Abstract
Background:
It has been reported that secreted frizzled-related protein-4 known as an antagonist of Wnt signaling pathway plays a role in luteinization process of rodent granulosa cells. The purpose of this study was twofold: 1) to determine whether recombinant human secreted frizzled-related protein-4 (rhSFRP-4) could directly induce terminal differentiation of rat Granulosa Cells (GCs) and 2) to understand how the modulation of β-catenin and Protein Kinase B (PKB)/AKT activity by exogenous SFRP-4 could be involved in steroidogenesis.
Methods:
GCs were firstly stimulated with Follicle-Stimulating Hormone (FSH) named as FSH-primed cells then were treated with luteinizing hormone (LH). Then estradiol (E2) and progesterone (P4) production levels were assessed in the absence or presence of rhSFRP-4 treatment. The expression levels of activated β-catenin, pAKTser 473 , pGSK3βser 9 were assessed by western blot or immunofluoresence.
Results:
In the presence of rhSFRP-4, there was 38% decreased E2 levels compared to untreated FSH-primed cells (p<0.05), and P4 production subsequently decreased. However, in GCs pre-treated with rhSFRP-4 prior to addition of FSH, P4 levels increased 2-fold compared with untreated cells (p<0.05). Unexpectedly, treatment with rhSFRP-4 prior to LH stimulation inhibited LH-induced P4 secretion. Treatment with low (0.5 ng/ml) but not high (50 ng/ml) concentrations of rhSFRP-4 led to significantly increased levels of pGSK3βser 9 (1.6-fold) and nuclear active β-catenin (2.8-fold) in GCs compared with untreated cells. Interestingly, pre-treating GCs with rhsFPR4 prior to LH stimulation resulted in a 38% decrease in pAKTser 473 levels compared with those in LH-treated cells (p<0.05).
Conclusion:
Taken together, our results showed that rhSFRP-4 could directly induce terminal differentiation in GCs via the modulation of β-catenin and PKB/AKT pathways and that it does so in a dose-dependent manner.
Keywords: Active β-catenin, GSK3β, PKB/AKT, Rat granulosa cell, Secreted frizzled-related protein-4 (SFRP-4)
Introduction
The ovary is a dynamic endocrine tissue that requires gonadotropins and steroids for follicular development, ovulation, and formation and regression of the corpus luteum. Previous studies showed that WNT signaling plays an important role in the development of rodent ovarian follicles 1. WNT proteins, a family of secreted glycoprotein ligands, act by binding to frizzled (Fzd) receptors, which are members of a specific class of seven-pass transmembrane receptors 2. The WNT pathway is implicated in many developmental processes, such as tissue differentiation, proliferation and apoptosis 3. In the absence of WNT ligands, β-catenin is targeted for degradation by a cytoplasmic complex that includes axin, adenomatous polyposis coli, and glycogen synthase kinase 3β (GSK3β) 4. In the canonical WNT pathway, the WNT/β-catenin signaling pathway, the second messenger, β-catenin, is stabilized by WNT binding to its receptors, leading to increased levels of β-catenin in the cytoplasm. Subsequently, β-catenin translocates to the nucleus where it acts as a transcriptional co-activator with members of the T-cell factor/lymphoid enhancer factor family to modulate the expression of a wide range of genes 4. WNT/Fzd activation of the β-catenin pathway is further modulated by proteoglycans and/or arrow/LRP-5/LRP-6 as co-receptors 5 and by antagonists such as Secreted Frizzled-Related Proteins (SFRPs) 6.
Recent studies provided evidence that in cultured rat Granulosa Cells (GCs), β-catenin activity can facilitate Follicle-Stimulating Hormone (FSH)-stimulated Cyp 19a1 expression via interaction with NR5A1 7 and can also facilitate proliferation via WNT2 8. Moreover, the conditional knockdown of β-catenin in primary mouse GCs confirmed that Cyp19a1 was a target for the β-catenin pathway 9. However, a recent study showed that co-treatment of mouse GCs with FSH and Wnt3a inhibits FSH-induced E2 production 10. Thus, the involvement of WNT/β-catenin signaling in FSH-induced nuclear β-catenin accumulation remains questionable.
SFRPs are the largest family of WNT inhibitors and comprise five members in humans, SFRP1-sFRP5. Biochemical studies have established that WNT proteins and SFRPs interact through cysteine-rich domains, which are postulated as the binding domains because of their homology with the WNT-binding region on Fzd receptors 11. This interaction impedes WNT binding to the Fzd receptor and further signal transduction.
It was demonstrated that Sfrp4 (the rat ortholog of human SFRP-4) is highly expressed in luteinized mouse GCs 12. The role of sFRP-4 in luteinization events is further highlighted by its down regulation in Fzd4-null mice leading to altered corpus luteum development and subsequent infertility 13. However, a recent study in human GCs demonstrated an inhibitory effect of Luteinizing Hormone/human Choriogonadotropin (LH/hCG) on SFRP-4 expression 14, which may indicate that the effect of SFRP-4 in luteinization events is species specific. sFRP-4 is also reportedly associated with apoptotic events in rodent corpus luteum regression 15 and ovarian surface epithelial cell apoptosis following ovulation 16.
Interestingly, sFRP-4 has also been shown to inhibit Protein Kinase B (PKB)/AKT activation 17. It has been demonstrated that Phosphoinositide 3-kinase (PI3K)/AKT activation plays a key role in GC survival, particularly for the survival of luteal cells 18,19. Although sFRP-4 expression is associated with luteinization events in rodent ovaries 20, its role in the terminal differentiation (i.e., luteinization) of GCs and the underlying molecular mechanism remains largely unknown. SFRPs could potentially have agonistic or antagonistic effects on WNT signaling in a concentration-dependent manner 21, therefore, in this study, both low and high concentrations of exogenous SFRP-4 were used to assess its effects on steroidogenesis and WNT/β-catenin or PKB/AKT activity in GCs. Additionally, because SFRP-4 potentially alters WNT and/or PI3K/AKT signaling, GCs were pre-treated with exogenous SFRP-4 to investigate whether this influences their response to gonadotropins.
Materials and Methods
Reagents and antibodies
Nutrient Mixture F-12 (DMEM+F12), penicillin-streptomycin, Bovine Serum Albumin (BSA), 3-(4,5-dimethylthiazol-2-yl)-3,5 diphenyltetrazolium bromide (MTT) and 4′,6-diamidino-2-phenylindole (DAPI) were obtained from Sigma-Aldrich (SaintLouis, USA). Fungizone and fetal bovine serum (FBS) were obtained from Gibco (Bristol, UK), and trypsin-EDTA was obtained from Fluka (Gillingham, UK). Pregnant Mare Serum Gonadotropin (PMSG) was provided by Intervet/Schering-Plough Animal Health (Utrecht, Netherlands). Recombinant human SFRP-4 (rhSFRP-4) was obtained from R&D Systems (Minneapolis, USA), whereas recombinant human FSH (Gonal-F) was obtained from Serono (Rockland, USA). Ovine Luteinizing Hormone (oLH) was a generous gift from Dr. Ariane de Agostini (Department of Pathology, Geneva University, Switzerland). Mouse monoclonal antiactive β-catenin (ABC) which is specific against nuclear β-catenin, and goat anti-mouse IgG-rhodamine were obtained from Millipore (Massachusetts, USA), whereas rabbit polyclonal anti-cleaved caspase-3 antibody was from Calbiochem (Massachusetts, USA), EMD Chemicals, goat anti-rabbit IgG-FITC, rabbit anti-goat IgG-HRP conjugated and IgG1 mouse isotypic control were from Vector Laboratories (Peterborough, UK). Rabbit polyclonal anti-GSK3β, goat polyclonal anti-pGSK3-βser9, rabbit monoclonal anti-AKT (pan) and anti-AKTser473, anti-rabbit IgG, were all obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The ELISA assay kits for the measurement of 17-β-estradiol (E2) and progesterone (P4) were obtained from DRG (Marburg, Germany).
