Abstract
One of the most well-recognized Cretaceous fossils is Citipati osmolskae (MPC-D 100/979), an oviraptorid dinosaur discovered in brooding position on a nest of unhatched eggs. The original description refers to a thin lens of white material extending from a manus ungual, which was proposed to represent original keratinous claw sheath that, in life, would have covered it. Here, we test the hypothesis that this exceptional morphological preservation extends to the molecular level. The fossil sheath was compared with that of extant birds, revealing similar morphology and microstructural organization. In living birds, the claw sheath consists primarily of two structural proteins; alpha-keratin, expressed in all vertebrates, and beta-keratin, found only in reptiles and birds (sauropsids). We employed antibodies raised against avian feathers, which comprise almost entirely of beta-keratin, to demonstrate that fossil tissues respond with the same specificity, though less intensity, as those from living birds. Furthermore, we show that calcium chelation greatly increased antibody reactivity, suggesting a role for calcium in the preservation of this fossil material.
Keywords: beta-keratin, scanning electron microscopy-energy dispersive X-ray spectroscopy, transmission electron microscopy, immunofluorescence, claw sheath
1. Introduction
In 1995, an oviraptorid dinosaur (MPC-D (Mongolian Paleontological Center-Dinosaur (formerly IGM)) 100/979) preserved upright over a nest of unhatched eggs [1,2] was recovered from the Djadokhta Formation of Mongolia, estimated to be Upper Campanian in age [3,4]. Its forelimbs encompass the eggs, and the hindlimbs are tucked underneath the body and lie parallel to the eggs [1]. The specimen is entombed by amorphous, massive sandstone; thus a dune-originated sand slide has been posited as cause of death [3].
The original description [1,2] noted a thin lens of material extending from the ungual of one of the manual digits (figure 1a, also Fig. 3 in [1]). This material differed in texture and colour from both the surrounding sandstone matrix and bone, and was proposed to be the remnants of a claw sheath, which covers the unguals of living animals, and consists primarily of keratin proteins. The location and structure of this material suggested the possibility that original organics may persist.
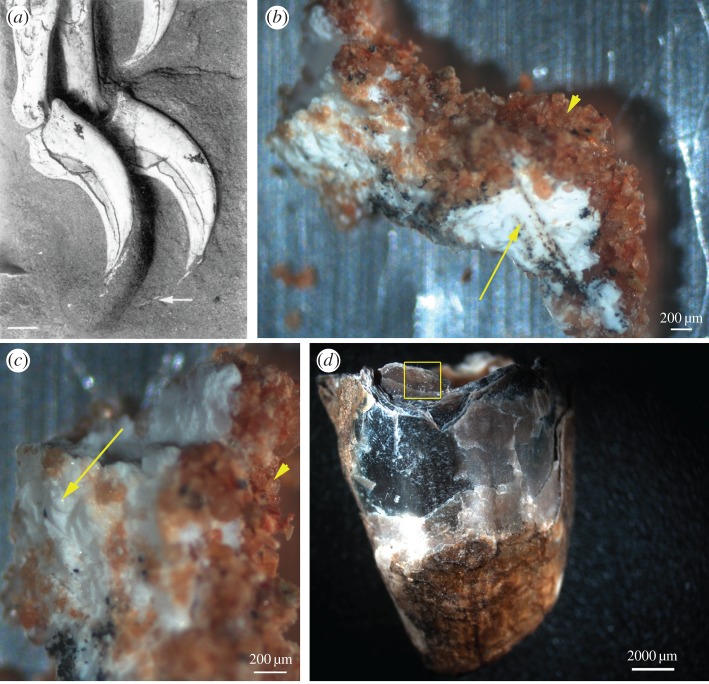
Figure 1.
Fossil and extant claw sheath material. (a) Citipati osmolskae (MPC-D 100/979) with exposed claw sheath (white arrow) extending off the ungual of one of the manual digits. Reprinted with permission from [1] (Copyright © 1995 Macmillan Publishers Ltd). Fossil claw sheath in (b) lower and (c) higher magnification clearly differentiated from surrounding sediment by colour and texture; sand (arrowhead) is red and granular, sheath is white, dense and cohesive. Higher magnification (c) reveals a fibrous, somewhat rugose texture (arrow). (d) Entire emu claw sheath, with boxed region showing approximate location sampled for comparative SEM analysis. (Online version in colour.)
Here we test this hypothesis using multiple techniques, including electron microscopy and in situ immunohistochemistry (IHC). We predicted that, if endogenous, microstructural features comparable with those of extant tissues would be retained, and the tissue would respond similarly to modern claw sheath material in immunochemical analyses.
