Abstract
Necrotizing enterocolitis (NEC) is the most frequent and lethal disease of the gastrointestinal tract of preterm infants. At present, NEC is thought to develop in the premature host in the setting of bacterial colonization, often after administration of non-breast milk feeds, and disease onset is thought to be due in part to a baseline increased reactivity of the premature intestinal mucosa to microbial ligands as compared with the full-term intestinal mucosa. The increased reactivity leads to mucosal destruction and impaired mesenteric perfusion and partly reflects an increased expression of the bacterial receptor Toll-like receptor 4 (TLR4) in the premature gut, as well as other factors that predispose the intestine to a hyper-reactive state in response to colonizing microorganisms. The increased expression of TLR4 in the premature gut reflects a surprising role for this molecule in the regulation of normal intestinal development through its effects on the Notch signalling pathway. This Review will examine the current approach to the diagnosis and treatment of NEC, provide an overview of our current knowledge regarding its molecular underpinnings and highlight advances made within the past decade towards the development of specific preventive and treatment strategies for this devastating disease.
Graphical abstract
Necrotizing enterocolitis is the most frequent and lethal gastrointestinal disease in premature infants. This Review outlines current approaches for the treatment and diagnosis of necrotizing enterocolitis and examines the progress made in our understanding of the molecular mechanisms of this disease as well as potential avenues for future treatment development.
Necrotizing enterocolitis (NEC) is the leading cause of death from gastrointestinal disease in premature infants, affecting newborn babies at a rate of 1–3 per 1000 births per year in North America1,2, with an average total treatment cost of US$500,000 per patient in the USA in current charges3,4. Importantly, the mechanisms leading to the development of NEC in premature infants and the lessons learned from management of patients with NEC could have broad implications to other neonatal inflammatory processes5,6. Despite several decades of experience in treating patients with NEC5,6, the overall mortality and approach to treatment have remained largely unchanged since the initial descriptions of the disease several decades ago3,7. Intensive research efforts over the past decade have begun to elucidate the molecular underpinnings of NEC and have identified several promising biologic strategies targeting the specific signalling pathways involved, which could potentially prevent and/or treat this disease in premature infants. Here, we will discuss current approaches to the diagnosis and treatment of NEC, review the current knowledge regarding its molecular pathophysiology and explore the advances made towards the development of specific preventive and treatment strategies.
Definitions and epidemiology of NEC
Epidemiology and trends in the incidence of NEC
The current occurrence of NEC is in fact a manifestation of the tremendous success achieved by neonatologists in their ability to keep premature infants alive at ever earlier gestational ages, with current global estimates of as many as 15 million babies born preterm every year, accounting for 11% of live births worldwide1,2,8. In the USA alone, the rate of prematurity is about 10% of all births, with rates as high as 13.23% in black individuals of non-Hispanic origin9. Large, population-based and hospital-based multicentre studies coordinated by neonatal research networks in Europe, North America, Australia and New Zealand have determined the incidence of NEC to be up to 13% among infants born ≤33 weeks of gestation or whose birth weight is ≤2,500 g1,10–18. Interestingly, the incidence of NEC among extremely preterm neonates in US academic centres has seen either a stabilization or even a decline to about 9% in the past 5 years analysed1, a trend that might reflect increased vigilance and the implementation of standardized feeding strategies19. Despite the fact that no predilection for sex, race or ethnicity has been conclusively established for NEC, a higher disease incidence is observed in male babies of African American decent than in any other single demographic, which could be related to the higher incidence of prematurity in this demographic than in the general US population9,10,12,17,20. Modal disease onset occurs between 27–34 weeks after conception, with the highest incidence (13%) among infants with a birth weight <1,000 g1,7. Furthermore, overall survival has not changed in the past five decades and the average mortality from NEC is 20–30%, with mortality as high as 50% in those infants requiring surgical management21. Although the majority of cases of NEC occur among premature infants, a small subset of babies born at term or shortly before (that is, ≥35 weeks of gestation) develop NEC-like gastrointestinal signs and symptoms, frequently in association with other conditions22.
Factors affecting the susceptibility for NEC development in premature infants
Despite the complex and multifactorial nature of the pathogenesis of NEC, three major risk factors have been implicated in its development: prematurity, bacterial colonization of the gut and formula-feeding23. Although no specific genetic predisposition has been clearly associated with NEC, studies evaluating concordance rates in monozygotic and dizygotic twins have found a familial predisposition for the disease24. Moreover, evidence suggests that genetic variants leading to upregulated expression of downstream signalling regulators of Toll-like receptor 4 (TLR4), an innate immune receptor that recognizes lipopolysaccharide found in Gram-negative bacteria, could lead to increased susceptibility to the disease. These signalling regulators include nuclear factor κB1 (REF. 25), single Ig IL-1-related receptor26, the co-receptor molecule lymphocyte antigen 96 and the small glycolipid transport protein ganglioside GM2 activator27. In addition, a single nucleotide polymorphism in the promoter region of IL18 (REF. 28) and genetic variants encoding proteins linked to the regulation of the immune phenotype shift from type 1 to type 2 T helper cells29 could all influence the risk of NEC development. Other important clinical factors associated with NEC are summarized in BOX 1.
Box 1. Factors linked to increased NEC incidence.
Factors related to the infant
Factors related to the mother
Illicit drug abuse (including opiates, cannabinoids and cocaine)161
Chorioamnionitis162
Vaginal delivery148
NEC, necrotizing enterocolitis.
Diagnosis of NEC
Clinical and radiographic markers
The cornerstone of effective NEC treatment relies on accurately diagnosing the disease, which can usually be established on the basis of readily available clinical, radiographic and laboratory data. The typical neonate with NEC is a premature infant who is thriving, yet suddenly presents with feeding intolerance, abdominal distension, bloody stools and signs of sepsis (that is, changes in heart rate, respiratory rate, temperature and blood pressure)5,7. An important consideration in the diagnosis of NEC is the gestational age at which these symptoms present, owing to the existence of an inverse relationship between gestational age and the onset and severity of symptoms in patients with NEC30,31. Specifically, an infant born at ~27 weeks of gestation will typically present with NEC at ~4–5 weeks of age and has a substantially higher risk of NEC development than an infant born at closer to 37 weeks of gestation, for whom onset typically occurs within the first 2 weeks after birth13. A late onset of NEC in the most premature infants might be related to delayed microbial colonization of the gut and establishment of virulent microbial agents, in part owing to the use of broad-spectrum antibiotics and prolonged hospital stay32–34. Signs of sepsis can be associated with high gastric residuals (defined as the volume that remains in the stomach before the next enteral feeding35) of ≥2 ml/kg or >50% of the previous feeding volume, which could indicate the presence of feeding intolerance35,36. Although feeding intolerance is the most common early gastrointestinal symptom associated with NEC37, some controversy persists as to the use of gastric residuals as an objective measure and their predictive value in the context of the disease progression, owing to the inherent variability in sampling gastric contents through a small nasogastric or orogastric tube, as well as to the lack of standardization in the procedure of obtaining gastric aspirates36,38.
For descriptive purposes and for disease stratification, the Bell scoring system has been widely utilized, which assesses the degree of NEC severity as mild (Bell stage I), moderate (Bell stage II) or severe (Bell stage III), as characterized in TABLE 1. The diagnosis of NEC can be established by plain abdominal radiography, which reveals intramural gas (so-called pneumatosis intestinalis) in the early stages of confirmed NEC (Bell stage II), whereas advanced cases of the disease have pneumoperitoneum (Bell stage III)5,39. Although no specific laboratory markers have been validated in making the diagnosis of NEC, neutropenia and thrombocytopenia are often present40–42. Consideration of alternative diagnoses is critical for infants who present with NEC and in whom overlapping signs and symptoms might be present, including those who have spontaneous intestinal perforation, ileus secondary to sepsis, sensitivity to cow milk, food protein intolerance, ischaemic bowel disease associated with heart disease or haematological disturbances (for example, polycythaemia).
Table 1.
Bell’s staging and suggested management for NEC
| Bell’s stage | Severity | Clinical signs and symptoms | Radiological | Treatment |
|---|---|---|---|---|
| I | Mild NEC, suspected NEC | Mild systemic signs and intestinal signs | Nonspecific |
|
| II | Moderate NEC |
|
Pneumatosis intestinalis, portal venous gas |
|
| III | Advanced NEC |
|
Pneumoperitoneum |
|
Biomarkers and noninvasive testing for the diagnosis of NEC
The relative nonspecificity of the readily available clinical and radiographic tests described earlier in the article suggest the need for additional molecular markers to improve early diagnosis of NEC in premature infants. In this regard, the presence of several molecules that are detected in the blood have been assessed for their value in establishing the diagnosis of NEC43–45 and a number of them have shown considerable promise, including acute-phase reactants (such as C-reactive protein) and proinflammatory cytokines (for example, TNFα, IL-6 and IL-8)45. In addition, organ-specific biomarkers, such as those that would indicate enterocyte injury or intestinal barrier impairment, include intestinal fatty acid-binding protein, liver fatty acid-binding protein, faecal calprotectin, trefoil factor 3 and claudin-3 (REFS 41,46). Among these circulating molecules, one of the most promising might be intestinal fatty acid-binding protein, a cytoplasmic protein involved in enterocyte lipid metabolism47,48 that is released into circulation and secreted into the urine after enterocyte damage, which has been suggested to be useful in the prediction of NEC development48 and to correlate with the extent of intestinal necrosis47. Furthermore, a promising strategy in the identification of progressive NEC that requires surgical intervention has been formulated as a novel algorithm combining 27 clinical parameters and three urine fibrinogen peptide biomarkers, FGA1826, FGA1883 and FGA2659. This algorithm was reported to accurately predict the need for surgery in infants with suspected NEC in 100% of the cases analysed as opposed to only 40.1% when using the clinical parameters alone49,50, but it remains to be validated independently and in larger population studies. These and other biomarkers, therefore, could be considered for use in association with noninvasive monitoring techniques that assess intestinal tissue perfusion (for example, near-infrared spectroscopy)51 to identify those infants at risk of developing NEC.
