Abstract
Background:
Genetic polymorphisms of drug metabolisms by cytochrome P450 (P450s) could affect drug response, attracting particular interest in the pharmacogenetics. Due to the importance of CYP2C19* 17 allele and its capability of super- fast metabolism and also lack of information about distribution of the alleles in Iranian population, this research aimed to use High Resolution Melting (HRM) method compared to PCR-RFLP for genotyping healthy Iranian population.
Methods:
Blood samples were collected from 100 healthy Iranian volunteers. DNA was extracted by salting out method. Real-time PCR was used for amplification of the CYP2C19 gene and the alleles were identified by HRM. Sequencing was used to confirm the amplified DNA fragments and data were analyzed using SPSS software ver.18.
Results:
The frequency of alleles CYP2C19*1/*1, CYP2C19*1/*17 and CYP2C19*17/*17 were estimated as 58.33, 29.1 and 11.1%, respectively. Specificity and sensitivity of HRM method were 90% and 100%, with respect to PCR-RFLP. Also, HRM analysis has been evaluated as a faster and more effective approach.
Conclusion:
Comparison of our results based on HRM analysis with PCR-RFLP showed that our developed method is rapid, accurate, fast and economic to study the CYP2C19*17 allele and it is appropriate for other similar population genetic studies.
Keywords: Cytochrome P-450 CYP2C19, Pharmacogenetics, Real-Time Polymerase Chain Reaction
Introduction
Different types of human body responses to a drug may be due to genetic discrepancy between the individuals 1. Single Nucleotide Polymorphisms (SNPs) are bases for pair mutations, some of which have clinical outcomes associated with development, health or metabolism of drugs. Genetic polymorphisms of drug metabolism by cytochrome P450 (P450s) could affect body responses to drug, attracting particular interest in pharmacogenetics 2–4. Cytochrome P450 is a large family of hemoproteins that catalyze the metabolism of a large number of xenobiotics and endobiotics 5. The function of these enzymes is to insert a single oxygen atom derived from molecular oxygen into the broad structure of very large organic compounds. The final product usually contains a hydroxylated water-soluble and polar derivative relevant to the substrate 6. Mutations of P450 genes can result in a variety of changes in the enzymes with high to low activity or even inactive enzyme that can affect the efficacy and toxicity of drugs. Polymorphisms of population in pharmacogenetics are divided into the phenotypes as poor metabolizer (PM), intermediate metabolizer (IM), Extensive Metabolizer (EM), and ultra-rapid metabolizer (UM) 7,8.
Since some P450s (CYP2C9, CYP2C19, CYP2D6, CYP3A5) are highly polymorphic, screening for identifying the mutations which leads to reduction or even abolition of drug metabolizing capacity can help to identify drug metabolizing phenotypes 9,10. In humans, 30 CYP enzymes are known as responsible for the metabolism of drugs and they comprise 1 to 4 groups. The members of CYP2 group and CYP2C subgroup include CYP2C8, CYP2C9, CYP2C19, CYP2C18 and the last three of them are important in treatment 11. CYP2C19 enzyme is essentially found in the liver while having a significant activity in the lining of the gastrointestinal tract by metabolizing of many drugs. CYP2C19 plays an important role in the metabolism of endogenous compounds such as arachidonic, estradiol and arachidonic acid and also some usual substrates including benzodiazepine diazepam, omeprazole, a proton pump inhibitor, and antidepressant amitriptyline. It is also responsible for metabolism of beta-adrenergic receptor blockers such as propranolol 11,12. In addition to metabolizing drugs and developing inactive metabolites, it is also activated in the activation process of some effective medications, including the conversion of proguanil anti-malaria drug into cycloguanil 13, conversion of tamoxifen to the two active metabolites including 4-hydroxy tamoxifen and 4-hydroxy methyl-N- dose tamoxifen 14 and the conversion of clopidogrel at first to its active form and then into 2-oxoclopidogrel 15,16. The reliable and fast genotyping methods for the detection of clinically relevant single nucleotide polymorphisms are intensely required, so identification of SNPs can be applied to select more reliable drugs for patients. Gene sequencing is the gold standard technique for diagnosis of mutations; however, it is time consuming and also requires intensive labor. Melting curve analysis of High Resolution Melting (HRM) is a powerful and affordable technique having many advantages especially in comparison to genotyping and sequencing analysis of SNP hydrolysis (TaqMan) 17 and RFLP. This method is fast and powerful; therefore, it is able to accurately and quickly determine the genotype of many samples and it can also be easily used by any unskillful laboratory staff with access to Real-Time PCR device 18.
