Abstract
Toads are chemically defended by bufadienolides, a class of cardiotonic steroids that exert toxic effects by binding to and disabling the Na+/K+-ATPases of cell membranes. Some predators, including a number of snakes, have evolved resistance to the toxic effects of bufadienolides and prey regularly on toads. Resistance in snakes to the acute effects of these toxins is conferred by at least two amino acid substitutions in the cardiotonic steroid binding pocket of the Na+/K+-ATPase. We surveyed 100 species of snakes from a broad phylogenetic range for the presence or absence of resistance-conferring mutations. We found that such mutations occur in a much wider range of taxa than previously believed. Although all sequenced species known to consume toads exhibited the resistance mutations, many of the species possessing the mutations do not feed on toads, much less specialize on that food source. This suggests that either there is little performance cost associated with these mutations or they provide an unknown benefit. Furthermore, the distribution of the mutation among major clades of advanced snakes suggests that the origin of the mutation reflects evolutionary retention more than dietary constraint.
Keywords: Na+/K+-ATPase, toxin resistance, bufadienolide, cardiotonic steroids, chemical ecology
1. Introduction
Many plants and animals are defended by toxic compounds, and circumvention of those defences has often involved the evolution of elaborate mechanisms for resistance to the toxins [1–5]. Among animals, some defensive compounds are synthesized from non-toxic precursors, whereas others are acquired and stored (sequestered) from environmental sources, primarily diet, and redeployed defensively [3,6–8]. Toads (Bufonidae) synthesize potent cardiotonic steroids known as bufadienolides (BDs) from cholesterol and store those toxins in high concentrations in their cutaneous glands [6]. Those toxins protect toads from the majority of predators, including many snakes that readily consume other species of frogs. Nonetheless, specialized bufophagy (the consumption of toads as a major (more than 80%) dietary component) or the sequestration of dietary BDs for defence occur in at least three genera of snakes on different continents (electronic supplementary material, table S1) [9–11]. Such systems of chemical defence and resistance between prey and predators exemplify the coevolutionary arms races that can drive molecular and physiological changes in the participating organisms [12].
Studies involving independently evolved systems of chemical defence and their circumvention by predators have shown that resistance to toxic dietary items can be accomplished by similar molecular changes that confer target-site insensitivity [11–15]. Furthermore, there is evidence that tight coupling between the resistance of predators and toxicity of prey in a coevolutionary arms race can result when the predator incurs a performance cost from resistance [12]. When a toxin targets a critical cellular function, phenotypes resistant to that toxin sometimes evolve through parallel or convergent mutations of the same gene across multiple species or populations [11–13,16–18].
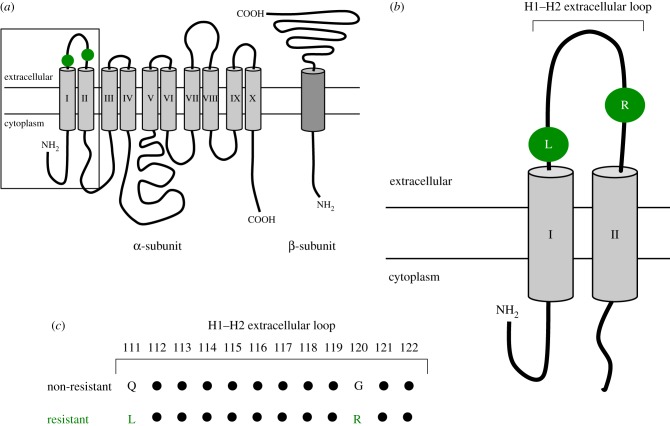
BDs and other cardiotonic steroids, including the plant-derived cardenolides (CDs) [19,20], target the ubiquitous cell-membrane enzyme Na+/K+-ATPase, inhibiting the transport of sodium ions out of the cell and potassium ions in. The Na+/K+-ATPase molecule consists of a catalytic α subunit, with 10 transmembrane segments, which carries out the functional activities of the enzyme, and a glycosylated β subunit, with one transmembrane segment, which provides structural stability (figure 1a) [22–25]. The H1–H2 extracellular loop of the α subunit comprises a significant portion of the binding pocket for cardiotonic steroids (figure 1b). However, some sites on the H3–H4 and the H5–H6 extracellular loops also contribute to the binding pocket to a lesser extent [13], and recent evidence suggests that mutations near the C-terminal end also confer resistance [26].
Figure 1.
(a) Schematic diagram of an unfolded Na+/K+-ATPase molecule, showing the location of the Q111 L and G120R substitutions on the H1–H2 extracellular loop. The box shows the area enlarged in b. (b) Enlargement of the H1–H2 extracellular loop and the first two transmembrane domains. (c) The amino acid sequence of the first extracellular loop, indicating the substitutions at positions 111 and 120 that confer resistance to bufadienolides in snakes. Redrawn from Köksoy [21]. For a more detailed structural reconstruction of the interactions of the Na+/K+-ATPase and a BD, see Laursen et al. [22]. (Online version in colour.)
Several studies have found that two amino acid substitutions, one at position 111 and the other at or near position 122 of the H1–H2 loop, confer lowered affinity for cardiotonic steroids in resistant insects, lizards, and snakes [11,13–15,27–30]. Dobler et al. [13] identified various amino acid replacements encoded by the gene for Na+/K+-ATPase, including replacements at two positions in the H1–H2 extracellular loop of several independent lineages of CD-resistant insects. Ujvari et al. [15] identified two similar amino acid substitutions in the H1–H2 extracellular loop of BD-resistant species of varanid lizards: a leucine (L) replaces a glutamine (Q) at position 111 (Q111 L) and an arginine (R) replaces a glycine (G) at position 120 (G120R). Together, the Q111 L and G120R substitutions were shown to confer significantly reduced affinity of Na+/K+-ATPase for BDs when transfected into human kidney cells [15]. These two substitutions were subsequently found to occur in several species of snakes that are resistant to BDs [11]. Mohammadi et al. [29] investigated the extent of resistance conferred at the whole-body level in a species of snake known to possess the Q111 L and G120R mutations but feeds only rarely on toads. Those snakes were able to withstand concentrations of intraperitoneally injected BDs 200-fold higher than the mouse LD50.
Site-directed mutagenesis of the gene that encodes for Na+/K+-ATPase in Drosophila melanogaster has revealed that the Q111 L substitution alone, in CD-resistant insects, contributes slightly to resistance, whereas the mutation at position 122 (N122H, with histidine replacing asparagine) contributes significantly [13]. The N122H substitution results in a substantial biochemical change, the replacement of a neutral amino acid by a positive one [13], so it is no surprise that this mutation significantly alters the sensitivity of insect Na+/K+-ATPases to CDs. Furthermore, because the G120R mutation in BD-resistant reptiles results in the same biochemical change two amino acid positions away from the N122H mutation of insects [11,15,29], it is safe to assume that the G120R mutation is likely to be the major contributor to lowered BD affinity in mutant reptile Na+/K+-ATPases [15].
