Abstract
Sexual selection theory predicts that because male reproductive success in mammals is limited by access to females, males will attempt to defend access to mates and exclude rivals from mating. In mammals, dominance rank is correlated with male reproductive success; however, the highest-ranking (alpha) male rarely monopolizes reproduction completely. To explain why, incomplete control models propose that alpha males simply cannot control other males' access to mates. If true, then dominance rank should be a key factor influencing subordinate (non-alpha) male mating success. Alternatively, the concession model states that alpha males can prevent other males from gaining access to mates but posits that they concede matings to subordinates in exchange for social favours. This predicts that a male's grooming interactions with the alpha should mediate his access to females. We test these predictions using 36 years of data, encompassing the tenures of eight alpha male chimpanzees at Gombe National Park, Tanzania. Incomplete control models were most strongly supported. At a given copulation event, the probability that the alpha was the male that mated was negatively associated with the number of males and sexually receptive females in the party. Additionally, as the number of males increased, high dominance rank was associated with an increased likelihood that a particular non-alpha male mated. The concession model, however, was also supported. The amount of time a male spent grooming with the alpha was positively associated with his likelihood of mating when the alpha was present in the party. As grooming is a major affiliative component of male social relationships, our results suggest that social bonds with dominant individuals are leveraged for mating access, particularly in species in which males form coalitions.
Keywords: reproductive skew, mating success, social bonds, grooming for tolerance, alpha male, Pan troglodytes schweinfurthii
1. Introduction
Sexual selection theory predicts that because male reproductive success in mammals is limited by access to females [1,2], males will attempt to defend access to mates and exclude rivals from mating. This prediction is generally supported across mammals, in which high-ranking individuals achieve the greatest reproductive success (reviewed in [3,4]). However, even in species with high reproductive skew, complete monopolization of females is rare. Reproductive skew theory has produced two general explanations for why dominant individuals do not monopolize reproduction within a group. Incomplete control models hypothesize that dominant individuals have limited control over access to mates and simply cannot prevent others from mating [5]. For example, in primates, many factors limit a dominant male's ability to monopolize mates, including female reproductive synchrony and the number of male competitors [6], as well as alternative reproductive strategies such as male–male coalitions and female mate choice (reviewed in [7]).
By contrast, the concession model [8] assumes that dominant individuals are capable of controlling access to mates but posits that they concede reproductive opportunities to lower-ranking individuals as an incentive for them to stay in the group or in exchange for social favours. Empirical support for the concession model among mammals is limited. In dwarf mongooses (Helogale parvula), subordinate females are hypothesized to receive breeding opportunities to discourage them from dispersing and, as predicted by the concession model, their reproductive success increases with age [9]. However, older individuals may be more capable of resisting efforts to suppress their breeding, so the incomplete control model cannot be ruled out [8]. Similarly, reproductive skew in male African lions (Panthera leo) is consistent with the concession model, in that coalitions of unrelated males have lower reproductive skew than those involving relatives, but female reproductive synchrony and differences in coalition size among unrelated and related males present more parsimonious explanations for this pattern [8,10].
Across primates, incomplete control models have received considerable empirical support in both comparative analyses [11,12] and studies of single populations (reviewed in [6]). By comparison, the concession model remains greatly understudied and has been supported in just a few species (Eulemur rufifrons: [13], Gorilla beringei: [14], Papio ursinus: [15], Theropithecus gelada: [16]). In one compelling example, dominant male geladas that tolerated the presence of subordinates benefited by receiving assistance in defending their reproductive unit from immigrant males, resulting in longer tenures [16]. However, few studies, in primates or mammals more generally, have investigated the concession model in species in which males reside in their natal groups for life. In these societies, there is no selective pressure for alpha males to provide ‘staying incentives’ (reproductive opportunities) to keep subordinates from dispersing; however, concessions may nevertheless be a beneficial strategy if males exchange services and/or form coalitions in mating or dominance contexts [8].
Chimpanzees (Pan troglodytes) live in large multi-male/multi-female groups (‘communities’) that exhibit high fission–fusion dynamics [17] in which members travel in subgroups (‘parties’) that frequently change in size and composition [18]. Adult males remain in their natal community for life [18], and alpha males sire 30–50% of offspring [19–22]. Male–male coalitions are a regular feature of chimpanzee social behaviour (reviewed in [23]) and are associated with increased fitness in non-alpha males [24]. Consistent with incomplete control models, the number of sexually receptive females [19,22] and male competitors [21] are both negatively associated with reproductive skew. Incomplete control is intensified by alternative reproductive strategies, including mating consortships [22,25] and male sexual coercion [26–28]. Female and male mate preferences [29,30], sperm competition [21,22], male-female socio-spatial relationships [31], and grooming-for-mating exchanges between males and females [28] also reduce male reproductive skew.
Only one study to date has directly investigated the existence of mating concessions in chimpanzees. Duffy et al. [32] found that one alpha male (at Kanyawara in Kibale National Park, Uganda), over a 22 month period, was least likely to interfere in the mating attempts of males that most often supported him in cooperative coalitions. This effect remained after controlling for subordinate male rank, which was not associated with mating success. These findings suggest a possible trade-off between an alpha male's incentive to monopolize access to females and his motivation to maintain alpha status [32]. However, despite providing evidence of concessions, this study analysed the behaviour of a single alpha male, preventing generalization across other individuals and populations.