In 2006, CYP2C19* 17 allele was detected with its superfast ability to metabolize drugs. Allele CYP2C19* 17 was identified by two SNPs in the promoter region. Cytosine is converted to thymine nucleotide in the -3402 or -806 region (C>T), where increases the CYP2C19 gene expression 19. The activity of the CYP2C19 enzyme has not been evenly distributed and polymorphism of the gene encoding this enzyme has been viewed. So far, at least 24 variants of this enzyme have been identified 20.
Polymerase Chain Reaction (PCR) is carried out to amplify a region of DNA before HRM analysis in which the mutation is considered 21,22. HRM process is simply a warming process in the presence of the color attached to the DNA that could distinguish the double strands of DNA from a single strand DNA. Mutation changes the temperature so the DNA strands get separated (melting) and therefore the wildtype and mutant DNA can be accurately separated. HRM procedure is based on Real time- PCR method in which the differences of the sequences in the nucleic acid are detected using the differences in melting curve and various fluorescent dyes that bind to double-stranded DNA, and they don’t lead to inhabitation of PCR at high concentrations 23,24. Melting curve is based on the fluorescent color attached to nucleic acids and their separation during the denaturing of double-stranded DNA is detectable by Real time- PCR 25. The main aim of this study was to determine the allele CYP2C19* 17 occurrence in healthy Iranian volunteers by HRM method and to critically compare this method with RFLP in terms of simplicity, cost and laboratory materials needed for analysis.
Materials and Methods
Subjects and DNA extraction
This was an experimental study that aimed to determine the frequency of -3402C> T or CYP2C19* 17 allele polymorphisms among 100 healthy volunteers of Iranian population by two HRM and PCR-RFLP methods. Informed written consent was taken from each participant and a specific questionnaire was filled out by them. Blood samples were collected using 5 ml syringe. Genomic DNA was extracted from white blood cells by salting out method 26.
Two different techniques (PCR-RFLP and HRM) were used in this study for CYP2C19* 17 genotyping. Primers for HRM were designed by Gene Runner software (version 3.05, 1994, Hastings Software Inc.) and their specificity for PCR was checked by nucleotide BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The primers used for RFLP method were received from Ragia G study 27. Sequences of the primers are demonstrated in table 1.
Table 1.
Sequences of primers for amplification of CYP2C19* 17 allele by RFLP and HRM methods
| Technique | Primer name | Sequence | Length (bp) | Tm |
|---|---|---|---|---|
| HRM | ||||
| CYP2C19*17-F | 5- TGACAAGACACAGACTGGGA -3 | 20 | 58.2 | |
| CYP2C19*17-R | 5- CGGGGTTTTAGCTTGCAGTT -3 | 20 | 59 | |
| PCR-RFLP | ||||
| 2C19-17-F | 5-TACAATGAAGGCACAATC-3 | 18 | 50 | |
| 2C19-17-R | 5-CAAGGAGCACAACTACTAGATA-3 | 22 | 54.7 | |
PCR-RFLP
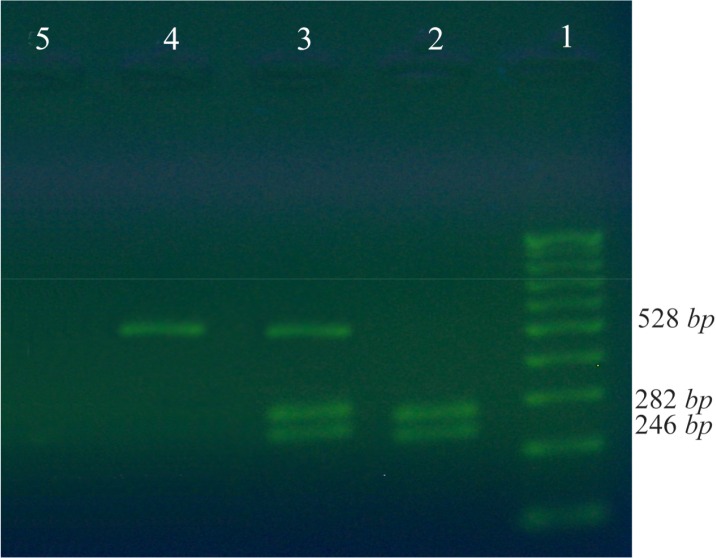
For PCR-RFLP analysis, PCR reactions were performed in 25 μl total volume by CYP2C19*17-F and CYP2C19*17-R primers for 100 samples. The reaction mixture contained 10 pM of each primer, PCR Master Mix containing 1 unit of Taq DNA polymerase, 0.2 mM of each of dNTPs, 1.5 mM of MgCl2 and 100 ng of DNA as template. All PCR reaction components were obtained from Fermentas Company. The amplification program was as follows: initial denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 94°C for 10 s, annealing at 55°C for 30 s and extension at 72°C for 1 min and an additional final extension at 72°C for 10 min. PCR products were analyzed by 1% agarose gel electrophoresis. Next, 10 μl of each PCR product was added to 20 μl of the restriction master mix which was composed of 2 μl of 10×buffer, 0.5 μl MnlI restriction enzyme that cuts the C allele of CYP2C19* and 18.5 μl of H2O. Digestion mixture was incubated at 37°C overnight and the digested products were analyzed by 2% agarose gel electrophoresis. By amplification, a 528 bp fragment was amplified and after digestion, two fragments with 282 bp and 246 bp lengths were produced. Finally, different detected genotypes (normal, mutant and heterozygote genotypes) were sequenced for confirmation and those were used as reference genotypes for HRM analysis in the next steps.
