Abstract
Background
Renal ischemia-reperfusion (IR) contributes to the development of acute renal failure (ARF). Oxygen free radicals are considered to be the principal components involved in the pathophysiological tissue alterations observed during renal IR.
Objectives
In this study, we compared the effects of melatonin (MEL) and erythropoietin (EPO), both known antioxidant and anti-inflammatory agents, on IR-induced renal injury in rats.
Materials and Methods
Wistar albino rats were unilaterally nephrectomized and then subjected to 45 minutes of renal pedicle occlusion followed by 24 hours of reperfusion. MEL (10 mg/kg, i.p) and EPO (5000 U/kg, i.p) were administered prior to the onset of ischemia. After 24 hours of reperfusion and following decapitation, blood samples were collected for the determination of the hemoglobin (Hb) and hematocrit (Hct) levels. Additionally, renal samples were taken for histological evaluation.
Results
Ischemia-reperfusion significantly decreased the observed Hb and Hct values. The histopathological findings in the IR group confirmed that there was an increase in the hyaline cast and thickening of the Bowman capsule basement membrane. Treatment with EPO or MEL significantly increased the Hb and Hct values. In the MEL + IR group, the histopathological changes were lower than those found in the EPO + IR group.
Conclusions
Treatment with EPO and MEL had a beneficial effect on renal IR injury. The results may also indicate that MEL protects against morphological damage better than EPO in renal IR injury.
Keywords: Melatonin, Erythropoietin, Ischemia Reperfusion Injury, Kidney
1. Background
Ischemia (cessation of blood flow) followed by reperfusion (re-establishment of the blood supply) causes serious damage to tissues and organs (1). Ischemia compromises the continuous supply of oxygen required by tissues to survive and maintain physiological function. Ischemia of the kidney is a common problem during kidney transplantation, partial nephrectomy, cardiopulmonary bypass, and hydronephrosis, which leads to renal dysfunction and injury (2). Additionally, when reperfusion takes place, additional renal reperfusion-related injury occurs. This involves the development of oxidative stress via the generation of superoxide anions (O2-) (3). The generation of reactive oxygen species (ROS) such as O2- and the hydroxyl radical (OH), as well as reactive nitrogen species (RNS) such as nitric oxide (NO) and peroxynitrite (OONO-), or a decline in antioxidant defenses lead to oxidative stress, which in turn plays a critical role in the development of renal ischemia-reperfusion (IR) injury and ischemic acute renal failure (ARF) (4).
Erythropoietin (EPO) is a hypoxia-inducible hematopoietic factor that is predominantly expressed in the kidney. It has multiple protective effects, including antioxidant, anti-apoptotic, pleiotropic, and anti-inflammatory effects (5, 6). The biological effects of EPO are mediated by binding to its specific cell surface receptor (EPOR), and the presence of functional EPOR in renal mesangial and tubular epithelial cells indicates a potential role for erythropoietin in the kidney (7, 8). It has also been revealed that the renal EPO level decreased following renal ischemia-reperfusion (9).
Melatonin (N-acetyl-5-methoxytryptamine) is the major product of the pineal gland, which functions as a regulator of sleep, circadian rhythm, and immune function. Melatonin (MEL) and its metabolites have potent antioxidant/anti-inflammatory properties, and they have proven to be highly effective in a variety of disorders linked to inflammation and oxidative stress (10, 11). MEL not only neutralizes RNS and ROS, but also acts through the stimulation of several antioxidative enzymatic systems and stabilizing cell membranes (12). Therefore, ROS were shown to contribute to the cellular damage induced by ischemia-reperfusion.
2. Objectives
The purpose of this study was to investigate and compare the efficiency of EPO and MEL in the reduction of injury caused by renal ischemia-reperfusion using both biochemical and histological parameters.
3. Materials and Methods
3.1. Animals
In this study, 40 male Wistar albino rats (weighing 200 - 300 g) were obtained from the experimental animal research center. The rats were housed at a temperature (21 ± 2°C) and humidity (60 ± 5%) controlled room in which a 12-12 hour light-dark cycle was maintained. They had free access to standard water and food. The study was approved by the ethics committee of Tabriz university.