Here, we present 36 years of data from Gombe National Park, Tanzania, to investigate mating skew in male chimpanzees. We characterize factors influencing variation in alpha male mating monopoly, and test the relative contributions of the incomplete control and concession models in explaining variation in subordinate male mating success. Incomplete control models predict that alpha male mating monopoly will be reduced in parties with more male competitors and sexually receptive females. The incomplete control model also predicts that among non-alpha males, mating success should increase with dominance rank. Alternatively, the concession model predicts that a subordinate male's access to females will be mediated by his grooming interactions with the alpha male, either in the context of a larger social bond (including coalitionary support of the alpha) or in exchange for mating tolerance.
2. Material and methods
(a). Study site and data collection
Gombe National Park, Tanzania, is located on the eastern shore of Lake Tanganyika. It consists of 35 km2 of evergreen riverine forest, deciduous woodland, and grassland [33]. The Kasekela chimpanzee community (P. t. schweinfurthii) has been studied continuously since 1960 and became fully habituated by 1966 through banana provisioning at a feeding station, which continued at low levels until 2000 [34]. Since 1973, Tanzanian field assistants have conducted full-day focal observations on members of the Kasekela community [18,34,35]. Observers follow each individual that travels independently of its mother approximately once per month, although the alpha male is often followed with greater frequency [36]. During these focal follows, one observer records party composition and female anogenital swelling size (an external indicator of sexual receptivity, with 0 indicating no swelling and 1 a maximal swelling) on check sheets. A second observer records a continuous narrative of the behaviour of the focal chimpanzee, while also recording conspicuous non-focal activities such as mating and submissive pant-grunt vocalizations.
A relational database of information extracted from the check sheets and narrative notes is maintained at Duke University and Arizona State University. In addition to behavioural data, the database also contains detailed demographic information. Ages were estimated based on size, appearance, and sightings of the mother (for details, see [37]).
(b). Analysis
We used behavioural data from 1976 to 2011, encompassing the tenures of eight alpha male chimpanzees (electronic supplementary material, table S1).
(i). Mating events
We identified successful copulations (intromission) that were observed during focal follows. We restricted our analyses to copulations involving males more than or equal to 12 years old (‘males’ hereafter) because at this age males can sire offspring and thus represent reproductive competition to the alpha [22]. We only analysed copulations with maximally swollen parous females, which receive the most attention from adult males [18,30]. Although typically conspicuous and accompanied by vocalizations, some mating events involving non-focal chimpanzees may have been missed; however, it is unlikely that copulations by particular individuals were systematically overlooked.
(ii). Dominance rank
We extracted all pant-grunt vocalizations (formal signals of submission in chimpanzees [18]) between males that were observed at either the feeding station or during focal follows. These interactions were used to calculate male Elo ratings [38,39], which have several advantages over other dominance metrics, including the ability to determine a subject's rank on any date while also accounting for demographic changes and missing data [40]. The hierarchies produced from Elo ratings have been validated against other widely used methods such as I&SI and the David's score [40]. We set k, which influences the amount that ratings change after an interaction, to 200, although this variable does not influence the average rating of a group [38]. We set the starting score of individuals entering the hierarchy at 1 000, and only included individuals with at least nine interactions [38].
(iii). Grooming
To quantify grooming interactions between the alpha male and each subordinate male, we calculated a dyadic grooming index at roughly annual periods from 1978 through 2011 (mean ± s.d. = 365.2 ± 37.0 days), with the intervals varying based on the start and end of each alpha male's tenure. We included dyads only if they had been present in the same party (and one was focal) for at least 30 h during a given period. On average, during each alpha's tenure, only one dyad did not meet this criterion (range = 0–3).
The grooming index equalled the amount of time that alpha male A spent grooming (giving, receiving, and mutual) with male B, divided by the amount of time the two spent in the same party. Because association time is expected to increase grooming time, this index measures the tendency for two individuals to groom, given that they are already together. Across the study period, the index was calculated based on either scan-samples or narrative notes during focal follows of either A or B (for details, see [41]). For years when both scans and narrative notes were available, the two methods were highly correlated (Pearson's r = 0.79, p < 0.0001, N = 638 dyadic grooming rates). Because the duration of some bouts in the narrative were uncertain, we only included those for which the observers had recorded precise start and end times (73.8%, N = 15 703 bouts).
We also used a second grooming index that equalled the number of minutes that alpha male A spent grooming with male B, divided by the total sum of minutes that A spent grooming with all males. This metric does not adjust for time spent together. The results were unaffected by which index was used; we present results from just the first index.
(iv). Statistical analyses
We conducted all statistical analyses in R v. 3.1.3 [42] using the lme4 (v. 1.7-1, [43]) and multcomp (v. 1.4-1, [44]) packages.