High-resolution melting curve PCR analysis
HRM experiments were performed by specific amplification of a 225 bp fragment with HRM-2C19-17-F and 2C19-17-R primers. HRM curve acquisition and analysis were performed on Rotor-Gene 6000 (Corbett, Australia). Reaction mixture contained 10 μl of 2× HRM Master Mix (Qiagen), 10 pM of each primer, and 50 ng of template DNA in final volume of 20 μl. PCR cycling parameters for real-time PCR and HRM curve acquisition were followed by one cycle of initial denaturation at 95°C for 5 min, 45 cycles of 95°C for 10 s, and 58°C for 50 s. For HRM preparation step, PCR products were heated at 72°C for 2 min and then cooled at 50°C for 1 min. HRM was performed by raising the temperature from 60°C to 90°C, with an increment of 0.1°C/s, in order to obtain the melting profile data in the next experiments. Initially, for HRM analysis, melting plots were normalized by adapting the start and end fluorescence signals of all samples to the same level. Then, normalized HRM data were subjected to gene scanning analysis to identify the temperature shift changes in melting curves, which indicate the presence of variation in target sequence.
Results
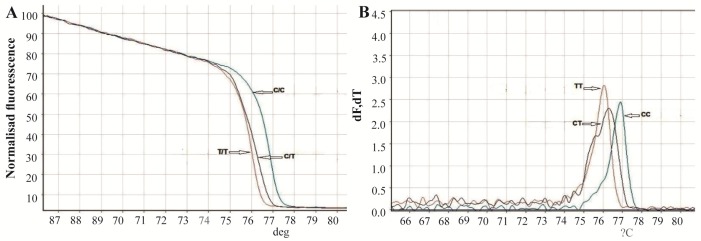
Forty six percent of all participants were men and 54% were women. Their age ranged from 19 to 57 years old. Because of the ethnic diversity in the country, the people were selected from different ethnics groups. All samples were successfully genotyped by HRM curve analysis and some results were approved by direct sequencing of PCR products. The resulting sequences were analyzed using BLAST and Chromas lite softwares and submitted to the GeneBank database (Accession numbers: KC689787, KC689788, KC-689786, KC677614). Melting of the PCR products was monitored by plotting the changes in fluorescence that occurred by gradual temperature-dependent releasing of a saturating double-strand DNA binding dye. Heterozygous DNA samples formed heteroduplexes, resulting in a different shape of the melting curve compared with a homozygous sample. Different genotypes of homozygous DNA samples, in contrast, were detected by a melting temperature (Tm) shift rather than an altered curve shape (Figure 1).
Figure 1.
HRM analysis of CYP2C19*17 allele. Results were analyzed in the normalized fluorescence versus temperature plot. A indicates the normalized plot and B the melting plot. Wild-type alleles consisted of a C nucleotide at position -3402 leading to amplicons with higher melting temperature (77°C), while the mutant alleles had T nucleotide, leading to amplicons with lower melting temperature (76.5°C). Heterozygous which contain both ingredient alleles, are heterodublex with different forms (B). Differentials in fluorescence and temperature between different profiles of samples are displayed in A after normalization. Each variant has a different melting profile.
The frequency of CYP2C19*1/*1(CC), CYP2C19*1/*17 (CT) and CYP2C19*17/*17 genotypes in all samples were 58.33%, 29.1% and 11.1%, respectively. In the present study, the allele frequency of CYP2C19*17 was 0.26 (Table 2). Figure 2 shows the agarose gel electrophoresis of PCR-RFLP results. In this study, RFLP was considered as the reference test to calculate the relative specificity and relative sensitivity of HRM method. Discrepant results were genotyped by sequencing method as a gold standard for further confirmation. In HRM results in comparison with RFLP (as the reference method), there were three false positives and no false negatives results; however, specificity and sensitivity of HRM method were 90% and 100%, respectively in comparison to RFLP.