Most researchers recognize two major families of keratins: alpha and beta. Alpha-keratins [5] are widely distributed across all vertebrates, consistent with an early evolutionary origin and comprise an alpha-helical central rod, producing a 10 nm intermediate filament [6]. Beta-keratins (or ‘corneous beta-proteins’ [7–10]) are insoluble, rigid, fibrous, structural proteins distinct from alpha-keratins in composition and structure. They are expressed only in reptiles and birds (sauropsids) [11–13], suggesting that this gene family originated after the divergence of sauropsids from other vertebrates [5,14–16]. The beta-keratins have in common a core of approximately 30 amino acids and produce filaments (microfibrils) 3 nm in diameter [17]. The core incorporates amino acids such as proline and valine [11,17–19] which confers hydrophobicity, therefore, increasing preservation potential.
Both alpha- and beta-keratins incorporate the amino acid cysteine, and thus contain sulfur [20]. However, alpha-keratins exist as both low- (‘soft’ keratins like skin), and high-sulfur proteins (‘hard’ keratins like hair and nails) [6,20,21], while all beta-keratins have a sulfur content and function similarly to the hard alpha-keratins [15,20]. As they mature, beta-keratins become increasingly cross-linked through formation of disulfide bonds [22], adding further rigidity and hardness to the structures they comprise, thus contributing to preservation potential. Because the outermost cornified layer of the claw sheath in modern birds is composed primarily of beta-keratin, it is harder, but is underlain by a more pliable and softer layer dominated by alpha-keratins [7,23,24].
The composition and structure of beta-keratin suggest that it may persist in fossil material [25–30]. Furthermore, because this protein is not found in humans and is vertebrate specific; its identification eliminates the alternative hypotheses of human or microbial contamination, and when used with adequate controls is a good indicator of endogeneity.
We performed scanning (SEM) and transmission electron microscopy (TEM) to compare microstructure of the oviraptorid claw sheath with homologous extant bird claw sheaths. Then, we employed an antibody raised against protein extracted from mature chicken feathers (comprised almost exclusively of beta-keratin [31,32]) to detect antibody–antigen complexes in immunohistochemical studies.
2. Material and methods
For additional details of material and methods, see the electronic supplementary material. Dinosaur claw samples were compared with extant emu and ostrich claw sheath. Experimental parameters were identical, but ancient and modern samples were treated in separate laboratory spaces, and all samples were always handled using gloves and sterile materials and instruments.
(a). Scanning electron microscopy coupled with energy dispersive X-ray spectroscopy
An undemineralized fragment of the fibrous material associated with the MPC-D 100/979 ungual (figure 1b,c) was subjected to SEM and compared with an approximately 2 mm fragment of emu claw sheath, taken from the dorsal proximal region (figure 1d, box).
Fossil material was not coated but emu claw images were captured after applying a 3–6 nm gold/palladium coating for charge compensation. EDS data were collected at 20 kV and 10 kV accelerating voltage (for extant and fossil material, respectively), a working distance of 10 mm and for 100–120 s.
(b). Transmission electron microscopy
After SEM, the same Citipati sheath fragment was embedded in LR white (hard grade acrylic resin, London Resin Company Ltd, lot no. 140916, batch no. 409081), and imaged using TEM.
Separately, the ostrich claw sheath was fixed in 10% neutral buffered formalin, washed in phosphate-buffered saline (PBS), embedded in LR white, sectioned and mounted following the protocol outlined in Moyer et al. [33]. Sectioned ostrich claws were stained with 15% methanolic uranyl acetate and Reynold's lead citrate prior to imaging. Fossil material was visualized without staining.
(c). In situ immunohistochemistry–immunofluorescence
Citipati samples were demineralized overnight in 0.5 M ethylenediaminetetraacetic acid (EDTA) pH 8.0 to chelate calcium (see Discussion) before conducting immunological tests. Tissue remaining after demineralization (electronic supplementary material, figure S4b) was collected and washed in PBS.
In a separate room, ostrich claw sheath, decalcified ostrich bone and alligator skin were fixed in 10% neutral buffered formalin for a minimum of 1 h followed by PBS wash. All samples were then embedded in LR white resin and sectioned to 200 nm using dedicated ultramicrotome diamond knives.
For complete details on antibodies, IHC methods and specificity controls, see [33]; electronic supplementary material, figures.
The primary antiserum was exposed to keratinous tissues from the two extant groups of archosauria: chicken feather and alligator skin (electronic supplementary material, figure S5). Both were positive for binding. Using extant phylogenetic bracketing, we applied the first-order assumption that dinosaur tissue would also show reactivity in a similar pattern, if epitopes were preserved [34]. This antiserum was applied at a final concentration of 1 : 500 for all test samples and controls.