3.2. Surgery and Experimental Protocol
Under anesthesia (75 mg/kg ketamine hydrochloride and 8 mg/kg xylazine, intraperitoneal injection), a right nephrectomy was performed. Next, the left renal pedicle (artery and vein) was occluded using an atraumatic microvascular clamp for 45 minutes to induce ischemia. It was then subjected to reperfusion for 24 hours.
The rats were divided into four groups. The sham group (n = 10) underwent a nephrectomy without occlusion. The other three groups were: IR group (ischemic control, n = 10), MEL + IR group (n = 10), and EPO + IR group (n = 10).
MEL (10 mg/kg; i.p) or a vehicle (1% alcohol in saline) was administered 10 minutes prior to the onset of ischemia. The MEL (Sigma, St. Louis, MO, USA) was dissolved in absolute ethanol and then diluted in saline to give a final alcohol concentration of 1% ethanol. EPO (NeoRecormon, Roche, Mannheim, Germany) was administered as a 5000 U/kg single dose intraperitoneally 10 min before the onset of ischemia.
3.3. Biochemical Analysis
The blood samples and left kidney tissues of the rats were obtained after 24 hours of reperfusion in each group. The kidneys were removed and weighed. The blood samples were centrifuged at approximately 4000 g for 10 min at 4°C. The Hb and Hct readings were also recorded for all the rats. The hemoglobin concentration in mg/mL was measured according to the cyanmethemoglobin method (13).
3.4. Histological Evaluation
The left renal tissues were fixed in 10% buffered formalin solution, dehydrated in ascending grades of alcohol, and embedded in paraffin. Sections of 5 μm were taken, stained with periodic acid Schiff (PAS), and then examined under a light microscope (Olympus BH-2, Tokyo, Japan) in a blinded manner by a pathologist. The renal tissues were evaluated in terms of the increase in the hyaline cast and thickening of the Bowman capsule basement membrane. The histological changes were scored on a four-point scale: (-) none, (+) mild, (++) moderate, or (+++) severe damage (14).
3.5. Statistical Analysis
All the data are presented as the mean ± standard deviation (SD). The significance testing between the groups was performed using a one-way analysis of variance (ANOVA) with SPSS version 19.0, while a multiple comparison post hoc test was used to determine significant differences between the groups. A P-value of less than 0.05 was considered to be statistically significant.
4. Results
The effect of EPO and MEL on renal ischemia-reperfusion injury was investigated in 45 minutes of renal ischemia followed by 24 hours of reperfusion. The biochemical analysis results are outlined in Table 1, while the results of the histological evaluation are shown in Tables 2 and 3.
Table 1. Biochemical Measurements After 24 hours of Reperfusion.
| Sham Group | IR Group | MEL + IR Group | EPO + IR Group | |
|---|---|---|---|---|
| RKW (% body wt) | 0.38 ± 0.03 | 0.41 ± 0.04 | 0.42 ± 0.05 | 0.43 ± 0.06 |
| Hct (%) | 44.00 ± 3.40 | 40.36 ± 4.43a | 46.10 ± 3.66b | 47.20 ± 3.46b |
| Hb (g/dl) | 14.66 ± 1.13 | 13.45 ± 1.48a | 15.36 ± 1.22b | 15.75 ± 1.10b |
Abbreviations: EPO, erythropoietin; Hb, hemoglobin; Hct, hematocrit; IR, ischemia reperfusion; MEL, melatonin;.RKW, relative kidney weight.
aSignificantly decreased when compared with the sham group (P < 0.05).
bSignificantly increased when compared with the IR group (P < 0.05).
Table 2. Comparison of the Thickening of the Bowman Capsule Basement Membrane After 24 hours of Reperfusion (Periodic Acid Schiff)a.
| Groups | Sham | IR | MEL | EPO |
|---|---|---|---|---|
| Thickening | + | ++++ | + | ++ |
aThe thickening of the Bowman capsule basement membrane was recorded as: (+) normal, (++) thick, (+++) very thick, or (++++) extremely thick (n = 7 for each group).