The use of Doppler ultrasonography to assess perfusion of the intestinal wall has been suggested to be an accurate screening tool to determine the need for surgical intervention by identifying the presence of bowel necrosis without perforation52,53. Although this strategy has the potential to be more sensitive and specific than detection of the presence of free air in the abdominal cavity by conventional radiology, the presence of intramural as well as intraluminal air might obscure a reliable interpretation of ultrasonographic images. Furthermore, available data regarding the use of Doppler ultrasonography remains confined to small clinical studies with sample sizes in the range of 26–62 patients52,53. This diagnostic strategy and others discussed here are promising; however, they remain incompletely proven, so the development of highly sensitive and specific diagnostic tools for NEC continues to be one of the most crucial areas of need in the field.
Medical and surgical treatment
Despite considerable advances in neonatal care, NEC remains a devastating disease that lacks a cure. Current management is largely nonspecific and includes the administration of broad-spectrum antibiotics, initiation of bowel rest and the provision of fluid and inotropic support to maintain cardiorespiratory function2,5,39,54–56. Surgical intervention is required in up to 50% of the NEC cases in large, population-based and hospital-based multicentre studies coordinated by neonatal research networks3,13,16,17 and typically includes the removal of necrotic intestine. In rare cases, the placement of a peritoneal drain and abdominal irrigation might be sufficient. Although several studies have reported that patients undergoing peritoneal drainage and laparotomy could have similar outcomes3,56,57, importantly, spontaneous intestinal perforation might resemble the initial presentation of perforated NEC, thus obscuring the interpretation of the findings from these studies. Several surgical guidelines have been published56,58–60. Given that up to 74% of infants initially managed with peritoneal drainage will require a subsequent laparotomy3,59, a commonly accepted approach has been to reserve primary peritoneal drainage for those patients with substantially elevated intra-abdominal pressure that impairs ventilation, or for extremely small infants under 750 g. Additional information based upon the Bell clinical staging criteria is provided in TABLE 1 (REF 39).
Outcomes of infants with NEC
The outcome of children with NEC is characterized by high overall morbidity ranging from 20–50%, as patients experience recurrence, intestinal strictures, short bowel syndrome, growth delay and neurodevelopmental impairment2,5. Infants with NEC have longer hospitalization stays, increased risk of death before discharge and accrue higher financial costs compared with premature infants without NEC3,10. In the long term, patients who survive NEC are frequently affected by neurodevelopmental impairment, demonstrated by their impaired performance in cognitive and developmental assessments such as the Bayley Scales of Infant Development, the Griffiths Quotient and the Stanford–Binet test61, underscoring the far-reaching sequelae of this disease6,62. A detailed list of complications and outcomes is presented in TABLE 2.
Table 2.
Complications and outcomes in patients with NEC
| Type of complication or outcome | Incidence | Associated factors |
|---|---|---|
| Recurrence | 4–10%56,194,195 | Nonoperative management, congenital heart disease2,56 |
| Mortality | 15–63%3,196 |
|
| Intestinal strictures | 12–35%197 |
|
| Stoma complications | 50%199 |
|
| Short Bowel Syndrome | 20–35%200 | |
| Neurodevelopmental impairment | 30–50%202,203 | NEC vs. no NEC (OR: 1.82). Surgical NEC versus medical NEC (OR: 2.34)202–204 |
| Growth delay | 10%62,201 |
|
NEC, necrotizing enterocolitis.
Pathogenesis of NEC
Considerable interest has been shown in advancing our understanding of the molecular mechanisms that lead to the development of NEC, to further the development of more precise diagnostic and treatment modalities for this devastating disease. The following paragraphs will review the current theories within the field that seek to explain how NEC develops, and will in particular highlight opportunities for drug discovery based upon the present understanding of its pathogenesis. On the basis of the work of many investigators, we now propose a unifying hypothesis for the development of NEC: that the intestine of the premature neonate exists in a hyper-reactive state relative to the full-term intestine, which favours NEC development upon colonization with an appropriate microbial milieu in a patient with a permissive genetic background.
The preterm gut: a susceptible environment for the development of NEC
To understand the reasons for which premature infants are at a particularly high risk of developing NEC compared with full-term infants, investigators have focused their efforts on comprehending the differences between the premature and the full-term intestinal tract. These studies have outlined important differences in bacterial colonization, micro-circulatory perfusion and the maturity of the gastrointestinal immune system5,63. Importantly, although none of these factors alone can completely explain the reasons for which NEC develops, taken together they provide a picture that explains the pathogenesis of this disease. These studies also suggest the possibility that a molecular determinant might have a role in distinguishing the premature from the full-term intestine. In this regard, increasing evidence suggests that TLR4 is expressed at higher levels in the premature than the full-term intestine in mice, humans and other species64. Activation of TLR4 on the lining of the premature intestine by the Gram-negative bacteria that colonize the premature gut leads to a number of deleterious effects, including increased enterocyte apoptosis, impaired mucosal healing and enhanced proinflammatory cytokine release, which in aggregate lead to the development of NEC65. Moreover, the translocation of Gram-negative bacteria through the gut mucosa leads to activation of TLR4 on the lining of the endothelium of the premature bowel mesentery, resulting in a reduction in blood flow and the development of intestinal ischaemia and necrosis66. In additional studies, the elevated expression of TLR4 in the premature gut is reflective of the surprising function TLR4 exhibits in the regulation of normal gut development in utero via its effects on the Notch signalling pathway and through its expression on intestinal stem cells positive for the leucine-rich repeat-containing G-protein coupled receptor 5 (REF. 64). As a consequence of this critical role in normal gut epithelial development, TLR4 is expressed at high levels in the developing gut; therefore, in the setting of a premature birth, intestinal TLR4 levels remain elevated67 as a consequence of the gut not having completed its full development, as well as perhaps through the ongoing activation by luminal microorganisms. Upon subsequent colonization of the gut by bacteria in the postnatal period, the deleterious consequences of exaggerated TLR4 signalling occur, leading to the development of NEC. We term this explanation for the pathogenesis of NEC, in which in utero TLR4 signalling that is required for gut differentiation becomes deleterious in the postnatal period, ‘the cross-switching hypothesis’. This hypothesis partially explains the reasons for which the premature infant is at risk of NEC development and why the disease occurs upon bacterial colonization. In further support of a role for TLR4 in NEC development, pharmacological inhibitors of TLR4 prevent NEC ex vivo in mice and human tissue68 and breast milk — which is long-known to be an effective preventive agent for patients at risk of NEC development69,70 (discussed in detail later in the article) — is a potent inhibitor of TLR4 signalling71.
Although TLR4 signalling offers an attractive pathway to explain the development of NEC, additional factors are known to differ between the premature and full-term host that might contribute to this disease. A comprehensive list of factors related to prematurity that increase the susceptibility and influence the pathogenesis of NEC are presented in BOX 2. The characteristics that predispose the premature intestine to NEC include increased molecular expression and signalling activity of key mediators such as TLR4 (REFS 65,72–75), immaturity of cellular and physiological processes such as decreased digestion and absorption of nutrients76,77 and impaired intestinal motility78–80. These factors have been identified both in humans with NEC and in experimental animal models of the disease, discussed later in this section. Among the most critical factors is the high baseline level of cellular endoplasmic reticulum stress within the premature intestine, which increases the likelihood of apoptosis in the epithelial lining compared with the intestinal epithelium of full-term infants81. In addition, the decreased number of mucus-producing goblet cells in the premature intestine results in deficient mechanical protection that leads to increased exposure of the vulnerable epithelia to pathogenic bacteria and toxic luminal contents64,82. The potential for injury resulting from insufficient mucus layer protection is heightened by the impaired clearance of luminal contents, owing to decreased motility78–80,83 and decreased digestion and absorption as a result of enterocyte immaturity76,77. Other important differences in the intestine of premature infants compared with full-term infants include increased microvascular tone within the intestinal mesentery66,84 and the presence of immature tight junctions85,86, all of which can render the bowel at risk of proinflammatory signalling, bacterial translocation and NEC development74,78,84. Notably, some of these important factors are linked to TLR4 signalling. For instance, TLR4 hyperactivation in the setting of prematurity leads to reduced goblet cell signalling via activated Notch pathways, and TLR4 activation also reduces endothelial nitric oxide synthase protein levels and activity within the vascular endothelium, leading to reduced mesenteric perfusion and potentially reduced motility66,84. Furthermore, T lymphocytes have been shown to participate in the adaptation of the premature intestinal mucosa to bacterial colonization and contribute to NEC development87–89. NEC is associated with lymphocyte imbalance within the intestinal mucosa, as TLR4 signalling in the intestinal epithelium leads to an upregulation of proinflammatory T helper 17 cells and a reduction in protective T regulatory cells, which can be reversed through the administration of retinoic acid in the diet89.