3. Results
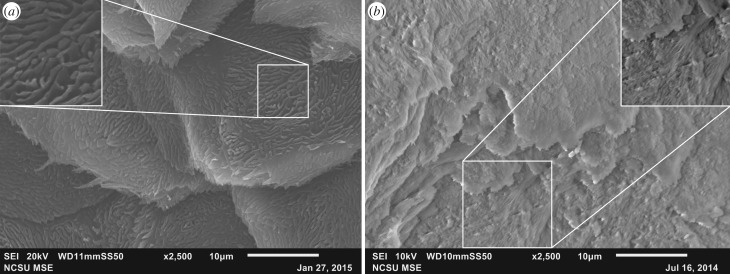
Location, gross morphology, microstructural features and molecular reactivity are similar between the fossil claw material and extant emu and ostrich claw sheaths, supporting the hypothesis that the original keratinous tissue was preserved in the Citipati specimen. In hand sample, fossil claw tissue was white and amorphous (figure 1b,c, arrow), contrasting with both the surrounding sandstone grains (figure 1b,c, arrowheads) and textured bone (figure 1a). SEM images show the presence of thin, plate-like layers, similar to those observed in extant emu claw sheaths (figure 2). Both fossil and extant claw possess rugosities over the surface, appearing as whorls in the emu claw (figure 2a, inset) while Citipati tissues show similar, but less pronounced ridges (figure 2b, inset).
Figure 2.
SEM images of (a) ventral surface of extant emu and (b) Citpati claw sheath material. Both show plate-like layering and rugose texture covering the surface. Sculpturing in emu occurs as a mat of finger-like projections (inset in a). A similar texture is observed in the fossil material (inset in b), but is less pronounced than in extant tissue. This may be preservational artefact, or may be due to sampling orientation, which was not noted when collected.
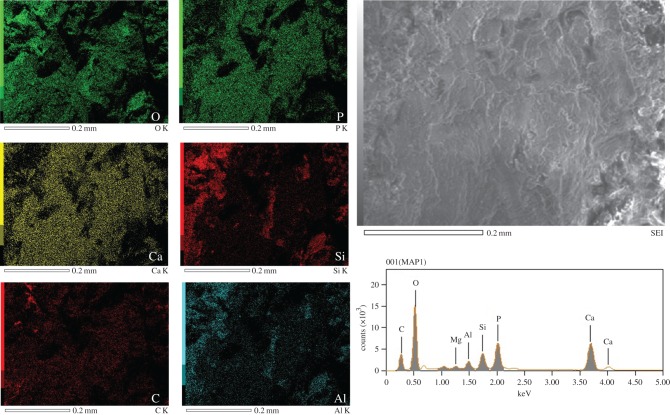
The elemental composition of the oviraptorid claw sheath was dominated by oxygen and calcium (figure 3, approx. 68% of total X-rays), but no calcium was detected in the emu tissue (electronic supplementary material, figure S2); rather carbon, oxygen and sulfur were most abundant (approx. 97% of X-rays detected). Other elements, including phosphorous, silicon, aluminium were detected in the fossil claw, which along with carbon, oxygen and calcium represented approximately 97% of the X-rays detected (figure 3). Quantitative data (table 1) showed that calcium was concentrated in the fossil claw over that detected in the surrounding sediments (electronic supplementary material, figure S3).
Figure 3.
SEM-EDS analyses of Citipati claw sheath material. These six elements represent approximately 97% (by mass) of the X-rays detected. Quantitative data show oxygen is dominant in both white claw sheath material and sediment; however, there was a relatively greater amount of calcium detected in the claw sheath than in the sediment. No calcium was detected in the emu claw (electronic supplementary material, figure S2). See table 1 for quantitative data. (Online version in colour.)
Table 1.
SEM-EDS quantitative data for the emu claw sheath (electronic supplementary material, figure S2), fossil Citipati claw sheath (figure 3) and sediment associated with the fossil (electronic supplementary material, figure S3), in decreasing abundance. Data are presented by weight (ms%) and molar (mol%) percentages. Intriguingly, iron is present in the sediment but is not detected in the fossil claw sheath, indicating little transfer between the oviraptorid claw sheath tissues and entombing sediments. Note the difference in relative abundance of calcium (italicized) in the fossil claw sheath compared to the extant emu claw sheath (see Discussion).
| emu |
Citipati |
sediment |
||||||
|---|---|---|---|---|---|---|---|---|
| element | ms% | mol% | element | ms% | mol% | element | ms% | mol% |
| C | 75.48 | 81.85 | O | 36.76 | 48.37 | O | 41.39 | 44.49 |
| O | 20.08 | 16.34 | Ca | 31.57 | 16.58 | C | 24.68 | 35.34 |
| S | 3.22 | 1.31 | C | 11.88 | 20.82 | Si | 18.64 | 11.41 |
| CI | 0.67 | 0.25 | P | 11.85 | 8.05 | Al | 7.2 | 4.59 |
| Na | 0.29 | 0.16 | Si | 4.76 | 3.57 | Ca | 2.37 | 1.02 |
| K | 0.26 | 0.09 | Al | 1.94 | 1.52 | Fe | 2.04 | 0.63 |
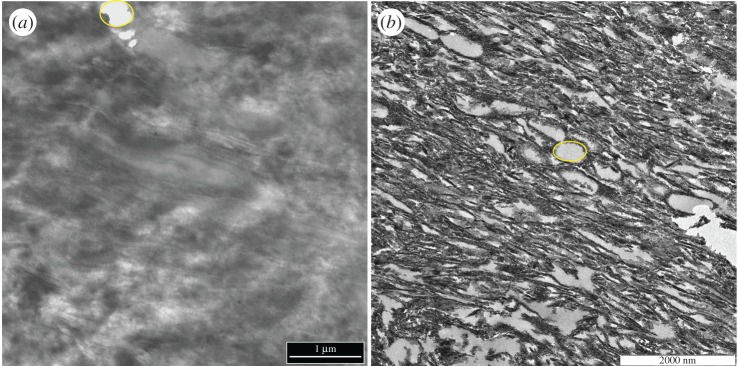
When the fossil and extant claw sheath tissues were observed under TEM (figure 4), both revealed parallel-oriented fibres which occasionally separate to form irregularly spaced openings (figure 4a,b, encircled voids). These do not reflect sectioning artefact but were part of the original structure (see Discussion).