Analysis 1: factors influencing the degree of alpha male mating monopoly. To characterize variation in alpha male mating monopoly, we ran a generalized linear mixed model (GLMM) on copulations between 1976 and 2011 (N = 4 994) when the alpha was in the party. For each mating, the model used a binomial error structure and a logit link function to estimate the probability that the copulating male was the alpha (Y/N). We included five main effects: (i) number of males in the party, (ii) number of maximally swollen parous females in the party, (iii) whether the female was closely related (mother, maternal sibling, or maternal niece, all of which can be identified via behavioural cues) to the alpha (Y/N), (iv) whether the alpha was the focal subject (Y/N), and (v) alpha identity. We predicted that being the focal subject (and therefore more likely to be observed and recorded) would increase the probability that the alpha was the copulating male. By contrast, alpha males should be less likely to mate with closely related females. After controlling for these factors, incomplete control models predict that the number of males and maximally swollen females present should reduce the likelihood that the alpha copulates. We included the identities of the male and female that mated as random effects. To determine which alphas differed in their tendency to monopolize matings, we conducted Tukey contrasts of the ‘alpha identity’ factor.
Analysis 2: factors influencing subordinate male mating success. To further investigate variation in mating success, we examined which non-alpha males successfully copulated when the alpha was present. The incomplete control model predicts that high male dominance rank should be positively associated with increased subordinate mating success. The concession model predicts that the amount of time that a male grooms with the alpha will be positively associated with mating success. We standardized the grooming index and dominance rank variables by applying Z-transformations within each alpha male's tenure, thereby controlling for variation in alpha gregariousness levels and average group Elo ratings.
Between 1978 and 2011 (for which dominance and grooming data were available), we identified all copulations by non-alpha males that occurred in parties that included the alpha male and at least two other males (N = 3 116). We used a random slope GLMM (with binomial error structure and a logit link function) to estimate the probability that a particular non-alpha male copulated (Y/N). Thus, each non-alpha male present for a given copulation was represented by a single row in the dataset. The model included three main effects: (i) the male's rate of grooming with the alpha (dyadic grooming index), (ii) the male's dominance rank (i.e. Elo rating), and (iii) whether the male was closely related (Y/N, as defined above) to the female that copulated. We also included male age because rank and grooming with the alpha may reflect age-specific patterns of dominance and association, respectively. Consistent with a previous study of males in this population [22], we divided age into six bins: 12 to 14 years, 15 to 19 years, 20 to 24 years, 25 to 29 years, 30 to 34 years, and more than or equal to 35 years. Additionally, we included random slopes of ‘alpha identity’ on (i) Elo rating and (ii) grooming rate, because the nature of these effects may vary between alphas [45]. We included male identity, female identity, alpha identity, and mating event as random effects.
Because an alpha might behave differently toward related females, we tested for an interaction between the maternal relatedness of a mating female and the alpha (Y/N) and our two main predictor variables, grooming rate and dominance rank. Lastly, because the number of male competitors in a party may influence (i) whether an alpha male can concede matings, and (ii) the consequences of male rank, we tested for an interaction between the two main predictor variables and the number of males in the party. Only the interaction between the number of males and dominance rank was significant. All other interactions did not improve model fit and were removed.
We tested for muliticollinearity using the corvif function [46]. All variance inflation factors were less than 1.3.
3. Results
(a). Analysis 1: factors influencing the degree of alpha male mating monopoly
There was significant variation in the degree to which alpha males monopolized matings with maximally swollen parous females (table 1), after controlling for (i) the number of males and maximally swollen parous females, and whether the alpha was (ii) the focal, or (iii) a close maternal relative of the female. An alpha male was more likely to be the copulating male if he was the focal subject. As predicted by incomplete control models, the number of males (β = −0.16, standard error (s.e.) = 0.03, p < 0.0001) and maximally swollen parous females in a party (β = −0.14, s.e. = 0.07, p < 0.05) were negatively associated with alpha male mating monopoly, as was the alpha's relatedness to the female (β = −1.88, s.e. = 0.27, p < 0.0001). Using Tukey contrasts, we found that alphas varied in the degree to which they monopolized matings (electronic supplementary material, table S2). GB, the male with the highest mating probability, was significantly more likely to acquire matings than was FR (β = 1.46, s.e. = 0.25, p < 0.001), the alpha male with the second-highest mating probability. FR had significantly higher mating probability than all of the remaining alpha males except WL. There were several other significant differences in mating monopoly among the remaining pairs.
Table 1.