Animals and GC culture
Immature Wistar female rats (22–23 days old, 50–55 g) were maintained using a 12:12 hr L:D photoperiod and were fed ad libitum on an ordinary laboratory diet. All experimentation was approved by the Animal Ethics Committee of the School of Biology, University of Tehran and was performed in accordance with the NIH guide for the care and use of laboratory animals.
In order to enable the isolation of a large number of GCs at the same stage of differentiation, immature female rats were injected intraperitoneally with 10 IU PMSG to induce follicular growth. All animals were killed by decapitation 48 hr after PMSG treatment, and their ovaries were removed. Follicles were punctured with a 30-gauge needle to isolate GCs and were then centrifuged at 400 g for 10 min. Cells were cultured overnight in DMEM+F-12 medium with 10% FBS, 100 IU penicillin, and 100 μg/ml streptomycin at 37°C in a 5% CO2 environment, and cell viability was assessed using a trypan blue exclusion technique. In all experiments, GCs were cultured in collagen type-I coated wells as previously described 22 and were left for 24 hr to adhere in the presence of 10% FBS. Subsequently, the original medium was replaced with a serum-free medium containing 0.2% BSA and either gonadotropins or rhSFRP-4 in isolation or combination.
MTT test for assessment of rhSFRP-4 effects on viability of GCs
GCs (100 μl, 1×104 cells/well) were seeded onto collagen type-I coated 96-well microtiter plates in the presence or absence of rhSFRP-4 (0.05, 0.5, 5, 50 ng/ml) for 24, 48, and 72 hr. Following this period, MTT reagent (5 mg/ml) in Phosphate-Buffered Saline (PBS) was added to the wells (1:10) and incubated at 37°C for 4 hr and MTT test was performed as described 23. A growth curve for GCs was constructed by plotting absorbance against rhSFRP-4-treatment time.
Experimental design for treatment of GCs with gonadotropins
GCs are known to luteinize in the presence of serum over several days of culture. Thus, isolated GCs were cultured for 14–16 hr in the presence of 10% FBS, and then treated for four days with gonadotropin in serum free medium. Briefly, GCs (1×105 cells/well) were seeded into coated 96-well microtiter plates, and on the following day, the medium was removed, the cells were washed with PBS and serum-free medium containing 0.2% BSA with or without FSH (50 ng/ml), and testosterone (T, 0.1 nM) was added for two days. Addition of these hormones promoted FSH receptor expression and E2 biosynthesis in GCs; thus, these stimulated cells were named “FSH-primed” GCs. Conditioned medium was removed after two days, centrifuged at 400 g, and maintained at −20°C before E2 detection using the ELISA assay. Next, FSH-primed GCs were treated with oLH (500 ng/ml) for two days, and conditioned medium was subsequently harvested and maintained at −20°C until P4 detection was performed using an ELISA assay. In another set of experiments, cells were pre-treated with rhSFRP-4 for 3 hr before addition of gonadotropin and were then stimulated with the gonadotropins for 48 or 96 hr and conditioned medium was used for E2 and P4 assessment as described 24. To avoid possible bias due to GC density in each well, the concentration of hormones in the conditioned media was adjusted according to its protein content, which was quantified using the Bradford assay. Protein concentration was determined using linear regression (Microsoft Excel), and hormone concentrations were expressed as pg/mg protein and ng/mg protein for E2 and P4, respectively.
Western blot analyses of active β-catenin, GSK3β and AKT/PKB activity
FSH-primed GCs (1×106 cells/well) were treated with rhSFRP-4 (0.5 or 50 ng/ml) for 48 hr and then stimulated with oLH for additional 48 hr. In parallel, in another set of experiments, cells were pre-treated with rhSFRP-4 for 3 hr before addition of gonadotropin and were then stimulated with the gonadotropins for 48 or 96 hr. Protein extracts were prepared by homogenization in RIPA buffer and western blot was performed as described 23. The membranes were incubated with anti-pGSK3βser9 antibody (1:100), stripped and reprobed with the following antibodies overnight at 4°C: total GSK3-β antibody (1:1000), active β-catenin antibody (1:500), p-AKTser473 antibody (1:1000), total-AKT antibody (1:1000) (Cell Signaling Technology, MA, USA), and β-actin antibody (1:1000) (Sigma Aldrich, USA). After washing three times with TBST, immunoreactive bands were visualized with ECL according to the manufacturer’s instruction. Intensity of bands of the exposed X-ray film was determined by densitometrycally scanning, and quantified using Al-phaEaseFC (Alpha Innotech, CA, USA) and normalized with β-Actin. The value for untreated cells was set as 1.0 and fold change was expressed relative to control.
Immunolocalization of active β-catenin and active caspase-3 in cells stimulated with gonadotropins and rhSFRP-4
GCs were treated in the presence or absence of rhSFRP-4 (0.5 or 50 ng/ml) with FSH+T for 48 hr or further stimulated with oLH for another 48 hr. Another set of cells was pre-treated with rhSFRP-4 for 3 hr before addition of gonadotropins. Immunolocalization was performed as described previously 22.
Statistical analysis
Quantitative results were analyzed using SPSS version 13 (Illinois, USA). The Shapiro-Wilk test was used to determine normality. All experiments were performed at least three times. ANOVA was used followed by post hoc LSD analysis. All data were presented as mean±SEM except for western blot results expressed as mean±SD. The p≤0.05 was considered as statistically significant.
Results
Effects of rhSFRP-4 on the viability of rat GCs
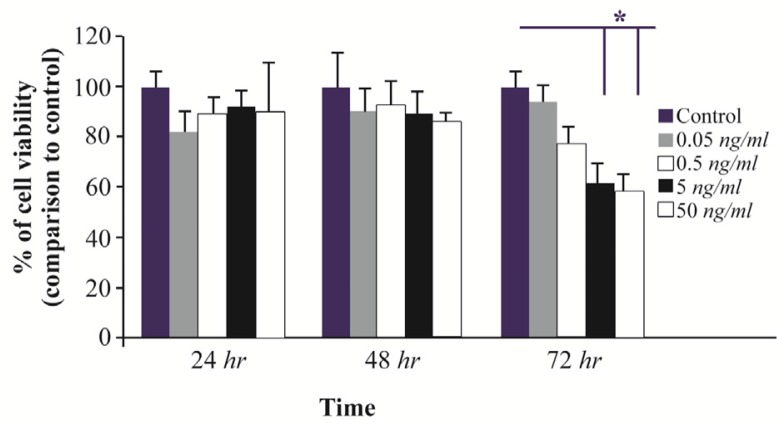
Previous studies reported an association between sFRP-4 and apoptotic events in the ovaries of rodents 15, but its role in cell survival has yet to be defined. In treated GCs, following 24 or 48 hr of treatment with rhSFRP-4 (0.05, 0.5, 5, and 50 ng/ml), cell viability was not significantly different from untreated cells (Figure 1). After 72 hr and compared to untreated cells, cell viability decreased by 20% following treatment with 0.5 ng/ml rhSFRP-4 and cell survival decreased by 40% (p<0.05) with 5 or 50 ng/ml rhSFRP-4 (Figure 1).