Figure 4.
TEM micrographs of (a) ostrich claw sheath and (b) Citipati claw sheath. In both samples, parallel fibres can be seen running diagonally, and identical voids (encircled regions) were observed among the fibres in both samples. We hypothesize that voids may represent structural remnants of original lipid droplets in developing claw sheath tissue [35,36]. Alternatively, these voids may represent amorphous corneous material in a poorly stainable, electron-lucent matrix ([10] and references therein). (Online version in colour.)
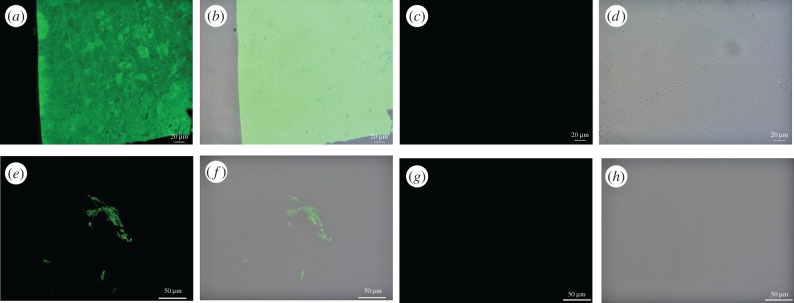
Electronic supplementary material, figure S5a,b show that the antibody cross reacts to alligator skin, localizing to the outermost layer (stratum corneum) of the epidermis, where beta-keratin dominates [18]. No binding was observed in the underlying layers, which are composed primarily of alpha-keratin [24], demonstrating specificity. By contrast, a wider distribution of binding was seen in the extant feather (electronic supplementary material, figure S5e,f) and claw sheath material (figure 5a,b), consistent with higher concentrations of this protein in these tissues. These data show that epitopes are conserved between these two extant archosaurian members. Binding was also observed in Citipati claw tissue (figure 5e,f), although reduced in intensity relative to extant counterparts.
Figure 5.
Immunohistochemical staining results for (a–d) ostrich claw sheath and (e–h) Citipati claw sheath exposed to antiserum raised against avian feathers [33]; (a, c, e and g) are imaged using a FITC filter; (b, d, f and h) are overlay images, superimposing fluorescent signal on a transmitted light image of sectioned tissue to reveal the localization of antibody–antigen complexes to tissue. Positive binding is observed in both extant and fossil claw sheath material. Controls using secondary antibody only are negative for both (c,d) ostrich and (g,h) fossil material. (Online version in colour.)
No binding was observed in any negative controls (figure 5; electronic supplementary materials, figures S5–S8, also see [33]).
4. Discussion
Micro- and ultrastructure of the Citipati claw material observed in both SEM and TEM are consistent with that of extant emu and ostrich claw sheaths in possessing plate-like morphology with regular rugosities on the surface. This sculpturing may serve to increase surface area, for greater adhesion to the underlying bone. In fact, similar rugosities on bony surfaces are used as an osteological correlate for the presence of a keratinous covering, e.g. on the rostrum of dinosaurs with beaks [37–39], or covering the bony shield, specifically the epiparietals of ceratopsians (e.g. [40,41]).
The voids between fibres of ostrich claw sheath tissue observed in TEM are also seen in Citipati, and may be remnants of lipid droplets which deform keratin fibres around them, forming rounded openings, which remain even after cornification in extant forming keratinous tissues [35,36,42,43]. Alternatively, these voids may represent amorphous corneous material, containing glycine-rich beta-keratins, in a poorly stainable, electron-pale matrix ([10] and references therein).
Although carbon was much higher in the emu claw sheath, consistent with its organic composition, carbon was also the third most abundant element in the fossil material, supporting the possibility of organic content remaining in these tissues.