Factors associated with the degree of alpha male mating monopoly. The alpha males in our study were observed to copulate with maximally swollen parous females between 47 and 303 times (mean ± s.d. = 166 ± 95). We conducted likelihood-ratio tests (LRT) to evaluate the significance of fixed effects. In each comparison, a single parameter was dropped and this reduced model was compared to the full model. Akaike Information Criterion (AIC) values in the table are model values, after the terms in the rows were dropped, one at a time. Higher AIC values indicate that dropping the particular term resulted in a model that was a worse fit to the data. The alpha ‘FG’ was the reference category for ‘alpha identity’ (ID). For Tukey contrasts between each pair of alphas, see electronic supplementary material, table S2. Variance components for the two random effects: female identity (n = 37; variance ± s.d. = 0.11 ± 0.33) and male identity (n = 27; variance ± s.d. = 148.5 ± 12.2).
| parameter | estimate | s.e. | Z | d.f. | AIC | LRT | p-values |
|---|---|---|---|---|---|---|---|
| intercept | −12.881 | 2.223 | −5.794 | ||||
| # males | −0.156 | 0.026 | −5.921 | 1 | 3135.92 | 36.58 | <0.0001 |
| # swollen parous females | −0.140 | 0.068 | −2.062 | 1 | 3103.65 | 4.32 | 0.038 |
| relatedness to female | −1.885 | 0.266 | −7.098 | 1 | 3157.97 | 58.64 | <0.0001 |
| alpha is focal? | 1.204 | 0.112 | 10.721 | 1 | 3218.92 | 119.59 | <0.0001 |
| AlphaID | 7 | 3376.85 | 289.52 | <0.0001 | |||
| AlphaID: FE | 0.114 | 0.607 | 0.187 | ||||
| AlphaID: KS | 0.366 | 0.585 | 0.626 | ||||
| AlphaID: SL | 0.520 | 0.598 | 0.869 | ||||
| AlphaID: FD | 1.328 | 0.577 | 2.301 | ||||
| AlphaID: WL | 1.833 | 0.566 | 3.240 | ||||
| AlphaID: FR | 2.179 | 0.577 | 3.778 | ||||
| AlphaID: GB | 3.634 | 0.549 | 6.623 |
(b). Analysis 2: factors influencing subordinate male mating success
The Elo rating and grooming index values of non-alpha males that successfully copulated were, on average, higher than the mean values of all non-alpha males in the party in which a given mating took place (Elo rating: +14%; grooming index: +27%; figure 1).
Figure 1.
Elo rating and grooming index values of the males that copulated, compared to the mean values of all non-alpha males present in the party. The values of non-alpha males that successfully copulated were, on average, higher than the mean values of all non-alpha males present in the party. Note that because the scales of these predictor variables differ substantially, these percentages are not directly comparable.
After controlling for maternal relatedness to the copulating female, grooming rate with the alpha, and male age, we found that a non-alpha male's dominance rank was positively associated with the likelihood that he was the copulating male (table 2). This was driven by a statistically significant interaction between rank and the number of males present (figure 2). Specifically, as the number of males increased, high dominance rank became a stronger predictor of mating success (GLMM: β = 0.08, s.e. = 0.02, p = 0.002). Male relatedness to the female was negatively associated with the probability of mating (β = −0.90, s.e. = 0.14, p < 0.0001). Male age was also a significant predictor of mating success (p < 0.0001), with 12- to 14-year-old males having the lowest success. Finally, after controlling for all of these factors, we found that a male's grooming rate with the alpha was positively and significantly associated with his likelihood of mating (β = 0.07, s.e. = 0.02, p = 0.01; table 2), supporting the concession model.
Table 2.
Factors associated with non-alpha male mating success. There were a total of 27 males in our sample, 24 of whom were observed to copulate. The number of males in the community during each alpha male tenure ranged from 8 to 13 (mean ± s.d. = 11 ± 1.6). When the alpha male was present in the party, these males successfully copulated between 4 and 469 times (mean ± s.d. = 130 ± 126). We conducted likelihood-ratio tests (LRT) to evaluate the significance of fixed effects. In each comparison, a single parameter or interaction was dropped and this reduced model was compared with the full model. The 12- to 14-year-old age bin was the reference category for ‘male age.’ For Tukey contrasts between each pair of age bins, see electronic supplementary material, table S3. Variance components for random effects: female identity (n = 35; mean ± s.d. = 0.00 ± 0.00), male identity (n = 27; mean ± s.d. = 1.94 ± 1.39), alpha identity (n = 8; mean ± s.d. = 0.003 ± 0.053), mating event (n = 3 116; mean ± s.d. = 0.00 ± 0.00). Variance components for random slopes: alpha identity on (a) dominance rank (mean ± s.d. = 0.012 ± 0.112) and (b) grooming rate (mean ± s.d. = 0.00 ± 0.00).
| parameter | estimate | s.e. | Z | d.f. | AIC | LRT | p-values |
|---|---|---|---|---|---|---|---|
| intercept | −2.829 | 0.292 | −9.706 | <0.0001 | |||
| grooming rate with alpha | 0.066 | 0.024 | 2.709 | 1 | 17 054.17 | 6.63 | 0.010 |
| dominance rank | 0.169 | 0.056 | 3.023 | 0.002 | |||
| relatedness to female | −0.900 | 0.140 | −6.428 | 1 | 17 098.36 | 50.82 | <0.0001 |
| # males | −0.339 | 0.023 | −14.800 | <0.0001 | |||
| dominance rank x # males | 0.078 | 0.024 | 3.168 | 1 | 17 057.41 | 9.87 | 0.002 |
| male age | 5 | 17 070.19 | 30.66 | <0.0001 | |||
| 15–19 | 0.335 | 0.099 | 3.397 | ||||
| 20–24 | 0.334 | 0.126 | 2.651 | ||||
| 25–29 | 0.242 | 0.124 | 1.951 | ||||
| 30–34 | 0.543 | 0.133 | 4.085 | ||||
| ≥35 | 0.420 | 0.142 | 2.960 |
Figure 2.