Figure 1.
The effects of exogenous SFRP4 on the viability of rat granulosa cells (GCs). GCs from immature rats were cultured and treated with rhSFRP-4 (0.05, 0.5, 5, and 50 ng/ml) for 24, 48, or 72 hr. Cell viability was assessed using MTT assay, and data were expressed relative to untreated cells (control). Bars represent mean± SEM of at least five independent experiments assayed in triplicate (*p<0.05, compared with untreated cells).
Combined treatment with FSH and rhSFRP-4 decreased E2 and P4 biosynthesis in rat GCs
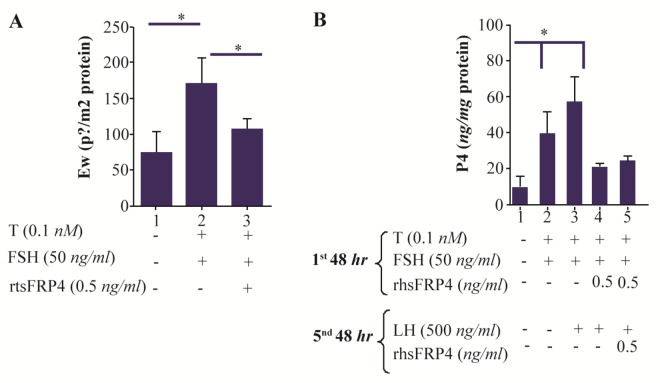
β-catenin, as a target of WNT signaling, is essential for FSH-induced E2 biosynthesis via the regulation of aromatase gene (Cyp19A1) transcription 7. It was found that E2 production increased by 2.3-fold (p< 0.05) in FSH-primed cells compared to untreated cells (Figure 2A). However, co-treatment of GCs with FSH+ T and rhSFRP-4 reduced E2 levels by 38% (p<0.05) compared to FSH-primed cells (Figure 2A). Similarly, P4 levels were reduced by 61% in GCs co-treated with FSH+T and rhSFRP-4, which were further stimulated with LH (Figure 2B).
Figure 2.
Exogenous SFRP4 decreases 17-beta-estradiol (E2) production in rat granulosa cells (GCs). GCs were cultured and treated with FSH (50 ng/ml) and testosterone (0.1 nM) in the presence or absence of rhSFRP-4 (0.5 ng/ml) for 48 hr. A. E2 levels and B. P4 levels were determined in conditioned media by ELISA and data were expressed relative to untreated cells (control) as pg/mg or ng/mg protein, respectively. Bars represent mean±SEM of four independent experiments assayed in triplicate. Superscript letters indicate statistically significant differences between treatment groups: *p<0.05.
rhSFRP-4 modulates P4 levels of GCs differently in the presence of FSH or LH
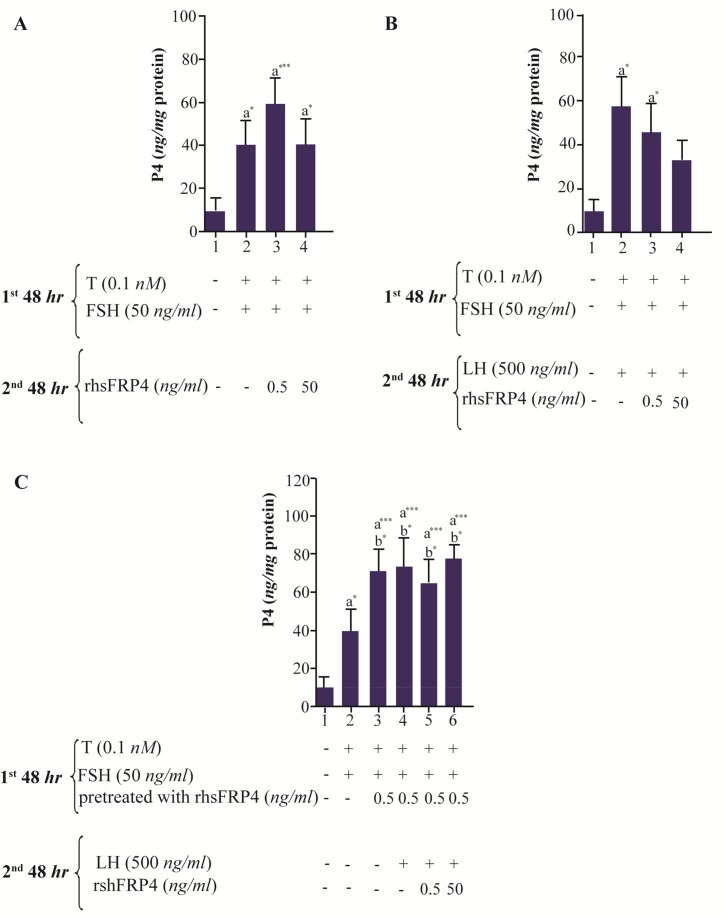
In a previous study, Sfrp4 expression was up-regulated in LH-stimulated GCs from rodent ovaries 12; however, the direct effect of exogenous SFRP-4 on P4 biosynthesis in GCs was not determined. Therefore, the direct effect of rhSFRP-4 was assessed by treatment of FSH-primed GCs with rhSFRP-4 alone (Figure 3A). Additionally, to determine whether rhSFRP-4 promotes the responsiveness of GCs to LH, it was also used in combination with LH (Figure 3B). Finally, GCs were pre-treated with rhSFRP-4 before the addition of FSH+ T and were further stimulated with LH with or without rhSFRP-4 (Figure 3C). A 5.5-fold increase in the levels of P4 was observed in FSH-primed cells compared with untreated cells (Figure 3A, column 2 compared with column 1, p<0.05). Levels of P4 in FSH-primed cells treated with rhSFRP-4 (0.5 ng/ml) increased 6.4 folds compared with untreated cells (Figure 3A, column 3 compared to column 1; p<0.001), whereas treatment with a higher concentration of rhSFRP-4 (50 ng/ml) produced P4 levels similar to those observed in FSH-primed cells (Figure 3A, column 4 compared to column 2). The combination of 0.5- or 50 ng/ml rhsFPR4 with LH increased P4 levels 4.8 and 3.4 folds relative to untreated cells, respectively (Figure 3B, columns 3 and 4 compared to column 1, p<0.05). However, there was no significant difference in the levels of P4 observed with LH+rhSFRP-4 or LH alone (Figure 3B).
