Yin P, Oxenham AJ, Shamma SA. 2010. Behavioral measures of auditory streaming in ferrets (Mustela putorius). J. Comp. Psychol. 124, 317–330. ( 10.1037/a0018273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nityananda V, Bee MA. 2011. Finding your mate at a cocktail party: frequency separation promotes auditory stream segregation of concurrent voices in multi-species frog choruses. PLoS ONE 6, e21191 ( 10.1371/journal.pone.0021191) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sussman ES, Horváth J, Winkler I, Orr M. 2007. The role of attention in the formation of auditory streams. Percept. Psychophys. 69, 136–152. ( 10.3758/BF03194460) [DOI] [PubMed] [Google Scholar]
- 57.Hoy RR. 1992. The evolution of hearing in insects as an adaptation to predation from bats. In The evolutionary biology of hearing (eds Webster D, Fay R, Popper A), pp. 115–129. New York, NY: Springer. [Google Scholar]
- 58.Brown WD, Wideman J, Andrade MCB, Mason AC, Gwynne DT. 1996. Female choice for an indicator of male size in the song of the black-horned tree cricket Oecanthus nigricornis (Orthoptera: Gryllidae: Oecanthinae). Evolution (N.Y) 50, 2400–2411. ( 10.2307/2410708) [DOI] [PubMed] [Google Scholar]
- 59.Wyttenbach RA, Farris HE. 2004. Psychophysics in insect hearing. Microsc. Res. Tech. 63, 375–387. ( 10.1002/jemt.20054) [DOI] [PubMed] [Google Scholar]
- 60.Raymond JE, Shapiro KL, Arenell KM. 1992. Temporary suppression of visual processing in an RSVP task: an attentional blink? J. Exp. Psychol. Hum. Percept. Perform. 18, 849–860. ( 10.1037/0096-1523.18.3.849) [DOI] [PubMed] [Google Scholar]
- 61.Marois R, Chun MM, Gore JC. 2000. Neural correlates of the attentional blink. Neuron 28, 299–308. ( 10.1016/S0896-6273(00)00104-5) [DOI] [PubMed] [Google Scholar]
- 62.Greenfield MD, Roizen I. 1993. Katydid synchronous chorusing is an evolutionary stable outcome of female choice. Nature 364, 618–620. ( 10.1038/364618a0) [DOI] [Google Scholar]
- 63.Fertschai I, Stradner J, Römer H. 2007. Neuroethology of female preference in the synchronously singing bushcricket Mecopoda elongata (Tettigoniidae; Orthoptera): why do followers call at all? J. Exp. Biol. 210, 465–476. ( 10.1242/jeb.02655) [DOI] [PubMed] [Google Scholar]
- 64.Buck J, Buck E, Hanson FE, Case JF, Mets L, Atta GJ. 1981. Control of flashing in fireflies IV. Free run pacemaking in a synchronic Pteroptyx. J. Comp. Physiol. A 144, 277–286. ( 10.1007/BF00612559) [DOI] [Google Scholar]
- 65.Chun MM, Jiang Y. 1998. Contextual cueing: implicit learning and memory of visual context guides spatial attention. Cogn. Psychol. 36, 28–71. ( 10.1006/cogp.1998.0681) [DOI] [PubMed] [Google Scholar]
- 66.de Fockert JW, Rees G, Frith CD, Lavie N. 2001. The role of working memory in visual selective attention. Science. 291, 1803–1806. ( 10.1126/science.1056496) [DOI] [PubMed] [Google Scholar]
- 67.Treue S. 2001. Neural correlates of attention in primate visual cortex. Trends Neurosci. 24, 295–300. ( 10.1016/S0166-2236(00)01814-2) [DOI] [PubMed] [Google Scholar]
- 68.Chittka L, Dyer AG, Bock F, Dornhaus A. 2003. Bees trade off foraging speed for accuracy. Nature 424, 388 ( 10.1038/424388a) [DOI] [PubMed] [Google Scholar]
- 69.Avarguès-Weber A, Deisig N, Giurfa M. 2011. Visual cognition in social insects. Annu. Rev. Entomol. 56, 423–443. ( 10.1146/annurev-ento-120709-144855) [DOI] [PubMed] [Google Scholar]
- 70.Wolfe JM. 1998. Visual search. In Attention (ed. H Pashler), London, UK: University College London Press.