Determining the mode of preservation is beyond the scope of this paper, but several aspects of our study suggest calcium may have played a role in the preservation of this oviraptorid claw sheath material. Calcium was not detected in the emu claw sheath, and was only minimally present in sediments directly surrounding the fossil, but was greatly elevated in the fossil claw sheath. The source of this calcium may be mobilization, possibly microbially mediated, either from the underlying bone [25] or from a calcium carbonate lens described in the Citipati burial sediments [44]; however, the detection of iron in the burial sediments but not in the Citipati claw sheath (table 1) suggests minimum transfer from sediments to tissue. Such rapid mineral deposition, regardless of source, may have acted to stabilize the keratinous sheath before its complete degradation [45,46]). Future taphonomic experiments will examine the mechanisms and role of calcium in the preservation of claw sheath tissue.
Another, but untestable hypothesis, is that dinosaur claw sheaths were biomineralized in life. Calcification of keratinous structures has been reported in alpha-keratinous structures in mammals [47,48], but the majority of keratinous structures in extant animals are not biomineralized and, to our knowledge, no beta-keratinous tissues are reported to undergo biomineralization in life. It is more parsimonious to assume the concentration of calcium in the oviraptorid claw sheath is diagenetic in origin. It may, nonetheless, have contributed to the molecular preservation we observed.
Although calcium may have played a role in preserving the organic residues detected by our methods, its removal greatly enhanced immunohistochemical results. This effect was demonstrated in experiments with other heavy metal ions, i.e. iron, associated with fossil soft tissues [49].
Because beta-keratin is not produced by mammals or microbes [50], which are the most likely sources for contamination, specific binding and localization of beta-keratin epitopes to these fossil tissues strongly supports the hypothesis that endogenous protein components are preserved in MPC-D 100/979. The reduced binding observed in the fossil sample relative to extant ostrich tissue may have resulted from: (i) less tissue incorporated into sections, (ii) the presence of fewer cross-reacting epitopes in this dinosaur in vivo because of evolutionary distance and/or (iii) the preservation of fewer reactive epitopes.
Binding was negative when antiserum was exposed to controls not expected to possess beta-keratin (e.g. decalcified ostrich bone; electronic supplementary material, figure S8c,d; human fingernail [33]); similarly, the Citipati claw sheath did not react when exposed to an antibody to human elastin (not present in any keratinous tissue) applied under the same parameters as beta-keratin antibodies (electronic supplementary material, figure S7a,b). These controls testify to the specificity of these antibodies, and refute the possibility that our antibodies were binding randomly, or that secondary antibodies were binding non-specifically. Furthermore, when antiserum was inhibited with purified feather extracts prior to exposure, antibody binding was greatly reduced or eliminated, supporting the hypothesis that these antibodies are specific to keratin epitopes remaining in the tissue (electronic supplementary material, figures S6c,d and S7c,d). Finally, localization of antibody–antigen complexes to the outermost layer of alligator skin shows this antiserum to be specific to and cross-reactive with beta-keratin epitopes in other tissues, but not with alpha-keratin (also see figure S3 in [33]), and demonstrates that specific antibodies can be used to delineate compositional differences in tissues.
Keratinous tissues preserved with fossils are rare, and in general, the amount of available working material is much less than that recovered from fossil bone or other biomineralized remains. This limits the methods that can be employed and requires methods that yield the most amount of information from the least destruction of irreplaceable, limited material. For these tissues, chemical extraction to recover proteins for electrophoresis and/or mass spectrometry to recover sequence information are precluded. However, in situ immunohistochemical analyses, when used with appropriate controls, can be employed to support endogeneity and inform on the presence of proteinaceous components also present in modern homologues. When antibodies support the presence of endogenous proteins, these can be used in immunoprecipitation to isolate and concentrate peptides for mass spectrometry and inform on epitope retrieval.
Because these keratinous tissues are often preserved as small, thin structures (compared with skeletal material), they are susceptible to destruction during preparation; our study calls for an increased awareness on the part of both palaeontologists and preparators that these originally proteinaceous structures may be preserved, particularly when other aspects of the fossil (e.g. articulation and three-dimensional preservation), suggest that exceptional taphonomic conditions prevailed. Molecular remains, even associated with minute tissues, have potential to elucidate aspects of the biology of extinct animals that are otherwise unattainable. Although conventional wisdom challenges the preservation of endogenous molecular remains, our combined data support the presence of original, proteinaceous material associated with this specimen, and add to the literature supporting molecular preservation in fossil materials across geological time.
5. Conclusion
Our data, obtained using electron microscopy and in situ immunofluorescence, support the preservation of original proteinaceous claw material in this approximately 75 million-year-old oviraptorid dinosaur, Citipati osmolskae (MPC-D 100/979). Using emu and ostrich claw tissues as modern comparisons, we have shown that fossil claw sheath material retains microstructure consistent with keratinous tissues. Antiserum specific for sauropsid beta-keratins demonstrates positive binding to these fossil tissues, while all appropriate controls are negative. This study adds to the growing body of evidence demonstrating that beta-keratins are an ideal target for molecular palaeontological studies. Although the amount of fossil material available to work with for keratinous remains is minute, as technology advances and sample preparation methods improve, we will be able to elucidate the early evolutionary history of beta-keratin, which remains uncertain when only data from extant material are studied.