Figure 3.
rhSFRP-4 modulates P4 biosynthesis. Granulosa cells from immature rats were cultured, hormonally stimulated, and treated sequentially with FSH for 48 hr (first 48 hr) and LH for another 48 hr (second 48 hr). A. rhSFRP-4 (0.5 or 50 ng/ml) was added alone, B. cells were simultaneously treated with FSH or LH in the presence of rhSFRP-4 (0.5 or 50 ng/ml), and C. cells were pre-treated with rhSFRP-4 3 hr before FSH treatment and then further stimulated with LH. P4 levels were measured using ELISA and data were expressed as ng/mg protein. Bars are the mean±SEM of three independent experiments assayed in triplicate. Superscript letters indicate statistically significant differences between treatment groups: a compared to untreated cells; b compared to FSH-primed cells (***p<0.001; *p<0.05).
Because the treatment of FSH-primed GCs with rhSFRP-4 alone increased levels of P4 (Figure 3A), an attempt was made to evaluate whether pre-treatment of GCs with rhSFRP-4 could further potentiate terminal differentiation of GCs in response to LH. Almost an 8-fold increase in P4 levels was observed compared to untreated cells (Figure 3C, columns 3–6 compared to column 1, p<0.001), and an almost 2-fold change in P4 compared to FSH-primed GCs (Figure 3C, columns 3–6 compared to column 2, p<0.05). Taken together, these results suggest that the effect of exogenous SFRP4 on GC terminal differentiation depends on how GCs were previously stimulated with gonadotropins and may be subsequently involved in signaling pathways.
Pre-treating FSH-primed GCs with rhSFRP-4 prior to addition of LH abolished LH-induced P4 production
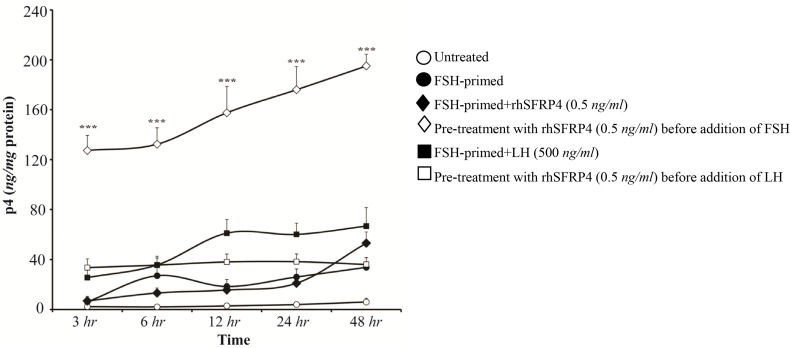
GCs are known to luteinize during the course of cell culture. In our experiments, P4 levels were measured after 4 days, and this may have introduced bias into the interpretation of our results. Therefore, a time kinetic study was also performed in which P4 levels were measured in harvested conditioned media at the following time points: 3, 6, 12, 24, and 48 hr. Untreated GCs produced low levels of P4 at all time-points, whereas levels increased gradually in FSH-primed cells, reaching their peak at 48 hr (Figure 4, empty vs. filled circles). Higher production of P4 was detected at 48 hr in FSH-primed cells treated with rhSFRP-4 (0.5 ng/ml) compared to untreated FSH-primed cells (Figure 4, filled lozenges vs. filled circles). However, treatment of FSH-primed cells with LH was more effective (Figure 4, filled lozenges vs. filled squares). In agreement with the results shown in figure 3C, pre-treatment of immature GCs with rhSFRP-4 prior to addition of FSH led to increased levels of P4 at all time-points compared with all other conditions (Figure 4, empty lozenges, p< 0.001). However, pre-treatment of FSH-primed GCs with rhSFRP-4 prior to addition of LH abolished LH-induced P4 biosynthesis (Figure 4, empty vs. filled squares). These results suggest that exogenous SFRP4 influences P4 production in a stage-specific manner.
Figure 4.
Pre-treating granulosa cells (GCs) with exogenous sFRP4 prior to LH treatment abolished LH-induced P4 production. rhSFRP-4 (0.5 ng/ml) was added to GCs prior to treatment with gonadotropins or simultaneously with FSH. Conditioned media were harvested after 3, 6, 12, 24, and 48 hr, and P4 levels were measured using ELISA. Data are expressed as ng/mg protein. Data are the mean±SD from three independent experiments assayed in triplicate (***p<0.001, compared with other conditions).
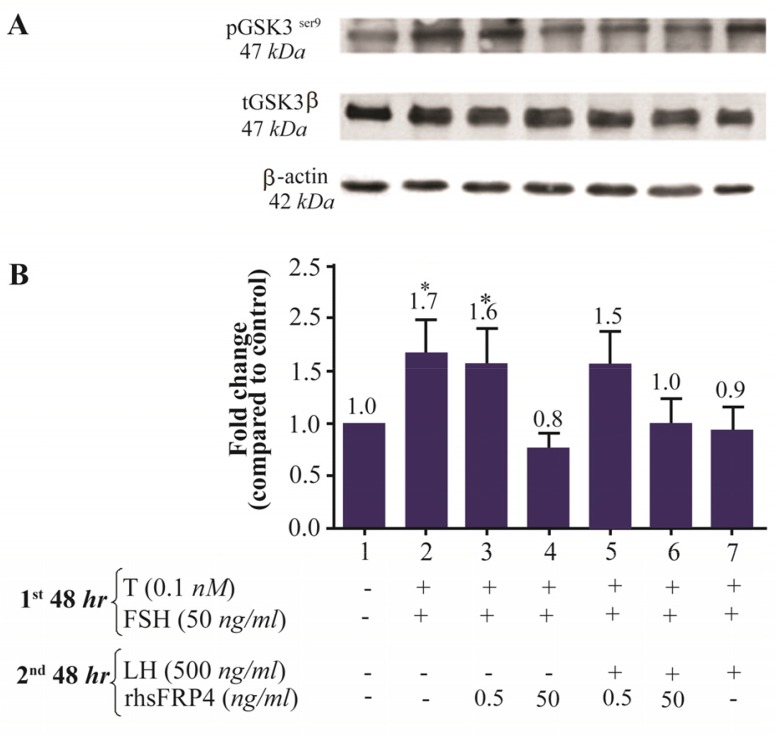
rhSFRP-4 modulates the levels of activated β-catenin and pGSK3βser9 in a dose-dependent manner
Using western blot analysis, the levels of nuclear β-catenin and the phosphorylation status of GSK3β were assessed as the two key molecules involved in WNT/β-catenin signaling. As shown in figure 5A, levels of pGSK3βser9 were similar, in FSH-primed GCs with or without a low dose treatment of rhSFRP-4 (0.5 ng/ml), i.e., levels increased by 1.6 and 1.7 folds compared with untreated cells, respectively (Figure 5B, columns 2–3 compared to column 1). However, a high dose of rhSFRP-4 (50 ng/ml) apparently antagonized the WNT/β-catenin pathway because levels of pGSK3βser9 decreased by 20% in comparison with untreated cells (Figures 5A and 5B, column 4 compared to column 1). Interestingly, LH-stimulated cells showed levels of pGSK3βser9 that were almost unchanged compared with untreated cells (Figures 5A and 5B, column 7 compared with column 1). In contrast, a low dose of rhSFRP-4 (0.5 ng/ml) increased levels of pGSK3βser9 1.5 folds, whereas 50 ng/ml rhSFRP-4 produced no effect (Figures 5A and 5B, column 5 compared to column 1).