Table 3. Comparison of the Hyaline Cast After 24 hours of Reperfusion (Periodic Acid Schiff)a.
| Groups | Sham | IR | MEL | EPO |
|---|---|---|---|---|
| Increase in hyaline cast | - | +++ | + | ++ |
aA minimum of 10 fields for each kidney slide were examined and assigned based on the severity of the hyaline cast increase using scores on a scale of: (–) none, (+) mild, (++) moderate, or (+++) severe damage (n = 7 for each group).
4.1. Effects of Ischemia-Reperfusion
The relative kidney weight in the IR group was higher than that in the sham group, although the difference was not statistically significant (P > 0.05). The Hb and Hct values in the IR group were significantly lower than those in the sham group (P < 0.05).
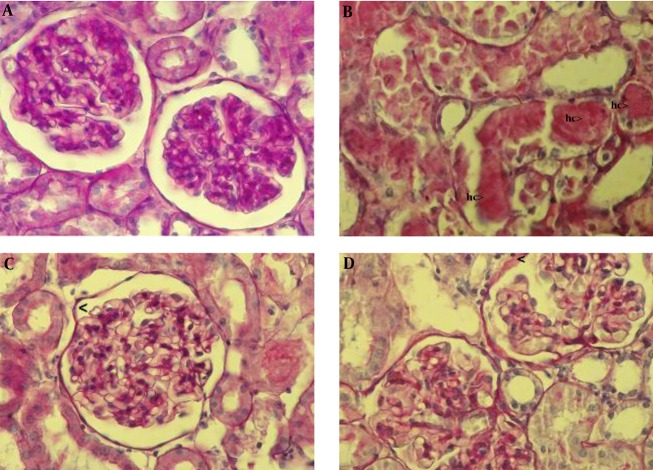
In the IR group, renal injury was very obvious. There was a thickening of the Bowman capsule basement membrane and an increase in the hyaline cast (Figure 1B).
Figure 1. Histopathological evaluation of rat kidneys after 45 minutes of ischemia followed by 24 hours reperfusion. The kidney sections were stained by periodic acid Schiff (PAS) and then examined using a light microscope. (A) The normal renal tissue structure in the sham group (40 × PAS). (B) Thickening of the Bowman capsule basement membrane and hyaline cast (hc) in the IR group (40 × PAS). (C) The normal Bowman capsule basement membrane in the MEL group (40 × PAS). (D) Thickening of the Bowman capsule basement membrane in the EPO group (40 × PAS).
4.2. Effects of Melatonin on Renal Ischemia-Reperfusion
The relative kidney weight in the MEL + IR group was higher than that in the IR group, although the difference was not statistically significant (P > 0.05). The Hb and Hct values in the MEL + IR group were significantly higher than those in the IR group (P < 0.001).
Melatonin pretreatment resulted in the marked attenuation of the increase in the hyaline cast, alongside the absence of a thickening of the Bowman capsule basement membrane induced by ischemia-reperfusion (Figure 1C).
4.3. Effects of Erythropoietin on Renal Ischemia-Reperfusion
The relative kidney weight in the EPO + IR group was higher than that in the IR group, although the difference was not statistically significant (P > 0.05). The Hb and Hct values in the EPO + IR group were significantly higher than those in the IR group (P < 0.001).
Erythropoietin pretreatment moderately prevented an increase in the hyaline cast and a thickening of the Bowman capsule basement membrane (Figure 1D).
5. Discussion
The results of this study indicate that melatonin has greater protective effects than erythropoietin on IR-induced renal injury in rats, since MEL attenuated the histopathological changes associated with renal IR injury better than EPO. Renal IR is a common result of clinical procedures such as partial nephrectomy, vascular surgery, or transplantation. Furthermore, renal IR injury is a leading cause of ARF, which is associated with high mortality rates. ARF is characterized by increased vascular resistance in the kidney, a low rate of filtration through the glomeruli, and tubular necrosis. These deleterious effects have been attributed to the generation of ROS during renal reperfusion (15).