Supplementary Material
Acknowledgements
We thank Mark Norell and Amy Davidson for providing the fossil sample for this study. We also thank Sue Brumfield at Montana State University and Toby Tung at NCSU (MSE) for their electron microscopy assistance.
Data accessibility
Supporting data can be found in the electronic supplementary material.
Authors' contributions
A.E.M. designed the experiment. A.E.M. and W.Z. performed analyses and collected data. A.E.M. and M.H.S. interpreted results and wrote the manuscript.
Competing interests
We have no competing interests.
Funding
This research was funded by grants to M.H.S. from the David and Lucile Packard Foundation and the NSF INSPIRE programme (EAR-1344198), and the National Science Foundation Graduate Research Fellowship Program (DGE-1252376) to A.E.M.
References
- 1.Norell MA, Clark JM, Chiappe LM, Dashzeveg D. 1995. A nesting dinosaur. Nature 378, 774–776. ( 10.1038/378774a0) [DOI] [Google Scholar]
- 2.Clark JM, Norell M, Chiappe LM. 1999. An oviraptorid skeleton from the Late Cretaceous of Ukhaa Tolgod, Mongolia, preserved in an avianlike brooding position over an oviraptorid nest. Am. Mus. Novit. 3265, 1–36. [Google Scholar]
- 3.Dingus L, Loope DB, Dashzeveg D, Swisher CC, Minjin C, Novacek MJ, Norell MA. 2008. The geology of Ukhaa Tolgod (Djadokhta Formation, Upper Cretaceous, Nemegt Basin, Mongolia). Am. Mus. Novit. 3616, 1 ( 10.1206/442.1) [DOI] [Google Scholar]
- 4.Dashzeveg D, Dingus L, Loope DB, Swisher CC, Dulam T, Sweeney MR. 2005. New stratigraphic subdivision, depositional environment, and age estimate for the Upper Cretaceous Djadokhta Formation, Southern Ulan Nur Basin, Mongolia. Am. Mus. Novit. 3498, 1 ( 10.1206/0003-0082(2005)498%5B0001:NSSDEA%5D2.0.CO;2) [DOI] [Google Scholar]
- 5.Wu D-D, Zhang Y-P. 2008. Molecular evolution of the keratin associated protein gene family in mammals, role in the evolution of mammalian hair. BMC Evol. Biol. 8, 1–15. ( 10.1186/1471-2148-8-241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moll R, Divo M, Langbein L. 2008. The human keratins: biology and pathology. Histochem. Cell Biol 129, 705–733. ( 10.1007/s00418-008-0435-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alibardi L. 2015. Immunolocalization of sulfhydryl oxidase in reptilian epidermis indicates that the enzyme participates mainly to the hardening process of the beta-corneous layer. Protoplasma 252, 1529–1536. ( 10.1007/s00709-015-0782-9) [DOI] [PubMed] [Google Scholar]
- 8.Holthaus KB, et al. 2015. Comparative genomics identifies epidermal proteins associated with the evolution of the turtle shell. Mol. Biol. Evol. 33, 726–737. ( 10.1093/molbev/msv265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strasser B, Mlitz V, Hermann M, Tschachler E, Eckhart L. 2015. Convergent evolution of cysteine-rich proteins in feathers and hair. BMC Evol. Biol. 15, 82 ( 10.1186/s12862-015-0360-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alibardi L. 2015. Immunolocalization of large corneous beta-proteins in the green anole lizard (Anolis carolinensis) suggests that they form filaments that associate to the smaller beta-proteins in the beta-layer of the epidermis. J. Morphol. 276, 1244–1257. ( 10.1002/jmor.20415) [DOI] [PubMed] [Google Scholar]
- 11.Greenwold MJ, Sawyer RH. 2013. Molecular evolution and expression of archosaurian β-keratins: diversification and expansion of archosaurian β-keratins and the origin of feather β-keratins. J. Exp. Zool. B. Mol. Dev. Evol. 320, 393–405. ( 10.1002/jez.b.22514) [DOI] [PubMed] [Google Scholar]
- 12.Greenwold MJ, Bao W, Jarvis ED, Hu H, Li C, Gilbert M, Zhang G, Sawyer RH. 2014. Dynamic evolution of the alpha (α) and beta (β) keratins has accompanied integument diversification and the adaptation of birds into novel lifestyles. BMC Evol. Biol. 14, 249 ( 10.1186/s12862-014-0249-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu P, et al. 2015. Topographical mapping of α- and β-keratins on developing chicken skin integuments: functional interaction and evolutionary perspectives. Proc. Natl Acad. Sci. USA 112, E6770–E6779. ( 10.1073/pnas.1520566112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alibardi L, Toni M, Dalla Valle L. 2007. Hard cornification in reptilian epidermis in comparison to cornification in mammalian epidermis. Exp. Dermatol. 16, 961–976. ( 10.1111/j.1600-0625.2007.00609.x) [DOI] [PubMed] [Google Scholar]