Figure 5.
Exogenous SFRP4 modulates GSK3β activity in rat granulosa cells in a dose-dependent manner. A. The western blot shows a representative result of three independent experiments. B. Quantification was performed using AlphaEaseFC software to conduct densitometric analysis of three independent experiments. β-actin levels were used as an internal control. Data were reported as the mean±SD of the fold change relative to untreated cells (which had an arbitrary value of 1.0). (*p<0.05, compared with untreated cells).
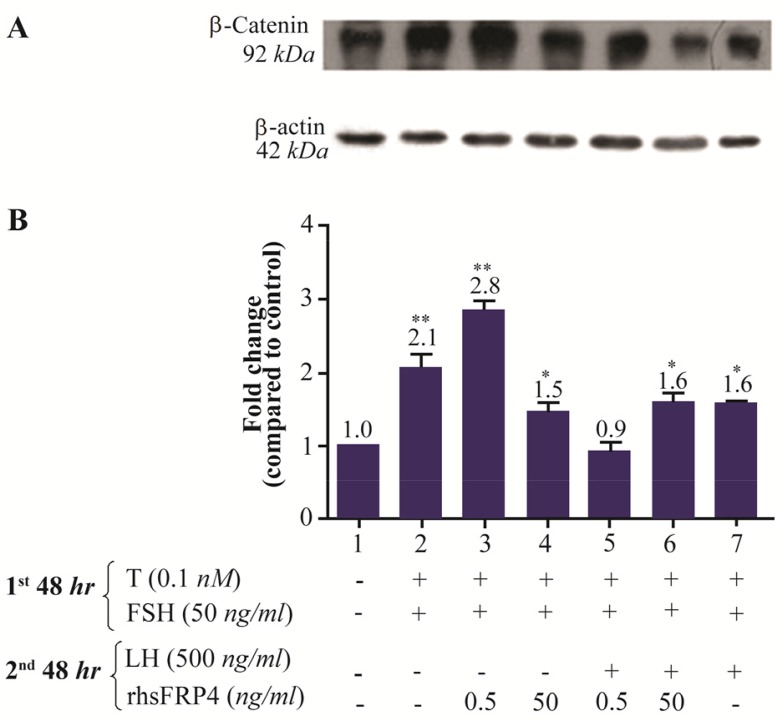
Additionally, levels of activated β-catenin increased 2.8 and 2.1 folds in FSH-primed cells with or without a 0.5 ng/ml treatment of rhSFRP-4, respectively (Figures 6A and 6B, columns 2–3 compared with column 1). However, the effect of rhSFRP-4 at 50 ng/ml on activated β-catenin levels was less substantial, i.e., a 1.5-fold increase compared to untreated cells (Figure 6A and 6B, column 4 compared to column 1). Similar levels of activated β-catenin (1.6-fold) was observed in in LH-treated cells without or with rhSFRP4 (50 ng/ml) (Figure 6A and 6B, columns 67 compared to column 1). However, in the presence of 0.5 ng/ml rhSFRP-4, the amount of activated β-catenin observed was similar to that in untreated cells (Figures 6A and 6B, column 5 compared to column 1).
Figure 6.
Exogenous SFRP4 modulates activated β-catenin levels in rat granulosa cells in a dose-dependent manner. A. The western blot shows a representative result of three independent experiments. B. Quantification was performed using AlphaEaseFC software to conduct densitometric analysis of three independent experiments. β-actin levels were used as an internal control. Data are reported as the mean±SD of the fold change relative to untreated cells (which were set with an arbitrary value 1.0).
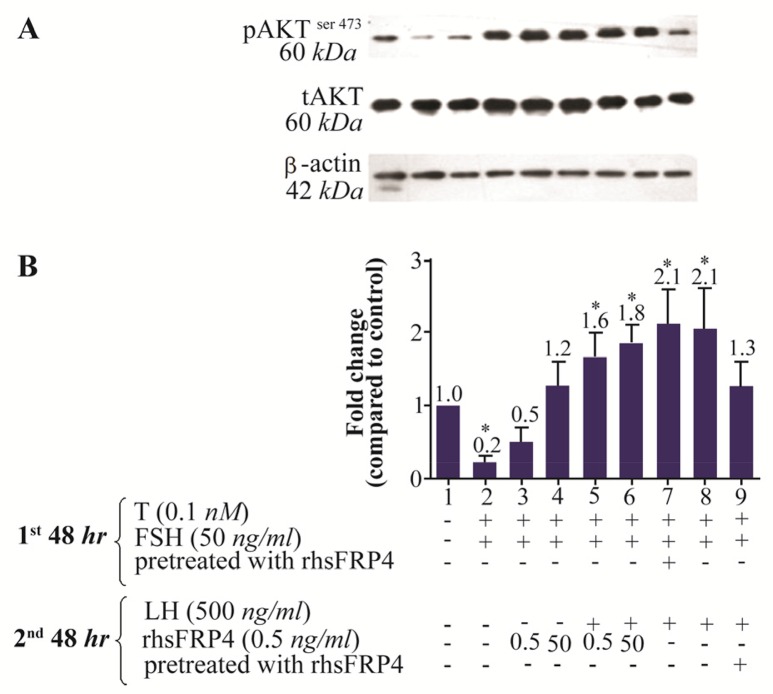
Pre-treating GCs with rhSFRP-4 prior to addition of LH reduced LH-induced pAKTser473 levels
SFRP4-mediated apoptosis purportedly involves the suppression of the PI3K and PKB/Akt survival pathways, through a mechanism independent of canonical WNT/β-catenin signaling 25. Therefore, an attempt was made to test whether pre-treatment of GCs with rhSFRP-4 prior to treatment with gonadotropins affected cell survival compared to simultaneous treatment with rhSFRP-4 and gonadotropins. In FSH-primed cells, levels of pAKTser473 decreased by 80% (p<0.05) and 50% without or with rhSFRP-4 (0.5 ng/ml), respectively (Figures 7A and 7B, columns 2–3 compared with column 1). However, there was no significant changes in the levels of pAKTser473 in the FSH-primed cells treated with rhSFRP-4 (50 ng/ml) compared to untreated FSH-primed cells (Figures 7A and 7B, column 4 compared to column 1). When combined with LH treatment, 0.5 and 50 ng/ml rhSFRP-4 resulted in a 1.6- and 1.8-fold increase in pAKTser473 levels, respectively (Figures 7A and 7B, columns 5–6). The maximum levels of pAKTser473 (2.1-fold) were observed following pre-treatment of GCs with rhSFRP-4 prior to the addition of FSH and subsequent stimulation with LH (Figures 7A and 7B, column 7). A similar increase of pAKTser473 levels (a 2.1-fold increase compared to untreated cells) was observed in LH-stimulated GCs (Figures 7A and 7B, column 8 compared to column 1). However, pre-treatment of cells with rhSFRP-4 before treatment with LH abolished LH-induced AKT activity (Figure 7B, column 9). These results indicate the occurrence of PI3K/AKT activity in GCs following gonadotropin treatment. This activity decreased in the presence of FSH, whereas it increased in LH-stimulated GCs. Interestingly, AKT activation was abolished where cells were pre-treated with rhSFRP-4 prior to the addition of LH.