Box 2. Factors related to prematurity that increase the susceptibility to NEC.
Increased expression of the innate immune receptor Toll-like receptor 4 (TLR4) in the apical surface of enterocytes65,72–75 and within intestinal stem cells81,123
Increased baseline levels of endoplasmic reticulum (ER) stress within intestinal crypts81
Decreased number of mucus-producing goblet cells181
Impaired regulation of microcirculatory perfusion of the gut66,84
Increased bile acid levels and decreased bile acid-binding protein in the intestinal lumen182
Inefficient antigen processing and presentation183
Impaired intestinal regeneration and healing75
Discontinuation of gut exposure to amniotic fluid
Increased levels of platelet-activating factor (increased production and decreased degradation)74,90,91 and increased expression of its receptor in the ileum90,91
Decreased FOXP3+ regulatory T cell levels in the small intestine87,89,184
Decreased levels of intraepithelial lymphocytes expressing the T cell receptor γδ88
Decreased intestinal expression of transforming growth factor β240,96
Although TLR4 is likely to have a critical role in the pathogenesis of NEC, other pathways have been shown to be important. Specifically, various investigators have identified roles for the increased expression and function of platelet-activating factor in the mucosal injury and barrier dysfunction associated with NEC74,90,91, whereas inhibitors of platelet-activating factor protect against NEC development in mouse and rat models74,92. Furthermore, the expression levels of the receptor for platelet-activating factor were also increased in mouse and rat ileum90,91. Infants with NEC have high circulating levels of platelet-activating factor90,93, associated with the increased expression of this protein as well as with deficient activity of platelet-activating factor acetylhydrolase, the enzyme involved in its degradation93,94. Consequently, the presence of this enzyme in human breast milk95 has been suggested to contribute to the protective effect associated with breastfeeding90. Additionally, platelet-activating factor has been demonstrated to induce TLR4 expression and signalling74,92.
Studies using isolated tissue from infants with NEC as well as from experimental mouse models have implicated a role for intestinal macrophages in the pathogenesis of NEC96. Incomplete development of macrophage tolerance to bacterial products within the intestinal lumen, which could breach the barrier as a result of injury to the mucosal epithelia, have been postulated to predispose the preterm human intestine to the development of the disease96. Specifically, intestinal macrophages present in the healthy intestinal mucosa of term infants have increased phagocytic and bactericidal activity, but do not produce inflammatory cytokines when challenged by bacterial products, an effect that has been ascribed to the inflammatory downregulation orchestrated by transforming growth factor β2 (REF. 96). On the other hand, macrophages present within NEC injury lesions are characterized by a highly inflammatory phenotype, resulting from increased expression of mothers against decapentaplegic homologue 7, an inhibitor of transforming growth factor β2 signalling97,98. Further studies have suggested a role for impaired Paneth cell function in the development of NEC99. Paneth cells are highly specialized secretory cells located within the intestinal crypts of Lieberkühn and are key components of the innate immune system of the small intestine through their release of antimicrobial peptides into the intestinal lumen100. Evidence from studies in animal models indicates that Paneth cell depletion in the presence of Klebsiella pneumonia can induce NEC-like pathology67,101–103. However, given that the newborn gut is reasonably deficient in Paneth cells at baseline, it is unclear what the functional relevance of Paneth cells might be in the disease pathogenesis, although additional studies into this potential cellular mechanism of NEC are clearly warranted.
The gut microbiota in NEC pathogenesis
Linking alterations in the intestinal microflora with the development of various gastrointestinal diseases, including NEC, has received tremendous interest32,104. An important factor to consider in the context of NEC is that colonization of the gut in the early neonatal period happens in two waves105. The first wave, which is similar in both term and preterm infants, is predominantly dependent upon the mode of childbirth105,106. The second wave of colonization in term infants is determined by feeding type, namely breastfeeding, which is rich in bifidobacteria and Bacteroides, or formula-feeding, which predominantly comprises streptococci, staphylococci and lactobacilli106. In the case of preterm infants the second wave of colonization is less influenced by the type of feeding and is characterized by high numbers of Clostridiaceae and Enterobacteriaceae and very low relative numbers of bifidobacteria and Bacteroidetes, in contrast with term infants105. In fact, 16S ribosomal RNA gene pyrosequencing has shown that the single most important determinant factor of the composition of the premature gut microbiota is the degree of prematurity107. A discernible patterned progression of colonizers from Bacilli to Gammaproteobacteria to Clostridium characterizes gut colonization in premature infants but the rate of assembly of the microbial population is dependent upon gestational age: that is, the more premature the infant, the slower the progression of bacterial colonization, yet the same pattern of colonization is followed107. These findings demonstrate that host biology is an essential modulator of microbiota composition and equilibrium, rather than a passive culture environment. Several investigators have shown a link between an abnormal gut microbiota in premature infants and the development of NEC32,85,105,108–111. Additionally, multiple reports have suggested that functional expression of TLRs is critical in the dynamic interaction between the host epithelium and the microbiota that enables successful intestinal adaptation to the commensal microbiota112–114. Furthermore, microbial colonization of the gut is required for the development of NEC115,116, as NEC occurs only after this event5 and can be treated in humans and animal models with broad-spectrum antibiotics that target enteric microorganisms2. However, whether abnormal bacteria represent a cause as opposed to a consequence of NEC is yet to be resolved115,117. Despite multiple reports of NEC outbreaks associated with certain bacteria, identification of a specific pathogen as the main aetiological factor remains elusive108,115. Several studies have shown that there is decreased diversity in the gut microbiota of infants diagnosed with NEC when compared with age-matched controls, although without a unified pattern except for the overabundance of strict anaerobes108,118,119.
Many of the studies described in the preceding paragraphs have been performed in animal models of NEC and validation of these results in human tissue wherever possible is important. Experimental models of NEC in mice and rats that employ a combination of hypoxia, administration of formula supplemented with bacteria isolated from human NEC stool and exposure to hypothermia have been the mainstay of many such studies and are roughly comparable to the human disease. Other animal model studies, involving clamping of the mesenteric artery or ablation of Paneth cells101,102, have relevance in certain scientific circumstances and are technically easier to perform. Larger animal models, especially the piglet model, share greater similarity to human NEC, but are technically more demanding and costlier to perform120. The benefits, drawbacks and challenges of individual models have been reviewed elsewhere120.
Strategies for NEC prevention
Given that NEC occurs in a well-defined population of patients — that is, those who are premature — there might be benefit in identifying specific preventive strategies that, if administered successfully to the appropriate patients, could reduce the incidence of NEC. In this regard, there has been tremendous interest in developing specific nutritional and pharmacological strategies to reduce the incidence of NEC. The most relevant and effective will be reviewed here.
Nutritional approaches for NEC prevention: the use of breast milk
Multiple randomized clinical trials have now validated the empirical observation that breast milk statistically significantly reduces the incidence of NEC69,121. Human milk contains a variety of beneficial bioactive factors, among which several have been shown to reduce NEC incidence and progression69,122. In BOX 3 we present a list of human milk components and the experimental evidence supporting their protective effects. Considerable research efforts have been deployed to identify these critical factors in the hope that new preventive strategies can be developed121. Although the precise mechanisms by which breast milk protects against NEC are not yet fully understood, emerging experimental evidence suggests that breast milk inhibits TLR4 signalling by preventing glycogen synthase kinase 3β activity71. Consequently, breast-milk-mediated downregulation of TLR4 signalling can reverse the inhibition in intestinal stem cell proliferation and mucosal healing, which are themselves inhibited by TLR464,71,123. Moreover, these effects were shown to be partially dependent upon activation of epidermal growth factor receptor signalling71. Whether the development of NEC in association with formula feeding represents the presence of an injurious component in infant formula, or the deficiency of a protective agent only present in breast milk remains to be determined37,69,124. The lack of availability of human breast milk (which can arise for a number of reasons, such as insufficient production by the mother of an infant) remains a major challenge in neonatal care37,69, and has led to the use of donor breast milk as a potential substitute or supplement to formula-feeding. Multiple reports support the use of donor human milk as a potentially effective strategy for reducing the incidence of NEC70,125. For those instances in which no human breast milk is available, emphasis has been placed on determining the best evidence-based strategies for formula-feeding37. Although no specific feeding regimen (that is, composition, volume and rate of feeding) has been validated to prevent NEC37, the use of standardized feeding guidelines (for example, patient-specific orders with set thresholds to manage feeding intolerance)126 have been implemented in multiple centres and have been proven to be effective to reduce the incidence and severity of the disease126.
Box 3. NEC-protective factors in human milk.
Secretory IgA173
NEC, necrotizing enterocolitis.