Adaptation to similar dietary toxins through the evolution of virtually identical amino acid substitutions across a range of taxa, spanning invertebrates and vertebrates, represents a striking example of convergent evolution. Previous studies have typically concluded that the resistance-conferring mutations co-occur with dietary specialization [11–14], suggesting that resistance to dietary toxins occurs only under strong selective pressure.
A recent survey of genetic resistance to cardiotonic steroids across the Metazoa [11] noted a strong correspondence in snakes between Q111 L and G120R mutations and predation on toads. Ujvari et al. [11] recovered three origins of resistance in their sample of 27 snakes and associated each origin with the assumption of toad-eating habits. Here, we expand the sample to 100 species of snakes, finding many more species that possess the resistance-conferring Q111 L and G120R substitutions. We reconstruct the ancestral states for these taxa and find, in contrast to the more limited taxon sampling of Ujvari et al. [11], that the mutations for resistance occur more widely among snakes than does either specialized or opportunistic bufophagy. Indeed, the mutations occur in some species with diets restricted to prey that lack cardiotonic steroids. This lack of close correspondence between the mutation and toad consumption suggests that, in contrast to similar studies of predators on amphibians defended by tetrodotoxin (which blocks sodium channels) [12], resistance to BDs does not impose a significant performance cost or there is some other, unknown benefit. Furthermore, our results suggest that target-site insensitivity is necessary, but may not be sufficient, to permit dietary specialization on toads or the sequestration of dietary BDs.
2. Material and methods
(a). Selection and classification of study taxa
Because our goal was to test whether a mutated Na+/K+-ATPase alone could explain the evolution of specialized bufophagous habits, we began by sequencing part of the ATP1a3 gene in previously unsequenced toad-eating species. Our criteria for toad-eating habits are restrictive and do not include diet data from general and secondary sources, which classify some species based upon anecdotal dietary records or assumptions of diet based on the predator's habitat. We based our dietary classification (electronic supplementary material, table S1) almost exclusively on dietary studies and other published reports in the primary literature. However, we also scored as (potentially) bufophagous those species that possess the resistance mutations and have a broad diet of amphibians, even in the absence of specific reports of toads in their diet. This conservative assumption maximizes the perceived co-occurrence of the mutations and bufophagy.
We also broadened our genetic sampling to include non-toad-eating sister taxa of the specialized and facultative toad-eaters, to determine whether the distribution of mutations for BD resistance closely correspond to toad-eating habits. Combined with other sequence data available in GenBank, we included data for 100 species of snakes (see electronic supplementary material). Although not shown in the figures, our phylogenetic analysis included 21 lizards (18 of them in the genus Varanus, plus one dactyloid, one agamid, and one scincid), as well as Sphenodon, Alligator, Gallus, and Xenopus, as outgroups.
(b). Sequence data
Ethanol-preserved tissue samples were obtained from the Louisiana State University Museum of Zoology. Other tissue samples were acquired from snakes collected in the field in Louisiana (permit no. LNHP-12-064), Utah (permit no. 1COLL9134), and Nebraska (permit no. 477) or from previously frozen and ethanol-preserved specimens in our laboratories at Utah State University and Kyoto University. DNA was isolated from tissues, using either the DNeasy Blood and Tissue Kit (Qiagen™) or the Phire direct polymerase chain reaction (PCR) kit (Thermo Fisher™). The H1–H2 extracellular loop of the ATP1a3 gene was amplified, using the primers (Integrated DNA Technologies®) ATP1a3Fwd (CGAGATGGCCCCAATGCTCTCA) and ATP1a3Rvs (TGGTAGTAGGAGAAGCAGCCGGT), which were modified from Moore et al. [31]. Amplified DNA product was purified, using the Wizard SV Gel and PCR clean-up system (Promega™). DNA was sequenced on an ABI 3730xo Genetic Analyzer, using BigDye Terminator kit v. 3.1. Sequences were aligned and analysed, using Geneious v. 6.1.8 (Biomatters Ltd.). Sequences were translated to amino acids, and the hydrophobicity and isoelectric points of each residue were calculated, using Geneious v. 6.1.8 (Biomatters Ltd.). Some sequences were obtained from GenBank (accession numbers KP238131-KP238176, XM_007437572, and AZIM01015831). DNA sequence data generated in this study have been submitted to GenBank with the accession numbers KU738063-KU738139, KU933521, and KU950321.
(c). Ancestral state reconstruction
Stochastic character mapping [32] was used to obtain probabilistic reconstructions of ancestral states for non-synonymous substitutions at amino acid positions 111 (second codon position, A versus T) and 120 (first codon position, A versus G or C; see Results). Character mapping was based on the time-calibrated squamate reptile phylogeny from Zheng & Weins [33], which we pruned to retain only the 121 species for which we had ATP1a3 sequences. Sequences were available for seven species that were not included in Zheng and Wiens' phylogeny, although congeners were included in that tree. Therefore, we considered the sequenced species to be equivalent to their congeners for the purposes of the analysis, placing them at the same point on the phylogeny. The included species were Conophis vittatus (replacing C. lineatus), Rhabdophis leonardi (replacing R. nuchalis), Cyclophiops semicarinatus (replacing C. major), Dendrelaphis subocularis (replacing D. tristis), D. punctulatus (replacing D. bifrenalis), Causus maculatus (replacing C. rhombeatus), and Vipera aspis (replacing V. ammodytes).
We first obtained the joint posterior distribution of ancestral character states and the transition matrix for each nucleotide position. We allowed all transition rates to differ and assumed a flat prior on the root states. Posteriors were inferred using Markov chain Monte Carlo (MCMC) with a 10 000 iteration burn-in, 100 000 sampling iterations, and a thinning interval of 50 iterations. Two chains were run to evaluate convergence of the MCMC algorithm to the stationary distribution, and results were combined for inference. Stochastic character maps were simulated over the posterior distribution of the transition matrix and thus account for uncertainty in transition rates. This analysis was conducted using the make.simmap function in the R package phytools [34].
In contrast to CD-resistant insects, in which position 122 of Na+/K+-ATPase has a positive amino acid, we always observed a shift from a neutral to positive amino acid at position 120 in BD-resistant snakes. Therefore, we asked whether such a pattern could be explained by chance under the null model that both changes would have an equivalent effect and are equally likely. Our calculations were made over the posterior distribution of the character state reconstructions, thus accounting for uncertainty in the number of changes.