Figure 7.
Exogenous SFRP4 differentially modulates PKB/AKT activity in rat granulosa cells (GCs) stimulated with FSH or LH. GCs were treated or pre-treated with rhSFRP-4 (0.5 ng/ml) combined with gonadotropin stimulation. A) The western blot shows a representative result of three independent experiments. B) Quantification was performed using AlphaEaseFC software for densitometric analysis of two independent experiments. β-actin levels were used as an internal control. Data are reported as the mean±SD of the fold change relative to untreated cells (set with the arbitrary value of 1.0) (*p<0.05, compared with untreated cells).
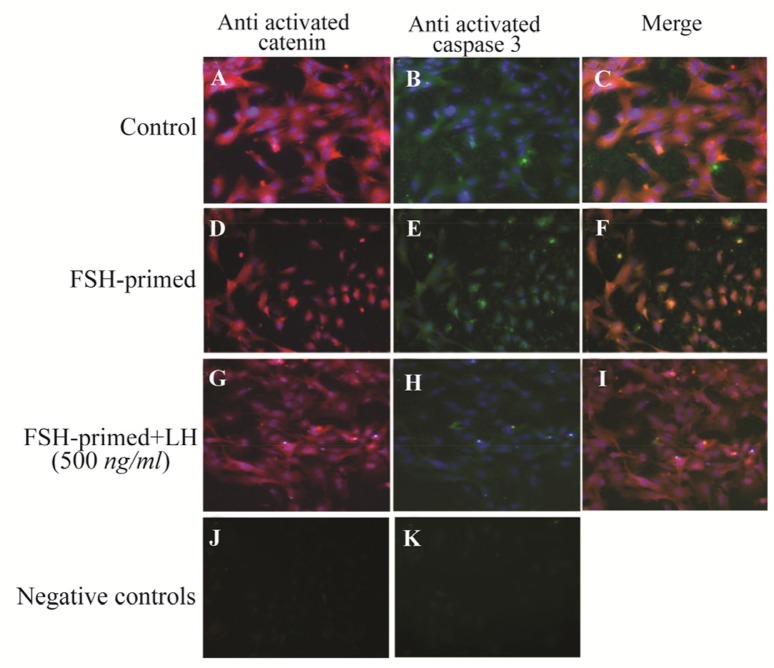
Activated β-catenin and cleaved caspase-3 were co-localized in FSH-primed but not LH-stimulated cells
Having observed decreased levels of pAKTser473 in FSH-primed GCs, with or without rhSFRP-4 treatment (Figure 7), caspase-3 activity was also investigated. In our previous study, the presence of nuclear β-catenin in the apoptotic GCs of rats was observed 22. Because increased levels of activated β-catenin was observed in FSH-primed GCs in the present study, it was decided to assess the potential co-localization of activated β-catenin and cleaved caspase-3 in these cells. In untreated cells, nuclear β-catenin and cleaved caspase-3 were not observed (Figure 8A–C). However, in FSH-primed GCs, nuclear β-catenin was co-localized with cleaved caspase-3 (Figure 8D–F). In accordance with increased AKT activity in LH-stimulated cells (Figure 7), nuclear β-catenin and cleaved caspase-3 were not observed in LH-stimulated GCs (Figure 8G–I).
Figure 8.
Active β-catenin and cleaved caspase-3 are co-localized in FSH-primed but not LH-stimulated granulosa cells (GCs). Cells were double immunostained with anti-active β-catenin (red) and anti-active casapse-3 (green). DNA was observed by using DAPI (blue). A–C) Cytoplasmic immunostaining of active β-catenin and active caspase-3 in untreated (control) GCs. D–F) Nuclear localization of active β-catenin and active caspase-3 in FSH-primed GCs. G–I) Cytoplasmic immunostaining of active β-catenin and active caspase-3 in LH-stimulated cells. J–K) The negative control was performed by using IgG1 mouse isotypic control as primary antibody. Images are at 200× magnification.
Discussion
In this study, the direct effect of exogenous SFRP-4 on the terminal differentiation of rat GCs was examined and the potential modulation of the WNT/β-catenin and PI3K/AKT pathways by this molecule was investigated. In a previous study, regulation of Cyp-19A1 (aromatase) activity by FSH/cAMP was shown to require a functional interaction between NR5A1 (Steroid factor 1) and CTNNB1 (β-catenin) 7. In agreement with this finding, bovine follicles with increased concentrations of E2 exhibited higher levels of β-catenin compared with low-E2 follicles, indicating that the FSH responsible for increased E2 content could result in accumulation of β-catenin 26. Accordingly, it was showed that treatment of cells with rhSFRP-4 led to decreased biosynthesis of E2 accompanied by lower levels of P4 in response to LH. This may be due to the attenuated autocrine effect of E2 on the induction of LH receptors in rat GCs, as previously reported 27. Interestingly, Law et al 28 revealed that β-catenin could be phosphorylated by PKA which leads to its interaction with both SF1 and TCF3 localized in the promoter of Lhcgr gene and activation of this gene 28. Moreover, dephosphorylated β-catenin at its N-terminal residues by canonical Wnt signaling pathway led to increased concentration of nuclear β-catenin which promoted increased expression of Star, Cyp11a1, and Cyp19a1 29.
Here, also similar levels of nuclear β-catenin were observed in FSH-primed cells treated with low concentrations of rhSFRP-4. This finding is consistent with our previous study, in which WNT-independent β-catenin nuclear localization was demonstrated in cells co-treated with FSH and rhSFRP-4 22. Evidence for this hypothesis is also provided by the recent study of Stapp et al 10, which showed that co-incubation of mouse GCs with canonical WNT3A and FSH did not affect the levels of total β-catenin, but did reduce the ability of FSH to stimulate steroidogenic enzymes and subsequently, E2 and P4 production. However, in the Stapp’s study, anti-nuclear β-catenin antibody was not used and therefore their conclusion regarding the non-effectiveness of WNT3A and FSH co-treatment and subsequent hormonal regulation through β-catenin needs more clarification.
LH-induced WNT signaling has been observed in rodent ovaries, particularly with increased expression of WNT4 and Fzd-1 20. Correspondingly, conditional knock-out of WNT4 in mouse ovaries significantly reduced FSH receptor expression and P4 levels in hormonally stimulated mice 29. Collectively, these data may suggest that WNT molecules regulate GC maturation and differentiation through a variety of molecular pathways and in a β-catenin-independent manner; however, these hypotheses will require further clarification.