Probiotics in the prevention of NEC
Probiotics are defined as live microorganisms that provide a health benefit to the host127,128. These agents have been shown to protect against NEC and reduce disease severity and overall mortality in premature infants127,128. The finding that a degree of perturbation in the normal gut microbial flora exists in patients with NEC supports a rationale of using probiotics to treat and prevent this disease105,115,116. Considering the vulnerability of premature infants, routine administration of probiotic agents has elicited substantial controversy regarding the type of agent to be used, dosing and timing128,129. A systematic review, analysing 24 trials, evaluated the efficacy and safety of probiotics for preventing NEC130 and suggested that oral administration of probiotics decreases all-cause mortality and incidence of severe NEC in preterm infants; however, the precise probiotic agent, timing and length of therapy still remains to be established128,130. Emerging consensus is that the use of probiotics in NEC could be effective in reducing the incidence of the disease without increasing rates of sepsis or other adverse events56,131,132. Mechanistic insights supporting the use of probiotics are scarce but are starting to emerge. Administration of the probiotic bacteria Lactobacillus rhamnosus was shown to increase enterocyte proliferation and differentiation of Paneth cells in enteroids grown in a 3D bioscaffold133. Furthermore, treatment with CpG-containing bacterial DNA, which bypasses the potential adverse effects of live bacteria, is effective against experimental NEC in mice and piglets, and acts by activating TLR9 and inhibiting TLR4 (REF. 134), providing a potential alternative to the use of live probiotics.
Novel pharmacological approaches
Certain biologic agents could have a role in preventing NEC or in treating NEC once the disease is established. Heparin-binding EGF-like growth factor has been identified as a biologic agent capable of preventing NEC in various animal models and of reversing the effects of established NEC, via positive effects on mucosal healing135, intestinal stem cell function136 and vascular perfusion84,137. A readily absorbed and nontoxic oligosaccharide that inhibits TLR4 was shown to prevent NEC in mice and piglets and to reduce intestinal inflammation in ex vivo human intestine obtained during the treatment of NEC68. Other investigators have established an important role for human milk oligosaccharides in NEC prevention and treatment138,139. Additionally, we and others showed that the administration of a simulated amniotic fluid might have benefit for the prevention or treatment of NEC, on the basis of the mucosal protection offered by amniotic fluid, which is rich in growth factors, and exerts anti-TLR4 effects140–142. A summary of biological approaches for the prevention of NEC is presented in FIG. 1. Randomized clinical trials are underway to determine the potential therapeutic and/or preventive strategies of some of these approaches. In particular, clinical trial NCT00437567 has been designed to evaluate the effect of the prebiotic galacto-oligosaccharide, a component of human milk, in the prevention of NEC143. Additionally, clinical trial NCT02405637 aims to evaluate the efficacy of synthetic amniotic fluid in preventing NEC among very-low-birth-weight infants144. Emerging evidence also suggests a prophylactic benefit against the development of NEC by oral administration of lactoferrin with or without probiotics to preterm infants at risk of NEC (gestational age <32 weeks or birth weight <1,500 g)121. Although these findings are encouraging, widespread use of these therapies cannot be recommended at this point, as the current evidence has been determined to be of moderate-to-low quality121 awaiting the completion of ongoing clinical trials145–147.
Figure 1. Factors that attenuate or prevent the development of NEC in experimental models.
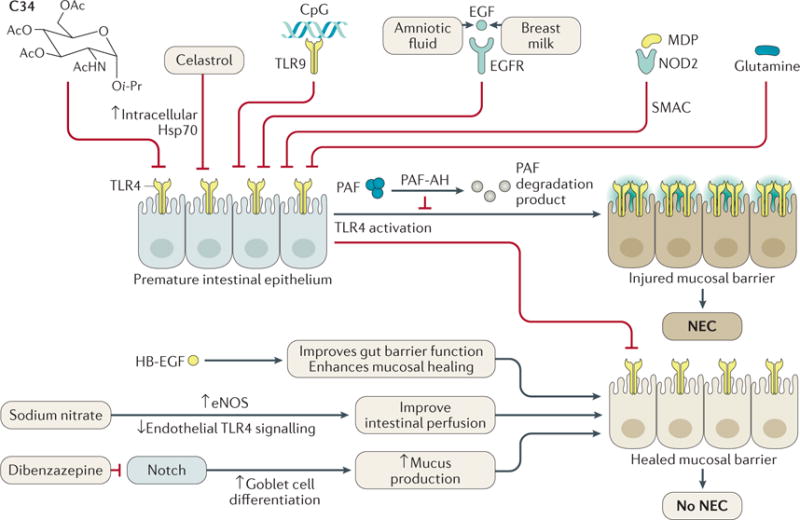
Activation of the innate immune receptor Toll-like receptor 4 (TLR4) plays an essential part in the development of necrotizing enterocolitis (NEC) by increasing enterocyte and intestinal stem cell apoptosis and impairing mucosal healing through decreased restitution and proliferation. These events lead to disruption of the epithelial barrier, which allows luminal bacteria to translocate and trigger a systemic inflammatory response, sepsis, multiple organ failure and death. Counter-regulatory factors can be exploited in order to dampen TLR4 signalling and expression to prevent the development of NEC. Natural factors include: epidermal growth factor (EGF)71,140,185,186, heparin-binding EGF-like growth factor (HB-EGF)186–188, nod-like receptor 2 (NOD2)189, Toll-like receptor 9 (TLR9)67,190, Platelet-activating factor acetylhydrolase (PAF-AH)90. Exogenous factors include: the small-molecule TLR4 inhibitor C34 (REF. 68), bacterial (CpG) DNA67,190, muramyl dipeptide (MDP)189, sodium nitrate66, glutamine191, celastrol (also known as tripterine)192 and dibenzazepine73. EGFR, epidermal growth factor receptor; eNOS, endothelial nitric oxide synthase; Hsp70, heat shock protein 70; PAF, platelet-activating factor; SMAC, second mitochondria-derived activator of caspase (also known as Diablo homolog, mitochondrial).
Conclusions
NEC is the most common and lethal gastrointestinal pathology that afflicts premature infants. Characterized by high morbidity and mortality, complex pathogenesis and devastating short-term and long-term sequelae, it has been dreaded by health-care providers and families for over a century. Only within the past decade have substantial strides been made in the understanding of the molecular mechanisms that determine NEC pathogenesis. These advances undoubtedly hold the promise to improve the development of effective preventive and diagnostic strategies to curtail the devastating consequences of the disease. Although substantial challenges lie ahead to translate the lessons learned at the experimental level, continued translational research efforts will certainly provide avenues to alleviate the healthcare and financial burden attributed to NEC.
Key points.
Necrotizing enterocolitis (NEC) is the most common and devastating gastrointestinal disease affecting premature infants; overall NEC mortality remains unchanged over the past 30 years owing to a lack of treatment options
The main risk factors for the development of NEC are prematurity, bacterial colonization and administration of formula feeds
The premature intestinal epithelium is predisposed to mounting an exaggerated inflammatory response to colonizing bacteria, leading to mucosal destruction and impaired mesenteric perfusion in the pathogenesis of NEC
The exaggerated inflammatory response is partially due to the increased expression of Toll-like receptor 4 (TLR4), which is expressed at high levels on the premature newborn intestinal epithelium
Increased expression of TLR4 in the intestinal epithelium of the premature gut reflects the surprising function that TLR4 plays in the regulation of normal gut development through effects on Notch signalling
Although no specific treatment for NEC exists, several potential biological targets have been identified, including growth factors, modifiers of perfusion and novel TLR4 inhibitors with potential translational importance
Acknowledgments
DJH is supported by R01GM078238 and R01DK083752 from the NIH.
Biographies
Diego F. Niño is currently a research fellow in the Division of Pediatric Surgery at Johns Hopkins University School of Medicine, Baltimore, Maryland, USA. He obtained his MD degree from Universidad Nacional de Colombia, Bogotá, Colombia and his PhD from University of California, San Diego, USA. His clinical and research interests are focused on paediatric gastrointestinal inflammatory diseases.
Chhinder P. Sodhi is Assistant Professor in the Division of Pediatric Surgery at Johns Hopkins University School of Medicine, Baltimore, Maryland, USA. He obtained his PhD degree from the Postgraduate Institute of Medical Education & Research, Chandigarh, India, and obtained postdoctoral training in molecular biology & mouse genetics at Northwestern University and the University of Wisconsin. His current major areas of research focus include necrotizing enterocolitis with a special interest in Toll-like 4 receptor signalling.
David J. Hackam is the Garrett Professor of Pediatric Surgery at the Johns Hopkins University, and Pediatric Surgeon-in-Chief at the Johns Hopkins Children’s Center. He obtained his MD degree from the University of Western Ontario in London, Ontario, Canada and his PhD from the University of Toronto, Canada. His surgical training was completed in Toronto, Canada and Pittsburgh, Pennsylvania, USA. Dr Hackam’s lab focuses on the mechanisms leading to the development of necrotizing enterocolitis (NEC), the effects of NEC on other organs in the body, and the development of strategies to treat this devastating disease.
Footnotes
Author contributions
All authors contributed to the researching, discussion, writing and editing of this manuscript.
Competing interests statement
The authors declare no competing interests.