- 15.Vandebergh W, Bossuyt F. 2012. Radiation and functional diversification of alpha keratins during early vertebrate evolution. Mol. Biol. Evol. 29, 995–1004. ( 10.1093/molbev/msr269) [DOI] [PubMed] [Google Scholar]
- 16.Greenwold M, Sawyer R. 2010. Genomic organization and molecular phylogenies of the beta (beta) keratin multigene family in the chicken (Gallus gallus) and zebra finch (Taeniopygia guttata): implications for feather evolution. BMC Evol. Biol. 10, 148 ( 10.1186/1471-2148-10-148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser RDB, Parry DAD. 2008. Molecular packing in the feather keratin filament. J. Struct. Biol. 162, 1–13. ( 10.1016/j.jsb.2008.01.011) [DOI] [PubMed] [Google Scholar]
- 18.Dalla Valle L, Nardi A, Gelmi C, Toni M, Emera D, Alibardi L. 2009. Beta-keratins of the crocodilian epidermis: composition, structure, and phylogenetic relationships. J. Exp. Zool. B Mol. Dev. Evol. 312, 42–57. ( 10.1002/jez.b.21241) [DOI] [PubMed] [Google Scholar]
- 19.Gregg K, Rogers GE. 1986. Feather keratin: composition, structure and biogenesis. In Biology of the integument (eds Bereiter-Hahn J, Maltosy AG, Richards KS), pp. 667–694. Berlin, Germany: Springer. [Google Scholar]
- 20.Fraser RDB, MacRae TP, Rogers GE. 1972. Keratins: their composition, structure, and biosynthesis. Springfield, IL: Thomas. [Google Scholar]
- 21.Marshall RC, Orwin DFG, Gillespie JM. 1991. Structure and biochemistry of mammalian hard keratin. Electron Microsc. Rev. 4, 47–83. ( 10.1016/0892-0354(91)90016-6) [DOI] [PubMed] [Google Scholar]
- 22.Fraser RD, Parry DA. 2011. The structural basis of the filament–matrix texture in the avian/reptilian group of hard beta-keratins. J. Struct. Biol. 173, 391–405. ( 10.1016/j.jsb.2010.09.020) [DOI] [PubMed] [Google Scholar]
- 23.Alibardi L. 2008. Microscopic analysis of lizard claw morphogenesis and hypothesis on its evolution. Acta Zool. 89, 169–178. ( 10.1111/j.1463-6395.2007.00312.x) [DOI] [Google Scholar]
- 24.Alibardi L. 2003. Immunocytochemistry and keratinization in the epidermis of crocodilians. Zool. Stud. 42, 346–356. [Google Scholar]
- 25.Schweitzer MH, Watt JA, Avci R, Forster CA, Krause DW, Knapp L, Rogers RR, Beech I, Marshall M. 1999. Keratin immunoreactivity in the Late Cretaceous bird Rahonavis ostromi. J. Vertebr. Paleontol. 19, 712–722. ( 10.1080/02724634.1999.10011183) [DOI] [Google Scholar]
- 26.Schweitzer MH, Watt JA, Avci R, Knapp L, Chiappe L, Norell M, Marshall M. 1999. Beta-keratin specific immunological reactivity in feather-like structures of the Cretaceous Alvarezsaurid, Shuvuuia deserti. J. Exp. Zool. 285, 146–157. ( 10.1002/(sici)1097-010x(19990815)285:2%3C146::aid-jez7%3E3.0.co;2-a) [DOI] [PubMed] [Google Scholar]
- 27.Moyer AE, Zheng W, Johnson E, Lamanna A, Li MC, Lacovara D-Q, Schweitzer KJ, H M. 2014. Melanosomes or microbes: testing an alternative hypothesis for the origin of microbodies in fossil feathers. Sci. Rep. 4, 4233 ( 10.1038/srep04233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schweitzer MH, Lindgren J, Moyer AE. 2015. Melanosomes and ancient coloration re-examined: a response to Vinther 2015 (DOI 10.1002/bies.201500018). BioEssays 37, 1174–1183. ( 10.1002/bies.201500061) [DOI] [PubMed] [Google Scholar]
- 29.Manning PL, et al. 2013. Synchrotron-based chemical imaging reveals plumage patterns in a 150 million year old early bird. J. Anal. At. Spectrom. 28, 1024–1030. ( 10.1039/C3JA50077B) [DOI] [Google Scholar]
- 30.Manning PL, et al. 2009. Mineralized soft-tissue structure and chemistry in a mummified hadrosaur from the Hell Creek Formation, North Dakota (USA). Proc. R. Soc. B 276, 3429–3437. ( 10.1098/rspb.2009.0812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng CS, et al. 2012. The chicken frizzle feather is due to an alpha-keratin (KRT75) mutation that causes a defective rachis. PLoS Genet. 8, e1002748 ( 10.1371/journal.pgen.1002748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alibardi L. 2013. Immunolocalization of alpha-keratins and feather beta-proteins in feather cells and comparison with the general process of cornification in the skin of mammals. Ann. Anat. 195, 189–198. ( 10.1016/j.aanat.2012.08.005) [DOI] [PubMed] [Google Scholar]