It is important to consider that follicular development also involves dramatic changes in cell morphology. GCs are epithelial cells held together by cadherins (E- and N-cadherin); β-catenin is known to bind directly to the cadherin cytoplasmic tail, while α-catenin binds to β-catenin and connects the complex to actin or actinin. E-cadherins are present between GCs of primary follicles, but they disappear in the fully developed preovulatory follicle, while α- and β-catenins are expressed in GCs 30. This suggests that localized nuclear β-catenin in FSH-primed cells may derive from the increased pool of free β-catenins caused by the absence of E-cadherin. However, additional signals capable of affecting β-catenin activation cannot be excluded because increased PKB/AKT activity was observed in the presence of exogenous SFRP4. AKT phosphorylates and thus inactivates GSK3β on Ser-9 leading to increased levels of hypophosphorylated β-catenin 31. Furthermore, a recent study showed that AKT also phosphorylates CTNNB1 directly at Ser-552, both in vivo and in vitro. AKT-mediated phosphorylation of CTNNB1 causes it to disassociate from cell-cell contacts and accumulate in the nucleus, thereby enabling the activation of target genes 32. Taken together, accumulation of β-catenin into the nucleus could be achieved by multiple pathways and it seems that its mass in the nucleus for hormonal regulation is more important than the signaling pathway that allowed its shuttling into nucleus 28.
Previous studies have shown that Sfrp4 levels increase substantially in luteinized rodent GCs 12, and decreased levels of sFRP-4 were observed in Fz-4 null mice with impairment of corpus luteum function 13. However, the role of sFRP-4 as a direct inducer of terminal differentiation in GCs was unconfirmed. In our study, it was shown, for the first time, that rhSFRP-4 plays a dual role in gonadotropin-induced GC differentiation. Here, it was demonstrated that P4 levels increased in GCs treated with a low dose of rhSFRP-4, while P4 levels decreased in FSH-primed GCs exposed to a high dose of rhSFRP-4. Although SFRPs have generally been described as antagonists of WNT signaling, several studies provide evidence to suggest they are also involved in positive regulation. SFRP1 purportedly has low- and high-affinity binding sites for Wg (the ortholog of WNT in Drosophila); binding to the low- or high-affinity sites would inhibit or promote Wg signaling, respectively 21. It has been suggested that SFRPs could sequester WNT ligands; thus, they could act as antagonists or in a dominant-negative manner via the formation of inactive complexes with Fzd receptors, thereby preventing signal activation 33. Alternatively, SFRPs could favor WNT–Fzd interaction by simultaneously binding to both molecules and synergizing signal activation 33. Therefore, it was speculated that the agonistic or antagonistic action of SFRP4 observed in our experiments may have been influenced by its concentration. This speculation is supported by our findings, specifically the dose-dependent dual role of rhSFRP-4 in WNT/β-catenin signaling activity, which was demonstrated by an increase and decrease in the levels of active β-catenin in GCs treated with low and high doses of rhSFRP-4, respectively.
In earlier in vivo studies, Sfrp4 overexpression in mouse mammary glands have stimulated the induction of apoptosis 25. SFRP-4 reportedly also inhibits PKB/AKT activation 17, and sFRP-4-mediated apoptosis is postulated to involve suppression of the PI3K/AKT survival pathway, possibly through a mechanism independent of canonical WNT signaling 25. Here, it was observed that long-term treatment (72 hr) of immature GCs with rhSFRP-4 reduced cell viability, which may have been due to its effect on PKB/AKT activity. The PKB/AKT pathway plays a key role in survival of GCs 18 and FSH-induced Lhcgr expression 28. Moreover, increased lifespan of luteal cells has been demonstrated in the GCs of Pten−/− mice 19. Here, reduced levels of pAKTser473 in FSH-primed GCs were shown, whereas pre-treatment or co-treatment with rhSFRP-4 increased pAKTser473 levels. In contrast, high levels of pAKTser473 accompanied LH stimulation; however, this effect was reduced when cells were pre-treated with rhSFRP-4 prior to LH stimulation and was accompanied by decreased LH-induced P4 production. Correspondingly, co-localization of nuclear β-catenin and active caspase-3 in FSH-primed GCs were observed in this study. However, nuclear β-catenin was not detected in LH-stimulated cells, which is consistent with a recent study that showed, during terminal differentiation of GCs, the WNT/β-catenin pathway is inactive and over-activation of CTNNB1 negatively affects LH-induced ovulation and luteinization 34. Taken together, these findings suggest that modulation of the PI3K/AKT pathway by exogenous sFRP4 may influence terminal differentiation of GCs.
Unexpectedly, in our study, pre-treatment of immature GCs with rhSFRP-4 prior to FSH stimulation resulted in a 2-fold increase in AKT activity, which was accompanied by decreased levels of E2 during the first 48 hr of culture. However, this effect did not influence the levels of P4 observed at the end of the culture period (4 days). In contrast, pre-treating FSH-primed cells with rhSFRP-4 before LH stimulation reduced AKT activity, and this was accompanied by the abolishment of LH-induced P4 biosynthesis. It has been demonstrated that FSH stimulation of rat GCs led to PI3Kinase/AKT activity which by phosphorylation of FOXO1 relieves repression of Lhcgr promoter activity 28. Altogether, it is tempting to speculate that Wnt/β-catenin initiation by low concentration of rhSFRP-4 prior to FSH stimulation may enhance the effect of FSH and estradiol biosynthesis. While blockage of Wnt/β-catenin signaling with high concentration of rhSFRP-4 inactivates required PI3Kinase/AKT activity for LH effect on target genes and P4 biosynthesis. Therefore, it seems that Wnt/β-catenin pathway is a key player for responsiveness of GCs to FSH but this pathway affects negatively the responsiveness of GCs to LH stimulation.
Conclusion
In summary, the complex role of SFRP-4 in steroidgenesis in rat GCs was demonstrated, which functions via the modulation of β-catenin and PKB/AKT activation. Our findings suggest the existence of tightly controlled crosstalk between the PI3K/AKT and WNT signaling pathways during GC maturation and terminal differentiation. Furthermore, our data suggest that SFRP-4 may have distinct functions depending on the concentration and the stage of GC differentiation at which gonadotropins are applied.
Acknowledgement
This work was supported by grant #268308/K6/012 from University College of Science, University of Tehran, Tehran, Iran, and by financial support from the Reproductive Biotechnology Research Center, Avicenna Research Institute, Tehran, Iran.