Table 2.
Allele and genotype frequency of CYP2C19*17 determined by HRM
| Gene | Genotype | Frequency (%) | Allele | Frequency (%) |
|---|---|---|---|---|
| CYP2C19 | CC (*1/*1) | 59 | -3402C (wild) | 148 (74) |
| CT (*1/*17) | 30 | -3402T (variant) | 52 (26) | |
| TT (*17/*17) | 11 | |||
Figure 2.
Restricted amplified fragments by Mnl1 enzyme. MnlI enzyme can digest the PCR products in at least 12 hr at the temperature of 37°C and this enzymatic activity can be stopped by incubation for 20 min at a temperature of 65°C. Variant -3402T, or allele CYP2C19*17 was resistant to MnlI digestion, therefore 528 bp fragment remained intact and it did not break, while the CYP2C19*1 allele was broken into two pieces of 246 and 282 bp. The digested products were separated on 2% agarose gel and the components were visible by Syber Green. 1 indicates the 100 bp DNA ladder; 2 is a wild type genotype (CC); 3 is a heterozygote CT and 4 is TT genotype; 5 is a negative control.
To calculate equilibrium of alleles in the population, Hardy-Weinberg equation and SPSS software were used. Results showed that the population did not follow the Hardy-Weinberg’s equilibrium.
Discussion
HRM PCR analysis of amplicons in real-time PCR provides the possibility to scan the genotypes of volunteers for CYP2C19*17 (Figure 1). In order to analyze the HRM, new primers were designed for production of shorter PCR products to distinguish the possible allele for a pair of nucleotides. Primers were tested to measure HRM analysis, and a reliable screening of volunteers with good sensitivity and without any interference between different CYP2C19 genotypes was conducted. The wild-type alleles consisted of a nucleotide C at -3402 situation so it generated a higher melting temperature amplicon (77°C), while the variant alleles including T nucleotide resulted in mutant with lower melting temperature (76.5°C). Heterozygotes included both; therefore, they had different effects. Accurate genotyping by HRM software brought different clusters with different melting temperatures and up to 0.1°C, the difference between the melting temperatures of amplicons and different genotypes was detected. In our study, the HRM method showed sensitivity just the same as RFLP but with slightly lower specificity in comparison with RFLP.
Frequency of CYP2C19*17 allele was higher in Iranian population in comparison with other studies (Table 3) 28–32. This study showed that a significant percentage of Iranian population (26%) had CYP2C19*17 allele; therefore, they can metabolize drugs that are metabolized by CYP2C19 enzyme much faster, so the drug concentration decreases in the blood and its therapeutic efficacy diminishes.
Table 3.
Comparison of CYP2C19*17 allele frequency among the population of various countries
| Population (Country) | Allele Frequency (%) CYP2C19*1 | Allele Frequency (%) CYP2C19*17 | References |
|---|---|---|---|
| Iran | 72.88 % | 25.65 % | Current study |
| China | 69.7 % | 1.2 % | 28 |
| Japan | 57.9 % | 1.3 % | 29 |
| Saudi Arabia | 73.1 % | 73.9 % | 30 |
| Mexico | 77.1 % | 14.29 % | 31 |
| South Korea | -- | 1.15 % | 32 |
Overall, the genotype of CYP2C19 focusing on the SNP at position -3402 was successfully determined by both PLFR test and HRM analysis. Considering the costs, tools, runtime and simplicity of evaluation of both methods, the HRM analysis can be considered as a more cost-benefit method. Additionally, the overall execution time of analysis related to CYP2C19 genotype by HRM was much less than PLFR. It seems that the assessment of melting curve analysis by precise software to distinguish the alleles is easier than the technique that requires strict separation of fluorescent signals between wild-type and variant probes.
In some studies, HRM technique was compared with other mutation detecting and genotyping methods. Zhang et al compared HRM and TaqMan procedures and showed that the results, precision, sensitivity, and specificity of both methods were the same and excellent. HRM has a comparative advantage to TaqMan and it is the ability to identify the undetermined mutations. The two methods were highly matched and the costs were almost the same, but the cost of applied probes in TaqMan method was more than HRM 33.
HRM with no need for labeled primers or labeled probes was used for the detection of the most common nonfunctional alleles of cytochrome P-450 (CYP) 2D6 in the Caucasian population that affect the metabolism of many commonly used drugs 34.
HRM was used to characterize the CYP2C8 polymorphism in Taiwanese population. Nine exons of the CYP2C8 gene were screened by HRM analysis. It is a fast, reliable, accurate and cost-benefit screening method for gene mutations, even with very similar cDNA sequences with 83% identities compared to CYP2C8 and CYP2C9 35.