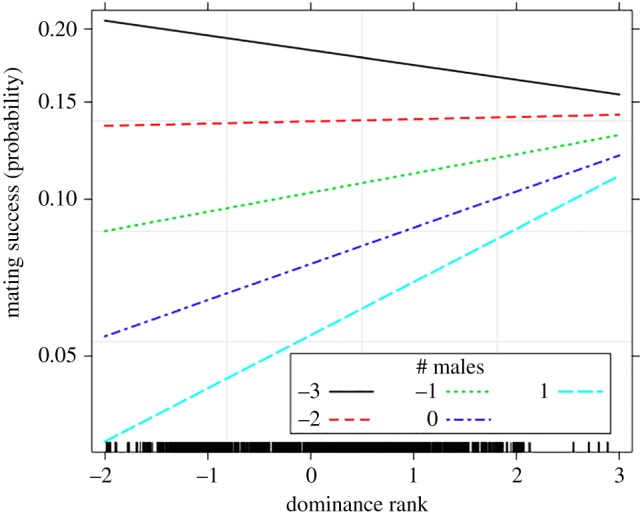
The effect of the interaction between dominance rank (Elo rating, Z-transformed) and the number of males in the party (Z-transformed) on mating success. Values above or below 1 reflect dominance ranks and male party sizes that are above or below average, respectively. As the number of males increased, high rank became a stronger predictor of non-alpha male mating success.
4. Discussion
Incomplete control and concession models present alternative mechanisms to explain reproductive and mating skew among social mammals [5,8]. Here, in a wild chimpanzee community, we found evidence supporting both hypotheses. The number of male competitors and maximally swollen parous females present when a copulation took place decreased the likelihood that it was the alpha male who mated, replicating previous studies of wild chimpanzees that supported incomplete control models [19,21,22]. Furthermore, when the alpha male was present, subordinate males had a higher likelihood of mating if they were high ranking, provided that the party was of average to large size (in smaller parties, rank was less important). This indicates that an alpha male's ability to monopolize access to females was compromised by his rivals' competitive ability. Age also predicted subordinate male mating success, a result that was driven mostly by males that were at least 15 years old being more successful than males that were 12 to 14 years old (i.e. adults and adolescents, approximately [47]). That the age effect held after accounting for rank, which has an inverted-U relationship with age [48], suggests that adult males are more successful than adolescents at using alternative mating strategies (e.g. coercion [26–28]). Compared to previous paternity data from this community [22], in which males aged 15 to 19 sired the most offspring, we found that males aged 30–34 had the highest mating success.
Our results also demonstrate that males who groomed more with the alpha male had increased mating success (when the alpha was also present). This suggests that alpha males are more tolerant of copulations by males with whom they groom frequently, supporting the existence of mating concessions in this population. Broadly, this finding supports the prediction that concessions are likely to be found in multi-male/multi-female societies in which males form coalitions [8]. In this respect, frequent grooming between the alpha and subordinate males may reflect attempts by the alpha to maintain the support of coalition partners [32,49]. Indeed, social support is important to the acquisition and maintenance of dominance rank in chimpanzees [24,50], in which males exhibit strong and durable social bonds (of which grooming is a critical component) and regularly form coalitions [18,23,51]. As a consequence, alpha male chimpanzees may face a trade-off between mating monopoly and tenure length [32]. Specifically, alpha males may trade or concede mating opportunities (and paternity) in exchange for coalitionary support, and consequently achieve longer tenures and greater lifetime reproductive success. The effectiveness of this strategy is likely to vary, however, in relation to the degree of control that an alpha has over mating access. Relatively large-bodied alpha males, in prime condition, should be less likely to exhibit mating concessions than smaller or past-prime alphas that depend on alliances to maintain their position in the dominance hierarchy. This would be consistent with previous research linking alpha male behavioural styles to body size [36].
Together with the prior study in the Kanyawara chimpanzee community [32], our results suggest that alpha male chimpanzees concede matings as a long-term reproductive strategy. This finding was consistent across populations, despite the use of a different metric (grooming rather than coalitions). Although coalitions are more directly linked to rank maintenance in chimpanzees [50], grooming plays a key role in male social relationships and male–male competition [23,50,51]. Moreover, rates of grooming and coalitions are positively correlated [52,53].
A stronger test of the concession model in chimpanzees would be to observe whether the effect of grooming on copulation probability only exists when the alpha is present; however, the alpha male is almost always present in parties containing maximally swollen parous females, therefore the sample size of copulations in his absence is prohibitively small. To test whether concessions involve a short-term exchange of grooming for tolerance [51], future studies should explore the impact of grooming directionality on non-alpha mating success and record the social context in which matings take place (e.g. [49]). These data would clarify whether there is a temporal association between grooming given and mating success. Finally, future work should also consider that the probability of conception in chimpanzees is highly variable within and between cycles [54]; the frequency of mating concessions may therefore vary in relation to female physiological status (e.g. periovulatory periods or conceptive cycles).