Subject categories
Health sciences/Gastroenterology/Gastrointestinal diseases/
Intestinal diseases/Infant necrotizing enterocolitis
[URI/692/4020/1503/1581/3189]
Health sciences/Risk factors
[URI/692/499]
Health sciences/Pathogenesis/Inflammation
[URI/692/420/256]
Health sciences/Health care/Therapeutics
[URI/692/700/565]
References
- 1.Stoll BJ, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papillon S, Castle SL, Gayer CP, Ford HR. Necrotizing enterocolitis: contemporary management and outcomes. Adv Pediatr. 2013;60:263–279. doi: 10.1016/j.yapd.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Stey A, et al. Outcomes and costs of surgical treatments of necrotizing enterocolitis. Pediatrics. 2015;135:e1190–1197. doi: 10.1542/peds.2014-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisquera JA, Cooper TR, Berseth CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics. 2002;109:423–428. doi: 10.1542/peds.109.3.423. [DOI] [PubMed] [Google Scholar]
- 5.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salhab WA, Perlman JM, Silver L, Sue Broyles R. Necrotizing enterocolitis and neurodevelopmental outcome in extremely low birth weight infants <1000 g. J Perinatol. 2004;24:534–540. doi: 10.1038/sj.jp.7211165. [DOI] [PubMed] [Google Scholar]
- 7.Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol. 2013;40:27–51. doi: 10.1016/j.clp.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blencowe H, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton BE, Martin JA, Osterman MJK, Curtain SC. Births: preliminary data for 2014. Natl Vital Stat Rep. 2015;64:1–19. [PubMed] [Google Scholar]
- 10.Holman RC, et al. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20:498–506. doi: 10.1111/j.1365-3016.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 11.Guillet R, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006;117:e137–e142. doi: 10.1542/peds.2005-1543. [DOI] [PubMed] [Google Scholar]
- 12.Horbar JD, et al. Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics. 2002;110:143–151. doi: 10.1542/peds.110.1.143. [DOI] [PubMed] [Google Scholar]
- 13.Yee WH, et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129:e298–e304. doi: 10.1542/peds.2011-2022. [DOI] [PubMed] [Google Scholar]
- 14.Luig M, Lui K, NSW & ACT NICUS Group Epidemiology of necrotizing enterocolitis—Part II: risks and susceptibility of premature infants during the surfactant era: a regional study. J Paediatr Child Health. 2005;41:174–179. doi: 10.1111/j.1440-1754.2005.00583.x. [DOI] [PubMed] [Google Scholar]
- 15.Luig M, Lui K, NSW & ACT NICUS Group Epidemiology of necrotizing enterocolitis—Part I: changing regional trends in extremely preterm infants over 14 years. J Paediatr Child Health. 2005;41:169–173. doi: 10.1111/j.1440-1754.2005.00582.x. [DOI] [PubMed] [Google Scholar]
- 16.Sankaran K, et al. Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J Pediatr Gastroenterol Nutr. 2004;39:366–372. doi: 10.1097/00005176-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Stoll BJ, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hossain S, et al. Outborns or inborns: where are the differences a comparison study of very preterm neonatal intensive care unit infants cared for in Australia and New Zealand and in Canada. Neonatology. 2016;109:76–84. doi: 10.1159/000441272. [DOI] [PubMed] [Google Scholar]
- 19.Patole SK, de Klerk N. Impact of standardised feeding regimens on incidence of neonatal necrotising enterocolitis: a systematic review and meta-analysis of observational studies. Arch Dis Child Fetal Neonatal Ed. 2005;90:F147–F151. doi: 10.1136/adc.2004.059741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llanos AR, et al. Epidemiology of neonatal necrotising enterocolitis: a population-based study. Paediatr Perinat Epidemiol. 2002;16:342–349. doi: 10.1046/j.1365-3016.2002.00445.x. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgibbons SC, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg. 2009;44:1072–1075. doi: 10.1016/j.jpedsurg.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Christensen RD, Lambert DK, Baer VL, Gordon PV. Necrotizing enterocolitis in term infants. Clin Perinatol. 2013;40:69–78. doi: 10.1016/j.clp.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Hackam DJ, Afrazi A, Good M, Sodhi CP. Innate immune signaling in the pathogenesis of necrotizing enterocolitis. Clin Dev Immunol. 2013;2013:475415. doi: 10.1155/2013/475415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhandari V, et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006;117:1901–1906. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 25.Sampath V, et al. The NFKB1 (g-24519delATTG) variant is associated with necrotizing enterocolitis (NEC) in premature infants. J Surg Res. 2011;169:e51–e57. doi: 10.1016/j.jss.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Sampath V, et al. SIGIRR genetic variants in premature infants with necrotizing enterocolitis. Pediatrics. 2015;135:e1530–e1534. doi: 10.1542/peds.2014-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou W, et al. Association of neonatal necrotizing enterocolitis with myeloid differentiation-2 and GM2 activator protein genetic polymorphisms. Mol Med Rep. 2015;12:974–980. doi: 10.3892/mmr.2015.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heninger E, et al. Genetic variants of the interleukin-18 promoter region (-607) influence the course of necrotising enterocolitis in very low birth weight neonates. Eur J Pediatr. 2002;161:410–411. doi: 10.1007/s00431-002-0968-y. [DOI] [PubMed] [Google Scholar]
- 29.Treszl A, et al. Lower prevalence of IL-4 receptor alpha-chain gene G variant in very-low-birth-weight infants with necrotizing enterocolitis. J Pediatr Surg. 2003;38:1374–1378. doi: 10.1016/s0022-3468(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 30.Beeby PJ, Jeffery H. Risk factors for necrotising enterocolitis: the influence of gestational age. Arch Dis Child. 1992;67:432–435. doi: 10.1136/adc.67.4_spec_no.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elfvin A, Dinsdale E, Wales PW, Moore AM. Low birthweight, gestational age, need for surgical intervention and gram-negative bacteraemia predict intestinal failure following necrotising enterocolitis. Acta Paediatr. 2015;104:771–776. doi: 10.1111/apa.12997. [DOI] [PubMed] [Google Scholar]
- 32.Collado MC, et al. Factors influencing gastrointestinal tract and microbiota immune interaction in preterm infants. Pediatr Res. 2015;77:726–731. doi: 10.1038/pr.2015.54. [DOI] [PubMed] [Google Scholar]
- 33.Orrhage K, Nord CE. Factors controlling the bacterial colonization of the intestine in breastfed infants. Acta Paediatr Suppl. 1999;88:47–57. doi: 10.1111/j.1651-2227.1999.tb01300.x. [DOI] [PubMed] [Google Scholar]
- 34.Claud EC, Walker WA. Bacterial colonization, probiotics, and necrotizing enterocolitis. J Clin Gastroenterol. 2008;42(Suppl. 2):S46–52. doi: 10.1097/MCG.0b013e31815a57a8. [DOI] [PubMed] [Google Scholar]
- 35.Cobb BA, Carlo WA, Ambalavanan N. Gastric residuals and their relationship to necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2004;113:50–53. doi: 10.1542/peds.113.1.50. [DOI] [PubMed] [Google Scholar]
- 36.Li YF, et al. Gastric residual evaluation in preterm neonates: a useful monitoring technique or a hindrance? Pediatr Neonatol. 2014;55:335–340. doi: 10.1016/j.pedneo.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Good M, Sodhi CP, Hackam DJ. Evidence-based feeding strategies before and after the development of necrotizing enterocolitis. Expert Rev Clin Immunol. 2014;10:875–884. doi: 10.1586/1744666X.2014.913481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fanaro S. Feeding intolerance in the preterm infant. Early Hum Dev. 2013;89(Suppl. 2):S13–S20. doi: 10.1016/j.earlhumdev.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Bell MJ, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maheshwari A, et al. Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr Res. 2014;76:100–108. doi: 10.1038/pr.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng PC. Biomarkers of necrotising enterocolitis. Semin Fetal Neonatal Med. 2014;19:33–38. doi: 10.1016/j.siny.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Berman L, Moss RL. Necrotizing enterocolitis: an update. Semin Fetal Neonatal Med. 2011;16:145–150. doi: 10.1016/j.siny.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Nantais-Smith L, Kadrofske M. Noninvasive biomarkers of necrotizing enterocolitis. J Perinat Neonatal Nurs. 2015;29:69–80. doi: 10.1097/JPN.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 44.Ng PC, Ma TP, Lam HS. The use of laboratory biomarkers for surveillance, diagnosis and prediction of clinical outcomes in neonatal sepsis and necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2015;100:F448–F452. doi: 10.1136/archdischild-2014-307656. [DOI] [PubMed] [Google Scholar]
- 45.Niemarkt HJ, et al. Necrotizing enterocolitis: a clinical review on diagnostic biomarkers and the role of the intestinal microbiota. Inflamm Bowel Dis. 2015;21:436–444. doi: 10.1097/MIB.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 46.Thuijls G, et al. Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Ann Surg. 2010;251:1174–1180. doi: 10.1097/SLA.0b013e3181d778c4. [DOI] [PubMed] [Google Scholar]