We next compared models of correlated versus independent evolution for the two amino acids. We fitted continuous-time Markov models for trait evolution, either with separate transition matrixes for each amino acid (independent evolution model) or with a single transition matrix for the pair of amino acids (dependent or correlated, evolution model) [34]. We obtained maximum-likelihood parameter estimates, using BayesTraitsV2 (25 starts were used for parameter estimation). Models were then compared, using a likelihood ratio test as described by Pagel [35].
3. Results
(a). Amino acid substitutions
Our analysis of Na+/K+-ATPase α3 subunit sequences for the H1–H2 extracellular loop revealed substitutions at several residues. In our survey of 100 species of snakes, we found two consistent amino acid substitutions in all taxa known to consume toads. At position 111, we found the Q111 L substitution and at position 120, the G120R substitution, a result that is consistent with previously published findings (figure 1c) [11]. These two substitutions always occurred together and usually involved the same nucleotide changes. Notably, in addition to the occurrence of the Q111 L and G120R substitutions in all bufophagous species included in our analysis, we found many instances of these same two substitutions (hereinafter, ‘resistance mutations’) across the phylogeny of snakes, including many species that rarely or never feed on toads. In addition to the Q111 L and G120R substitutions, several other substitutions occurred at various positions among different groups (electronic supplementary material, figure S1). However, these changes are not known to alter the biochemical properties of the binding pocket to any significant extent, and thus are believed to be of no major functional consequence [11].
(b). Ancestral state reconstruction
Stochastic character mapping supported multiple gains and losses of the resistance mutations across the tree (figure 2). We found evidence of 11 (95% credible intervals [CIs] 2–18) transitions from A to T (with eight reversals, 95% CIs 2–19) at position 2 of amino acid 111 and 11 (95% CIs 1–33) transitions from A to G (with 10 reversals, 95% CIs 1–32) at position 1 of amino acid 120. Given the total number of transitions and the paucity of taxa with only one of the resistance mutations, we can confidently reject the null model that changes at amino acid 111 and 120 are equivalent (binomial probability: median across MCMC replicates, p = 0.0005; mean across MCMC replicates = 0.0511). Moreover, a model of correlated or dependent evolution for amino acid positions 111 and 120 was preferred relative to a null model of independent evolution (likelihood ratio = 88.85, χ2 with 4 d.f., p < 0.001).
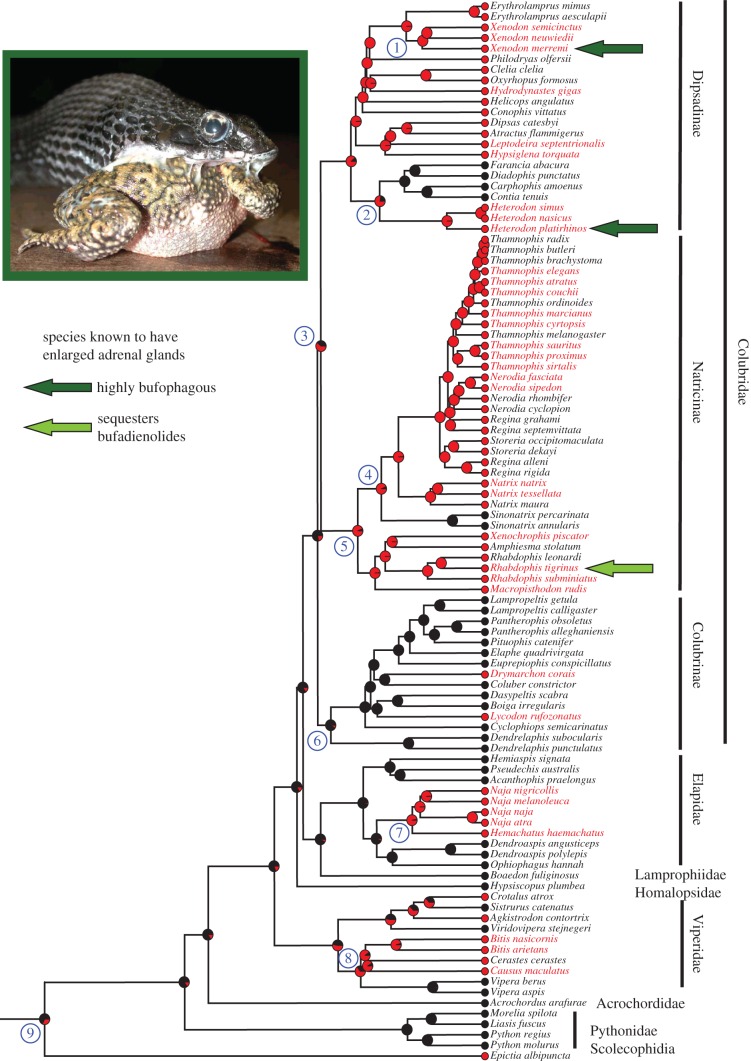
Figure 2.
Ancestral state reconstruction of the mutations to Na+/K+-ATPase that confer resistance to bufadienolides in snakes. Coloured circles beside terminal taxa indicate the amino acid composition of Na+/K+-ATPase at the H1–H2 extracellular loop (red = resistant L111 and R120, and black = non-resistant Q111 and G120). The names of bufophagous species are shown in red, and non-bufophagous species are shown in black (electronic supplementary material table S1) [10,35,36]. Inferred ancestral states are denoted by pie charts that indicate the posterior probability of having (red) versus not having (black) the resistance mutations. Selected higher taxa are identified, and circled numbers identify nodes that are discussed in the text. Note that the resistance mutations to Na+/K+-ATPase are not limited to taxa that prey on toads (as shown by the number of black species names beside red circles). Two highly bufophagous species (Heterodon platirhinos and Xenodon merremii) and one that sequesters BDs (Rhabdophis tigrinus), indicated by green arrows, possess enlarged adrenal glands that may function in countering the effects of chronic exposure to bufadienolides. Inset: Heterodon platirhinos feeding on the toad Anaxyrus terrestris. Note the viscous skin secretion of the toad, which is rich in bufadienolides.