- 71.Avargues-Weber A, Dyer AG, Ferrah N, Giurfa M. 2014. The forest or the trees: preference for global over local image processing is reversed by prior experience in honeybees. Proc. R. Soc. B 282, 20142384 ( 10.1098/rspb.2014.2384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heisenberg M. 1998. What do the mushroom bodies do for the insect brain? An introduction. Learn. Mem. 5, 1–10. ( 10.1101/lm.5.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zars T. 2000. Behavioral functions of the insect mushroom bodies. Curr. Opin. Neurobiol. 10, 790–795. ( 10.1016/S0959-4388(00)00147-1) [DOI] [PubMed] [Google Scholar]
- 74.Davis RL. 1993. Mushroom bodies and Drosophila learning. Neuron 11, 1–14. ( 10.1016/0896-6273(93)90266-T) [DOI] [PubMed] [Google Scholar]
- 75.Xi W, Peng Y, Guo J, Ye Y, Zhang K, Yu F, Guo A. 2008. Mushroom bodies modulate salience-based selective fixation behavior in Drosophila. Eur. J. Neurosci. 27, 1441–1451. ( 10.1111/j.1460-9568.2008.06114.x) [DOI] [PubMed] [Google Scholar]
- 76.van Swinderen B. 2007. Attention-like processes in Drosophila require short-term memory genes. Science 315, 1590–1593. ( 10.1126/science.1137931) [DOI] [PubMed] [Google Scholar]
- 77.Miller SM, Ngo TT, van Swinderen B. 2012. Attentional switching in humans and flies: rivalry in large and miniature brains. Front. Hum. Neurosci. 5, 1–17. ( 10.3389/fnhum.2011.00188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koenig S, Wolf R, Heisenberg M. 2016. Vision in flies: measuring the attention span. PLoS ONE 11, e0148208 ( 10.1371/journal.pone.0148208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Swinderen B, Greenspan RJ. 2003. Salience modulates 20–30 Hz brain activity in Drosophila. Nat. Neurosci. 6, 579–586. ( 10.1038/nn1054) [DOI] [PubMed] [Google Scholar]
- 80.van Swinderen B. 2012. Competing visual flicker reveals attention-like rivalry in the fly brain. Front. Integr. Neurosci. 6, 1–12. ( 10.3389/fnint.2012.00096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vialatte F-B, Maurice M, Dauwels J, Cichocki A. 2010. Steady-state visually evoked potentials: focus on essential paradigms and future perspectives. Prog. Neurobiol. 90, 418–438. ( 10.1016/j.pneurobio.2009.11.005) [DOI] [PubMed] [Google Scholar]
- 82.Paulk AC, Stacey JA, Pearson TWJ, Taylor GJ, Moore RJD, Srinivasan MV, van Swinderen B. 2014. Selective attention in the honeybee optic lobes precedes behavioral choices. Proc. Natl Acad. Sci. USA 111, 5006–5011. ( 10.1073/pnas.1323297111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paulk AC, Kirszenblat L, Zhou Y, van Swinderen B. 2015. Closed-loop behavioral control increases coherence in the fly brain. J. Neurosci. 35, 10 304–10 315. ( 10.1523/JNEUROSCI.0691-15.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Bivort BL, Van Swinderen B. 2016. Evidence for selective attention in the insect brain. Curr. Opin. Insect Sci. 15, 9–15. ( 10.1016/j.cois.2016.02.007) [DOI] [PubMed] [Google Scholar]
- 85.Bailey NW, Zuk M. 2009. Field crickets change mating preferences using remembered social information. Biol. Lett. 5, 449–451. ( 10.1098/rsbl.2009.0112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang S, Bock F, Si A, Tautz J, Srinivasan MV. 2005. Visual working memory in decision making by honey bees. Proc. Natl Acad. Sci. USA 102, 5250–5255. ( 10.1073/pnas.0501440102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown MF, Mckeon D, Curley T, Weston B, Lambert C, Lebowitz B. 1998. Working memory for color in honeybees. Anim. Learn. Behav. 26, 264–271. ( 10.3758/BF03199220) [DOI] [Google Scholar]
- 88.Menzel R. 1979. Behavioural access to short-term memory in bees. Nature 281, 368–369. ( 10.1038/281368a0) [DOI] [PubMed] [Google Scholar]
- 89.Raine NE, Chittka L. 2007. Flower constancy and memory dynamics in bumblebees (Hymenoptera: Apidae: Bombus). Entomol. Gen. 29, 179–199. [Google Scholar]
- 90.Stowers JR, Fuhrmann A, Hofbauer M, Streinzer M, Schmid A, Dickinson MH, Straw AD. 2014. Reverse engineering animal vision with virtual reality and genetics. Computer (Long. Beach. Calif). 47, 38–45. ( 10.1109/MC.2014.190) [DOI] [Google Scholar]
- 91.Grover D, Katsuki T, Greenspan RJ. 2016. Flyception: imaging brain activity in freely walking fruit flies. Nat. Methods 13, 569–572. ( 10.1038/nmeth.3866) [DOI] [PubMed] [Google Scholar]
- 92.Lavie N. 1995. Perceptual load as a necessary condition for selective attention. J. Exp. Psychol. Hum. Percept. Perform. 21, 451–468. ( 10.1037/0096-1523.21.3.451) [DOI] [PubMed] [Google Scholar]
- 93.Proulx MJ, Egeth HE. 2006. Target–nontarget similarity modulates stimulus-driven control in visual search. Psychon. Bull. Rev. 13, 524–529. ( 10.3758/BF03193880) [DOI] [PubMed] [Google Scholar]
- 94.Nityananda V, Chittka L. 2015. Modality-specific attention in foraging bumblebees. R. Soc. open sci. 2, 150324 ( 10.1098/rsos.150324) [DOI] [PMC free article] [PubMed] [Google Scholar]