Despite the evidence of mating concessions in two chimpanzee communities, the effect of concessions on tenure length is likely to be small. This is because incomplete control models remain a more dominant explanation for the broad patterns of male reproductive skew in chimpanzees. Specifically, these models are consistently supported in several communities in which the number of sexually swollen females and male competitors reduce male mating skew (this study) and reproductive skew [19,21,22]. Several other factors (see Introduction) also suggest that alpha male chimpanzees typically have limited control over access to mates, and some degree of control is a prerequisite for being able to concede mating opportunities. This general lack of control therefore limits the long-term impact of concessions on tenure length.
Nevertheless, ceding matings to subordinates appears to be a strategy available to alpha males, and our results suggest that concessions should be included among the many factors that influence male mating skew in chimpanzees. To understand variability in the impact of concessions on reproductive skew and tenure length among social mammals more broadly, we recommend that future studies focus on species in which males form cooperative coalitions.
Supplementary Material
Acknowledgements
We thank the Tanzanian Wildlife Research Institute, Tanzania National Parks, and the Tanzania Commission for Science and Technology for research permissions. We thank Jane Goodall and the Gombe Stream Research Centre staff for their efforts over six decades to collect these data. We thank numerous research assistants at Duke University and the University of Minnesota, including Joann Schumacher-Stankey, for data entry. We thank Kevin Langergraber, Joan Silk, Steffen Foerster, Joseph Feldblum, and two anonymous reviewers for constructive feedback on previous versions of the manuscript.
Ethics
This research adhered to all the laws and guidelines of Tanzania, Arizona State University, and Duke University.
Data accessibility
Bray J, Pusey A, Gilby I (2016) Data from: Incomplete control and concessions explain mating skew in male chimpanzees. Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.r4d09 [55].
Authors' contributions
J.B. and I.C.G. designed the study; J.B. analysed the data and drafted the manuscript; A.E.P. and I.C.G. revised the manuscript; all authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by the Jane Goodall Institute, the National Science Foundation (9021946, 9319909, 0452315, 1052693, 0431141), the National Institutes of Health (R01-AI058715, R00-HD057992), Harris Steel, the Windibrow Foundation, the University of Minnesota and Duke University. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under grant no. 026257-001.
References
- 1.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man 1871–1971, pp. 136–179. Chicago, IL: Aldine Publishing Company. [Google Scholar]
- 2.Clutton-Brock TH, Parker GA. 1992. Potential reproductive rates and the operation of sexual selection. Q. Rev. Biol. 67, 437–456. ( 10.1086/417793) [DOI] [Google Scholar]
- 3.Ellis L. 1995. Dominance and reproductive success among nonhuman animals—a cross-species comparison. Ethol. Sociobiol. 16, 257–333. ( 10.1016/0162-3095(95)00050-U) [DOI] [Google Scholar]
- 4.Alberts SC. 2012. Magnitude and sources of variation in male reproductive performance. In The evolution of primate societies, pp. 412–431. Chicago, IL: University of Chicago Press. [Google Scholar]
- 5.Reeve HK, Emlen ST, Keller L. 1998. Reproductive sharing in animal societies: reproductive incentives or incomplete control by dominant breeders? Behav. Ecol. 9, 267–278. ( 10.1093/beheco/9.3.267) [DOI] [Google Scholar]
- 6.Port M, Kappeler PM. 2010. The utility of reproductive skew models in the study of male primates, a critical evaluation. Evol. Anthropol. 19, 46–56. ( 10.1002/evan.20243) [DOI] [Google Scholar]
- 7.Setchell JM. 2008. Alternative reproductive tactics in primates. In Alternative reproductive tactics, pp. 373–393. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Clutton-Brock TH. 1998. Reproductive skew, concessions and limited control. Trends Ecol. Evol. 13, 288–292. ( 10.1016/S0169-5347(98)01402-5) [DOI] [PubMed] [Google Scholar]
- 9.Creel SR, Waser PM. 1991. Failures of reproductive suppression in dwarf mongooses (Helogale parvula): accident or adaptation? Behav. Ecol. 2, 7–15. ( 10.1093/beheco/2.1.7) [DOI] [Google Scholar]
- 10.Packer C, Gilbert DA, Pusey AE, O'Brien SJ. 1991. A molecular genetic analysis of kinship and cooperation in African lions. Nature 351, 562–565. ( 10.1038/351562a0) [DOI] [Google Scholar]