SNPs of VKORC (1173T/C, rs9934438) and CYP2C9 (1075A/C, rs1057910) are major contributory factors in sensitivity of warfarin in Chinese population. Two genomic loci could prevent from bleeding or thrombosis events in warfarin treatment of individuals. HRM with the advantages of simliplicity, speed, high sensitivity and low cost was used as a diagnostic assay for genotyping rs9934438 and rs1057910 36. Cheng et al demonstrated that formation of hetero-duplex requires full recognition of hemi/homozygote of all genes and implied that HRM analysis is a fast, sensitive and selective method for screening hemi/homo-zygous 37.
SNPs of diagnostic markers were important for the detection and differentiation of Bacillus anthracis (B. anthracis) while Derzelle et al quickly detected all B. anthracis using HRM and shift-Tm (Melting Temperature shift) without any expensive labeled probes 38.
Mutations in the CDKL5 gene that is mainly seen in women with epileptic are detected by costly, time-consuming and difficult methods. However, Laure Raymond et al compared HRM and denatured High Performance Liquid Chromatography (dHPLC) methods for scanning this gene and indicated that HRM detected point mutations, small deletion and insertions faster and more sensitive than dHPLC. HRM is an easy, affordable, commissioning, sensitive, harmless and rapid mutation screening method to identify mutations in large heterogeneous genes on a general term like gene coding sequence of CDKL5 39. In another study, Yi-Ching Lin et al successfully detected eleven common mutations of gene CYP21A2 by HRM method using 6 amplicons. So, this method was selected as the best for screening CAH (congenital adrenal hyperplasia) an autosomal recessive disease 40,41.
Vorkas PA et al applied the combination of Real-Time and HRM curve analysis as a successful, fast, simple and cost benefit method to detect 20 exons of BRCA gene for the clinical mutations 42. Manikandan M et al demonstrated that HRM analysis is an effective tool with high specificity for knockout studies of miR-TS-SNP deletion mutations 43.
Naing et al investigated the Ehlers-Danlos syndrome, which is a dominant autosome disorder of vascular type developed by mutations in procollagen type 3 (Col3A1) through HRM for screening mutations after the PCR as a highly sensitive screening tool for mutations in large genes, in combination with the small loop genotyping method (SAG) of genomic DNA as a rapid diagnosis method for Col3A1 gene mutation with high specificity 44.
HRM was also compared with phylogenetic analysis and findings showed that it was fast and affordable. The classification of fowl adeno viruses (FAdv) was carefully carried out with high sensitivity and speed 45.
High sensitivity and specificity of HRM compared with the results of probe hybridization method for usual genotyping single nucleotide polymorphisms (C677T) of MTHFR gene implied that HRM is a quick and affordable method. Many methods have been introduced for genotyping MTHFR genes such as RFLP, real-time PCR with a fluorescent probe, Tagman, Mini Sequencing and mass spectrometry, but HRM have special advantages. Allelic-specific primers or probes are not required for specific targets. Only two primers are required to identify the allelic transitions. In addition to the use of short amplicons for maximum difference of melting temperature (TM) between wild-type and mutant homozygotes, better results were obtained with shorter amplicons. Comparison of the costs and time of implementation showed that HRM with the high success rate and compliance and shorter amplicons is the best method for genotyping SNPs of (C677T) RFHTM gene. HRM implementation time was about an hour that is lower than probe hybridization. But if the amount and quality of the starting DNA is low, then success rate will be less in HRM. A standard amount of DNA is required for the HRM method. HRM is also an effective technique that can be used for identification of mutations in known and unknown genes 46.
The excellence of HRM over SSGE (sensitive structure gel electrophoresis method) for screening the mutations of BRCA genes was evaluated. The mutation spectrum with HRM technique had a higher sensitivity compared to SSGE. Also, HRM was faster and more sensitive and specific 47.
Cai et al showed that all SNPs cannot be determined by short amplicons particularly SNPs Class 3 and 4, since a small change is observed between the two homozygous and their TM. Therefore, ASE-HRM method was used with the primer ASE (Allele Specific Extension) by adding the heat stages to primer ASE that is an easy and sensitive method for genotyping in the closed pipe, and it can check out all kinds of SNPs 48.