4. Discussion
Our results demonstrate that the mutations at positions 111 and 120 in the H1–H2 extracellular loop of the Na+/K+-ATPase α3 subunit, which confer resistance to toad toxins in snakes [11,15,29], occur in a much wider range of species than previously believed, including many species that do not regularly, if ever, prey on toads (electronic supplementary material, table S1). The first extracellular loop (amino acid positions 111–122) comprises a major part of the cardiotonic steroid binding pocket, and the non-resistant gene codes for the amino acids glutamine at position 111 and glycine at position 120. The observed substitution of leucine at position 111 results in a biochemical residue change from polar-neutral to non-polar-neutral, which increases the hydrophobicity of the residue but does not change the isoelectric point (electronic supplementary material, figure S1). The substitution of arginine at position 120 results in a biochemical residue change from non-polar-neutral to polar-positive, which increases the isoelectric point and decreases the hydrophobicity of the residue (electronic supplementary material, figure S1). Although the physiological effect of the G120R substitution has not been specifically tested, it is biochemically similar to the residue change caused by the N122H substitution in milkweed insects (change from a neutral to positively charged residue), which has been shown to confer significantly lower binding affinity to CDs [13]. Furthermore, the combined substitutions of Q111 L and G120R have been shown to confer a much lower affinity to BDs than the wild-type gene in vertebrate cells [15]. Although the Q111 L substitution does contribute to resistance in cardenolide-resistant invertebrates [13], evidence from insect phylogenies suggests that it is a necessary intermediate step that leads to the later appearance of other substitutions in the H1–H2 extracellular loop [4]. A similar relationship presumably explains the consistent co-occurrence of substitution Q111 L with G120R in BD-resistant snakes.
The distribution of the resistance mutations Q111 L and G120R among colubroid snakes, including their widespread appearance in many species that do not prey on toads, raises a number of important issues that were obscured by the limited taxon sampling of previous studies. The occurrence of such mutations among non-toad-eating species suggests that possession of these mutations does not carry a high performance cost. Paradoxically, however, investigations of the ion transport activities of some resistant Na+/K+-ATPases have shown that the mutations do in fact lower the enzyme's function [36,37]. It appears that in insects, the deleterious effects of the lowered enzyme function are circumvented by the presence of a secondary Na+/K+-ATPase isoform [37]. It is thus likely that in snakes Na+/K+-ATPase isoforms α1 and α2 might be contributing similarly. This contrasts sharply with the limited distribution of mutations for resistance in snakes that consume amphibians defended by tetrodotoxin (TTX) [12]. TTX targets voltage-gated sodium channels, and the mutations that confer resistance to TTX also impart a substantial countervailing cost, as measured by locomotor performance [38,39]. Several point mutations have been documented among TTX-resistant snakes [12], and their occurrence is tightly linked to the consumption of TTX-defended amphibians. In the best-documented case, involving the natricine snake Thamnophis sirtalis and the newt Taricha granulosa, geographical differences in the level of TTX in the newt are mirrored by differences in the level of innate resistance of the sodium channels in the snake [40]. Populations of snakes that consume low levels of TTX exhibit low levels of resistance.
In contrast to such close correspondence between prey toxicity and sodium channel resistance to TTX, our data document the occurrence of BD-resistance mutations in Na+/K+-ATPase among many species that are not known to consume toads at all, including specialized predators on molluscs (Dipsas catesbyi, Storeria occipitomaculata), crayfishes (Regina spp.), fishes (Nerodia rhombifer, Helicops angulatus), and mammals (Crotalus atrox, Agkistrodon contortrix; electronic supplementary material, table S1). It is possible that the same mutation protects a few of these taxa against other toxins that exert yet unknown pharmacological effects (as in some molluscs [41] and other invertebrates [42–46]). However, most non-toad-eating species that possess the resistance mutations appear not to derive any benefit nor incur a substantial cost from the mutated form of Na+/K+-ATPase. Future studies should address that inference directly.
Our study includes relatively broad taxon sampling of the three largest subfamilies of colubrid snakes. The sister lineages Natricinae and Dipsadinae (figure 2) both include large numbers of taxa that possess the resistance mutations. The species in both lineages consume a wide variety of prey, and both subfamilies include species that are highly bufophagous or sequester BDs. Our results indicate a 0.61 probability that resistance to BDs originated in the common ancestor of both of these groups (figure 2, node 3) and was independently lost in two extant lineages. Interestingly, both these subfamilies include early branches that are highly bufophagous or sequester BDs, suggesting the possibility that consumption of toads characterized early members of both radiations, and possibly their common ancestor. Arguably the most highly bufophagous genus of snakes, Heterodon of North America, lies near the base of the Dipsadinae, although members of the sister lineage of Heterodon (which includes Farancia, Diadophis, Carphophis, and Contia) lack the resistance mutations, most likely through secondary loss of those residues (figure 2, node 2). The other highly bufophagous lineage of dipsadines, the genus Xenodon (sensu lato, including the nominal genera Waglerophis and Lystrophis [47]), is nested more deeply within the Dipsadinae, as a member of a diverse South American radiation (figure 2, node 1).
Among the Natricinae, the overwhelming majority of species possesses the resistance mutations. A basal split (figure 2, node 5) separates an exclusively Asian lineage from one that includes a few Asian species, as well as a number in Europe and the large North American tribe Thamnophiini. The exclusively Asian lineage includes the genus Rhabdophis, most members of which possess defensive structures known as nuchal or nucho-dorsal glands [48]. Some species accumulate high concentrations of BDs from toads consumed as prey and release the toxins to deter the snakes' predators [3,49]. Furthermore, female Rhabdophis tigrinus can transfer prey toxins to their embryos before oviposition [50], and a recent field study demonstrated that females preferentially forage for toads during the reproductive season, presumably increasing the likelihood of provisioning their embryos with BDs [51]. Therefore, although toads comprise a numerically small element of the diet in this species [52], the snakes often retain high levels of BDs in their tissues. The sampled species of Natricinae exhibit only a single example of likely secondary loss of the resistance mutations, the East Asian genus Sinonatrix (figure 2, node 4). That genus of highly aquatic fish-eaters (electronic supplementary material, table S1), is sister to the West Asian, European, and North American natricines, all of which possess the resistance mutations and one of which, Natrix natrix of Europe, frequently consumes toads.
The third broadly sampled colubrid subfamily, the Colubrinae, displays a different evolutionary picture. Of 15 species sampled, only two distantly related species possess the resistance mutation (and are known to feed on toads). Our analysis suggests a 91% probability that the ancestor of the Colubrinae (figure 2, node 6) lacked the mutation. Thus, although all species in our sample that are known to prey on toads possess the resistance mutations, the recovered pattern is one in which only a few members of the ancestrally non-resistant Colubrinae evolved resistance, whereas many members of the ancestrally resistant Natricinae and Dipsadinae retained the mutations despite consuming prey other than toads. These results argue that historical contingency plays a strong role in the origin of genetic resistance to BDs and suggest that the performance cost of retaining the mutations is low, even when it confers no evident advantage.