- 47.Heida FH, et al. Intestinal fatty acid-binding protein levels in Necrotizing Enterocolitis correlate with extent of necrotic bowel: results from a multicenter study. J Pediatr Surg. 2015;50:1115–1118. doi: 10.1016/j.jpedsurg.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 48.Schurink M, et al. Intestinal fatty acid-binding protein as a diagnostic marker for complicated and uncomplicated necrotizing enterocolitis: a prospective cohort study. PLoS ONE. 2015;10:e0121336. doi: 10.1371/journal.pone.0121336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sylvester KG, et al. A novel urine peptide biomarker-based algorithm for the prognosis of necrotising enterocolitis in human infants. Gut. 2014;63:1284–1292. doi: 10.1136/gutjnl-2013-305130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sylvester KG, et al. Urine protein biomarkers for the diagnosis and prognosis of necrotizing enterocolitis in infants. J Pediatr. 2014;164:607–612.e7. doi: 10.1016/j.jpeds.2013.10.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore JE. Newer monitoring techniques to determine the risk of necrotizing enterocolitis. Clin Perinatol. 2013;40:125–134. doi: 10.1016/j.clp.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Faingold R, et al. Necrotizing enterocolitis: assessment of bowel viability with color doppler US. Radiology. 2005;235:587–594. doi: 10.1148/radiol.2352031718. [DOI] [PubMed] [Google Scholar]
- 53.Yikilmaz A, et al. Prospective evaluation of the impact of sonography on the management and surgical intervention of neonates with necrotizing enterocolitis. Pediatr Surg Int. 2014;30:1231–1240. doi: 10.1007/s00383-014-3613-8. [DOI] [PubMed] [Google Scholar]
- 54.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 55.Shah D, Sinn JK. Antibiotic regimens for the empirical treatment of newborn infants with necrotising enterocolitis. Cochrane Database Syst Rev. 2012;8:CD007448. doi: 10.1002/14651858.CD007448.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Downard CD, et al. Treatment of necrotizing enterocolitis: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. J Pediatr Surg. 2012;47:2111–2122. doi: 10.1016/j.jpedsurg.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Hall NJ, Eaton S, Pierro A. Royal Australasia of Surgeons Guest Lecture. Necrotizing enterocolitis: prevention, treatment, and outcome. J Pediatr Surg. 2013;48:2359–2367. doi: 10.1016/j.jpedsurg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Rao SC, Basani L, Simmer K, Samnakay N, Deshpande G. Peritoneal drainage versus laparotomy as initial surgical treatment for perforated necrotizing enterocolitis or spontaneous intestinal perforation in preterm low birth weight infants. Cochrane Database Syst Rev. 2011:CD006182. doi: 10.1002/14651858.CD006182.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Raval MV, Hall NJ, Pierro A, Moss RL. Evidence-based prevention and surgical treatment of necrotizing enterocolitis-a review of randomized controlled trials. Semin Pediatr Surg. 2013;22:117–121. doi: 10.1053/j.sempedsurg.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 60.Rees CM, et al. Peritoneal drainage or laparotomy for neonatal bowel perforation? A randomized controlled trial. Ann Surg. 2008;248:44–51. doi: 10.1097/SLA.0b013e318176bf81. [DOI] [PubMed] [Google Scholar]
- 61.Henry MC, Moss RL. Neonatal necrotizing enterocolitis. Semin Pediatr Surg. 2008;17:98–109. doi: 10.1053/j.sempedsurg.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 62.Hintz SR, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115:696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 63.Tanner SM, et al. Pathogenesis of necrotizing enterocolitis: modeling the innate immune response. Am J Pathol. 2015;185:4–16. doi: 10.1016/j.ajpath.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sodhi CP, et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired β-catenin signaling in necrotizing enterocolitis. Gastroenterology. 2010;138:185–196. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu P, Sodhi CP, Hackam DJ. Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing enterocolitis. Pathophysiology. 2014;21:81–93. doi: 10.1016/j.pathophys.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yazji I, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci USA. 2013;110:9451–9456. doi: 10.1073/pnas.1219997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gribar SC, et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol. 2009;182:636–646. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neal MD, et al. Discovery and validation of a new class of small molecule Toll-like receptor 4 (TLR4) inhibitors. PLoS ONE. 2013;8:e65779. doi: 10.1371/journal.pone.0065779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Section on B. Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 70.Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2014;4:CD002971. doi: 10.1002/14651858.CD002971.pub3. [DOI] [PubMed] [Google Scholar]
- 71.Good M, et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 2015;8:1166–1179. doi: 10.1038/mi.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leaphart CL, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007;179:4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 73.Sodhi CP, et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology. 2012;143:708–718.e5. doi: 10.1053/j.gastro.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caplan MS, Simon D, Jilling T. The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:145–151. doi: 10.1053/j.sempedsurg.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Cetin S, et al. Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J Biol Chem. 2004;279:24592–24600. doi: 10.1074/jbc.M313620200. [DOI] [PubMed] [Google Scholar]
- 76.Reisinger KW, et al. Breast-feeding improves gut maturation compared with formula feeding in preterm babies. J Pediatr Gastroenterol Nutr. 2014;59:720–724. doi: 10.1097/MPG.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 77.Lebenthal A, Lebenthal E. The ontogeny of the small intestinal epithelium. J Parenter Enteral Nutr. 1999;23:S3–S6. doi: 10.1177/014860719902300502. [DOI] [PubMed] [Google Scholar]
- 78.Berseth CL. Gastrointestinal motility in the neonate. Clin Perinatol. 1996;23:179–190. [PubMed] [Google Scholar]
- 79.Berseth CL. Gestational evolution of small intestine motility in preterm and term infants. J Pediatr. 1989;115:646–651. doi: 10.1016/s0022-3476(89)80302-6. [DOI] [PubMed] [Google Scholar]
- 80.Young H, Beckett E, Bornstein J, Jadcherla S. In: Pediatric Neurogastroenterology Clinical Gastroenterology. Faure C, Di Lorenzo C, Thapar N, editors. Humana Press; 2013. pp. 23–35. Ch. 3. [Google Scholar]
- 81.Afrazi A, et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J Biol Chem. 2014;289:9584–9599. doi: 10.1074/jbc.M113.526517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001;73:1131S–1141S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- 83.Hackam DJ, Upperman JS, Grishin A, Ford HR. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:49–57. doi: 10.1053/j.sempedsurg.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 84.Watkins DJ, Besner GE. The role of the intestinal microcirculation in necrotizing enterocolitis. Semin Pediatr Surg. 2013;22:83–87. doi: 10.1053/j.sempedsurg.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anand RJ, Leaphart CL, Mollen KP, Hackam DJ. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock. 2007;27:124–133. doi: 10.1097/01.shk.0000239774.02904.65. [DOI] [PubMed] [Google Scholar]
- 86.Liu Z, Li N, Neu J. Tight junctions, leaky intestines, and pediatric diseases. Acta Paediatr. 2005;94:386–393. doi: 10.1111/j.1651-2227.2005.tb01904.x. [DOI] [PubMed] [Google Scholar]
- 87.Dingle BM, et al. FoxP3+ regulatory T cells attenuate experimental necrotizing enterocolitis. PLoS ONE. 2013;8:e82963. doi: 10.1371/journal.pone.0082963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weitkamp JH, et al. Small intestinal intraepithelial TCRγδ+ T lymphocytes are present in the premature intestine but selectively reduced in surgical necrotizing enterocolitis. PLoS ONE. 2014;9:e99042. doi: 10.1371/journal.pone.0099042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Egan CE, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest. 2016;126:495–508. doi: 10.1172/JCI83356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frost BL, Caplan MS. Necrotizing enterocolitis: pathophysiology, platelet-activating factor, and probiotics. Semin Pediatr Surg. 2013;22:88–93. doi: 10.1053/j.sempedsurg.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 91.Maheshwari A. Immunologic and hematological abnormalities in necrotizing enterocolitis. Clin Perinatol. 2015;42:567–585. doi: 10.1016/j.clp.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Soliman A, et al. Platelet-activating factor induces TLR4 expression in intestinal epithelial cells: implication for the pathogenesis of necrotizing enterocolitis. PLoS ONE. 2010;5:e15044. doi: 10.1371/journal.pone.0015044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rabinowitz SS, et al. Platelet-activating factor in infants at risk for necrotizing enterocolitis. J Pediatr. 2001;138:81–86. doi: 10.1067/mpd.2001.110132. [DOI] [PubMed] [Google Scholar]
- 94.Chatterton DE, Nguyen DN, Bering SB, Sangild PT. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int J Biochem Cell Biol. 2013;45:1730–1747. doi: 10.1016/j.biocel.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 95.Furukawa M, Narahara H, Yasuda K, Johnston JM. Presence of platelet-activating factor-acetylhydrolase in milk. J Lipid Res. 1993;34:1603–1609. [PubMed] [Google Scholar]