- 33.Moyer AE, Zheng W, Schweitzer MH. 2016. Keratin durability has implications for the fossil record: results from a 10 year feather degradation experiment. PLoS ONE 11, e0157699 ( 10.1371/journal.pone.0157699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witmer LM. 1995. The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In Functional morphology in vertebrate paleontology, pp. 19–33. New York; NY: Cambridge University Press. [Google Scholar]
- 35.El-Gendy SAA, Derbalah A, El-Magd MERA. 2012. Macro-microscopic study on the toepad of ostrich (Struthio camelus). Vet. Res. Commun. 36, 129–138. ( 10.1007/s11259-012-9522-1) [DOI] [PubMed] [Google Scholar]
- 36.Zaghloul D, El-Gendy S. 2014. Anatomical, light and scanning electron microscopical study of ostrich (Struthio camelus) integument. J. Vet. Anat. 7, 33–54. [Google Scholar]
- 37.Lautenschlager S, Witmer LM, Altangerel P, Rayfield EJ. 2013. Edentulism, beaks, and biomechanical innovations in the evolution of theropod dinosaurs. Proc. Natl Acad. Sci. USA 110, 20 657–20 662. ( 10.1073/pnas.1310711110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norell MA, Makovicky PJ, Currie PJ. 2001. Palaeontology. The beaks of ostrich dinosaurs. Nature 412, 873–874. ( 10.1038/35091139) [DOI] [PubMed] [Google Scholar]
- 39.Ostrom JH. 1961. Cranial morphology of the Hadrosaurian dinosaurs of North America. Bull. Am. Mus. Nat. Hist. 122, 33–186. [Google Scholar]
- 40.Sereno PC, Shichin C, Zhengwu C, Chenggang R. 1988. Psittacosaurus meileyingensis (Ornithischia: Ceratopsia), a new psittacosaur from the Lower Cretaceous of northeastern China. J. Vertebr. Paleontol. 8, 366–377. ( 10.1080/02724634.1988.10011725) [DOI] [Google Scholar]
- 41.Horner JR, Goodwin MB. 2008. Ontogeny of cranial epi-ossifications in Triceratops. J. Vertebr. Paleontol. 28, 134–144. ( 10.1671/0272-4634(2008)28%5B134:OOCEIT%5D2.0.CO;2) [DOI] [Google Scholar]
- 42.Alibardi L. 2008. Cornification in developing claws of the common Australian skink (Lampropholis guichenoti) (Squamata, Lacertidae). Ital. J. Zool. 75, 327–336. ( 10.1080/11250000801973334) [DOI] [Google Scholar]
- 43.Carver WE, Sawyer RH. 1988. Avian scale development: XI. Immunoelectron microscopic localization of alpha and beta keratins in the scutate scale. J. Morphol. 195, 31–43. ( 10.1002/jmor.1051950104) [DOI] [PubMed] [Google Scholar]
- 44.Dingus L, Loope DB, Dashzeveg D, Swisher CC, Minjin C, Novacek MJ, Norell M.2008. The geology of Ukhaa Tolgod (Djadokhta Formation, Upper Cretaceous, Nemegt Basin, Mongolia). Am. Mus. Novit. 3616 , 1–40. ( ) [DOI]
- 45.Daniel JC, Chin K. 2010. The role of bacterially mediated precipitation in the permineralization of bone. Palaios 25, 507–516. ( 10.2110/palo.2009.p09-120r) [DOI] [Google Scholar]
- 46.Briggs DEG. 1995. Experimental taphonomy. Palaios 10, 539–550. ( 10.2307/3515093) [DOI] [Google Scholar]
- 47.Pautard FGE. 1963. Mineralization of keratin and its comparison with the enamel matrix. Nature 199, 9531–9535. ( 10.1038/199531a0) [DOI] [PubMed] [Google Scholar]
- 48.Bragulla HH, Homberger DG. 2009. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 214, 516–559. ( 10.1111/j.1469-7580.2009.01066.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schweitzer MH, Zheng W, Cleland TP, Goodwin MB, Boatman E, Theil E, Marcus MA, Fakra SC. 2014. A role for iron and oxygen chemistry in preserving soft tissues, cells and molecules from deep time. Proc. R. Soc. B 281, 20132741 ( 10.1098/rspb.2013.2741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuchs E. 1995. Keratins and the skin. Annu. Rev. Cell Dev. Biol. 11, 123–153. ( 10.1146/annurev.cb.11.110195.001011) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting data can be found in the electronic supplementary material.