Two other large and widespread families, Viperidae and Elapidae, possess bufophagous genera, both of which occur in Africa. Viperids exhibit greater variation in the distribution of the resistance mutations than other major lineages. However, the bufophagous viper Causus is basal to a radiation of African Viperinae (figure 2, node 8), of which all sequenced taxa retain those mutations. The Eurasian genus Vipera diverged before the African radiation and lacks the mutations. The other major lineage of vipers, the Crotalinae, exhibits a diversity of Na+/K+-ATPase sequences. However, our sampling of Viperidae is limited, and further sequencing is needed to resolve the origins of resistance in this family with confidence. Among the Elapidae, the bufophagous African genus Hemachatus is sister to the diverse radiation of African and Asian Naja (figure 2, node 7), which includes a number of facultative toad-eaters. Unfortunately, our sampling includes only a single species of Lamprophiidae, a large and ecologically diverse African colubroid radiation that is sister to the Elapidae. The only sequenced lamprophiid, Boaedon fuliginosus, lacks the resistance mutations, but further sampling of this family is desirable, especially considering that one species of the lamprophiid genus Crotaphopeltis feeds largely on toads [9].
It is possible that some generally non-toad-eating taxa may rarely encounter and consume bufonids, either as adults or as juveniles. (Information on the diets of young snakes is especially limited.) However, it is doubtful that rare instances of toad consumption would have driven the evolution of the resistance mutations, especially in the varied dietary specialists discussed above. It is more likely that species belonging to ancestrally resistant lineages may occasionally consume bufonids when encountered, despite a diet consisting primarily of other prey. Such a scenario is not inconsistent with our conclusions. Conversely, however, we predict that non-resistant snakes would rarely, if ever, survive such a rare predatory event.
Recent time-calibrated phylogenetic studies of toads and snakes support the hypothesis that toads arrived on all occupied continents prior to, or coincident with, the evolution of specialized bufophagous snakes. The Bufonidae are believed to have arisen in South America, after the breakup of Gondwana approximately 88 Ma, and dispersed north and then west across the Bering Strait, establishing lineages in Eurasia and Africa before returning to the Americas across a North Atlantic land connection about 43 Ma [53]. The presence of BDs in the most basal genus of bufonids, Melanophryniscus, is uncertain, although its species are chemically defended by the indolealkylamine bufotenine and by alkaloids sequestered from arthropod prey [54]. Skin extracts from Melanophryniscus appear to inhibit Na+/K+-ATPase [55]. BDs are present in Atelopus [55], which is sister to all remaining bufonids in both the New and Old Worlds. Furthermore, a more derived clade of Neotropical bufonids (Rhinella) diverged from a North American ancestor about 41 Ma, so the presence of BD-defended toads in South America clearly predates the origin of the bufophagous South American genus Xenodon about 18 Ma. Similarly, the origin of the notably bufophagous taxa Heterodon (North America, 30 Ma), Rhabdophis (East Asia, 29 Ma), Natrix natrix (Europe, 13 Ma), Hemachatus (Africa, 23 Ma), and Causus (Africa, 30 Ma) all postdate the completion of the bufonid diaspora [33].
Importantly, dates vary for the divergence of the related colubrid subfamilies Natricinae and Dipsadinae, both of which include relatively basal bufophagous genera and large numbers of genera that possess the resistance mutations. Indeed, our analysis indicated a fairly high probability that the ancestor of Natricinae + Dipsadinae (figure 2, node 3) possessed those mutations. A detailed analysis of the Natricinae suggested that they originated in eastern Asia approximately 35 Ma (figure 2, node 5), later establishing lineages in Africa, Europe, and North America [56]. Consistent with that scenario, the most basal dipsadines, Thermophis and Stichophanes, occur in Asia, whereas all other members of the subfamily are limited to the New World, including the highly bufophagous Heterodon and Xenodon. More recently, Zheng & Wiens [33] dated the divergence of Natricinae and Dipsadinae to about 48 Ma. These two divergence estimates bracket the date for the re-entry of toads into the New World from Eurasia (about 43 Ma; [38]) and are reasonably consistent with an origin of the mutations in the ancestor of Natricinae + Dipsadinae in Asia while in sympatry with bufonids.
The earliest lineage of snakes in which we found the resistance mutations is Epictia albipuncta (Leptotyphlopidae; figure 2, node 9), our only sample from the large but poorly understood radiation of Scolecophidia. That possibly paraphyletic group of five families and about 400 species [33,57] underwent an initial split into three major lineages about 123–125 Ma [57], long before the origin of toads. Given the presence of the resistance mutations in some varanid lizards [11,15], it is possible that the mutations may have been present in the common ancestor of all snakes. Alternatively, resistance may have evolved independently in scolecophidians, nearly all of which specialize on a diet of social insects, either ants or termites [8]. Ants are defended by a diverse array of alkaloids [42,43], and some termites possess terpene toxins [43–46], although the diversity of toxins in the early life-history stages on which scolecophidians largely feed is not well known. The pharmacological effects of most of ant and termite compounds are also poorly understood, and thus it is uncertain whether the same mutations to Na+/K+-ATPase that confer resistance to BDs would protect against those prey. However, evidence of resistance to both BDs and alkaloids has been demonstrated in Heterodon platirhinos, a highly bufophagous species that is also resistant to TTX in newts, although the mechanism for its resistance to TTX remains unknown [58]. Clearly, additional sequencing of diverse scolecophidian species is needed, together with a better understanding of the physiological effects of their prey's defensive toxins.
Although our sampling efforts were biased toward certain lineages, our data are sufficient to demonstrate that the presence of BD resistance mutations across the phylogeny of snakes does not correspond closely to the consumption of toads. Nonetheless, it is true that all bufophagous snakes in our sample possess the resistance mutations. Comparison of the physiological effects of a controlled dose of the BD gamabufotalin on a species with the resistance mutations, Nerodia rhombifer, and one that lacks the mutations, Pituophis catenifer, revealed sharply different responses [29]. N. rhombifer, a specialized predator on fishes, exhibited no signs of distress when subjected to mass-specific doses 20 times greater than those that proved lethal to P. catenifer. This suggests that the resistance mutations are indeed protective against the acute effects of BD consumption. Furthermore, the widespread occurrence of the resistance mutations among non-toad-eating snakes suggests that possession of the resistance mutations does not impose a large performance cost. However, the inclusion of toads as an occasional prey item among the genetically resistant species, together with the relative rarity of species that either specialize on a diet of toads or sequester dietary BDs, suggests that the cost of chronic exposure to those toxins poses a greater physiological challenge and may require additional mechanisms of resistance.