- 96.Maheshwari A, et al. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology. 2011;140:242–253. doi: 10.1053/j.gastro.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Namachivayam K, et al. Smad7 inhibits autocrine expression of TGF-β2 in intestinal epithelial cells in baboon necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2013;304:G167–G180. doi: 10.1152/ajpgi.00141.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.MohanKumar K, et al. Smad7 interrupts TGF-β signaling in intestinal macrophages and promotes inflammatory activation of these cells during necrotizing enterocolitis. Pediatr Res. 2016;79:951–961. doi: 10.1038/pr.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McElroy SJ, Underwood MA, Sherman MP. Paneth cells and necrotizing enterocolitis: a novel hypothesis for disease pathogenesis. Neonatology. 2013;103:10–20. doi: 10.1159/000342340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Underwood MA. Paneth cells and necrotizing enterocolitis. Gut Microbes. 2012;3:562–565. doi: 10.4161/gmic.21738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang C, et al. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis Model Mech. 2012;5:522–532. doi: 10.1242/dmm.009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tanabe H, et al. Mouse paneth cell secretory responses to cell surface glycolipids of virulent and attenuated pathogenic bacteria. Infect Immun. 2005;73:2312–2320. doi: 10.1128/IAI.73.4.2312-2320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wolfs TG, et al. Localization of the lipopolysaccharide recognition complex in the human healthy and inflamed premature and adult gut. Inflamm Bowel Dis. 2010;16:68–75. doi: 10.1002/ibd.20995. [DOI] [PubMed] [Google Scholar]
- 104.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 105.Vongbhavit K, Underwood MA. Prevention of necrotizing enterocolitis through manipulation of the intestinal microbiota of the premature infant. Clin Ther. 2016;38:716–732. doi: 10.1016/j.clinthera.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arboleya S, et al. Deep 16S rRNA metagenomics and quantitative PCR analyses of the premature infant fecal microbiota. Anaerobe. 2012;18:378–380. doi: 10.1016/j.anaerobe.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 107.La Rosa PS, et al. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci USA. 2014;111:12522–12527. doi: 10.1073/pnas.1409497111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carlisle EM, Morowitz MJ. The intestinal microbiome and necrotizing enterocolitis. Curr Opin Pediatr. 2013;25:382–387. doi: 10.1097/MOP.0b013e3283600e91. [DOI] [PubMed] [Google Scholar]
- 109.Torrazza RM, Neu J. The altered gut microbiome and necrotizing enterocolitis. Clin Perinatol. 2013;40:93–108. doi: 10.1016/j.clp.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Elgin TG, Kern SL, McElroy SJ. Development of the neonatal intestinal microbiome and its association with necrotizing enterocolitis. Clin Ther. 2016;38:706–715. doi: 10.1016/j.clinthera.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 111.Patel RM, Denning PW. Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr Res. 2015;78:232–238. doi: 10.1038/pr.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 113.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 114.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 115.Coggins SA, Wynn JL, Weitkamp JH. Infectious causes of necrotizing enterocolitis. Clin Perinatol. 2015;42:133–154. ix. doi: 10.1016/j.clp.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Neu J. Preterm infant nutrition, gut bacteria, and necrotizing enterocolitis. Curr Opin Clin Nutr Metab Care. 2015;18:285–288. doi: 10.1097/MCO.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grishin A, Papillon S, Bell B, Wang J, Ford HR. The role of the intestinal microbiota in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2013;22:69–75. doi: 10.1053/j.sempedsurg.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brower-Sinning R, et al. Mucosa-associated bacterial diversity in necrotizing enterocolitis. PLoS ONE. 2014;9:e105046. doi: 10.1371/journal.pone.0105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McElroy SJ. The role of bacteria in necrotizing enterocolitis: understanding the forest for the trees. Neonatology. 2015;108:196–197. doi: 10.1159/000437205. [DOI] [PubMed] [Google Scholar]
- 120.Lu P, et al. Animal models of gastrointestinal and liver diseases. Animal models of necrotizing enterocolitis: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;306:G917–G928. doi: 10.1152/ajpgi.00422.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pammi M, Abrams SA. Oral lactoferrin for the prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2015;2:CD007137. doi: 10.1002/14651858.CD007137.pub4. [DOI] [PubMed] [Google Scholar]
- 122.Lonnerdal B. Bioactive proteins in human milk: mechanisms of action. J Pediatr. 2010;156:S26–30. doi: 10.1016/j.jpeds.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 123.Neal MD, et al. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J Biol Chem. 2012;287:37296–37308. doi: 10.1074/jbc.M112.375881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou P, Li Y, Ma LY, Lin HC. The role of immunonutrients in the prevention of necrotizing enterocolitis in preterm very low birth weight infants. Nutrients. 2015;7:7256–7270. doi: 10.3390/nu7095334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sullivan S, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156:562–567.e1. doi: 10.1016/j.jpeds.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 126.Gephart SM, McGrath JM, Effken JA, Halpern MD. Necrotizing enterocolitis risk: state of the science. Adv Neonatal Care. 2012;12:77–87. doi: 10.1097/ANC.0b013e31824cee94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Neu J. Probiotics and necrotizing enterocolitis. Clin Perinatol. 2014;41:967–978. doi: 10.1016/j.clp.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Robinson J. Cochrane in context: probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid Based Child Health. 2014;9:672–674. doi: 10.1002/ebch.1977. [DOI] [PubMed] [Google Scholar]
- 129.Fleming P, Hall NJ, Eaton S. Probiotics and necrotizing enterocolitis. Pediatr Surg Int. 2015;31:1111–1118. doi: 10.1007/s00383-015-3790-0. [DOI] [PubMed] [Google Scholar]
- 130.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid Based Child Health. 2014;9:584–671. doi: 10.1002/ebch.1976. [DOI] [PubMed] [Google Scholar]
- 131.Floch MH, et al. Recommendations for probiotic use — 2015 update: proceedings and consensus opinion. J Clin Gastroenterol. 2015;49(Suppl. 1):S69–S73. doi: 10.1097/MCG.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 132.Houghteling PD, Walker WA. From birth to “immunohealth, ” allergies and enterocolitis. J Clin Gastroenterol. 2015;49(Suppl. 1):S7–S12. doi: 10.1097/MCG.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shaffiey SA, et al. Intestinal stem cell growth and differentiation on a tubular scaffold with evaluation in small and large animals. Regen Med. 2016;11:45–61. doi: 10.2217/rme.15.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Good M, et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am J Physiol Gastrointest Liver Physiol. 2014;306:G1021–G1032. doi: 10.1152/ajpgi.00452.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Su Y, Yang J, Besner GE. HB-EGF promotes intestinal restitution by affecting integrin-extracellular matrix interactions and intercellular adhesions. Growth Factors. 2013;31:39–55. doi: 10.3109/08977194.2012.755966. [DOI] [PubMed] [Google Scholar]
- 136.Chen CL, et al. Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab Invest. 2012;92:331–344. doi: 10.1038/labinvest.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang J, Su Y, Zhou Y, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) therapy for intestinal injury: application and future prospects. Pathophysiology. 2014;21:95–104. doi: 10.1016/j.pathophys.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Newburg DS. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. J Anim Sci. 2009;87:26–34. doi: 10.2527/jas.2008-1347. [DOI] [PubMed] [Google Scholar]
- 139.Jantscher-Krenn E, et al. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut. 2012;61:1417–1425. doi: 10.1136/gutjnl-2011-301404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Good M, et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci USA. 2012;109:11330–11335. doi: 10.1073/pnas.1200856109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jain SK, et al. Amniotic fluid-borne hepatocyte growth factor protects rat pups against experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G361–G369. doi: 10.1152/ajpgi.00272.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ostergaard MV, et al. Modulation of intestinal inflammation by minimal enteral nutrition with amniotic fluid in preterm pigs. J Parenter Enteral Nutr. 2014;38:576–586. doi: 10.1177/0148607113489313. [DOI] [PubMed] [Google Scholar]
- 143.US National Library of Medicine. ClinicalTrials.gov. 2007 https://clinicaltrials.gov/ct2/show/NCT00437567.
- 144.US National Library of Medicine. ClinicalTrials.gov. 2015 https://clinicaltrials.gov/ct2/show/NCT02405637.
- 145.US National Library of Medicine. ClinicalTrials.gov. 2012 https://clinicaltrials.gov/ct2/show/NCT01525316.
- 146.ISRCTN registry. BioMed Central. 2013 http://www.isrctn.com/ISRCTN88261002.
- 147.ISRCTN registry. BioMed Central. 2012 http://www.isrctn.com/ISRCTN66482337.