In the present study, the Hb and Hct values were significantly reduced by renal IR. It was revealed that the renal EPO level decreased after renal ischemia-reperfusion. EPO is a hematopoietic growth factor that increases the values of Hb and Hct. Thus, this effect of IR may be due to the decrease in the renal EPO level following renal IR. We explored how EPO and MEL significantly increased the Hb and Hct values. Labonia et al. (16) reported that the mean Hb and Hct values were significantly increased by melatonin treatment.
Our data showed that both EPO and MEL increased the relative kidney weight. The pleiotropic growth factor EPO promotes the proliferation, survival, and differentiation of erythroid progenitor cells in the bone marrow. A single pre-ischemia administration of erythropoietin stimulated the early initiation of tubular regeneration, as evidenced by the increased tubular mitosis observed at 24 hours, within the proximal tubules (17). Moussavian et al. (18) indicated that MEL has a pleiotropic nature. The MEL receptors are clearly widespread and multifunctional, allowing MEL to act as a physiological regulator, pleiotropic, and promotor of cell repair.
In our study, the histological evaluation showed that IR caused an increase in the hyaline cast and a thickening of the Bowman capsule basement membrane. EPO treatment attenuated the histopathological changes associated with renal IR injury. On the other hand, the attenuating effect of EPO on the morphological changes in the renal tissue caused by IR injury has previously been reported (19, 20). Sener et al. (21) reported that melatonin has protective effects on IR-induced renal injury, while the histopathological changes are reversed by MEL treatment. Additionally, they proposed that melatonin appears to play a cytoprotective role in the kidney injured by ischemia-reperfusion.
5.1. Conclusion
In conclusion, ROS are considered to be the principal components involved in the pathophysiological tissue alterations observed during renal IR. The administration of EPO or MEL, both known antioxidant agents, appears to have beneficial effects on IR-induced renal injury, as indicated by the lower degrees of the histopathological changes and renal dysfunction. However, MEL pretreatment exerted more nephroprotective effect than EPO pretreatment, since MEL was effective in reversing renal IR due to its potent antioxidant effects. These results may indicate that MEL protects against morphological damage better than EPO in renal IR injury. However, further investigations are needed to explore whether the combination of EPO and MEL treatment has a synergistic effect of protection against IR-induced renal injury.
Footnotes
Authors’ Contribution:Shokofeh Banaei was responsible for the study design, as well as the biochemical analysis and writing the manuscript; Nasser Ahmadiasl was reasonable for the study’s design and the statistical analysis; Alireza Alihemmati conducted the histological evaluation; All authors read and approved the final manuscript.
Conflict of Interest:The authors declare that they have no conflict of interest.
Funding/Support:This study was financially supported by the drug applied research center of Tabriz University of Medical Sciences.