In parallel invertebrate systems, a variety of mechanisms confer resistance to CDs, including detoxification by enzymes, excretion and exclusion from sensitive tissues, in addition to target-site insensitivity [1,2,4,5]. As such, target-site insensitivity may not always be required as a precursor for tolerance to toxic prey. Furthermore, several resistance mechanisms against environmental toxins can exist within a single species. For example, dichlorodiphenyltrichloroethane resistance in insects can be achieved by amino acid substitutions in the voltage-gated sodium channel [17,59] and also through increased expression of detoxifying enzymes [17,60]. This further suggests that, although the resistance mutations of Na+/K+-ATPase may be sufficient to protect a snake from the acute effects of BD ingestion, additional physiological adaptations may be required to protect highly bufophagous taxa and those that sequester BDs against the chronic effects of toxin exposure. Some CD-resistant insects show tissue-specific increases of mutant Na+/K+-ATPase expression that reflect the relative exposure of different tissues to CDs [14]. A similar mechanism in bufophagous snakes may involve a specific endocrine response to BDs. Mohammadi et al. [10] demonstrated that three independently evolved snakes that specialize on toads possess highly enlarged adrenal glands in comparison with their non-toad-eating sister taxa (figure 2). Adrenal corticosteroid hormones exert diverse physiological effects, and aldosterone in particular has been linked to increased expression of Na+/K+-ATPase in cardiac cells [61].
5. Conclusion
Although target-site insensitivity clearly plays an essential role in the survival of toad-eating snakes, it is not tightly coupled to bufophagy. The occurrence of the resistance mutations among species with varied diets suggests that the mutations impose little performance cost. To understand the evolution of highly specialized bufophagous snakes, it will be necessary to move beyond target-site insensitivity and investigate the complex, interconnected physiological pathways that confer resistance to chronic BD exposure.
Supplementary Material
Acknowledgements
We thank Caleb D. McMahan, Bryan M. Kluever, Andrew M. Durso, and Lorin A. Neuman-Lee for assistance in collecting snakes in the field. We especially thank the Louisiana State University Museum of Natural Science for providing the tissues that made this study possible.
Data accessibility
Supplementary material can be found in the online version of this article. Sequence data are available on GenBank through accession numbers: KU738063-KU738139, KU933521, and KU950321. Ancestral state reconstruction data available in Dryad at doi:10.5061/dryad.4085r [62].
Authors' contributions
S.M. and A.H.S. designed the research; S.M., J.G., and H.T. performed the research and collected data; Z.G., S.M., and A.H.S. analysed the data; A.M. contributed to the intellectual development of the research; S.M., A.H.S., Z.G., and J.G. wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by a SICB Grant-in-Aid of Research (to S.M.), a USU Ecology Center Research Grant (to S.M.), a USU Center for Women and Gender Research Grant (to S.M.), the National Science Foundation (EAPSI SP15039 to S.M.), and a grant from the Japan Society for the Promotion of Science (Scientific Research C, no. 26440213 to A.M.).
References
- 1.Scudder GGE, Meredith J. 1982. The permeability of the midgut of three insects to cardiac glycosides. J. Insect Physiol. 28, 689–694. ( 10.1016/0022-1910(82)90147-0) [DOI] [Google Scholar]
- 2.Despres L, David J-P, Gallet C. 2007. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 22, 298–307. ( 10.1016/j.tree.2007.02.010) [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson DA, Mori A, Savitzky AH, Burghardt GM, Wu X, Meinwald J, Schroeder FC. 2007. Dietary sequestration of defensive steroids in nuchal glands of the Asian snake Rhabdophis tigrinus. Proc. Natl Acad. Sci. USA 104, 2265–2270. ( 10.1073/pnas.0610785104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petschenka G, Fandrich S, Sander N, Wagschal V, Boppré M, Dobler S. 2013. Stepwise evolution of resistance to toxic cardenolides via genetic substitutions in the Na+/K+-ATPase of milkweed butterflies (Lepidoptera: Danaini). Evolution 67, 2753–2761. ( 10.1111/evo.12152) [DOI] [PubMed] [Google Scholar]
- 5.Petschenka G, Pick C, Wagschal V, Dobler S. 2013. Functional evidence for physiological mechanisms to circumvent neurotoxicity of cardenolides in an adapted and a non-adapted hawk-moth species. Proc. R. Soc. B 280, 20123089 ( 10.1098/rspb.2012.3089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santa Coloma A, Garraffo HM, Pignataro OP, Charreau EH, Gros EG. 1984. Biosynthesis of bufadienolides in toads. V. The origin of the cholesterol used by toad parotoid glands for biosynthesis of bufadienolides. Steroids 44, 11–22. ( 10.1016/S0039-128X(84)80012-4) [DOI] [PubMed] [Google Scholar]
- 7.Opitz SE, Müller C. 2009. Plant chemistry and insect sequestration. Chemoecology 19, 117–154. ( 10.1007/s00049-009-0018-6) [DOI] [Google Scholar]
- 8.Savitzky AH, Mori A, Hutchinson DA, Saporito RA, Burghardt GM, Lillywhite HB, Meinwald J. 2012. Sequestered defensive toxins in tetrapod vertebrates: principles, patterns, and prospects for future studies. Chemoecology 22, 141–158. ( 10.1007/s00049-012-0112-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keogh JS, Branch WR, Shine R. 2000. Feeding ecology, reproduction and sexual dimorphism in the colubrid snake Crotaphopeltis hotamboeia in southern Africa. Afr. J. Herpetol. 49, 129–137. ( 10.1080/21564574.2000.9635439) [DOI] [Google Scholar]
- 10.Mohammadi S, McCoy KA, Hutchinson DA, Gauthier DT, Savitzky AH. 2013. Independently evolved toad-eating snakes exhibit sexually dimorphic enlargement of adrenal glands. J. Zool. 290, 237–245. ( 10.1111/jzo.12038) [DOI] [Google Scholar]