- 148.Guthrie SO, et al. Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003;23:278–285. doi: 10.1038/sj.jp.7210892. [DOI] [PubMed] [Google Scholar]
- 149.Cristofalo EA, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163:1592–1595.e1. doi: 10.1016/j.jpeds.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 150.Boyd CA, Quigley MA, Brocklehurst P. Donor breast milk versus infant formula for preterm infants: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2007;92:F169–F175. doi: 10.1136/adc.2005.089490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fisher JG, et al. Serious congenital heart disease and necrotizing enterocolitis in very low birth weight neonates. J Am Coll Surg. 2015;220:1018–1026.e14. doi: 10.1016/j.jamcollsurg.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 152.Dollberg S, Lusky A, Reichman B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study. J Pediatr Gastroenterol Nutr. 2005;40:184–188. doi: 10.1097/00005176-200502000-00019. [DOI] [PubMed] [Google Scholar]
- 153.Bergholz R, Boettcher M, Reinshagen K, Wenke K. Complex gastroschisis is a different entity to simple gastroschisis affecting morbidity and mortality-a systematic review and meta-analysis. J Pediatr Surg. 2014;49:1527–1532. doi: 10.1016/j.jpedsurg.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 154.Sinkey RG, et al. Sonographic markers associated with adverse neonatal outcomes among fetuses with gastroschisis: an 11-year, single-center review. Am J Obstet Gynecol. 2016;214:275.e1–7. doi: 10.1016/j.ajog.2015.09.081. [DOI] [PubMed] [Google Scholar]
- 155.Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/ or low birth weight infants. Cochrane Database Syst Rev. 2010:CD003481. doi: 10.1002/14651858.CD003481.pub4. [DOI] [PubMed] [Google Scholar]
- 156.Cotten CM, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sood BG, et al. The risk of necrotizing enterocolitis after indomethacin tocolysis. Pediatrics. 2011;128:e54–62. doi: 10.1542/peds.2011-0265. [DOI] [PubMed] [Google Scholar]
- 158.Patel RM, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA. 2016;315:889–897. doi: 10.1001/jama.2016.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Desfrere L, et al. Increased incidence of necrotizing enterocolitis in premature infants born to HIV-positive mothers. AIDS. 2005;19:1487–1493. doi: 10.1097/01.aids.0000183123.09206.07. [DOI] [PubMed] [Google Scholar]
- 160.Schmitz T, Weizsaecker K, Feiterna-Sperling C, Eilers E, Obladen M. Exposure to HIV and antiretroviral medication as a potential cause of necrotizing enterocolitis in term neonates. AIDS. 2006;20:1082–1083. doi: 10.1097/01.aids.0000222089.74905.0f. [DOI] [PubMed] [Google Scholar]
- 161.Stout G, et al. Necrotizing enterocolitis during the first week of life: a multicentered case-control and cohort comparison study. J Perinatol. 2008;28:556–560. doi: 10.1038/jp.2008.36. [DOI] [PubMed] [Google Scholar]
- 162.Hallstrom M, Koivisto AM, Janas M, Tammela O. Frequency of and risk factors for necrotizing enterocolitis in infants born before 33 weeks of gestation. Acta Paediatr. 2003;92:111–113. doi: 10.1111/j.1651-2227.2003.tb00479.x. [DOI] [PubMed] [Google Scholar]
- 163.Wada Y, Lonnerdal B. Bioactive peptides derived from human milk proteins—mechanisms of action. J Nutr Biochem. 2014;25:503–514. doi: 10.1016/j.jnutbio.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 164.Amin HJ, et al. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J Pediatr. 2002;140:425–431. doi: 10.1067/mpd.2002.123289. [DOI] [PubMed] [Google Scholar]
- 165.Polycarpou E, et al. Enteral L-arginine supplementation for prevention of necrotizing enterocolitis in very low birth weight neonates: a double-blind randomized pilot study of efficacy and safety. J Parenter Enteral Nutr. 2013;37:617–622. doi: 10.1177/0148607112471561. [DOI] [PubMed] [Google Scholar]
- 166.Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha1, 2Galβ1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. 2003;278:14112–14120. doi: 10.1074/jbc.M207744200. [DOI] [PubMed] [Google Scholar]
- 167.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. 2005;25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553. [DOI] [PubMed] [Google Scholar]
- 168.Pacheco AR, Barile D, Underwood MA, Mills DA. The impact of the milk glycobiome on the neonate gut microbiota. Annu Rev Anim Biosci. 2015;3:419–445. doi: 10.1146/annurev-animal-022114-111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sherman MP. Lactoferrin and necrotizing enterocolitis. Clin Perinatol. 2013;40:79–91. doi: 10.1016/j.clp.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Buccigrossi V, et al. Lactoferrin induces concentration-dependent functional modulation of intestinal proliferation and differentiation. Pediatr Res. 2007;61:410–414. doi: 10.1203/pdr.0b013e3180332c8d. [DOI] [PubMed] [Google Scholar]
- 171.Manzoni P, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302:1421–1428. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 172.Sharma D, Shastri S. Lactoferrin and neonatology – role in neonatal sepsis and necrotizing enterocolitis: present, past and future. J Matern Fetal Neonatal Med. 2016;29:763–770. doi: 10.3109/14767058.2015.1017463. [DOI] [PubMed] [Google Scholar]
- 173.Rogier EW, et al. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci USA. 2014;111:3074–3079. doi: 10.1073/pnas.1315792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dvorak B. Milk epidermal growth factor and gut protection. J Pediatr. 2010;156:S31–35. doi: 10.1016/j.jpeds.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nair RR, Warner BB, Warner BW. Role of epidermal growth factor and other growth factors in the prevention of necrotizing enterocolitis. Semin Perinatol. 2008;32:107–113. doi: 10.1053/j.semperi.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 176.Dvorak B, et al. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol. 2002;282:G156–G164. doi: 10.1152/ajpgi.00196.2001. [DOI] [PubMed] [Google Scholar]
- 177.Yu X, Radulescu A, Zorko N, Besner GE. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology. 2009;137:221–230. doi: 10.1053/j.gastro.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Michalsky MP, Lara-Marquez M, Chun L, Besner GE. Heparin-binding EGF-like growth factor is present in human amniotic fluid and breast milk. J Pediatr Surg. 2002;37:1–6. doi: 10.1053/jpsu.2002.29415. [DOI] [PubMed] [Google Scholar]
- 179.Lara-Marquez ML, Mehta V, Michalsky MP, Fleming JB, Besner GE. Heparin-binding EGF-like growth factor down regulates proinflammatory cytokine-induced nitric oxide and inducible nitric oxide synthase production in intestinal epithelial cells. Nitric Oxide. 2002;6:142–152. doi: 10.1006/niox.2001.0393. [DOI] [PubMed] [Google Scholar]
- 180.Yu Y, et al. Erythropoietin protects epithelial cells from excessive autophagy and apoptosis in experimental neonatal necrotizing enterocolitis. PLoS ONE. 2013;8:e69620. doi: 10.1371/journal.pone.0069620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Karadag A, et al. Protective effects of dexpanthenol in an experimental model of necrotizing enterocolitis. J Pediatr Surg. 2015;50:1119–1124. doi: 10.1016/j.jpedsurg.2014.10.053. [DOI] [PubMed] [Google Scholar]
- 182.Halpern MD, Dvorak B. Does abnormal bile acid metabolism contribute to NEC? Semin Perinatol. 2008;32:114–121. doi: 10.1053/j.semperi.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hunter CJ, Upperman JS, Ford HR, Camerini V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC) Pediatr Res. 2008;63:117–123. doi: 10.1203/PDR.0b013e31815ed64c. [DOI] [PubMed] [Google Scholar]
- 184.Liu Y, Tran DQ, Fatheree NY, Marc Rhoads J. Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G177–G186. doi: 10.1152/ajpgi.00038.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Coursodon CF, Dvorak B. Epidermal growth factor and necrotizing enterocolitis. Curr Opin Pedriatr. 2012;24:160–164. doi: 10.1097/MOP.0b013e3283504ddb. [DOI] [PubMed] [Google Scholar]
- 186.Rowland KJ, et al. The role of growth factors in intestinal regeneration and repair in necrotizing enterocolitis. Sem Pediatr Surg. 2013;22:101–111. doi: 10.1053/j.sempedsurg.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Feng J, et al. Heparin-binding epidermal growth factor-like growth factor reduces intestinal apoptosis in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2006;41:742–747. doi: 10.1016/j.jpedsurg.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 188.Feng J, et al. Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2006;41:144–149. doi: 10.1016/j.jpedsurg.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 189.Richardson WM, et al. Nucleotide-binding oligomerization domain-2 inhibits toll-like receptor-4 signaling in the intestinal epithelium. Gastroenterology. 2010;139:904–917. doi: 10.1053/j.gastro.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–53. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 191.Zhou W, et al. Glutamine downregulates TLR-2 and TLR-4 expression and protects intestinal tract in preterm neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2014;49:1057–1063. doi: 10.1016/j.jpedsurg.2014.02.078. [DOI] [PubMed] [Google Scholar]
- 192.Afrazi A, et al. Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium. J Immunol. 2009;188:4543–4557. doi: 10.4049/jimmunol.1103114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Neu J. Necrotizing enterocolitis: the search for a unifying pathogenic theory leading to prevention. Pediatr Clin North Am. 1996;43:409–432. doi: 10.1016/S0031-3955(05)70413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Thyoka M, et al. Advanced necrotizing enterocolitis part 2: recurrence of necrotizing enterocolitis. Eur J Pediatr Surg. 2012;22:13–16. doi: 10.1055/s-0032-1306264. [DOI] [PubMed] [Google Scholar]
- 195.Stringer MD, et al. Recurrent necrotizing enterocolitis. J Pediatr Surg. 1993;28:979–981. doi: 10.1016/0022-3468(93)90496-8. [DOI] [PubMed] [Google Scholar]
- 196.Thyoka M, et al. Advanced necrotizing enterocolitis part 1: mortality. Eur J Pediatr Surg. 2012;22:8–12. doi: 10.1055/s-0032-1306263. [DOI] [PubMed] [Google Scholar]
- 197.Heida FH, et al. Risk factors associated with postnecrotizing enterocolitis strictures in infants. J Pediatr Surg. 2015 doi: 10.1016/j.jpedsurg.2015.09.015. http://dx.doi.org/10.1016/j.jpedsurg.2015.09.015. [DOI] [PubMed]
- 198.Kastenberg ZJ, Sylvester KG. The surgical management of necrotizing enterocolitis. Clin Perinatol. 2013;40:135–148. doi: 10.1016/j.clp.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 199.Aguayo P, Fraser JD, Sharp S, St Peter SD, Ostlie DJ. Stomal complications in the newborn with necrotizing enterocolitis. J Surg Res. 2009;157:275–278. doi: 10.1016/j.jss.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 200.Amin SC, Pappas C, Iyengar H, Maheshwari A. Short bowel syndrome in the NICU. Clin Perinatol. 2013;40:53–68. doi: 10.1016/j.clp.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Cole CR, et al. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics. 2008;122:e573–e582. doi: 10.1542/peds.2007-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schulzke SM, Deshpande GC, Patole SK. Neurodevelopmental outcomes of very low-birth-weight infants with necrotizing enterocolitis: a systematic review of observational studies. Arch Pediatr Adolesc Med. 2007;161:583–590. doi: 10.1001/archpedi.161.6.583. [DOI] [PubMed] [Google Scholar]
- 203.Lee I, et al. The impact of prenatal and neonatal infection on neurodevelopmental outcomes in very preterm infants. J Perinatol. 2014;34:741–747. doi: 10.1038/jp.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wadhawan R, et al. Neurodevelopmental outcomes of extremely low birth weight infants with spontaneous intestinal perforation or surgical necrotizing enterocolitis. J Perinatol. 2014;34:64–70. doi: 10.1038/jp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]