Conclusion
In the near future, pharmacogenetics will play an important role in determining the dosage of drugs used for the treatment of the patients and the treatment process will be individualized and adverse effects or failures of drugs will be minimized. According to the perceived high frequency of the allele in Iranian population, further clinical studies on this allele are essential. In the Iranian population considering the larger study population as well as other drugs that are metabolized by CYP2C19, it would be beneficial to evaluate clinical aspects of allele CYP2C19*17. Different characteristics such as GC content, length, sequence or heterozygosity due to differences in melting curve are identified by HRM technique and the results can be used to screen mutations, genotyping, methylation and other applied researches. In some methods such as screening for mutations and SNPs genotyping, the nucleotide difference leads to differences in melting curve, although in point mutations and SNP, the content of this difference is minor, and the temperature difference changes the nucleotide G/T, G/A, C/T and C/A between 0.5 and 1°C and C/G at 0.2 to 0.5°C and T/A at less than 0.2°C. The HRM curve analysis is sensitive and can be used to screen mutations in several parallel reactions, but depends on good PCR, instruments and dyes.
Acknowledgement
We thank the research deputy of Zanjan University of Medical Sciences (ZUMS) for financial support of this project.
Footnotes
Conflict of Interest
The authors declare no conflict of interests.
References
- 1. Meyer UA. Pharmacogenetics-five decades of therapeutic lessons from genetic diversity. Nat Rev Genet 2004; 5 (9): 669– 676. [DOI] [PubMed] [Google Scholar]
- 2. Solus JF, Arietta BJ, Harris JR, Sexton DP, Steward JQ, McMunn C, et al. Genetic variation in eleven phase I drug metabolism genes in an ethnically diverse population. Pharmacogenomics 2004; 5 (7): 895– 931. [DOI] [PubMed] [Google Scholar]
- 3. Tamási V , Vereczkey L , Falus A , Monostory K. Some aspects of interindividual variations in the metabolism of xenobiotics. Inflamm Res 2003; 52 (8): 322– 333. [DOI] [PubMed] [Google Scholar]
- 4. Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, et al. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and anti-depressants. Clin Pharmacol Ther 2006; 79 (1): 103– 113. [DOI] [PubMed] [Google Scholar]
- 5. Wrighton SA, Stevens JC. The human hepatic cytochromes P450 involved in drug metabolism. Crit Rev Toxicol 1992; 22 (1): 1– 21. [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene 2006; 25 (11): 1679– 1691. [DOI] [PubMed] [Google Scholar]
- 7. Ramazani A, Ghaffari M, Fakhri A. Genetic polymorphisms of CYP2C8 in a healthy Iranian population. Br J Med Med Res 2014; 4 (10): 2081– 2088. [Google Scholar]
- 8. Samimifar MS, Ramazani A. Frequency of two genetic polymorphisms of CYP1A2 gene in Iranian population. Annu Res Rev Biol 2014; 4 (11): 1769– 1776. [Google Scholar]
- 9. Ingelman-Sundberg M. Pharmacogenetics: an opportunity for a safer and more efficient pharmacotherapy. J Intern Med 2001; 250 (3): 186– 200. [DOI] [PubMed] [Google Scholar]
- 10. Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J 2005; 5 (1): 6– 13. [DOI] [PubMed] [Google Scholar]
- 11. Bibi Z. Role of cytochrome P450 in drug interactions. Nutr Metab (Lond) 2008; 5: 27. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Honig PK, Woosley RL, Zamani K, Conner DP, Cantilena LR. Changes in the pharmacokinetics and electrocardiographic pharmacodynamics of terfenadine with concomitant administration of erythromycin. Clin Pharmacol Ther 1992; 52 (3): 231– 238. [DOI] [PubMed] [Google Scholar]
- 13. Ward SA, Helsby NA, Skjelbo E, Brøsen K, Gram LF, Breckenridge AM. The activation of the biguanide anti-malarial proguanil co-segregates with the mephenytoin oxidation polymorphism--a panel study. Br J Clin Pharmacol 1991; 31 (6): 689– 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast cancer Res Treat 2004; 85 (2): 151– 159. [DOI] [PubMed] [Google Scholar]