- 11.Kutsukake N, Nunn CL. 2006. Comparative tests of reproductive skew in male primates: the roles of demographic factors and incomplete control. Behav. Ecol. Sociobiol. 60, 695–706. ( 10.1007/s00265-006-0213-1) [DOI] [Google Scholar]
- 12.Ostner J, Nunn CL, Schülke O. 2008. Female reproductive synchrony predicts skewed paternity across primates. Behav. Ecol. 19, 1150–1158. ( 10.1093/beheco/arn093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Port M, Johnstone RA, Kappeler PM. 2010. Costs and benefits of multi-male associations in redfronted lemurs (Eulemur fulvus rufus). Biol. Lett. 6, 620–622. ( 10.1098/rsbl.2010.0091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoinski TS, Rosenbaum S, Ngaboyamahina T, Vecellio V, Ndagijimana F, Fawcett K. 2009. Patterns of male reproductive behaviour in multi-male groups of mountain gorillas: examining theories of reproductive skew. Behaviour 146, 1193–1215. ( 10.1163/156853909X419992) [DOI] [Google Scholar]
- 15.Henzi SP, Clarke PMR, van Schaik CP, Pradhan GR, Barrett L. 2010. Infanticide and reproductive restraint in a polygynous social mammal. Proc. Natl Acad. Sci. USA 107, 2130–2135. ( 10.1073/pnas.0913294107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder-Mackler N, Alberts SC, Bergman TJ. 2012. Concessions of an alpha male? Cooperative defence and shared reproduction in multi-male primate groups. Proc. R. Soc. B 279, 3788–3795. ( 10.1098/rspb.2012.0842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aureli F, et al. 2008. Fission-fusion dynamics: new research frameworks. Curr. Anthropol. 49, 627–654. ( 10.1086/586708) [DOI] [Google Scholar]
- 18.Goodall J. 1986. The chimpanzees of Gombe: patterns of behavior. Cambridge, MA: Belknap Press. [Google Scholar]
- 19.Boesch C, Kohou G, Néné H, Vigilant LL. 2006. Male competition and paternity in wild chimpanzees of the Taï forest. Am. J. Phys. Anthropol. 130, 103–115. ( 10.1002/ajpa.20341) [DOI] [PubMed] [Google Scholar]
- 20.Inoue E, Inoue-Murayama MM, Vigilant LL, Takenaka O, Nishida T. 2008. Relatedness in wild chimpanzees: influence of paternity, male philopatry, and demographic factors. Am. J. Phys. Anthropol. 137, 256–262. ( 10.1002/ajpa.20865) [DOI] [PubMed] [Google Scholar]
- 21.Newton-Fisher NE, Emery Thompson M, Reynolds V, Boesch C, Vigilant LL. 2009. Paternity and social rank in wild chimpanzees (Pan troglodytes) from the Budongo Forest, Uganda. Am. J. Phys. Anthropol. 142, 417–428. ( 10.1002/ajpa.21241) [DOI] [PubMed] [Google Scholar]
- 22.Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. 2009. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim. Behav. 77, 873–885. ( 10.1016/j.anbehav.2008.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitani JC. 2009. Cooperation and competition in chimpanzees: current understanding and future challenges. Evol. Anthropol. 18, 215–227. ( 10.1002/evan.20229) [DOI] [Google Scholar]
- 24.Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, Pusey AE. 2013. Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol. 67, 373–381. ( 10.1007/s00265-012-1457-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tutin CEG. 1979. Mating patterns and reproductive strategies in a community of wild chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol. 6, 29–38. ( 10.1007/BF00293242) [DOI] [Google Scholar]
- 26.Muller MN, Kahlenberg SM, Emery Thompson M, Wrangham RW. 2007. Male coercion and the costs of promiscuous mating for female chimpanzees. Proc. R. Soc. B 274, 1009–1014. ( 10.1098/rspb.2006.0206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldblum JT, Wroblewski EE, Rudicell RS, Hahn BH, Paiva T, Cetinkaya-Rundel M, Pusey AE, Gilby IC. 2014. Sexually coercive male chimpanzees sire more offspring. Curr. Biol. 24, 2855–2860. ( 10.1016/j.cub.2014.10.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaburu SSK, Newton-Fisher NE. 2015. Trading or coercion? Variation in male mating strategies between two communities of East African chimpanzees. Behav. Ecol. Sociobiol. 69, 1039–1052. ( 10.1007/s00265-015-1917-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stumpf RM, Boesch C. 2006. The efficacy of female choice in chimpanzees of the Taï Forest, Côte d'Ivoire. Behav. Ecol. Sociobiol. 60, 749–765. ( 10.1007/s00265-006-0219-8) [DOI] [Google Scholar]
- 30.Muller MN, Emery Thompson M, Wrangham RW. 2006. Male chimpanzees prefer mating with old females. Curr. Biol. 16, 2234–2238. ( 10.1016/j.cub.2006.09.042) [DOI] [PubMed] [Google Scholar]
- 31.Langergraber KE, Mitani JC, Watts DP, Vigilant L. 2013. Male–female socio-spatial relationships and reproduction in wild chimpanzees. Behav. Ecol. Sociobiol. 67, 861–873. ( 10.1007/s00265-013-1509-6) [DOI] [Google Scholar]
- 32.Duffy KG, Wrangham RW, Silk JB. 2007. Male chimpanzees exchange political support for mating opportunities. Curr. Biol. 17, R586–R587. ( 10.1016/j.cub.2007.06.001) [DOI] [PubMed] [Google Scholar]