References
- 1.Anaya-Prado R, Toledo-Pereyra LH, Lentsch AB, Ward PA. Ischemia/reperfusion injury. J Surg Res. 2002;105(2):248–58. doi: 10.1006/jsre.2002.6385. [DOI] [PubMed] [Google Scholar]
- 2.Troppmann C, Gillingham KJ, Benedetti E, Almond PS, Gruessner RW, Najarian JS, et al. Delayed graft function, acute rejection, and outcome after cadaver renal transplantation. The multivariate analysis. Transplantation. 1995;59(7):962–8. doi: 10.1097/00007890-199504150-00007. [DOI] [PubMed] [Google Scholar]
- 3.Masztalerz M, Wlodarczyk Z, Czuczejko J, Slupski M, Kedziora J. Superoxide anion as a marker of ischemia-reperfusion injury of the transplanted kidney. Transplant Proc. 2006;38(1):46–8. doi: 10.1016/j.transproceed.2005.12.084. [DOI] [PubMed] [Google Scholar]
- 4.Nath KA, Norby SM. Reactive oxygen species and acute renal failure. Am J Med. 2000;109(8):665–78. doi: 10.1016/s0002-9343(00)00612-4. [DOI] [PubMed] [Google Scholar]
- 5.Ebert BL, Bunn HF. Regulation of the erythropoietin gene. Blood. 1999;94(6):1864–77. [PubMed] [Google Scholar]
- 6.Siren AL, Ehrenreich H. Erythropoietin--a novel concept for neuroprotection. Eur Arch Psychiatry Clin Neurosci. 2001;251(4):179–84. doi: 10.1007/s004060170038. [DOI] [PubMed] [Google Scholar]
- 7.Genc S, Koroglu TF, Genc K. Erythropoietin and the nervous system. Brain Res. 2004;1000(1-2):19–31. doi: 10.1016/j.brainres.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Westenfelder C, Biddle DL, Baranowski RL. Human, rat, and mouse kidney cells express functional erythropoietin receptors. Kidney Int. 1999;55(3):808–20. doi: 10.1046/j.1523-1755.1999.055003808.x. [DOI] [PubMed] [Google Scholar]
- 9.Plotnikov EY, Chupyrkina AA, Jankauskas SS, Pevzner IB, Silachev DN, Skulachev VP, et al. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim Biophys Acta. 2011;1812(1):77–86. doi: 10.1016/j.bbadis.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27(2):189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- 11.Mayo JC, Sainz RM, Tan DX, Hardeland R, Leon J, Rodriguez C, et al. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J Neuroimmunol. 2005;165(1-2):139–49. doi: 10.1016/j.jneuroim.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36(1):1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 13.Mahoney JJ, Vreman HJ, Stevenson DK, Van Kessel AL. Measurement of carboxyhemoglobin and total hemoglobin by five specialized spectrophotometers (CO-oximeters) in comparison with reference methods. Clin Chem. 1993;39(8):1693–700. [PubMed] [Google Scholar]
- 14.Kiris I, Kapan S, Kilbas A, Yilmaz N, Altuntas I, Karahan N, et al. The protective effect of erythropoietin on renal injury induced by abdominal aortic-ischemia-reperfusion in rats. J Surg Res. 2008;149(2):206–13. doi: 10.1016/j.jss.2007.12.752. [DOI] [PubMed] [Google Scholar]
- 15.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190(3):255–66. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Labonia W, Rubio D, Arias C. Melatonin corrects reticuloendothelial blockade and iron status in haemodialysed patients. Nephrology (Carlton). 2005;10(6):583–7. doi: 10.1111/j.1440-1797.2005.00488.x. [DOI] [PubMed] [Google Scholar]
- 17.Sharples EJ, Yaqoob MM. Erythropoietin in experimental acute renal failure. Nephron Exp Nephrol. 2006;104(3):83–8. doi: 10.1159/000094546. [DOI] [PubMed] [Google Scholar]
- 18.Moussavian MR, Scheuer C, Schmidt M, Kollmar O, Wagner M, von Heesen M, et al. Multidrug donor preconditioning prevents cold liver preservation and reperfusion injury. Langenbecks Arch Surg. 2011;396(2):231–41. doi: 10.1007/s00423-010-0668-4. [DOI] [PubMed] [Google Scholar]
- 19.Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, et al. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15(8):2115–24. doi: 10.1097/01.ASN.0000135059.67385.5D. [DOI] [PubMed] [Google Scholar]
- 20.Vesey DA, Cheung C, Pat B, Endre Z, Gobe G, Johnson DW. Erythropoietin protects against ischaemic acute renal injury. Nephrol Dial Transplant. 2004;19(2):348–55. doi: 10.1093/ndt/gfg547. [DOI] [PubMed] [Google Scholar]
- 21.Sener G, Sehirli AO, Keyer-Uysal M, Arbak S, Ersoy Y, Yegen BC. The protective effect of melatonin on renal ischemia-reperfusion injury in the rat. J Pineal Res. 2002;32(2):120–6. doi: 10.1034/j.1600-079x.2002.1848.x. [DOI] [PubMed] [Google Scholar]