References
- 1. Vainio S, Heikkilä M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature 1999; 397 (6718): 405– 409. [DOI] [PubMed] [Google Scholar]
- 2. Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 1996; 382 (6588): 225– 230. [DOI] [PubMed] [Google Scholar]
- 3. Nusse R, Varmus HE. Wnt Genes. Cell 1992; 69 (7): 1073– 1087. [DOI] [PubMed] [Google Scholar]
- 4. Novak A, Dedhar S. Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci 1999; 56 (5–6): 523– 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mi K, Johnson GV. Role of the intracellular domains of LRP5 and LRP6 inactivating the Wnt canonical pathway. J Cell Biochem 2005; 95 (2): 328– 338. [DOI] [PubMed] [Google Scholar]
- 6. Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci 2008; 121 (Pt 6): 737– 746. [DOI] [PubMed] [Google Scholar]
- 7. Parakh TN, Hernandez JA, Grammer JC, Weck J, Hunzicker-Dunn M, Zeleznik AJ, et al. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires beta-catenin. Proc Natl Acad Sci USA 2006; 103 (33): 12435– 12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang HX, Li TY, Kidder GM. WNT2 regulates DNA synthesis in mouse granulose cells through beta-catenin. Biol Reprod 2010; 82 (5): 865– 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hernandez Gifford JA, Hunzicker-Dunn ME, Nilson JH. Condition aldeletion of beta-catenin mediated by Amhr-2cre in mice causes female infertility. Biol Reprod 2009; 80 (6): 1282– 1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stapp AD, Gómez BI, Gifford CA, Hallford DM, Hernandez Gifford JA. Canonical WNT signaling inhibits follicle stimulating hormone mediated steroidogenesis in primary cultures of rat granulosa cells. PloS One 2014; 9 (1): e86432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, et al. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA 1997; 94 (7): 2859– 2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsieh M, Mulders SM, Friis RR, Dharmarajan A, Richards JS. Expression and localization of secreted frizzled-related protein-4 in the rodent ovary: evidence for selective up-regulation in luteinized granulose cells. Endocrinology 2003; 144 (10): 4597– 4606. [DOI] [PubMed] [Google Scholar]
- 13. Hsieh M, Boerboom D, Shimada M, Lo Y, Parlow AF, Luhmann UF, et al. Mice null for Frizzled4 (Fzd4−/−) are infertile and exhibit impaired corpora lutea formation and function. Biol Reprod 2005; 73 (6): 1135– 1146. [DOI] [PubMed] [Google Scholar]
- 14. Maman E, Yung Y, Cohen B, Konopnicki S, Dal Canto M, Fadini R, et al. Expression and regulation of sFRP family members in human granulose cells. Mol Hum Reprod 2011; 17 (7): 399– 404. [DOI] [PubMed] [Google Scholar]
- 15. Guo K, Wolf V, Dharmarajan AM, Feng Z, Bielke W, Saurer S, et al. Apoptosis-associated gene expression in the corpus luteum of the rat. Biol Reprod 1998; 58 (3): 739– 746. [DOI] [PubMed] [Google Scholar]
- 16. Drake JM, Friis RR, Dharmarajan AM. The role of sFRP4, a secreted frizzled-related protein, in ovulation. Apoptosis 2003; 8 (4): 389– 397. [DOI] [PubMed] [Google Scholar]
- 17. Constantinou T, Baumann F, Lacher MD, Saurer S, Friis R, Dharmarajan A. SFRP-4 abrogates Wnt-3a-induced beta-catenin and Akt/PKB signaling and reverses a Wnt-3a-imposed inhibition of in vitro mammary differentiation. J Mol Signal 2008; 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson AL, Bridgham JT, Swenson JA. Activation of the Akt/proteinkinase B signaling pathway is associated with granulose cell survival. Biol Reprod 2001; 64 (5): 1566– 1574. [DOI] [PubMed] [Google Scholar]
- 19. Fan HY, Liu Z, Cahill N, Richards JS. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol 2008; 22 (9): 2128– 2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsieh M, Johnson MA, Greenberg NM, Richards JS. Regulated expression of Wnts and Frizzled sat specific stages of follicular development in the rodent ovary. Endocrinology 2002; 143 (3): 898– 908. [DOI] [PubMed] [Google Scholar]
- 21. Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, et al. Secreted frizzled-related protein-1 binds directly to Wingless and isabiphasic modulator of Wnt signaling. J Biol Chem 2000; 275 (6): 4374– 4382. [DOI] [PubMed] [Google Scholar]
- 22. Hossein G, Khanmohammadi M, Jarooghi N, Kazemnejad S. Evidence for an association between Wnt-independent β-catenin intracellular localization and ovarian apoptotic events in normal and PCO-induced rat ovary. Prog Biol Sci 2011; 1 (2): 1– 10. [Google Scholar]
- 23. Jannesari-Ladani F, Hossein G, Monhasery N, Shahoei SH, Mood N. Wnt5a influences Viability, migration, adhesion, colony formation, E- and N-cadherin expression of human ovarian cancer cell line SKOV-3. Folia Biol (Praha) 2014; 60 (2): 57– 67. [PubMed] [Google Scholar]
- 24. Hossein G, Arabzadeh S, Hossein-Rashidi B, Hosseini MA. Relations between steroids and AMH: impact of basal and intrafollicular steroids to AMH ratios on oocyte yield and maturation rate in women with or without polycystic ovary undergoing in vitro fertilization. Gynecol Endocrinol 2012; 28 (6): 413– 417. [DOI] [PubMed] [Google Scholar]
- 25. Lacher MD, Siegenthaler A, Jäger R, Yan X, Hett S, Xuan L, et al. Role of DDC-4/sFRP-4, a secreted frizzled-related protein, at the onset of apoptosis in mammary involution. Cell Death Differ 2003; 10 (5): 528– 538. [DOI] [PubMed] [Google Scholar]
- 26. Castañon BI , Stapp AD , Gifford CA , Spicer LJ , Hallford DM , Hernandez Gifford JA. Follicle-stimulating hormone regulation of estradiol production: possible involvement of WNT2 and β-catenin in bovine granulosa cells. J Anim Sci 2012; 90 (11): 3789– 3797. [DOI] [PubMed] [Google Scholar]
- 27. Kessel B, Liu YX, Jia XC, Hsueh AJ. Autocrine role of estrogens in the augmentation of luteinizing hormone receptor formation in cultured rat granulose cells. Biol Reprod 1985; 32 (5): 1038– 1050. [DOI] [PubMed] [Google Scholar]
- 28. Law NC, Weck J, Kyriss B, Nilson JH, Hunzicker-Dunn M. Lhcgr expression in granulosa cells: roles for PKA-phosphorylated β-catenin, TCF3, and FOXO1. Mol Endocrinol 2013; 27 (8): 1295– 1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boyer A, Lapointe E, Zheng X, Cowan RG, Li H, Quirk SM, et al. WNT4 is required for normal ovarian follicle development and female fertility. FASEB J 2010; 24 (8): 3010– 3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sundfeldt K, Piontkewitz Y, Billig H, Hedin L. E-cadherin-catenin complex in the rat ovary: cell-specific expression during folliculogenesis and luteal formation. J Reprod Fertil 2000; 118 (2): 375– 385. [DOI] [PubMed] [Google Scholar]
- 31. Desbois-Mouthon C, Cadoret A, Blivet-Van Eggelpoël MJ, Bertrand F, Cherqui G, Perret C, et al. Insulin and IGF-1 stimulate the beta-catenin pathway through two signaling cascades involving GSK-3beta inhibition and Ras activation. Oncogene 2001; 20 (2): 252– 259. [DOI] [PubMed] [Google Scholar]
- 32. Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 2007; 282 (15): 11221– 11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Espada J, Calvo MB, Díaz-Prado S, Medina V. Wnt signalling and cancer stem cells. Clin Transl Oncol 2009; 11 (7): 411– 427. [DOI] [PubMed] [Google Scholar]
- 34. Fan HY, O’Connor A, Shitanaka M, Shimada M, Liu Z, Richards JS. Beta-catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol Endocrinol 2010; 24 (8): 1529– 1542. [DOI] [PMC free article] [PubMed] [Google Scholar]