- 15. Savi P, Herbert JM, Pflieger AM, Dol F, Delebassee D, Combalbert J, et al. Importance of hepatic metabolism in the antiaggregating activity of the thienopyridine clopidogrel. Biochem Pharmacol 1992; 44 (3): 527– 532. [DOI] [PubMed] [Google Scholar]
- 16. Clarke TA, Waskell LA. The metabolism of clopidogrel is catalyzed by human cytochrome P450 3A and is inhibited by atorvastatin. Drug Metab Dispos 2003; 31 (1): 53– 59. [DOI] [PubMed] [Google Scholar]
- 17. Temesvári M , Paulik J , Kóbori L , Monostory K. High-resolution melting curve analysis to establish CYP2C19*2 single nucleotide polymorphism: Comparison with hydrolysis SNP analysis. Mol Cell Probes 2011; 25 (2–3): 130– 133. [DOI] [PubMed] [Google Scholar]
- 18. Wittwer CT, Reed GH, Gundry CN, Vandersteen JG, Pryor RJ. High-resolution genotyping by amplicon melting analysis using LCGreen. Clin Chem 2003; 49 (6 Pt 1): 853– 860. [DOI] [PubMed] [Google Scholar]
- 19. Desta Z, Zhao X, Shin JG, Flockhart DA. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 2002; 41 (12): 913– 958. [DOI] [PubMed] [Google Scholar]
- 20. Sistonen J, Sajantila A, Lao O, Corander J, Barbujani G, Fuselli S. CYP2D6 worldwide genetic variation shows high frequency of altered activity variants and no continental structure. Pharmacogenet Genomics 2007; 17 (2): 93– 101. [DOI] [PubMed] [Google Scholar]
- 21. Izadi A, Moslemi E, Tabatabaei Panah A, Kheiri Manjili H. Brucella spp. detection in dairy products using nested and hemi nested PCR techniques. Ann Biol Res 2014; 5 (1): 124– 131. [Google Scholar]
- 22. Krypuy M, Newnham GM, Thomas DM, Conron M, Dobrovic A. High resolution melting analysis for the rapid and sensitive detection of mutations in clinical samples: KRAS codon 12 and 13 mutations in non-small cell lung cancer. BMC Cancer 2006; 6: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Izadi A, Moslemi E, Poorhosseini SM, Yassaee VR, Kheiri HR, Elikai HR. UBD identify in paraffin tissues in patients with colorectal cancer. J Isfahan Med School 2014; 32 (291): 1– 10. [Google Scholar]
- 24. Robertson T, Bibby S, O’Rourke D, Belfiore T, Lambie H, Noormohammadi A. Characterization of Chlamydiaceae species using PCR and high resolution melt curve analysis of the 16S rRNA gene. J Appl Microbiol 2009; 107 (6): 2017– 2028. [DOI] [PubMed] [Google Scholar]
- 25. Reed GH, Kent JO, Wittwer CT. High-resolution DNA melting analysis for simple and efficient molecular diagnostics. Pharmacogenomics 2007; 8 (6): 597– 608. [DOI] [PubMed] [Google Scholar]
- 26. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16 (3): 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ragia G, Arvanitidis KI, Tavridou A, Manolopoulos VG. Need for reassessment of reported CYP2C19 allele frequencies in various populations in view of CYP2C19*17 discovery: the case of Greece. Pharmacogenomics 2009; 10 (1): 43– 49. [DOI] [PubMed] [Google Scholar]
- 28. Arvanitidis K, Ragia G, Iordanidou M, Kyriaki S, Tavridou A, Manolopoulos VG. Genetic polymorphisms of drug-metabolizing enzymes CYP2D6, CYP2C9, CYP2C19 and CYP3A5 in the Greek population. Fundam Clin Pharmacol 2007; 21 (4): 419– 426. [DOI] [PubMed] [Google Scholar]
- 29. Sugimoto K, Uno T, Yamazaki H, Tateishi T. Limited frequency of the CYP2C19*17 allele and its minor role in a Japanese population. Br J Clin Pharmacol 2008; 65 (3): 437– 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al-Jenoobi FI, Alkharfy KM, Alghamdi AM, Bagulb KM, Al-Mohizea AM, Al-Muhsen S, et al. CYP2C19 genetic polymorphism in Saudi Arabians. Basic Clin Pharmacol Toxicol 2013; 112 (1): 50– 54. [DOI] [PubMed] [Google Scholar]
- 31. Favela-Mendoza AF, Martinez-Cortes G, Hernandez-Zaragoza M, Salazar-Flores J, Muñoz-Valle JF, Martinez-Sevilla VM, et al. Genetic variability of CYP2C19 in a Mexican population: contribution to the knowledge of the inheritance pattern of CYP2C19*17 to develop the ultrarapid metabolizer phenotype. J Genet 2015; 94 (1): 3– 7. [DOI] [PubMed] [Google Scholar]
- 32. Kim KA, Song WK, Kim KR, Park JY. Assessment of CYP2C19 genetic polymorphisms in a Korean population using a simultaneous multiplex pyrosequencing method to simultaneously detect the CYP2C19*2, CYP2C19*3, and CYP2C19*17 alleles. J Clin Pharm Ther 2010; 35 (6): 697– 703. [DOI] [PubMed] [Google Scholar]