- 11.Ujvari B, et al. 2015. Widespread convergence in toxin resistance by predictable molecular evolution. Proc. Natl Acad. Sci. USA 112, 11 911–11 916. ( 10.1073/pnas.1511706112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman CR, Brodie ED Jr, Pfrender ME. 2009. The evolutionary origins of beneficial alleles during the repeated adaptation of garter snakes to deadly prey. Proc. Natl Acad. Sci. USA 106, 13 415–13 420. ( 10.1073/pnas.0901224106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobler S, Dalla S, Wagschal V, Agrawal AA. 2012. Community-wide convergent evolution in insect adaptation to toxic cardenolides by substitutions in the Na, K-ATPase. Proc. Natl Acad. Sci. USA 109, 13 040–13 045. ( 10.1073/pnas.1202111109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhen Y, Aardema ML, Medina EM, Schumer M, Andolfatto P. 2012. Parallel molecular evolution in an herbivore community. Science 337, 1634–1637. ( 10.1126/science.1226630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ujvari B, Mun HC, Conigrave AD, Bray A, Osterkamp J, Halling P, Madsen T. 2013. Isolation breeds naivety: island living robs Australian varanid lizards of toad-toxin immunity via four-base-pair mutation. Evolution 67, 289–294. ( 10.1111/j.1558-5646.2012.01751.x) [DOI] [PubMed] [Google Scholar]
- 16.Zakon HH. 2002. Convergent evolution on the molecular level. Brain. Behav. Evol. 59, 250–261. ( 10.1159/000063562) [DOI] [PubMed] [Google Scholar]
- 17.Baxter SW, Badenes-Pérez FR, Morrison A, Vogel H, Crickmore N, Kain W, Wang P, Heckel DG, Jiggins CD. 2011. Parallel evolution of Bacillus thuringiensis toxin resistance in Lepidoptera. Genetics 189, 675–679. ( 10.1534/genetics.111.130971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storz JF. 2016. Causes of molecular convergence and parallelism in protein evolution. Nat. Rev. Genet. 17, 239–250. ( 10.1038/nrg.2016.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krenn L, Kopp B. 1998. Bufadienolides from animal and plant sources. Phytochemistry 48, 1–29. ( 10.1016/S0031-9422(97)00426-3) [DOI] [PubMed] [Google Scholar]
- 20.Schoner W, Scheiner-Bobis G. 2007. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am. J. Cardiovasc. Drugs 7, 173–189. ( 10.2165/00129784-200707030-00004) [DOI] [PubMed] [Google Scholar]
- 21.Köksoy AA. 2002. Na+, K+-ATPase: a review. J. Ankara. Med. Sch. 24, 73–82. [Google Scholar]
- 22.Laursen M, Gregersen JL, Yatime L, Nissen P, Fedosova NU. 2015. Structures and characterization of digoxin-and bufalin-bound Na+, K+-ATPase compared with the ouabain-bound complex. Proc. Natl Acad. Sci. USA 112, 1755–1760. ( 10.1073/pnas.1422997112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose AM, Valdes R. 1994. Understanding the sodium pump and its relevance to disease. Clin. Chem. 40, 1674–1685. [PubMed] [Google Scholar]
- 24.Morth JP, Pedersen BP, Toustrup-Jensen MS, Sørensen TLM, Petersen J, Andersen JP, Vilsen B, Nissen P. 2007. Crystal structure of the sodium-potassium pump. Nature 450, 1043–1049. ( 10.1038/nature06419) [DOI] [PubMed] [Google Scholar]
- 25.Schwinger RH, Bundgaard H, Müller-Ehmsen J, Kjeldsen K. 2003. The Na, K-ATPase in the failing human heart. Cardiovasc. Res. 57, 913–920. ( 10.1016/S0008-6363(02)00767-8) [DOI] [PubMed] [Google Scholar]
- 26.Pierce AA, de Roode JC, Tao L. 2016. Comparative genetics of Na+/K+-ATPase in monarch butterfly populations with varying host plant toxicity. Biol. J. Linn. Soc. 119, 194–200. ( 10.1111/bij.12797) [DOI] [Google Scholar]
- 27.Croyle ML, Woo AL, Lingrel JB. 1997. Extensive random mutagenesis analysis of the Na+/K+-ATPase alpha subunit identifies known and previously unidentified amino acid residues that alter ouabain sensitivity Implications for ouabain binding. Eur. J. Biochem. 248, 488–495. ( 10.1111/j.1432-1033.1997.00488.x) [DOI] [PubMed] [Google Scholar]
- 28.Dostanic-Larson I, Loreny JN, Van Huysse JW, Neumann JC, Moseley AE, Lingrel JB. 2006. Physiological role of the α1- and α2-isoforms of the Na+-K+-ATPase and biological significance of their cardiac glycoside binding site. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R524–R528. ( 10.1152/ajpregu.00838.2005) [DOI] [PubMed] [Google Scholar]
- 29.Mohammadi S, Brodie ED Jr, Neuman-Lee LA, Savitzky AH. 2016. Mutations to the cardiotonic steroid binding site of Na+/K+-ATPase are associated with high level of resistance to gamabufotalin in a natricine snake. Toxicon 114, 13–15. ( 10.1016/j.toxicon.2016.02.019) [DOI] [PubMed] [Google Scholar]
- 30.Petschenka G, Offe JK, Dobler S. 2012. Physiological screening for target site insensitivity and localization of Na+/K+-ATPase in cardenolide-adapted Lepidoptera. J. Insect Physiol. 58, 607–612. ( 10.1016/j.jinsphys.2011.12.012) [DOI] [PubMed] [Google Scholar]
- 31.Moore DJ, Halliday DCT, Rowell DM, Robinson AJ, Keogh JS. 2009. Positive Darwinian selection results in resistance to cardioactive toxins in true toads (Anura: Bufonidae). Biol. Lett. 5, 513–516. ( 10.1098/rsbl.2009.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bollback JP. 2006. SIMMAP: stochastic character mapping of discrete traits on phylogenies. BMC Bioinformatics 7, 88 ( 10.1186/1471-2105-7-88) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Y, Wiens JJ. 2016. Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4 162 species. Mol. Phylogenet. Evol. 94, 537–547. ( 10.1016/j.ympev.2015.10.009) [DOI] [PubMed] [Google Scholar]
- 34.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 35.Pagel M. 1994. Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B 255, 37–45. ( 10.1098/rspb.1994.0006) [DOI] [Google Scholar]
- 36.Kockskämper J., Gisselmann G., Glitsch HG. 1997. Comparison of ouabain-sensitive and -insensitive Na/K pumps in HEK293 cells. Biochim. Biophys. Acta 1325, 197–208 ( 10.1016/S0005-2736(96)00259-3) [DOI] [PubMed] [Google Scholar]
- 37.Dalla S, Dobler S. In press. Gene duplications circumvent trade-offs in enzyme function: insect adaptation to toxic host plants. Evolution in press. ( 10.1111/evo.13077) [DOI] [PubMed] [Google Scholar]
- 38.Brodie ED III, Brodie ED Jr. 1999. Costs of exploiting poisonous prey: evolutionary trade-offs in a predator–prey arms race. Evolution 53, 626–631. ( 10.2307/2640799) [DOI] [PubMed] [Google Scholar]