- 33.Clutton-Brock TH, Gillett JB. 1979. A survey of forest composition in the Gombe National Park, Tanzania. Afr. J. Ecol. 17, 131–158. ( 10.1111/j.1365-2028.1979.tb00250.x) [DOI] [Google Scholar]
- 34.Wilson ML. 2012. Long-term studies of the chimpanzees of Gombe National Park, Tanzania. In Long-term field studies of primates, pp. 357–384. Berlin, Germany: Springer. [Google Scholar]
- 35.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49, 227–267. ( 10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 36.Foster MW, Gilby IC, Murray CM, Johnson A, Wroblewski EE, Pusey AE. 2009. Alpha male chimpanzee grooming patterns: implications for dominance ‘style’. Am. J. Primatol. 71, 136–144. ( 10.1002/ajp.20632) [DOI] [PubMed] [Google Scholar]
- 37.Gilby IC, Machanda ZP, Mjungu DC, Rosen J, Muller MN, Pusey AE, Wrangham RW. 2015. ‘Impact hunters’ catalyse cooperative hunting in two wild chimpanzee communities. Phil. Trans. R. Soc. B 370, 20150005 ( 10.1098/rstb.2015.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albers PCH, de Vries H. 2001. Elo-rating as a tool in the sequential estimation of dominance strengths. Anim. Behav. 61, 489–495. ( 10.1006/anbe.2000.1571) [DOI] [Google Scholar]
- 39.Kaburu SSK, Inoue S, Newton-Fisher NE. 2013. Death of the alpha: within-community lethal violence among chimpanzees of the Mahale Mountains National Park. Am. J. Primatol. 75, 789–797. ( 10.1002/ajp.22135) [DOI] [PubMed] [Google Scholar]
- 40.Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, Widdig A, Engelhardt A. 2011. Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Anim. Behav. 82, 911–921. ( 10.1016/j.anbehav.2011.07.016) [DOI] [Google Scholar]
- 41.Foerster S, McLellan K, Schroepfer-Walker K, Murray CM, Krupenye C, Gilby IC, Pusey AE. 2015. Social bonds in the dispersing sex: partner preferences among adult female chimpanzees. Anim. Behav. 105, 139–152. ( 10.1016/j.anbehav.2015.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.R Core Team. 2015. R: A language and environment for statistical computing. Vienna, Austria. [Google Scholar]
- 43.Bates D, Maecher M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 44.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom. J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 45.Schielzeth H, Forstmeier W. 2009. Conclusions beyond support: overconfident estimates in mixed models. Behav. Ecol. 20, 416–420. ( 10.1093/beheco/arn145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuur AF, Ieno EN, Elphick CS. 2010. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 1, 3–14. ( 10.1111/j.2041-210X.2009.00001.x/full) [DOI] [Google Scholar]
- 47.Sandel AA, Reddy RB, Mitani JC. 2016. Adolescent male chimpanzees do not form a dominance hierarchy with their peers. Primates. ( 10.1007/s10329-016-0553-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foerster S, Franz M, Murray CM, Gilby IC, Feldblum JT, Walker KK, Pusey AE. 2016. Chimpanzee females queue but males strive for social status. Sci. Rep. 6, 35404 ( 10.1038/srep35404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watts DP. 1998. Coalitionary mate guarding by male chimpanzees at Ngogo, Kibale National Park, Uganda. Behav. Ecol. Sociobiol. 44, 43–55. ( 10.1007/s002650050513) [DOI] [Google Scholar]
- 50.Muller MN, Mitani JC. 2006. Conflict and cooperation in wild chimpanzees. Adv. Study Behav. 35, 275–331. ( 10.1016/S0065-3454(05)35007-8) [DOI] [Google Scholar]
- 51.Kaburu SSK, Newton-Fisher NE. 2015. Egalitarian despots: hierarchy steepness, reciprocity and the grooming-trade model in wild chimpanzees, Pan troglodytes. Anim. Behav. 99, 61–71. ( 10.1016/j.anbehav.2014.10.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watts DP. 2002. Reciprocity and interchange in the social relationships of wild male chimpanzees. Behaviour 139, 343–370. ( 10.1163/156853902760102708) [DOI] [Google Scholar]
- 53.Koyama NF, Caws C, Aureli F. 2006. Interchange of grooming and agonistic support in chimpanzees. Int. J. Primatol. 27, 1293–1309. ( 10.1007/s10764-006-9074-8) [DOI] [Google Scholar]
- 54.Emery Thompson M. 2005. Reproductive endocrinology of wild female chimpanzees (Pan troglodytes schweinfurthii): methodological considerations and the role of hormones in sex and conception. Am. J. Primatol. 67, 137–158. ( 10.1002/ajp.20174) [DOI] [PubMed] [Google Scholar]
- 55.Bray J, Pusey AE, Gilby IC. 2016 doi: 10.5061/dryad.r4d09. Data from: Incomplete control and concessions explain mating skew in male chimpanzees. Dryad Digital Repository. ( ) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Bray J, Pusey A, Gilby I (2016) Data from: Incomplete control and concessions explain mating skew in male chimpanzees. Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.r4d09 [55].