- 33. Zhang L, Cui G, Li Z, Wang H, Ding H, Wang DW. Comparison of high-resolution melting analysis, TaqMan allelic discrimination assay, and Sanger sequencing for clopidogrel efficacy genotyping in routine molecular diagnostics. J Mol Diagn 2013; 15 (5): 600– 606. [DOI] [PubMed] [Google Scholar]
- 34. Pindurová E , Zourková A , Zrůstová J , Juřica J , Pavelka A. Alternative reliable method for cytochrome P450 2D6 poor metabolizers genotyping. Mol Biotechnol 2013; 53 (1): 29– 40. [DOI] [PubMed] [Google Scholar]
- 35. Chang CC, Lin PC, Lin CH, Yeh KT, Hung HY, Chang JG. Rapid identification of CYP2C8 polymorphisms by high resolution melting analysis. Clin Chim Acta 2012; 413 (1–2): 298– 302. [DOI] [PubMed] [Google Scholar]
- 36. Chen C, Li S, Lu X, Tan B, Huang C, Qin L. High resolution melting method to detect single nucleotide polymorphism of VKORC1 and CYP2C9. Int J Clin Exp Pathol 2014; 7 (5): 2558– 2564. [PMC free article] [PubMed] [Google Scholar]
- 37. Cheng J, Yim OS, Low PS, Tay SK, Yap EP, Lai PS. Detection of hemi/homozygotes through heteroduplex formation in high-resolution melting analysis. Anal Biochem 2011; 410 (1): 158– 160. [DOI] [PubMed] [Google Scholar]
- 38. Derzelle S, Mendy C, Laroche S, Madani N. Use of high-resolution melting and melting temperature-shift assays for specific detection and identification of Bacillus anthracis based on single nucleotide discrimination. J Microbiol Methods 2011; 87 (2): 195– 201. [DOI] [PubMed] [Google Scholar]
- 39. Millat G, Chanavat V, Julia S, Crehalet H, Bouvagnet P, Rousson R. Validation of high-resolution DNA melting analysis for mutation scanning of the LMNA gene. Clin Biochem 2009; 42 (9): 892– 898. [DOI] [PubMed] [Google Scholar]
- 40. Lin YC, Lin YC, Liu TC, Chang JG, Lee HH. High-resolution melting curve (HRM) analysis to establish CYP21A2 mutations converted from the CYP21A1P in congenital adrenal hyperplasia. Clin Chim Acta 2011; 412 (21–22): 1918– 1923. [DOI] [PubMed] [Google Scholar]
- 41. Ramazani A, Kahrizi K, Razaghiazar M, Mahdieh N, Koppens P. The frequency of eight common point mutations in CYP21 gene in Iranian patients with congenital adrenal hyperplasia. Iran Biomed J 2008; 12 (1): 49– 53. [PubMed] [Google Scholar]
- 42. Vorkas PA, Christopoulos K, Kroupis C, Lianidou ES. Mutation scanning of exon 20 of the BRCA1 gene by high-resolution melting curve analysis. Clin Biochem 2010; 43 (1–2): 178– 185. [DOI] [PubMed] [Google Scholar]
- 43. Manikandan M, Raksha G, Munirajan AK. Haploinsufficiency of tumor suppressor genes is driven by the cumulative effect of microRNAs, microRNA binding site polymorphisms and microRNA polymorphisms: An in silico approach. Cancer Inform 2012; 11: 157– 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Naing BT, Watanabe A, Shimada T. A novel mutation screening system for Ehlers-Danlos Syndrome, vascular type by high-resolution melting curve analysis in combination with small amplicon genotyping using genomic DNA. Biochem Biophys Res Commun 2011; 405 (3): 368– 372. [DOI] [PubMed] [Google Scholar]
- 45. Marek A, Günes A, Schulz E, Hess M. Classification of fowl adenoviruses by use of phylogenetic analysis and high-resolution melting-curve analysis of the hexon L1 gene region. J Virol Methods 2010; 170 (1–2): 147– 154. [DOI] [PubMed] [Google Scholar]
- 46. Sinthuwiwat T, Poowasanpetch P, Wongngamrungroj A, Promso S, Auewarakul C, Mooney S, et al. High-resolution melting curve analysis for genotyping of common SNP in MTHFR gene using fixed-cell suspension. Mol Cell Probes 2008; 22 (5–6): 329– 332. [DOI] [PubMed] [Google Scholar]
- 47. de Juan Jiménez I , Cardeñosa EE , Suela SP , González EB , Trejo DS , Lluch OF, et al. Advantage of high-resolution melting curve analysis over conformation-sensitive gel electrophoresis for mutational screening of BRCA1 and BRCA2 genes. Clin Chim Acta 2011; 412 (7–8): 578– 582. [DOI] [PubMed] [Google Scholar]
- 48. Cai Y, Yuan Y, Lin Q, Chan P. Allele-specific extension allows base-pair neutral homozygotes to be discriminated by high-resolution melting of small amplicons. Anal Biochem 2010; 406: 29– 33. [DOI] [PubMed] [Google Scholar]