- 39.Brodie ED III, Feldman CR, Hanifin CT, Motychak JE, Mulcahy DG, Williams BL, Brodie ED Jr. 2005. Parallel arms races between garter snakes and newts involving tetrodotoxin as the phenotypic interface of coevolution. J. Chem. Ecol. 31, 343–356. ( 10.1007/s10886-005-1345-x) [DOI] [PubMed] [Google Scholar]
- 40.Brodie ED Jr, Ridenhour BJ, Brodie ED III. 2002. The evolutionary response of predators to dangerous prey: hotspots and coldspots in the geographic mosaic of coevolution between newts and snakes. Evolution 56, 2067–2082. ( 10.1111/j.0014-3820.2002.tb00132.x) [DOI] [PubMed] [Google Scholar]
- 41.Schroeder FC, Gonzàlez A, Eisner T, Meinwald J. 1999. Miriamin, a defensive diterpene from the eggs of a land slug (Arion sp.). Proc. Natl Acad. Sci. USA 96, 13 620–13 625. ( 10.1073/pnas.96.24.13620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones TH, Torres JA, Spande TF, Garraffo HM, Blum MS, Snelling RR. 1996. Chemistry of venom alkaloids in some Solenopsis (Diplorhoptrum) species from Puerto Rico. J. Chem. Ecol. 22, 1221–1236. ( 10.1007/BF02266962) [DOI] [PubMed] [Google Scholar]
- 43.Jones TH, Gorman JS, Snelling RR, Delabie JH, Blum MS, Garraffo HM, Jain P, Daly JW, Spande TF. 1999. Further alkaloids common to ants and frogs: decahydroquinolines and a quinolizidine. J. Chem. Ecol. 25, 1179–1193. ( 10.1023/A:1020898229304) [DOI] [Google Scholar]
- 44.Mizuno T, Kojima Y. 2015. A blindsnake that decapitates its termite prey. J. Zool. 297, 220–224. ( 10.1111/jzo.12268) [DOI] [Google Scholar]
- 45.Moore BP. 1968. Studies on the chemical composition and function of the cephalic gland secretion in Australian termites. J. Insect Physiol. 14, 33–39. ( 10.1016/0022-1910(68)90131-5) [DOI] [Google Scholar]
- 46.Prestwich GD, Jones RW, Collins MS. 1981. Terpene biosynthesis by nasute termite soldiers (Isoptera: Nasutitermitinae). Insect Biochem. 11, 331–336. ( 10.1016/0020-1790(81)90011-1) [DOI] [Google Scholar]
- 47.Zaher H, Grazziotin FG, Cadle JE, Murphy RW, Moura-Leite JCD, Bonatto SL. 2009. Molecular phylogeny of advanced snakes (Serpentes: Caenophidia) with an emphasis on South American Xenodontines: a revised classification and descriptions of new taxa. Pap. Avulsos. Zool. (São Paulo) 49, 115–153. ( 10.1590/S0031-10492009001100001) [DOI] [Google Scholar]
- 48.Mori A, Burghardt GM, Savitzky AH, Roberts KA, Hutchinson DA, Goris RC. 2012. Nuchal glands: a novel defensive system in snakes. Chemoecology 22, 187–198. ( 10.1007/s00049-011-0086-2) [DOI] [Google Scholar]
- 49.Hutchinson DA, Savitzky AH, Mori A, Burghardt GM, Meinwald J, Schroeder FC. 2012. Chemical investigations of defensive steroid sequestration by the Asian snake Rhabdophis tigrinus. Chemoecology 22, 199–206. ( 10.1007/s00049-011-0078-2) [DOI] [Google Scholar]
- 50.Hutchinson DA, Savitzky AH, Mori A, Meinwald J, Schroeder FC. 2008. Maternal provisioning of sequestered defensive steroids by the Asian snake Rhabdophis tigrinus. Chemoecology 18, 181–190. ( 10.1007/s00049-008-0404-5) [DOI] [Google Scholar]
- 51.Kojima Y, Mori A. 2015. Active foraging for toxic prey during gestation in a snake with maternal provisioning of sequestered chemical defenses. Proc. R. Soc. B 282, 2014–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mori A, Vincent SE. 2008. An integrative approach to specialization: relationships among feeding morphology, mechanics, behaviour, performance and diet in two syntopic snakes. J. Zool. 275, 47–56. ( 10.1111/j.1469-7998.2007.00410.x) [DOI] [Google Scholar]
- 53.Pramuk JB, Robertson T, Sites JW, Noonan BP. 2008. Around the world in 10 million years: biogeography of the nearly cosmopolitan true toads (Anura: Bufonidae). Glob. Ecol. Biogeogr. 17, 72–83. [Google Scholar]
- 54.Jeckel AM, Grant T, Saporito RA. 2015. Sequestered and synthesized chemical defenses in the poison frog Melanophryniscus moreirae. J. Chem. Ecol. 41, 505–512. ( 10.1007/s10886-015-0578-6) [DOI] [PubMed] [Google Scholar]
- 55.Flier J, Edwards MW, Daly JW, Myers CW. 1980. Widespread occurrence in frogs and toads of skin compounds interacting with the ouabain site of Na+, K+-ATPase. Science 208, 503–505. ( 10.1126/science.6245447) [DOI] [PubMed] [Google Scholar]
- 56.Guo P, Liu Q, Xu Y, Jiang K, Hou M, Ding L, Pyron RA, Burbrink FT. 2012. Out of Asia: natricine snakes support the Cenozoic Beringian dispersal hypothesis. Mol. Phylogenet. Evol. 63, 825–833. ( 10.1016/j.ympev.2012.02.021) [DOI] [PubMed] [Google Scholar]
- 57.Hedges SB, Marion AB, Lipp KM, Marin J, Vidal N. 2014. A taxonomic framework for typhlopid snakes from the Caribbean and other regions (Reptilia, Squamata). Caribbean Herpetol. 49, 1–61. [Google Scholar]
- 58.Feldman CR, Durso AM, Hanifin CT, Pfrender ME, Ducey PK, Stokes AN, Barnett KE, Brodie ED III, Brodie ED Jr. 2016. Is there more than one way to skin a newt? Convergent toxin resistance in snakes is not due to a common genetic mechanism. Heredity 116, 84–91. ( 10.1038/hdy.2015.73) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davies TGE, Field LM, Usherwood PNR, Williamson MS. 2007. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 59, 151–162. ( 10.1080/15216540701352042) [DOI] [PubMed] [Google Scholar]
- 60.Daborn PJ, et al. 2002. A single P450 allele associated with insecticide resistance in Drosophila. Science 297, 2253–2256. ( 10.1126/science.1074170) [DOI] [PubMed] [Google Scholar]
- 61.Ikeda U, Hyman R, Smith TW, Medford RM. 1991. Aldosterone-mediated regulation of Na+, K+-ATPase gene expression in adult and neonatal rat cardiocytes. J. Biol. Chem. 266, 12 058–12 066. [PubMed] [Google Scholar]
- 62.Mohammadi S, Gompert Z, Gonzalez J, Takeuchi H, Mori A, Savitzky AH. 2016. Data from: Toxin-resistant isoforms of Na+/K+-ATPase in snakes do not closely track dietary specialization on toads. Dryad Digital Repository. ( 10.5061/dryad.4085r) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary material can be found in the online version of this article. Sequence data are available on GenBank through accession numbers: KU738063-KU738139, KU933521, and KU950321. Ancestral state reconstruction data available in Dryad at doi:10.5061/dryad.4085r [62].